Abstract
Cu-SSZ-13 has been generally considered as the predominant commercial selective catalytic reduction (SCR) catalyst in the NH3-SCR reaction because of its superior activity and durability. However, in real applications, SCR catalysts readily undergo hydrothermal aging and sulfur poisoning. In this work, the deactivation and regeneration of a commercial Cu-SSZ-13 catalyst was investigated for SO2 exposures during hydrothermal aging and the effect of different regeneration temperatures was compared. By using XRD, SEM, H2-temperature programmed reduction (TPR), X–ray photoelectron spectra (XPS) and NH3-temperature programmed desorption (TPD) analysis, it was found that SO2 poisoning influenced the chabazite (CHA) structure even if regeneration cannot restore its original structure, the redox ability and ammonia storage performance also influenced by sulfation and the regeneration process. Moreover, the extent of a decrease in redox ability was more severe than acidity, suggesting that the amount of isolated Cu2+ and Cu+ reduction was responsible for irreversible deactivation over the Cu-SSZ-13 catalyst. Combined with the analysis of Ea values and pre-exponential factor of the SCR reaction, a more likely explanation for the irreversible deactivation was that active sites were lost mostly in sulfated and regenerated process sites.
1. Introduction
Selective catalytic reduction with ammonia (NH3-SCR) has been successfully developed as a promising technology for reducing NOx emitted in combustion gas emissions from automotive sources [1,2,3]. Cu-SSZ-13, with superior activity and durability, has been generally considered as the predominant commercial SCR catalyst applied in North American, European and Chinese diesel vehicles [4,5,6]. A disadvantage of Cu-SSZ-13 catalysts is their sensitivity to sulfur. SO2 severely deactivates the NH3-SCR performance over a Cu-SSZ-13 catalyst in diesel exhaust after-treatment systems. A problem, however, is that SO2 is an inevitable compound in diesel exhausts, even in ultra-low sulfur diesel which contains less than 10 ppm sulfur under CHINA VI regulation; accumulated lifetime exposure of catalysts in a heavy-duty system may reach to kilograms of sulfur [7,8,9,10].
Numerous studies have been carried out to learn about the reversible and irreversible damage of Cu-SSZ-13 such as dealumination of the framework structure, accumulation and distribution of different state copper species when Cu-SSZ-13 is subjected to sulfur poisoning [11,12,13]. These changes are closely related with deactivation of the Cu-SSZ-13 catalyst. It is generally considered that the sulfur poisoning of Cu-zeolite catalyst is caused by SO2 interaction with the Cu-sites, including adsorption of SO2 or a chemical reaction between the SO2 and Cu-sites. Another mechanism for catalyst deactivation induced by SO2 is at least partially due to the formation of ammonium sulfate on the catalyst, which restricts reactants access to the active sites [8,12,13]. It is known that the detrimental effect of SO2 on the NOx conversion is different from hydrothermal aging. The catalytic activity of sulfur poisoned Cu-SSZ-13 can be recovered by high temperature treatment [9,14,15]. When sulfur poisoning of the SCR catalyst is studied, the co-effect of hydrothermal aging and sulfur poisoning is always ignored. In fact, if the regeneration of diesel particulate filter (DPF) is present in the exhaust after-treatment system, the SO2 formation from substances accumulated on DPF will take place, which goes through the SCR catalysts together [16,17,18]. Therefore, the regeneration of Cu-SSZ-13 catalyst should be taken into account during SO2 poisoning and hydrothermal aging.
In this work, the deactivation and regeneration of a commercial Cu-SSZ-13 catalyst was investigated for SO2 exposures during hydrothermal aging and the effect of different regeneration temperatures was compared. By using different characterization techniques, we investigated the changes of the framework structure, the acid and active sites of the Cu-SSZ-13 with different regeneration temperature. All conclusions would help to understand the impact of the reversible and irreversible deactivation of Cu-SSZ-13 over long-term SO2 exposure, thereby providing guidance for applying Cu-SSZ-13 in the diesel after-treatment system.
2. Results and Discussion
2.1. X-ray Powder Diffraction
The framework of the Cu-SSZ-13 catalysts in fresh, sulfated and regenerated state was characterized by XRD measurements. As shown in Figure 1, all the samples exhibited typical CHA topological structure (PDF data file 52-0784) [18,19,20]. No noticeable diffraction peaks assigned to CuO (2θ = 35.6° and 38.8°) or Cu2O (2θ = 36.4°) [21] species could be observed because of the lower content of copper oxides (~3%) in our catalyst than the requirement of the laboratory-based XRD (5%). Notably, compared with F-Cu, the strongest diffraction peaks centered at about 9.6° shifted to a higher angle range for S-Cu and R-450, while with the increase of regeneration temperature, the position of the main diffraction peak over the catalysts was almost the same as F-Cu. The lattice parameter corresponding to the sharp peak was calculated via Bragg’s Law [22] and the results were plotted in Figure 2. The d-spacing of the zeolite decreased from 9.19 to 9.14 Å after sulfated treatment, and still reduced to 9.11 Å after regeneration at 450 °C, which was a hint for lattice contraction. However, the matrix of the catalysts was expanding when the regeneration temperature was up to 500 °C and higher, and the d-spacing maintained basically as 9.17 or 9.19 Å.
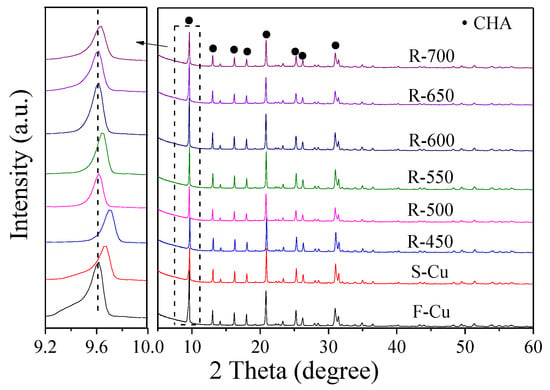
Figure 1.
XRD profiles of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
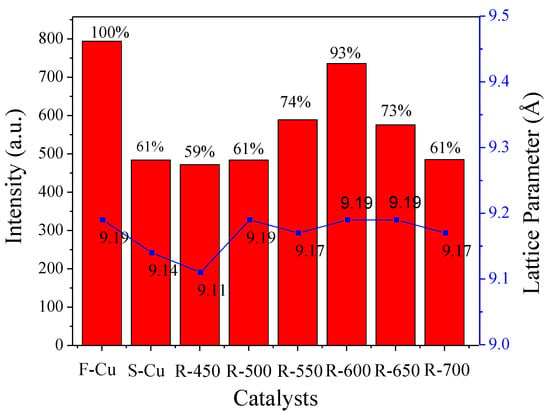
Figure 2.
The relative crystallinity and lattice parameter of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
As is known, the peak intensity has been reported to correlate with the crystallinity of the zeolites [22,23]. The changes in samples crystallinity are listed in Figure 2. It should be noted that the crystallinity of F-Cu sample was assumed as 100%, and the crystallinity evolution of the sulfated and regenerated samples were determined by using the sharp peak 9.6° of the CHA zeolite. As the pattern showed, the S-Cu sample was only crystalline in 61%, indicating that the harsh SO2 treatment at 450 °C had an obvious influence on CHA structure. After regeneration at 450 and 500 °C, the crystallinity of the samples remained the same. Furthermore, increasing regeneration temperature, such as 550 or 600 °C, brought a considerable modification of the zeolite structure. This demonstrated that 600 °C regeneration treatment can be able to remove sulfur from the crystalline structure. In addition, the peak intensity of the catalyst obviously decreased when the regeneration temperature exceeded 600 °C, this may have been due to the harsh hydrothermal exposure at 650 or 700 °C decreasing the crystallinity of the zeolites.
2.2. Nitrogen Physisorption Analyses
The N2 physisorption results of the investigated samples are summarized in Table 1. After sulfur treatment, a 11.4% loss of surface area was found on S-Cu relative to F-Cu, and the total pore volume decreased from 0.2747 to 0.2478 mL/g. Apart from the aggregation of sulfur species in the micropores, it may also be associated with a slight destruction of micropores. This finding was in accordance with Wijayanti et al. [24] and Shen et al. [25]. Meanwhile, the moderate increase of the pore size was observed on S-Cu. When S-Cu underwent thermal treatment with increasing temperature, the specific surface area and total pore volume of samples increased significantly. It was clear that the total pore volume of R-650 and R-700 was basically consistent with F-Cu, indicating that the agglomeration that blocked the “channel” of catalysts was gone after regeneration at 650 or 700 °C. However, a decrease of specific surface area was still 4.5% on R-650 and R-700 with regard to F-Cu, which may have been associated with the decreased structural integrity of Cu-SSZ, as with XRD discussed above. A similar result was obtained by Shen et al [18].

Table 1.
Textural properties over Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
2.3. Scanning Electron Microscopy
The structural changes of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states can be observed in the SEM micrographs as displayed in Figure 3, where morphological changes were evident. It can be seen that the F-Cu sample was cube-shaped grains and showed a perfect degree of crystallization. When F-Cu was sulfated with SO2 feed, the grain morphology broke severely. After regeneration, the damage of the cubic shape and quantity retained to a certain extent, suggesting that the irreversible deterioration was existed, which was verified by XRD and N2 physisorption results.
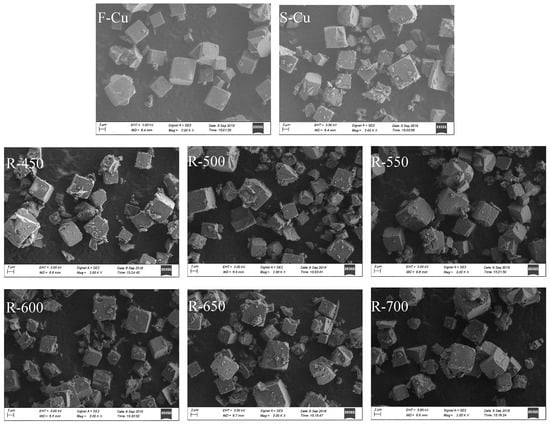
Figure 3.
SEM images of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated state.
The elemental mapping and energy dispersive X-ray spectroscopy (EDX) analysis results of studied samples are illustrated in Figure 4. It was clear that the particles which were attributed to the sulfur species on the zeolite catalysts could be observed after F-Cu treating by SO2 feed, and the amount of SO2 monotonically decreased with regeneration temperature increasing. As reported by Wijayanti et al. [26], copper sulfates were predominant after poisoning at 400 °C. Wang et al. [27] confirmed by the diffuse reflectance infrared Fourier transform spectroscopy (DRIFTs) and thermo gravimetric analyzer (TGA) that the formation of copper sulfate on the sulfated Cu–CHA catalysts. Shen et al. [28] reported that copper sulfate was completely decomposed at 750 °C, and sulfur species over our sample could be completely removed when the temperature reached 700 °C, so it was reasonably believed that there were copper sulfates present. No ammonium sulfate was presented because our samples underwent sulfur poisoning in air without NH3.
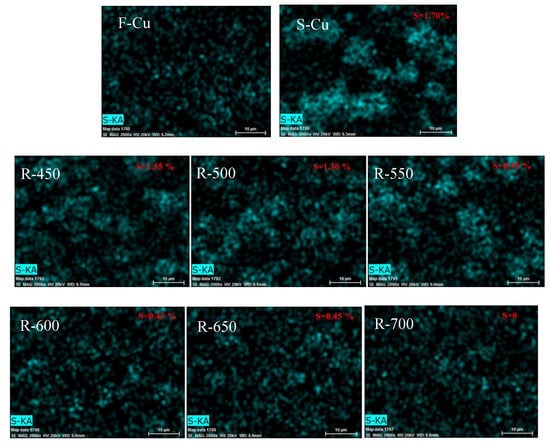
Figure 4.
The elemental mapping and EDX results of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
2.4. X–ray Photoelectron Spectra
X-ray photoelectron spectroscopy (XPS) was carried out to evaluate the surface characteristics of catalysts in fresh, sulfated and regenerated states. Figure 5a shows the XPS spectra of Cu 2p bands of the catalysts. According to literature [21,29,30], the Cu 2p3/2 and Cu 2p1/2 peaks ascribed to Cu2+ appeared at about 933.3 and 955.5 eV, with two shakeup satellite peaks located at about 939.9 and 944.7 eV, while peaks assigned to Cu+ species were located at about 932.4 and 952.6 eV. Furthermore, the peak at around 938.3 eV pertained to Cu2+ in CuO, and the one at 944.1 eV represented the existence of dispersed Cu2+ species on the surface. The atomic ratio of surface Cu and integral computation of each Cu species of all samples calculated by Gaussian deconvolution are displayed in Table 2. It can be seen that the atomic ratio of surface Cu was smaller than those in bulk (~3%), indicating that there were more than one kind of framework sites on Cu-SSZ-13. In other words, not all copper species can be detected by the XPS technique. This phenomenon was also evidenced by other scholars [21,31]. It was noticed that Cu2+ species in the bulk CuO was observed for all samples, which was not in accordance with the XRD analysis above. This may be because concentrations so low are not probably detected by XRD. In comparison with the fresh Cu-SSZ-13, the amount of Cu2+, Cu+, Cu2+ in CuO and dispersed Cu2+ species decreased significantly after sulfur treatment, indicating a great loss of surface Cu species in the catalyst. However, the appreciable decrease was also observed for the regeneration catalysts, suggesting that the loss of surface copper species was irreversible. Additionally, the ratio of Cu+/Cu2+ was calculated by the area ratio of corresponding peaks. The results in Table 2 shows that the ratio of Cu+/Cu2+ was the highest for F-Cu (0.89), demonstrating that more Cu+ existed on the surface of the fresh sample, whereas the ratio decreased on the surface of the sulfured and regenerated samples. It was worthwhile to note that the ratio of Cu+/Cu2+ increased with regeneration temperature rising and reached to the maximum (0.84) for R-650, from which can be inferred that the high temperature regeneration can achieve removal of sulfur species and formation of Cu+ species. While the amount of Cu+ ions decreased with the temperature increasing to 700 °C, indicated that the regeneration of Cu-SSZ-13 was possible without resorting to so high temperatures.

Figure 5.
Cu 2p (a) and S 2p (b) X-ray photoelectron spectra (XPS) spectra of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated state.

Table 2.
The atomic ratio of surface Cu and integral areas of Cu 2p and S 2p determined from XPS spectra over Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
The XPS spectra of S 2p peaks are exhibited in Figure 5b. As shown, the peak at about 169.2 eV was originated to the S 2p signal of SO42−, which demonstrated the presence of sulfur species on both of the sulfated and regenerated state catalysts [32,33,34]. There was no SO32− species on the poisoning catalysts surface because of the presence of excessive O2 during the sulfating process [34]. Table 2 reveals that large amounts of sulfur species formed on Cu-SSZ-13 after sulfur treatment. After the regeneration process, the amount of sulfur complexes decreased monotonically with increasing temperature, and the sulfur species vanished when the regeneration temperature reached 700 °C. These results were in good agreement with EDX. Despite the possible formation of CuSO4 or H2SO4 existing by SO2 reacting with H2O and O2 on the Cu-SSZ-13 catalysts as suggested by Yang et al. [35], the bonding strength between surface Cu (Cu2+, Cu+ or Cu+/H+) and S was so weak that it was broken in the regeneration process. Thus, the SCR activity of the Cu-SSZ-13 catalyst recovered well after the regeneration.
2.5. H2-Temperature Programmed Reduction (TPR) Measurements
In order to further investigate the Cu species in fresh, sulfated and regenerated catalysts, the H2-TPR was measured. As presented in Figure 6, compared with the fresh Cu-SSZ-13, the catalysts after sulfating and regeneration exhibited diverse reduction behaviors, indicating the sulfur had a significant influence on the distribution of Cu species. It can be seen that there were four reduction peaks in the profiles. As reported by Shen et al. [28], the reduction of Cu2+ is around 200–300 °C, the reduction of the bulk CuO is at about 300−400 °C, and the peak above 400 °C is ascribed to the reduction from Cu+, which was also confirmed by Fan et al. [36] and Gao et al. [37]. Wijayanti et al. [13] find only one peak at around 413 °C in Cu-SSZ-13 and proposes that the peak originates from the reduction of Cu2+. Besides, a recent investigation by Chen et al. [38] shows that the peaks centered at about 230 and 425 °C stand for the reduction of isolated Cu2+ ions at CHA cages and double six-rings (D6Rs) to Cu+, the peak at approximately 300 °C can be assigned to the one-step reduction of CuO to Cu0, the peaks at about 650 and 865 °C indicate the reduction of two different types of Cu+ into Cu0, respectively. In this study, we interpreted the first peak (α) from the fresh sample at lower temperature as an indication of the reduction of isolated Cu2+ located in different cationic sites to Cu+. The peak β appearing at a moderate temperature stood for the reduction of CuO to Cu0 and low-stability Cu+ into Cu0, respectively. The peak γ centered at around 770 °C came from the reduction of high-stability Cu+ to Cu0. As is known, the reduction of Cu+ species contains the initial Cu+ species existing in zeolites and Cu+ species produced by Cu2+ ions reduction [13,36,37,38].

Figure 6.
Temperature programmed reduction (TPR) profiles of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
It is worth noting that the position of the reduction peaks over S-Cu sample shifted obviously compared with F-Cu. As shown in Figure 6, there were three reduction peaks at about 442, 529 and 695 °C, which can be attributed to the deactivated copper species. This is because the position of the reduction peaks is close to those observed from the reduction of CuSO4·5H2O [18]. An obvious H2 consumption peak was seen above 800 °C, which can be assigned to the sulfates or other surface SO2 groups created during sulfur and regenerated treatment. Furthermore, it was clearly seen that the position of the reduction peaks changed after hydrothermal regeneration. By combining the reduced diffraction peak intensity of the regenerated catalysts in the XRD discussion above, it can be concluded that the coordination environment or redox ability of copper ions has been changed. Figure 7 displays the variation of all copper species amount calculated from TPR results. A previous investigation by Wijayanti et al. [13] shows that the reduction of copper and sulfur species overlap, which was why it is not possible to resolve the hydrogen consumption owing to copper reduction in the sulfur poisoned and hydrothermally regenerated samples. Shen et al. [18] demonstrates that the amounts of CuO in CHA catalyst are basically unchanged either reacted with SO2 or SO3. According to this literature, we illustrated that the reduced Cu2+ as well as Cu+ was due to sulfur species deterioration and copper sulfate formation. It was noticeable that more than 50% reduction of active copper species was found on S-Cu relative to F-Cu, which was due to the formation of the SO3 under H2O + SO2 + O2 conditions. This result was in line with the study of Shen et al. [18] that a higher sulfate formation rate was obtained in the presence of SO3 because of the higher oxidizing ability of SO3 than that of SO2. Apparently, the amount of Cu species under a low oxidation state (Cu+) declined more severely compared to isolated Cu2+ ion after vulcanization and regeneration, which was consistent with the periodic density functional theory study of Yang et al [35]. In addition, the H2 consumption of the samples declined significantly in Figure 7 after hydrothermal treatment in the presence of SO2, which was likely due to some generated Cu–SO4 complex on the surface dissolved in water during the cooling process.
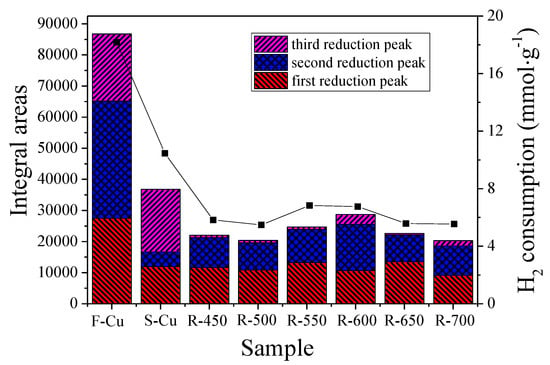
Figure 7.
Cu species amount of catalysts and H2 total consumption based on the H2-TPR analysis.
2.6. NH3-TPD Measurements
To confirm the density and strength of acid sites in fresh, sulfated and regenerated Cu-SSZ-13 catalysts, a NH3-temperature programmed desorption (TPD) measurement was carried out. As shown in Figure 8, there were two distinct regions at 250−400 and 400−800 °C for F-Cu sample, and the two NH3 desorption peak could be fitted to three peaks (A, B and C) after deconvolution because of the asymmetry of the second peak. Combined with the previous studies [11,36,39,40], the peak A at about 319 °C was related to the moderate structural Brønsted acid sites (Si–O(H)–Al). The peak B appearing at 474 °C represented the adsorbed NH3 molecules at Lewis acid sites created by isolated Cu2+ species, whereas the peak C centered at ~587 °C was attributed to ammonium species adsorbed at strong Brønsted acid sites and strong Lewis acids. It was worth noting that the catalyst desorbed more ammonia after sulfur poisoning than the fresh catalyst. As is known [41], Cu-SSZ-13 can oxidize SO2 to SO3 due to the strong oxidizability, which can lead to more pronounced sulfate formations such as H2SO4, CuSO4 and Al2(SO4)3. These sulfur species may bind more ammonia, which can explain the higher ammonia reserves over the S-Cu sample. Furthermore, the NH3 desorption peaks of sulfured samples appeared at a higher temperature, suggesting the ammonia adsorbed at the sulfur sites was more cohesive. Additionally, compared with the NH3 desorption of S-Cu (Figure 9), the acidity amount decreased with the regenerated temperature increasing to 550 °C, which was induced by the decomposition of the ammonia sulfate species and unrecovered active sites. When the regeneration temperature of the catalyst was up to 700 °C, the acidity was still less than the F-Cu sample. This illustrated that the sulfating and regeneration process had an influence on the Brønsted acid sites, which was consistent with the XRD results.
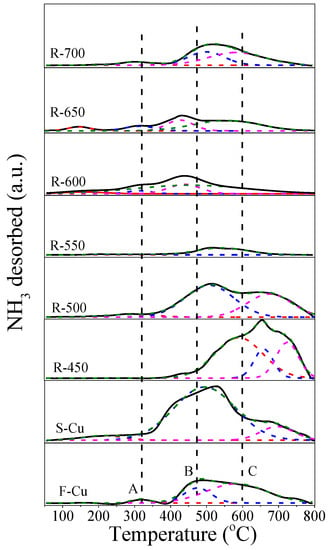
Figure 8.
Temperature programmed desorption (TPD) profiles of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.

Figure 9.
The acidity of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated state.
2.7. Catalytic Performance
Figure 10 shows the results of the catalytic activities for NH3-SCR of NOx over Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states. For the SO2 exposure, 500 ppm of SO2 was added to the humid aging atmosphere, and the catalyst was held at 450 °C for 10 h. A regeneration treatment was carried out over the sulfated catalysts at 450−700 °C for 30 min in aging atmosphere without SO2 aimed to remove sulfur and restore activity. As displayed in Figure 10, the F-Cu catalyst exhibited high activity with ~90% NO conversion from 175 to 450 °C. After exposure to SO2, the catalytic activity in the entire studied temperature range considerably decreased except 350–450 °C. Compared to the F-Cu sample, the NO conversion of S-Cu decreased from 100% to ~20% at 200 °C, and reached about 90% as high as 300 °C, which likely resulted from the competitive adsorption of NH3 between sulfur species and active sites over the catalyst. It can be clearly seen that the catalytic activity of the sulfated catalysts in the NH3-SCR reaction gradually recovered with the regeneration temperature increasing, among which R-650 recovered the most between 150 and 200 °C. Particularly noteworthy, when the regeneration temperature increased to 700 °C, the activity decreased about 10% relative to that of R-650 at 150 and 175 °C. Furthermore, the activity of R-650 reached the level of F-Cu above 200 °C, however, the activity did not reach its original SCR performance from 150 to 200 °C. These phenomena can be understood in terms of irreversible and reversible deactivation. According to the definitions by Hammershøi et al. [17], the irreversible deactivation is the deactivation measured after regeneration at different temperatures, and the difference in deactivation after SO2 exposure and regeneration is the reversible deactivation.
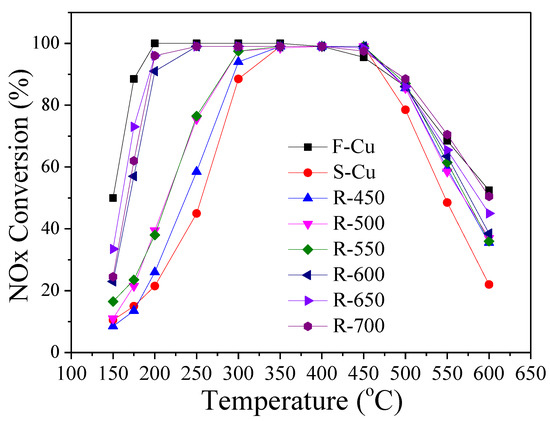
Figure 10.
Selective catalytic reduction (SCR) catalytic performance of Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
According to Figure 10, the NOx conversion of both the sulfated and regenerated states of the catalysts below 250 °C was lower than that of F-Cu in all measurements. Furthermore, the irreversible deactivations in Figure 11 show that there was a clear trend of less extensive deactivation of the catalyst at higher regenerated temperature, except the low temperature (150 and 175 °C) performance of R-700 catalyst. It was notable to see that the irreversible deactivation of catalysts at 150 and 175 °C decreased slightly when the regeneration temperature was below 600 °C. Since the decomposition product of CuSO4 is CuO, and CuO in zeolite exhibits less activity than copper ions [21,28], it was reasonable to believe that the severe irreversible deactivation was due to abundant CuO over catalysts at this regeneration temperature range (450–550 °C). As the regeneration temperature continued to rise (600–650 °C), the irreversible deactivation of catalysts at 150 and 175 °C declined significantly. It should be considered that the increased contents of isolated Cu2+ or Cu+ were due to copper sulfate decomposition and the migration effect (CuO to isolated Cu2+ or Cu+) [28]. These changes of copper species content was confirmed by our H2-TPR results. On the contrary, when the regeneration temperature reached 700 °C, the low temperature irreversible deactivation increased by nearly 20%. This phenomenon was correlated with the decreased contents of surface Cu2+ and Cu+ [18,41] which values were calculated from XPS. Additionally, a different trend was observed for the irreversible deactivation of the regenerated states of the catalyst when the reaction temperature was 550 and 600 °C. As displayed in Figure 11, it can be seen that the deactivation attained more than 20% compared to S-Cu after being treated at 450 °C. When regeneration temperature was between 500−600 °C, a slight decrease or no influence was observed. A significant decrease of the deactivation was detected as the regeneration temperature continued to rise (650–700 °C). According to literature by Chen et al. [21], the isolated Cu2+ ions probably made a contribution to the high-temperature activity. Thus, the above results of activity recovery were related to decomposition and desorption of the H2SO4 and weak-bond Cu–SOx species at 450 °C, and in order to further decompose and make poisoned Cu2+ recover, it was essential to increase the regeneration temperature to 700 °C. This conclusion was also confirmed by our EDX and XPS discussion. The results presented above clearly displayed that sulfur primarily influenced the low-temperature activity of F-Cu sample for SCR, and the irreversible deactivation was related to the decomposition of Cu-sulfate species and migration (CuO to isolated Cu2+ and Cu+) reactions.
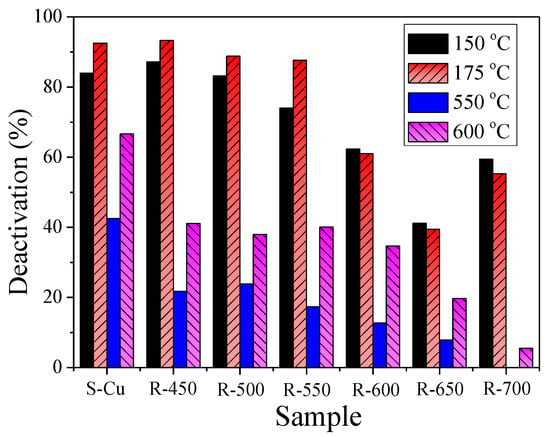
Figure 11.
Deactivation evaluated at 150, 175, 550 and 600 °C of the sulfated and regenerated states of the Cu-SSZ-13 catalyst.
Does the property (structure, redox ability and ammonia storage performance) of Cu-SSZ-13 remain the same after sulfation and the regeneration process? This question could be answered by XRD, SEM, H2-TPR and NH3-TPD results. As shown in Figure 2,3, the crystallinity and morphology of S-Cu and R-Cu were not as the same as that of F-Cu, illustrating that the CHA structure was influenced by SO2 and could not be restored by regeneration. According to the H2-TPR results, the property of Cu2+ and Cu+ on the sulfated and regenerated catalysts were different from that of F-Cu. Moreover, the acid contents of these samples also differed from F-Cu, as displayed in the NH3-TPD results. It is reasonably believed that the redox ability and ammonia storage performance can be influenced by sulfation and the regeneration process even if all the copper sulfate decomposed. Notably, the extent of decrease in redox ability was more severe than acidity, suggesting that the amount of isolated Cu2+ and Cu+ reduction was responsible for irreversible deactivation over Cu-SSZ-13 catalyst.
The Arrhenius plots for the SCR reaction over the fresh, sulfated and regenerated catalysts are displayed in Figure 12. During SO2 poisoning and regeneration under humid conditions, the slopes of sulfated and regenerated states of the catalyst were different from that of fresh catalyst. It can be seen that the slope of S-Cu catalyst was smaller compared to F-Cu, while the slopes of regenerated catalysts were bigger than the fresh catalyst when regeneration temperatures were between 600−700 °C. Thus, our interpretation was that apparent activation energies (Ea) of the samples differed, indicating distinguishing catalytic centers and rate-limiting steps exist. Another parameter that can contribute to the difference in rate constants is the pre-exponential factor. It was seen that the pre-exponential factor of F-Cu, R-600, R-650 and R-700 are bigger than other samples, which was well consistent with the NH3-SCR results. Since the Ea values and pre-exponential factor of the SCR reaction was well correlated with the amount of Cu2+ and Cu+ redox ability, it is also demonstrated that a loss of active sites was essential for the irreversible deactivation over sulfated and regenerated Cu-SSZ-13 catalyst.
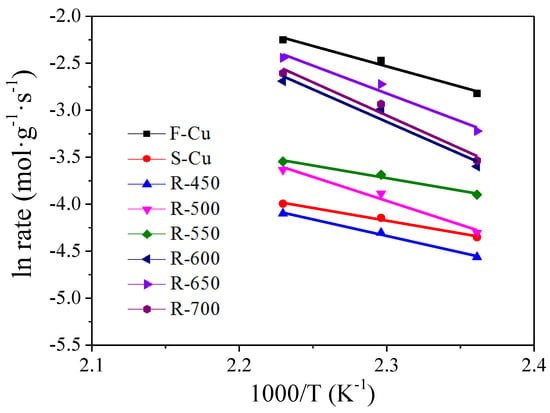
Figure 12.
Arrhenius plots of the SCR reaction rates over Cu-SSZ-13 catalysts in fresh, sulfated and regenerated states.
3. Experimental
3.1. Catalyst Preparation
Cu-SSZ-13 catalysts were commercial SCR catalysts. The samples underwent sulfur poisoning by exposing it to 500 ppm SO2 with 10% H2O in air at 450 °C for 10 h. The total flow rate and the gas hourly space velocity (GHSV) were 300 mL·min–1 and 60 000 h–1, respectively. After sulfur treatment, regeneration was carried out in situ in the reactor at different temperatures in the presence of 10% H2O and air. The regeneration temperatures were 450, 500, 550, 600, 650 and 700 °C, and the time was 30 min.
The fresh Cu-SSZ-13 was denoted as F-Cu, the sulfur poisoning sample was named as S-Cu, and regenerated catalysts were denoted as R-X, where X indicates 450, 500, 550, 600, 650 and 700 °C.
3.2. Catalyst Characterization
X–ray diffraction measurement was carried out on an X’pert Pro rotatory diffractometer (Panalytical Company, The Netherlands) operating at 40 mA and 40 kV using Cu Kα as radiation source (λ = 0.1542 nm). The data of 2θ from 10° to 90° were collected with a step size of 0.03°.
The Brunner-Emmet-Teller (BET) surface area was measured at −196 °C on an ASAP 2050 physical adsorption instrument (Micromeritics Corp., Norcross, GA, USA) by using the nitrogen adsorption method. The samples were pretreated in vacuum at 300 °C for 4 h before experiments. The surface area was determined by Langmuir method.
Scanning electron microscopy (SEM) images were taken with a ZEISS Sigma 500 field emission microscope. The samples were fixed onto a sample holder using carbon tape and then covered with Au spray to make them conductive.
X–ray photoelectron spectra (XPS) were recorded with a Thermo ESCALAB 250Xi spectrometer (ThermoFisher Scientific, Waltham, MA, USA using Al Kα radiation (1486.6 eV). The binding energies were calibrated using C1s peak of contaminant carbon (B.E. = 284.6 eV) as standard, and quoted with a precision of ± 0.2 eV. The surface composition of the samples in terms of atomic ratios was calculated, and Gaussian–Lorentzian and Shirley background was applied for peak analysis.
Temperature programmed reduction (TPR) and NH3 temperature programmed desorption (NH3–TPD) were conducted on a Quantachrome: Chem BET chemisorption analyzer supplied by Micromeritics Company. Each time, 50 mg of the sample were heated from room temperature to 800 °C at a rate of 10 °C·min–1. A mixture gas consisting of H2 and N2 with H2 content of 5vol.% was used as reductant at a flow rate of 60 mL·min–1. Before detection by the thermal conductivity detector (TCD), the gas was purified by a trap containing CaO + NaOH materials in order to remove the H2O and CO2.
3.3. Activity Evaluation
The catalytic activity of the catalysts was evaluated by in a fixed–bed quartz reactor (I.D. = 6 mm) at atmospheric pressure. The reaction conditions were controlled as follows: 200 ppm NO, 200 ppm NH3, 12% O2, 5% H2O, 200 ppm CO, 4.5% CO2, 50 ppm C3H6 and N2 as balance gas. The total flow rate and the gas hourly space velocity (GHSV) were 300 mL·min–1 and 300,000 h–1, respectively. The effluent gas, including NO, NO2 and O2 was continuously analyzed by an online flue gas analyzer. The results for steady–state activity of catalysts were recorded after about 20 min at each temperature. The NOx conversion were calculated accordingly [3], whereas NOx = NO + NO2.
If we assume that the SCR reaction is first order in NO, the rate constant is according to [7,17]:
where F is the total molar flow rate, W is catalyst weight, and X is NOx conversion.
The deactivation is expressed as the relative rate constant of a sulfated or regenerated catalyst (k) with respect to the rate constant for the fresh catalyst [7,17]:
According to the definitions in [18], the SCR reaction rate is given by the following equation:
where XNOx is the conversion of NOx, FNOx is the volumetric flow rate of NOx.
4. Conclusions
The deactivation and regeneration behaviors of a commercial Cu-SSZ-13 catalyst in the NH3-SCR reaction were evaluated in a simulated real diesel engine exhaust before and after exposure to 500 ppm SO2 with 10% H2O in air at 450 °C, and regenerated at 450−700 °C in this atmosphere. It could be seen that sulfur removal was accompanied by the recovery of the NOx conversion efficiency, however, the low-temperature (below 350 °C) activity did not restore its original SCR performance even if the regeneration temperature was as high as 700 °C, and the irreversible deactivation was consistent with decomposition of Cu-sulfate species and migration (CuO to isolated Cu2+ and Cu+) reactions. For the sulfated and regenerated catalysts, the crystallinity and morphological were not as the same as that of F-Cu, illustrating that SO2 poisoning influenced the CHA structure. Besides, the redox ability and ammonia storage performance can be influenced by sulfation and the regeneration process even if all the copper sulfate decomposed. Notably, the extent of decrease in redox ability was more severe than acidity, suggesting that the amount of isolated Cu2+ and Cu+ reduction was responsible for irreversible deactivation over the Cu-SSZ-13 catalyst. The analysis of Ea values and pre-exponential factor of the SCR reaction demonstrated that a loss of active sites was essential for the irreversible deactivation over sulfated and regenerated Cu-SSZ-13 catalyst.
Author Contributions
Conceptualization, (Y.W.) Yan Wang; investigation, (Y.W.) Yan Wang, R.F., C.Z., (Y.W.) Yu Wang, Z.D., R.W., X.G. and W.L.; data curation, (Y.W.) Yan Wang, R.F., X.G.; writing—review and editing, (Y.W.) Yan Wang, Z.L.; supervision, Z.L., R.W.; project administration, Z.L.
Funding
This research was funded by the Science and Technology Projects of China Northern Rare Earth (Group) High–tech Co., Ltd. (Grant No. BFXT–2019–D–028) and Natural Science Foundation of Inner Mongolia (Grant No. 2017MS0209).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Zhao, H.W.; Zhao, Y.N.; Liu, M.K.; Li, X.H.; Ma, Y.H.; Yong, X.; Chen, H.; Li, Y.D. Phosphorus modification to improve the hydrothermal stability of a Cu-SSZ-13 catalyst for selective reduction of NOx with NH3. Appl. Catal. B Environ. 2019, 252, 230–239. [Google Scholar] [CrossRef]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal. B Environ. 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Li, Z.Q.; Wang, Y.; Fan, R.R.; Zhang, C.; Wang, Y.; Guo, X.; Wang, R.; Zhang, S.L. Ce and Zr Modified WO3-TiO2 Catalysts for Selective Catalytic Reduction of NOx by NH3. Catalysts 2018, 8, 375. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts, 1st ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local Environment and Nature of Cu Active Sites in Zeolite-Based Catalysts for the Selective Catalytic Reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Hua, L.; Shuai, S.; Wang, J. Ammonia Storage and Slip in a Urea Selective Catalytic Reduction Catalyst under Steady and Transient Conditions. Ind. Eng. Chem. Prod. Res. Dev. 2011, 50, 11863–11871. [Google Scholar] [CrossRef]
- Hammershøia, P.S.; Jensen, A.D.; Janssens, T.V.W. Impact of SO2-poisoning over the lifetime of a Cu-CHA catalyst for NH3-SCR. Appl. Catal. B Environ. 2018, 238, 104–110. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. Chemical deSOx: An effective way to recover Cu-zeolite SCR catalysts from sulfur poisoning. Catal. Today 2016, 267, 10–16. [Google Scholar] [CrossRef]
- Cheng, Y.; Montreuil, C.; Cavataio, G.; Lambert, C. Sulfur tolerance and DeSOx studies on diesel SCR catalysts. SAE Int. J. Fuels Lubr. 2008, 1, 471–476. [Google Scholar] [CrossRef]
- Dahlin, S.; Nilsson, M.; Bäckström, D.; Bergman, S.L.; Bengtsson, E.; Bernasek, S.L.; Pettersson, L.J. Multivariate analysis of the effect of biodiesel-derived contaminants on V2O5-WO3/TiO2 SCR catalysts. Appl. Catal. B: Environ. 2016, 183, 377–385. [Google Scholar] [CrossRef]
- Shan, Y.L.; Shi, X.Y.; Yan, Z.D.; Li, J.J.; Yu, Y.B.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2019, 320, 84–90. [Google Scholar] [CrossRef]
- Shih, A.J.; Khurana, I.; Li, H.; González, J.; Kumar, A.; Paolucci, C.; Lardinois, T.M.; Jones, C.B.; Caballero, J.D.A.; Kamasamudram, K.; et al. Spectroscopic and kinetic responses of Cu-SSZ-13 to SO2 exposure and implications for NOx selective catalytic reduction. Appl. Catal. A Gen. 2019, 574, 122–131. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; An, H.; Yezerets, A. Impact of different forms of feed sulfur on small-pore Cu-zeolite SCR catalyst. Catal. Today 2014, 231, 75–82. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156–157, 371–377. [Google Scholar] [CrossRef]
- Sappok, A.G.; Wong, V.W. Physical Characterization of Ash Species in Diesel Exhaust Entering After Treatment Systems; SAE International 2007-01-0318; Massachusetts Institute of Technology, Sloan Automotive Laboratory: Cambridge, MA, USA, 2007. [Google Scholar]
- Hammershøi, P.S.; Jangjou, Y.; Epling, W.S.; Jensen, A.D.; Janssens, T.V.W. Reversible and irreversible deactivation of Cu-CHA NH3-SCR catalysts by SO2 and SO3. Appl. Catal. B Environ. 2018, 226, 38–45. [Google Scholar] [CrossRef]
- Shen, M.Q.; Zhang, Y.; Wang, J.Q.; Wang, C.; Wang, J. Nature of SO3 poisoning on Cu/SAPO-34 SCR catalysts. J. Catal. 2018, 358, 277–286. [Google Scholar] [CrossRef]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.J.; Dong, C.Y.; Albert, K.B.; Liu, P.; Wang, J.Y.; Zhu, D.J.; Chen, H.P.; et al. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, Y.H.; Zhang, R.R. Ammonia selective catalytic reduction of NO over Ce–Fe/Cu–SSZ-13 catalysts. RSC Adv. 2015, 5, 85453–85459. [Google Scholar] [CrossRef]
- Chen, B.H.; Xu, R.N.; Zhang, R.D.; Liu, N. Economical Way to Synthesize SSZ-13 with Abundant Ion-Exchanged Cu+ for an Extraordinary Performance in Selective Catalytic Reduction (SCR) of NOx by Ammonia. Environ. Sci. Technol. 2014, 48, 13909–13916. [Google Scholar] [CrossRef]
- Su, W.K.; Li, Z.G.; Peng, Y.; Li, J.H. Correlation of the changes in the framework and active Cu sites for typical Cu/CHA zeolites (SSZ-13 and SAPO-34) during hydrothermal aging. Phys. Chem. Chem. Phys. 2015, 17, 29142–29149. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.S.; Cavataio, G.; McCabe, R.W.; Fu, L.X.; Li, J.H. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Wijayanti, K.; Andonova, S.; Kumar, A.; Li, J.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34. Appl. Catal. B Environ. 2015, 166–167, 568–579. [Google Scholar] [CrossRef]
- Shen, M.; Wen, H.; Hao, T.; Yu, T.; Fan, D.; Wang, J.; Li, W.; Wang, J. Deactivation mechanism of SO2 on Cu/SAPO-34 NH3-SCR catalysts: Structure and active Cu2+. Catal. Sci. Technol. 2015, 5, 1741–1749. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.P.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.Q.; Yu, T.; Shen, M.Q.; Wang, W.L.; Li, W. The effect of sulfate species on the activity of NH3-SCR over Cu/SAPO-34. Appl. Catal. B Environ. 2017, 204, 239–249. [Google Scholar] [CrossRef]
- Shen, M.Q.; Li, X.H.; Wang, J.Q.; Wang, C.; Wang, J. Nature Identification of Cu Active Sites in Sulfur-Fouled Cu/SAPO-34 Regeneration. Ind. Eng. Chem. Res. 2018, 57, 3501–3509. [Google Scholar] [CrossRef]
- Lin, Q.J.; Feng, X.; Zhang, H.L.; Lin, C.L.; Liu, S.; Xu, H.D.; Chen, Y.Q. Hydrothermal deactivation over CuFe/BEA for NH3-SCR. J. Ind. Eng. Chem. 2018, 65, 40–50. [Google Scholar] [CrossRef]
- Li, Y.H.; Deng, J.L.; Song, W.Y.; Liu, J.; Zhao, Z.; Gao, M.L.; Wei, Y.C.; Zhao, L. Nature of Cu Species in Cu–SAPO-18 Catalyst for NH3–SCR: Combination of Experiments and DFT Calculations. J. Phys. Chem. C 2016, 120, 14669–14680. [Google Scholar] [CrossRef]
- Hajjar, R.; Millot, Y.; Man, P.P.; Che, M.; Dzwigaj, S. Two kinds of framework Al sites studied in BEA zeolite by X-ray diffraction, Fourier transform infrared spectroscopy, NMR techniques, and V probe. J. Phys. Chem. C 2008, 112, 20167–20175. [Google Scholar] [CrossRef]
- Muzio, L.; Bogseth, S.; Himes, R.; Chien, Y.C.; Dunn-Rankin, D. Ammonium bisulfate formation and reduced load SCR operation. Fuel 2017, 206, 180–189. [Google Scholar] [CrossRef]
- Li, J.H.; Peng, Y.; Chang, H.Z.; Li, X.; Crittenden, J.C.; Hao, J.M. Chemical poison and regeneration of SCR catalysts for NOx removal from stationary sources. Front. Environ. Sci. Eng. 2016, 10, 413–427. [Google Scholar] [CrossRef]
- Xu, L.W.; Wang, C.Z.; Chang, H.A.; Wu, Q.R.; Zhang, T.; Li, J.H. New Insight into SO2 Poisoning and Regeneration of CeO2−WO3/TiO2 and V2O5−WO3/TiO2 Catalysts for Low-Temperature NH3−SCR. Environ. Sci. Technol. 2018, 52, 7064–7071. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.P.; Du, X.S.; Ran, J.Y.; Wang, X.M.; Chen, Y.R.; Zhang, L. Understanding SO2 Poisoning over Different Copper Species of Cu-SAPO-34 Catalyst: A Periodic DFT Study. J. Phys. Chem. C 2018, 122, 21468–21477. [Google Scholar] [CrossRef]
- Fan, D.Q.; Wang, J.; Yu, T.; Wang, J.Q.; Hu, X.Q.; Shen, M.Q. Catalytic deactivation mechanism research over Cu/SAPO-34 catalysts for NH3-SCR (I): The impact of hydrothermal aging time. Chem. Eng. J. 2018, 176, 285–293. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and Evaluation of Cu-SAPO-34 Catalysts for Ammonia Selective Catalytic Reduction. 1. Aqueous Solution Ion Exchange. ACS Catal. 2013, 3, 2083–2093. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, S.J.; Liu, P.; Li, T. The influence of phosphorus on the catalytic properties, durability, sulfur resistance and kinetics of Cu-SSZ-13 for NOx reduction by NH3-SCR. Appl. Catal. B Environ. 2018, 237, 116–127. [Google Scholar] [CrossRef]
- Ming, S.J.; Chen, Z.; Fan, C.; Pang, L.; Guo, W.; Albert, K.B.; Liu, P.; Li, T. The effect of copper loading and silicon content on catalytic activity and hydrothermal stability of Cu-SAPO-18 catalyst for NH3-SCR. Appl. Catal. A Gen. 2018, 559, 47–56. [Google Scholar] [CrossRef]
- Luo, J.Y.; Gao, F.; Kamasamudram, K.; Currier, N.; Peden, C.H.F.; Yezerets, A. New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: Nature of acidic sites probed by NH3 titration. J. Catal. 2017, 348, 291–299. [Google Scholar] [CrossRef]
- Su, W.K.; Li, Z.G.; Zhang, Y.N.; Meng, C.C.; Li, J.H. Identification of sulfate species and their influence on SCR performance of Cu/CHA catalyst. Catal. Sci. Technol. 2017, 7, 1523–1528. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).