Abstract
The application of flow reactors in multiphase catalytic reactions represents a promising approach for enhancing the efficiency of this important class of chemical reactions. We developed a simple approach to improve the reactor productivity of multiphase catalytic reactions performed using a flow chemistry unit with a packed bed reactor. Specifically, a tube-in-tube membrane contactor (sparger) integrated in-line with the flow reactor has been successfully applied to the aerobic oxidation of benzyl alcohol to benzaldehyde utilizing a heterogeneous palladium catalyst in the packed bed. We examined the effect of sparger hydrodynamics on reactor productivity quantified by space time yield (STY). Implementation of the sparger, versus segmented flow achieved with the built in gas dosing module (1) increased reactor productivity 4-fold quantified by space time yield while maintaining high selectivity and (2) improved process safety as demonstrated by lower effective operating pressures.
Keywords:
flow chemistry; continuous reactor; tube-in-tube; multiphase catalysis; oxidation; aerobic 1. Introduction
Multiphase catalytic reactions (e.g., hydrogenation and oxidation) are important in the production of petroleum-derived products, commodity and fine chemicals, and pharmaceuticals [1,2,3]. Such reactions using conventional reactors (e.g., trickle-beds, bubble columns) are challenging due to insufficient heat- and mass transfer, low interfacial areas, and potential process safety concerns [3,4,5,6]. Flow reactor technology is a promising alternative to achieve safer and more efficient on-demand manufacturing that can be scaled with parallel reactors [3,4,5,7,8,9,10]. Key benefits include (i) enhanced heat and mass transfer due to inherently large surface area-to-volume ratios; and (ii) small volumes which mitigate safety issues [3,4,5,7,8,9,10,11].
Performing aerobic, selective oxidation of alcohols to aldehydes using flow reactors could greatly reduce economic and environmental costs associated with chemical processing [2,8,12]. Several factors must be considered to acquire these benefits, including the type of oxidant and catalyst. Using catalytic amounts of oxygen gas as the terminal oxidant, water is typically the only byproduct and it avoids the use of stoichiometric amounts of harsh oxidizing agents [13]. Both homogeneous and heterogeneous catalysts have the ability to facilitate selective oxidations using gas oxidants; however, heterogeneous catalysts are advantageous due to the ease of product separation in continuous reactors [2,12,14,15,16,17,18,19,20]. In these solid-liquid-gas reactions, hydrodynamics and wetting are important considerations [5,21].
Hii and co-workers demonstrated selective oxidation of primary and secondary alcohols using a commercially available catalyst and flow reactor [19]. Using 0.1–1 M substrate concentrations of benzyl alcohol, complete conversion and >99% selectivity was achieved [19]. However, the dilute conditions inherently limited reactor productivity. Alternatively, Gavriilidis and co-workers built a tube-in-tube membrane reactor for selective oxidation of neat benzyl alcohol to benzaldehyde [22]. The role of the tube-in-tube membrane reactor was to facilitate the gas oxidant diffusion into the reacting liquid to feed to the catalytic packed bed [8,10,22,23,24,25,26,27,28,29]. They found that the tube-in-tube reactor improved the catalyst contact with both benzyl alcohol and oxygen simultaneously, resulting in increased conversion and selectivity. This result indicated that the reactor performance was limited by oxygen permeation. Diluting the catalyst bed with silica beads to increase the catalyst contact time also improved selectivity; however, the maximum selectivity obtained was less than 80% in all cases [22]. From these studies, the authors indicated that improving reactor productivity while maintaining selective oxidation was desirable.
We investigated the use of a tube-in-tube membrane contactor with a commercial continuous reactor to improve reactor productivity of a multiphase (solid-liquid-gas) catalytic reactions. Specifically, we introduced a tube-in-tube membrane contactor (sparger) to a flow reactor for examination of the aerobic oxidation of benzyl alcohol by a heterogeneous palladium catalyst using oxygen or air as a model reaction. It is important to note that the reactor is the specific focus of this investigation, rather than the choice of substrate or catalyst. The effect of the sparger on hydrodynamics and resulting selectivity and reactor productivity are discussed.
2. Results and Discussion
Selective aerobic oxidation of benzyl alcohol to benzaldehyde via a commercial palladium-based catalyst was selected as a model system to examine the effect of gas-liquid contact in heterogeneous catalytic reactions performed in flow. Figure 1 depicts the full reaction pathway for the oxidation of benzyl alcohol. Use of noble metals such as palladium (Pd) were known to facilitate aerobic oxidation of alcohols, and Pd has been shown to be more active than Pt or Ru for selective oxidation of benzyl alcohol [30]. Furthermore, the mechanism of the reaction on the Pd surface has been well characterized [16]. Even with an active Pd catalyst, achieving selective aldehyde production was challenging since the thermodynamics favor the over-oxidized products [16,21]. The catalyst and reactor conditions affected the conversion and selectivity to the desired aldehyde product [21].
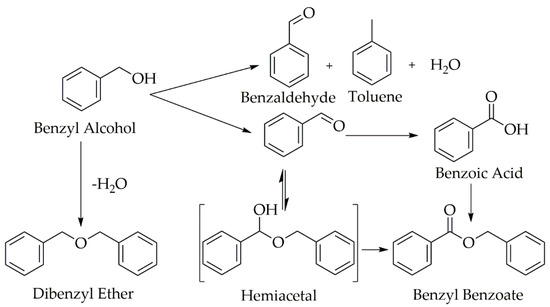
Figure 1.
Reaction mechanism for the oxidation of benzyl alcohol.
The reaction was first carried out continuously using the ThalesNano X-Cube™ reactor (Figure S1, Budapest, Hungary). An external reciprocating syringe pump upstream of an HPLC pump was added for the liquid stream to achieve precise residence times in the packed bed. The gas dosing module within the X-Cube™ maintained segmented flow with a fixed 1:19 volumetric gas bubble to liquid slug ratio throughout the process. In these initial experiments, the oxidation of benzyl alcohol was examined over 8 min of total residence time with 10 passes while monitoring the conversion of benzyl alcohol and selectivity to benzaldehyde. With air as the oxidant, the highest conversion of benzyl alcohol was 2.1% achieved after 8 passes with selectivity averaging 76% across the process (Figure S2).
To determine if availability of oxygen at the catalyst surface was limiting conversion, oxygen was used as the oxidant in place of air. The conversion of benzyl alcohol using oxygen was 60% higher than air: 3.4% compared to 2.1% after 6 min of residence time (Figure S2). Similarly, the conversion increased with overall residence time when using oxygen; this increase in conversion was likely due to the higher concentration of oxygen present in the liquid phase. Notably, the use of oxygen decreased the selectivity by 25% compared to air. The observation of decreasing benzaldehyde selectivity with increasing benzyl alcohol conversion can be attributed to over-oxidation to benzyl benzoate and was consistent with previous reports for Pd catalysts [30].
Conversion in a three-phase packed bed reactor can be affected by fluid hydrodynamics, such as fluid flow regime, on catalyst wetting. Increasing the oxygen-to-reagent ratio in such reactors could improve conversion and selectivity [21]. Therefore, a tube-in-tube membrane contactor (sparger) was introduced as an alternative to the gas dosing module of the X-Cube™ in order to increase the oxygen-to-reagent ratio. The sparger contained a semipermeable inner tube constructed with Teflon AF-2400, which was highly gas-permeable to facilitate transport of oxygen from the gas into the liquid phase [31]. The sparger was configured such that the gas stream in the inner tube ran countercurrent to the liquid stream in the outer PTFE shell (Figure 2). This configuration was chosen based on modeling demonstrating a high saturation fraction [24]. The sparger was integrated in line with the existing X-Cube™ between the syringe and HPLC pumps. The liquid feed containing dissolved gas from the sparger was pumped directly into the X-Cube™.
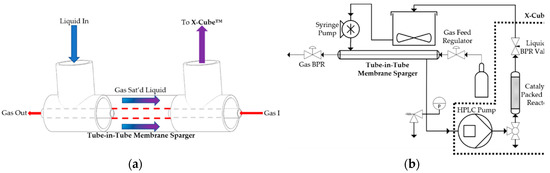
Figure 2.
(a) Diagram of the tube-in-tube membrane sparger. The gas, red arrows, flows through the center tube counter-currently to the liquid fluid, blue arrows, in the shell. The gas diffuses through the membrane dissolving into the liquid, the blue arrows transition to purple to indicate a gas rich liquid stream. The gas rich liquid stream is fed to the packed bed. (b) Flow diagram of the continuous process with the external syringe pump and sparger added to the X-Cube™.
The integration of the sparger unit resulted in a significant increase in conversion and selectivity. The maximum conversion achieved after 10 passes was 10.9 ± 1.3% using air and 31.7 ± 6.1% using oxygen (Figure 3). At 2.8 bar, the sparger exhibited a 5-fold improvement using air and a 9-fold improvement using oxygen in conversion compared to the X-Cube™ gas dosing module with a fixed 1:19 volumetric gas to liquid ratio (dictated by the X-Cube™ system). The increase in conversion of benzyl alcohol indicated that the sparger improved the effective oxygen supplied to the catalyst surface compared to the gas dosing module. The maximum conversion of 31.7 ± 6.1% was consistent with a gas-limited system described by Yang and Jensen based on mass transfer modeling [24]. This result confirmed that the sparger serves to facilitate oxygen solubilization into the liquid stream according to Henry’s law, which is a similar result to previous reports for industrially relevant gas/liquid reactions [25,26,28,32]. Conversion can be increased by diluting the substrate; however, dilution decreases the reactor productivity [10,26]. While conversion was an important metric for evaluating the efficiency of a chemical process, productivity/space time yield (STY) was an important consideration for reactor/process design. Reactor productivity/STY were especially relevant for continuous processes that incorporate recycling unreacted materials. Often, there exists a trade-off between selectivity/yield and productivity [33,34]. Practical examples include a time-dependent racemization process [35] and vigorously exothermic reductions [36].
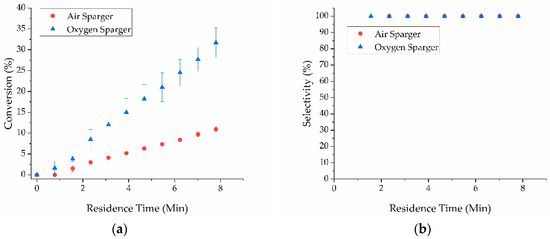
Figure 3.
(a) Conversion of benzyl alcohol; (b) selectivity to benzaldehyde for the sparger modified X-Cube™ and external pump system for both air (red circles) and pure oxygen (blue triangles) at 2.8 bar.
It should be noted that 10% conversion was achieved in only 8 min total residence time, which equates to a STY of 22,000 g∙L−1∙h−1 with air and 64,000 g∙L−1∙h−1 with oxygen. Hii and co-workers previously reported achieving 100% conversion using a ruthenium based catalyst. To compare across different process conditions (2.5-fold higher pressure, ~50-fold lower benzyl alcohol concentration, different temperature, and different catalyst) we use STY. We note they only achieved a STY of approximately 1800 g∙L−1∙h−1, due in large part to the 50-fold lower benzyl alcohol concentration [19]. This work focused on productivity rather than obtaining 100% conversion; we were able to obtain a 10-fold higher STY using a different metal catalyst and increasing the substrate concentration at lower overall operating pressure compared to previous work [19]. Therefore, implementing the sparger in line with the X-Cube™ provided a straightforward approach to increasing reactor productivity at lower operating pressures from 25 bar [19] to 2.8 bar (this work).
Additionally, use of the sparger increased the reaction selectivity to 100% for both air and pure oxygen with no observable over-oxidation over ten passes (Figure 3). By comparison, over-oxidation was observed with the gas dosing module at the same pressure (Figure S2). The improvement in selectivity with the sparger was attributed to solubilization of the oxidant in the liquid phase. Specifically, segmented flow at a fixed 1:19 gas-liquid ratio resulted in poor gas-liquid mass transfer. Overall, this led to sequential presentation of the gas phase oxidant and then the reactant. This sequential presentation promoted overoxidation due to high concentration of oxygen on the catalyst surface [5,16,21,37]. In contrast with the segmented flow achieved by the X-Cube™ gas dosing module, the sparger solubilized the gas in the liquid phase before entering the packed bed, resulting in simultaneous presentation of the oxidant and reaction thus preventing overoxidation. This simultaneous presentation achieved a higher selectivity to benzaldehyde, even at lower pressures.
Next, the effect of pressure using the sparger was examined. The reaction pressure was increased to 9.7 bar and the conversion and selectivity were examined. At a residence time of four minutes, the conversion was increased from 5.2% to 11.7% with air (Figure 4a); however, the selectivity decreased to 84.8% (Figure 4b). The resulting STY was calculated to be 40,000 g∙L−1∙h−1. Similar results were observed using oxygen as well: conversion increased from 15.0% to 17.5% and selectivity decreased to 90% with a calculated STY of 54,000 g∙L−1∙h−1 (Figure 4). These results suggested that at higher pressure, the resulting higher solubilized oxygen content enhanced reaction kinetics which improved conversion, but also promoted overoxidation which reduced selectivity. Therefore, operating pressure with the sparger was an important consideration to achieving selective oxidation.
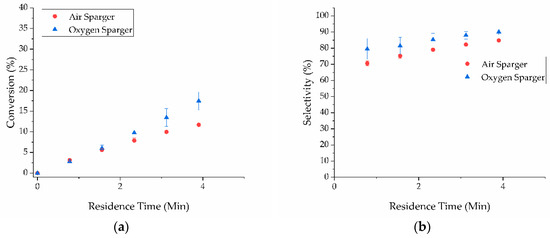
Figure 4.
(a) Conversion of benzyl alcohol; (b) selectivity to benzaldehyde for the sparger modified X-Cube™ and external pump system for both air (red circles) and pure oxygen (blue triangles) at 9.7 bar.
The selectivity with the sparger using air at low (2.8 bar) pressure was approximately 10% better than the X-Cube™ at high (9.7 bar) pressures using oxygen as the oxidant (Table 1). Figure S3 contains the data for the X-Cube™ at high (9.7 bar) pressure. At low (2.8 bar) pressure, the productivity of the sparger with air increased 4-fold over the X-Cube™ with oxygen. Comparing the X-Cube™ at high (9.7 bar) pressure with the sparger at low (2.8 bar) pressure, there was a trade-off between conversion and selectivity indicated by the ~17% decrease in throughput and STY (Table 1). Importantly, using the sparger at lower pressure was beneficial because (1) lowering the operating pressure mitigated safety hazards and (2) improving the selectivity to 100% which reduced the complexity of separation. Overall, this example of a tube-in-tube membrane sparger integrated in-line with the X-Cube™ offered a simple alternative to the gas dosing module, with segmented flow, resulting in a significant increase in STY as well as improved selectivity at low (2.8 bar) pressure.

Table 1.
Conversion, selectivity, throughput, and space time yield (STY) of the sparged process with air and non-sparged process with pure oxygen.
3. Materials and Methods
Benzyl alcohol (99% pure) and triethylene glycol dimethyl ether (99% pure) were received from Acros Organics. Toluene (99.9% pure) and palladium on activated carbon (10 wt% loading) were received from Sigma-Aldrich (St. Louis, MO, USA) Oxygen (Ox R, 99.999% pure) and air (Ultra Zero, 21% pure) were supplied by Praxair (Danbury, CT, USA). For lower pressure reactions, house air was used. All chemicals were used as received from commercial sources without any purification.
A commercially-available reactor, the X-Cube™ (ThalesNano, Budapest, Hungary), was employed to perform the reactions. An external reciprocating syringe pump (Chemtrix, Echt, The Netherlands) was added in line as the primary pump in order to maintain consistent liquid flow. A catalyst cartridge, packed with 100 mg of catalyst, was installed into the X-Cube™ mantle. Typically, the pump inlet and the reactor outlet lines were placed in a 40 mL solution of 50 vol.% benzyl alcohol (4.8 M) and 10 vol.% triglyme (0.55 M, the internal standard) in toluene. Oxygen or air was introduced to the reaction solution before entering the packed bed. The liquid flow rate into the system was 1 mL/min and the reactor was set to 100 °C. The liquid back pressure was maintained by the X-Cube™ BPR valve. The solution was then passed 10 times; each pass was characterized by gas chromatography (FID detector, HP-5MS column, 6890N, Agilent, Santa Clara, CA, USA) and the identity of the constituents in solution were confirmed with GC-mass spectroscopy (MS Detector 5973, HP-5MS column, 6890N, Agilent, Santa Clara, CA, USA).
Gas introduction was controlled using the gas dosing module in the X-Cube™ or a tube-in-tube membrane sparger depending on the experiment. The gas dosing module was set to maintain a 1:19 gas bubble to liquid ratio within the reactor. The membrane sparger was developed to increase the oxygen available for reaction and integrated with the existing system (Figure 2), replacing the gas dosing module. The sparger was configured with gas in the inner tube (Teflon AF-2400, Biogeneral, San Diego, CA, USA) flowing counter-currently to the liquid stream in the outer shell (PTFE, Restek, Bellefonte, PA, USA). The inner tubing had an OD and ID of 1.0 and 0.8 mm and the outer tubing had an OD and ID of 0.125 and 0.063 inches, respectively. The tubing was joined by two 1/8-inch tee joints (Swagelok, Solon, OH, USA) and the inner tubing was fitted into special 1/16” tubing with a bore of 0.040” (VICI, Houston, TX, USA). The gas flow rate, measured at 1227 mL/min, was controlled by a pressure differential between the cylinder regulator (Praxair, Danbury, CT, USA), and a back pressure regulator (Swagelok, Solon, OH, USA) at the outlet of the inner tube. A pressure relief valve (Swagelok, Solon, OH, USA) was added to prevent over pressurization of the sparger. The liquid from the sparger was fed to the X-Cube™ and the oxidation reaction and characterization was carried out as described above.
The system was characterized by benzyl alcohol conversion and selectivity. Conversion (Xi) was based on the consumption of benzyl alcohol and was calculated according to Equation (1):
where Nalcohol,in and Nalcohol,out were the mols of benzyl alcohol at the inlet and outlet, respectively. The selectivity (S) of the desired product, benzaldehyde, relative to the undesired side products, typically benzyl benzoate, was calculated according to Equation (2):
where Naldehyde was the number of mols of benzaldehyde produced and Nproducts was the total moles of all products. The amount of product produced, throughput (TH, g/h), was calculated based on Equation (3):
where MWaldehyde represented the molecular weight of benzaldehyde and trxn was the overall reaction time in hours. Space-time yield (STY, g/(L*h)) was calculated by Equation (4):
where Vr is the packed bed reactor volume (0.78 mL, CatCart 4 mm × 62 mm).
4. Conclusions
We have investigated a simple approach to improve multiphase catalytic reactions performed in a flow chemistry unit. Specifically, we integrated a tube-in-tube membrane contactor (sparger) to the X-Cube™ reactor and examined oxidation of benzyl alcohol using a heterogeneous palladium catalyst. The sparger increased the conversion 9-fold using oxygen and 5-fold using air as the oxidant compared to the gas-dosing module of the X-Cube™ at low (2.8 bar) pressure operation. Furthermore, the sparger increased the reaction selectivity of benzaldehyde up to 100% compared to ~60–80% without the sparger. The sparger facilitated these improvements by enabling the simultaneous addition of the dissolved gas oxidant and the reagents to the catalyst in the same phase through facilitated gas solubilization. The sparger also allowed for safer operation of these gas-liquid processes since the sparged process was performed at lower pressure and achieved similar results, i.e. throughput within 20%, compared to the segmented flow process at higher pressures. Thus, sparger implementation offered opportunities to improve process safety while also improving reactor productivity as the space time yield increased by 4-fold using air with the sparger compared to oxygen with the X-Cube™ at 2.8 bar operation. Overall, we anticipated this approach can be broadly applied to other multiphase catalytic reactions.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/9/1/95/s1, Figure S1: The schematic diagram of the X-Cube™ reactor’s components (provided by ThalesNano), Figure S2: (a) conversion of benzyl alcohol; (b) selectivity to benzaldehyde for the X-Cube™ and external pump system for both air (red circles) and pure oxygen (blue triangles) at 2.8 bar, Figure S3: (a) Conversion of benzyl alcohol; (b) selectivity to benzaldehyde for the X-Cube™ and external pump system for both air (red circles) and pure oxygen (blue triangles) at 9.7 bar.
Author Contributions
Conceptualization, M.B., S.E.G.III, C.T. and B.F.G.; Investigation, M.B. and A.L., Validation, M.B., S.E.G.III, A.L., C.T. and B.F.G.; Formal Analysis, M.B. and C.T.; Resources, C.T. and B.F.G.; Writing—Original Draft Preparation, M.B. and S.G.; Writing—Review & Editing, C.T. and B.F.G.; Visualization, M.B. and C.T.; Supervision, C.T. and B.F.G.; Project Administration, C.T. and B.F.G.; Funding Acquisition, C.T. and B.F.G.
Funding
This research was partially supported by VCU College of Engineering and the Center for Rational Catalyst Synthesis an Industry/University Cooperative Research Center funded in part by the National Science Foundation (Industry/University Collaborative Research Center IIP 1464630 grant ID 17-3171).
Acknowledgments
The financial support of the VCU College of Engineering and National Science Foundation, USA (IIP 1464630 grant ID 17-3171) is gratefully acknowledged. The authors thank Evan Pfab for his assistance with experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pieber, B.; Kappe, C.O. Aerobic Oxidations in Continuous Flow. Top. Organomet. Chem. 2016, 57, 97–136. [Google Scholar] [CrossRef]
- Gavriilidis, A.; Constantinou, A.; Hellgardt, K.; Hii, K.K.; Hutchings, G.J.; Brett, G.L.; Kuhn, S.; Marsden, S.P. Aerobic oxidations in flow: Opportunities for the fine chemicals and pharmaceuticals industries. React. Chem. Eng. 2016, 1, 595–612. [Google Scholar] [CrossRef]
- Mills, P.L.; Quiram, D.J.; Ryley, J.F. Microreactor technology and process miniaturization for catalytic reactions-A perspective on recent developments and emerging technologies. Chem. Eng. Sci. 2007, 62, 6992–7010. [Google Scholar] [CrossRef]
- Losey, M.W.; Schmnidt, M.A.; Jensen, K.F. Microfabricated multiphase packed-bed reactors: Characterization of mass transfer and reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Hessel, V.; Angeli, P.; Gavriilidis, A.; Löwe, H. Gas-liquid and gas-liquid-solid microstructured reactors: Contacting principles and applications. Ind. Eng. Chem. Res. 2005, 44, 9750–9769. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef] [PubMed]
- Geyer, K.; Codée, J.D.C.; Seeberger, P.H. Microreactors as tools for synthetic Chemists—The chemists’ round-bottomed flask of the 21st century? Chem. Eur. J. 2006, 12, 8434–8442. [Google Scholar] [CrossRef]
- Gemoets, H.P.L.; Su, Y.; Shang, M.; Hessel, V.; Luque, R.; Noël, T. Liquid phase oxidation chemistry in continuous-flow microreactors. Chem. Soc. Rev. 2016, 45, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Compagnoni, M. Chemical reaction engineering, process design and scale-up issues at the frontier of synthesis: Flow chemistry. Chem. Eng. J. 2016, 296, 56–70. [Google Scholar] [CrossRef]
- Jensen, K.F.; Reizman, B.J.; Newman, S.G. Tools for chemical synthesis in microsystems. Lab Chip 2014, 14, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Caron, S.; Dugger, R.W.; Ruggeri, S.G.; Ragan, J.A.; Brown Ripin, D.H. Large-scale oxidations in the pharmaceutical industry. Chem. Rev. 2006, 106, 2943–2989. [Google Scholar] [CrossRef] [PubMed]
- Zotova, N.; Roberts, F.J.; Kelsall, G.H.; Jessiman, A.S.; Hellgardt, K.; Hii, K.K. (Mimi) Catalysis in flow: Au-catalysed alkylation of amines by alcohols. Green Chem. 2012, 14, 226–232. [Google Scholar] [CrossRef]
- Dijksman, A.; Marino-González, A.; Mairata i Payeras, A.; Arends, I.W.C.E.; Sheldon, R.A. Efficient and Selective Aerobic Oxidation of Alcohols into Aldehydes and Ketones Using Ruthenium/TEMPO as the Catalytic System. J. Am. Chem. Soc. 2001, 123, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, E.; Hofmann, J.P.; Parker, S.F.; Sankar, M.; Lari, G.M.; Kondrat, S.A.; Knight, D.W.; Bethell, D.; Weckhuysen, B.M.; Hutchings, G.J. In situ spectroscopic investigation of oxidative dehydrogenation and disproportionation of benzyl alcohol. Phys. Chem. Chem. Phys. 2013, 15, 12147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Y.; Guo, Z.; Wang, X.; Yang, Y. Promoted aerobic oxidation of benzyl alcohol on CNT supported platinum by iron oxide. Chem. Commun. 2011, 47, 7473. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Medlin, J. Benzyl alcohol oxidation on Pd (111): Aromatic binding effects on alcohol reactivity. Langmuir 2014, 30, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127–141. [Google Scholar] [CrossRef]
- Albonetti, S.; Mazzoni, R.; Cavani, F. Homogeneous, Heterogeneous and Nanocatalysis; The Royal society of Chemistry: London, UK, 2015; ISBN 9781782621652. [Google Scholar]
- Zotova, N.; Hellgardt, K.; Kelsall, G.H.; Jessiman, A.S.; Hii, K.K. (Mimi) Catalysis in flow: The practical and selective aerobic oxidation of alcohols to aldehydes and ketones. Green Chem. 2010, 12, 2157. [Google Scholar] [CrossRef]
- Miedziak, P.; Sankar, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Knight, D.W.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Oxidation of benzyl alcohol using supported gold-palladium nanoparticles. Catal. Today 2011, 164, 315–319. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Galvanin, F.; Morad, M.; Cao, E.; Cattaneo, S.; Sankar, M.; Dua, V.; Hutchings, G.; Gavriilidis, A. Hydrodynamic effects on three phase micro-packed bed reactor performance—Gold-palladium catalysed benzyl alcohol oxidation. Chem. Eng. Sci. 2016, 149, 129–142. [Google Scholar] [CrossRef]
- Wu, G.; Constantinou, A.; Cao, E.; Kuhn, S.; Morad, M.; Sankar, M.; Bethell, D.; Hutchings, G.J.; Gavriilidis, A. Continuous heterogeneously catalyzed oxidation of benzyl alcohol using a tube-in-tube membrane microreactor. Ind. Eng. Chem. Res. 2015, 54, 4183–4189. [Google Scholar] [CrossRef]
- O’Brien, M.; Taylor, N.; Polyzos, A.; Baxendale, I.R.; Ley, S.V. Hydrogenation in flow: Homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas–liquid contact at elevated pressure. Chem. Sci. 2011, 2, 1250–1257. [Google Scholar] [CrossRef]
- Yang, L.; Jensen, K.F. Mass transport and reactions in the tube-in-tube reactor. Org. Process Res. Dev. 2013, 17, 927–933. [Google Scholar] [CrossRef]
- Wu, G.; Cao, E.; Kuhn, S.; Gavriilidis, A. A Novel Approach for Measuring Gas Solubility in Liquids Using a Tube-in-Tube Membrane Contactor. Chem. Eng. Technol. 2017, 1–6. [Google Scholar] [CrossRef]
- Petersen, T.P.; Polyzos, A.; O’Brien, M.; Ulven, T.; Baxendale, I.R.; Ley, S.V. The oxygen-mediated synthesis of 1,3-butadiynes in continuous flow: Using teflon AF-2400 to effect gas/liquid contact. ChemSusChem 2012, 5, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.; Wu, G.; Corredera, A.; Ellis, P.; Bethell, D.; Hutchings, G.J.; Kuhn, S.; Gavriilidis, A. Continuous Heterogeneously Catalyzed Oxidation of Benzyl Alcohol in a Ceramic Membrane Packed-Bed Reactor. Org. Process Res. Dev. 2015, 19, 1973–1979. [Google Scholar] [CrossRef]
- Skowerski, K.; Czarnocki, S.J.; Knapkiewicz, P. Tube-in-tube reactor as a useful tool for homo- and heterogeneous olefin metathesis under continuous flow mode. ChemSusChem 2014, 7, 536–542. [Google Scholar] [CrossRef]
- Brzozowski, M.; O’Brien, M.; Ley, S.V.; Polyzos, A. Flow chemistry: Intelligent processing of gas-liquid transformations using a tube-in-tube reactor. Acc. Chem. Res. 2015, 48, 349–362. [Google Scholar] [CrossRef]
- Feng, J.; Ma, C.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Li, D.; Du, Y.; Morgan, D.J.; Hutchings, G.J. Au–Pd nanoalloys supported on Mg–Al mixed metal oxides as a multifunctional catalyst for solvent-free oxidation of benzyl alcohol. Dalton Trans. 2013, 42, 14498–14508. [Google Scholar] [CrossRef]
- Greene, J.F.; Preger, Y.; Stahl, S.S.; Root, T.W. PTFE-Membrane Flow Reactor for Aerobic Oxidation Reactions and Its Application to Alcohol Oxidation. Org. Process Res. Dev. 2015, 19, 858–864. [Google Scholar] [CrossRef]
- Koos, P.; Gross, U.; Polyzos, A.; O’Brien, M.; Baxendale, I.; Ley, S.V. Teflon AF-2400 mediated gas–liquid contact in continuous flow methoxycarbonylations and in-line FTIR measurement of CO concentration. Org. Biomol. Chem. 2011, 9, 6903–6908. [Google Scholar] [CrossRef] [PubMed]
- Schweidtmann, A.M.; Clayton, A.D.; Holmes, N.; Bradford, E.; Bourne, R.A.; Lapkin, A.A. Machine learning meets continuous flow chemistry: Automated optimization towards the Pareto front of multiple objectives. Chem. Eng. J. 2018, 352, 277–282. [Google Scholar] [CrossRef]
- Jumbam, D.N.; Skilton, R.A.; Parrott, A.J.; Bourne, R.A.; Poliakoff, M. The Effect of Self-Optimisation Targets on the Meethylation of Alcohols Using Dimethyl Carbonate in Supercritical CO2. J. Flow Chem. 2012, 2, 24–27. [Google Scholar] [CrossRef]
- Erdmann, V.; Mackfeld, U.; Rother, D.; Jakoblinnert, A. Enantioselective, continuous (R)- and (S)-2-butanol synthesis: Ahieving high space-time yields with recombinant E. coli cells in a micro-aqueous, solvent-free reaction system. J. Biotechnol. 2014, 191, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Schotten, C.; Howard, J.L.; Jenkins, R.L.; Codina, A.; Browne, D.L. A continuous flow-batch hybrid reactor for commodity chemical synthesis enabled by inline NMR and temperature monitoring. Tetrahedon 2018, 74, 5503–5509. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, J.C.F.; Friend, C.M.; Madix, R.J. Origin of the selectivity in the gold-mediated oxidation of benzyl alcohol. Surf. Sci. 2012, 606, 1129–1134. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).