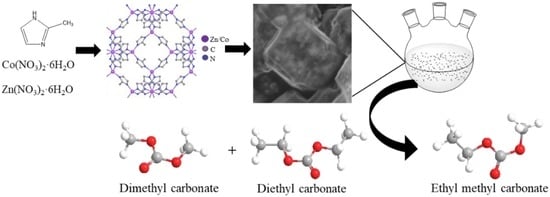
Zn-Co@N-Doped Carbon Derived from ZIFs for High-Efficiency Synthesis of Ethyl Methyl Carbonate: The Formation of ZnO and the Interaction between Co and Zn
Abstract
1. Introduction
2. Results and Discussion
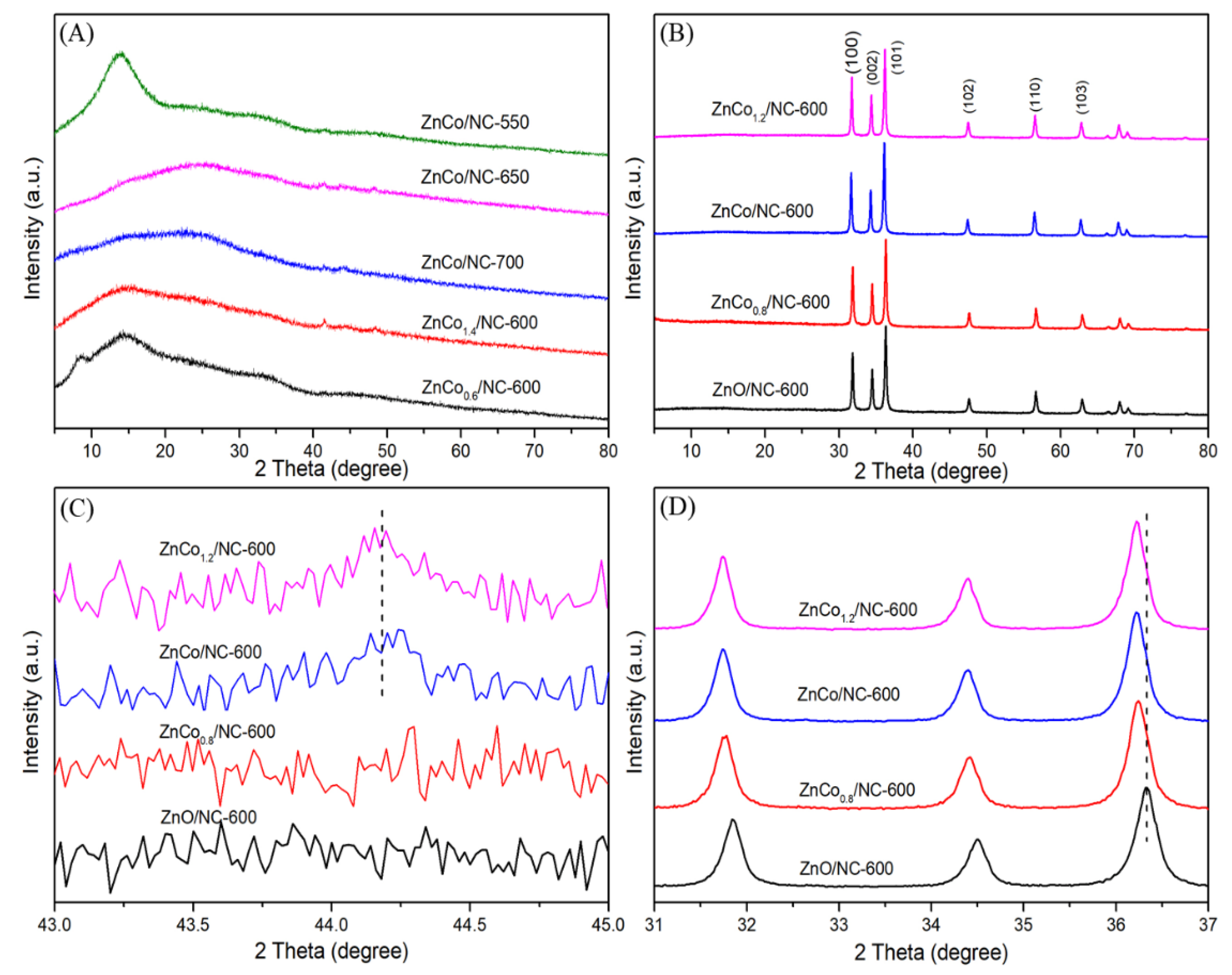
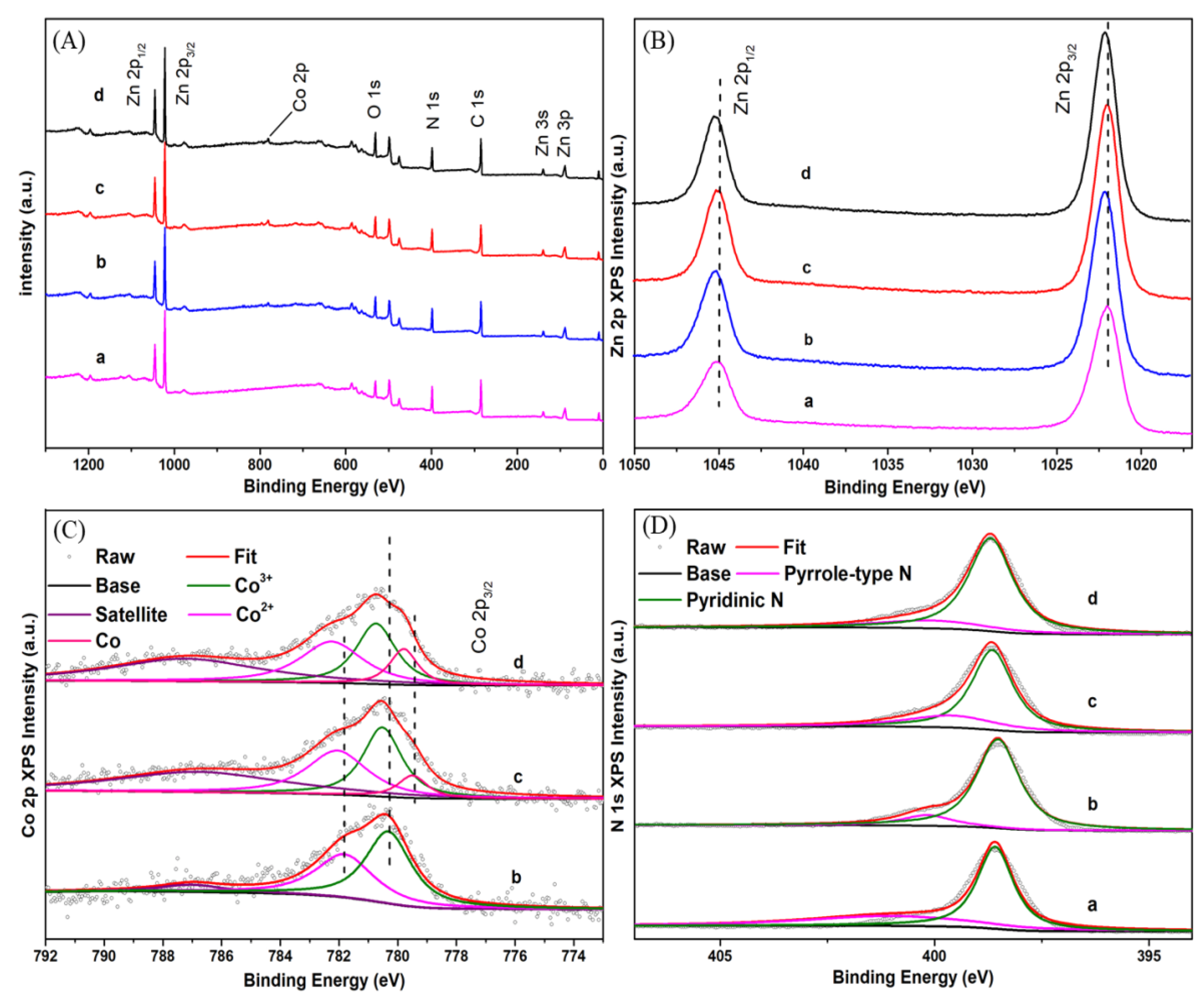
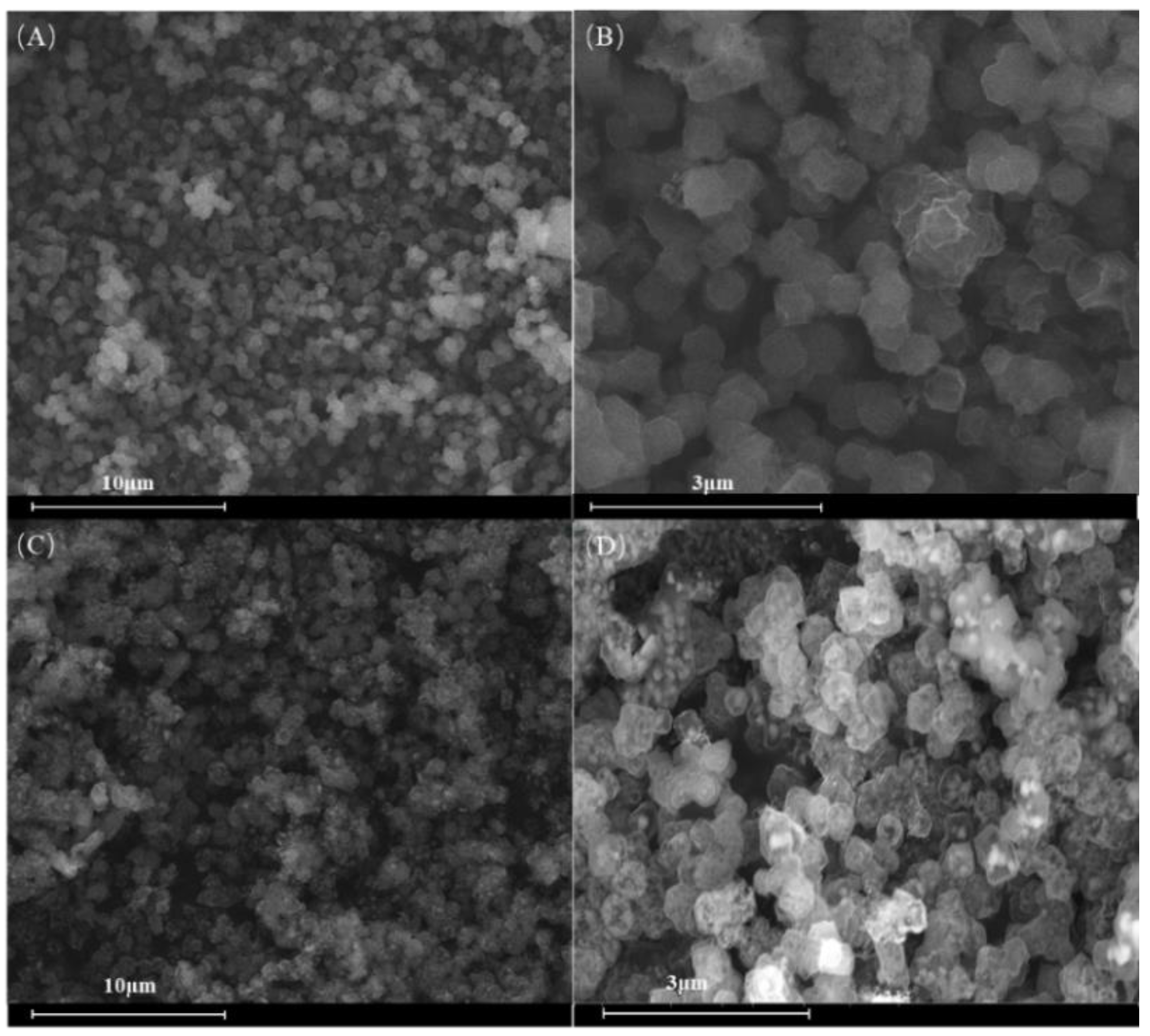
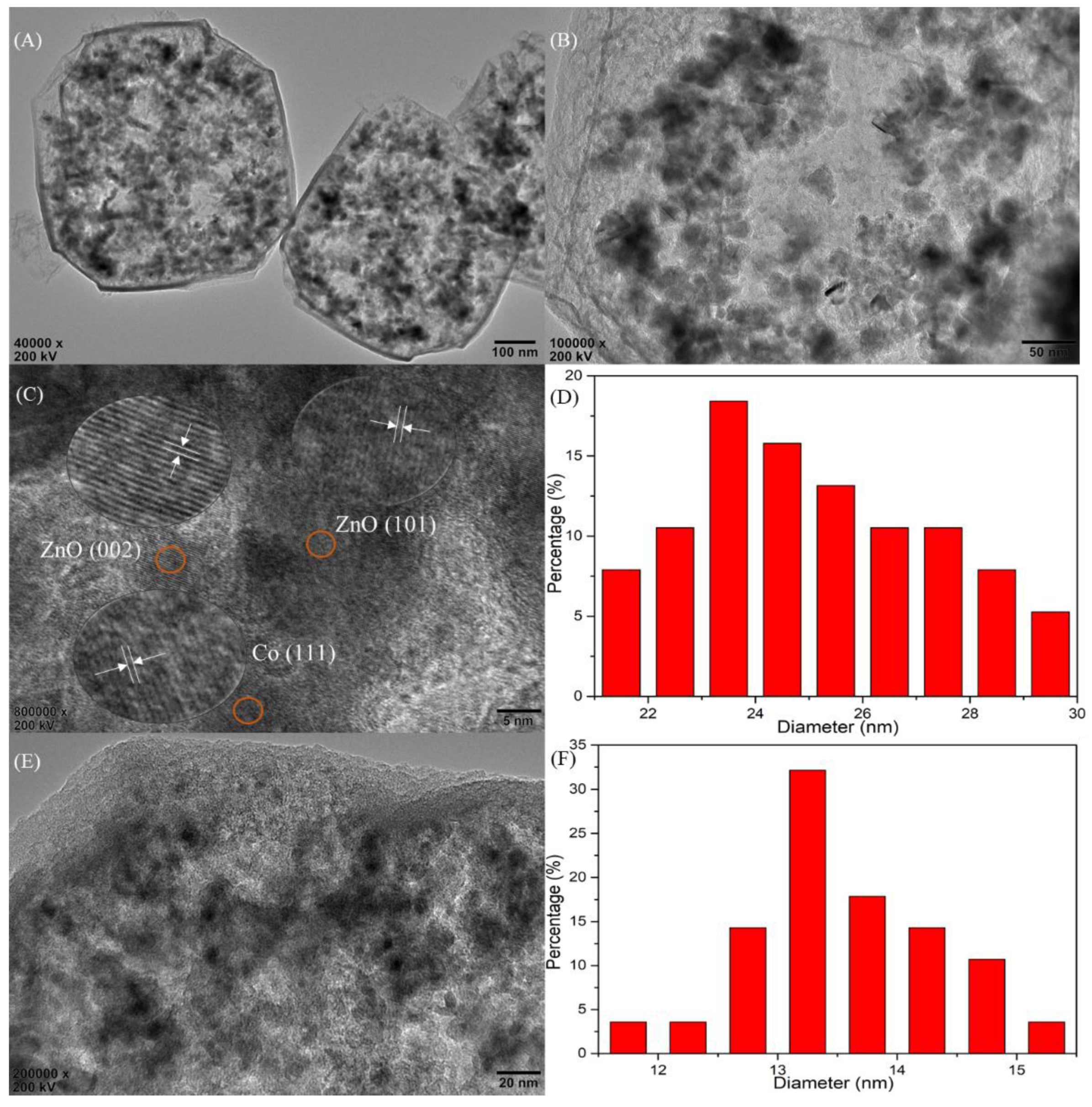
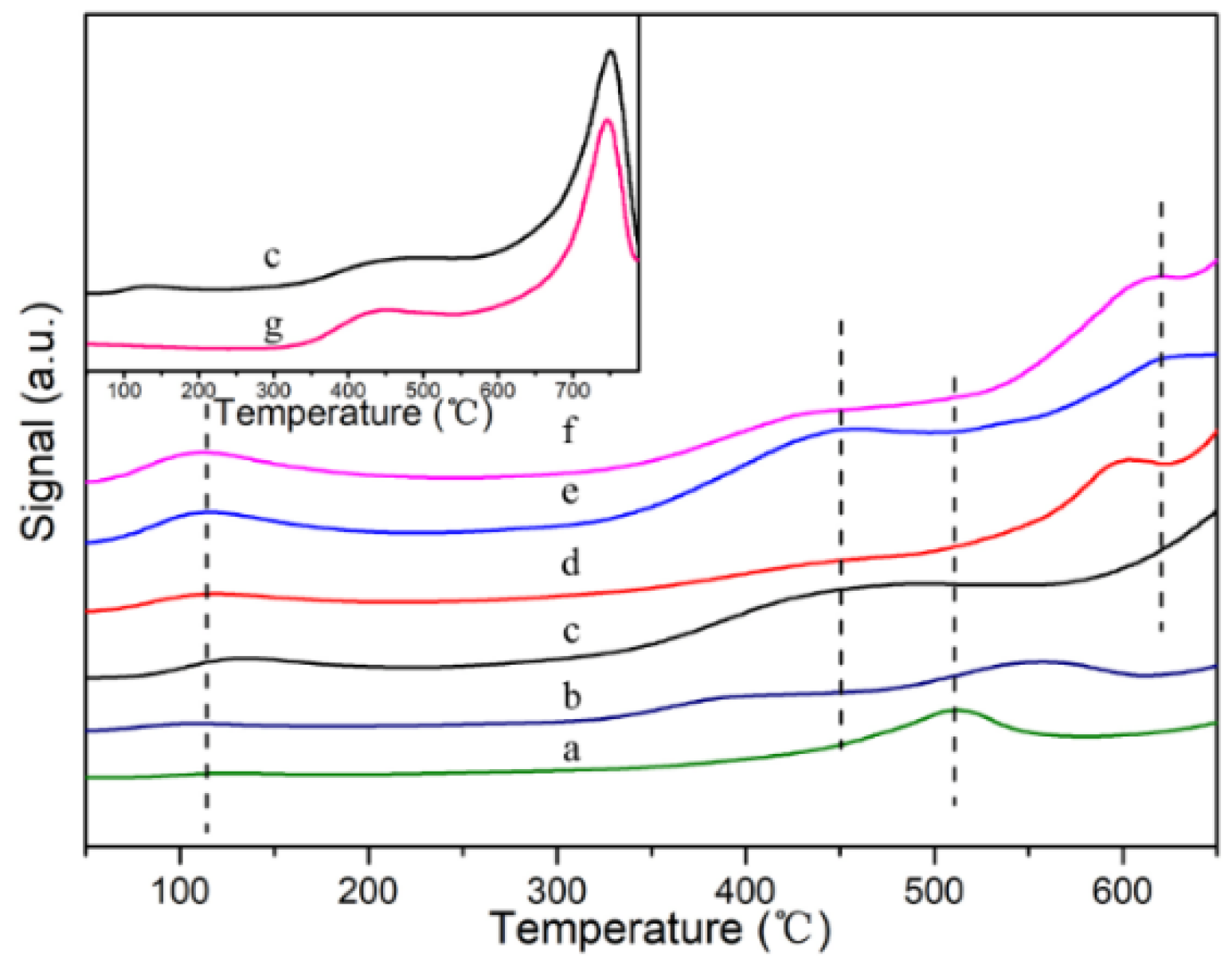
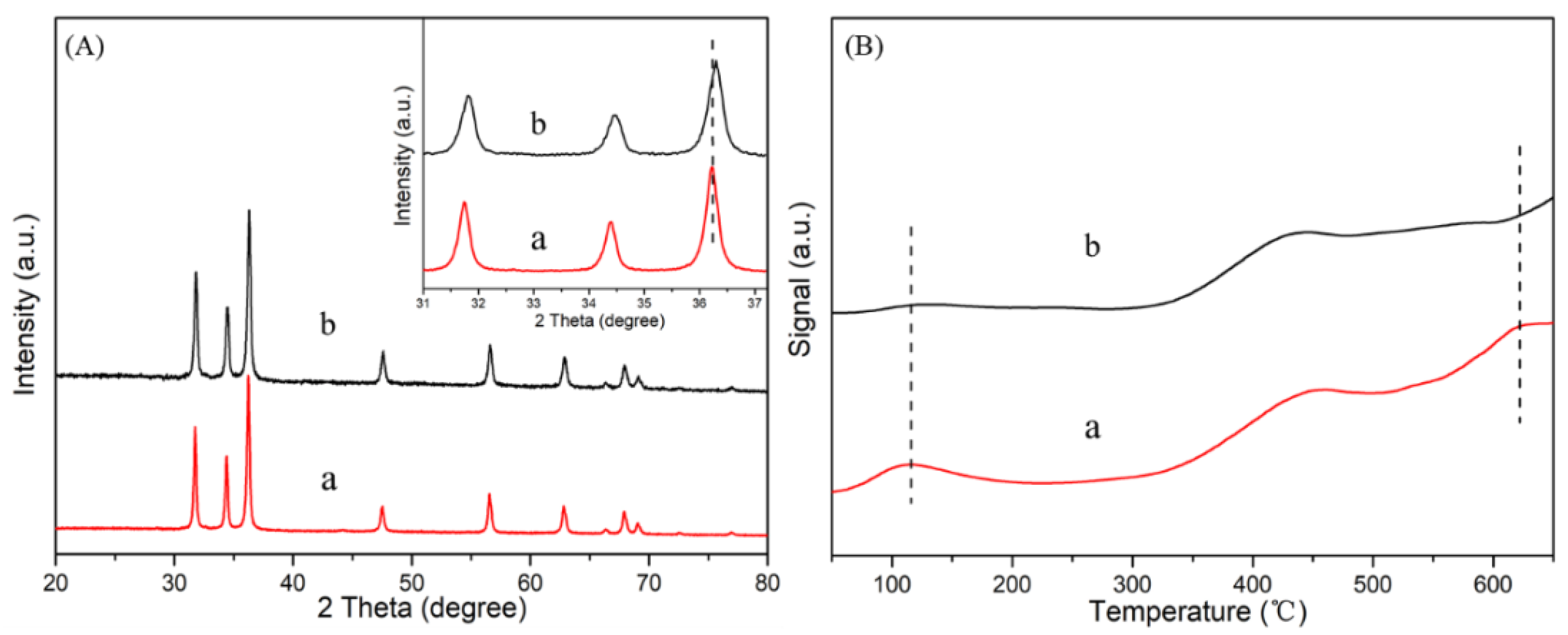
2.1. Characterization of Catalysts
2.2. Synthesis of EMC across Different Zn-Co@N-Doped Carbon Materials
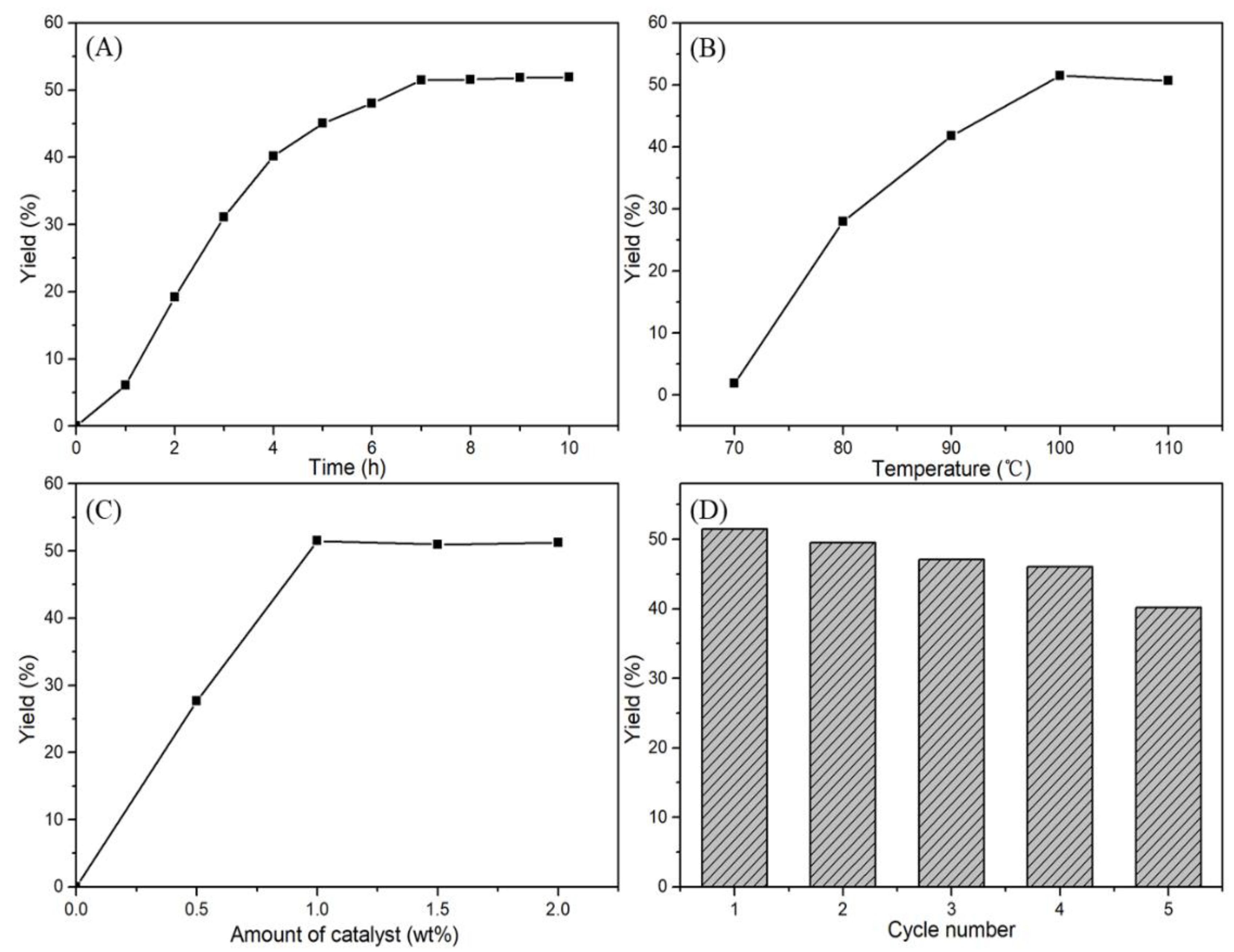
2.3. Effects of Reaction Conditions
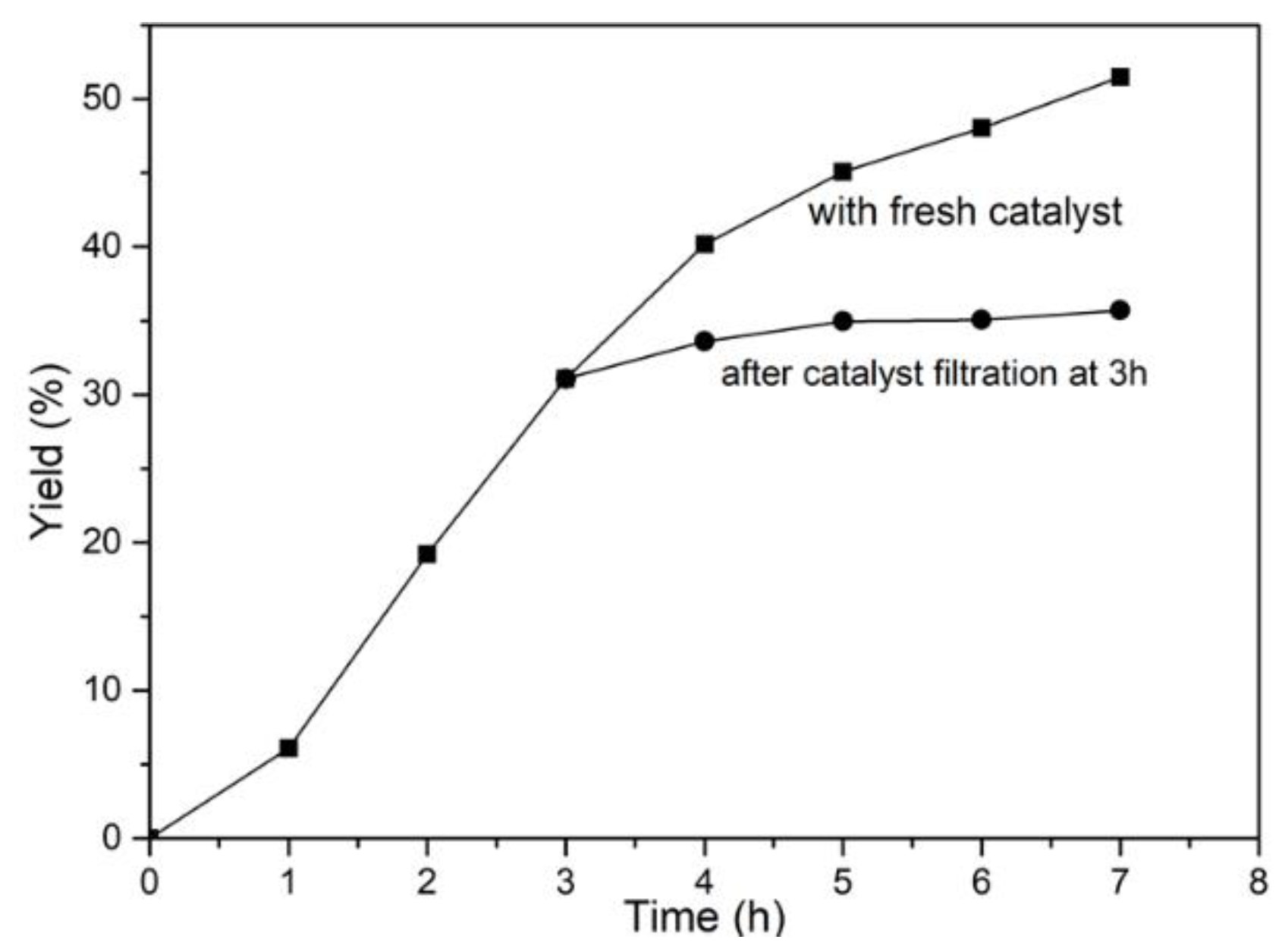
2.4. Recycling of the Catalyst
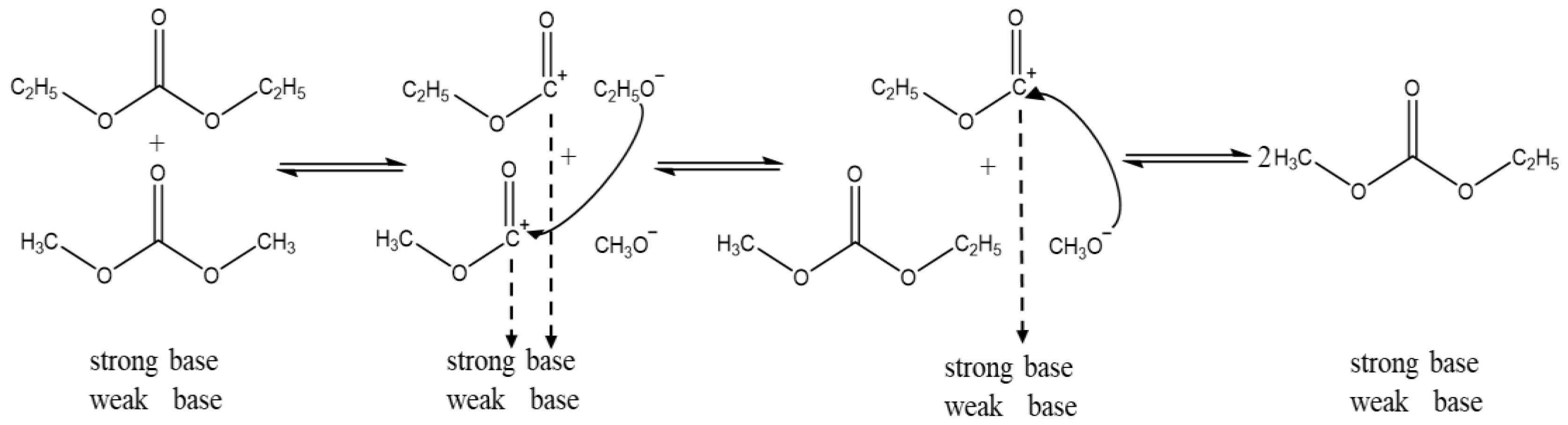
2.5. Reaction Mechanism
3. Materials and Methods
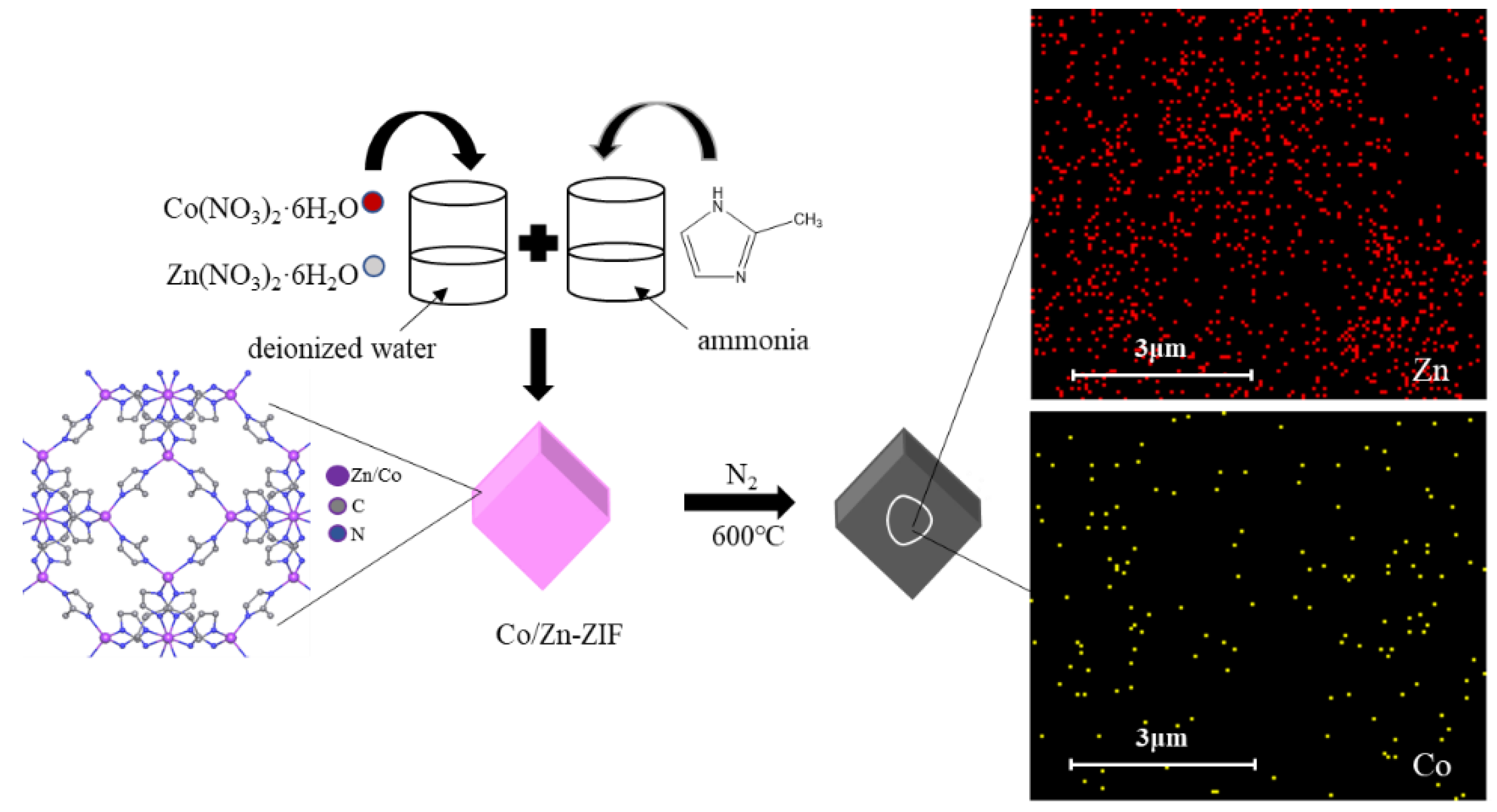
3.1. Catalyst Preparation
3.2. Characterization of Catalysts
3.3. Reaction Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ding, M.S.; Xu, K.; Zhang, S.S.; Amine, K.; Henriksen, G.L.; Jow, T.R. Change of Conductivity with Salt Content, Solvent Composition, and Temperature for Electrolytes of LiPF6 in Ethylene Carbonate-Ethyl Methyl Carbonate. J. Electrochem. Soc. 2001, 148, A1196–A1204. [Google Scholar] [CrossRef]
- Ding, M.S. Liquid Phase Boundaries, Dielectric Constant, and Viscosity of PC-DEC and PC-EC Binary Carbonates. J. Electrochem. Soc. 2003, 150, A455–A462. [Google Scholar] [CrossRef]
- Ue, M.; Mori, S. Mobility and Ionic Association of Lithium Salts in a Propylene Carbonate-Ethyl Methyl Carbonate Mixed Solvent. J. Electrochem. Soc. 1995, 142, 2577–2581. [Google Scholar] [CrossRef]
- Hall, D.S.; Self, J.; Dahn, J.R. Dielectric Constants for Quantum Chemistry and Li-Ion Batteries: Solvent Blends of Ethylene Carbonate and Ethyl Methyl Carbonate. J. Phys. Chem. C 2015, 119, 22322–22330. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, Z.; Li, J. LiPF6 and lithium difluoro (oxalato) borate/ethylene carbonate + dimethyl carbonate + ethyl(methyl)carbonate electrolyte for Li4Ti5O12 anode. J. Power Sources 2013, 230, 148–154. [Google Scholar] [CrossRef]
- Keller, T.; Holtbruegge, J.; Górak, A. Transesterification of dimethyl carbonate with ethanol in a pilot-scale reactive distillation column. Chem. Eng. J. 2012, 180, 309–322. [Google Scholar] [CrossRef]
- Murugan, C.; Bajaj, H.C. Synthesis of diethyl carbonate from dimethyl carbonate and ethanol using KF/Al2O3 as an efficient solid base catalyst. Fuel Process. Technol. 2011, 92, 77–82. [Google Scholar] [CrossRef]
- Zielinska-Nadolska, I.; Warmuzinski, K.; Richter, J. Zeolite and other heterogeneous catalysts for the transesterification reaction of dimethyl carbonate with ethanol. Catal. Today 2006, 114, 226–230. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, J.; Jian, C. Experimental Isobaric Vapor-Liquid Equilibrium for Binary Systems of Ethyl Methyl Carbonate + Methanol, + Ethanol, + Dimethyl Carbonate, or + Diethyl Carbonate at 101.3 kPa. J. Chem. Eng. Data 2010, 55, 4896–4902. [Google Scholar] [CrossRef]
- Palani, A.; Gokulakrishnan, N.; Palanichamy, M.; Pandurangan, A. Transesterification of dimethyl carbonate with diethyl carbonate over Al-Zn-MCM-41 and Al-MCM-41 molecular sieves. Appl. Catal. A 2006, 304, 152–158. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.; Sun, M.; Gao, C. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate. Catal. Commun. 2014, 54, 86–90. [Google Scholar] [CrossRef]
- Zhuo, G.; Shen, Z.; Jiang, X. Synthesis of ethyl methyl carbonate by homogeneous catalysis. Chin. J. Catal. 2004, 25, 171–172. [Google Scholar]
- Shen, Z.; Jiang, X.; Zhao, W. A New Catalytic Transesterification for the Synthesis of Ethyl Methyl Carbonate. Catal. Lett. 2003, 91, 63–67. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, J.; Liu, G.; Liu, Y.; Wang, Z.; Zhang, W.; Jia, M. Efficient porous carbon-supported MgO catalysts for the transesterification of dimethyl carbonate with diethyl carbonate. J. Mol. Catal. A Chem. 2010, 327, 32–37. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Ma, X.; He, Y.; Alshammari, A.S.; Deng, Y. Binary Mg–Fe oxide as a highly active and magnetically separable catalyst for the synthesis of ethyl methyl carbonate. RSC. Adv. 2015, 5, 25849–25856. [Google Scholar] [CrossRef]
- Chen, Y.; Han, J.; Zhang, H. Facile synthesis and characterization of acid-base bifunctionalized mesoporous silica. Appl. Surf. Sci. 2008, 254, 5967–5974. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Wang, S.; Zhang, J.; Yang, Y. Magnesium Aluminum Spinel as an Acid-Base Catalyst for Transesterification of Diethyl Carbonate with Dimethyl Carbonate. Catal. Lett. 2014, 144, 1602–1608. [Google Scholar] [CrossRef]
- Chen, F.; Bai, D.; Wang, Y.; Jiang, D.; He, Y. A family of ssa-type copper-based MOFs constructed from unsymmetrical diisophthalates: Synthesis, characterization and selective gas adsorption. Mater. Chem. Front. 2017, 1, 2283–2291. [Google Scholar] [CrossRef]
- Xue, Z.Z.; Zhang, D.; Pan, J.; Han, S.D.; Li, J.H.; Wang, G.M. A porous copper-organic framework with intersecting channels and gas adsorption properties. Dalton Trans. 2017, 46, 13952–13956. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Adsorptive removal and separation of chemicals with metal-organic frameworks: Contribution of pi-complexation. J. Hazard. Mater. 2017, 325, 198–213. [Google Scholar] [CrossRef]
- Luo, F.; Yan, C.; Dang, L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X.; Han, Y.; Hu, T.L.; O’Keeffe, M.; et al. UTSA-74: A MOF-74 Isomer with Two Accessible Binding Sites per Metal Center for Highly Selective Gas Separation. J. Am. Chem. Soc. 2016, 138, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Titanium-based zeolitic imidazolate framework for chemical fixation of carbon dioxide. Green Chem. 2016, 18, 4855–4858. [Google Scholar] [CrossRef]
- Chen, B.L.; Yang, Z.X.; Zhu, Y.Q.; Xia, Y.D. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Cai, W.; Chu, C.C.; Liu, G.; Wang, Y.X. Metal-Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of ZIF-8 under oxidative and inert environments: A practical perspective on using ZIF-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Park, K.; Ni, Z.; Côté, A.P.; Choi, J.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, J.; Liang, S.; Hu, S.; Liu, H.; Jiang, T.; Han, B. Metal-organic frameworks as an acid catalyst for the synthesis of ethyl methyl carbonate via transesterification. J. Mol. Catal. A Chem. 2009, 308, 68–72. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.; Bats, N. Catalysis of Transesterification by a Nonfunctionalized Metal-Organic Framework: Acido-Basicity at the External Surface of ZIF-8 Probed by FTIR and ab Initio Calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, H.P.; Wang, G.Y.; Yao, Z.G.; Tang, Y.R.; Zheng, S.S. Zeolitic imidazolate framework as efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate. J. Mol. Catal. A Chem. 2013, 366, 43–47. [Google Scholar] [CrossRef]
- Gai, P.; Zhang, H.; Zhang, Y.; Liu, W.; Zhu, G.; Zhang, X.; Chen, J. Simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid based on nitrogen doped porous carbon nanopolyhedra. J. Mater. Chem. B 2013, 1, 2742. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-Organic Frameworks and their derived nanostructures for Electrochemical Energy Storage and Conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Wu, M.; Ye, H.L.; Zhao, F.Q.; Zeng, B.Z. High-Quality Metal-Organic Framework ZIF-8 Membrane Supported on Electrodeposited ZnO/2-methylimidazole Nanocomposite: Efficient Adsorbent for the Enrichment of Acidic Drugs. Sci. Rep. 2017, 7, 39778. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Hu, M.; Wang, H.; Huang, H.S.; Fujita, T.; Wu, K.C.; Chen, L.C.; Yamauchi, Y.; Ariga, K. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem. Commun. (Camb.) 2012, 48, 7259–7261. [Google Scholar] [CrossRef]
- Jiang, H.L.; Liu, B.; Lan, Y.Q.; Kuratani, K.; Akita, T.; Shioyama, H.; Zong, F.; Xu, Q. From metal-organic framework to nanoporous carbon: Toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 2011, 133, 11854–11857. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yao, J.; Liu, Q.; Wang, K.; Chen, F.; Wang, H. Facile synthesis of zeolitic imidazolate framework-8 from a concentrated aqueous solution. Microporous Mesoporous Mater. 2014, 184, 55–60. [Google Scholar] [CrossRef]
- Han, Y.; Qi, P.; Li, S.; Feng, X.; Zhou, J.; Li, H.; Su, S.; Li, X.; Wang, B. A novel anode material derived from organic-coated ZIF-8 nanocomposites with high performance in lithium ion batteries. Chem. Commun. (Camb.) 2014, 50, 8057–8060. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y. Chemoselective hydrogenation of functionalized nitroarenes using MOF-derived co-based catalysts. J. Mol. Catal. A Chem. 2016, 420, 56–65. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Cai, F.; Pan, D.; Xiao, G. High-efficiency and low-cost Li/ZnO catalysts for synthesis of glycerol carbonate from glycerol transesterification: The role of Li and ZnO interaction. Appl. Catal. A 2017, 532, 77–85. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, S.; Gao, L.; Xiao, G. Metal-organic frameworks derived bimetallic Cu-Co catalyst for efficient and selective hydrogenation of biomass-derived furfural to furfuryl alcohol. Mol. Catal. 2017, 436, 128–137. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Collinge, G.; Xiang, Y.; Barbosa, R.; McEwen, J.S.; Kruse, N. CO-induced inversion of the layer sequence of a model CoCu catalyst. Surf. Sci. 2016, 648, 74–83. [Google Scholar] [CrossRef]
- PELs, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Wang, W.; Huang, Y.; Liu, X.; Miao, S.; Liu, J.; Zhang, T. Co-N-C Catalyst for C-C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Apesteguía, C.R. Base catalysis for the systhesis of α, β-unsaturated ketones from the vapor-phase aldol consendation of acetone. Appl. Catal. A 1996, 137, 149–166. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Di Cosimo, J.I. Acid-base properties and active site requirements for elimination reactions on alkali-promoted MgO catalysts. Catal. Today 2000, 63, 53–62. [Google Scholar] [CrossRef]
- Seemala, B.; Cai, C.M.; Kumar, R.; Wyman, C.E.; Christopher, P. Effects of Cu-Ni Bimetallic Catalyst Composition and Support on Activity, Selectivity, and Stability for Furfural Conversion to 2-Methyfuran. ACS Sustain. Chem. Eng. 2018, 6, 2152–2161. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.B.; Nadagouda, M.N.; Varma, R.S. Hydroxylation of Benzene via C–H Activation Using Bimetallic CuAg@g-C3N4. ACS Sustain. Chem. Eng. 2017, 5, 3637–3640. [Google Scholar] [CrossRef]
| Entry | Catalyst | SBET (m2/g) | Vp (cm3/g) | dp (nm) |
|---|---|---|---|---|
| 1 | Co/Zn-ZIF | 1168.3 | 0.074 | 10.15 |
| 2 | ZnO/NC-600 | 715.6 | 0.144 | 5.38 |
| 3 | ZnCo0.8/NC-600 | 275.0 | 0.295 | 11.15 |
| 4 | ZnCo/NC-600 | 254.8 | 0.274 | 11.72 |
| 5 | ZnCo1.2/NC-600 | 200.8 | 0.535 | 12.18 |
| Entry | Catalysts | n(DMC):n(DEC) | Yield (%) | Selectivity (%) |
|---|---|---|---|---|
| 1 | ZnO/NC-600 | 1:1 | 29.50 | ~100 |
| 2 | ZnCo0.6/NC-600 | 1:1 | 2.26 | ~100 |
| 3 | ZnCo0.8/NC-600 | 1:1 | 43.58 | ~100 |
| 4 | ZnCo/NC-600 | 1:1 | 51.50 | ~100 |
| 5 | ZnCo1.2/NC-600 | 1:1 | 42.53 | ~100 |
| 6 | ZnCo1.4/NC-600 | 1:1 | -- | -- |
| 7 | ZnCo/NC-550 | 1:1 | 0.91 | ~100 |
| 8 | ZnCo/NC-650 | 1:1 | 9.33 | ~100 |
| 9 | ZnCo/NC-700 | 1:1 | 1.82 | ~100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.-N.; Wang, Y.; Pan, D.-H.; Song, X.-H.; Xu, S.-Q.; Gao, L.-J.; Xiao, G.-M. Zn-Co@N-Doped Carbon Derived from ZIFs for High-Efficiency Synthesis of Ethyl Methyl Carbonate: The Formation of ZnO and the Interaction between Co and Zn. Catalysts 2019, 9, 94. https://doi.org/10.3390/catal9010094
Miao Y-N, Wang Y, Pan D-H, Song X-H, Xu S-Q, Gao L-J, Xiao G-M. Zn-Co@N-Doped Carbon Derived from ZIFs for High-Efficiency Synthesis of Ethyl Methyl Carbonate: The Formation of ZnO and the Interaction between Co and Zn. Catalysts. 2019; 9(1):94. https://doi.org/10.3390/catal9010094
Chicago/Turabian StyleMiao, Ya-Nan, Yuan Wang, Dong-Hui Pan, Xiang-Hai Song, Si-Quan Xu, Li-Jing Gao, and Guo-Min Xiao. 2019. "Zn-Co@N-Doped Carbon Derived from ZIFs for High-Efficiency Synthesis of Ethyl Methyl Carbonate: The Formation of ZnO and the Interaction between Co and Zn" Catalysts 9, no. 1: 94. https://doi.org/10.3390/catal9010094
APA StyleMiao, Y.-N., Wang, Y., Pan, D.-H., Song, X.-H., Xu, S.-Q., Gao, L.-J., & Xiao, G.-M. (2019). Zn-Co@N-Doped Carbon Derived from ZIFs for High-Efficiency Synthesis of Ethyl Methyl Carbonate: The Formation of ZnO and the Interaction between Co and Zn. Catalysts, 9(1), 94. https://doi.org/10.3390/catal9010094