Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts
Abstract
1. Introduction
2. Results and Discussion
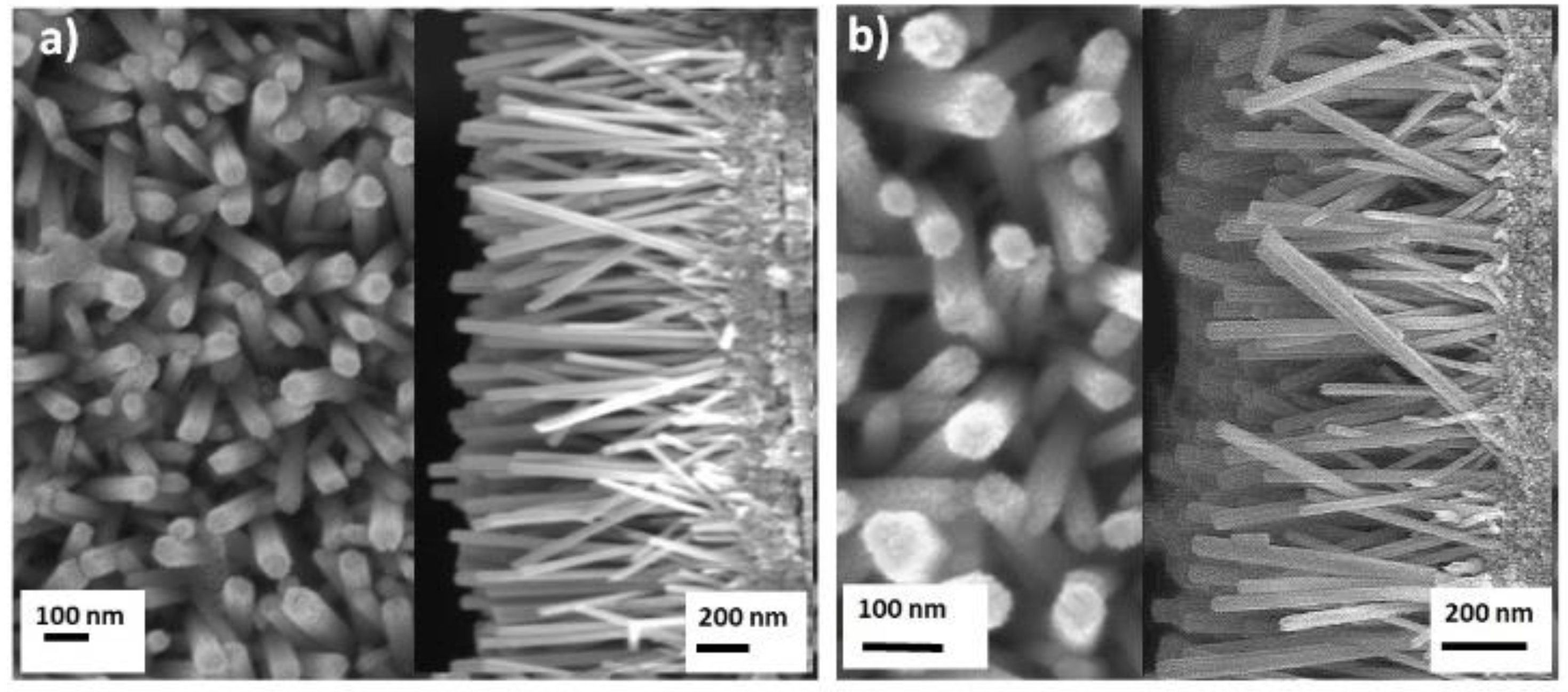
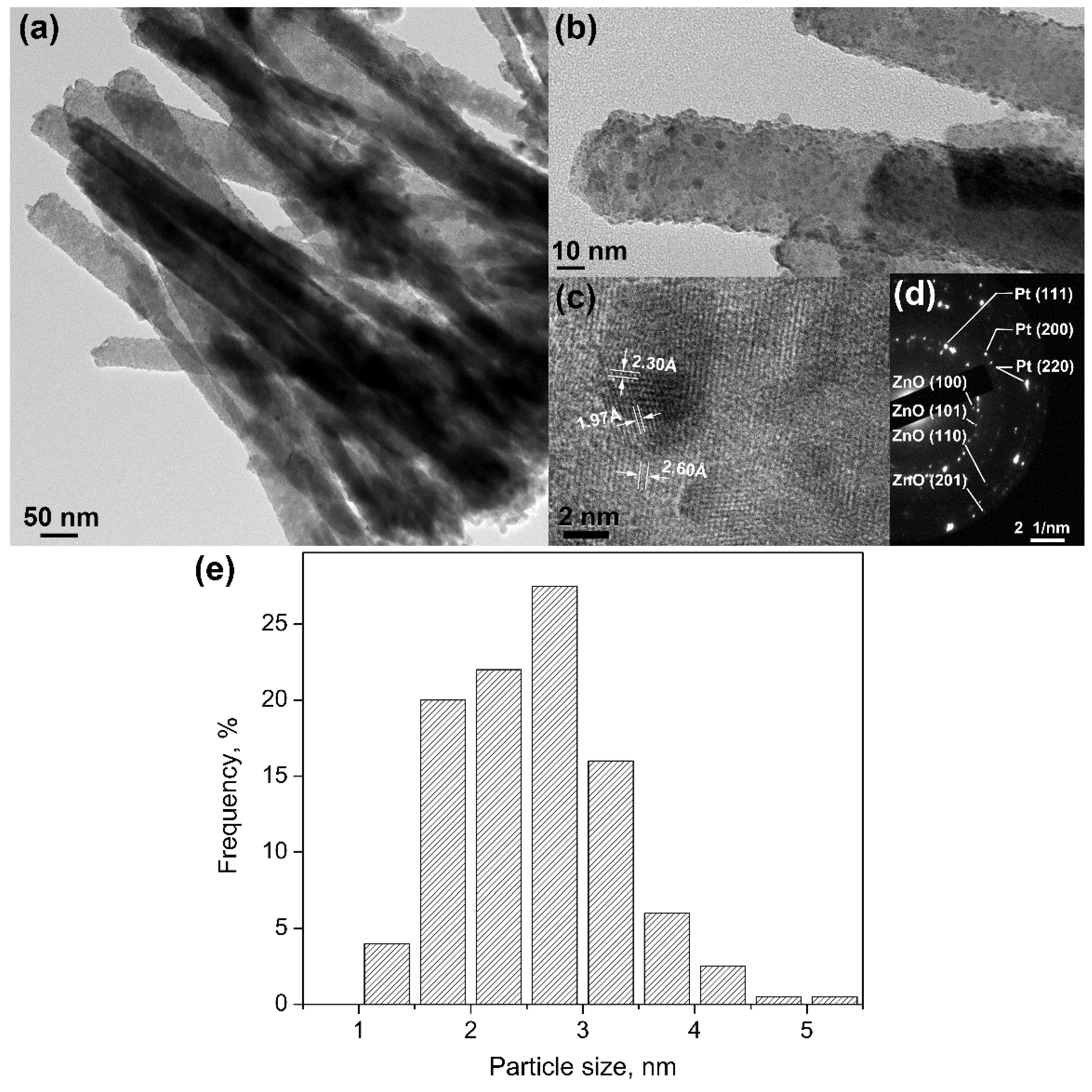
2.1. Morphology and Crystal Structure of Designed Photocatalysts
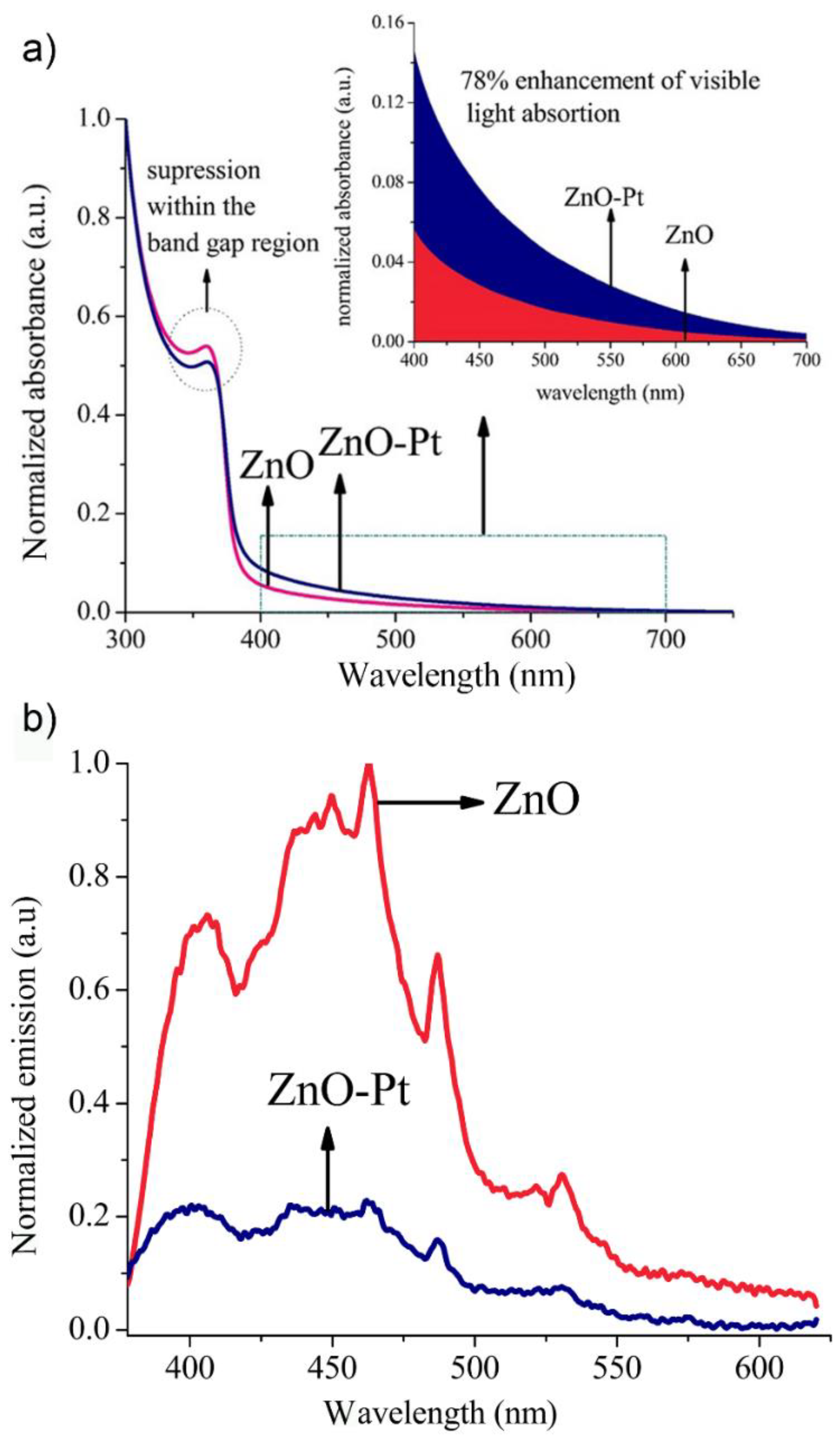
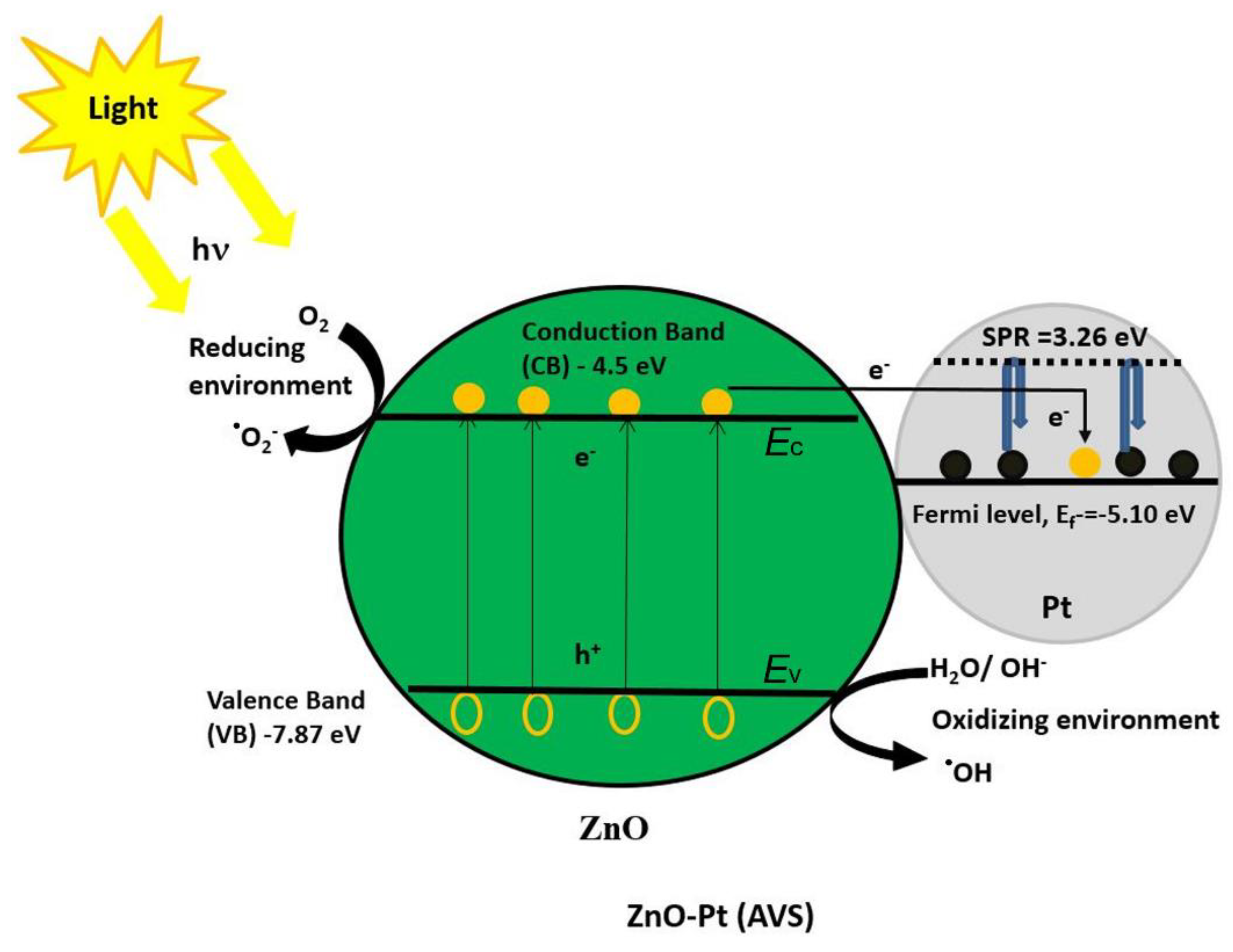
2.2. Modification of Optical Properties of ZnO/Pt Nanocomposite
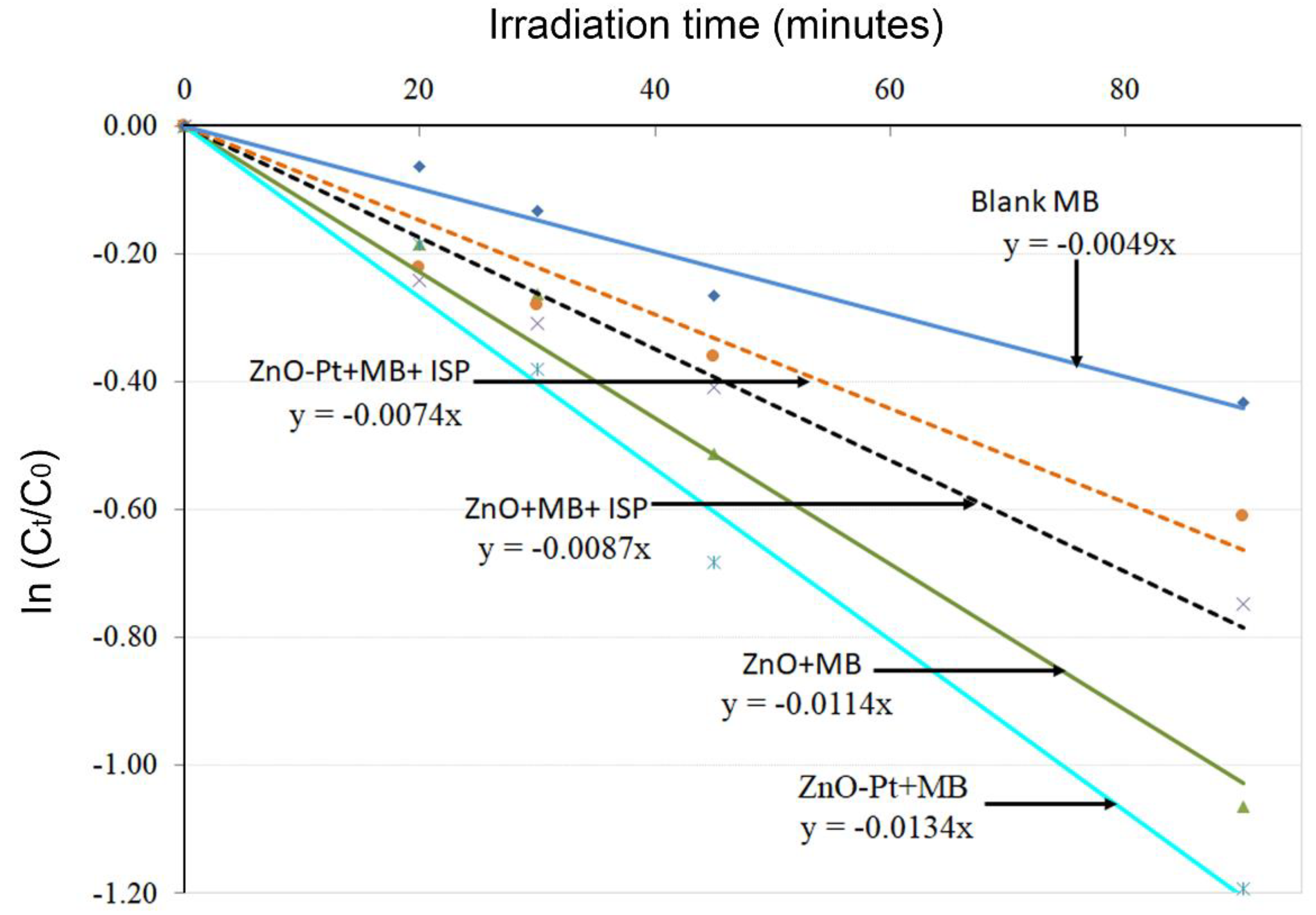
2.3. Photocatalytic Activity Test for ZnO and ZnO-Pt
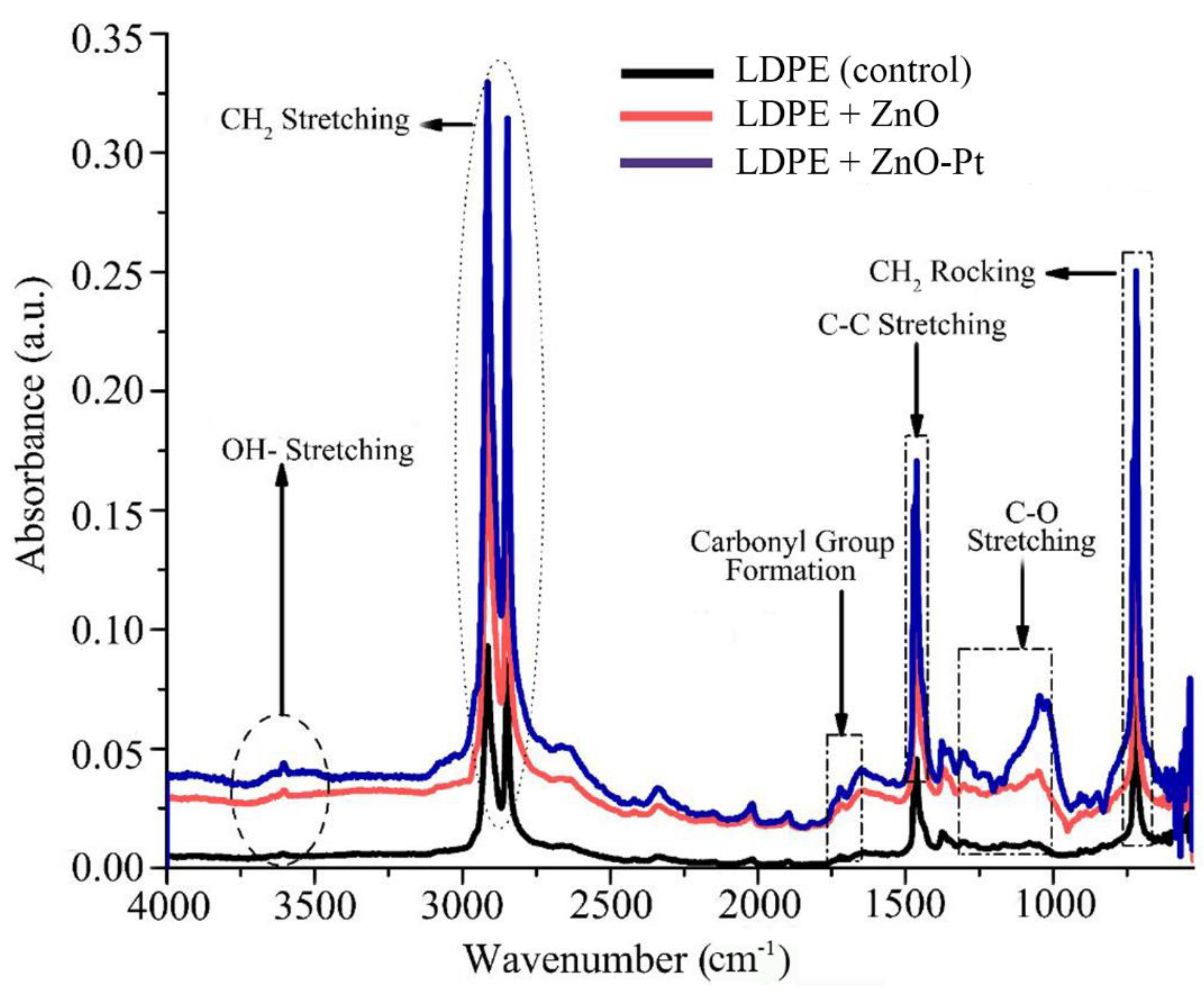
2.4. Photocatalytic Degradation of Low Density Polyethylene Films
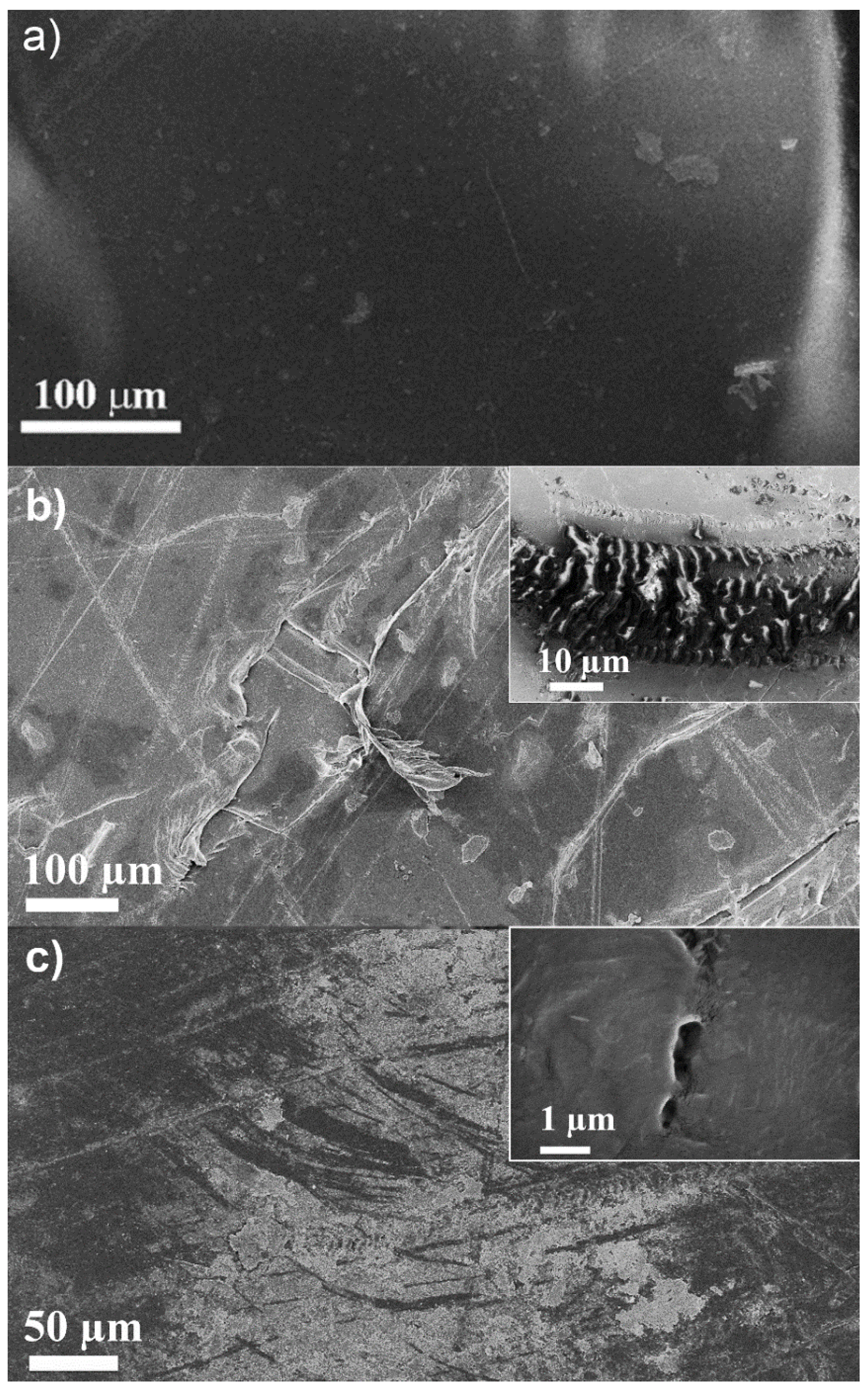
2.5. Microstructure Analysis of Non-Irradiated and Irradiated Low Density Polyethylene Films
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Nanostructured Catalysts
3.3. Deposition of Platinum Nanoparticles on ZnO Nanorods
3.4. Photocatalysis Test Setup
3.5. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. London Ser. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Wu, W.-M.; Yang, J.; Criddle, C.S. Microplastics pollution and reduction strategies. Front. Environ. Sci. Eng. 2016, 11, 6. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, H.; Abdelrasoul, A. Challenges and treatment of microplastics in water. In Water Challenges of an Urbanizing World; Glavan, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, occurrence, and degradation of microplastics in the aqueous environment. In Freshwater Microplastics. The Handbook of Environmental Chemistry; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 58, pp. 51–57. [Google Scholar]
- Zhou, D.; Chen, L.; Li, J.; Wu, F. Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: A state-of-the-art minireview. Chem. Eng. J. 2018, 346, 726–738. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Qazi, I.A.; Arshad, M.; Khan, Z.; Voice, T.C.; Mehmood, C.T. Photocatalytic degradation of low density polyethylene (LDPE) films using titania nanotubes. Environ. Nanotechnol. Monit. Manag. 2016, 5, 44–53. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Li, S.; Xu, S.; He, L.; Xu, F.; Wang, Y.; Zhang, L. Photocatalytic degradation of polyethylene plastic with polypyrrole/TiO2 nanocomposite as photocatalyst. Polym. Plast. Technol. Eng. 2010, 49, 400–406. [Google Scholar] [CrossRef]
- Laxman, K.; Al Rashdi, M.; Al Sabahi, J.; Al Abri, M.; Dutta, J. Supported versus colloidal zinc oxide for advanced oxidation processes. Appl. Surf. Sci. 2017, 411, 285–290. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Bora, T.; Dutta, J. Plasmonic photocatalyst design: Metal-semiconductor junction affecting photocatalytic efficiency. J. Nanosci. Nanotechnol. 2019, 19, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Lin, H.Y.; Cheng, C.L.; Chen, Y.F. Giant enhancement of bandgap emission of ZnO nanorods by platinum nanoparticles. Nanotechnology 2006, 17, 4391–4394. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, R.; Laxman, K.; Dastgir, S.; Dutta, J. Role of bonding mechanisms during transfer hydrogenation reaction on heterogeneous catalysts of platinum nanoparticles supported on zinc oxide nanorods. Appl. Surf. Sci. 2016, 377, 200–206. [Google Scholar] [CrossRef]
- Bora, T.; Zoepfl, D.; Dutta, J. Importance of plasmonic heating on visible light driven photocatalysis of gold nanoparticle decorated zinc oxide nanorods. Sci. Rep. 2016, 6, 26913. [Google Scholar] [CrossRef] [PubMed]
- Chichagov, A.; Belonozhko, A.; Lopatin, A.; Dokina, T.; Samokhvalova, O.; Ushakovskaya, T.; Shilova, Z. Information computing system from structural data on minerals (Mincryst). Kristallografiya 1990, 35, 610–616. [Google Scholar]
- Sun, F.; Qiao, X.; Tan, F.; Wang, W.; Qiu, X. One-step microwave synthesis of Ag/ZnO nanocomposites with enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2012, 47, 7262–7268. [Google Scholar] [CrossRef]
- Yang, T.; Wang, M.; Duan, C.; Hu, X.; Huang, L.; Peng, J.; Huang, F.; Gong, X. Inverted polymer solar cells with 8.4% efficiency by conjugated polyelectrolyte. Energy Environ. Sci. 2012, 5, 8208–8214. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, W.; Wang, P.; Zhang, X.; Zhao, Z. In situ enhancement of NBE emission of Au–ZnO composite nanowires by SPR. Cryst. Eng. Comm. 2013, 3301–3304. [Google Scholar] [CrossRef]
- Anandan, S.; Vinu, A.; Sheeja Lovely, K.L.P.; Gokulakrishnan, N.; Srinivasu, P.; Mori, T.; Murugesan, V.; Sivamurugan, V.; Ariga, K. Photocatalytic activity of La-doped ZnO for the degradation of monocrotophos in aqueous suspension. J. Mol. Catal. A Chem. 2007, 266, 149–157. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Xie, Y.; Fan, Q.; Yu, J.C.; Shu, Q.; Wang, C. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Flyunt, R.; Knolle, W.; Kahnt, A.; Halbig, C.E.; Lotnyk, A.; Häupl, T.; Prager, A.; Eigler, S.; Abel, B. High quality reduced graphene oxide flakes by fast kinetically controlled and clean indirect UV-induced radical reduction. Nanoscale 2016, 8, 7572–7579. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, R.; Babu, S.G.; Ramachandran, R.; Neppolian, B. Bismuth oxyiodide incorporated reduced graphene oxide nanocomposite material as an efficient photocatalyst for visible light assisted degradation of organic pollutants. Appl. Surf. Sci. 2017, 418, 163–170. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Mouallif, I.; Latrach, A.; Benali, C.M.H.; Barbe, N. FTIR study of HDPE structural changes, moisture absorption and mechanical properties variation when exposed to sulphuric acid aging in various temperatures. In 20ÈME Congrès Français de Mécanique, AFM; Maison de la Mécanique: Besançon, France, 2011; pp. 2011–25044. [Google Scholar]
- Luongo, J.P. Infrared study of oxygenated groups formed in polyethylene during oxidation. J. Polym. Sci. 1960, 42, 139–150. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, C.; Zhang, S.; Chen, G.; Yang, M. Photo-oxidative degradation of polyethylene/montmorillonite nanocomposite. Polym. Degrad. Stab. 2003, 81, 497–500. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Photocatalytic degradation of polystyrene plastic under fluorescent light. Environ. Sci. Technol. 2003, 37, 4494–4499. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef] [PubMed]
- Myint, M.T.Z.; Kumar, N.S.; Hornyak, G.L.; Dutta, J. Hydrophobic/hydrophilic switching on zinc oxide micro-textured surface. Appl. Surf. Sci. 2013, 264, 344–348. [Google Scholar] [CrossRef]
- Baral, A.; Khanuja, M.; Islam, S.S.; Sharma, R.; Mehta, B.R. Identification and origin of visible transitions in one dimensional (1D) ZnO nanostructures: Excitation wavelength and morphology dependence study. J. Lumin. 2017, 183, 383–390. [Google Scholar] [CrossRef]
| Catalyst | Carbonyl index (CI) | Vinyl index (VI) |
|---|---|---|
| LDPE (control) | 0.71 | 0.51 |
| LDPE + ZnO | 1.38 | 1.12 |
| LDPE + ZnO-Pt | 1.49 | 1.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. https://doi.org/10.3390/catal9100819
Tofa TS, Ye F, Kunjali KL, Dutta J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts. 2019; 9(10):819. https://doi.org/10.3390/catal9100819
Chicago/Turabian StyleTofa, Tajkia Syeed, Fei Ye, Karthik Laxman Kunjali, and Joydeep Dutta. 2019. "Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts" Catalysts 9, no. 10: 819. https://doi.org/10.3390/catal9100819
APA StyleTofa, T. S., Ye, F., Kunjali, K. L., & Dutta, J. (2019). Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts, 9(10), 819. https://doi.org/10.3390/catal9100819







