Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst
Abstract
1. Introduction
2. Results and Discussions
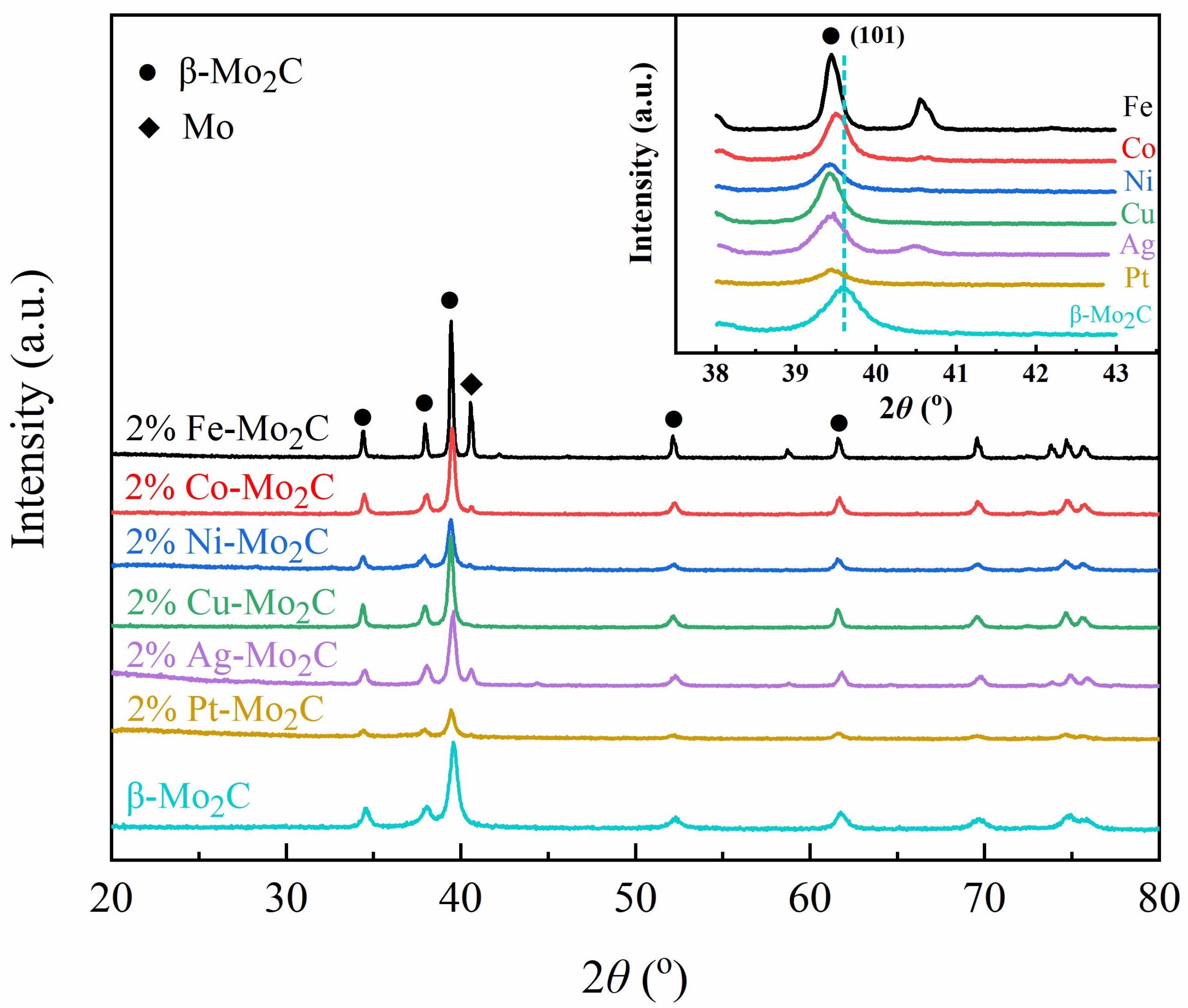
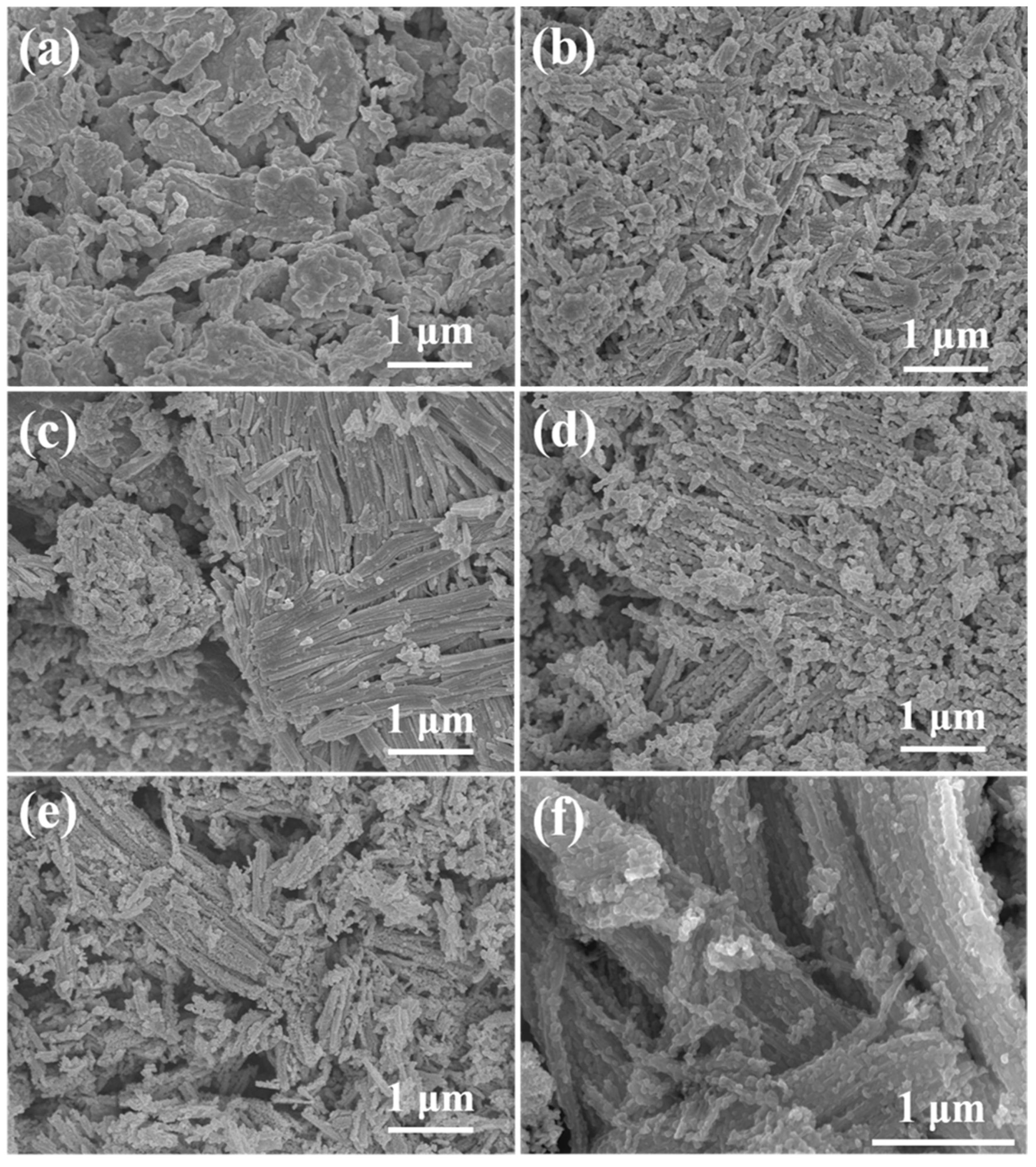
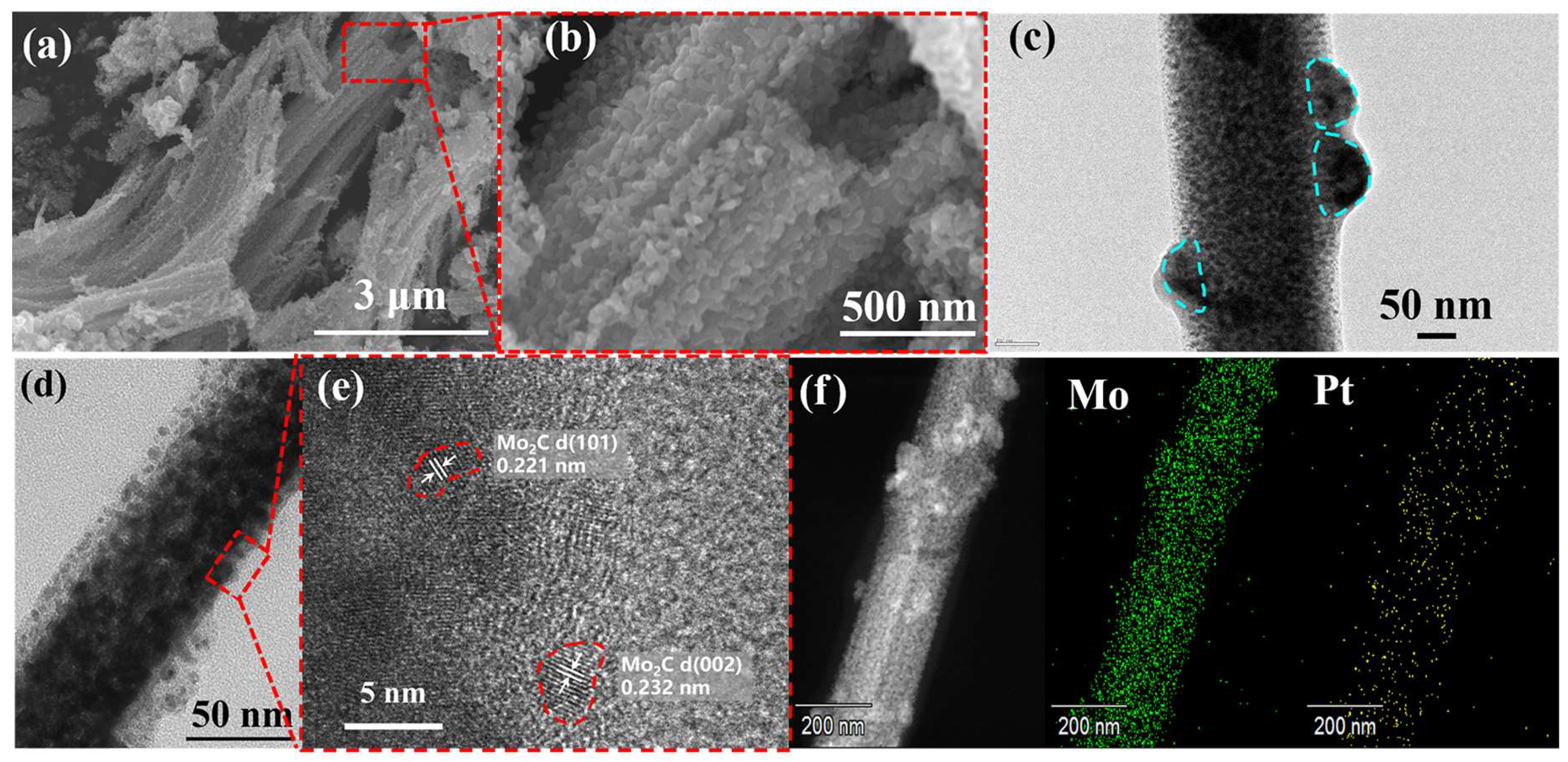
2.1. Characterization of as-Prepared Catalysts
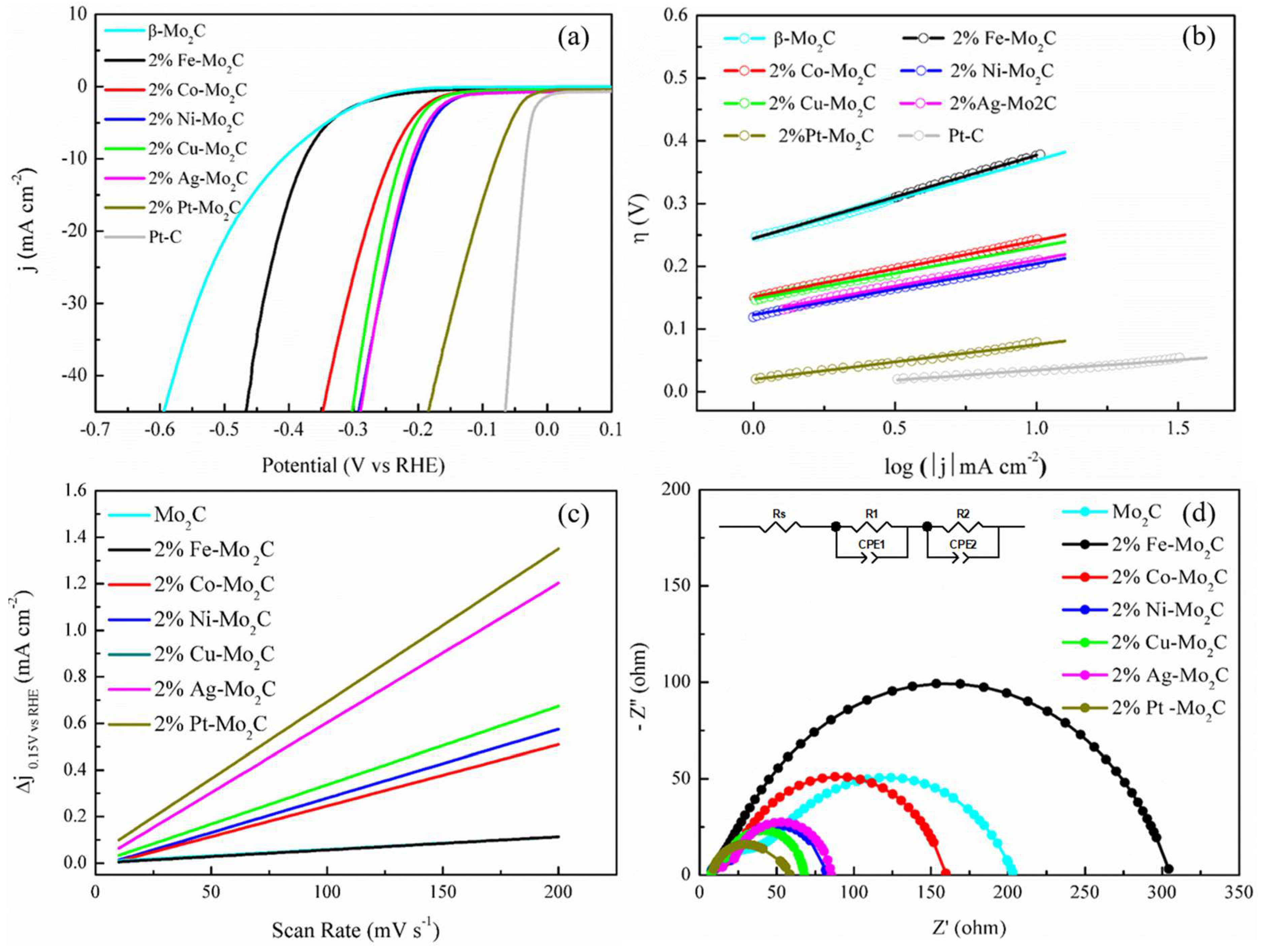
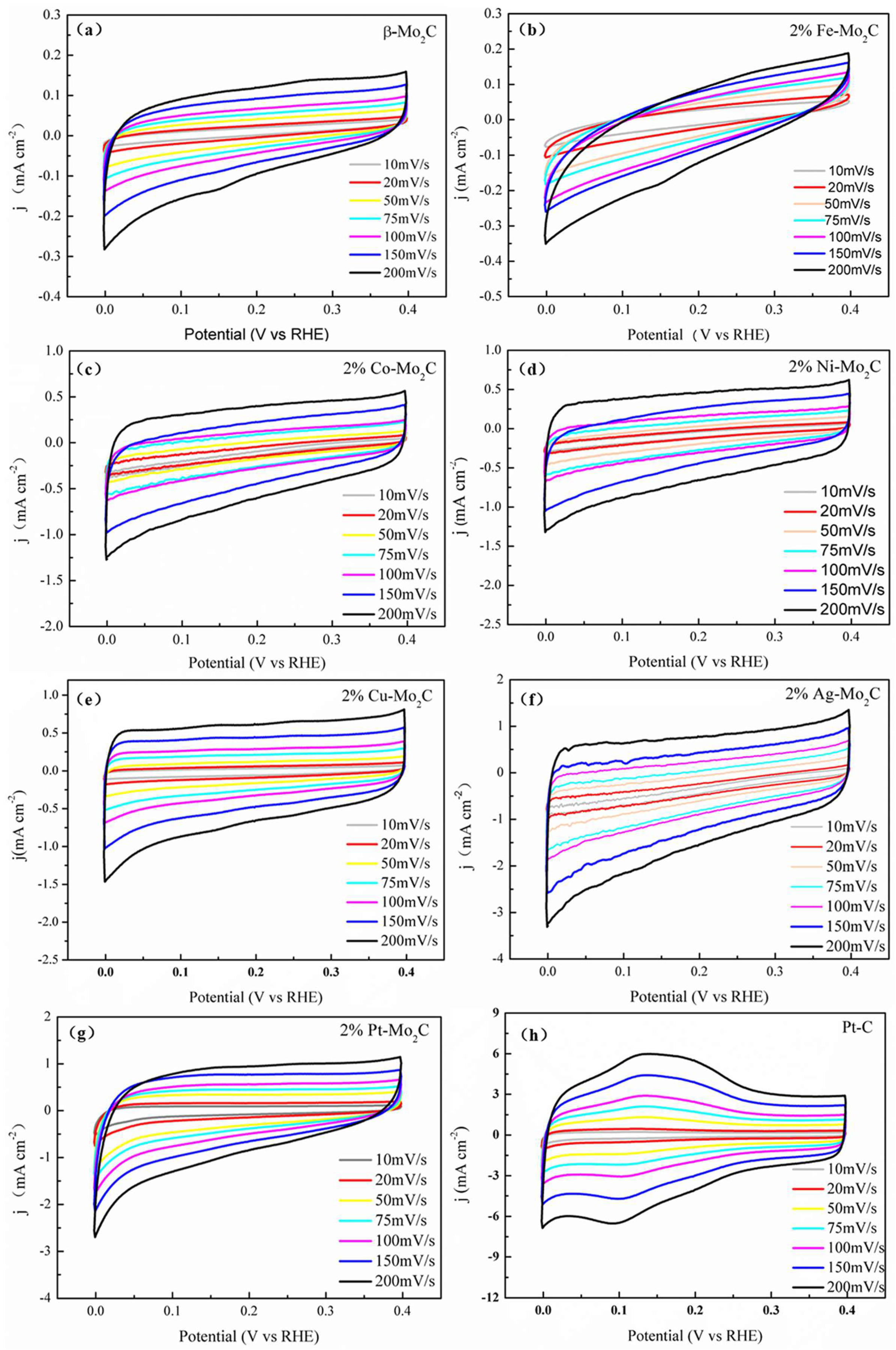
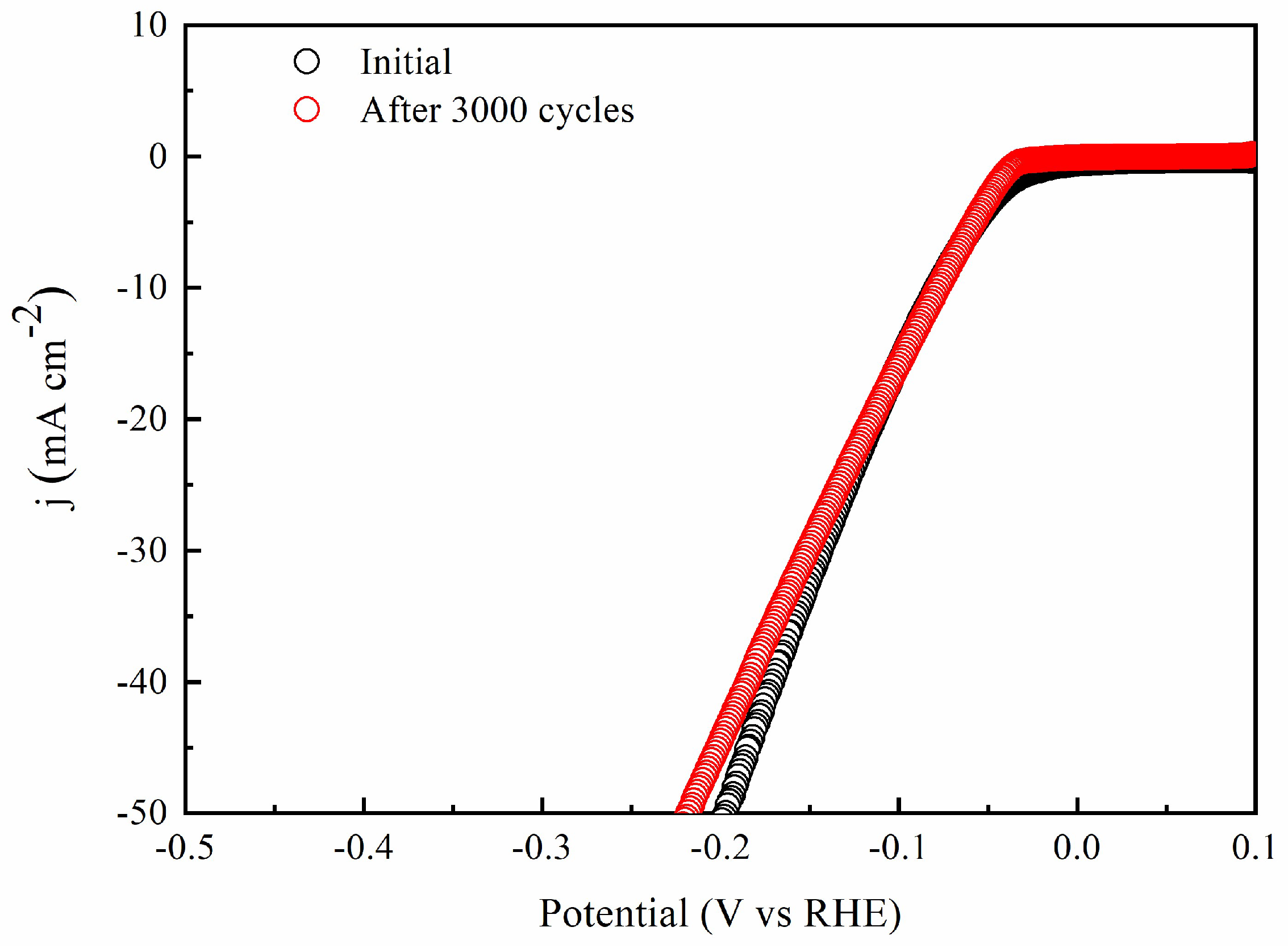
2.2. HER Catalytic Performance of Various Metal Doped Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Electrode Preparation and Its Performance Test for HER
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Pei, J.; He, C.T.; Wan, J.; Ren, H.; Zhu, Y.; Wang, Y.; Dong, J.; Tian, S.; Cheong, W.C.; et al. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew. Chem. Int. Edit. 2017, 56, 16086–16090. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum carbide as alternative catalyst for hydrogen production-a review. Renew. Sust. Energ. Rev. 2017, 75, 1101–1129. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.M.; Okyay, M.S.; Ahmad, I.; Kim, S.J.; Park, N.; Jeong, H.Y.; Baek, J.B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; Shi, C.; Wen, X.D.; Ma, D. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Zhou, W.; Gao, R.; Xu, W.; Ye, Y.; Lin, L.; Wen, X.; Liu, P.; Chen, B.; et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guan, G.; Phanthong, P.; Hao, X.; Huang, W.; Tsutsumi, A.; Kusakabe, K.; Abudula, A. Catalytic activity and stability of nickel-modified molybdenum carbide catalysts for steam reforming of methanol. J. Phys. Chem. C 2014, 118, 9485–9496. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Zuo, Z.; Huang, W.; Phanthong, P.; Li, X.; Kusakabe, K.; Abudula, A. Embedded structure catalyst: A new perspective from noble metal supported on molybdenum carbide. RSC Adv. 2015, 5, 15002–15005. [Google Scholar] [CrossRef]
- Cao, J.; Ma, Y.; Guan, G.; Hao, X.; Ma, X.; Wang, Z.; Kusakabe, K.; Abudula, A. Reaction intermediate species during the steam reforming of methanol over metal modified molybdenum carbide catalysts. Appl. Catal. B 2016, 189, 12–18. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, G.; Hao, X.; Zuo, Z.; Huang, W.; Phanthong, P.; Kusakabe, K.; Abudula, A. Highly-efficient steam reforming of methanol over copper modified molybdenum carbide. RSC Adv. 2014, 4, 44175–44184. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Liu, Z.; Loh, K.P. A graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lun, Z.; Xia, G.; Zheng, F.; He, M.; Chen, Q. Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571. [Google Scholar] [CrossRef]
- Lee, J.S.; Yeom, M.H.; Park, K.Y.; Nam, I.S.; Chung, J.S.; Kim, Y.G.; Moon, S.H. Preparation and benzene hydrogenation activity of supported molybdenum carbide catalysts. J. Catal. 1991, 128, 126–136. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew. Chem. Int. Ed. 2014, 53, 6705–6709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.W.; Shi, C.; Ma, D. Highly dispersed copper over β-Mo2C as an efficient and stable catalyst for the reverse water gas shift (RWGS) reaction. ACS Catal. 2017, 7, 912–918. [Google Scholar] [CrossRef]
- Blekkan, E.A.; Pham-Huu, C.; Ledoux, M.J.; Guille, J. Isomerization of n-heptane on an oxygen-modified molybdenum carbide catalyst. Ind. Eng. Chem. Res. 1994, 33, 1657–1664. [Google Scholar] [CrossRef]
- Liao, L.; Wang, S.; Xiao, J.; Bian, X.; Zhang, Y.; Scanlon, M.D.; Hu, X.; Tang, Y.; Liu, B.; Girault, H.H. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. 2012, 124, 12875–12878. [Google Scholar] [CrossRef]
- Lin, H.; Liu, N.; Shi, Z.; Guo, Y.; Tang, Y.; Gao, Q. Cobalt-doping in molybdenum-carbide nanowires toward efficient electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2016, 26, 5590–5598. [Google Scholar] [CrossRef]
- Wang, J.; Cao, J.; Ma, Y.; Li, X.; Xiaokaiti, P.; Hao, X.; Yu, T.; Abudula, A.; Guan, G. Decomposition of formic acid for hydrogen production over metal doped nanosheet-like MoC1-x catalysts. Energy Convers. Manag. 2017, 147, 166–173. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; Ma, Y.; Li, X.; Xiaokaiti, P.; Hao, X.; Abudula, A.; Guan, G. Hydrogen production from formic acid over morphology-controllable molybdenum carbide catalysts. J. Alloys Compd. 2018, 735, 1463–1471. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Mo, Q.; Gao, B.; Liu, B.; Wang, L.; Zhang, Y.; Gao, Q.; Tang, Y. Mesoporous and skeletal molybdenum carbide for hydrogen evolution reaction: diatomite-type structure and formation mechanism. Chem. Electro. Chem. 2017, 4, 2169–2177. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. 2014, 126, 6525–6528. [Google Scholar] [CrossRef]
- Michalsky, R.; Zhang, Y.J.; Peterson, A.A. Trends in the hydrogen evolution activity of metal carbide catalysts. ACS Catal. 2014, 4, 1274–1278. [Google Scholar] [CrossRef]

| Catalysts | Mo 3d5/2 (eV) | ||||
|---|---|---|---|---|---|
| Mo2+ (Mo2C) | Mo4+ (MoO2) | Moδ+ (MoOxCy) | Mo6+ (MoO3) | Mo0 (Mo) | |
| β-Mo2C | 228.60 | 229.30 | 232.20 | 233.30 | ̶ |
| 2% Fe-Mo2C | 228.40 | 229.10 | 231.60 | 232.70 | 228.00 |
| 2% Co-Mo2C | 228.50 | 229.20 | 231.70 | 232.80 | 228.10 |
| 2% Ni-Mo2C | 228.58 | 229.20 | 231.80 | 232.90 | ̶ |
| 2% Cu-Mo2C | 228.58 | 229.20 | 232.00 | 232.60 | ̶ |
| 2% Ag-Mo2C | 228.40 | 229.10 | 231.60 | 232.70 | 228.20 |
| 2% Pt-Mo2C | 228.00 | 228.90 | 230.60 | 232.30 | ̶ |
| Catalyst | M/Moa | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| β-Mo2C | \ | 20.7 | 0.09 | 16.3 |
| 2% Fe-Mo2C | \ | 3.2 | 0.01 | 50.7 |
| 2% Co-Mo2C | 0.5 | 9.7 | 0.06 | 44.1 |
| 2% Ni-Mo2C | \ | 15.3 | 0.08 | 33.4 |
| 2% Cu-Mo2C | \ | 14.9 | 0.07 | 19.9 |
| 2% Ag-Mo2C | 1.9 | 14.3 | 0.1 | 32.9 |
| 2% Pt-Mo2C | 0.2 | 48.3 | 0.06 | 3.0 |
| Catalyst | η10 (mV) | ηonset (mV) | Tafel Slope (mV/dec) | Rct(a) (Ω) | Cdl(b) (mF/cm2) | j0(c) (mA/cm2) |
|---|---|---|---|---|---|---|
| Mo2C | 410 | 245 | 124 | 148.40 | 0.54 | 1.1 × 10−2 |
| 2% Fe-Mo2C | 377 | 226 | 132 | 290.90 | 0.56 | 1.4 × 10−2 |
| 2% Co-Mo2C | 243 | 150 | 89 | 129.90 | 2.63 | 2.0 × 10−2 |
| 2% Ni-Mo2C | 205 | 120 | 81 | 64.04 | 2.96 | 2.8 × 10−2 |
| 2% Cu-Mo2C | 227 | 146 | 84 | 52.33 | 3.37 | 1.7 × 10−2 |
| 2% Ag-Mo2C | 210 | 108 | 83 | 61.73 | 6.00 | 3.0 × 10−2 |
| 2% Pt-Mo2C | 79 | 32 | 55 | 54.19 | 6.59 | 4.4 × 10−1 |
| 20% Pt/C | 34 | 23 | 32 | \ | 28.2 | 8.5 × 10−1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Ma, Y.; Zhou, Y.; Liu, C.; Qin, Y.; Fang, Y.; Guan, G.; Li, X.; Zhang, Z.; Wang, T. Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst. Catalysts 2018, 8, 294. https://doi.org/10.3390/catal8070294
Chen M, Ma Y, Zhou Y, Liu C, Qin Y, Fang Y, Guan G, Li X, Zhang Z, Wang T. Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst. Catalysts. 2018; 8(7):294. https://doi.org/10.3390/catal8070294
Chicago/Turabian StyleChen, Meng, Yufei Ma, Yanqiang Zhou, Changqing Liu, Yanlin Qin, Yanxiong Fang, Guoqing Guan, Xiumin Li, Zhaoshun Zhang, and Tiejun Wang. 2018. "Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst" Catalysts 8, no. 7: 294. https://doi.org/10.3390/catal8070294
APA StyleChen, M., Ma, Y., Zhou, Y., Liu, C., Qin, Y., Fang, Y., Guan, G., Li, X., Zhang, Z., & Wang, T. (2018). Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst. Catalysts, 8(7), 294. https://doi.org/10.3390/catal8070294