Improving Productivity of Multiphase Flow Aerobic Oxidation Using a Tube-in-Tube Membrane Contactor
Abstract
1. Introduction
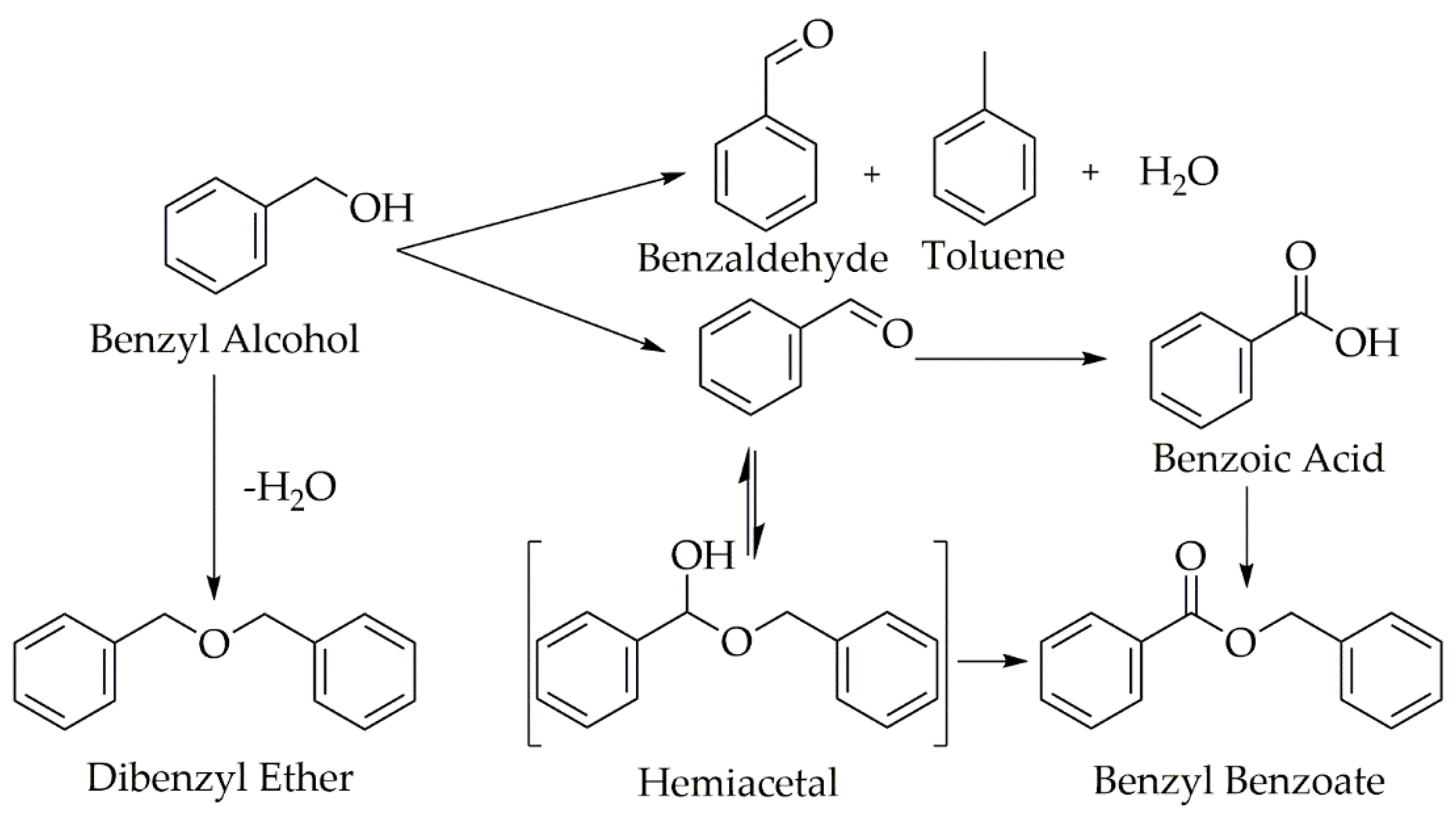
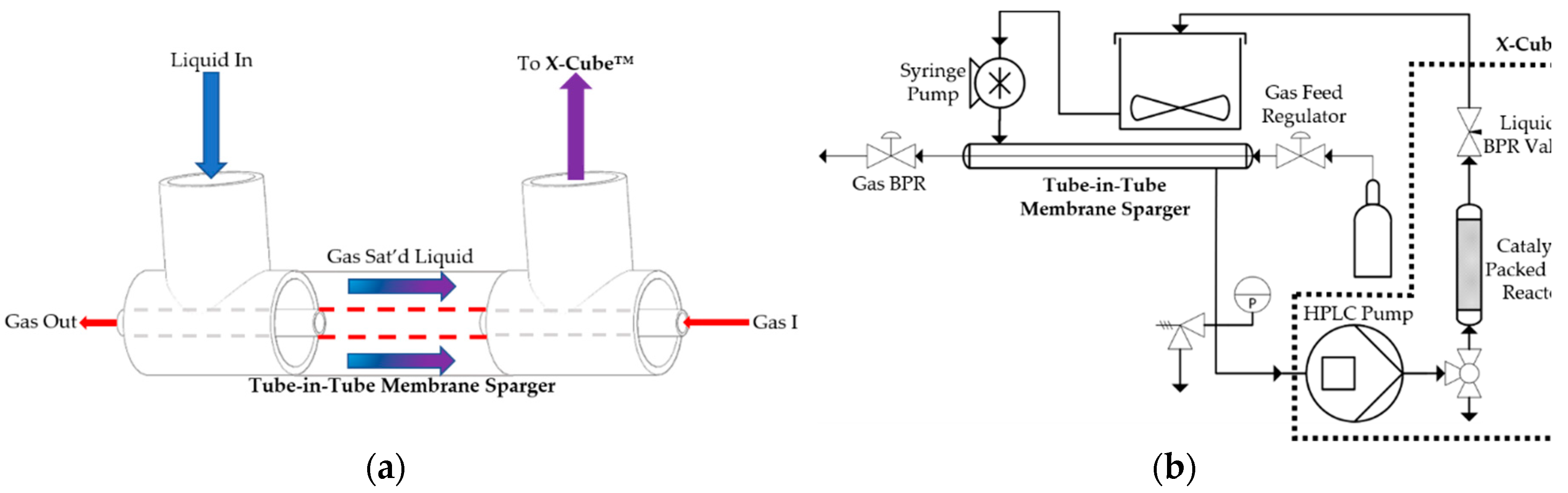
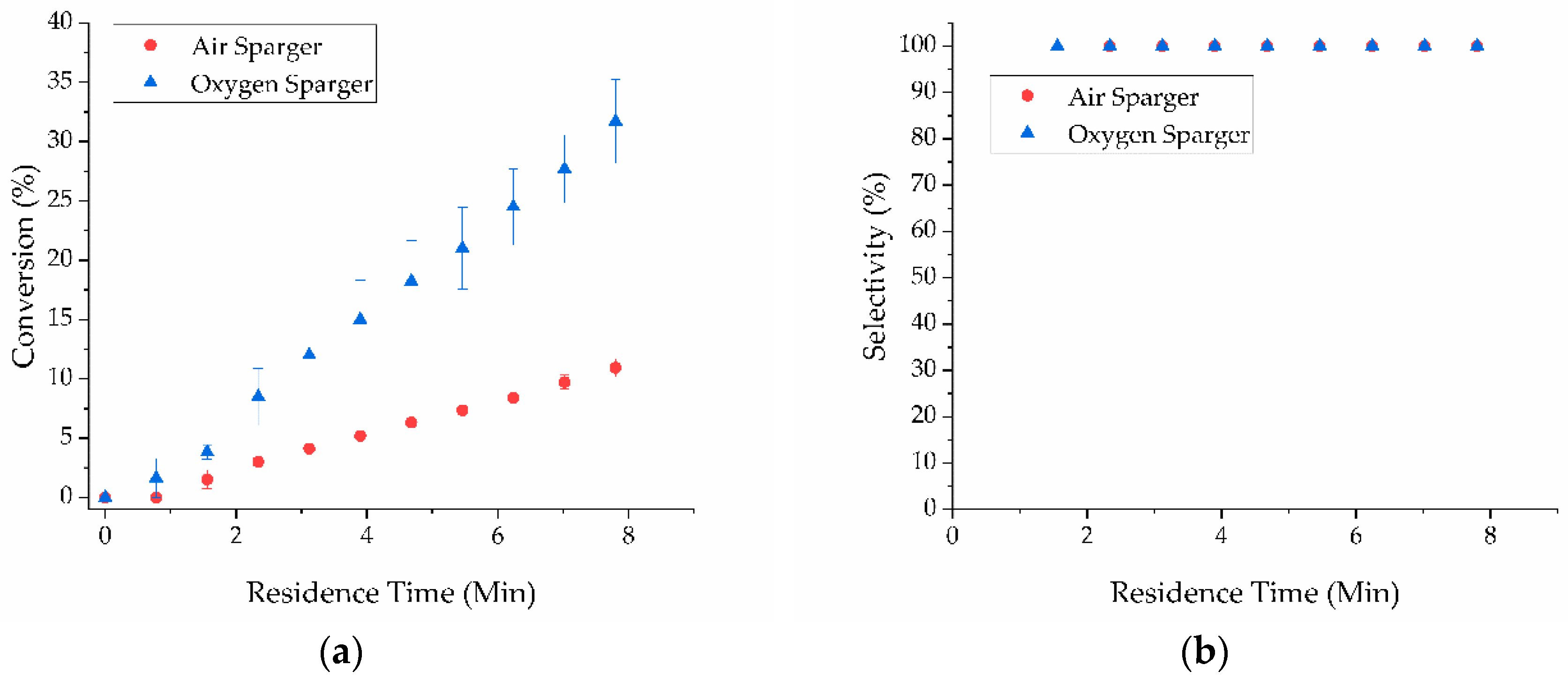
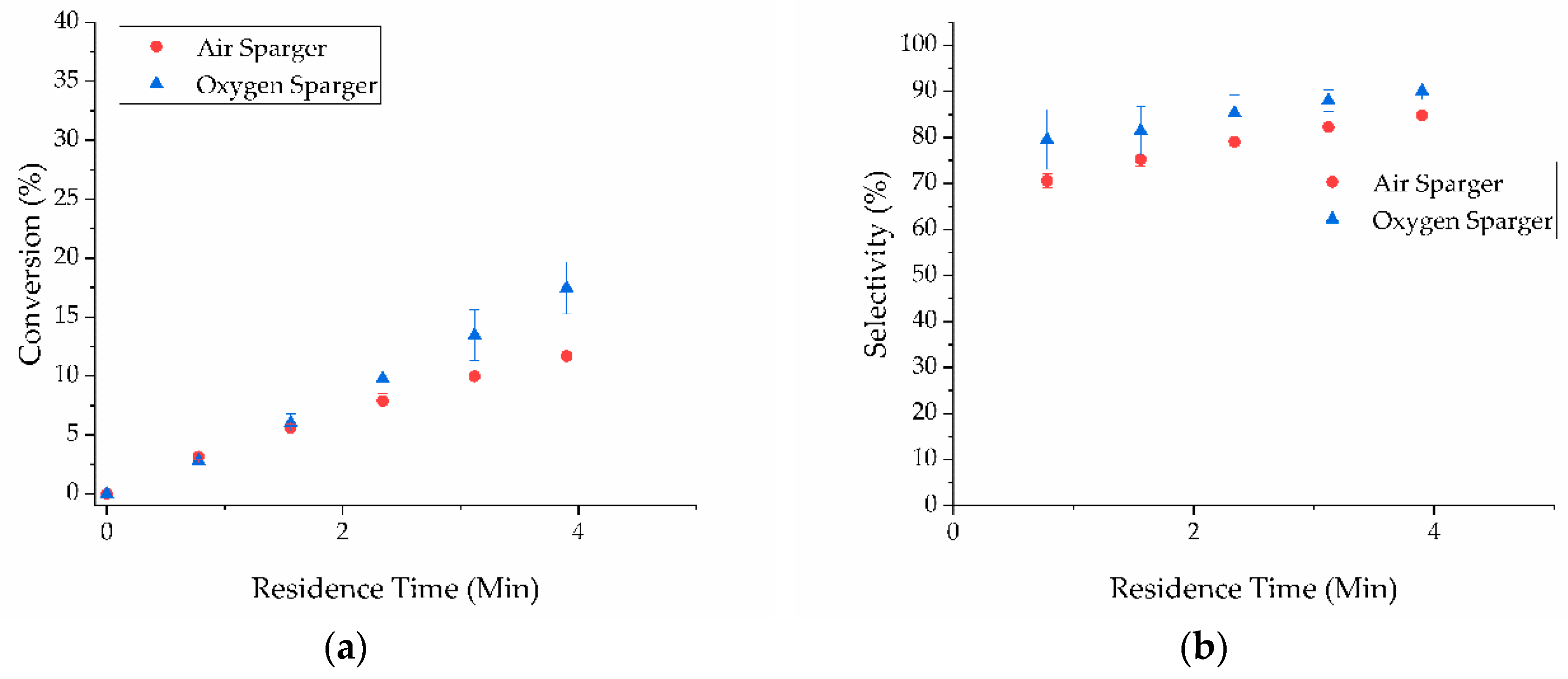
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pieber, B.; Kappe, C.O. Aerobic Oxidations in Continuous Flow. Top. Organomet. Chem. 2016, 57, 97–136. [Google Scholar] [CrossRef]
- Gavriilidis, A.; Constantinou, A.; Hellgardt, K.; Hii, K.K.; Hutchings, G.J.; Brett, G.L.; Kuhn, S.; Marsden, S.P. Aerobic oxidations in flow: Opportunities for the fine chemicals and pharmaceuticals industries. React. Chem. Eng. 2016, 1, 595–612. [Google Scholar] [CrossRef]
- Mills, P.L.; Quiram, D.J.; Ryley, J.F. Microreactor technology and process miniaturization for catalytic reactions-A perspective on recent developments and emerging technologies. Chem. Eng. Sci. 2007, 62, 6992–7010. [Google Scholar] [CrossRef]
- Losey, M.W.; Schmnidt, M.A.; Jensen, K.F. Microfabricated multiphase packed-bed reactors: Characterization of mass transfer and reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Hessel, V.; Angeli, P.; Gavriilidis, A.; Löwe, H. Gas-liquid and gas-liquid-solid microstructured reactors: Contacting principles and applications. Ind. Eng. Chem. Res. 2005, 44, 9750–9769. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef] [PubMed]
- Geyer, K.; Codée, J.D.C.; Seeberger, P.H. Microreactors as tools for synthetic Chemists—The chemists’ round-bottomed flask of the 21st century? Chem. Eur. J. 2006, 12, 8434–8442. [Google Scholar] [CrossRef]
- Gemoets, H.P.L.; Su, Y.; Shang, M.; Hessel, V.; Luque, R.; Noël, T. Liquid phase oxidation chemistry in continuous-flow microreactors. Chem. Soc. Rev. 2016, 45, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Compagnoni, M. Chemical reaction engineering, process design and scale-up issues at the frontier of synthesis: Flow chemistry. Chem. Eng. J. 2016, 296, 56–70. [Google Scholar] [CrossRef]
- Jensen, K.F.; Reizman, B.J.; Newman, S.G. Tools for chemical synthesis in microsystems. Lab Chip 2014, 14, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Caron, S.; Dugger, R.W.; Ruggeri, S.G.; Ragan, J.A.; Brown Ripin, D.H. Large-scale oxidations in the pharmaceutical industry. Chem. Rev. 2006, 106, 2943–2989. [Google Scholar] [CrossRef] [PubMed]
- Zotova, N.; Roberts, F.J.; Kelsall, G.H.; Jessiman, A.S.; Hellgardt, K.; Hii, K.K. (Mimi) Catalysis in flow: Au-catalysed alkylation of amines by alcohols. Green Chem. 2012, 14, 226–232. [Google Scholar] [CrossRef]
- Dijksman, A.; Marino-González, A.; Mairata i Payeras, A.; Arends, I.W.C.E.; Sheldon, R.A. Efficient and Selective Aerobic Oxidation of Alcohols into Aldehydes and Ketones Using Ruthenium/TEMPO as the Catalytic System. J. Am. Chem. Soc. 2001, 123, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, E.; Hofmann, J.P.; Parker, S.F.; Sankar, M.; Lari, G.M.; Kondrat, S.A.; Knight, D.W.; Bethell, D.; Weckhuysen, B.M.; Hutchings, G.J. In situ spectroscopic investigation of oxidative dehydrogenation and disproportionation of benzyl alcohol. Phys. Chem. Chem. Phys. 2013, 15, 12147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Y.; Guo, Z.; Wang, X.; Yang, Y. Promoted aerobic oxidation of benzyl alcohol on CNT supported platinum by iron oxide. Chem. Commun. 2011, 47, 7473. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Medlin, J. Benzyl alcohol oxidation on Pd (111): Aromatic binding effects on alcohol reactivity. Langmuir 2014, 30, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Albonetti, S.; Mazzoni, R.; Cavani, F. Homogeneous, Heterogeneous and Nanocatalysis; The Royal society of Chemistry: London, UK, 2015; ISBN 9781782621652. [Google Scholar]
- Zotova, N.; Hellgardt, K.; Kelsall, G.H.; Jessiman, A.S.; Hii, K.K. (Mimi) Catalysis in flow: The practical and selective aerobic oxidation of alcohols to aldehydes and ketones. Green Chem. 2010, 12, 2157. [Google Scholar] [CrossRef]
- Miedziak, P.; Sankar, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Knight, D.W.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Oxidation of benzyl alcohol using supported gold-palladium nanoparticles. Catal. Today 2011, 164, 315–319. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Galvanin, F.; Morad, M.; Cao, E.; Cattaneo, S.; Sankar, M.; Dua, V.; Hutchings, G.; Gavriilidis, A. Hydrodynamic effects on three phase micro-packed bed reactor performance—Gold-palladium catalysed benzyl alcohol oxidation. Chem. Eng. Sci. 2016, 149, 129–142. [Google Scholar] [CrossRef]
- Wu, G.; Constantinou, A.; Cao, E.; Kuhn, S.; Morad, M.; Sankar, M.; Bethell, D.; Hutchings, G.J.; Gavriilidis, A. Continuous heterogeneously catalyzed oxidation of benzyl alcohol using a tube-in-tube membrane microreactor. Ind. Eng. Chem. Res. 2015, 54, 4183–4189. [Google Scholar] [CrossRef]
- O’Brien, M.; Taylor, N.; Polyzos, A.; Baxendale, I.R.; Ley, S.V. Hydrogenation in flow: Homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas–liquid contact at elevated pressure. Chem. Sci. 2011, 2, 1250–1257. [Google Scholar] [CrossRef]
- Yang, L.; Jensen, K.F. Mass transport and reactions in the tube-in-tube reactor. Org. Process Res. Dev. 2013, 17, 927–933. [Google Scholar] [CrossRef]
- Wu, G.; Cao, E.; Kuhn, S.; Gavriilidis, A. A Novel Approach for Measuring Gas Solubility in Liquids Using a Tube-in-Tube Membrane Contactor. Chem. Eng. Technol. 2017, 1–6. [Google Scholar] [CrossRef]
- Petersen, T.P.; Polyzos, A.; O’Brien, M.; Ulven, T.; Baxendale, I.R.; Ley, S.V. The oxygen-mediated synthesis of 1,3-butadiynes in continuous flow: Using teflon AF-2400 to effect gas/liquid contact. ChemSusChem 2012, 5, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.; Wu, G.; Corredera, A.; Ellis, P.; Bethell, D.; Hutchings, G.J.; Kuhn, S.; Gavriilidis, A. Continuous Heterogeneously Catalyzed Oxidation of Benzyl Alcohol in a Ceramic Membrane Packed-Bed Reactor. Org. Process Res. Dev. 2015, 19, 1973–1979. [Google Scholar] [CrossRef]
- Skowerski, K.; Czarnocki, S.J.; Knapkiewicz, P. Tube-in-tube reactor as a useful tool for homo- and heterogeneous olefin metathesis under continuous flow mode. ChemSusChem 2014, 7, 536–542. [Google Scholar] [CrossRef]
- Brzozowski, M.; O’Brien, M.; Ley, S.V.; Polyzos, A. Flow chemistry: Intelligent processing of gas-liquid transformations using a tube-in-tube reactor. Acc. Chem. Res. 2015, 48, 349–362. [Google Scholar] [CrossRef]
- Feng, J.; Ma, C.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Li, D.; Du, Y.; Morgan, D.J.; Hutchings, G.J. Au–Pd nanoalloys supported on Mg–Al mixed metal oxides as a multifunctional catalyst for solvent-free oxidation of benzyl alcohol. Dalton Trans. 2013, 42, 14498–14508. [Google Scholar] [CrossRef]
- Greene, J.F.; Preger, Y.; Stahl, S.S.; Root, T.W. PTFE-Membrane Flow Reactor for Aerobic Oxidation Reactions and Its Application to Alcohol Oxidation. Org. Process Res. Dev. 2015, 19, 858–864. [Google Scholar] [CrossRef]
- Koos, P.; Gross, U.; Polyzos, A.; O’Brien, M.; Baxendale, I.; Ley, S.V. Teflon AF-2400 mediated gas–liquid contact in continuous flow methoxycarbonylations and in-line FTIR measurement of CO concentration. Org. Biomol. Chem. 2011, 9, 6903–6908. [Google Scholar] [CrossRef] [PubMed]
- Schweidtmann, A.M.; Clayton, A.D.; Holmes, N.; Bradford, E.; Bourne, R.A.; Lapkin, A.A. Machine learning meets continuous flow chemistry: Automated optimization towards the Pareto front of multiple objectives. Chem. Eng. J. 2018, 352, 277–282. [Google Scholar] [CrossRef]
- Jumbam, D.N.; Skilton, R.A.; Parrott, A.J.; Bourne, R.A.; Poliakoff, M. The Effect of Self-Optimisation Targets on the Meethylation of Alcohols Using Dimethyl Carbonate in Supercritical CO2. J. Flow Chem. 2012, 2, 24–27. [Google Scholar] [CrossRef]
- Erdmann, V.; Mackfeld, U.; Rother, D.; Jakoblinnert, A. Enantioselective, continuous (R)- and (S)-2-butanol synthesis: Ahieving high space-time yields with recombinant E. coli cells in a micro-aqueous, solvent-free reaction system. J. Biotechnol. 2014, 191, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Schotten, C.; Howard, J.L.; Jenkins, R.L.; Codina, A.; Browne, D.L. A continuous flow-batch hybrid reactor for commodity chemical synthesis enabled by inline NMR and temperature monitoring. Tetrahedon 2018, 74, 5503–5509. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, J.C.F.; Friend, C.M.; Madix, R.J. Origin of the selectivity in the gold-mediated oxidation of benzyl alcohol. Surf. Sci. 2012, 606, 1129–1134. [Google Scholar] [CrossRef]
| System | Pressure (Bar) | % Conversion | % Selectivity | Throughput (g/h) | STY (g/(L*h)) |
|---|---|---|---|---|---|
| X-Cube™ O2 | 9.7 | 17.5 ± 1.4 | 88.0 ± 1.8 | 60.3 | 77,000 |
| Sparger O2 | 2.8 | 31.7 ± 6.1 | 100 ± 0.0 | 49.7 | 64,000 |
| 9.7 | 17.5 ± 3.8 | 90.1 ± 3.1 | 42.4 | 54,000 | |
| Sparger Air | 2.8 | 10.9 ± 1.9 | 100 ± 0.0 | 17.1 | 22,000 |
| 9.7 | 11.7 ± 0.5 | 84.8 ± 1.2 | 31.1 | 40,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkholder, M.; Gilliland, S.E., III; Luxon, A.; Tang, C.; Gupton, B.F. Improving Productivity of Multiphase Flow Aerobic Oxidation Using a Tube-in-Tube Membrane Contactor. Catalysts 2019, 9, 95. https://doi.org/10.3390/catal9010095
Burkholder M, Gilliland SE III, Luxon A, Tang C, Gupton BF. Improving Productivity of Multiphase Flow Aerobic Oxidation Using a Tube-in-Tube Membrane Contactor. Catalysts. 2019; 9(1):95. https://doi.org/10.3390/catal9010095
Chicago/Turabian StyleBurkholder, Michael, Stanley E. Gilliland, III, Adam Luxon, Christina Tang, and B. Frank Gupton. 2019. "Improving Productivity of Multiphase Flow Aerobic Oxidation Using a Tube-in-Tube Membrane Contactor" Catalysts 9, no. 1: 95. https://doi.org/10.3390/catal9010095
APA StyleBurkholder, M., Gilliland, S. E., III, Luxon, A., Tang, C., & Gupton, B. F. (2019). Improving Productivity of Multiphase Flow Aerobic Oxidation Using a Tube-in-Tube Membrane Contactor. Catalysts, 9(1), 95. https://doi.org/10.3390/catal9010095






