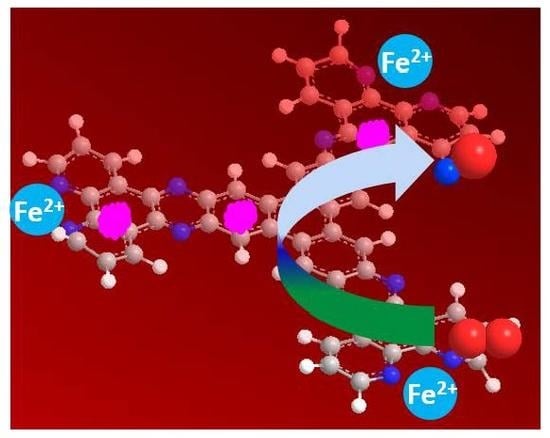
An Iron-Based Catalyst with Multiple Active Components Synergetically Improved Electrochemical Performance for Oxygen Reduction Reaction
Abstract
1. Introduction
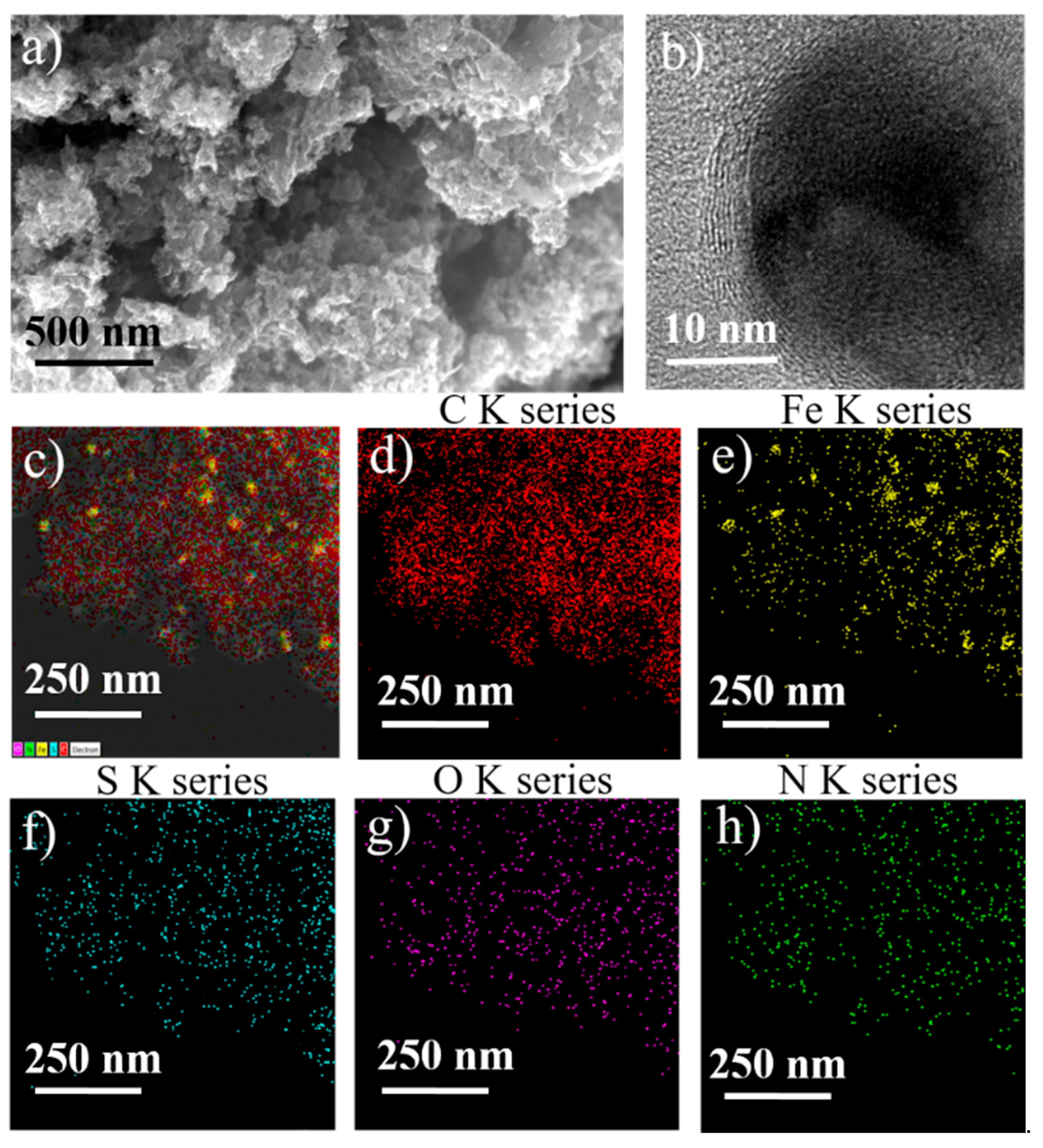
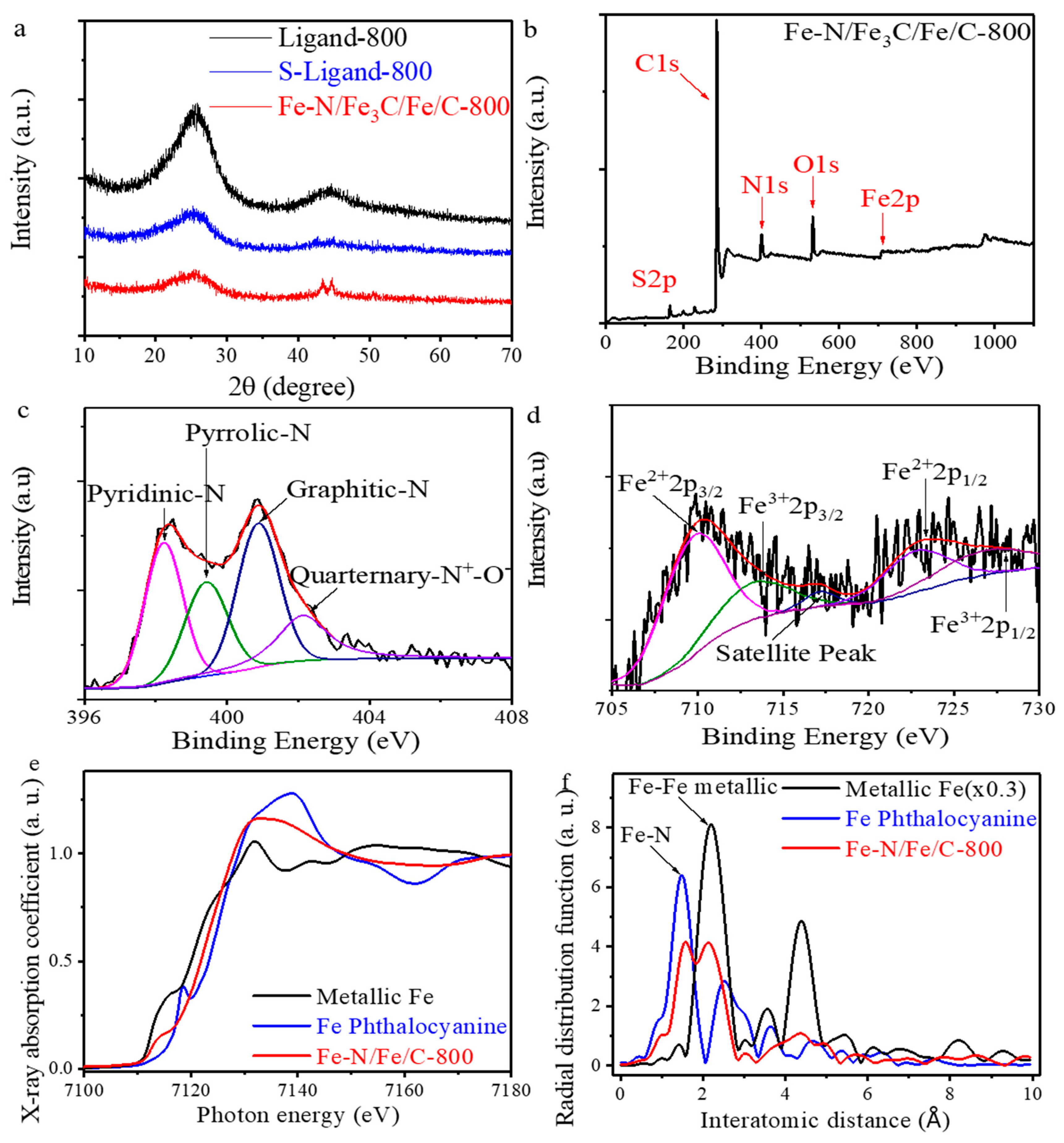
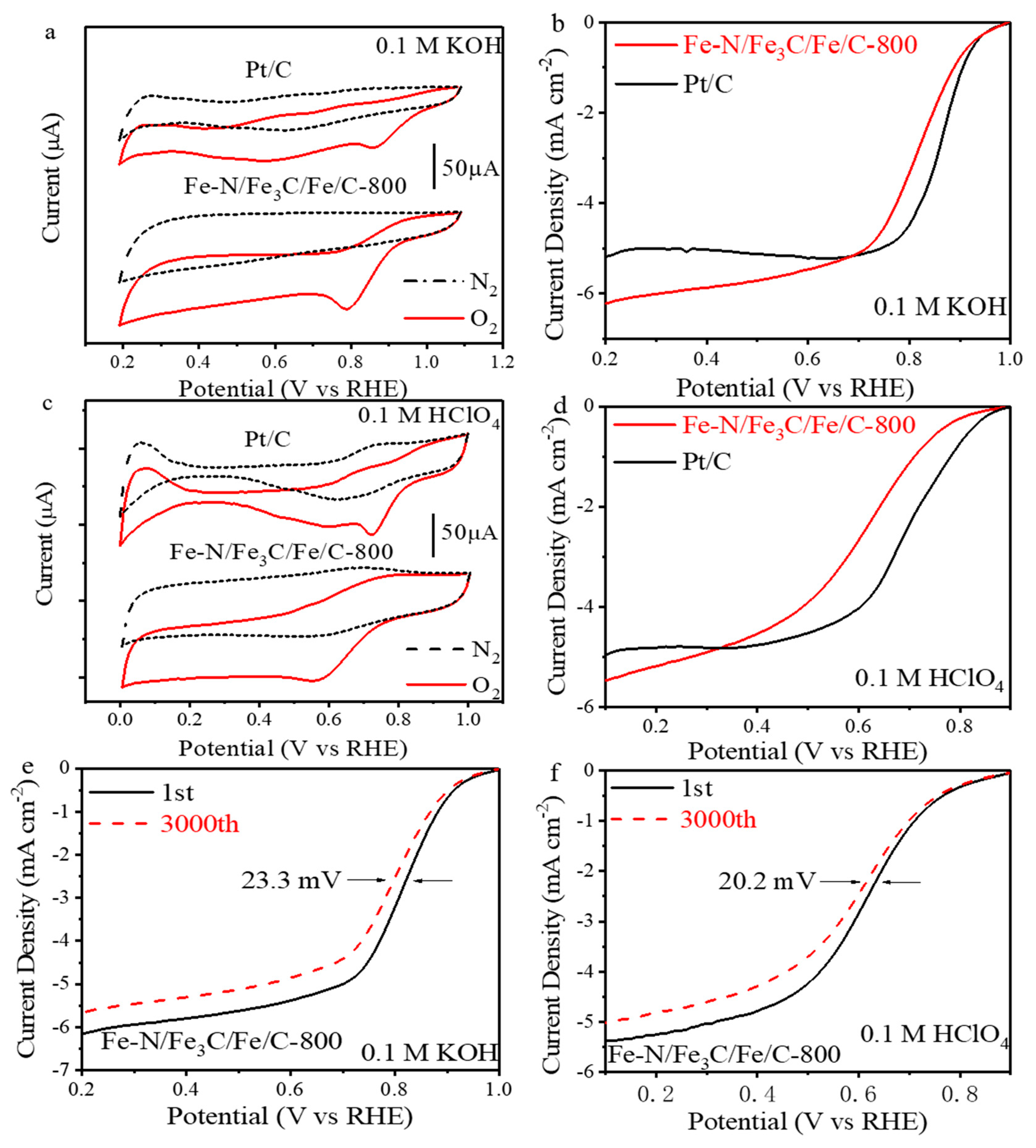
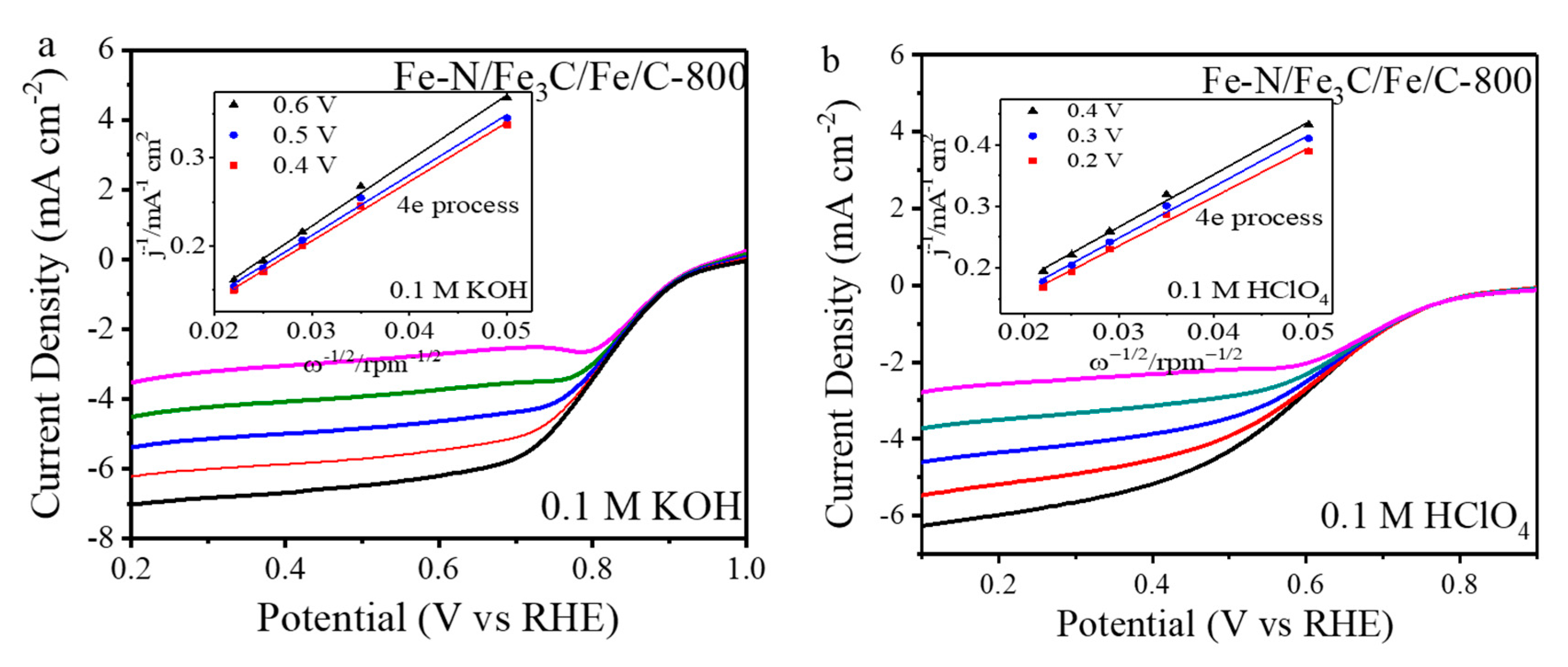
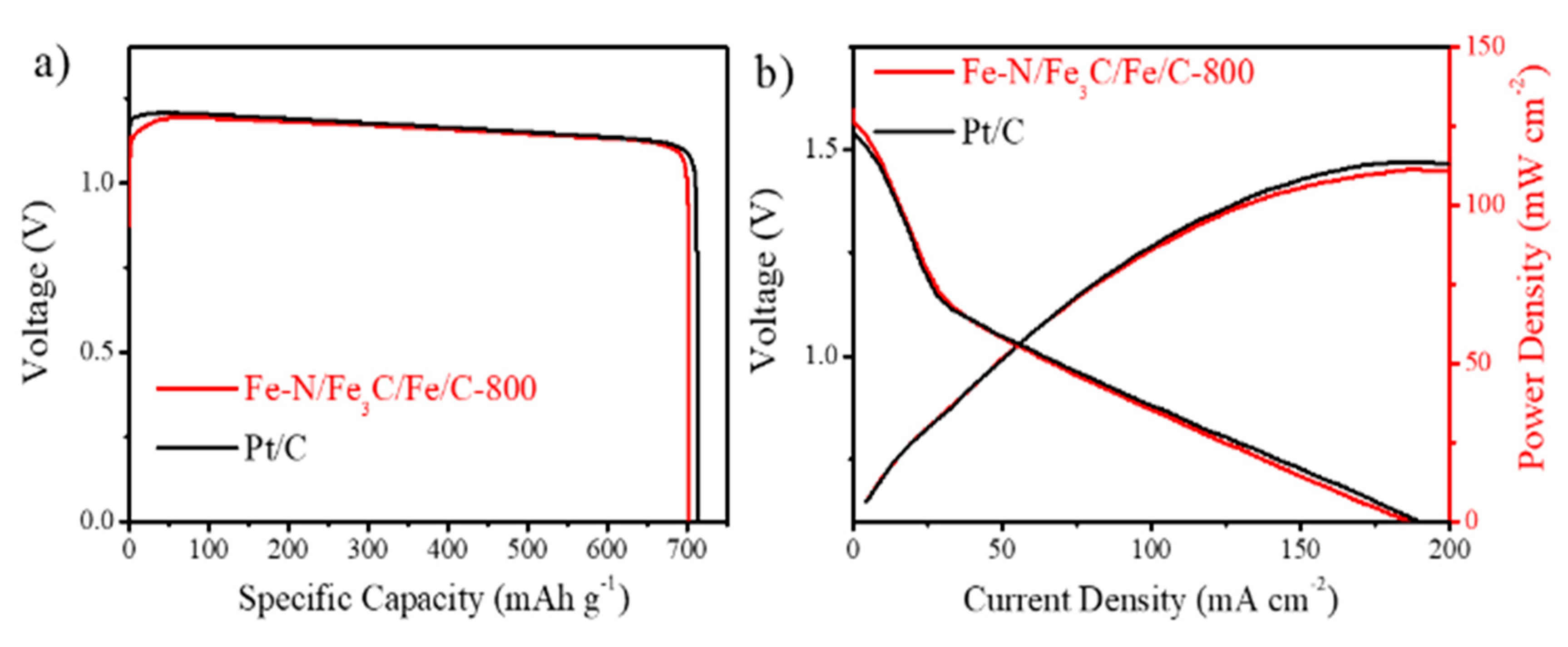
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Tri (dipyrido[3,2-a:2′,3′-c] phenazinyl) Phenylene
3.3. Synthesis of Catalysts
3.4. Characterization of Catalysts
3.5. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Su, D.S.; Sun, G.Q. Nonprecious-Metal Catalysts for Low-Cost Fuel Cells. Angew. Chem. Int. Ed. 2011, 50, 11570–11572. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Morozan, A.; Sougrati, M.T.; Lefevre, M.; Chenitz, R.; Dodelet, J.P.; Jones, D.; Jaouen, F. Optimized Synthesis of Fe/N/C Cathode Catalysts for PEM Fuel Cells: A Matter of Iron-Ligand Coordination Strength. Angew. Chem. Int. Ed. 2013, 52, 6867–6870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Zhang, L.; Sun, X. Phosphorus-Doped Co3O4 Nanowire Array: A Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Goenaga, G.A.; Call, A.V.; Liu, D.J. Cobalt imidazolate framework as precursor for oxygen reduction reaction electrocatalysts. Chem.-A Eur. J. 2011, 17, 2063–2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lai, Y.J.; Song, L.; Zhou, Z.Y.; Liu, J.G.; Wang, Q.; Yang, X.D.; Chen, C.; Shi, W.; Zheng, Y.P.; et al. S-Doping of an Fe/N/C ORR Catalyst for Polymer Electrolyte Membrane Fuel Cells with High Power Density. Angew. Chem. Int. Ed. 2015, 54, 9907–9910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Zhang, B.S.; Liu, X.; Wang, D.W.; Su, D.S. Unravelling the Structure of Electrocatalytically Active Fe-N Complexes in Carbon for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2014, 53, 10673–10677. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A.W. Noble-Metal-Free Fe-N/C Catalyst for Highly Efficient Oxygen Reduction Reaction under Both Alkaline and Acidic Conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Wu, D.Q.; Feng, X.L.; Mullen, K. Nitrogen-Doped Ordered Mesoporous Graphitic Arrays with High Electrocatalytic Activity for Oxygen Reduction. Angew. Chem. Int. Ed. 2010, 49, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.; Stevenson, K.J. Influence of nitrogen doping on oxygen reduction electrocatalysis at carbon nanofiber electrodes. J. Phys. Chem. B 2005, 109, 4707–4716. [Google Scholar] [CrossRef] [PubMed]
- Matter, P.H.; Zhang, L.; Ozkan, U.S. The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J. Catal. 2006, 239, 83–96. [Google Scholar] [CrossRef]
- Gong, K.P.; Du, F.; Xia, Z.H. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.Y.; Zheng, C.; Deng, W.J.; Chen, X.A.; Wei, H.F.; Liu, M.L.; Huang, S.M. Nitrogen-doped porous carbon plates derived from fallen camellia flower for electrochemical energy storage. J. Solid State Electr. 2016, 21, 1165–1174. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefevre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.H.; Yu, L.; Chen, X.Q.; Wang, G.X.; Jin, L.; Pan, X.L.; Deng, J.; Sun, G.Q.; Bao, X.H. Iron Encapsulated within Pod-like Carbon Nanotubes for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.X.; Liu, X.J.; Yue, X.Y.; Jia, J.B.; Guo, S.J. Bamboo-like Carbon Nanotube/Fe3C Nanoparticle Hybrids and Their Highly Efficient Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Arul, N.S.; Han, J.I.; Chen, P.C. Fabrication of β-Ni(OH)2//γ-Fe2O3 nanostructures for high-performance asymmetric supercapacitors. J. Solid State Electr. 2017, 22, 293–302. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Yan, X.; Yu, S.; Tang, Y.; Sun, D.; Xu, L.; Xue, C. Triangular AgAu@Pt core-shell nanoframes with a dendritic Pt shell and enhanced electrocatalytic performance toward the methanol oxidation reaction. Nanoscale 2018, 10, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Schwab, M.G.; Zhi, L.J.; Mugnaioli, E.; Kolb, U.; Feng, X.L.; Mullen, K. Direct Access to Metal or Metal Oxide Nanocrystals Integrated with One-Dimensional Nanoporous Carbons for Electrochemical Energy Storage. J. Am. Chem. Soc. 2010, 132, 15030–15037. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Li, C.L.; Li, Y.Q.; Tade, M.O.; Dai, S.; Yamauchi, Y. Synthesis of Nitrogen-Doped Mesoporous Carbon Spheres with Extra-Large Pores through Assembly of Diblock Copolymer Micelles. Angew. Chem. Int. Ed. 2015, 54, 588–593. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-Treated Ordered Mesoporous Carbons as Catalytic Materials for Oxygen Reduction Reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, H.M.; Zhong, H.X.; Zhang, J.L. Nitrogen-doped carbon xerogel: A novel carbon-based electrocatalyst for oxygen reduction reaction in proton exchange membrane (PEM) fuel cells. Energy Environ. Sci. 2011, 4, 3389–3394. [Google Scholar] [CrossRef]
- Wu, Z.S.; Chen, L.; Liu, J.Z.; Parvez, K.; Liang, H.W.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.L.; Mullen, K. High-Performance Electrocatalysts for Oxygen Reduction Derived from Cobalt Porphyrin-Based Conjugated Mesoporous Polymers. Adv Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Zhou, W.; Tade, M.O.; Shao, Z. B-Site Cation-Ordered Double-Perovskite Oxide as an Outstanding Electrode Material for Supercapacitive Energy Storage Based on the Anion Intercalation Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 9415–9423. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.H.; Ci, S.Q.; Zhang, F.; Feng, X.L.; Cui, S.M.; Mao, S.; Luo, S.L.; He, Z.; Chen, J.H. Nitrogen-Enriched Core-Shell Structured Fe/Fe3C-C Nanorods as Advanced Electrocatalysts for Oxygen Reduction Reaction. Adv. Mater. 2012, 24, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, P.; Gao, W.; Ma, S.G.; Zhang, G.Q.; Cao, R.G.; Cho, J.; Wang, H.L.; Wu, G. Graphene/Graphene-Tube Nanocomposites Templated from Cage-Containing Metal-Organic Frameworks for Oxygen Reduction in Li-O2 Batteries. Adv. Mater. 2014, 26, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Li, S.L.; Tang, Y.J.; Han, M.; Dai, Z.H.; Bao, J.C.; Lan, Y.Q. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: EFFICIENT bifunctional electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Chen, X.T.; Cao, Y.; Chen, Y.Z.; Wang, F.; Chen, Y.G.; Liu, Y. Pyridinic and pyrrolic nitrogen-rich ordered mesoporous carbon for efficient oxygen reduction in microbial fuel cells. RSC Adv. 2017, 7, 14669–14677. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Waghmare, U.V.; Hegde, M.S. Origin of activation of Lattice Oxygen and Synergistic Interaction in Bimetal-Ionic Ce0.89Fe0.1Pd0.01O2-delta Catalyst. Chem. Mater. 2009, 21, 4880–4891. [Google Scholar] [CrossRef]
- Strickland, K.; Elise, M.W.; Jia, Q.Y.; Tylus, U.; Ramaswamy, N.; Liang, W.T.; Sougrati, M.T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal-nitrogen coordination. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J. Inner-Sphere Heterogeneous Electrode Reactions. Electrocatalysis and Photocatalysis: The Challenge. J. Am. Chem. Soc. 2010, 132, 7559–7567. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sa, Y.J.; Jeong, H.Y.; Joo, S.H. Roles of Fe−Nx and Fe−Fe3C@C Species in Fe−N/C Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 9567–9575. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the Role of Metals in Nitrogen-Doped Carbon Electrocatalysts for Oxygen Reduction. Angew. Chem. Int. Ed. 2015, 54, 10102–10120. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. Engl. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Hou, Y.; Wen, Z.H.; Guo, X.R.; Chen, J.H.; He, Z. Porous Carbon Nanosheets Codoped with Nitrogen and Sulfur for Oxygen Reduction Reaction in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2015, 7, 18672–18678. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, J.; Wu, Z.X.; Xin, H.L.; Wang, D.L. Synergistic enhancement of nitrogen and sulfur co-doped graphene with carbon nanosphere insertion for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 7727–7731. [Google Scholar] [CrossRef]
- Jiang, R.Z.; Chu, D. Comparative study of CoFeNx/C catalyst obtained by pyrolysis of hemin and cobalt porphyrin for catalytic oxygen reduction in alkaline and acidic electrolytes. J. Power Sources 2014, 245, 352–361. [Google Scholar] [CrossRef]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient Metal-Free Electrocatalysts for Oxygen Reduction: Polyaniline-Derived N- and O-Doped Mesoporous Carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q.F. Hollow Spheres of Iron Carbide Nanoparticles Encased in Graphitic Layers as Oxygen Reduction Catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.Q.; Jiao, Y.F.; Song, J.X.; Zhang, K.; Li, J.; Zhang, Z. Fe/Fe3C@graphitic carbon shell embedded in carbon nanotubes derived from Prussian blue as cathodes for Li–O2 batteries. Mater. Chem. Front. 2018, 2, 376–384. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yu, D.S.; Dai, L.M. Polyelectrolyte Functionalized Carbon Nanotubes as Efficient Metal-free Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Song, M.; Wang, J.; Liu, X. Recent Progress in Nitrogen-Doped Metal-Free Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2018, 8, 196. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Xue, Y.H.; Cao, D.P.; Huang, L.; Chen, J.F.; Dai, L.M. Highly Efficient Electrocatalysts for Oxygen Reduction Based on 2D Covalent Organic Polymers Complexed with Non-precious Metals. Angew. Chem. Int. Ed. 2014, 53, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kim, J.; Ishizuka, T.; Honsho, Y.; Saeki, A.; Seki, S.; Ihee, H.; Jiang, D.L. Photoconductive Sheets with Extremely High Carrier Mobility and Conduction Anisotropy from Triphenylene-Fused Metal Trigon Conjugates. J. Am. Chem. Soc. 2009, 131, 7287–7292. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Song, X.; Li, P.; Wang, S.; Wu, Z.; Liu, X. An Iron-Based Catalyst with Multiple Active Components Synergetically Improved Electrochemical Performance for Oxygen Reduction Reaction. Catalysts 2018, 8, 243. https://doi.org/10.3390/catal8060243
Zhang J, Song X, Li P, Wang S, Wu Z, Liu X. An Iron-Based Catalyst with Multiple Active Components Synergetically Improved Electrochemical Performance for Oxygen Reduction Reaction. Catalysts. 2018; 8(6):243. https://doi.org/10.3390/catal8060243
Chicago/Turabian StyleZhang, Jian, Xiaoming Song, Ping Li, Shuai Wang, Zexing Wu, and Xien Liu. 2018. "An Iron-Based Catalyst with Multiple Active Components Synergetically Improved Electrochemical Performance for Oxygen Reduction Reaction" Catalysts 8, no. 6: 243. https://doi.org/10.3390/catal8060243
APA StyleZhang, J., Song, X., Li, P., Wang, S., Wu, Z., & Liu, X. (2018). An Iron-Based Catalyst with Multiple Active Components Synergetically Improved Electrochemical Performance for Oxygen Reduction Reaction. Catalysts, 8(6), 243. https://doi.org/10.3390/catal8060243