Abstract
Laminarinases exhibit potential in a wide range of industrial applications including the production of biofuels and pharmaceuticals. In this study, we present the genetic and biochemical characteristics of FLamA and FLamB, two laminarinases derived from a metagenomic sample from a hot spring in the Azores. Sequence comparison revealed that both genes had high similarities to genes from Fervidobacterium nodosum Rt17-B1. The two proteins showed sequence similarities of 62% to each other and belong to the glycoside hydrolase (GH) family 16. For biochemical characterization, both laminarinases were heterologously produced in Escherichia coli and purified to homogeneity. FLamA and FLamB exhibited similar properties and both showed highest activity towards laminarin at 90 °C and pH 6.5. The two enzymes were thermostable but differed in their half-life at 80 °C with 5 h and 1 h for FLamA and FLamB, respectively. In contrast to other laminarinases, both enzymes prefer β-1,3-glucans and mixed-linked glucans as substrates. However, FLamA and FLamB differ in their catalytic efficiency towards laminarin. Structure predictions were made and showed minor differences particularly in a kink adjacent to the active site cleft. The high specific activities and resistance to elevated temperatures and various additives make both enzymes suitable candidates for application in biomass conversion.
1. Introduction
β-1,3-Glucans are non-cellulosic carbohydrates that are widespread in nature. They can be found in the cell walls of fungi (pachyman), in reproductive structures of plants (callose) or as exopolysaccharide from bacteria (curdlan) [1]. β-1,3-Glycosidic linkages are also present in mixed-linked β-glucans from cereals (e.g., barley) or lichens [2]. Moreover, β-1,3-glucans are one of the most abundant carbohydrates in marine ecosystems [3]. In micro- and macroalgae, β-1,3-glucans are structurally diverse and serve as storage glucans. In brown algae, the β-1,3-glucan is named laminarin and represents up to 25% of the dry weight, depending on species, season, and growing conditions [4]. Due to the significantly higher production yields than terrestrial biomass, a high carbohydrate content, and the lack of hemicellulose and lignin, macroalgae biomass is a promising feedstock for new biorefinery concepts [5,6]. For industrial utilization of this feedstock, robust and efficient enzymes like laminarinases are required [7]. Furthermore, laminarinases could be applied for yeast extract production, as a biocontrol agent against fungal plant pathogens [8], and for partial hydrolysis of β-1,3-glucans for the production of antiviral and antitumor therapeutics [9].
For the complete enzymatic hydrolysis of β-1,3-glucans endo-acting β-1,3-glucanases (EC 3.2.1.39) and β-1,3(4)-glucanases (EC 3.2.1.6), both known as laminarinases, exo-β-1,3-glucosidases (EC 3.2.1.58) are required. These enzymes and their corresponding substrates have in common that they both are widely distributed among plants, fungi, and bacteria from many different habitats. On the basis of amino acid sequence similarities, all endo-acting laminarinases from plants can be assigned to the glycoside hydrolase family GH 17, whereas most of the bacterial laminarinases belong to GH 16 [10]. According to the Carbohydrate-Active Enzymes database (CAZy), around 40 β-1,3-glucanases from bacteria are already characterized (August 2019). Nevertheless, laminarinases with high stability at varying conditions and temperatures are desired for industrial applications [11]. To obtain highly stable and efficient enzymes for industrial application, thermophilic organisms represent an excellent resource. Moreover, these enzymes allow reactions at elevated process temperatures, which do not only reduce microbial contamination, but also increase the solubility and diffusion rates of the catalysts for complex polymeric substrates [12].
In this study, an environmental sample from an Azorean hot spring (São Miguel, Portugal) was used as a source for novel laminarinase-encoding genes. Recently, this environmental sample has been proven to be an excellent source for unique thermo-active enzymes [13]. By sequence-based screening, two putative genes were identified with sequence similarities to parts of the genomic sequence of the thermophilic bacterium Fervidobacterium nodosum Rt17-B1, which was completely sequenced in 2007 [14]. Fervidobacterium nodosum which was isolated from a hot spring in New Zealand, is able to ferment a wide range of carbohydrates [15] and is considered as a good source for novel carbohydrate degrading enzymes. So far, only one highly active and thermostable cellulase from this bacterium has been characterized [16].
Here, we describe the recombinant production and purification of the two thermoactive laminarinases FLamA and FLamB originating from Fervidobacterium sp. To estimate their potential relevance for industrial applications, both recombinant enzymes were characterized in detail.
2. Results
2.1. Sequence Analysis of FLamA and FLamB
By sequence-based screening, two new putative laminarinase encoding genes were identified. Metagenomic DNA from a hot spring of the Azores, which was known to contain genomic DNA of a Fervidobacterium strain, was used as template DNA for PCR. The primers for the amplification of the two genes encoding for FLamA and FLamB were based on two putative laminarinase genes of the complete genome sequence of Fervidobacterium nodosum Rt17-B1 (GenBank: CP000771). The amplified DNA fragment for flamA showed 99% sequence similarity to putative laminarinase genes from the genomes of F. nodosum Rt17-B1 and F. pennivorans DSM 9078 (GenBank: NC_017095). The corresponding amino acid sequence is annotated as a multispecies endo-β-1,3-glucanase found in various Fervidobacterium species (GenBank: WP_011994743). In comparison to that, the amplified DNA fragment for flamB showed 95% and 67% sequence similarity to GenBank sequences of two other putative laminarinase genes from F. nodosum Rt17-B1 and F. pennivorans DSM 9078, respectively. Due to the 99% similarity of the amino acid sequence to next hits in the database, flamB was annotated in GenBank with the accession number LT882624.
In the genome of F. nodosum Rt17-B1, the highly similar genes of flamA and flamB are embedded in two different operons. By comparing these operons, we identified putative open reading frames for proteins with the same predicted functions (Figure 1). Among both gene clusters, the related proteins revealed high sequence similarities of 62–89% on amino acid level (100% query coverage). FlamA and flamB showed 62% sequence similarity to each other. Adjacent to the putative laminarinase encoding genes, we identified genes for putative proteins involved in ABC transporter system and genes for putative alanine racemases and GH 3 proteins. Moreover, a truncated gene encoding a Transposase_20 and a repeat region nearby one of the predicted operons indicated that the two related operons originate from a gene duplication event of a DNA cassette around 20 kb in size.
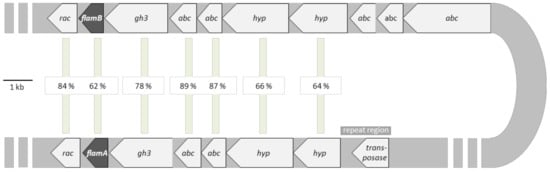
Figure 1.
Predicted structural organization of the two operons containing flamA and flamB based on the highly similar genes in the genome of Fervidobacterium nodosum. Sequence identities of similar genes are shown in the center. Predicted genes: rac—putative gene for alanine racemase; flamA and flamB—genes for β-1,3-glucanases; gh3—putative genes for glycoside hydrolase of family GH 3; abc—putative genes for ABC transporter proteins; transposase—truncated gene of a Transposase_20 with a repeat region; hyp—hypothetical genes.
Structure predictions for FLamA and FLamB revealed a sandwich-like β-jelly roll fold, which is characteristic for laminarinases of class GH 16 (Figure S1). The structural prediction of both proteins was based on the catalytic residue of Thermotoga maritima, with 58% and 60% sequence identity to flamA and flamB, respectively. The comparison of both predictions demonstrated the high structural similarities of FLamA and FLamB with only small differences in a loop adjacent to the cleft of the active site (highlighted in color).
The sequences of both proteins showed similarities to those of laminarinases from other thermophilic bacteria. Indeed, FLamA and FLamB were similar to catalytic domains of experimentally verified β-1,3-glucanases from T. maritima, T. petrophila, and T. neapolitana with a 54–55% and 58–59% identity, respectively. Moreover, FLamA and FLamB were 55% and 58% similar to the catalytic domain of a β-1,3-glucanase from the hyperthermophilic Archaeon Pyrococcus furiosus. The phylogenetic relationship among both enzymes and other biochemically characterized GH 16 family members of bacteria is shown in Figure 2. Concerning the predicted tree based on the homologous region of the catalytic domain, FLamA and FLamB form a solid clade with the β-1,3-glucanases of the Archaeon P. furiosus and members of the same eubacterial order Thermotogales, which includes some of the most extremely thermophilic species currently known.
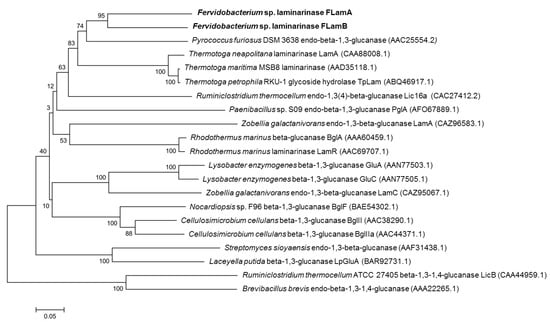
Figure 2.
Phylogenetic tree of biochemically characterized GH 16 enzymes. For construction of the tree, sequences of the catalytic domains were used. GenBank accession numbers are indicated in brackets. Bootstrap values are designated on each branch of the tree.
2.2. Recombinant Production of FLamA and FLamB
Both genes were expressed in E. coli C43(DE3) at 37 °C over 4 h of induction. The obtained proteins harboring a C-terminal 6xHis affinity tag were purified via affinity chromatography and size exclusion chromatography. Both FLamA and FLamB were purified to homogeneity with a factor of 178 and 593 and a final yield of 30% and 18%, respectively (Table S1). The SDS-PAGE revealed a molecular weight of approximately 30 kDa for both proteins, which was slightly smaller than the predicted molecular size of 34.9 kDa and 34.1 kDa for FLamA and FLamB, respectively (Figure 3). Domain prediction revealed that FLamA and FLamB consisted, in each case, of one single GH 16 domain without any further known structural elements.
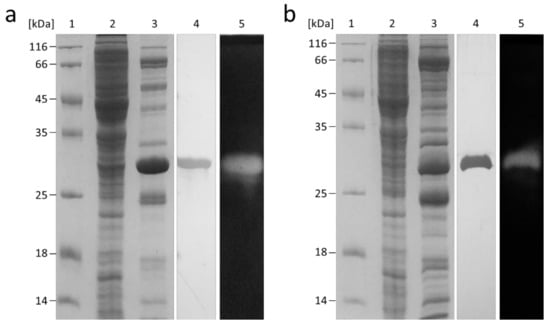
Figure 3.
SDS-PAGE analysis of the His-tagged β-1,3-glucanases FLamA (a) and FLamB (b). Line 1, molecular weight marker; line 2, crude extract; line 3, eluate after the Ni-NTA affinity chromatography; line 4, purified enzymes after size exclusion chromatography; line 5, zymogram for activity staining of the purified enzyme.
2.3. Substrate Specificity of FLamA and FLamB
The substrate specificity of FLamA and FLamB was tested towards a number of complex carbohydrates in a range from 40 to 100 °C (Figure 4, Table 1). Significant differences between FLamA and FLamB were detected in the specific activities towards β-1,3-glucans. Highest activities of both enzymes were measured at 90 °C towards laminarin, whereby FLamB showed a higher specific activity than FLamA, with 876 U/mg and 609 U/mg, respectively. Compared to that, highest activities towards amorphous curdlan were revealed at 70 °C with 78% and 94% relative activity. For undissolved curdlan, the highest activity was determined at 80 °C, with even lower relative activities of 44% and 50% for FLamA and FLamB, respectively.
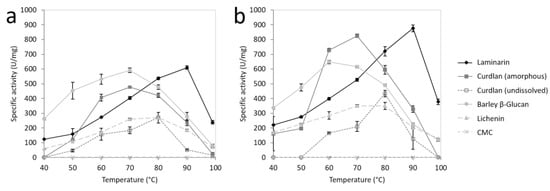
Figure 4.
The temperature profiles of recombinant FLamA (a) and FLamB (b) depending on substrates laminarin, amorphous curdlan, unsolved curdlan, barley β-glucan, lichenin, and carboxymethyl cellulose (CMC).

Table 1.
Substrate specificity of FLamA and FLamB at the optimal temperatures for each substrate.
In comparison to β-1,3-glucans, the specific activities towards mixed-linked glucans did not differ substantially between FLamA and FLamB. The specific activities of FLamA and FLamB towards barley β-glucan from barley were in the same range with 592 U/mg and 648 U/mg, respectively. This was determined at optimal temperatures of 70 °C (FLamA) and 60 °C (FLamB). For both enzymes, 44% and 52% residual activities towards barley β-glucan were detected at 40 °C. Moreover, both enzymes showed, at 40 °C, higher activities towards mixed-linked β-glucan (262 U/mg and 336 U/mg) than towards laminarin (124 U/mg and 220 U/mg). The specific activities towards lichenin at optimal conditions (80 °C and 70 °C) were with 271 U/mg and 350 U/mg for FLamA and FLamB, lower than towards the other tested substrates. Hydrolysis of the β-1,4-glucan CMC was not observed.
2.4. Degradation Pattern and Enzyme Kinetics
The hydrolysis products of laminarin and barley β-glucan were investigated by HPLC analysis. The FLamA and FLamB produced similar degradation patterns. Hydrolysis of laminarin (Figure 5a) mainly results in laminaribiose, glucose-mannitol units (DP2), and glucose (DP1) and to a lesser extent laminaritriose (DP3) and higher oligosaccharides. The degradation pattern of barley β-glucan was different (Figure 5b). Laminaritriose was the major product and to a lesser extent DP1 and DP2 were detected. The produced oligosaccharides indicated that FLamA and FLamB are β-1,3-glucanases with endo-acting mode. Moreover, the shifted product variation with barley β-glucan suggested that both enzymes hydrolyzed the β-1,3-glycosidic linkages in mixed-linked glucans and to a lesser extent the β-1,4-building blocks.
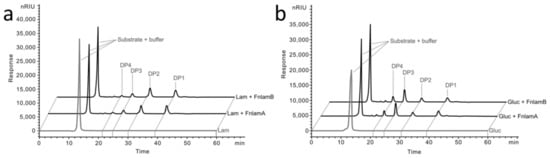
Figure 5.
HPLC analysis of products from polysaccharide degradation. Hydrolysis products from laminarin (a) and barley β-glucan (b) after 18 h of incubation at 70 °C. DP: degree of polymerization. DP1: glucose. Lam: laminarin. Gluc: barley β-glucan.
The kinetic parameters were determined in the presence of laminarin. At optimal conditions (90 °C, pH 6.5) the Km value of FLamA was 2.01 mg ml−1 and with that higher than that of FLamB (1.64 mg ml−1). Moreover, the catalytic efficiency values (kcat/Km) were 494.3 ml s−1 mg−1 and 806.3 ml s−1 mg−1 for FLamA and FLamB, respectively. The results indicated that FLamB has a 61% higher catalytic efficiency on laminarin than FLamA, which is in agreement with the determined substrate specificities.
2.5. Effects of pH and Temperature
The effect of pH was analyzed in a range of pH 2 to 12. Measurements revealed similar pH spectra for FLamA and FLamB (Figure 6). Both enzymes showed activity in a range between pH 5 and 9. Optimal activity was detected at pH 6.5. Concerning the stability of FLamA, there was a decrease by approximately 8% in activity over the entire pH range after 24 h (Figure S3). In the same pH range, FLamB showed at least 82% residual activity.
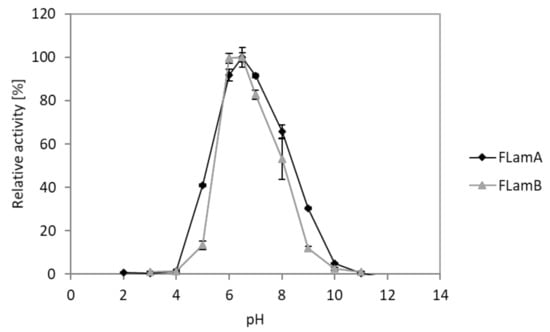
Figure 6.
Influence of pH on the activity of recombinant FLamA and FLamB towards laminarin.
To examine the temperature stability of the two thermoactive enzymes, the residual activities after heat incubation at 70, 80, and 90 °C were measured (Figure 7). FLamA and FLamB exhibited more than 80% and 40% residual activity over 24 h at 70 °C, respectively. At 80 °C, the enzymes had a half-life time of 5 h (FLamA) and 1 h (FLamB). By incubating the enzymes at 90 °C after 30 min, no residual activity was detected. FLamA was, in the tested temperature range, more stable than FLamB.
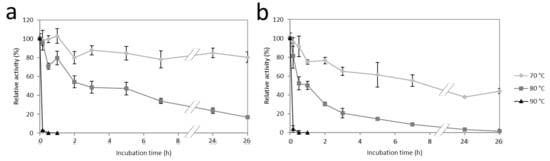
Figure 7.
Influence of temperature on the stability of FLamA (a) and FLamB (b).
2.6. Effects of Metal Ions and Chemical Additives
The effect of various additives on the activities of FLamA and FLamB was investigated. For both enzymes, the examined additives had similar influences. Among the tested metal ions (5 mM), Ag+, Al3+, Cr3+, Cu2+, Zn2+, and Fe2+ completely inhibited FLamA and FLamB (Table 2a). Both Co2+ and Ni2+ significantly reduced the activity of the enzymes. The same effect was observed by the addition of 5 mM SDS, cetyltrimethylammonium bromide (CTAB), and Pefabloc (Table 2b). In contrast, the reducing agents dithiothreitol (DTT) and β-mercaptoethanol had positive effects on enzyme activities.

Table 2.
Influence of metal ions (a) and different reagents (b) on laminarinase activity of FLamA and FLamB.
3. Discussion
The two genes flamA and flamB revealed high sequence similarities of 62% (100% query coverage) with each other. Two highly similar genes within the genome of Fervidobacterium nodosum were located in two different operons. The pattern of the operons and adjacent genes as well as the presence of a truncated transposase gene suggest that these structures were generated by a duplication of an around 20 kb genome section. Moreover, it has been shown that flamA and flamB encode for active laminarinases possessing nearly the same biochemical characteristics, indicating that both genes form a multigene family.
There are other bacterial species which express more than one gene encoding for laminarinases. Nevertheless, these genes contain various modular architectures and the proteins possess different domains and functions like in the marine bacterium Zobellia galactanivorans [17]. In Lysobacter enzymogenes, two laminarinases are present which have high sequence identities in part of the catalytic domain (86%) but differentiate in an additional carbohydrate binding module (CBM) in one of the proteins [18]. Similar relationships were also observed for related laminarinases from Streptomyces sioyaensis [19]. So far, however, only members of the family Fervibacteriaceae are known to harbor two similar genes, such as flamA and flamB, which encode for laminarinases with similar sequence and function.
It has been shown that gene duplication probably generates no benefit by enhanced gene dosage of laminarinases or adjacent genes, such as those for the ABC transporter system. The increase of gene dosages may cause higher energy consumption leading to significant reduced fitness of the organism [20,21]. Therefore, it is more likely that gene duplication leads to redundancy and, thus, facilitates mutations in one of the copies and possibly results in new benefiting gene functions [22]. A similar operon composition occurs in the closely related F. pennivorans, where two genes exist with 100% and 70% sequence identity to flamA and flamB, respectively. This demonstrates that flamA is highly conserved in both species, whereas differences in flamB were probably generated by point mutations. This may lead to beneficial properties, like obviously higher catalytic activities of FLamB towards β-1,3-glucans. If there is no selective advantage, duplicated genes normally become inactivated by mutations and these pseudogenes will disappear from the genome [23].
Phylogenetic analysis of the catalytic residues of FLamA, FLamB, and other characterized laminarinases revealed a solid clade with close a relationship between enzymes from the bacterial order Thermotogales, including FLamA and FLamB, and the Archaeon Pyrococcus furiosus (Figure 2). Thermotogales are assumed to have interchanged large numbers of genes with Archaea and Firmicutes by horizontal gene transfer (HGT) [14]. An extraordinarily high number of insertion sequence elements for example in the genome of F. nodosum demonstrate the great influence of foreign genes. The high sequence similarity of FLamA, FLamB, and the laminarinases of the bacterium Thermotoga to one from the Archaeon P. furiosus suggests that an ancestral gene was exchanged by HGT.
Regarding the temperature optima of these enzymes, FLamA and FLamB are both thermoactive enzymes showing highest activity at 90 °C. This optimum is similar to that of the other examined laminarinases from the phylum Thermotogae [24,25] which are to our knowledge the ones with the highest temperature optima among reported β-1,3-glucanases from eubacteria and plants. However, LamA from the Archaeon P. furiosus showed among all characterized laminarinases activity at the highest temperatures of 100–105 °C (Table 3) [26]. Both FLamA and FLamB showed high storage stabilities at 4 °C in a broad pH range of 3 to 11 which enables an easy handling of both laminarinases. Their stability at 70 to 90 °C is also comparable to those of the other laminarinases operating at high temperatures (Table 3). All in all, FLamA and FLamB have significantly high temperature stabilities, which are a decisive criterion for industrial application.

Table 3.
Comparison of biochemically characterized laminarinases from thermophilic bacteria and Archaea.
The investigation of the substrate specificities of FLamA and FLamB revealed different temperature optima for the tested substrates (Figure 4). Schwarz et al. [27] observed related characteristics of a laminarinase of Clostridium thermocellum. The optimal temperature towards curdlan is lower in consideration to the fact that curdlan forms at temperatures above 80 °C irreversible high-set gels [28], which might be less accessible for enzymes. Nevertheless, the reasons for the different temperature optima can only be speculated.
So far, published thermostable β-1,3-linkage hydrolyzing enzymes generally possessed strong preferences either for β-1,3-glucans or mixed-linked glucans (Table 3). Both FLamA and FLamB take an intermediate position between those groups by degrading approximately both types of substrates with activity in the same range. Additionally, FLamA and FLamB exhibit higher substrate affinities towards mixed-linked glucans in comparison to similar enzymes from Thermotoga neapolitana [25] and P. furiosus [26]. Ilari et al. [36] investigated a deletion mutant and were able to show that the loss of a five amino acid kink at the entrance of the catalytic cleft leads to higher activity towards mixed-linked glucans. This effect was also observed in other laminarinases missing these residues [34,37]. Even in FLamA and FLamB, these amino acid residues are missing (Figure S2). Thus, differences in the kink possibly explain the altered substrate preferences between those enzymes.
The described substrate spectra and hydrolysis products suggest that FLamA and FLamB are able to cleave β-1,3 glycosidic bonds in an endo-acting mode. Nevertheless, the hydrolysis of β-1,4 glycosidic bonds in mixed-linked glucans is also possible. Similar product patterns of laminarin and barley β-glucan were observed with a laminarinase form Caldicellulosiruptor sp., when the authors proved that the enzyme was able to degrade β-1,4 glyosidic bonds adjacent to 3-O-substituted glucopyranose units [32].
For industrial application, detection of inhibitory effects and comparison with other enzymes the influences of different metal ions and additives were tested. For FLamA and FLamB similar effects were observed. The inhibitory effects of metal ions observed in this study are well known for many glucosidases, probably due to the redox effects on the amino acids [38]. The SDS had a negative influence on the activity of the enzymes by disturbing hydrophobic interactions and generating protein denaturation. Moreover, the detergent CTAB blocked the catalytic cleft of a related laminarinase by hydrophobic interactions [38], which may lead to reduced activities of FLamA and FLamB as well. Pefabloc, a serine protease inhibitor, negatively influenced the enzyme activity probably by binding irreversiblely to serine residues nearby the catalytic cleft. In contrary, the reducing properties of DTT and β-mercaptoethanol might positively influence a cysteine residue adjacent to the nucleophile of the active site and therefore increase the laminarinase activity.
Although FLamA and FLamB share many biochemical properties, the two enzymes exhibit differences in substrate specificities, particularly towards β-1,3-glucans. In comparison to the specific activities of FLamA, FLamB exhibits approximately 40% and more than 70% higher activity towards laminarin and amorphous curdlan, respectively (Table 1). These differences were reflected in the kinetic parameters as well. In the analysis of Labourel et al. [39], significant differences in substrate specificity were caused by an additional loop in protein structure which leads to higher affinities towards mixed-linked glucans. Nevertheless, according to structural predictions, those considerable differences were not observed between FLamA and FLamB (Figure S1). Only minor structural modifications particularly in a loop adjacent to the catalytic cleft possibly result in an upwardly more opened cleft of FLamB. Based on the observations of Labourel et al. [39] and Jeng et al. [40] concerning the enzyme-substrate complexes, the enlarged opening might improve the access for β-1,3-glucans that possess helical conformation, whereas the affinity of FLamB towards mixed-linked glucans with linear conformation is not affected. These results and further investigations will help to improve activities of β-1,3-glucananases towards β-1,3-glucans.
4. Materials and Methods
4.1. Cloning of the Endo-β-1,3-Glucanase Encoding Genes flamA and flamB
Metagenomic DNA was extracted from environmental samples taken from different locations at the hot spring Caldeirão at Furnas Valley (Azores, Portugal) followed by the production and sequencing of a 454 shotgun library as described previously [41]. For the two genes flamA and flamB, encoding for endo-β-1,3-glucanases from Fervidobacterium, no signal peptides were predicted by SignalP [42]. For cloning into the StarGate system (IBA Lifesciences) the two genes were amplified by PCR using the metagenomic DNA as a template and the following primers (primer extending sequences are indicated in boldface):
- flamA-for: AGCGGCTCTTCAATGAAAGTTAAATATTTCTCAAATATT
- flamA-rev: AGCGGCTCTTCTCCCCTCATTTTCAAGCTTGTATAC
- flamB-for: AGCGGCTCTTCAATGAGAGAAAAGTTGCTGT
- flamB-rev: AGCGGCTCTTCTCCCCTCTTCATCTAATGTATACAC
The PCR products were cloned into the destination vector pASG-IBA33 according to the producer instructions resulting in recombinant fusion genes with C-terminal sequences encoding for hexahistidine tags. After transformation of Escherichia coli TOP10 and selection on LB medium containing 100 µg/mL ampicillin and 50 µL/mL X-gal, plasmids of recombinant clones were isolated. The inserts were sequenced for verification. Subsequently, the vectors were used to transform E. coli C43(DE3) for protein production.
4.2. Sequence Comparison and Phylogenetic Analysis
From the GenBank database, amino acid sequences of characterized β-1,3-glucanases and β-1,3(4)-glucanases of the family GH 16 were selected. Multiple sequence alignment was performed using ClustalX. Homologous sequence regions were selected and applied in a second multiple sequence alignment. Using MEGA6, a phylogenetic tree was calculated by the neighbor joining method. Bootstrap analysis with resampling of the dataset was performed (n = 100) to test the reliability of the tree. Structure prediction of both proteins were done by SWISS-MODEL using the crystal structure of the laminarinase from Thermotoga maritima (PDB ID: 3azx) as a template. Structures of FLamA and FLamB were visualized and compared in the UCFS Chimera program by applying the Needleman–Wunsch algorithm and the scoring matrix Blosum62.
4.3. Heterologous Expression of the flamA and flamB Genes and Purification of the Endo-β-1,3-Glucanases
Escherichia coli C43(DE3) harboring the plasmids pASG-IBA33::flamA or pASG-IBA33::flamB were grown in LB media (100 µg/mL ampicillin) at 37 °C and 160 rpm. Gene expression was induced at OD600 0.6 by adding anhydrotetracycline to a final concentration of 200 ng/µL. After four hours of induction cells were harvested by centrifugation at 9000 × g at 4 °C for 20 min. The resulting cell pellet was stored at −20 °C.
For purification, 0.2 g cells were resuspended per 1 mL lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8) and disrupted by three passages through a French pressure cell with constant pressure of 1,000 psi (French Pressure Cell Press, SLM-Aminco). Cell debris was removed by centrifugation (20,000 × g, 4 °C, 30 min) and supernatant was loaded onto a 1 ml Ni-NTA Superflow column (Qiagen). Proteins were eluted by an increasing imidazole gradient according to the manufacturer’s instructions. Eluted fractions were pooled, washed three times with buffer G (50 mM Na-phosphate buffer, pH 7.2, 150 mM NaCl) by ultrafiltration in an Amicon filter unit (Amicon Ultra-15, 1000 MWCO, Merck Millipore). For final purification via size exclusion chromatography, protein solutions were loaded onto a Superose 12 column (GE Healthcare) previously equilibrated with buffer G. Protein fractions containing the purified β-1,3-glucanases were pooled and stored at 4 °C.
Protein samples were analyzed on a 12.5% SDS-PAGE (12.5%) [43]. Additionally, β-1,3-glucanase activity was determined by zymogram technique. For this, proteins were applied to a denaturing SDS-PAGE and were subsequently renaturated by incubation for 1 h in 1% (v/v) Triton X-100 and three successive washings for 5 min in 50 mM sodium phosphate buffer (pH 6.5). Then, the gel slices were incubated for 20 min at 80 °C on an agarose gel containing 0.1% (w/v) curdlan. For visualization agarose slides were stained for 1 h with 1% (w/v) Congo Red and destained in 1 M NaCl. To increase the contrast, the slides were finally overlaid with 0.1 M acetic acid.
Protein concentrations were determined according to Bradford [44], with bovine serum albumin as the standard.
4.4. β-Glucanase Activity Assay
The standard assay was carried out at 90 °C for 7 min in 500 µL reaction mixture using 0.25% (w/v) laminarin from Laminaria digitata (Merck) as substrate in 20 mM sodium phosphate buffer (pH 6.5) and 50 µL enzyme sample. In advance, it was ensured that product formation per min was constant in the time interval and with a linear correlation to preclude instability effects of the enzymes. Additionally, blank experiments without enzymes were performed by default for all measurement series. The hydrolytic activities of the purified enzymes FLamA and FLamB were detected by measuring the reducing sugars with 3,5-dinitrosalicylic acid (DNS) according to Miller [45] with glucose as the standard. In brief, after enzyme reaction 500 µl reaction mixture were mixed with 500 µL DNS reagent (1% (w/v) DNS, 30% (w/v) potassium sodium tartrate, 0.4 M NaOH) and were incubated for 5 min at 100 °C. Samples were subsequently cooled on ice to room temperature and absorption was measured at 546 nm. All measurements were done in triplicates. One unit of enzyme activity was defined as the amount of enzyme required to release 1 µmol of reducing sugars per minute.
The influence of temperature was examined by performing the standard assay at temperatures from 20 to 100 °C. To investigate the temperature stability of FLamA and FLamB, the enzymes were preincubated with a concentration of 0.1 mg/mL in 20 mM sodium phosphate buffer (pH 6.5) at 70, 80, and 90 °C. Samples were taken in time intervals up to 26 h and residual activities were measured by using the standard assay.
To investigate the influence of the pH on enzyme activity, a standard assay was performed using Britton–Robinson buffer (50 mM) in a range of pH 2–11 in the reaction mixture [46]. The pH stabilities of both enzymes were tested by preincubation of the enzymes with a concentration of 0.01 mg/mL in 50 mM Britton–Robinson buffer pH 3–11 for 24 h at 4 °C. Residual activity was determined with the standard assay by dilution the incubation mixtures in Britton–Robinson buffer at pH 6. Enzyme activity previous to incubation was defined as 100%.
Additionally, the influences of metal ions on enzyme activity were analyzed by using a standard assay, but with 20 mM maleate buffer (pH 6.5) and the addition of 5 mM AgNO3, AlCl3, CaCl2, CoCl2, CrCl3, CuCl2, FeCl2, KCl, MgCl2, NaCl, NiCl2, RbCl, SrCl2 or ZnCl2. Furthermore, the influences of 3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate (CHAPS), SDS, Triton X-100, Tween 20, Tween 80, guanidine hydrochloride, urea, dithiothreitol (DTT), β-mercaptoethanol, EDTA, iodoacetic acid, Pefabloc, cetyltrimethylammonium bromide (CTAB) and sodium azide were examined by the standard assay procedure. All additives were tested in a concentration of 5 mM under standard conditions.
To measure the specific activities of FLamA and FLamB, substrates were used in a final concentration of 0.25% (w/v). The CMC and lichenin were obtained from Merck and β-glucan (barley) and curdlan (Alcaligenes faecalis) from Megazyme. In case of curdlan an undissolved and a dissolved (amorphous) form was tested. To achieve an amorphous type of curdlan, 0.2 g were first solubilized in 6 mL alkaline solution (0.6 M NaOH) and subsequently neutralized with HCl to a concentration of 0.5% (w/v) and pH 6.5 in 20 mM sodium phosphate buffer.
Kinetic parameters were determined by performing the standard assay with twelve different substrate concentrations varying from 0 to 25 mM. The Michaelis constant Km and the maximum reaction rate at maximum substrate concentration vmax were calculated by non-linear regression applying the Michaelis–Menten equation.
4.5. Determination of the Hydrolysis Products
For determination of the hydrolysis products of laminarin and barley β-glucan, 0.25% (w/v) substrate were incubated with FLamA or FLamB in standard reaction mixtures at 70 °C for 18 h. After the inactivation of the enzymes at 100 °C for 10 min, samples were centrifuged (20.000 × g, 10 min) and the supernatant was filtered using a 0.22 µm membrane filter unit. Hydrolysis products were analyzed by high-performance liquid chromatography (HPLC) under the following conditions: Hi-Plex Na column (Agilent Technologies), 80 °C, 0.2 mL/min flow rate, water as mobile phase, RI detector (Agilent Technologies). Laminaritetraose, laminaritriose, laminaribiose (all from Megazymes) and glucose (Merck) were used as standards.
5. Conclusions
Laminarinases are enzymes which could be applied in diverse fields, from biomass conversion, over yeast extract production, agents against fungal plant pathogens to the production of antiviral and antitumor therapeutics from β-1,3-glucans. The biochemical characterization of the two laminarinases FLamA and FLamB derived from a Fervidobacterium species revealed high specific activities and resistance to elevated temperatures and various additives which make both enzymes suitable candidates for application under harsh conditions. Moreover, the comparative analysis of both enzymes showed differences in their thermal stability and catalytic efficiency towards β-1,3-glucans, like laminarin and curdlan. In conclusion, these results will contribute to our knowledge of sequence-function correlations and will potentially help to improve activity and stability of laminarinases and other related glucanases.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/10/830/s1, Figure S1: Structural models of FLamA and FLamB, Figure S2: Amino acid alignment of the catalytic domains of FLamA and FLamB with other members of GH 16, Figure S3: Laminarinase activity of FLamA and FLamB after incubation at pH 3-11 and 4 °C for 24 h, Table S1: Purification of the recombinant FLamA and FLamB from E. coli.
Author Contributions
C.B. designed the study and performed the experimental work. C.S., G.S. and G.A. supervised this study. C.B. prepared the manuscript. C.S., J.C. and G.A. reviewed and edited the manuscript before submission.
Funding
This research was funded by BMBF (Bundesministerium für Bildung und Forschung, Germany) in the project LIPOMAR (Lipids and surfactants from marine biomass), FKZ 031A261.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stone, B.A. Chemistry, biochemistry, and biology of (1-3)-β-glucans and related polysaccharides. In Chemistry of β-Glucans, 1st ed.; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2009; pp. 5–46. [Google Scholar]
- Ebringerová, A.; Hromádková, Z.; Heinze, T. Hemicellulose. In Polysaccharides, I. Structure, Characterisation and Use; Heinze, T.T., Ed.; Springer-Verlag GmbH: Berlin/Heidelberg, Germany, 2005; pp. 1–67. [Google Scholar]
- Painter, T.J. Algal polysaccharides. In The Polysaccharides; Aspinall, G.O., Ed.; Academic Press (Molecular biology): New York, NY, USA, 1983; pp. 195–285. Volume 2. [Google Scholar]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: an untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.-H.; Boraston, A.B.; Czjzek, M. A sweet new wave: structures and mechanisms of enzymes that digest polysaccharides from marine algae. Curr. Opin. Struct. Biol. 2014, 28, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Yao, G.; Yang, P.; Li, N.; Luo, H.; Bai, Y.; Wang, Y.; Yao, B. Cloning, characterization, and antifungal activity of an endo-1,3-β-D-glucanase from Streptomyces sp. S27. Appl. Microbiol. Biotechnol. 2010, 85, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011, 2, 115–119. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front. Bioeng. Biotechnol. 2015, 3, 348. [Google Scholar] [CrossRef]
- Krahe, M.; Markl, H.; Antranikian, G. Fermentation of extremophilic microorganisms. FEMS Microbiol. Rev. 1996, 18, 271–285. [Google Scholar] [CrossRef]
- Schroeder, C.; Elleuche, S.; Blank, S.; Antranikian, G. Characterization of a heat-active archaeal beta-glucosidase from a hydrothermal spring metagenome. Enzyme Microb. Technol. 2014, 57, 48–54. [Google Scholar] [CrossRef]
- Zhaxybayeva, O.; Swithers, K.S.; Lapierre, P.; Fournier, G.P.; Bickhart, D.M.; DeBoy, R.T.; Nelson, K.E.; Nesbø, C.L.; Doolittle, W.F.; Gogarten, J.P.; et al. On the chimeric nature, thermophilic origin, and phylogenetic placement of the Thermotogales. Proc. Natl. Acad. Sci. USA 2009, 106, 5865–5870. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.C.; Morgan, H.W.; Daniel, R.M. Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch. Microbiol. 1985, 141, 63–69. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Tang, R.; Yu, S.; Zheng, B.; Feng, Y. A novel thermostable cellulase from Fervidobacterium nodosum. J. Mol. Catal. B: Enzym. 2010, 66, 294–301. [Google Scholar] [CrossRef]
- Labourel, A.; Jam, M.; Legentil, L.; Sylla, B.; Hehemann, J.H.; Ferrières, V.; Czjzek, M.; Michel, G. Structural and biochemical characterization of the laminarinase ZgLamCGH16 from Zobellia galactanivorans suggests preferred recognition of branched laminarin. Acta Crystallogr. D 2015, 71 Pt 2, 173–184. [Google Scholar] [CrossRef]
- Palumbo, J.D.; Sullivan, R.F.; Kobayashi, D.Y. Molecular Characterization and Expression in Escherichia coli of Three β-1,3-Glucanase Genes from Lysobacter enzymogenes Strain N4-7. J. Bacteriol. 2003, 185, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-Y.; Huang, J.-W.; Meng, M.; Cheng, C.-W. Isolation and biochemical characterization of an endo-1,3-β-glucanase from Streptomyces sioyaensis containing a C-terminal family 6 carbohydrate-binding module that binds to 1,3-β-glucan. Microbiology 2002, 148 Pt 4, 1151–1159. [Google Scholar] [CrossRef]
- Qian, W.; Zhang, J. Gene dosage and gene duplicability. Genetics 2008, 179, 2319–2324. [Google Scholar] [CrossRef]
- Wagner, A. Energy Constraints on the Evolution of Gene Expression. Mol. Boil. Evol. 2005, 22, 1365–1374. [Google Scholar] [CrossRef]
- Francino, M.P. An adaptive radiation model for the origin of new gene functions. Nat. Genet. 2005, 37, 573–578. [Google Scholar] [CrossRef]
- Bratlie, M.S.; Johansen, J.; Sherman, B.T.; Huang, D.W.; Lempicki, R.A.; Drabløs, F. Gene duplications in prokaryotes can be associated with environmental adaptation. BMC Genom. 2010, 11, 588. [Google Scholar] [CrossRef]
- Cota, J.; Alvarez, T.M.; Citadini, A.P.; Santos, C.R.; Neto, M.D.O.; Oliveira, R.R.; Pastore, G.M.; Ruller, R.; Prade, R.A.; Murakami, M.T.; et al. Mode of operation and low-resolution structure of a multi-domain and hyperthermophilic endo-β-1,3-glucanase from Thermotoga petrophila. Biochem. Biophys. Res. Commun. 2011, 406, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Zverlov, V.V.; Volkov, I.Y.; Velikodvorskaya, T.V.; Schwarz, W.H. Thermotoga neapolitana bglB gene, upstream of lamA, encodes a highly thermostable beta-glucosidase that is a laminaribiase. Microbiology 1997, 143 Pt 11, 3537–3542. [Google Scholar] [CrossRef]
- Gueguen, Y.; Voorhorst, W.G.B.; van der Oost, J.; de Vos, W.M. Molecular and biochemical characterization of an endo-β -1,3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 1997, 272, 31258–31264. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.H.; Schimming, S.; Staudenbauer, W.L. Isolation of a Clostridium thermocellum gene encoding a thermostable β-1,3-glucanase (laminarinase). Biotechnol. Lett. 1988, 10, 225–230. [Google Scholar] [CrossRef]
- Kasai, N.; Harada, T. Ultrastructure of Curdlan. In Fiber Diffraction Methods; French, A.D., Ed.; American Chemical Society (ACS symposium series): Washington, DC, USA, 1980; pp. 363–383. Volume 141. [Google Scholar]
- Meng, D.D.; Wang, B.; Ma, X.Q.; Ji, S.Q.; Lu, M.; Li, F.L. Characterization of a thermostable endo-1,3(4)-beta-glucanase from Caldicellulosiruptor sp. strain F32 and its application for yeast lysis. Appl. Microbiol. Biotechnol. 2016, 100, 4923–4934. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Uchimura, K.; Kubota, T.; Nunoura, T.; Deguchi, S. Biochemical and genetic characterization of beta-1,3 glucanase from a deep subseafloor Laceyella putida. Appl. Microbiol. Biotechnol. 2016, 100, 203–214. [Google Scholar] [CrossRef]
- Masuda, S.; Endo, K.; Koizumi, N.; Hayami, T.; Fukazawa, T.; Yatsunami, R.; Fukui, T.; Nakamura, S. Molecular identification of a novel beta-1,3-glucanase from alkaliphilic Nocardiopsis sp. strain F96. Extremophiles 2006, 10, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Spilliaert, R.; Hreggvidsson, G.O.; Kristjansson, J.K.; Eggertsson, G.; Palsdottir, A. Cloning and Sequencing of a Rhodothermus marinus Gene, bglA, Coding for a Thermostable beta-Glucanase and its Expression in Escherichia coli. JBIC J. Boil. Inorg. Chem. 1994, 224, 923–930. [Google Scholar] [CrossRef]
- Krah, M.; Misselwitz, R.; Politz, O.; Thomsen, K.K.; Welfle, H.; Borriss, R. The laminarinase from thermophilic eubacterium Rhodothermus marinus. Conformation, stability, and identification of active site carboxylic residues by site-directed mutagenesis. Eur. J. Biochem. 1998, 257, 101–111. [Google Scholar] [CrossRef]
- Fuchs, K.-P.; Zverlov, V.V.; Velikodvorskaya, G.A.; Lottspeich, F.; Schwarz, W.H. Lic16A of Clostridium thermocellum, a non-cellulosomal, highly complex endo-beta-1,3-glucanase bound to the outer cell surface. Microbiology 2003, 149 Pt 4, 1021–1031. [Google Scholar] [CrossRef]
- Woo, C.-B.; Kang, H.-N.; Lee, S.-B. Molecular cloning and anti-fungal effect of endo-β-1,3-glucanase from Thermotoga maritima. Food Sci. Biotechnol. 2014, 23, 1243–1246. [Google Scholar] [CrossRef]
- Ilari, A.; Fiorillo, A.; Angelaccio, S.; Florio, R.; Chiaraluce, R.; Van Der Oost, J.; Consalvi, V. Crystal structure of a family 16 endoglucanase from the hyperthermophile Pyrococcus furiosus- structural basis of substrate recognition. FEBS J. 2009, 276, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, O.J.; Piotukh, K.; Ponnuswamy, M.N.; Planas, A.; Borriss, R.; Heinemann, U. Structural basis for the substrate specificity of a Bacillus 1,3-1,4-beta-glucanase. J. Mol. Biol. 2006, 357, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.D.C.; Giese, E.C.; Moretti, M.M.D.S.; Gomes, A.C.D.S.; Perrone, M.B.O.M.; Da Silva, R.; Martins, D.A.B. Effect of Metal Ions, Chemical Agents and Organic Compounds on Lignocellulolytic Enzymes Activities. In Enzyme Inhibitors and Activators; IntechOpen: London, UK, 2017; pp. 139–164. [Google Scholar]
- Labourel, A.; Jam, M.; Jeudy, A.; Hehemann, J.-H.; Czjzek, M.; Michel, G. The β-glucanase ZgLamA from Zobellia galactanivorans evolved a bent active site adapted for efficient degradation of algal laminarin. J. Biol. Chem. 2014, 289, 2027–2042. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.-Y.; Wang, N.-C.; Lin, C.-T.; Shyur, L.-F.; Wang, A.H.-J. Crystal structures of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with inhibitors: essential residues for β-1,3- and β-1,4-glucan selection. J. Biol. Chem. 2011, 286, 45030–45040. [Google Scholar] [CrossRef] [PubMed]
- Sahm, K.; John, P.; Nacke, H.; Wemheuer, B.; Grote, R.; Daniel, R.; Antranikian, G. High abundance of heterotrophic prokaryotes in hydrothermal springs of the Azores as revealed by a network of 16S rRNA gene-based methods. Extremophiles 2013, 17, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).