Comparative Study between Pristine Ag and Ag Foam for Electrochemical Synthesis of Syngas with Carbon Dioxide and Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrode Characterization
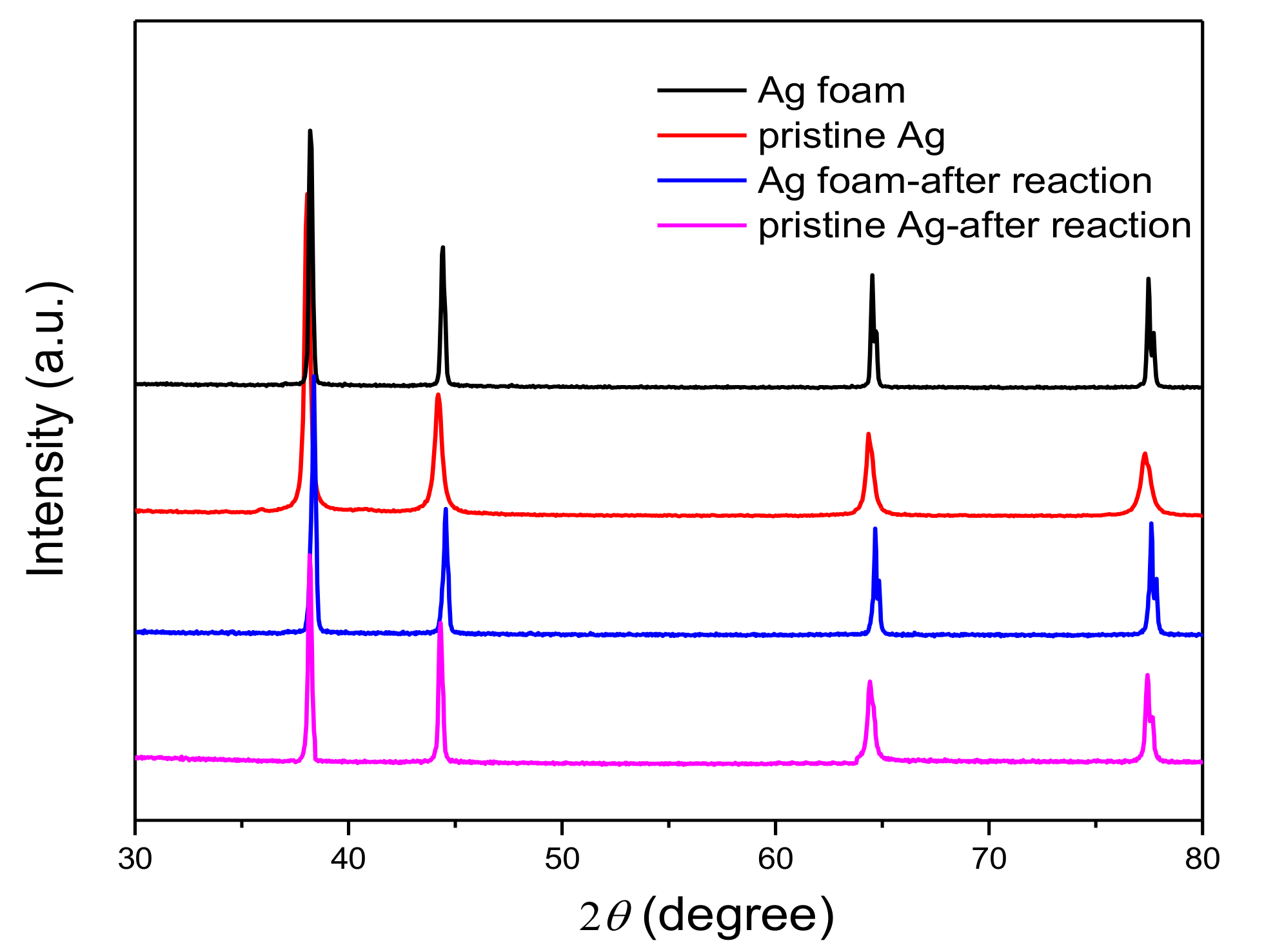
2.1.1. XRD
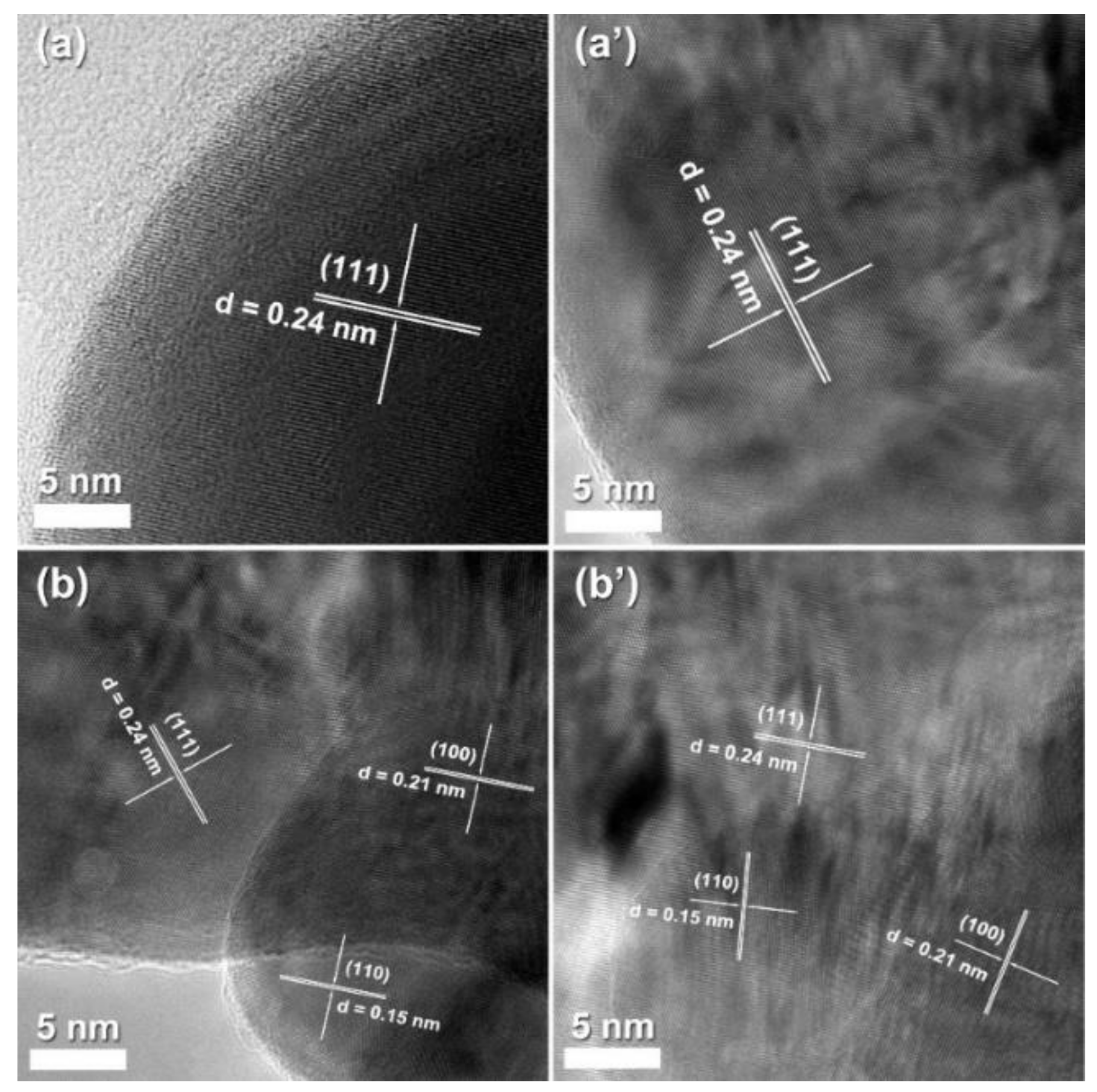
2.1.2. FESEM and High-Resolution TEM (HR-TEM)
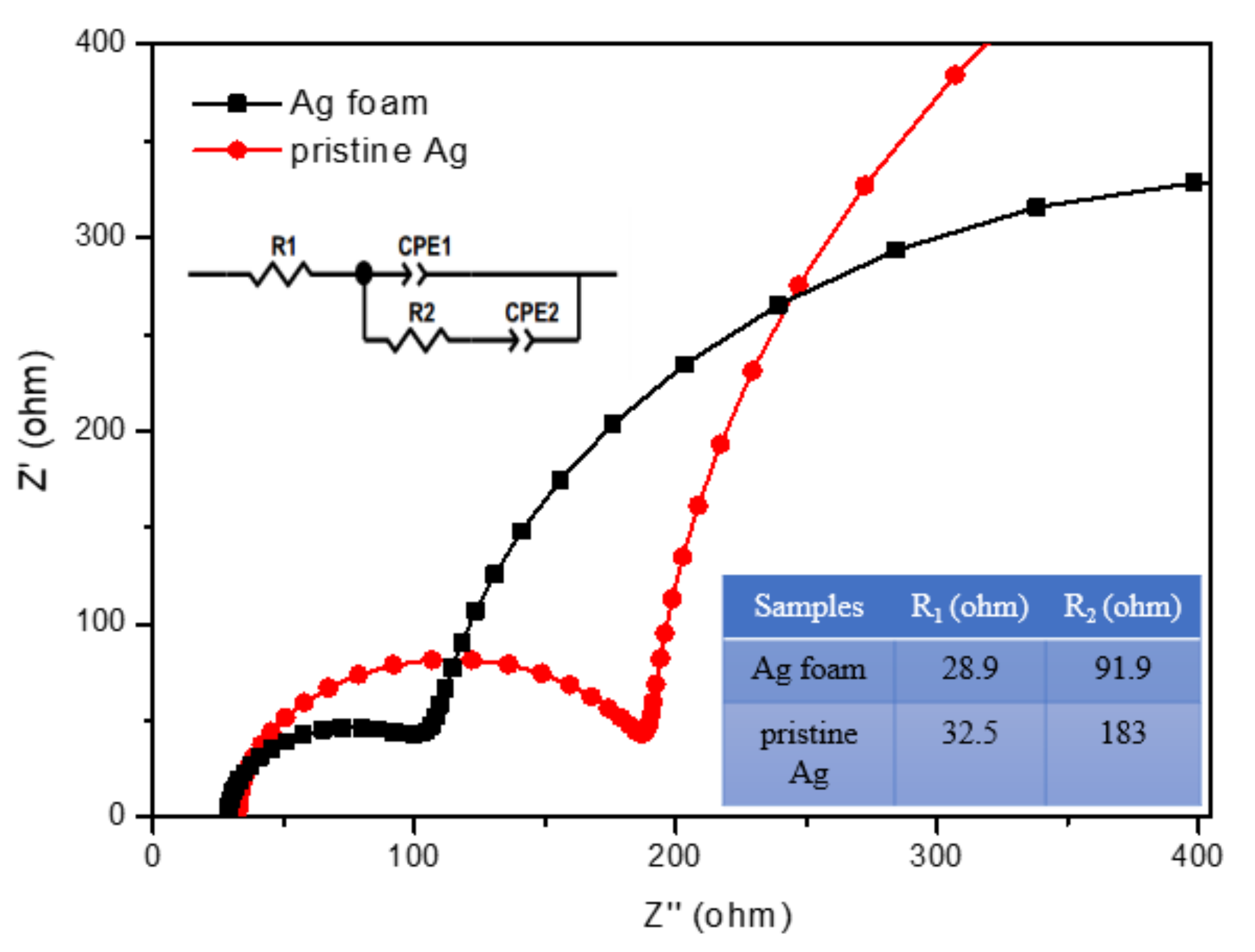
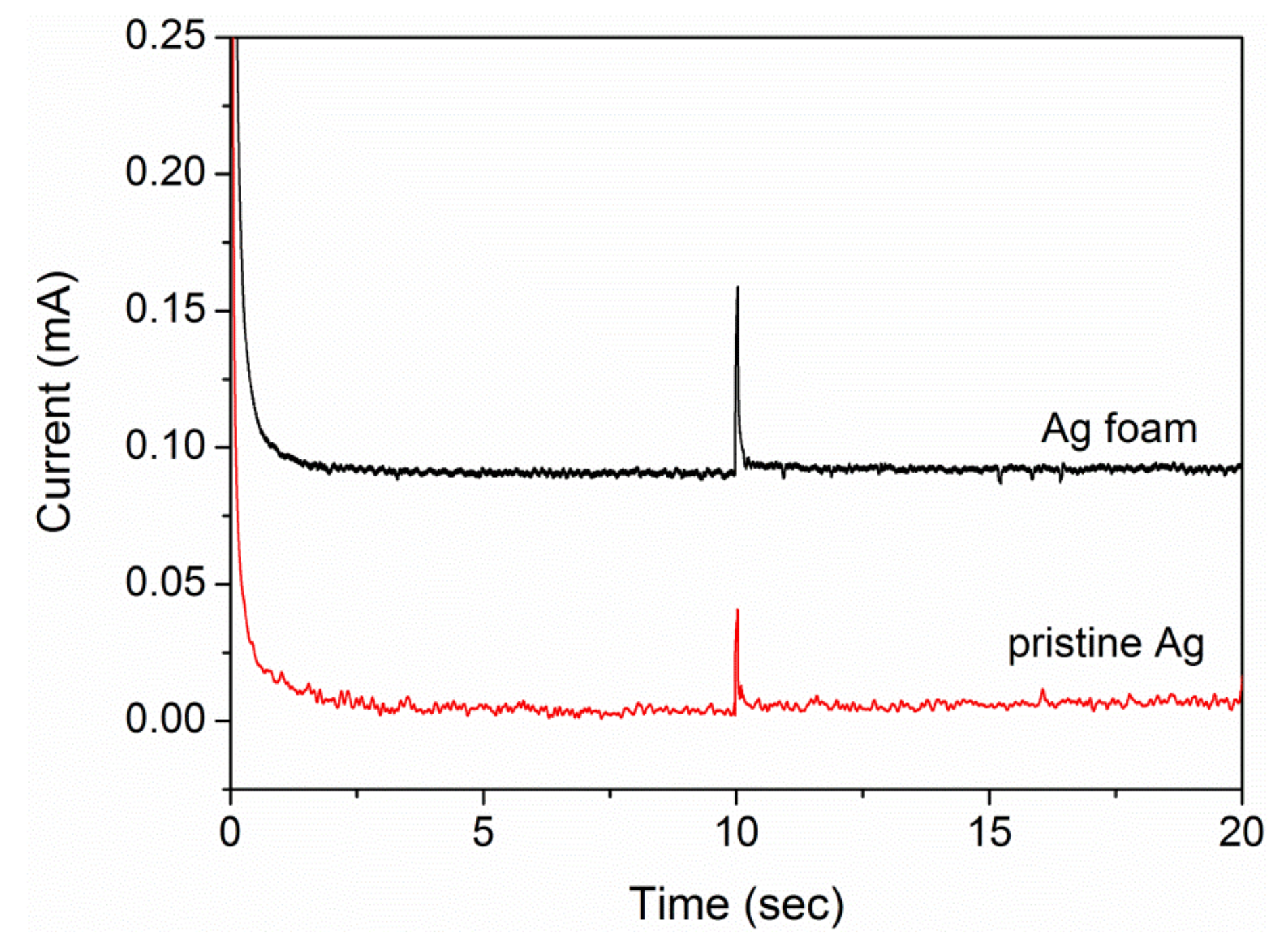
2.1.3. Roughness Factor and Uncompensated Solution Resistance
2.2. Electrode Selectivity
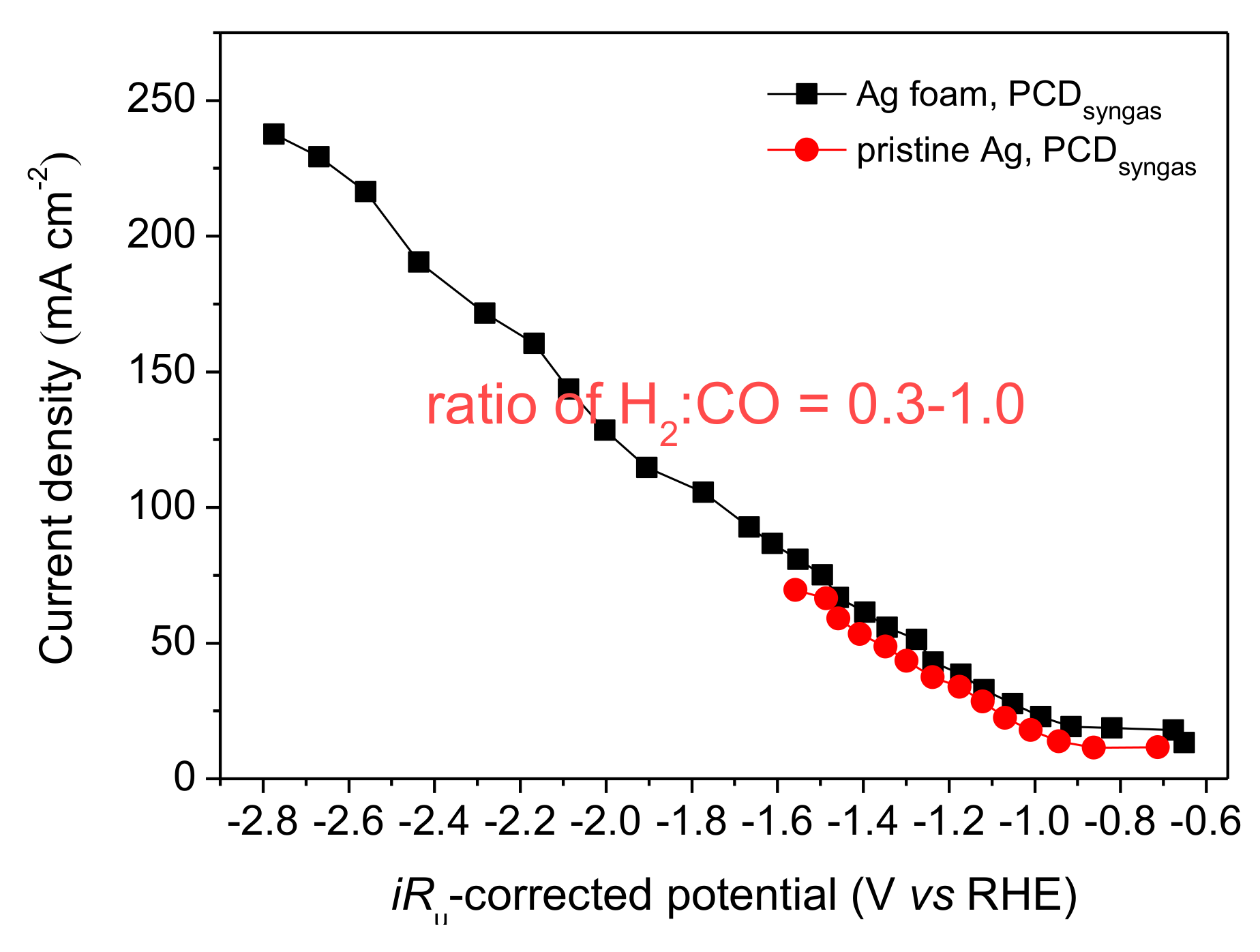
2.3. Electrode Activity
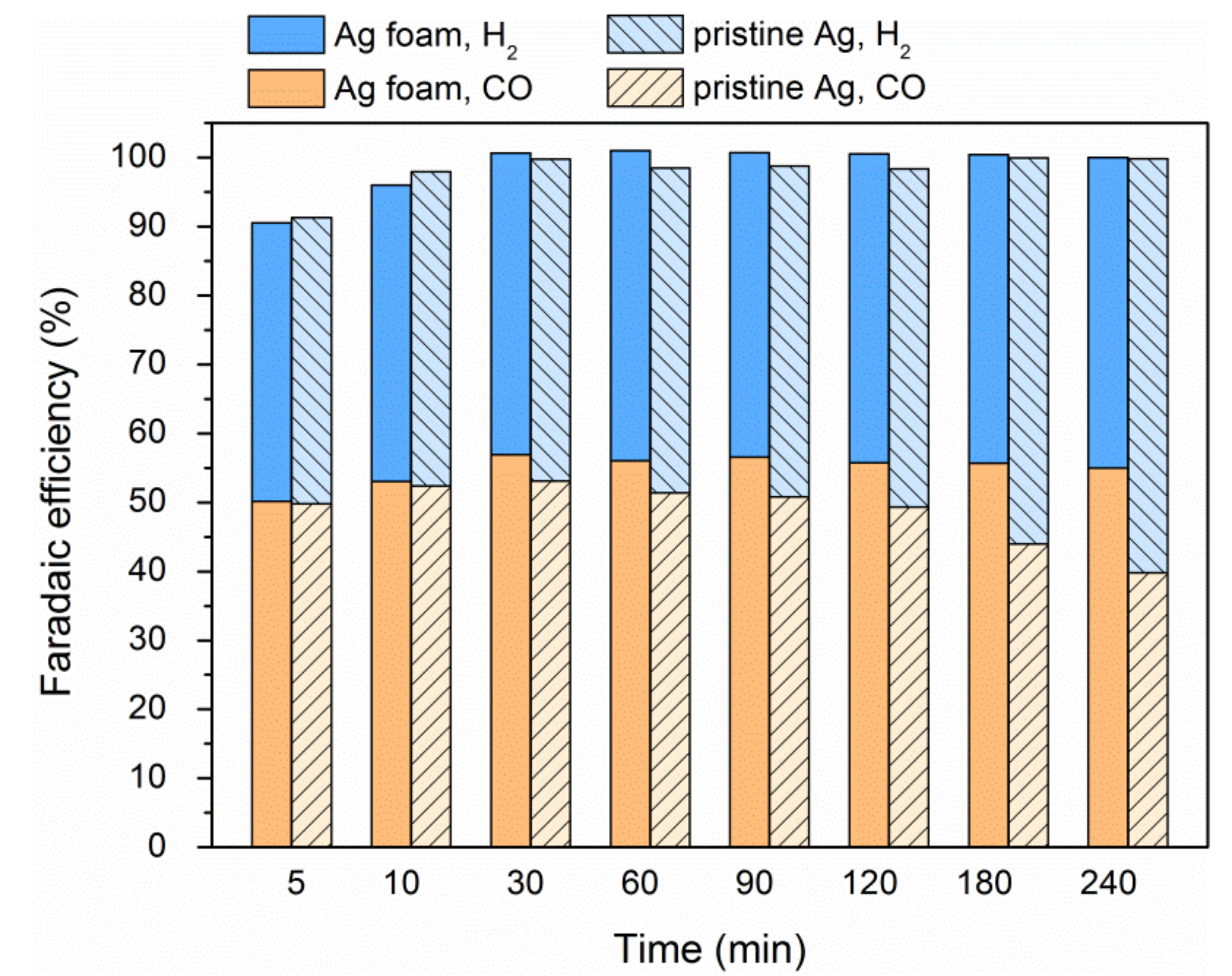
2.4. Electrode Stability
3. Experimental
3.1. Materials and Reagents
3.2. Electrode Characterization
3.3. Electrochemical Reduction of CO2
3.4. Analytical Procedures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.P.; Zhang, Y.T.; Chen, B.L.; Yu, S.X.; Zhou, H.W.; Qu, K.G.; Kong, Y.X.; Huang, X.Q.; Zhang, X.X.; Lu, J.X. Electrocarboxylation of dichlorobenzenes on a silver electrode in DMF. Catalysts 2017, 7, 274. [Google Scholar] [CrossRef]
- Zhang, S.H.; Shen, Y.; Shao, P.J.; Chen, J.M.; Wang, L.D. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas. Environ. Sci. Technol. 2018, 52, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ye, J.H.; Zhu, N.; Hong, H.L.; Ma, L.; Yang, J.R.; Zhang, J.B. Amine phase-transfer chemistry: A green and sustainable approach to the nonbiological mineralization of CO2. ACS Sustain. Chem. Eng. 2018, 6, 7105–7108. [Google Scholar] [CrossRef]
- Zhang, S.H.; Lu, Y.Q. Surfactants facilitating carbonic anhydrase enzyme-mediated CO2 absorption into a carbonate solution. Environ. Sci. Technol. 2017, 51, 8537–8543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Du, M.E.; Shao, P.J.; Wang, L.D.; Ye, J.X.; Chen, J.; Chen, J.M. Carbonic anhydrase enzyme-MOFs composite with a superior catalytic performance to promote CO2 absorption into tertiary amine solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef]
- Qiu, J.P.; Tang, J.T.; Shen, J.; Wu, C.W.; Qian, M.Q.; He, Z.Q.; Chen, J.M.; Song, S. Preparation of a silver electrode with a three-dimensional surface and its performance in the electrochemical reduction of carbon dioxide. Electrochim. Acta 2016, 203, 99–108. [Google Scholar]
- Wang, J.L.; Zhou, S.Y.; Bei, K.; Zhang, D.; Chou, I.M.; Chen, Z.H.; Lin, C.M.; Pan, Z.Y. Using a fused silica capillary cell and in situ raman spectroscopy to develop a setup for measurement of the volume expansion of carbon dioxide + n-hexane. Energy Fuel 2017, 31, 6314–6319. [Google Scholar] [CrossRef]
- Ma, L.; Fan, S.; Zhen, D.X.; Wu, X.M.; Liu, S.S.; Lin, J.J.; Huang, S.Q.; Chen, W.; He, G.H. Electrochemical reduction of CO2 in proton exchange membrane reactor: The function of buffer layer. Ind. Eng. Chem. Res. 2017, 56, 10242–10250. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y.; Jin, F.M. Effect of CO2 bubbling into aqueous solutions used for electrochemical reduction of CO2 for energy conversion and storage. J. Phys. Chem. C 2014, 119, 55–61. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, J.J.; Yang, M.P.; Meng, W.J.; Wang, H.; Lu, J.X. CuO nanoparticles supported on TiO2 with high efficiency for CO2 electrochemical reduction to ethanol. Catalysts 2018, 8, 171. [Google Scholar] [CrossRef]
- Foit, S.R.; Vinke, I.C.; de Haart, L.G.J.; Eichel, R.A. Power-to-syngas: An enabling technology for the transition of the energy system? Angew. Chem.-Int. Ed. 2017, 56, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Li, Y.N.; Zhang, X.; Liu, Y.Y.; Qiao, J.L.; Zhang, J.J.; Wilkinson, D.P. Novel hierarchical SnO2 microsphere catalyst coated on gas diffusion electrode for enhancing energy efficiency of CO2 reduction to formate fuel. Appl. Energy 2016, 175, 536–544. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, L.; Guo, R.R.; Zeng, S.; Wang, H.; Lu, J.X. Electroreduction of CO2 into ethanol over an active catalyst: Copper supported on titania. Catalysts 2017, 7, 220. [Google Scholar] [CrossRef]
- Kang, P.; Chen, Z.F.; Nayak, A.; Zhang, S.; Meyer, T.J. Single catalyst electrocatalytic reduction of CO2 in water to H2 + CO syngas mixtures with water oxidation to O2. Energy Environ. Sci. 2014, 7, 4007–4012. [Google Scholar] [CrossRef]
- Costentin, C.; Drouet, S.; Robert, M.; Saveant, J.M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 2012, 338, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.M.; Zhu, Y.P.; Chen, P.; Ma, T.Y. Recent advances in transition-metal-mediated electrocatalytic CO2 reduction: From homogeneous to heterogeneous systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef]
- Ross, M.B.; Dinh, C.T.; Li, Y.; Kim, D.; De Luna, P.; Sargent, E.H.; Yang, P. Tunable Cu enrichment enables designer syngas electrosynthesis from CO2. J. Am. Chem. Soc. 2017, 139, 9359–9363. [Google Scholar] [CrossRef]
- Fu, Q.; Mabilat, C.; Zahid, M.; Brisse, A.; Gautier, L. Syngas production via high-temperature steam/CO2 co-electrolysis: An economic assessment. Energy Environ. Sci. 2010, 3, 1382. [Google Scholar] [CrossRef]
- Sheng, W.C.; Kattel, S.; Yao, S.Y.; Yan, B.H.; Liang, Z.X.; Hawxhurst, C.J.; Wu, Q.Y.; Chen, J.G.G. Electrochemical reduction of CO2 to synthesis gas with controlled CO/H2 ratios. Energy Environ. Sci. 2017, 10, 1180–1185. [Google Scholar] [CrossRef]
- Back, S.; Yeom, M.S.; Jung, Y. Active sites of Au and Ag nanoparticle catalysts for CO2 electroreduction to CO. ACS Catal. 2015, 5, 5089–5096. [Google Scholar] [CrossRef]
- Hernández, S.; Amin Farkhondehfal, M.; Sastre, F.; Makkee, M.; Saracco, G.; Russo, N. Syngas production from electrochemical reduction of CO2: Current status and prospective implementation. Green Chem. 2017, 19, 2326–2346. [Google Scholar] [CrossRef]
- Oloman, C.; Li, H. Electrochemical processing of carbon dioxide. ChemSusChem 2008, 1, 385–391. [Google Scholar] [CrossRef]
- Verma, S.; Hamasaki, Y.; Kim, C.; Huang, W.; Lu, S.; Jhong, H.-R.M.; Gewirth, A.A.; Fujigaya, T.; Nakashima, N.; Kenis, P.J.A. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 2017, 3, 193–198. [Google Scholar] [CrossRef]
- Schmid, B.; Reller, C.; Neubauer, S.S.; Fleischer, M.; Dorta, R.; Schmid, G. Reactivity of copper electrodes towards functional groups and small molecules in the context of CO2 electro-reductions. Catalysts 2017, 7, 161. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- He, Z.Q.; Liu, T.; Tang, J.T.; Zhou, C.Y.; Wen, L.S.; Chen, J.M.; Song, S. Highly active, selective and stable electroreduction of carbon dioxide to carbon monoxide on a silver catalyst with truncated hexagonal bipyramidal shape. Electrochim. Acta 2016, 222, 1234–1242. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.L.; Zhang, Z.Y.; Zhang, M.Y.; Mu, J.B.; Guo, Z.C.; Liu, Y.C. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Peterson, A.A.; Nørskov, J.K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef]
- Gao, D.F.; Zhang, Y.; Zhou, Z.W.; Cai, F.; Zhao, X.F.; Huang, W.G.; Li, Y.S.; Zhu, J.F.; Liu, P.; Yang, F.; et al. Enhancing CO2 electroreduction with the metal-oxide interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Electrocatalytic reduction of CO2 to small organic molecule fuels on metal catalysts. Cheminform 2010, 1056, 55–76. [Google Scholar]
- Zhao, S.; Jin, R.X.; Jin, R.C. Opportunities and challenges in CO2 reduction by gold- and silver-based electrocatalysts: From bulk metals to nanoparticles and atomically precise nanoclusters. ACS Energy Lett. 2018, 3, 452–462. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. Controlling the product syngas H2:CO ratio through pulsed-bias electrochemical reduction of CO2 on copper. ACS Catal. 2016, 6, 4739–4745. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hu, Y.; Ma, L.B.; Zhu, G.Y.; Wang, Y.R.; Xue, X.L.; Chen, R.P.; Yang, S.Y.; Jin, Z. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, X.G.; Jiang, K.; Wu, D.Y.; Cai, W.B. Boosting formate production in electrocatalytic CO2 reduction over wide potential window on Pd surfaces. J. Am. Chem. Soc. 2018, 140, 2880–2889. [Google Scholar] [CrossRef]
- Qiu, J.P.; Tong, Y.W.; Zhao, D.M.; He, Z.Q.; Chen, J.M.; Song, S. Electrochemical reduction of CO2 to methanol at TiO2 nanotube electrodes. Acta Phys.-Chim. Sin. 2017, 33, 1411–1420. [Google Scholar]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Rivera, S.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal. 2015, 5, 4293–4299. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Senanayake, S.D.; Zhang, Y.; Xu, W.; Polyansky, D.E. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction. ACS Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, Y.; Al-Enizi, A.M.; Zheng, G. Tuning of CO2 reduction selectivity on metal electrocatalysts. Small 2017, 13, 1701809. [Google Scholar] [CrossRef]
- Bui, L.; Chakrabarti, R.; Bhan, A. Mechanistic origins of unselective oxidation products in the conversion of propylene to acrolein on Bi2Mo3O12. ACS Catal. 2016, 6, 6567–6580. [Google Scholar] [CrossRef]
- Ma, M.; Trzesniewski, B.J.; Xie, J.; Smith, W.A. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts. Angew. Chem.-Int. Ed. 2016, 55, 9748–9752. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeon, H.S.; Eom, T.; Jee, M.S.; Kim, H.; Friend, C.M.; Hwang, Y.J. Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. [Google Scholar] [CrossRef] [PubMed]
- Delacourt, C.; Ridgway, P.L.; Kerr, J.B.; Newman, J. Design of an electrochemical cell making syngas (CO + H2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 2008, 155, B42–B49. [Google Scholar] [CrossRef]
- Yano, J.; Yamasaki, S. Pulse-mode electrochemical reduction of carbon dioxide using copper and copper oxide electrodes for selective ethylene formation. J. Appl. Electrochem. 2008, 38, 1721–1726. [Google Scholar] [CrossRef]





| Facets | ECO (eV) | ECOOH (eV) |
|---|---|---|
| Ag(111) | −0.15 | −1.15 |
| Ag(110) | −0.33 | −1.44 |
| Ag(100) | −0.35 | −1.62 |
| Sample | Roughness Factor (cm2 cm−2) | Potential (V vs. RHE) | PCDCO (mA cm−2) | SACO (10−5 A cm−2) |
|---|---|---|---|---|
| Ag foam | 44.5 | −0.53 | 1.20 | 2.70 |
| −0.60 | 2.97 | 6.68 | ||
| −0.65 | 7.38 | 16.59 | ||
| pristine Ag | 33.4 | −0.56 | 0.34 | 1.02 |
| −0.61 | 1.15 | 3.46 | ||
| −0.68 | 3.11 | 9.32 |
| Morphology of Ag Electrodes | Electrolyte | Selective Production of CO | |||
|---|---|---|---|---|---|
| Applied Potential V (vs. RHE) | Faradaic Efficiency (%) | Current Density (mA cm−2) | Ref. | ||
| Nanoparticles | 0.5 M KHCO3 | −0.75 | 79 | 1 | 43 |
| Nanoporous | 0.5 M KHCO3 | −0.60 | 92 | 18 | 30 |
| Nanocorals | 0.1 M KHCO3 | −0.60 | 95 | 6.62 | 39 |
| Oxide-derived | 0.1 M KHCO3 | −0.80 | 89 | 1.15 | 44 |
| Compact grains | 0.1 M KHCO3 | −1.1 | 88.87 | 5.73 | 7 |
| Truncated hexagonal bipyramidal | 0.1 M KHCO3 | −0.93 | 89.4 | 4.92 | 27 |
| Plate | 0.1 M KHCO3 | −1.12 | 79.0 | 22.93 | In our work |
| Foam | 0.1 M KHCO3 | −1.12 | 82.9 | 27.43 | In our work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhong, N.; Fang, J.; Tang, S.; Ye, X.; He, Z.; Song, S. Comparative Study between Pristine Ag and Ag Foam for Electrochemical Synthesis of Syngas with Carbon Dioxide and Water. Catalysts 2019, 9, 57. https://doi.org/10.3390/catal9010057
Yu Y, Zhong N, Fang J, Tang S, Ye X, He Z, Song S. Comparative Study between Pristine Ag and Ag Foam for Electrochemical Synthesis of Syngas with Carbon Dioxide and Water. Catalysts. 2019; 9(1):57. https://doi.org/10.3390/catal9010057
Chicago/Turabian StyleYu, Yan, Na Zhong, Jinhui Fang, Shasha Tang, Xincheng Ye, Zhiqiao He, and Shuang Song. 2019. "Comparative Study between Pristine Ag and Ag Foam for Electrochemical Synthesis of Syngas with Carbon Dioxide and Water" Catalysts 9, no. 1: 57. https://doi.org/10.3390/catal9010057
APA StyleYu, Y., Zhong, N., Fang, J., Tang, S., Ye, X., He, Z., & Song, S. (2019). Comparative Study between Pristine Ag and Ag Foam for Electrochemical Synthesis of Syngas with Carbon Dioxide and Water. Catalysts, 9(1), 57. https://doi.org/10.3390/catal9010057