Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal
Abstract
:1. Introduction
2. Results
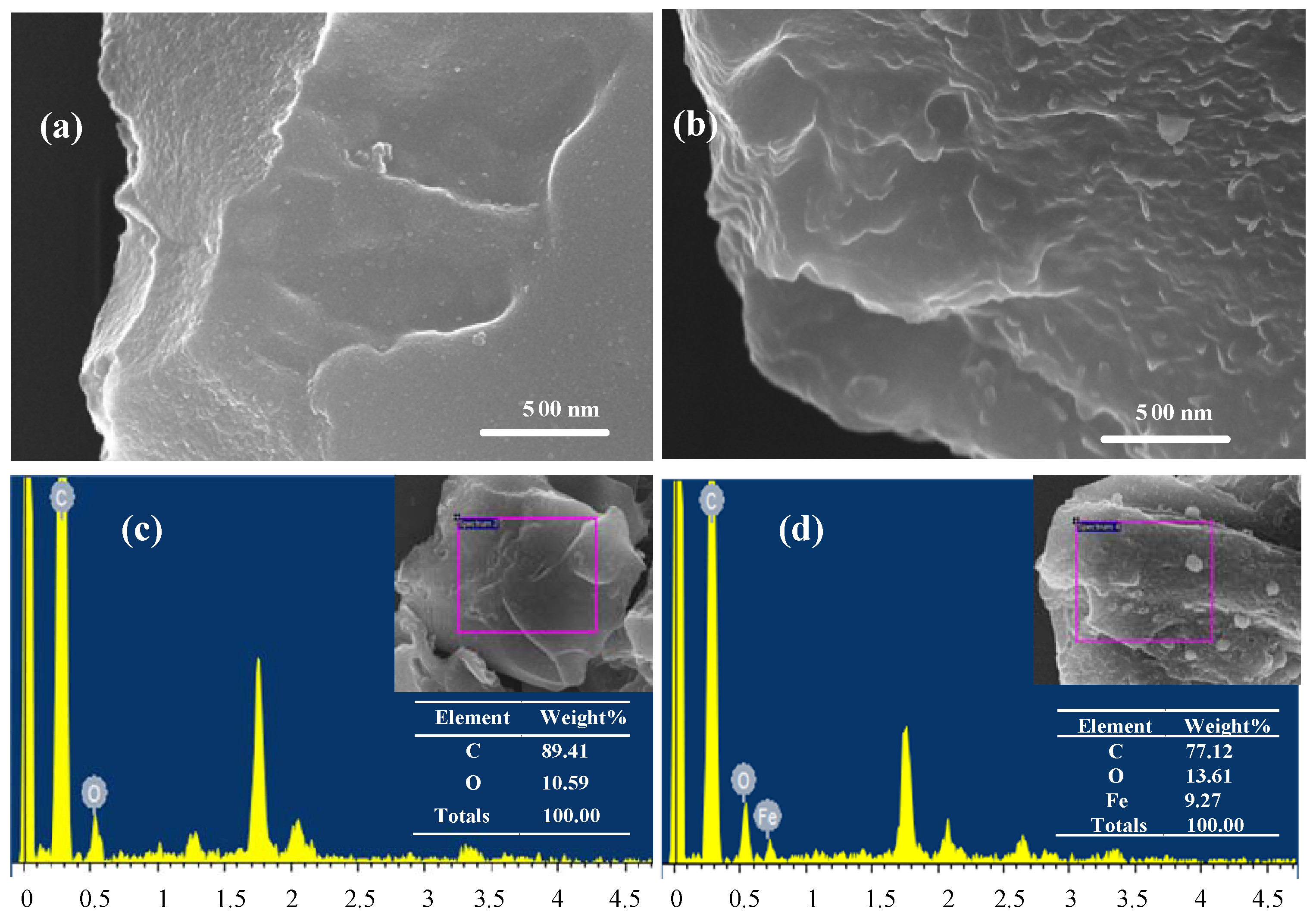
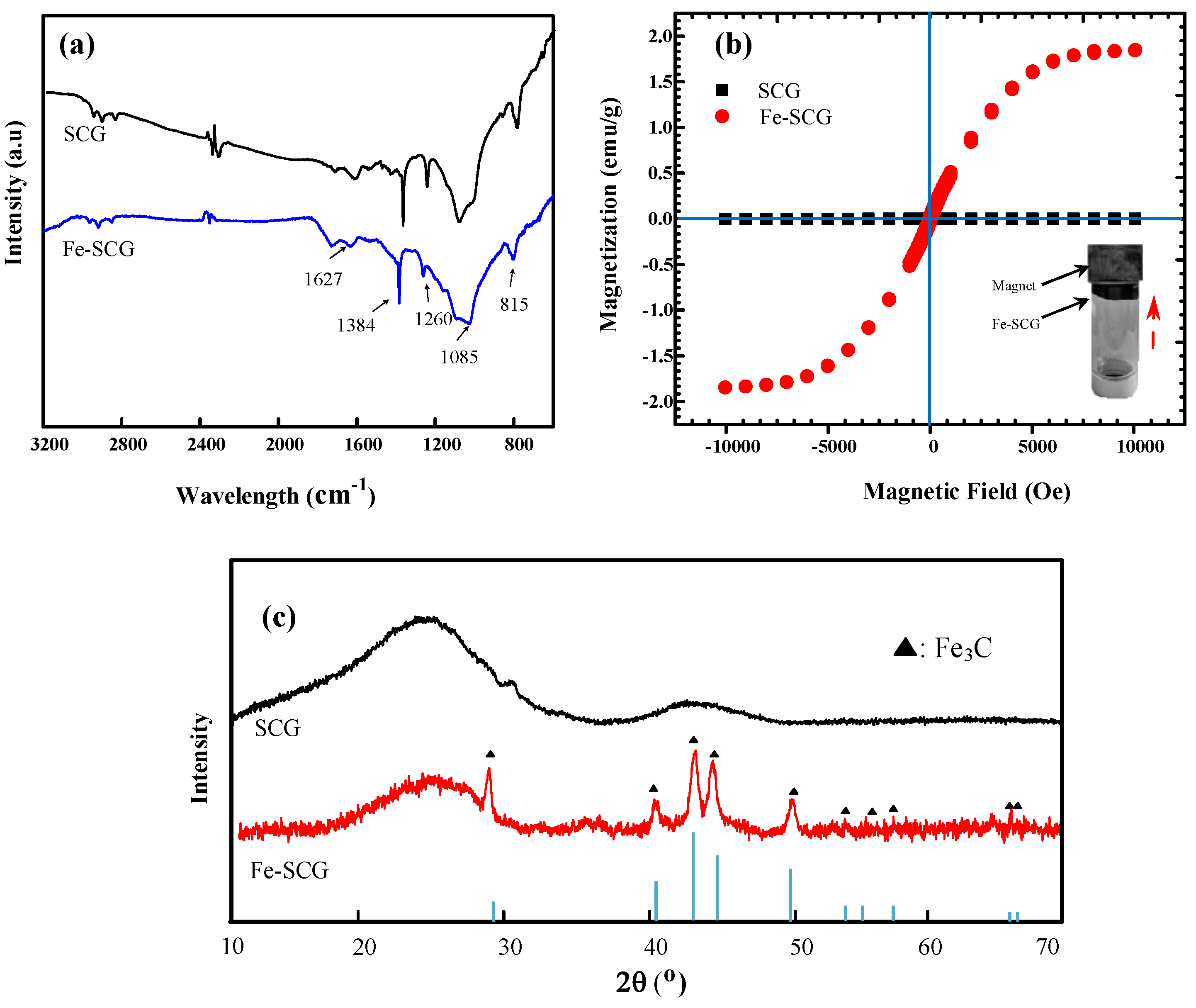
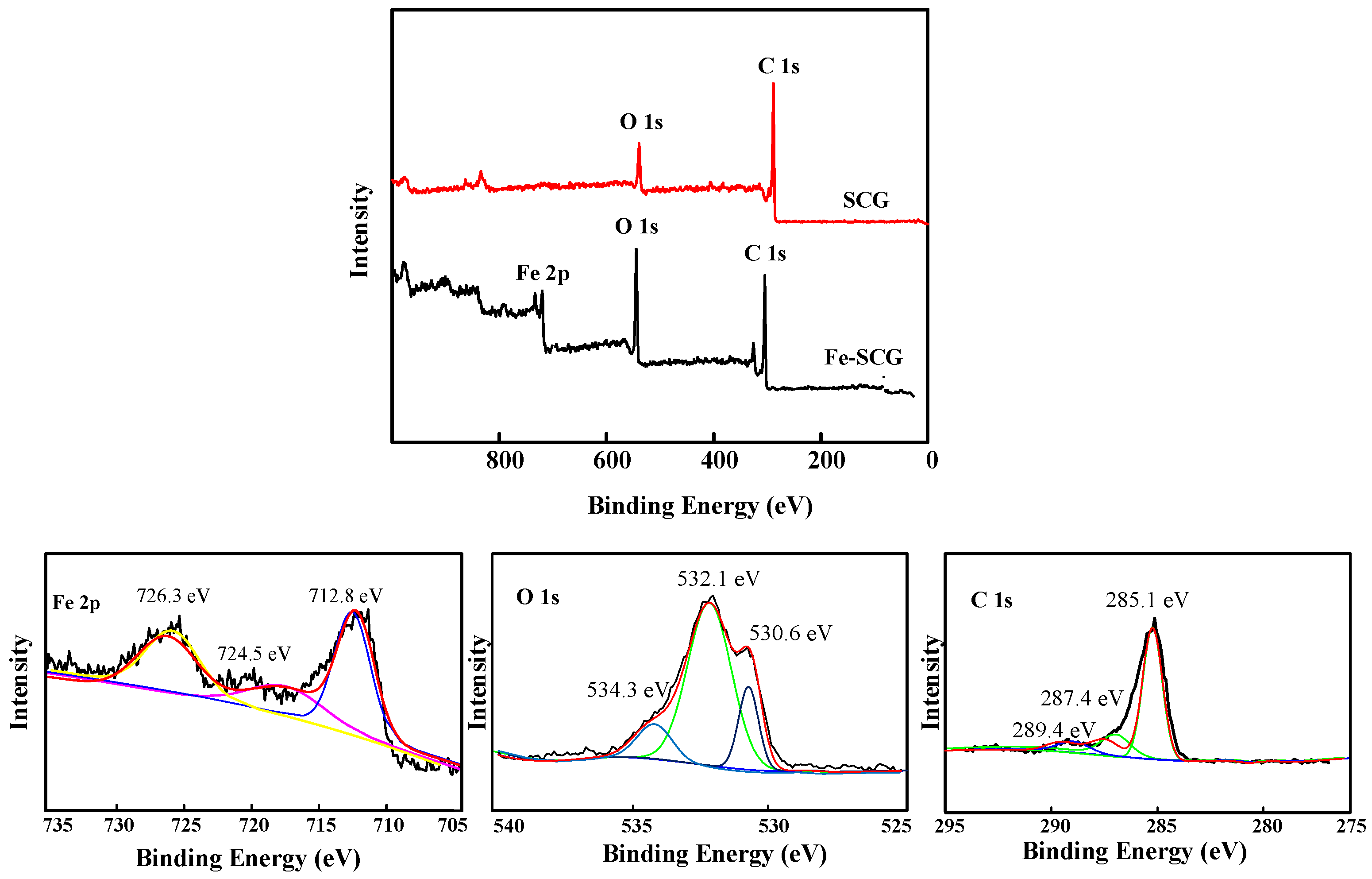
2.1. Characterization of Biochar
2.2. Adsorption Isotherms
2.3. Effect of Different Materials on Tetracycline (TC) Degradation
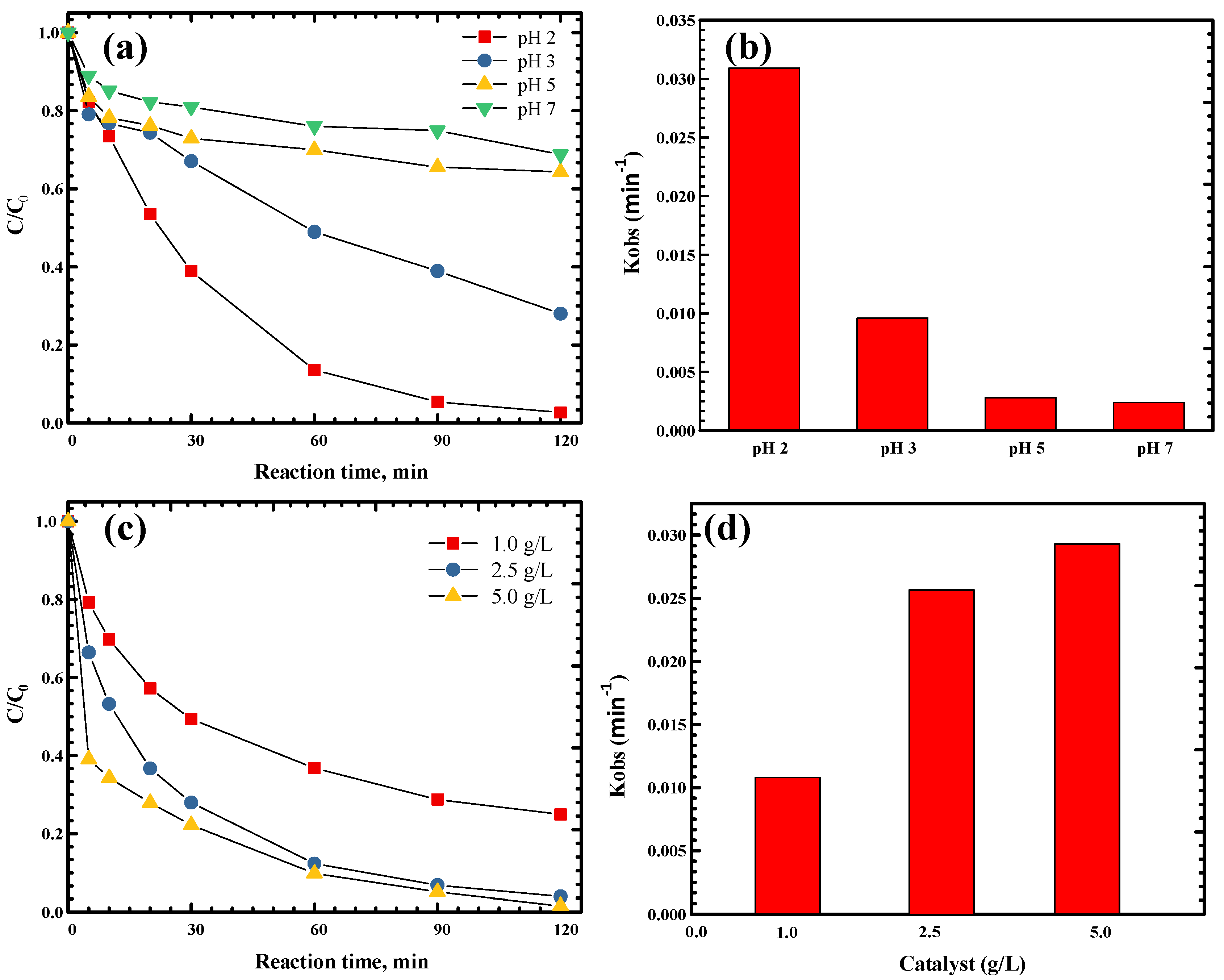
2.4. Effect of the Initial pH on TC Degradation
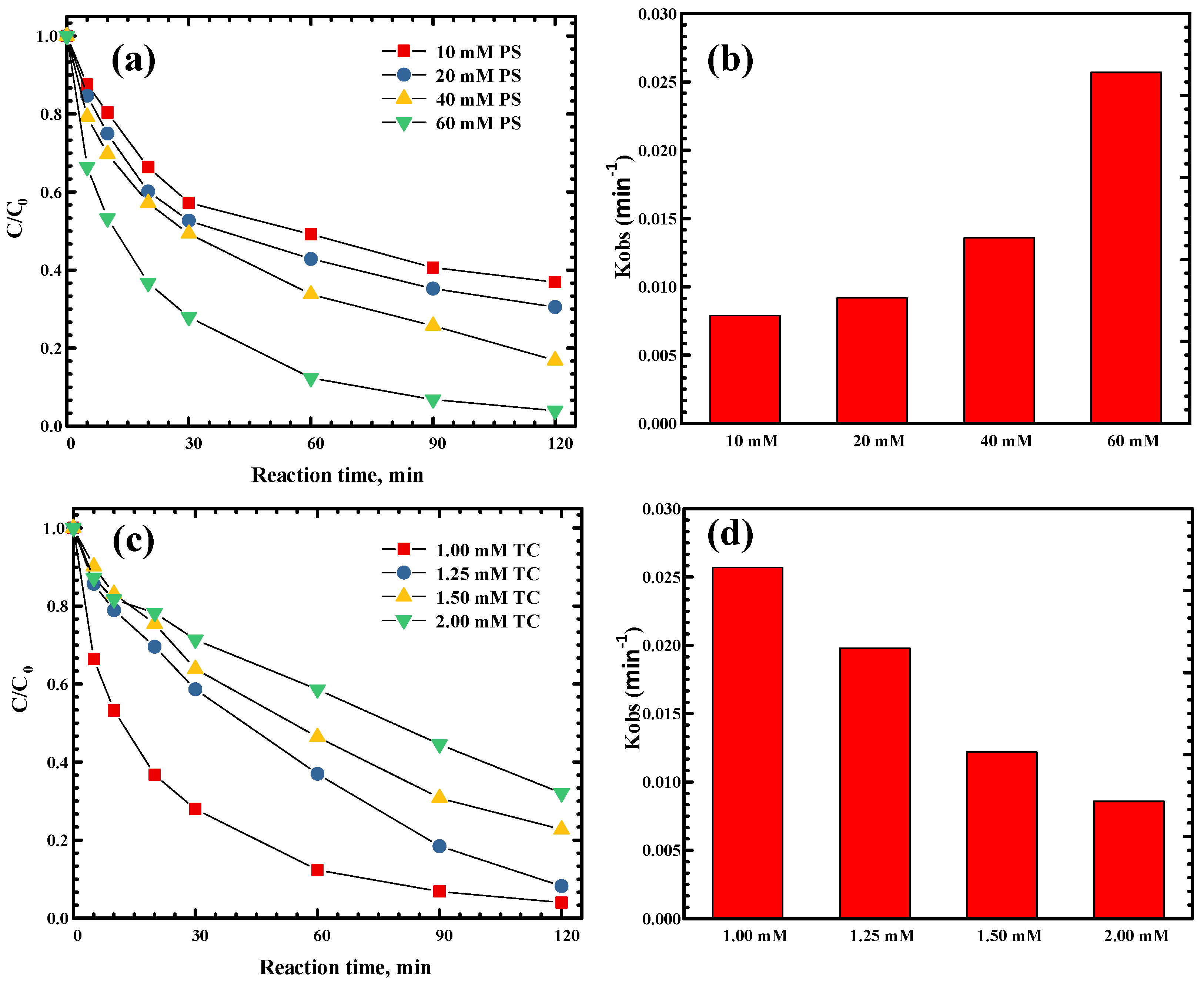
2.5. Effect of Catalyst Concentrations on TC Degradation
2.6. Effect of the Persulfate Activator on TC Degradation
2.7. Effect of Initial TC Concentration on TC Degradation
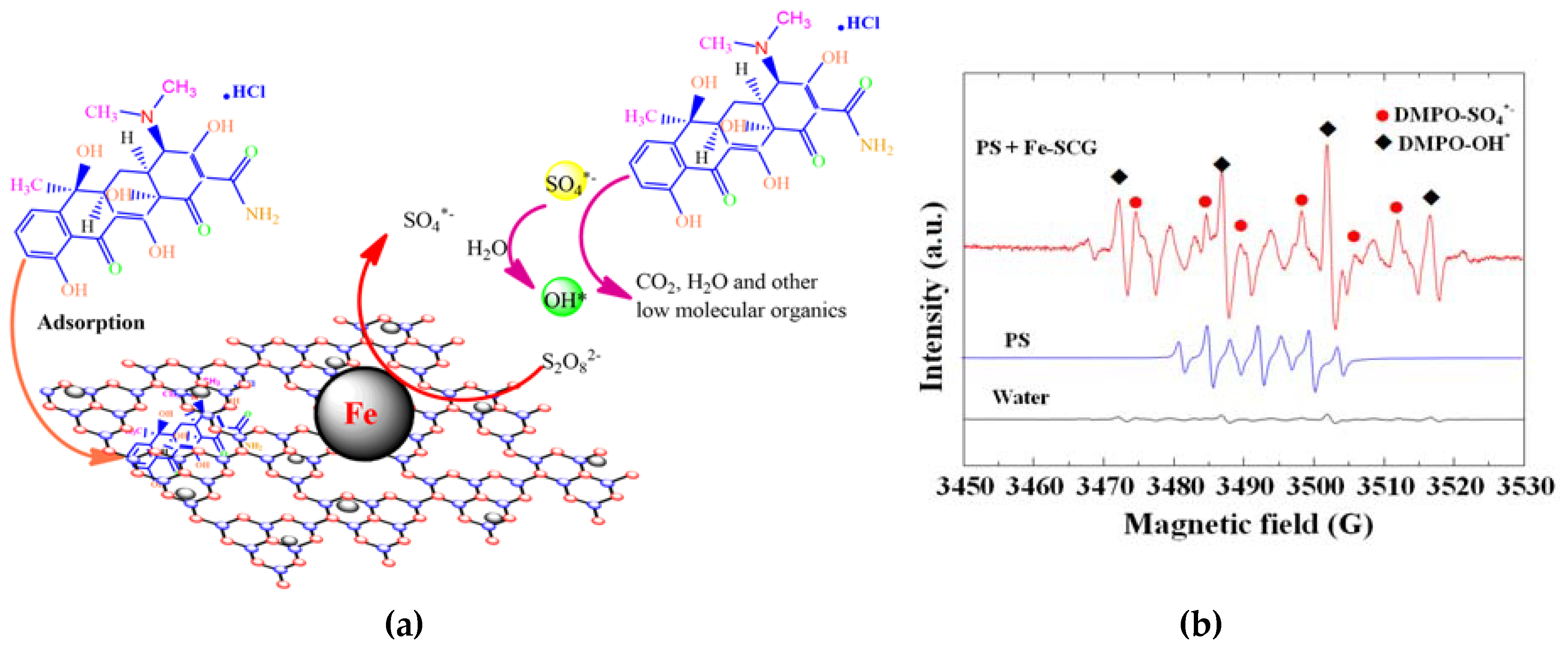
2.8. Reaction Mechanism of TC Degradation by Fe-SCG Catalyst in Presence of Persulfate
3. Materials and Methods
3.1. Iron-Modified Biochar Preparation
3.2. Chemical Preparation
3.3. Batch Experimental Design
3.4. Biochar Characteristic Analysis
3.5. Tetracycline Analysis
4. Future Research
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: http://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=AC57BF3229D37F12700322840FC9902D?sequence=1 (accessed on 25 October 2018).
- United State Food & Drug Administration (FDA). The 2016 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals 2017. Available online: https://www.fda.gov/downloads/forindustry/userfees/animaldruguserfeeactadufa/ucm588085.pdf (accessed on 1 June 2018).
- Sayğılı, G.A.; Sayğılı, H.; Koyuncu, F.; Güzel, F. Development and physicochemical characterization of a new magnetic nanocomposite as an economic antibiotic remover. Process Saf. Environ. Prot. 2015, 94, 441–451. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Weinberg, H.S.; Meyer, M.T. Trace analysis of trimethoprim and sulfonamide, macrolide, quinolone, and tetracycline antibiotics in chlorinated drinking water using liquid chromatography electrospray tandem mass spectrometry. Anal. Chem. 2007, 79, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.; Mesdaghinia, A.; Nasseri, S.; Mahvi, A.H.; Alimohammadi, M.; Gharibi, H. Assessment of tetracycline contamination in surface and groundwater resources proximal to animal farming houses in Tehran, Iran. J. Environ. Health Sci. Eng. 2016, 14, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Bui, X.T.; Luu, V.P.; Nguyen, P.D.; Guo, W.; Ngo, H.H. Removal of antibiotics in sponge membrane bioreactors treating hospital wastewater: Comparison between hollow fiber and flat sheet membrane systems. Bioresour. Technol. 2017, 240, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes–A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.D.; Chen, C.W.; Hung, C.M. Persulfate activation with rice husk-based magnetic biochar for degrading PAEs in marine sediments. Environ. Sci. Pollut. Res. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, I.; Yahia Cherif, L.; Madi, K.; Aissani-Benissad, F.; Fourcade, F.; Amrane, A. The feasibility of combining an electrochemical treatment on a carbon felt electrode and a biological treatment for the degradation of tetracycline and tylosin–application of the experimental design methodology. Sep. Sci. Technol. 2018, 53, 337–348. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Ultrasound assisted dispersion of Bi2Sn2O7-C3N4 Nanophotocatalyst over various amount of zeolite Y for enhanced solar-light photocatalytic degradation of tetracycline in aqueous solution. Ultrason. Sonochem. 2018, 43, 61–72. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Neisi, A.; Mohammadi, M.J.; Takdastan, A.; Babaei, A.A.; Yari, A.R.; Farhadi, M. Assessment of tetracycline antibiotic removal from hospital wastewater by extended aeration activated sludge. Desalin. Water Treat. 2017, 80, 380–386. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Kumara, A.V.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Selmi, T.; Sanchez-Sanchez, A.; Gadonneix, P.; Jagiello, J.; Seffen, M.; Sammouda, H.; Celzard, A.; Fierro, V. Tetracycline removal with activated carbons produced by hydrothermal carbonisation of Agave americana fibres and mimosa tannin. Ind. Crops Prod. 2018, 115, 146–157. [Google Scholar] [CrossRef]
- Ramos-Vargas, S.; Alfaro-Cuevas-Villanueva, R.; Huirache-Acuña, R.; Cortés-Martínez, R. Removal of fluoride and arsenate from aqueous solutions by aluminum-modified guava seeds. Appl. Sci. 2018, 8, 1807. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Removal of methylene blue from aqueous solution by bone char. Appl. Sci. 2018, 8, 1903. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S.; Ferreira, M.E. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 2009, 249, 267–272. [Google Scholar] [CrossRef]
- International Coffee Organization (ICO). Coffee Production. 2018. Available online: www.ico.org (accessed on 1 June 2018).
- Kim, M.S.; Min, H.G.; Koo, N.; Park, J.; Lee, S.H.; Bak, G.I.; Kim, J.G. The effectiveness of spent coffee grounds and its biochar on the amelioration of heavy metals-contaminated water and soil using chemical and biological assessments. J. Environ. Manag. 2014, 146, 124–130. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Abureesh, M.A.; Gazi, M. Bifunctional composite from spent “Cyprus coffee” for tetracycline removal and phenol degradation: Solar-Fenton process and artificial neural network. Int. J. Biol. Macromol. 2016, 90, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Shi, S.; Liu, J.; Su, S.; Liang, Q.; Zeng, X.; Li, T. Study of the effect of pyrolysis temperature on the Cd2+ adsorption characteristics of biochar. Appl. Sci. 2018, 8, 1019. [Google Scholar] [CrossRef]
- Cho, D.W.; Yoon, K.; Kwon, E.E.; Biswas, J.K.; Song, H. Fabrication of magnetic biochar as a treatment medium for As (V) via pyrolysis of FeCl3-pretreated spent coffee ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.A.; Ifebajo, A.O.; Nisar, N.; Ajayi, O.A. High-performance magnetic chicken bone-based biochar for efficient removal of rhodamine-B dye and tetracycline: Competitive sorption analysis. Water Sci. Technol. 2017, 76, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, Y.G.; Sakyi, N.Y. Simultaneous absorption and oxidation of nitric oxide and sulfur dioxide by aqueous solutions of sodium persulfate activated by temperature. Ind. Eng. Chem. Res. 2013, 52, 11702–11711. [Google Scholar] [CrossRef]
- Jafari, A.J.; Kakavandi, B.; Jaafarzadeh, N.; Kalantary, R.R.; Ahmadi, M.; Babaei, A.A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Pap, S.; Knudsen, T.Š.; Radonić, J.; Maletić, S.; Igić, S.M.; Sekulić, M.T. Utilization of fruit processing industry waste as green activated carbon for the treatment of heavy metals and chlorophenols contaminated water. J. Clean. Prod. 2017, 162, 958–972. [Google Scholar] [CrossRef]
- Hao, F.; Zhao, X.; Ouyang, W.; Lin, C.; Chen, S.; Shan, Y.; Lai, X. Molecular structure of corncob-derived biochars and the mechanism of atrazine sorption. Agron. J. 2013, 105, 773–782. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Martin, S.; Chenitz, R.; Pan, C.; Xing, W.; Bjerrum, N.J.; Li, Q. Fe3C-based oxygen reduction catalysts: Synthesis, hollow spherical structures and applications in fuel cells. J. Mater. Chem. A 2015, 3, 1752–1760. [Google Scholar] [CrossRef]
- Domínguez, C.; Perez-Alonso, F.J.; Salam, M.A.; Al-Thabaiti, S.A.; Peña, M.A.; Barrio, L.; Rojas, S. Effect of the N content of Fe/N/graphene catalysts for the oxygen reduction reaction in alkaline media. J. Mater. Chem. A 2015, 3, 24487–24494. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Duan, L.; Yang, H.; Gao, J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J. Mol. Liq. 2016, 222, 487–494. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbali, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study. Process Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
- Fujioka, N.; Suzuki, M.; Kurosu, S.; Kawase, Y. Linkage of iron elution and dissolved oxygen consumption with removal of organic pollutants by nanoscale zero-valent iron: Effects of pH on iron dissolution and formation of iron oxide/hydroxide layer. Chemosphere 2016, 144, 1738–1746. [Google Scholar] [CrossRef]
- Khataee, A.; Salahpour, F.; Fathinia, M.; Seyyedi, B.; Vahid, B. Iron rich laterite soil with mesoporous structure for heterogeneous Fenton-like degradation of an azo dye under visible light. J. Ind. Eng. Chem. 2015, 26, 129–135. [Google Scholar] [CrossRef]
- Safari, G.H.; Nasseri, S.; Mahvi, A.H.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 2015, 13, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bruton, T.A.; Doyle, F.M.; Sedlak, D.L. In situ chemical oxidation of contaminated groundwater by persulfate: Decomposition by Fe(III)-and Mn(IV)-containing oxides and aquifer materials. Environ. Sci. Technol. 2014, 48, 10330–10336. [Google Scholar] [CrossRef] [PubMed]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of base activation of persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.J.; Jeong, J.; Lee, J.; Park, N.B.; Lee, C. Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 2015, 266, 28–33. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R.-A. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.; Ali, H.S.; Murtaza, B.; Khan, H.M.; Imran, M.; Muhammad, N. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82−. Chem. Eng. J. 2019, 356, 199–209. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herrmann-Geppert, I.; Fiechter, S.; Zehl, G.; Zizak, I.; Dorbandt, I.; Schmeißer, D.; Bogdanoff, P. Effect of iron-carbide formation on the number of active sites in Fe–N–C catalysts for the oxygen reduction reaction in acidic media. J. Mater. Chem. A 2014, 2, 2663–2670. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.-T.; Hung, C.-M.; Nguyen, T.-B.; Chang, J.-H.; Wang, T.-H.; Wu, C.-H.; Lin, Y.-L.; Chen, C.-W.; Dong, C.-D. Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal. Catalysts 2019, 9, 49. https://doi.org/10.3390/catal9010049
Nguyen V-T, Hung C-M, Nguyen T-B, Chang J-H, Wang T-H, Wu C-H, Lin Y-L, Chen C-W, Dong C-D. Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal. Catalysts. 2019; 9(1):49. https://doi.org/10.3390/catal9010049
Chicago/Turabian StyleNguyen, Van-Truc, Chang-Mao Hung, Thanh-Binh Nguyen, Jih-Hsing Chang, Tsing-Hai Wang, Chung-Hsin Wu, Yi-Li Lin, Chiu-Wen Chen, and Cheng-Di Dong. 2019. "Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal" Catalysts 9, no. 1: 49. https://doi.org/10.3390/catal9010049
APA StyleNguyen, V.-T., Hung, C.-M., Nguyen, T.-B., Chang, J.-H., Wang, T.-H., Wu, C.-H., Lin, Y.-L., Chen, C.-W., & Dong, C.-D. (2019). Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal. Catalysts, 9(1), 49. https://doi.org/10.3390/catal9010049







