Application of TiO2-Cu Composites in Photocatalytic Degradation Different Pollutants and Hydrogen Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- two commercial titanium dioxides: Evonik Aeroxide P25 (89% anatase and 11% rutile photocatalyst—abbreviated as P25) and Aldrich anatase (AA);
- copper precursor: CuCl2; (0.3 mol L−1 solution);
- for stabilizing the particle size of copper: trisodium citrate (Na3C3H5O(COO)3, Alfa-Aesar, 0.63 × 10−4 mol L−1);
- for reducing copper nanoparticles: sodium borohydride (NaBH4, Merck, 0.15 mol L−1 as a 4 °C solution);
- for the study of hydrogen-producing ability: crystalline oxalic acid (C2H2O4, 50 mmol L−1 solution);
- for the investigation of photocatalytic activity, methyl orange (125 µmol L−1 solution) and ketoprofen (C7H6O3 in 0.5 mmol L−1 solution);
- distilled water to prepare the above-mentioned solutions.
2.2. Synthesis of the Composites
2.3. Characterization Methods and Instrumentation
2.4. Determination of Photocatalytic Activity
2.4.1. Methyl Orange Degradation
2.4.2. Ketoprofen Degradation
2.4.3. Rhodamine B Degradation
2.5. Determination of Photocatalytic Hydrogen Production Capacity
3. Results and Discussion
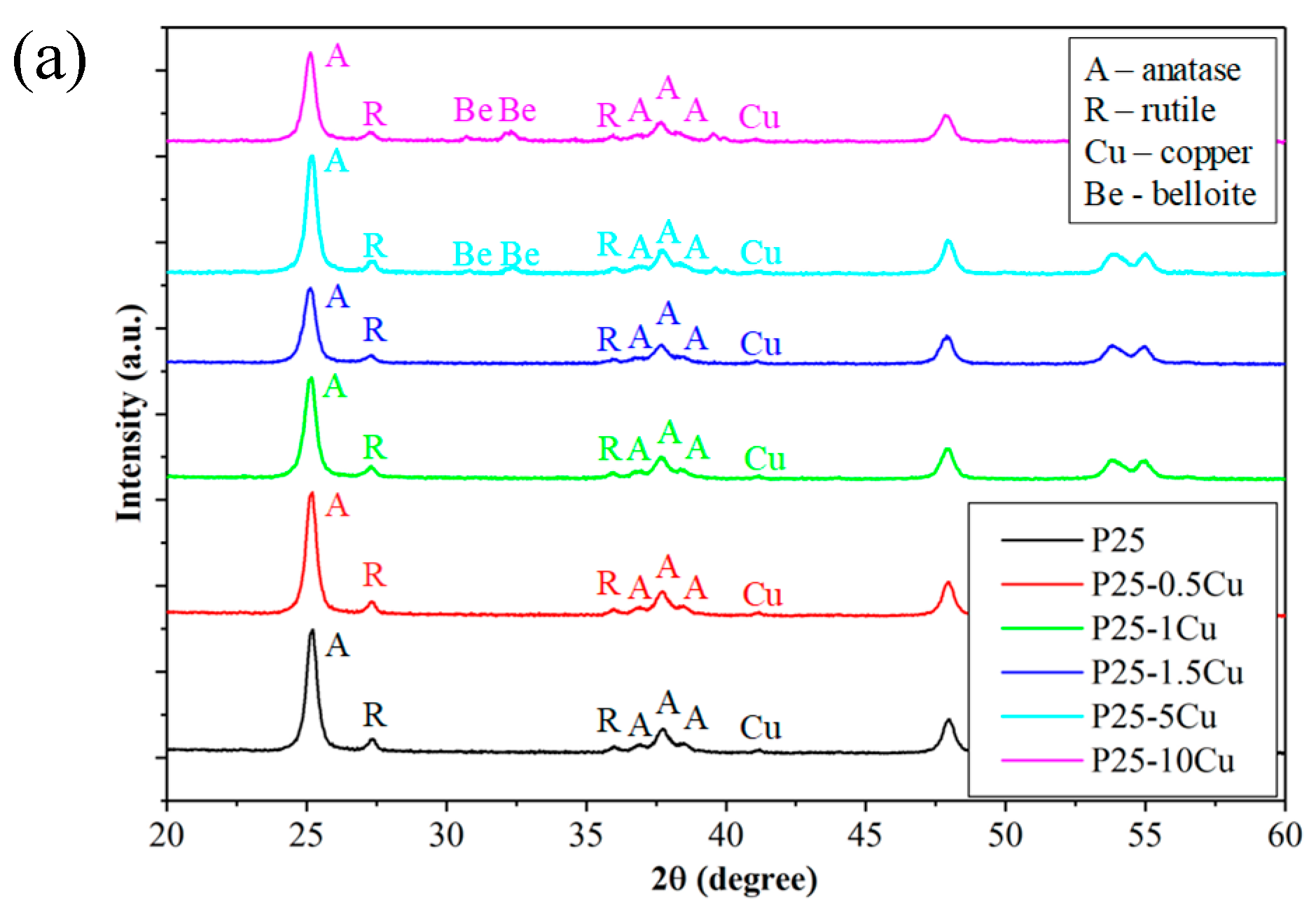
3.1. X-ray Diffraction
3.2. Transmission Electron Microscopy
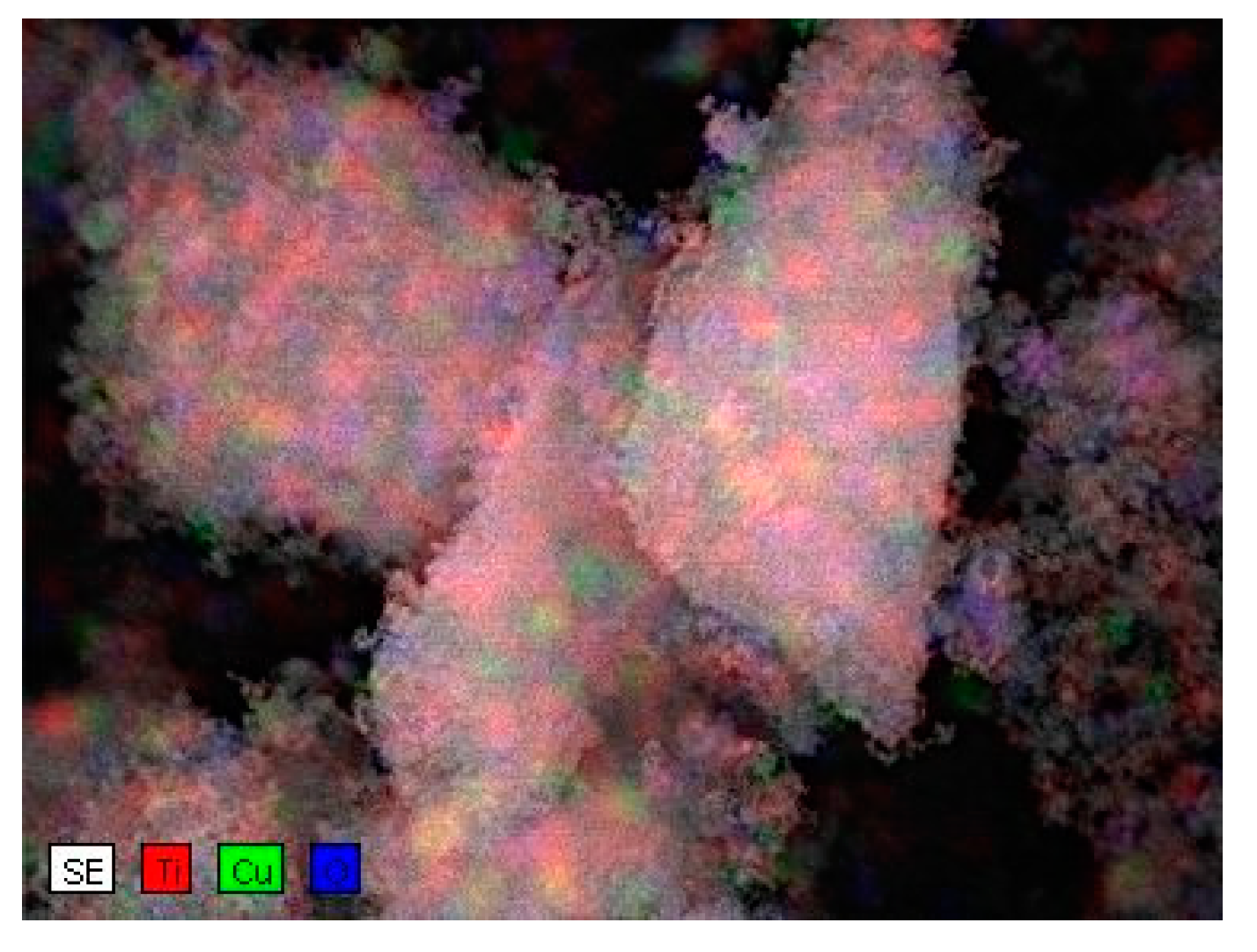
3.3. Energy-Dispersive X-Ray Spectroscopy (EDX)
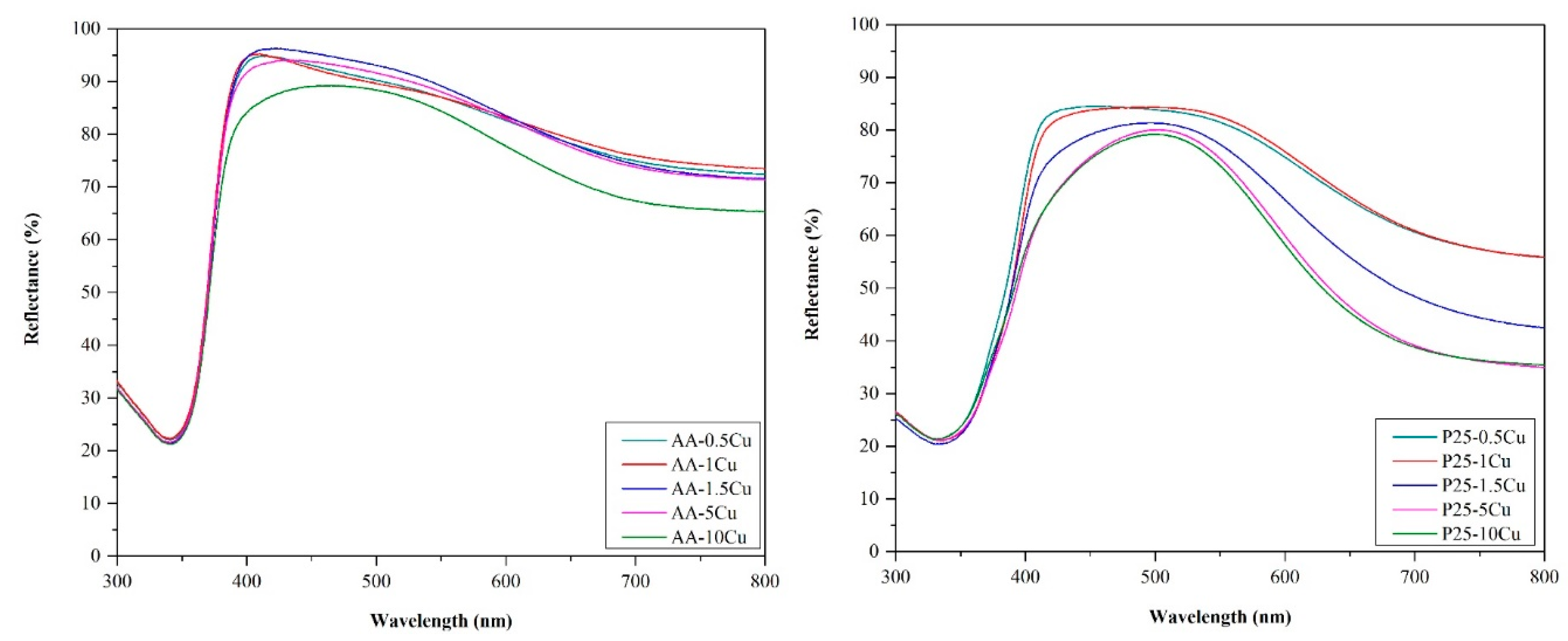
3.4. Diffuse Refllectance Spectroscopy
- On the surface of P25 particles, copper was anchored more efficiently, as demonstrated in the previous sections.
- It is interesting that the composites based on AA did not change their band-gap energy. Usually, if a composite component is present in a higher concentration, it should also be visible in the optical properties (in the case of P25, it was visible in the spectra). Therefore, the key to explain this anomaly lies in the number of contacts between Cu containing particles and TiO2. P25 is made of smaller crystallites (25–40 nm) than AA (~80 nm); therefore, the ratio between the Cu and titania particles is totally different, as demonstrated in our recent papers [47,48]. More precisely, as the anatase particles are twice as large in AA than in P25, therefore, the possibility of contacting a Cu containing particle is high in AA, while in the case of P25, it is low. In the latter case, it could be imagined that any kind of inhomogeneity in Cu particle distribution will be visible in the DRS spectra, as well, as it was in our case.
3.5. Photocatalytic Performance of the Composites
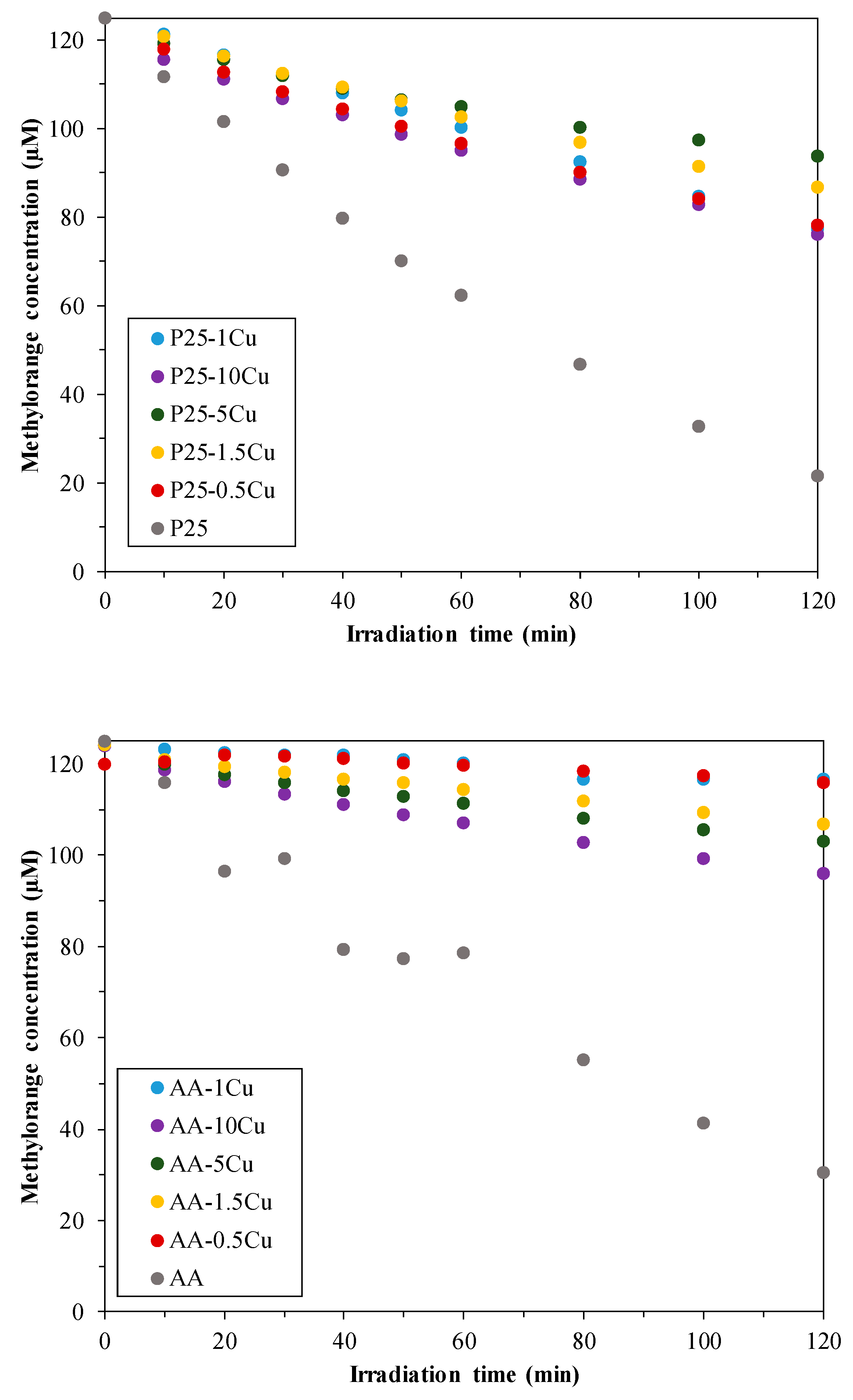
3.5.1. Photodegradation of Methyl Orange
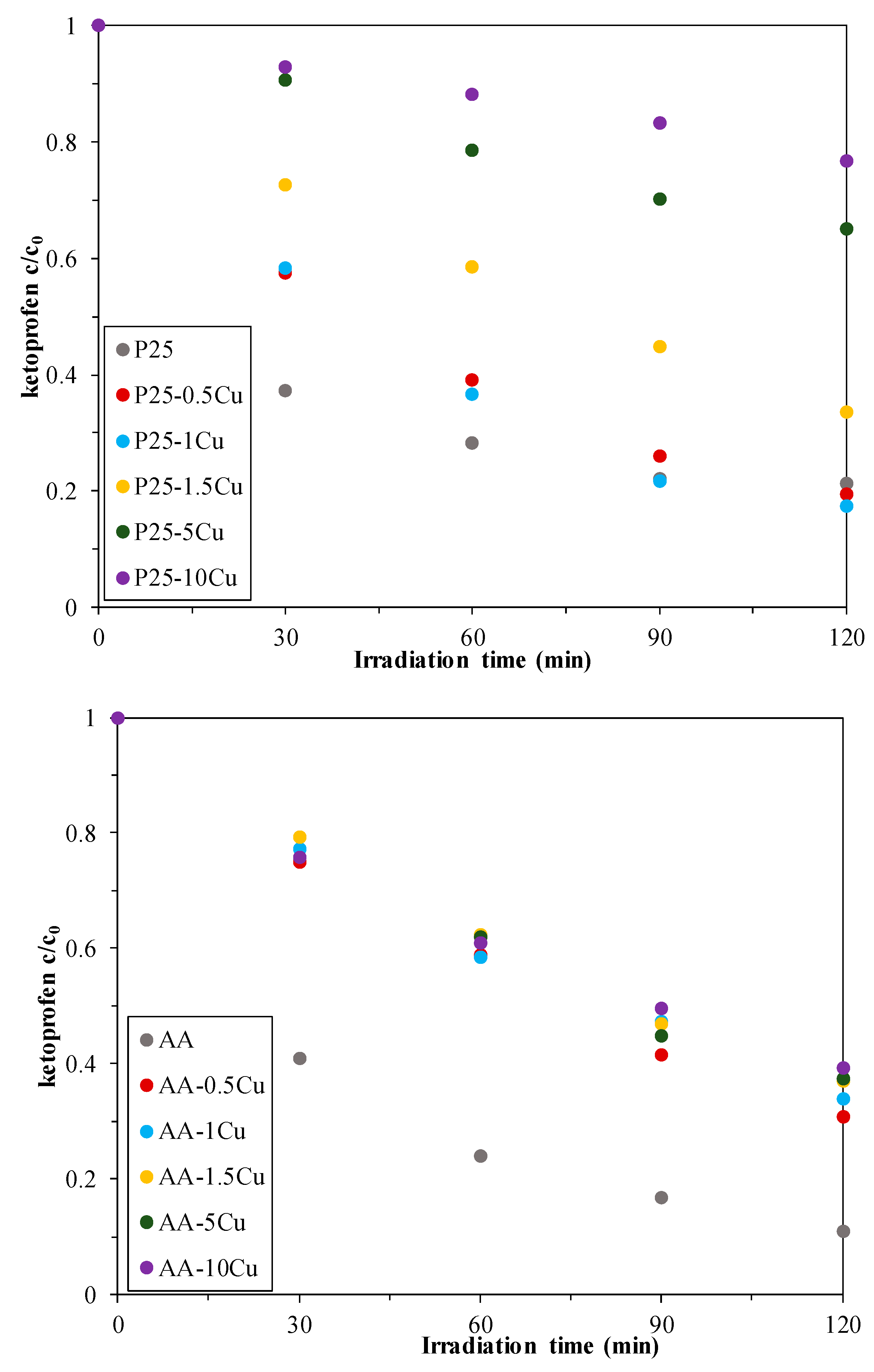
3.5.2. Photodegradation of Ketoprofen
3.5.3. Photodegradation of Rhodamine B
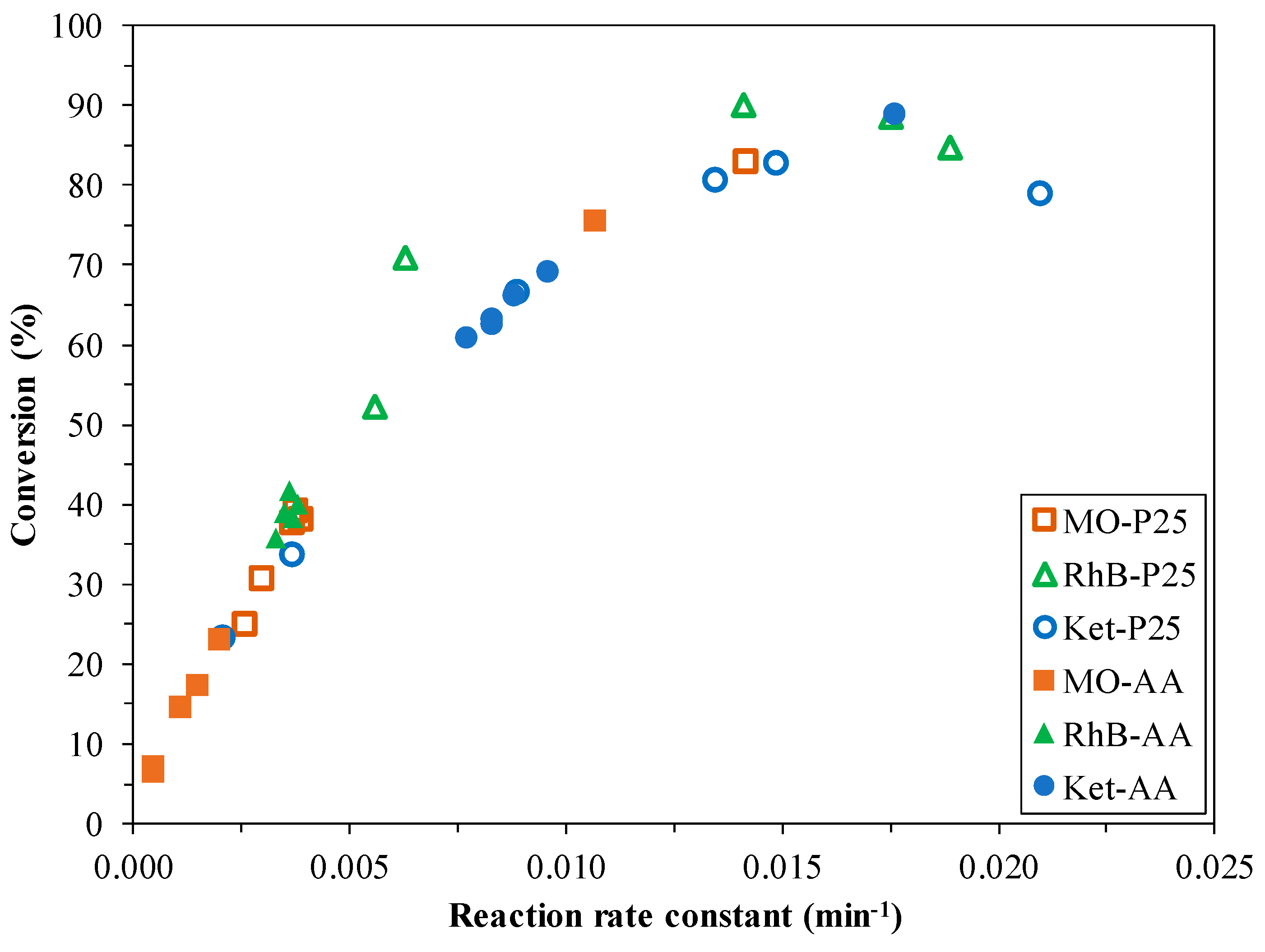
3.5.4. The Comparison of Pseudo-First Order Kinetics and the Achieved Degradation Efficiencies
- It is known that ketoprofen can react with holes directly. This is a plausible approach as the holes are available on the surface of the titania nanoparticles. By the oxidation via holes in the first step, a carbocation is obtained, which can be easily decarboxylated.
- Ketoprofen can uptake the generated photoelectrons, as well. This can occur in two ways:
- ○
- First, the ketoprofene molecule captures a photoelectron, forming carbanionic intermediate at the surface of titania (where no Cu is present), which can react further with a hole if one is formed in the vicinity of the organic molecule. This scenario can also lead to a fast decarboxylation of the molecule.
- ○
- The photoelectron can be captured, as well, directly from the Cu itself. The resultant carbanionic intermediate results in a metastable radical, which can be transformed into several products, as well.
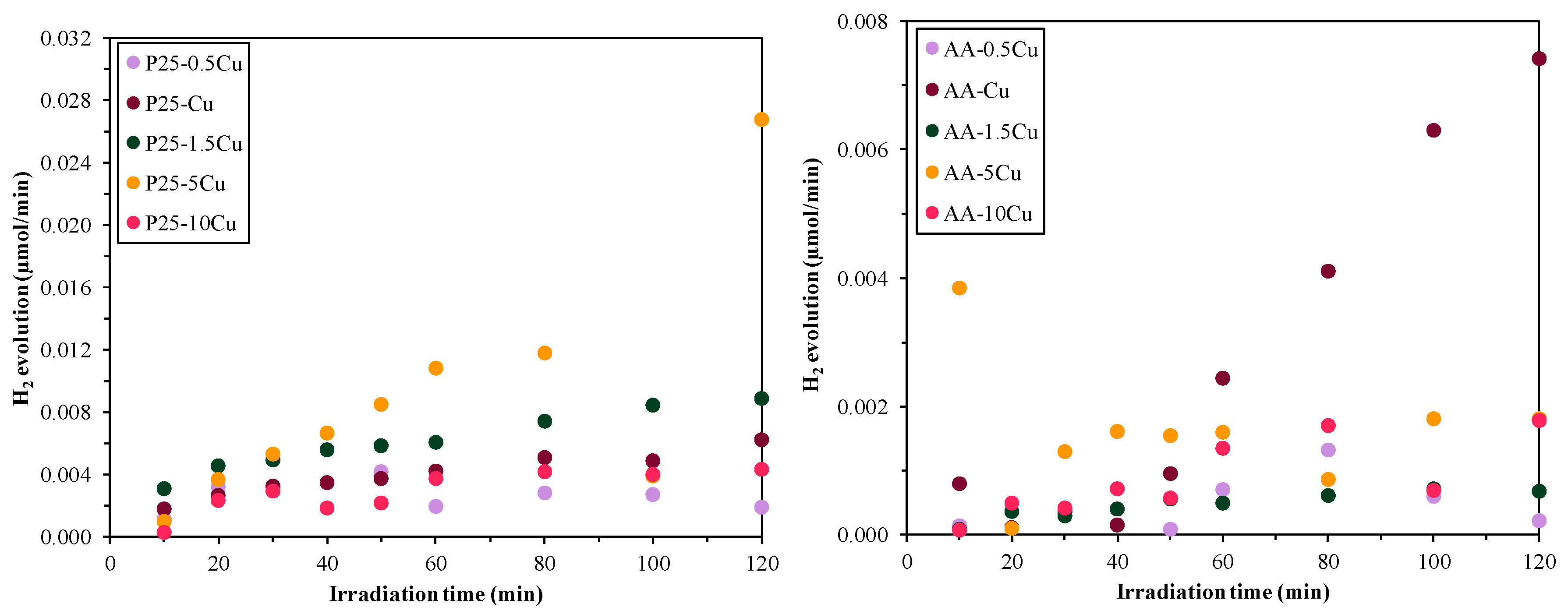
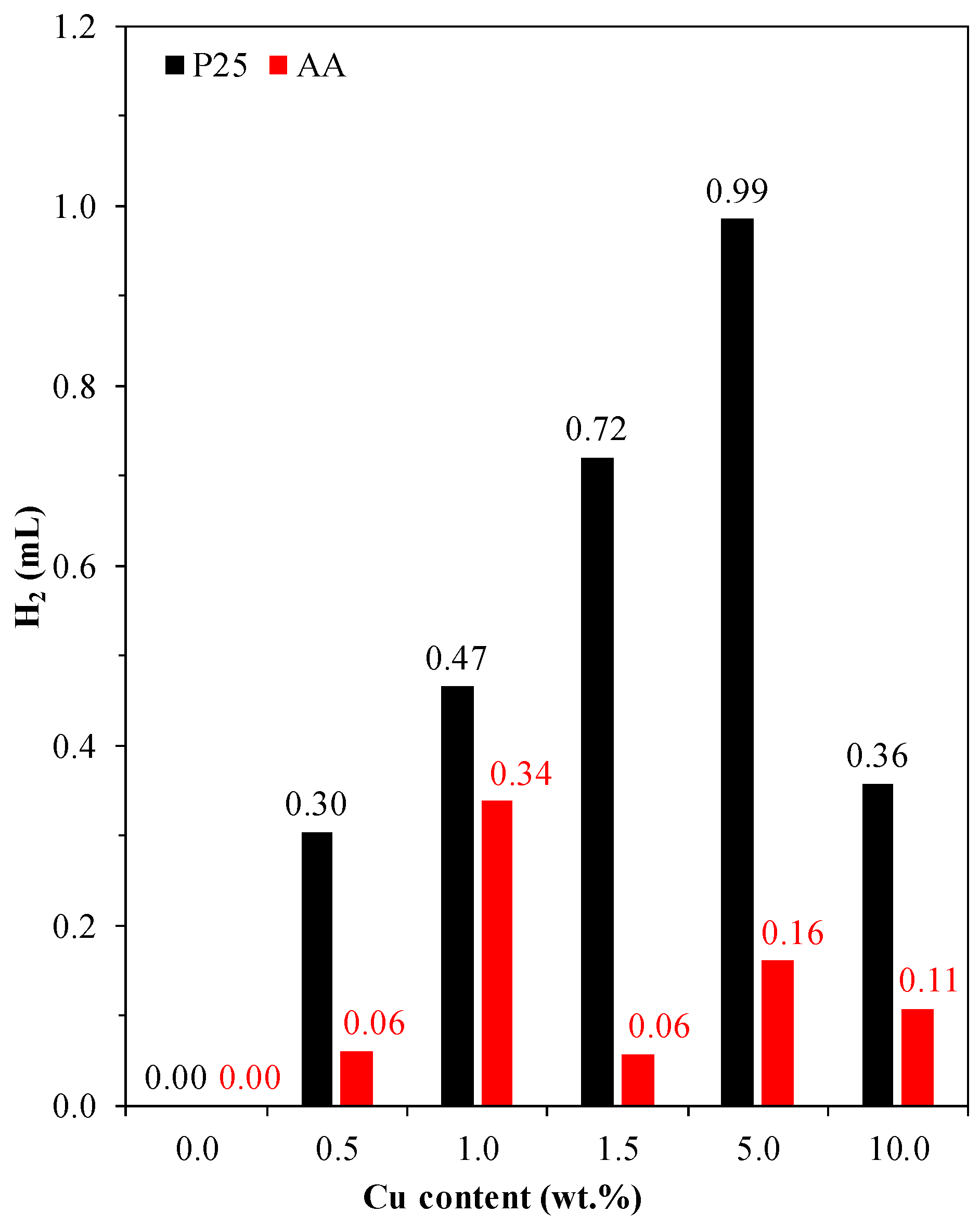
3.5.5. Photocatalytic H2 Production
3.5.6. Structural and Mechanistic Insights on the Behavior of Cu Containing TiO2 Photocatalysts
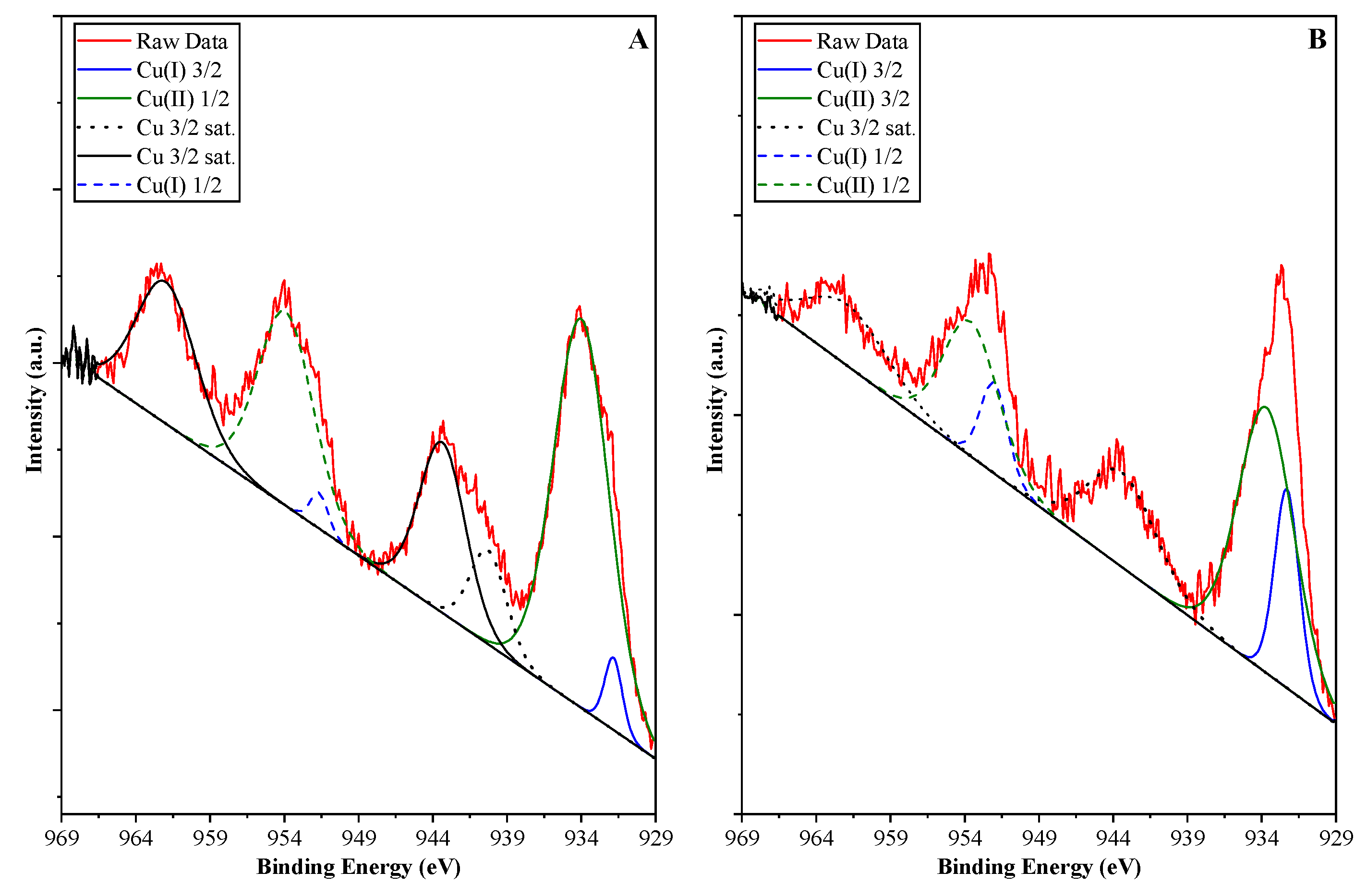
- Belloite will be transformed to elemental Cu as the experiment is carried out under reductive atmosphere (nitrogen), and the holes are scavenged by the oxalic acid. Moreover, the formation of pure CuCl2 (soluble in water) or Cu(OH)2 cannot be excluded, as well, due to migration of the Cl− from belloite to a Cu2+ center. And if CuCl2 will be formed again, then its reduction to elemental Cu will be possible again as the electrons will be consumed by ionic Cu, while the formed hydroxide could block the surface of the clusters/particles.
- If scenario (i) occurs, an increase in Cu content would be inevitable, thus possibly releasing a higher amount of H2.
- Cu can be oxidized by the formed holes, as well; therefore, the formation of oxides (CuO and Cu2O) also cannot be excluded.
- If scenario (iii) occurs, then we have an oxide/oxide heterojunction, which induces another variable in our system.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khetan, A.S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Amin, M.; Rashid, M. Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma. Chem. Eng. J. 2016, 313, 1033–1041. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef]
- Watcharenwong, A.; Chanmanee, W.; Tacconi, N.R.; Chenthamarakshan, C.R.; Kajitvichyanukul, R.; Rajeshwar, K. Anodic growth of nanoporous WO3 films: Morphology, photoelectrochemical response and photocatalytic activity for methylene blue and hexavalent chrome conversion. J. Electroanal. Chem. 2008, 612, 112–120. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Farbodn, M.; Ghaffari, N.M.; Kazeminezhad, I. Fabrication of single phase CuO nanowires and effect of electric field on their growth and investigation of their photocatalytic properties. Ceram. Int. 2014, 40, 517–521. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environmental remediation. ChemSusChem 2014, 7, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Arabatzis, I.M.; Stergiopoulos, T.; Andreeva, D.; Kitova, S.; Neophytides, S.G.; Falaras, P. Characterization and photocatalytic activity of Au/TiO2 thin films for azo-dye degradation. J. Catal. 2003, 220, 127–135. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and characterization of Ag–TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Yu, L.; Shao, Y.; Li, D. Direct combination of hydrogen evolution from water and methane conversion in a photocatalytic system over Pt/TiO2. Appl. Catal. B Environ. 2017, 204, 216–223. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Martinelli, A.; Alberti, S.; Caratto, V.; Lova, P.; Locardi, F.; Pampararo, G.; Villa, S.; Ferretti, M. Structural studies on copper and nitrogen doped nanosized anatase. Zeitschrift für Kristallographie 2018, 233, 867–876. [Google Scholar] [CrossRef]
- Alaoui, O.T.; Herissan, A.; Quoca, C.L.; Zekri, M.M.; Sorgues, S.; Remita, H.; Colbeau-Justin, C. Elaboration, charge-carrier lifetimes and activity of Pd-TiO2 photocatalysts obtained by gamma radiolysis. J. Photochem. Photobiol. A 2012, 242, 34–43. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; Ruiz de Larramendi, I.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.W.; Allaker, R.P.; Reip, P.; Oxford, J.; Ahmad, Z.; Ren, G. A review of nanoparticle functionality and toxicity on the central nervous system. J. R. Soc. Interface 2010, 7, 411–422. [Google Scholar] [CrossRef]
- Jafari, A.; Pourakbar, L.; Farhadi, K.; Mohamadgolizad, L.; Goosta, Y. Biological synthesis of silver nanoparticles and evaluation of antibacterial and antifungal properties of silver and copper nanoparticles. Turk. J. Biol. 2015, 39, 556–561. [Google Scholar] [CrossRef]
- Welch, C.M.; Hyde, M.E.; Banks, C.E.; Compton, R.G. The detection of nitrate using in-situ copper nanoparticle deposition at a boron doped diamond electrode. Anal. Sci. 2005, 21, 1421–1430. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Ranu, B.C.; Dey, R.; Chatterjee, T.; Ahammed, S. Copper nanoparticle-catalyzed carbon-carbon and carbon-heteroatom bond formation with a greener perspective. ChemSusChem 2012, 5, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Saha, A.; Jana, R. Microwave-assisted simple and efficient ligand free copper nanoparticle catalyzed aryl-sulfur bond formation. Adv. Synth. Catal. 2007, 349, 2690–2696. [Google Scholar] [CrossRef]
- Shephard, D.S.; Maschmeyer, T.; Sankar, G.; Thomas, J.M.; Ozkaya, D.; Johnson, B.F.G.; Raja, R.; Oldroyd, R.D.; Bell, R.G. Preparation, characterisation and performance of encapsulated copper ± ruthenium bimetallic catalysts derived from molecular cluster carbonyl precursors. Chem. Eur. J. 1998, 4, 1214–1224. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Ángeles Lillo-Ródenas, M.; Román-Martínez, M. Cu/TiO2 photocatalysts for the conversion of acetic acid into biogas and hydrogen. Catal. Today 2017, 287, 78–84. [Google Scholar] [CrossRef]
- Babu, B.; Mallikarjuna, K.; Reddy, C.V.; Park, J. Facile synthesis of Cu@TiO2 core shell nanowires for efficient photocatalysis. Mater. Lett. 2016, 176, 265–269. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Wu, J.C.S.; Chou, H.-Y. Effects of sol–gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction. J. Catal. 2004, 221, 432–440. [Google Scholar] [CrossRef]
- Yin, R.; Ling, L.; Xiang, Y.; Yang, Y.; Bokare, A.D.; Shang, C. Enhanced photocatalytic reduction of chromium (VI) by Cu-doped TiO2 under UV-A irradiation. Sep. Purif. Technol. 2018, 190, 53–59. [Google Scholar] [CrossRef]
- Turkten, N.; Cinar, Z.; Tomruk, A.; Bekbolet, M. Copper-doped TiO2 photocatalysts: Application to drinking water by humic matter degradation. Environ. Sci. Pollut. Res. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, D.H.; Kim, S.J.; Lee, K.S. The photocatalytic activity of 2.5 wt% Cu-doped TiO2 nano powders synthesized by mechanical alloying. J. Alloys Compd. 2006, 415, 51–55. [Google Scholar] [CrossRef]
- Sahu, M.; Biswas, P. Single-step processing of copper-doped titania nanomaterials in a flame aerosol reactor. Nanoscale Res. Lett. 2011, 6, 411. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.-M.; He, J.; Wang, L.-Y.; Lei, J.-D. Combining the photocatalysis and absorption properties of core-shell Cu-BTC@TiO2 microspheres: Highly efficient desulfurization of thiophenic compounds from fuel. Materials 2018, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Mogyorósi, K.; Kmetykó, Á.; Czirbus, N.; Veréb, G.; Sipos, P.; Dombi, A. Comparison of the substrate dependent performance of Pt-, Au- and Ag-doped TiO2 photocatalysts in H2-production and in decomposition of various organics. React. Kinet. Catal. L 2009, 98, 215–225. [Google Scholar] [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Jos Delgado, J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 photocatalysts for H2 production from ethanol and glycerol aqueous solutions. ChemCatChem 2011, 3, 574–577. [Google Scholar] [CrossRef]
- Wu, N.-L.; Lee, M.-S. Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int. J. Hydrog. Energy 2004, 29, 1601–1605. [Google Scholar] [CrossRef]
- Kotesh Kumar, M.; Yeon Do, J.; Prabhakar Vattikuti, S.V.; Kumar Reddy, P.A.; Kang, M. Solar light response with noble metal-free highly active copper(II) phosphate/titanium dioxide nanoparticle/copper(II) oxide nanocomposites for photocatalytic hydrogen production. J. Alloys Compd. 2018, 750, 292–303. [Google Scholar]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Suave, J.; Amorim, S.M.; Moreira, R.F.P.M. TiO2-graphene nanocomposite supported on floating autoclaved cellular concrete for photocatalytic removal of organic compounds. J. Environ. Chem. Eng. 2017, 5, 3215–3223. [Google Scholar] [CrossRef]
- Martínez, C.; Vilarino, S.; Fernández, M.I.; Faria, J.; Canle, M.L.; Santaballa, J.A. Mechanism of degradation of ketoprofen by heterogeneous photocatalysis in aqueous solution. Appl. Catal. B 2013, 142, 633–646. [Google Scholar] [CrossRef]
- Jankunaite, D.; Tichonovas, M.; Buivydiene, D.; Radziuniene, I.; Racys, V.; Krugly, E. Removal of diclofenac, ketoprofen, and carbamazepine from simulated drinking water by advanced oxidation in a model reactor. Water Air Soil Pollut. 2017, 228, 353. [Google Scholar] [CrossRef]
- Djouadi, L.; Khalaf, H.; Boukhatem, H.; Boutoumi, H.; Kezzime, A.; Santaballa, A.; Canle, M. Degradation of aqueous ketoprofen by heterogeneous photocatalysis using Bi2S3/TiO2–Montmorillonite nanocomposites under simulated solar irradiation. Appl. Clay Sci. 2018, 166, 27–37. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-ray Powder Diffractometry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996; pp. 47–95. [Google Scholar]
- Kubelka, P.; Munk, F. An article on optics of paint layers. Zeitschrift für Technische Physik 1931, 12, 593–601. [Google Scholar]
- Magyari, K.; Pap, Z.; Tóth, Z.R.; Kása, Z.; Licarete, E.; Vodnar, D.C.; Hernadi, K.; Baia, L. The impact of copper oxide nanoparticles on the structure and applicability of bioactive glasses. J. Sol-Gel Sci. Technol. 2019, 91, 634–643. [Google Scholar] [CrossRef]
- Kovács, G.; Baia, L.; Vulpoi, A.; Radu, T.; Karácsonyi, É.; Dombi, A.; Hernádi, K.; Danciu, V.; Simon, S.; Pap, Z. TiO2/WO3/Au nanoarchitectures’ photocatalytic activity, “from degradation intermediates to catalysts’ structural peculiarities”, Part I: Aeroxide P25 based composites. Appl. Catal. B Environ. 2014, 147, 508–517. [Google Scholar] [CrossRef]
- Kovács, G.; Fodor, S.; Vulpoi, A.; Schrantz, K.; Dombi, A.; Hernádi, K.; Danciu, V.; Pap, Z.; Baia, L. Polyhedral Pt vs. spherical Pt nanoparticles on commercial titanias: Is shape tailoring a guarantee of achieving high activity? J. Catal. 2015, 325, 156–167. [Google Scholar] [CrossRef]
- Fodor, S.; Kovács, G.; Hernádi, K.; Danciu, V.; Baia, L.; Pap, Z. Shape tailored Pd nanoparticles’ effect on the photocatalytic activity of commercial TiO2. Catal. Today 2017, 284, 137–145. [Google Scholar] [CrossRef]


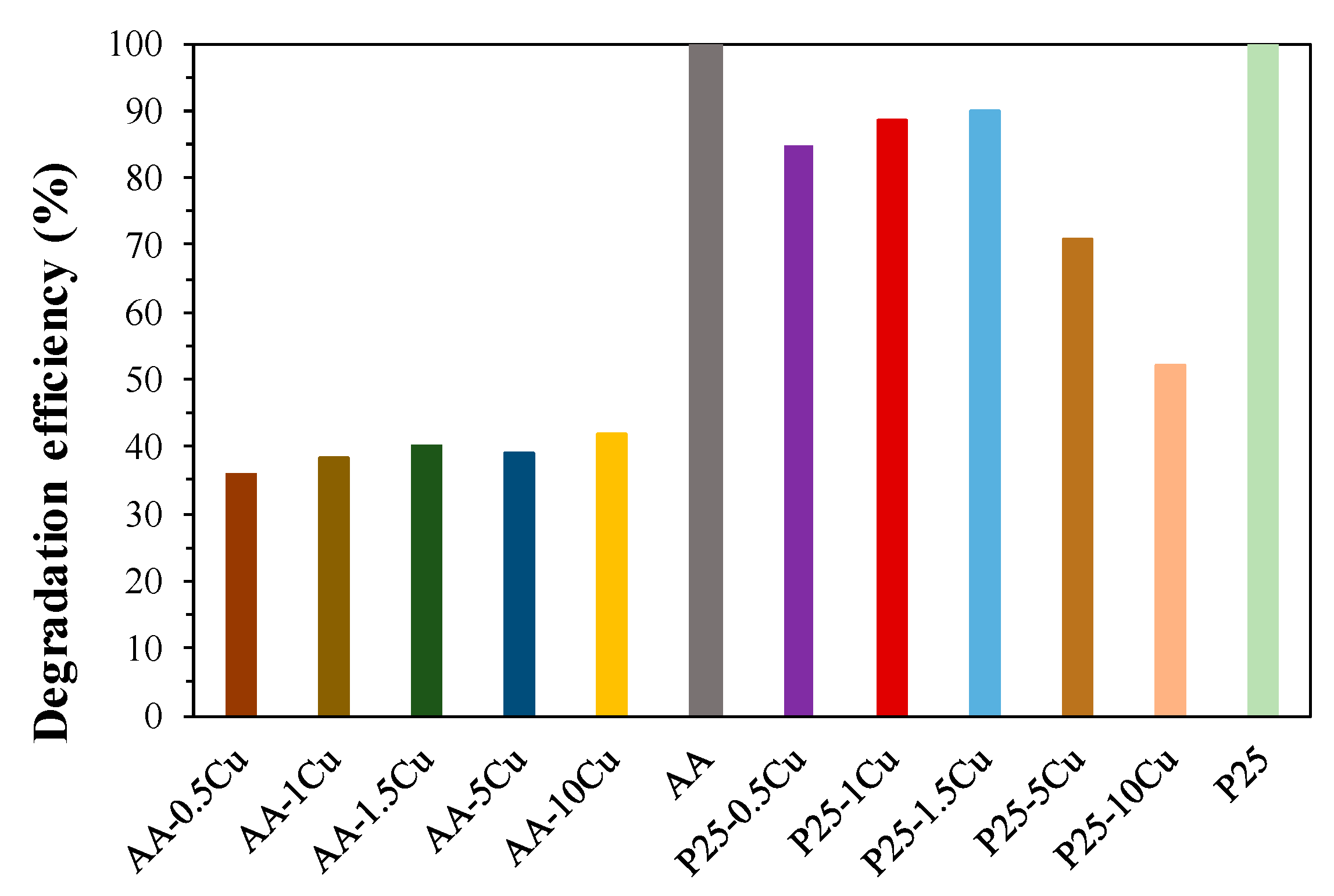

| Composite Name | Cu (%) | Band Gap (eV) | Methyl Orange Degradation (%) | Ketoprofen Degradation (%) | Rhodamine B Degradation (%) | |
|---|---|---|---|---|---|---|
| Theoretical | Real (EDX) | |||||
| AA-0.5Cu | 0.5 | 0.45 | 3.19 | 7.1 | 69.1 | 35.7 |
| AA-1Cu | 1.0 | 0.94 | 3.21 | 6.6 | 66.1 | 38.4 |
| AA-1.5Cu | 1.5 | 0.69 | 3.18 | 14.5 | 63.1 | 40.1 |
| AA-5Cu | 5.0 | 2.35 | 3.18 | 17.4 | 62.6 | 39 |
| AA-10Cu | 10.0 | 3.95 | 3.18 | 23.1 | 60.8 | 41.8 |
| P25-0.5Cu | 0.5 | 0.5 | 2.97 | 37.5 | 80.5 | 84.8 |
| P25-1Cu | 1.0 | 1.1 | 2.95 | 38.1 | 82.6 | 88.6 |
| P25-1.5Cu | 1.5 | 1.9 | 2.91 | 30.5 | 66.4 | 90.1 |
| P25-5Cu | 5.0 | 4.7 | 2.83 | 24.9 | 33.5 | 70.9 |
| P25-10Cu | 10.0 | 6.5 | 2.85 | 39.1 | 23.2 | 52.2 |
| AA | - | - | 3.24 | 75.6 | 88.9 | 100 |
| P25 | - | - | 3.04 | 82.8 | 78.7 | 100 |
| Sample Name | Methyl Orange | Rhodamine B | Ketoprofen | H2 Production Capacity (mL) | |||
|---|---|---|---|---|---|---|---|
| Rate Constant (min−1) | Degradation (%) | Rate Constant (min−1) | Degradation (%) | Rate Constant (min−1) | Degradation (%) | ||
| P25 | 0.0142 | 82.8 | n.a. | 100 | 0.021 | 78.7 | 0 |
| P25-0.5Cu | 0.0037 | 37.5 | 0.0189 | 84.8 | 0.0135 | 80.5 | 0.304 |
| P25-1Cu | 0.0039 | 38.1 | 0.0175 | 88.6 | 0.0149 | 82.6 | 0.466 |
| P25-1.5Cu | 0.003 | 30.5 | 0.0141 | 90.1 | 0.0089 | 66.4 | 0.721 |
| P25-5Cu | 0.0026 | 24.9 | 0.0063 | 70.9 | 0.0037 | 33.5 | 0.985 |
| P25-10Cu | 0.0038 | 39.1 | 0.0056 | 52.2 | 0.0021 | 23.2 | 0.358 |
| AA | 0.0107 | 75.6 | n.a. | 100 | 0.0176 | 88.9 | 0 |
| AA-0.5Cu | 0.0005 | 7.1 | 0.0033 | 35.7 | 0.0096 | 69.1 | 0.061 |
| AA-1Cu | 0.0005 | 6.6 | 0.0037 | 38.4 | 0.0088 | 66.1 | 0.338 |
| AA-1.5Cu | 0.0011 | 14.5 | 0.0038 | 40.1 | 0.0083 | 63.1 | 0.057 |
| AA-5Cu | 0.0015 | 17.4 | 0.0035 | 39.0 | 0.0083 | 62.6 | 0.161 |
| AA-10Cu | 0.002 | 23.1 | 0.0036 | 41.8 | 0.0077 | 60.8 | 0.108 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hampel, B.; Pap, Z.; Sapi, A.; Szamosvolgyi, A.; Baia, L.; Hernadi, K. Application of TiO2-Cu Composites in Photocatalytic Degradation Different Pollutants and Hydrogen Production. Catalysts 2020, 10, 85. https://doi.org/10.3390/catal10010085
Hampel B, Pap Z, Sapi A, Szamosvolgyi A, Baia L, Hernadi K. Application of TiO2-Cu Composites in Photocatalytic Degradation Different Pollutants and Hydrogen Production. Catalysts. 2020; 10(1):85. https://doi.org/10.3390/catal10010085
Chicago/Turabian StyleHampel, Boglárka, Zsolt Pap, Andras Sapi, Akos Szamosvolgyi, Lucian Baia, and Klara Hernadi. 2020. "Application of TiO2-Cu Composites in Photocatalytic Degradation Different Pollutants and Hydrogen Production" Catalysts 10, no. 1: 85. https://doi.org/10.3390/catal10010085
APA StyleHampel, B., Pap, Z., Sapi, A., Szamosvolgyi, A., Baia, L., & Hernadi, K. (2020). Application of TiO2-Cu Composites in Photocatalytic Degradation Different Pollutants and Hydrogen Production. Catalysts, 10(1), 85. https://doi.org/10.3390/catal10010085









