Influence of Pluronic® P123 Addition in the Synthesis of Bulk Ni Promoted MoS2 Catalyst. Application to the Selective Hydrodesulfurization of Sulfur Model Molecules Representative of FCC Gasoline
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalysts Characterizations
2.2. Catalytic Performances in the Transformation of Model Molecules Representative of FCC Gasoline
3. Materials and Methods
3.1. Catalyst Preparation
3.2. NiMoS Characterizations
3.3. Catalytic Performances
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Directive 2009/30/CE L140/88. Available online: https://eur-lex.europa.eu/legal-content/FR/TXT/HTML/?uri=CELEX:32009L0030 (accessed on 21 July 2019).
- Available online: TransportPolicy.net https://www.transportpolicy.net/?s=sulfur (accessed on 23 April 2019).
- Chapuis, T.; Hudebine, D.; Souchon, V. Catalysis by Transition Metal Sulfides: From Molecular Theory to Industrial Application; Technip Edition: Paris, France, 2013; pp. 547–578. [Google Scholar]
- Sun, Y.; Wang, H.; Prins, R. Hydrodesulfurization with classic Co–MoS2 and Ni–MoS2/-Al2O3 and new Pt–Pd on mesoporous zeolite catalysts. Catal. Today 2010, 150, 213–217. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Purón, H.; Torres, D.; de Llobet, S.; Moliner, R.; Suelves, I.; Millan, M. Carbon nanofibres coated with Ni decorated MoS2 nanosheets as catalyst for vacuum residue hydroprocessing. Appl. Catal. B 2014, 148–149, 357–365. [Google Scholar] [CrossRef]
- Wang, S.; An, C.; Yuan, J. Synthetic Fabrication of Nanoscale MoS2-Based Transition Metal Sulfides. Materials 2010, 3, 401–433. [Google Scholar] [CrossRef]
- Alvarez, L.; Espino, J.; Ornelas, C.; Rico, J.L.; Cortez, M.T.; Berhault, G.; Alonso, G. Comparative study of MoS2 and Co/MoS2 catalysts prepared by ex situ/insitu activation of ammonium and tetraalkylammonium thiomolybdates. J. Mol. Catal. A Chem. 2004, 210, 105–117. [Google Scholar] [CrossRef]
- Liu, H.; Yin, C.; Li, B.L.X.; Li, Y.; Chai, Y.; Liu, C. Effect of Calcination Temperature of Unsupported NiMo Catalysts on the Hydrodesulfurization of Dibenzothiophene. Energy Fuels 2014, 28, 2429–2436. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Yin, C.; Chai, Y.; Li, Y.; Liu, D.; Li, B.L.X.; Wang, Y.; Li, X. Preparation of highly active unsupported nickel–zinc–molybdenumcatalysts for the hydrodesulfurization of dibenzothiophene. Appl. Catal. B 2015, 174–175, 264–276. [Google Scholar] [CrossRef]
- Song, W.; Lai, W.; Chen, Z.; Cao, J.; Wang, H.; Lian, Y.; Yang, W.; Jiang, X. Fabrication of 3D Porous Hierarchical NiMoS Flowerlike Architectures for Hydrodesulfurization Applications. ACS Appl. Nano Mater. 2018, 1, 442–454. [Google Scholar] [CrossRef]
- Fuentes, S.; Diaz, G.; Pedraza, F.; Rojas, H.; Rosas, N. The influence of a new preparation method on the catalytic properties of CoMo and NiMo sulfides. J. Catal. 1988, 113, 535–539. [Google Scholar] [CrossRef]
- Lai, W.; Chen, Z.; Zhu, J.; Yang, L.; Zheng, J.; Yi, X.; Fang, W. A NiMoS flower-like structure with self-assembled nanosheets as high-performance hydrodesulfurization catalysts. Nanoscale 2016, 8, 3823–3833. [Google Scholar] [CrossRef]
- Lamic, A.F.; Daudin, A.; Brunet, S.; Legens, C.; Bouchy, C.; Devers, E. Effect of H2S partial pressure on the transformation of a model FCC gasoline olefin over unsupported molybdenum sulfide-based catalysts. Appl. Catal. A 2008, 344, 198–204. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, J.; Liu, J.; Pan, A.; Tan, Y.; Chen, T.; Fang, G. PVP-assisted synthesis of MoS2 nanosheets with improved lithium storage properties. CrystEngComm 2013, 15, 4998–5002. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Sun, A. Surfactant-assisted fabrication of MoS2 nanospheres. J. Mater. Sci. 2010, 45, 182–187. [Google Scholar] [CrossRef]
- Gu, P.; Zhao, C.; Wen, T.; Ai, Y.; Zhang, S.; Chen, W.; Wang, J.; Hu, B.; Wang, X. Highly U(VI) immobilization on polyvinyl pyrrolidine intercalated molybdenum disulfide: Experimental and computational studies. Chem. Eng. J. 2019, 35, 1563–1575. [Google Scholar] [CrossRef]
- Afanasiev, P. Synthesis of finely divided molybdenum sulfide nanoparticles in propylene carbonate solution. J. Solid State Chem. 2014, 213, 158–164. [Google Scholar] [CrossRef]
- Tang, G.; Wang, Y.; Chen, W.; Tang, H.; Li, C. Hydrothermal synthesis and characterization of novel flowerlike MoS2 hollow microspheres. Mater. Lett. 2013, 100, 15–18. [Google Scholar] [CrossRef]
- Tang, G.; Sun, J.; Wei, C.; Wu, K.; Ji, X.; Liu, S.; Tang, H.; Li, C. Synthesis and characterization of flowerlike MoS2 nanostructures through CTAB-assisted hydrothermal process. Mater. Lett. 2012, 86, 9–12. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Sun, A. Surfactant-assisted preparation of hexagonal molybdenum disulfide nanoparticles. Mater. Lett. 2009, 63, 2591–2593. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Zhang, J.; Yin, C.; Zhao, Y.; Yin, S.; Liu, C.; Suna, W. PVP-assisted synthesis of unsupported NiMo catalysts with enhanced hydrodesulfurization activity. Fuel Process. Technol. 2017, 160, 93–101. [Google Scholar] [CrossRef]
- Liu, H.; Yin, C.; Li, X.; Chai, Y.; Li, Y.; Liu, C. Effect of NiMo phases on the hydrodesulfurization activities of dibenzothiophene. Catal. Today 2017, 282, 222–229. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, L.; Bai, Z.; Liu, H.; Liu, Y.; Liu, C. A novel porous ammonium nickel molybdate as the catalyst precursor towards deep hydrodesulfurization of gas oil. Fuel 2013, 107, 873–878. [Google Scholar] [CrossRef]
- Genuit, D.; Afanasiev, P.; Vrinat, M. Solution syntheses of unsupported Co(Ni)–Mo–S hydrotreating catalysts. J. Catal. 2005, 235, 302–317. [Google Scholar] [CrossRef]
- Yoosuk, B.; Song, C.; Kim, J.H.; Ngamcharussrivichai, C.; Prasassarakich, P. Effects of preparation conditions in hydrothermal synthesis of highly active unsupported NiMo sulfide catalysts for simultaneous hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. Catal. Today 2010, 149, 52–61. [Google Scholar] [CrossRef]
- Leyral, G.; Ribes, M.; Courthéoux, L.; Uzio, D.; Pradel, A. Synthesis and Structuring of Ni MoS2 by using an Ionic Liquid. Eur. J. Inorg. Chem. 2012, 4967–4971. [Google Scholar] [CrossRef]
- Xiong, Y.; Xie, Y.; Li, Z.; Li, X.; Zhang, R. Micelle-assisted fabrication of necklace-shaped assembly of inorganic fullerene-like molybdenum disulfide nanospheres. Chem. Phys. Lett. 2003, 382, 180–185. [Google Scholar] [CrossRef]
- Xu, J.; Huang, T.; Fan, Y. Highly efficient NiMo/SiO2-Al2O3 hydrodesulfurizatio ncatalyst prepared from gemini surfactant-dispersed Mo precursor. Appl. Catal. B 2017, 203, 839–850. [Google Scholar] [CrossRef]
- Afanasiev, P.; Xia, G.F.; Berhault, G.; Jouguet, B.; Lacroix, M. Surfactant-Assisted Synthesis of Highly Dispersed Molybdenum Sulfide. Chem. Mater. 1999, 11, 3216–3219. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Leyral, G.; Brillouet, S.; Rousseau, J.; Richard, F.; Mamede, A.S.; Courtheoux, L.; Pradel, A.; Ribes, M.; Brunet, S. Effect of the presence of ionic liquid during the NiMoS bulk preparation in the transformation of decanoic acid. Appl. Catal. A 2017, 532, 120–132. [Google Scholar] [CrossRef]
- Joensen, P.; Frindt, R.F.; Morrison, S.R. Single-layer MoS2. Mater. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Ishutenko, D.I.; Mozhaev, A.A.; Maslakov, K.I.I.; Pimerzin, A.A. Effects of composition and morphology of active phase of CoMo/Al2O3 catalysts prepared using Co2Mo10–heteropolyacid and chelating agents on their catalytic properties in HDS and HYD reactions. J. Catal. 2014, 312, 152–169. [Google Scholar] [CrossRef]
- Santos, A.S.d.; Girard, E.; Leflaive, P.; Brunet, S. Competitive adsorptions between thiophenic compounds over a CoMoS/Al2O3 catalyst under deep HDS of FCC gasoline. Appl. Catal. A 2019, 570, 292–298. [Google Scholar] [CrossRef]
- Brunet, S.; Mey, D.; Pérot, G.; Bouchy, C.; Diehl, F. On the hydrodesulfurization of FCC gasoline: A review. Appl. Catal. A 2005, 278, 143–172. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R.R. Structure-function relation in molybdenum sulfide catalysts: The “rim-edge” model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
- Sadakane, O. Desulfurization method for catalytically cracked gasoline. Eur. Pat. EP0745660A1, 4 December 1996. [Google Scholar]
- Hatanaka, S. Hydrodesulfurization of Selective Catalytic Cracked Gasoline. Catal. Surv. Asia 2005, 9, 87–93. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Cordporation: Waltham, MA, USA, 1992. [Google Scholar]
- Guichard, B.; Roy-Auberger, M.; Devers, E.; Legens, C.; Raybaud, P. Aging of Co(Ni)MoP/Al2O3 catalysts in working state. Catal. Today 2008, 130, 97–108. [Google Scholar] [CrossRef]
- Ninh, T.K.T.; Massin, L.; Laurenti, D.; Vrinat, M. A new approach in the evaluation of the support effect for NiMo hydrodesulfurization catalysts. Appl. Catal. A 2011, 407, 29–39. [Google Scholar] [CrossRef]
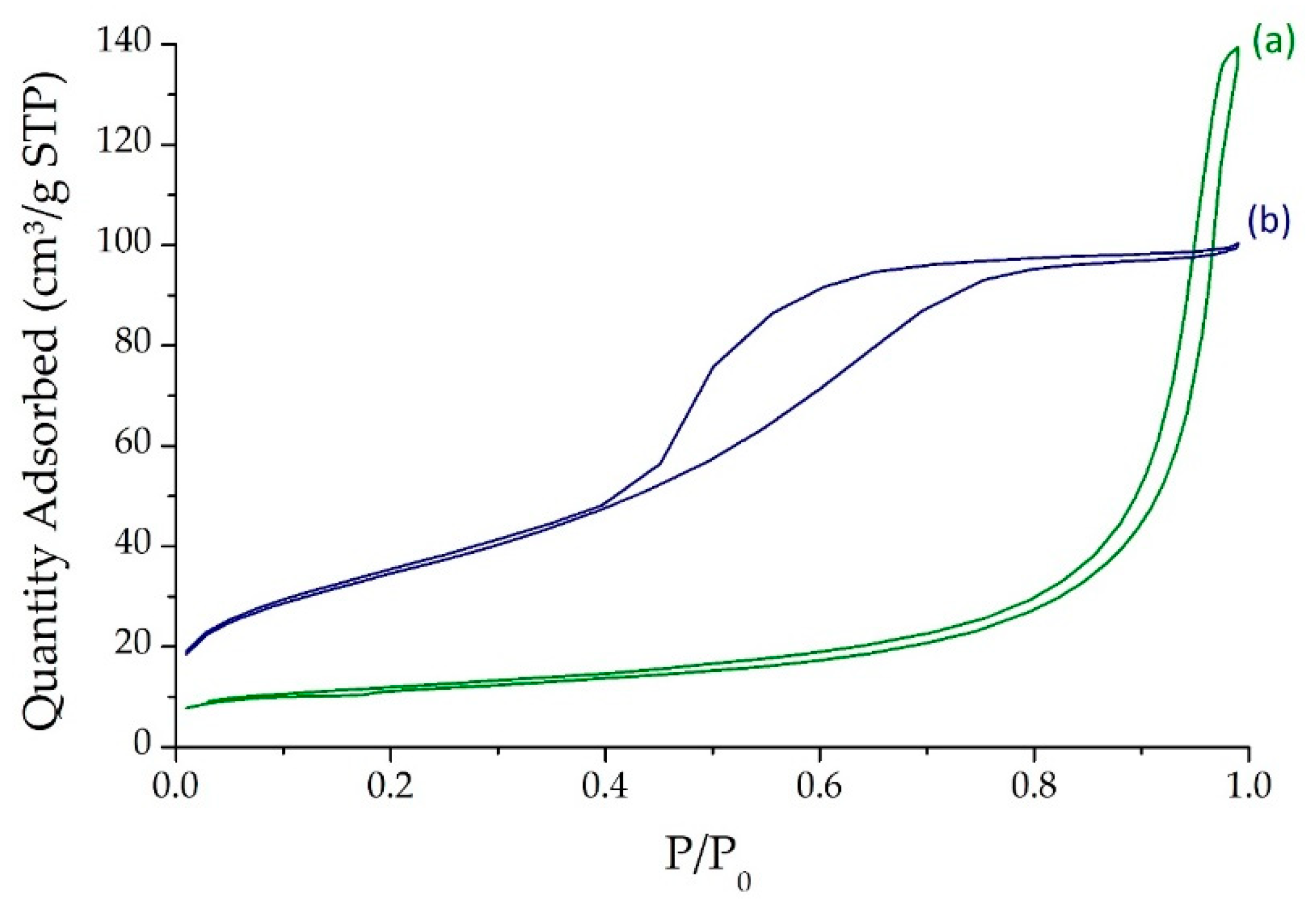
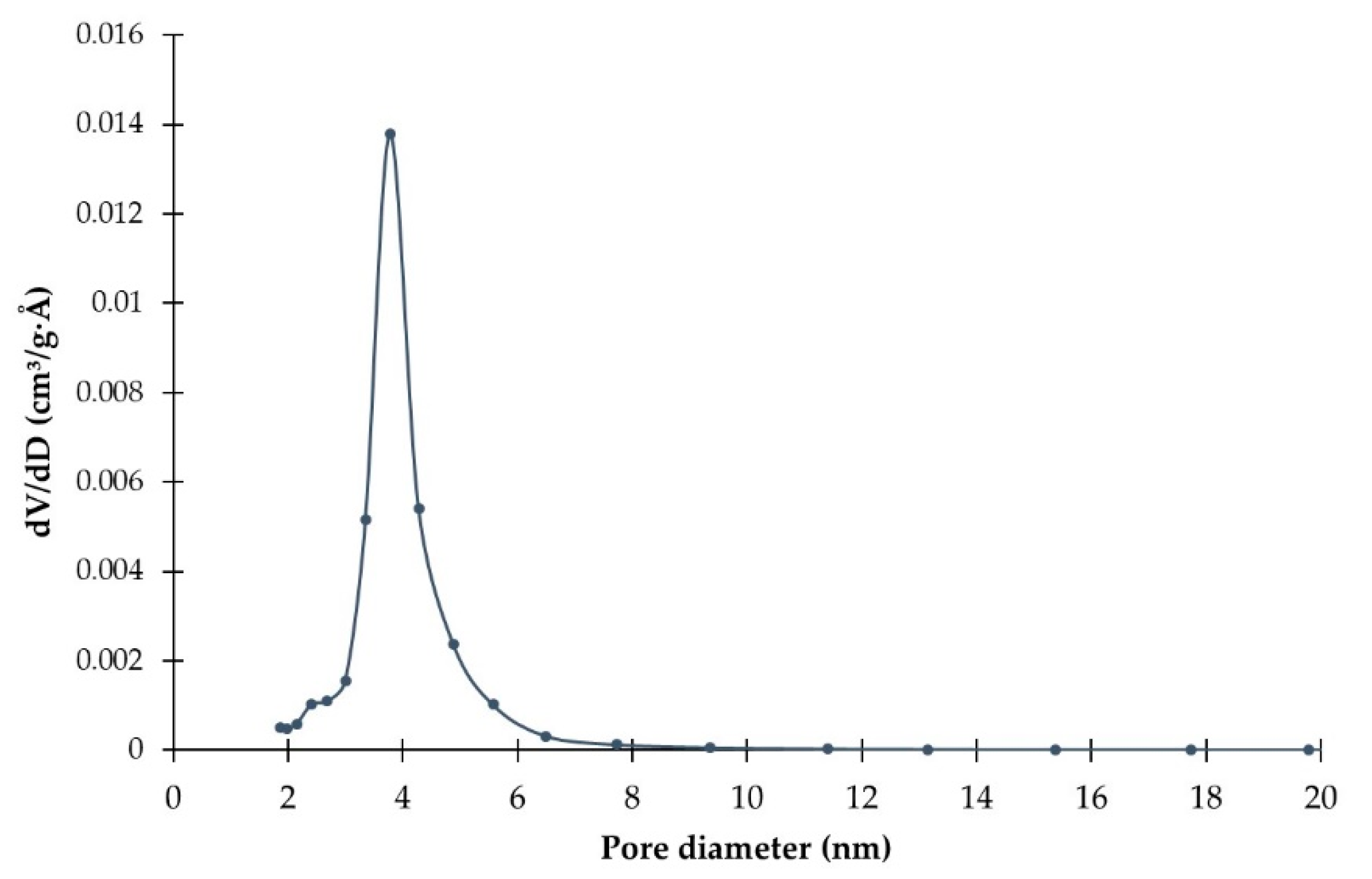
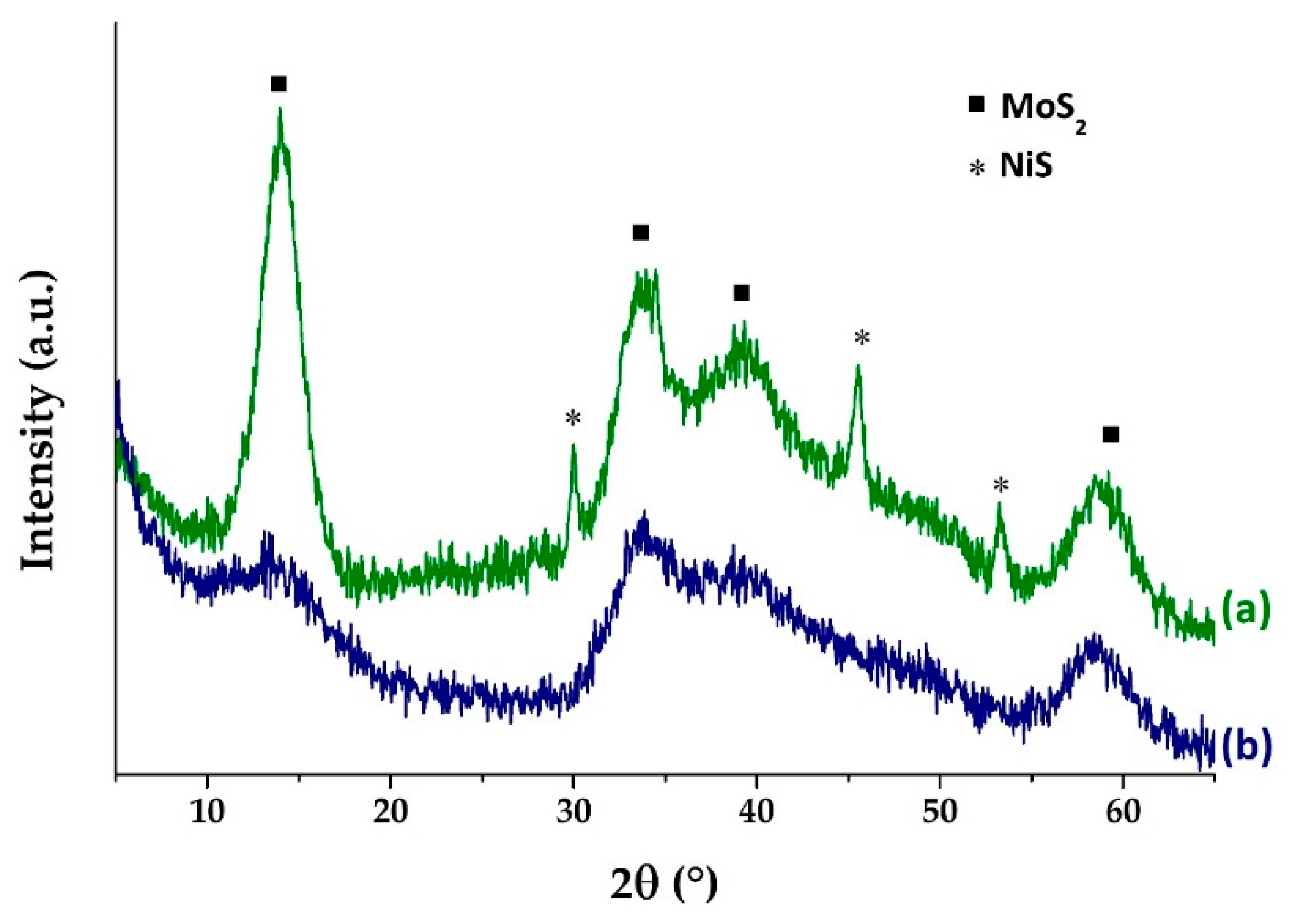
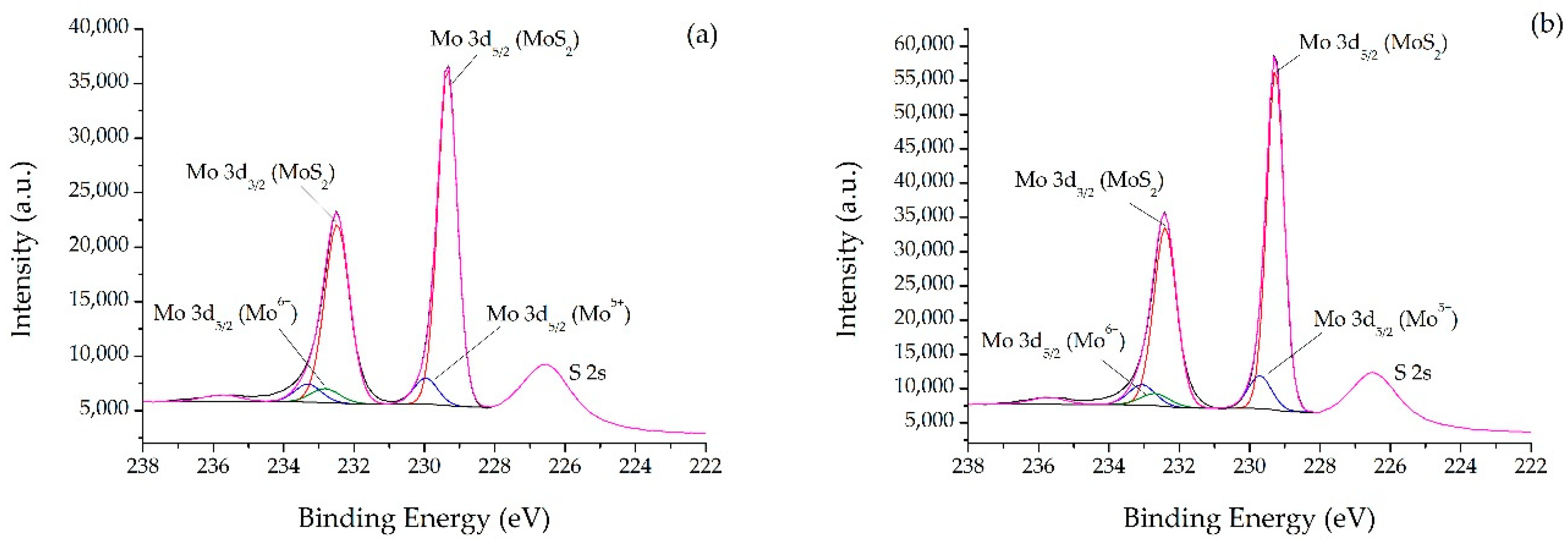
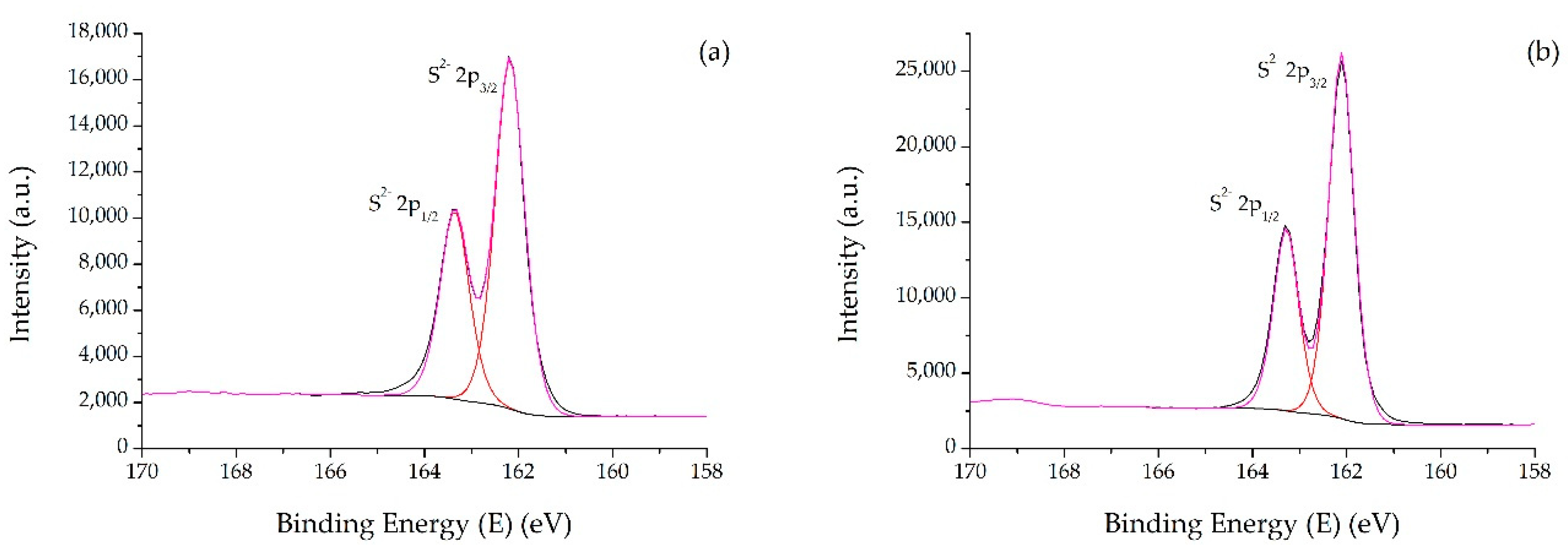
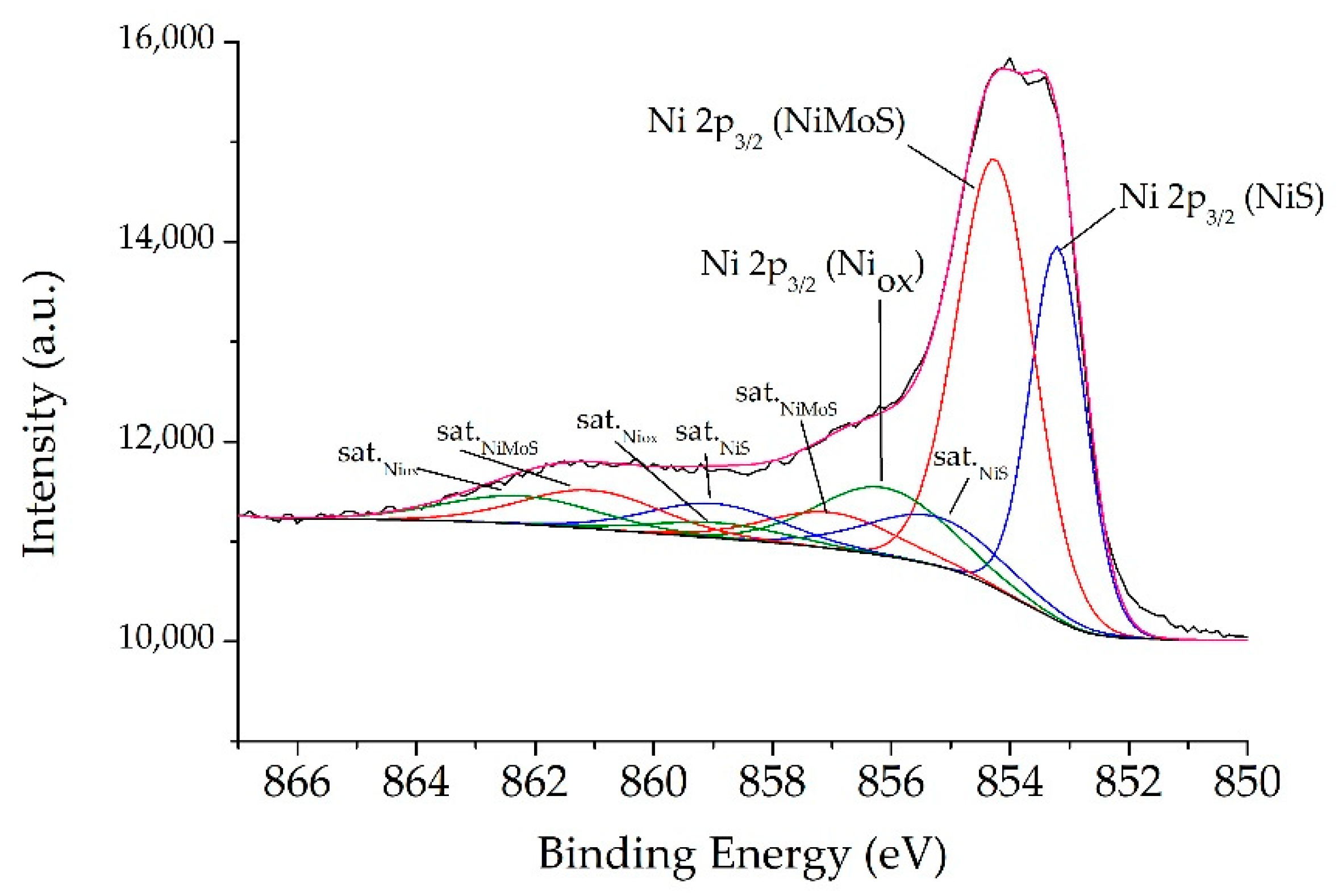
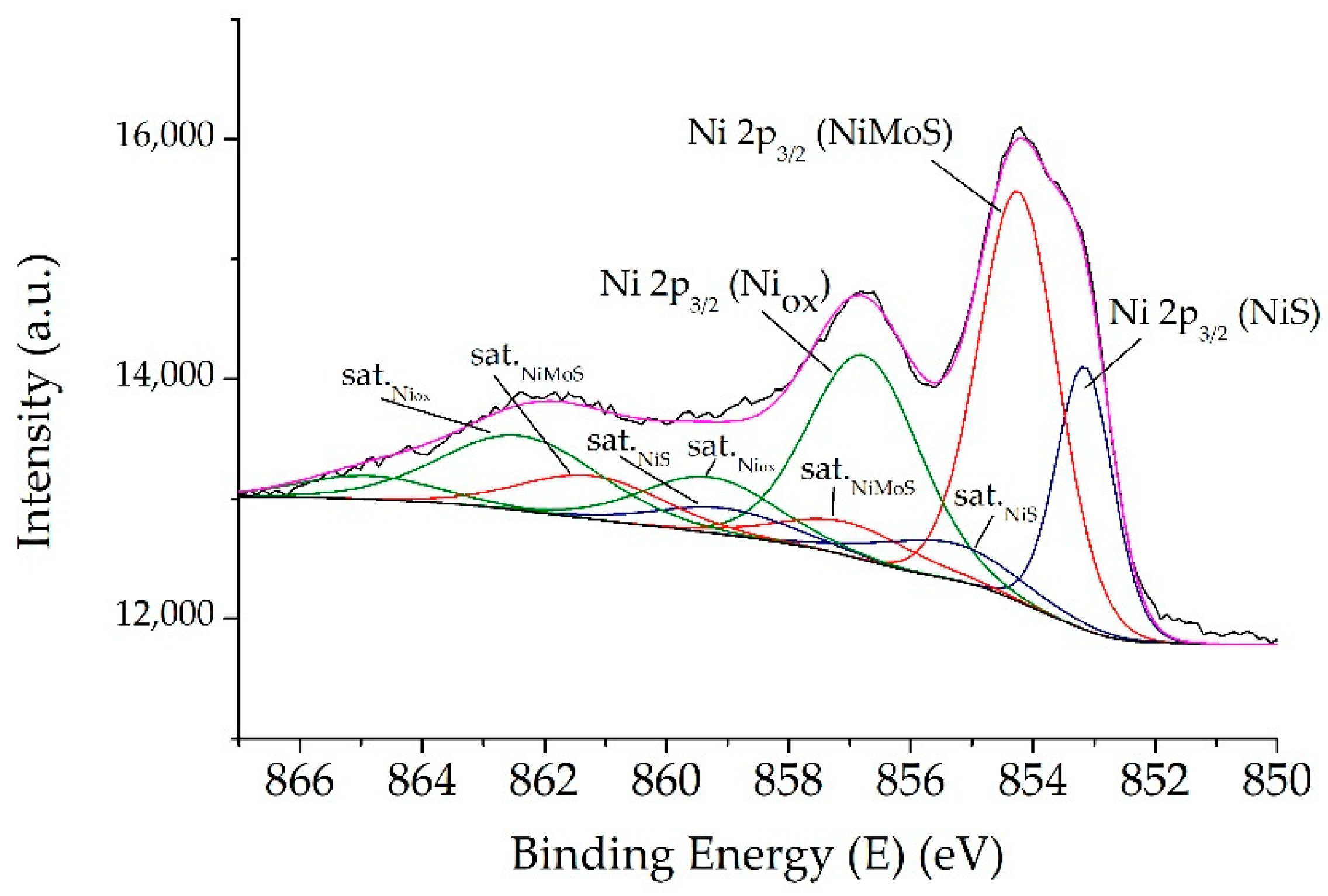
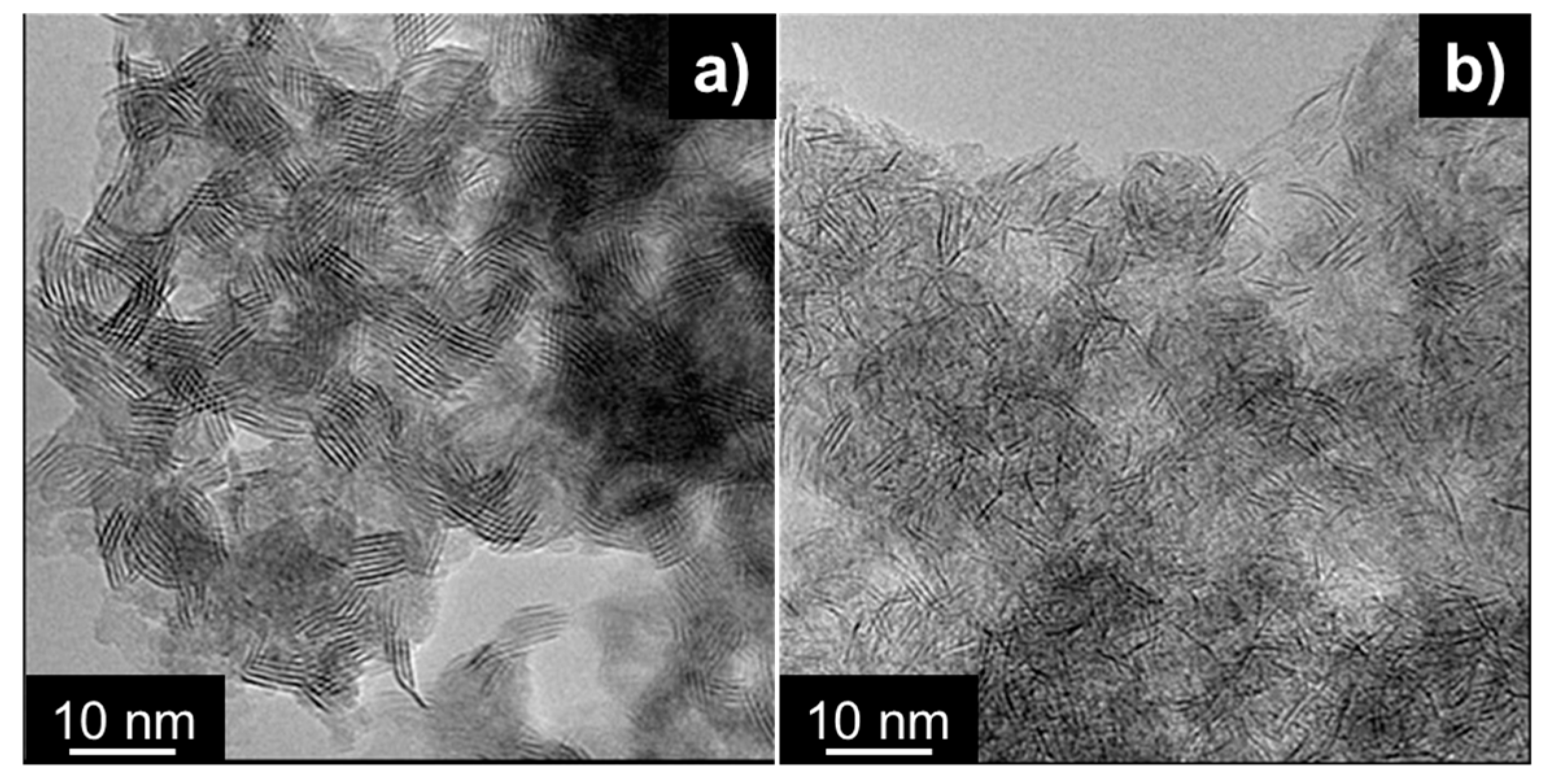
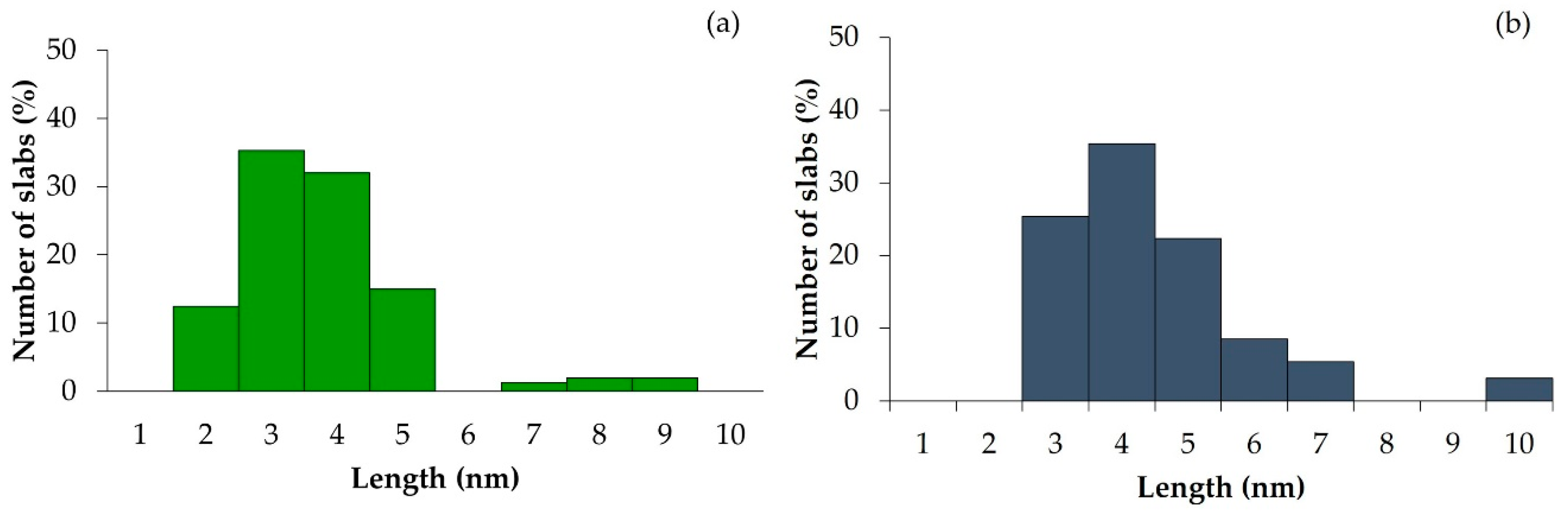
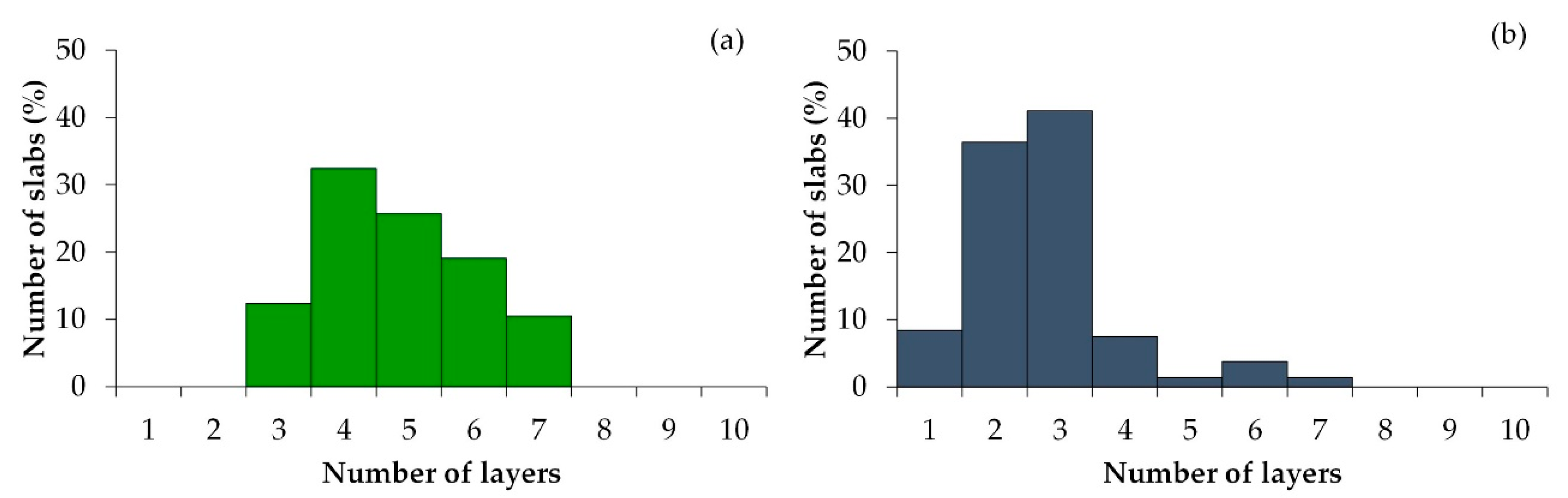
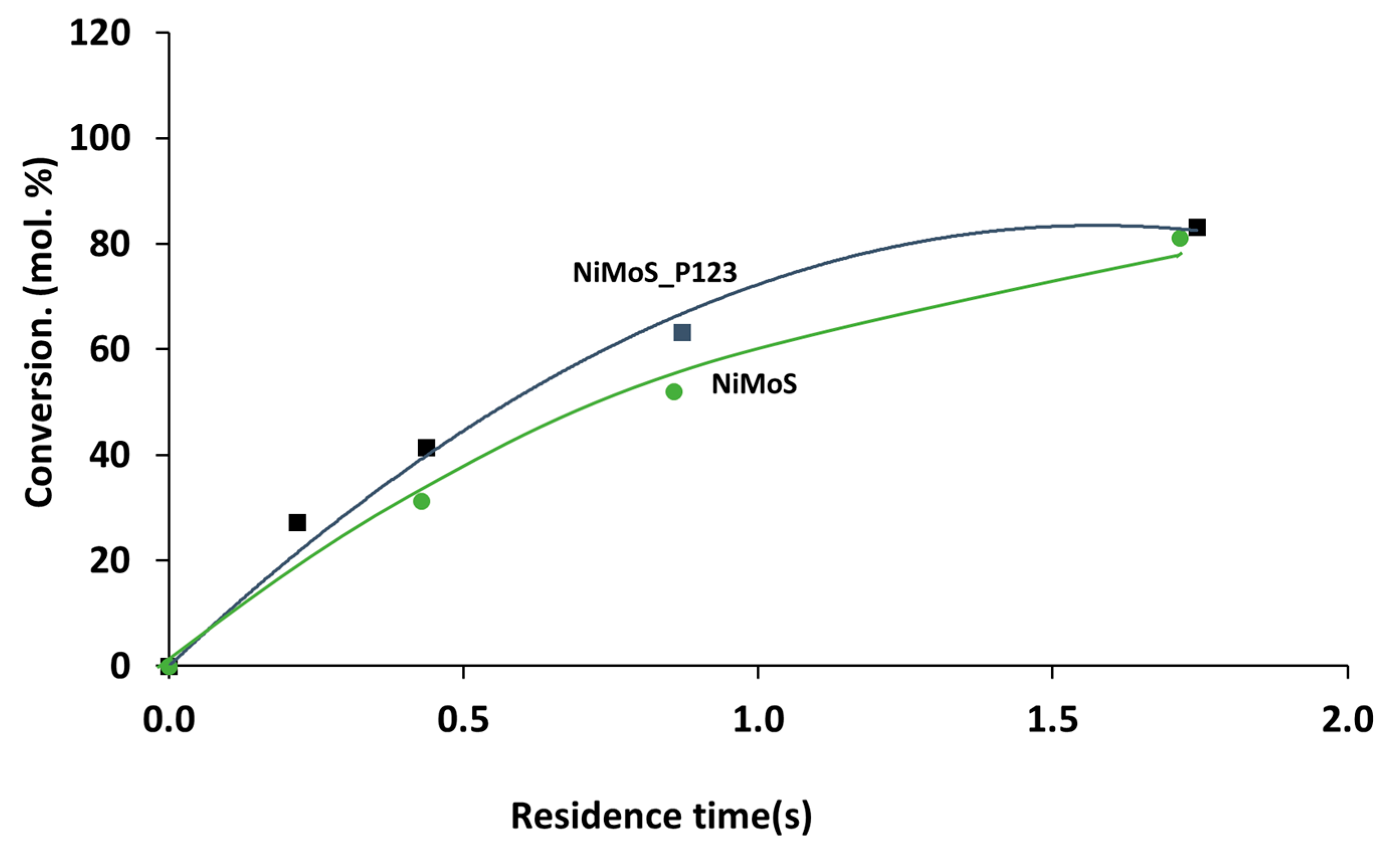
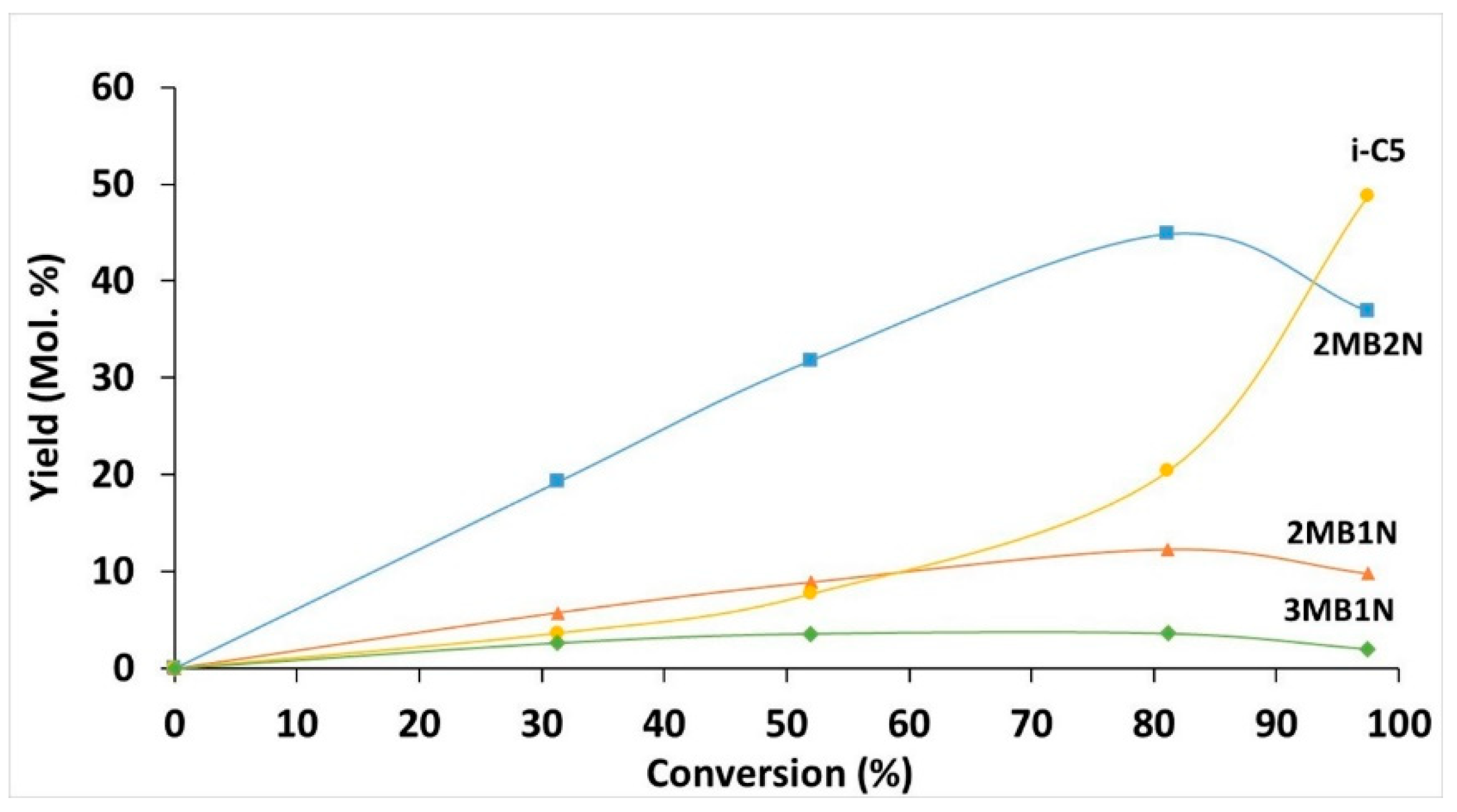
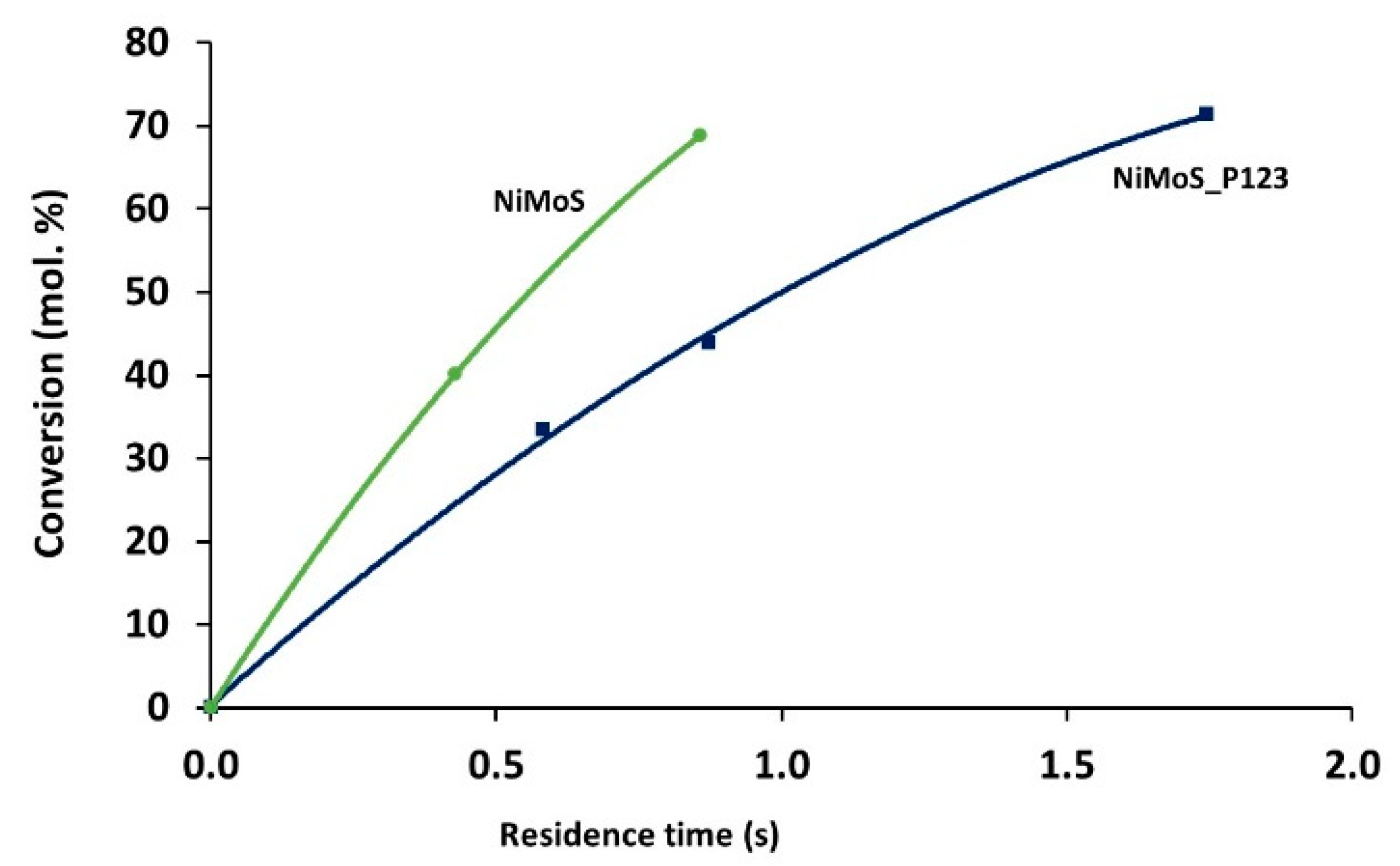
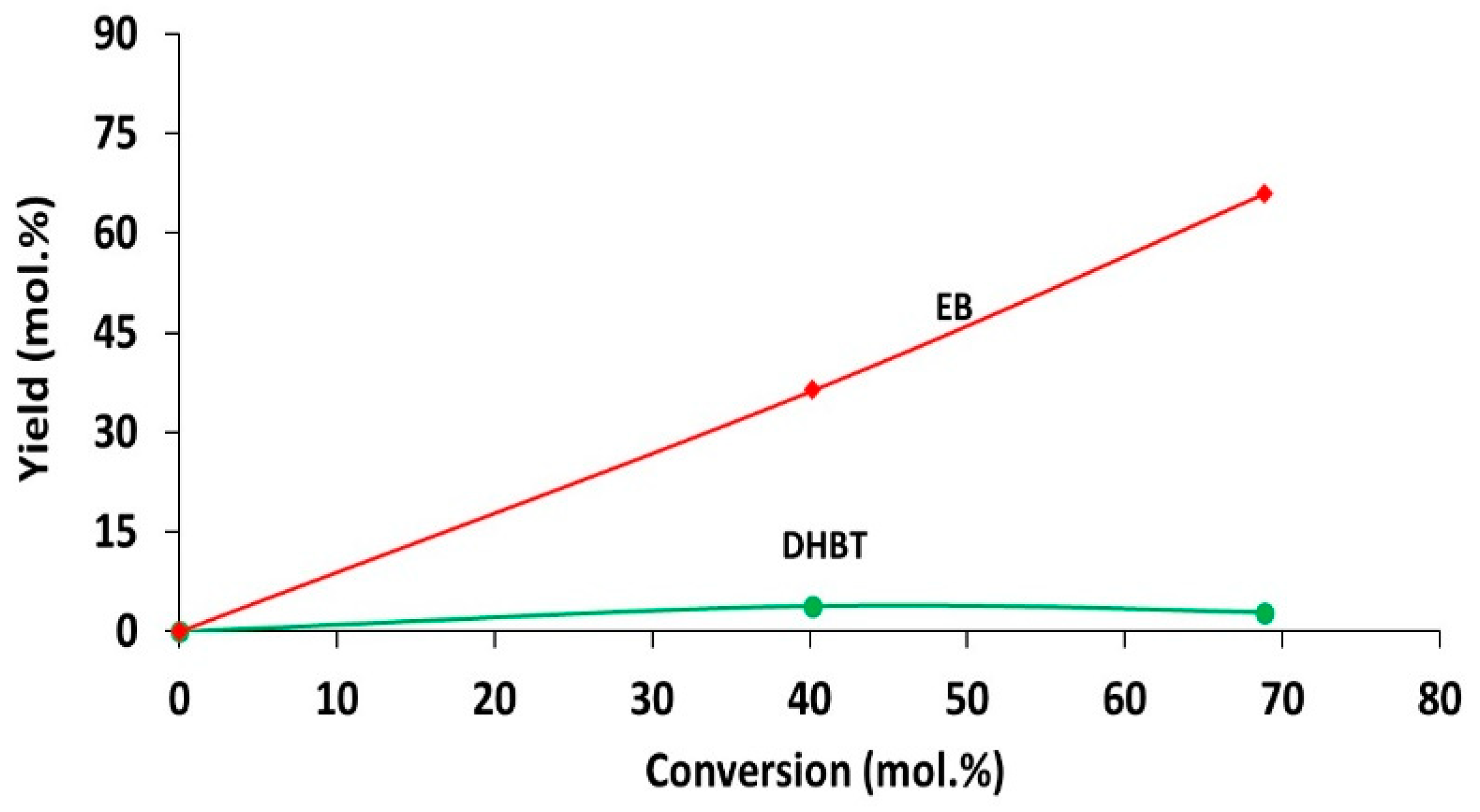
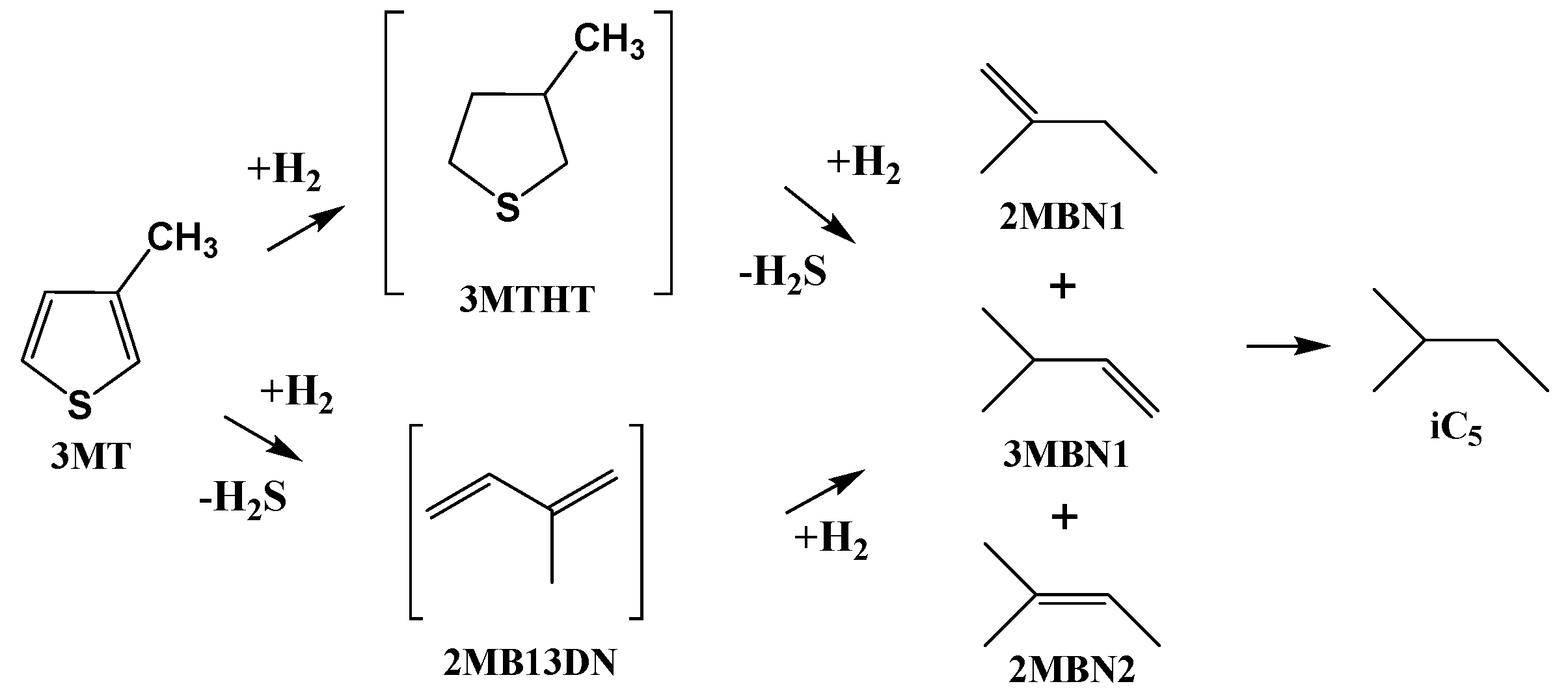
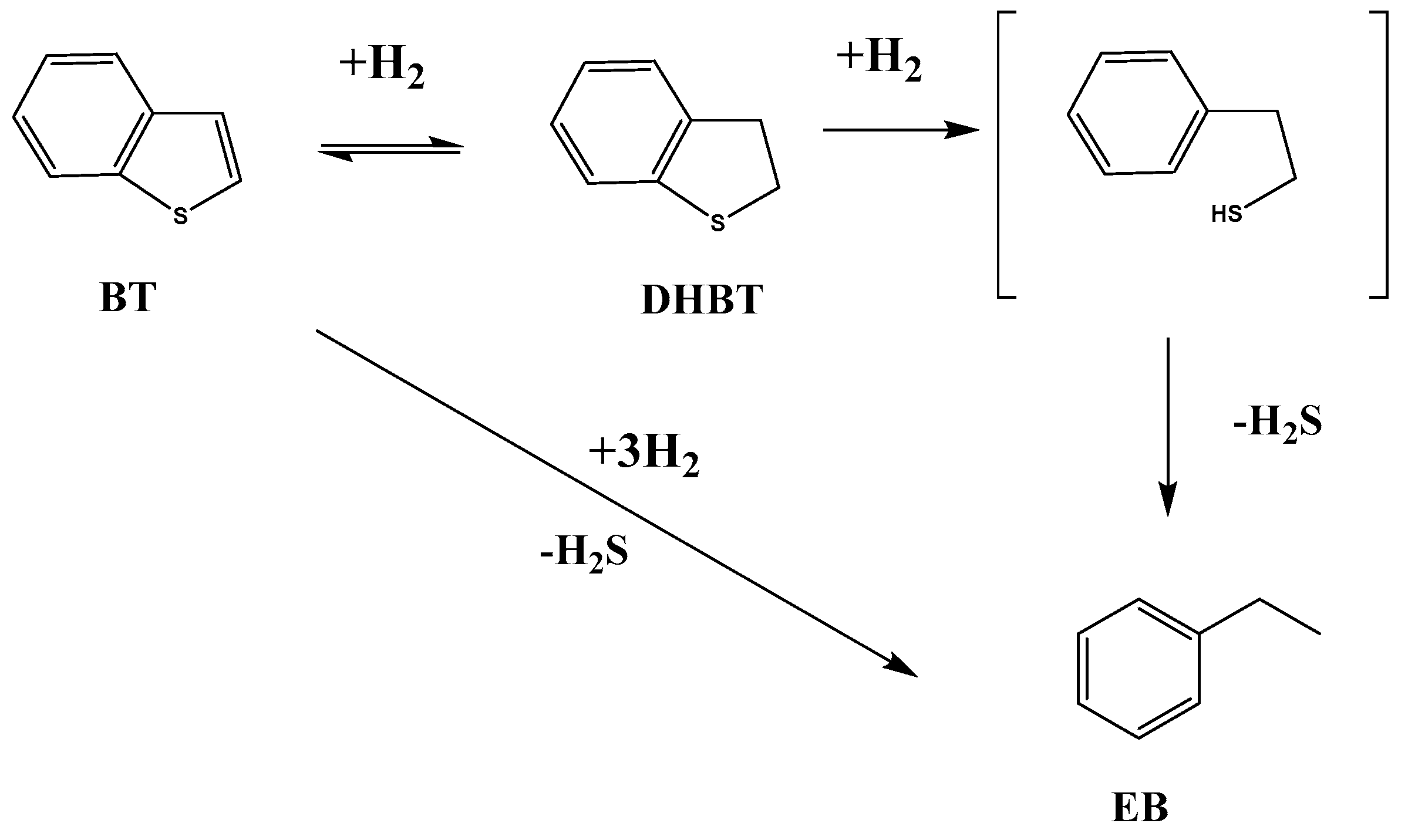
| Sample | SBET (m2·g) | C* | H* | S* | Ni** | Mo** | Ni/Mo** |
|---|---|---|---|---|---|---|---|
| NiMoS a | 0.6 | 0.1 | 34.8 | ||||
| NiMoS b | 31 | 0.6 | 0.1 | 34.6 | 17.6 | 34.6 | 0.5 |
| NiMoS-P123 a | 26.9 | 5.1 | 21.5 | ||||
| NiMoS-P123 b | 126 | 9.4 | 1.1 | 30.9 | 12.9 | 27.6 | 0.5 |
| Mo Fraction (rel.%) | Ni Fraction (rel.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalyst | S/Mo | Ni/(Ni+Mo) | Mo5+ | Mo6+ | MoS2 | NiMoS | NiS | Niox | (Ni/Mo)slab* |
| NiMoS | 2.5 | 0.37 | 9 | 5 | 86 | 48 | 35 | 17 | 0.56 |
| NiMoS-P123 | 2.3 | 0.28 | 11 | 5 | 84 | 38 | 21 | 41 | 0.45 |
| XRD | TEM | ||||||
|---|---|---|---|---|---|---|---|
| Catalyst | S a (nm) | St b | St b | L c (nm) | Lmin (nm) | Lmax (nm) | (fe/fc)Mo |
| NiMoS | 3 | 6 | 4.8 | 3.8 | 2.2 | 9.3 | 4.4 |
| NiMoS-P123 | - | - | 2.7 | 3.3 | 1.1 | 10.2 | 3.7 |
| 3MT | BT | |||||
|---|---|---|---|---|---|---|
| mmol·gcata−1 ·h−1 | 10−2 mmol ·m²·h−1 | 10−21 mmol·atMo−1·h−1 | mmol·gcata−1·h−1 | 10−2 mmol·m²·h−1 | 10−21 mmol·atMo−1·h−1 | |
| NiMoS | 2.2 | 7.1 | 1.2 | 4.2 | 13.6 | 2.2 |
| NiMoS-P123 | 3.1 | 2.5 | 2.2 | 3.7 | 2.9 | 2.6 |
| Catalyst | iC5 % | 3MB1N % | 2MB1N % | 2MB2N % | iC5/=C5 % | ||
|---|---|---|---|---|---|---|---|
| NiMoS | 11.7 | 8.4 | 18.4 | 61.5 | 0.13 | ||
| NiMoS-P123 | 13.2 | 5.7 | 18.2 | 62.9 | 0.15 |
| Catalyst | DHBT % | EB % |
|---|---|---|
| NiMoS | 3.9 | 96.1 |
| NiMoS-P123 | 6.8 | 93.2 |
| Pressure (kPa) | Sulfidation | 3MT | BT |
|---|---|---|---|
| PH2S | 10 | 0 | 0 |
| P3MT | 0 | 200 | 0 |
| PBT | 0 | 0 | 200 |
| PH2 | 90 | 1360 | 1360 |
| PnC7 | 0 | 637 | 637 |
| PTOT | 100 | 2000 | 2000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetier, V.; Pena, D.; Carvalho, A.; Courthéoux, L.; Flaud, V.; Girard, E.; Uzio, D.; Brunet, S.; Lacroix-Desmazes, P.; Pradel, A. Influence of Pluronic® P123 Addition in the Synthesis of Bulk Ni Promoted MoS2 Catalyst. Application to the Selective Hydrodesulfurization of Sulfur Model Molecules Representative of FCC Gasoline. Catalysts 2019, 9, 793. https://doi.org/10.3390/catal9100793
Hetier V, Pena D, Carvalho A, Courthéoux L, Flaud V, Girard E, Uzio D, Brunet S, Lacroix-Desmazes P, Pradel A. Influence of Pluronic® P123 Addition in the Synthesis of Bulk Ni Promoted MoS2 Catalyst. Application to the Selective Hydrodesulfurization of Sulfur Model Molecules Representative of FCC Gasoline. Catalysts. 2019; 9(10):793. https://doi.org/10.3390/catal9100793
Chicago/Turabian StyleHetier, Valentin, Diego Pena, Alexandre Carvalho, Laurence Courthéoux, Valérie Flaud, Etienne Girard, Denis Uzio, Sylvette Brunet, Patrick Lacroix-Desmazes, and Annie Pradel. 2019. "Influence of Pluronic® P123 Addition in the Synthesis of Bulk Ni Promoted MoS2 Catalyst. Application to the Selective Hydrodesulfurization of Sulfur Model Molecules Representative of FCC Gasoline" Catalysts 9, no. 10: 793. https://doi.org/10.3390/catal9100793
APA StyleHetier, V., Pena, D., Carvalho, A., Courthéoux, L., Flaud, V., Girard, E., Uzio, D., Brunet, S., Lacroix-Desmazes, P., & Pradel, A. (2019). Influence of Pluronic® P123 Addition in the Synthesis of Bulk Ni Promoted MoS2 Catalyst. Application to the Selective Hydrodesulfurization of Sulfur Model Molecules Representative of FCC Gasoline. Catalysts, 9(10), 793. https://doi.org/10.3390/catal9100793







