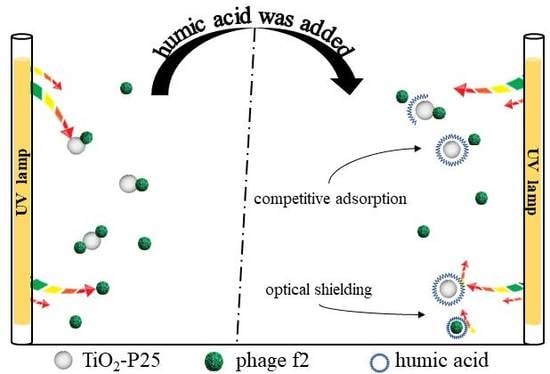
Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Humic Acid on Individual Units of PMR System
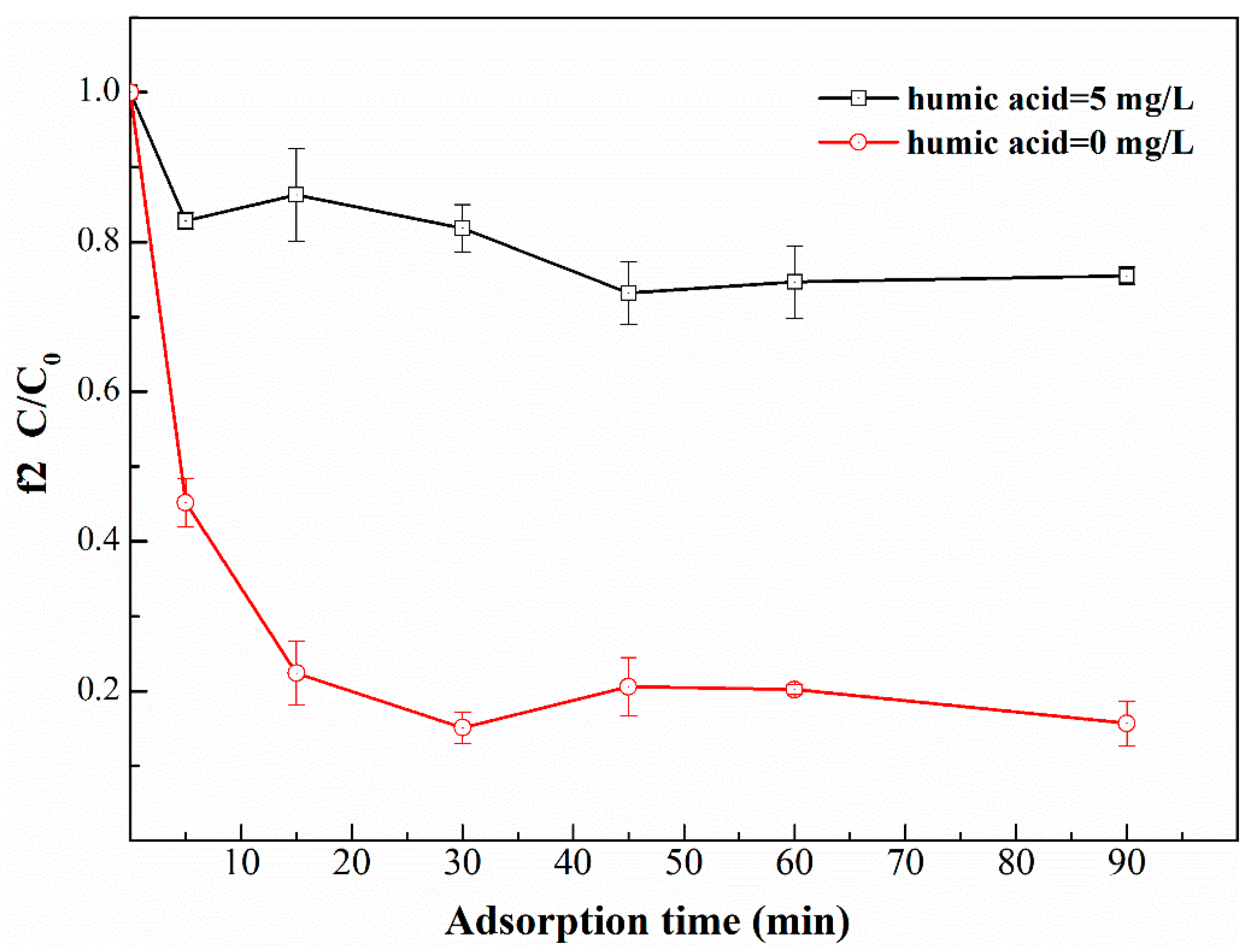
2.1.1. Effect on Adsorption Unit
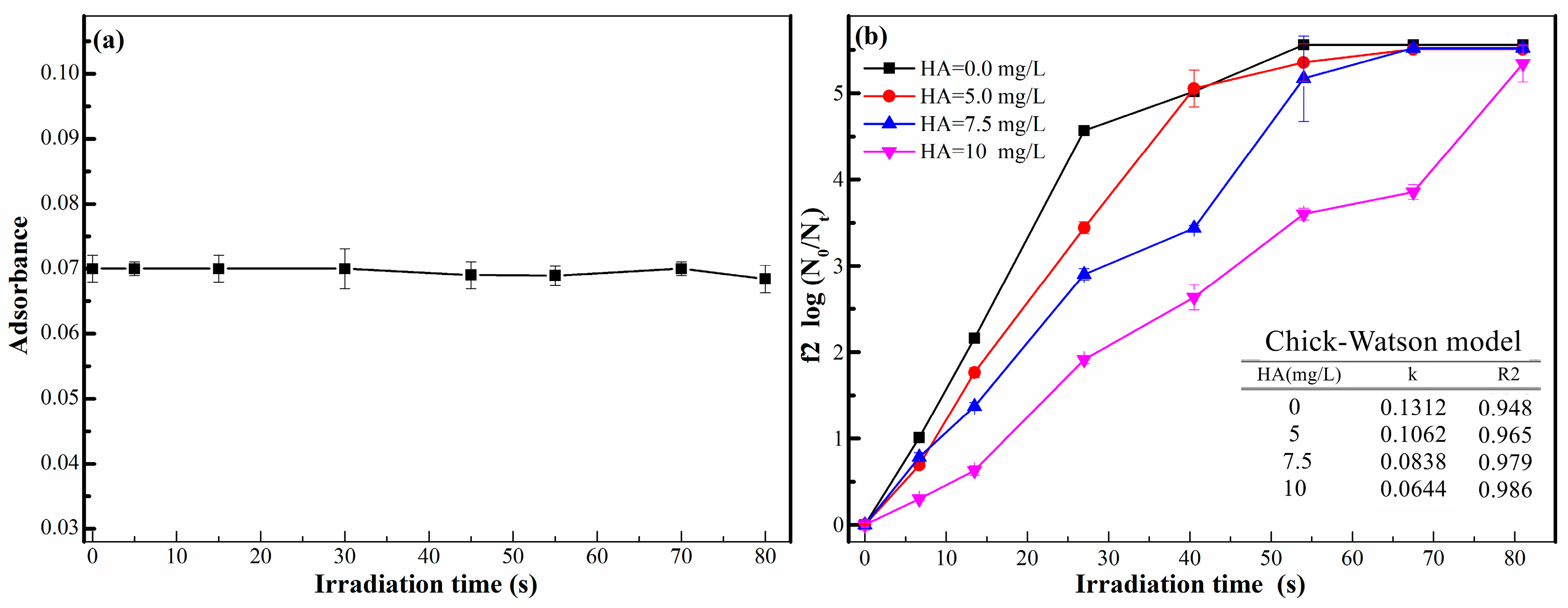
2.1.2. Effect on UV Disinfection
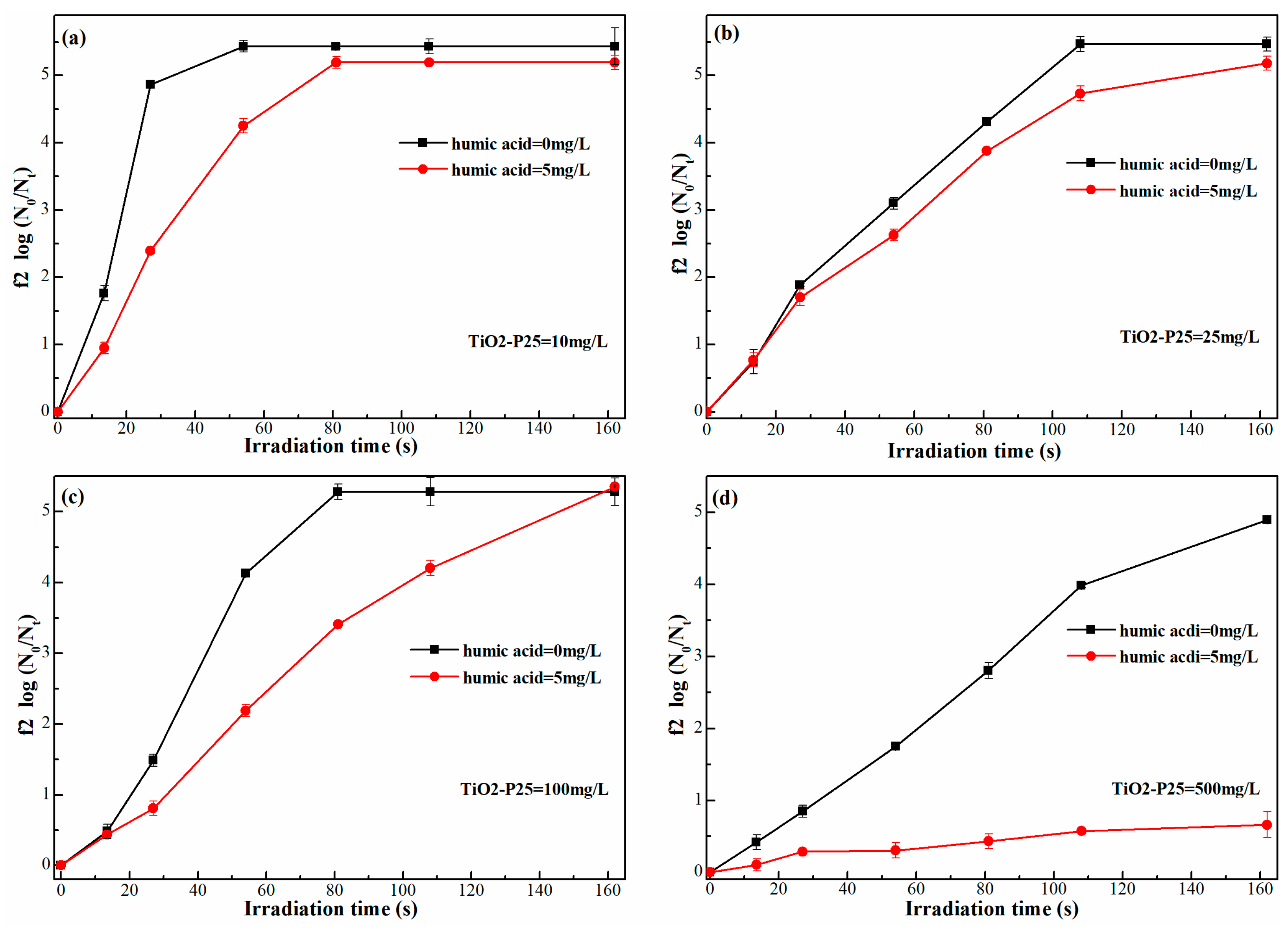
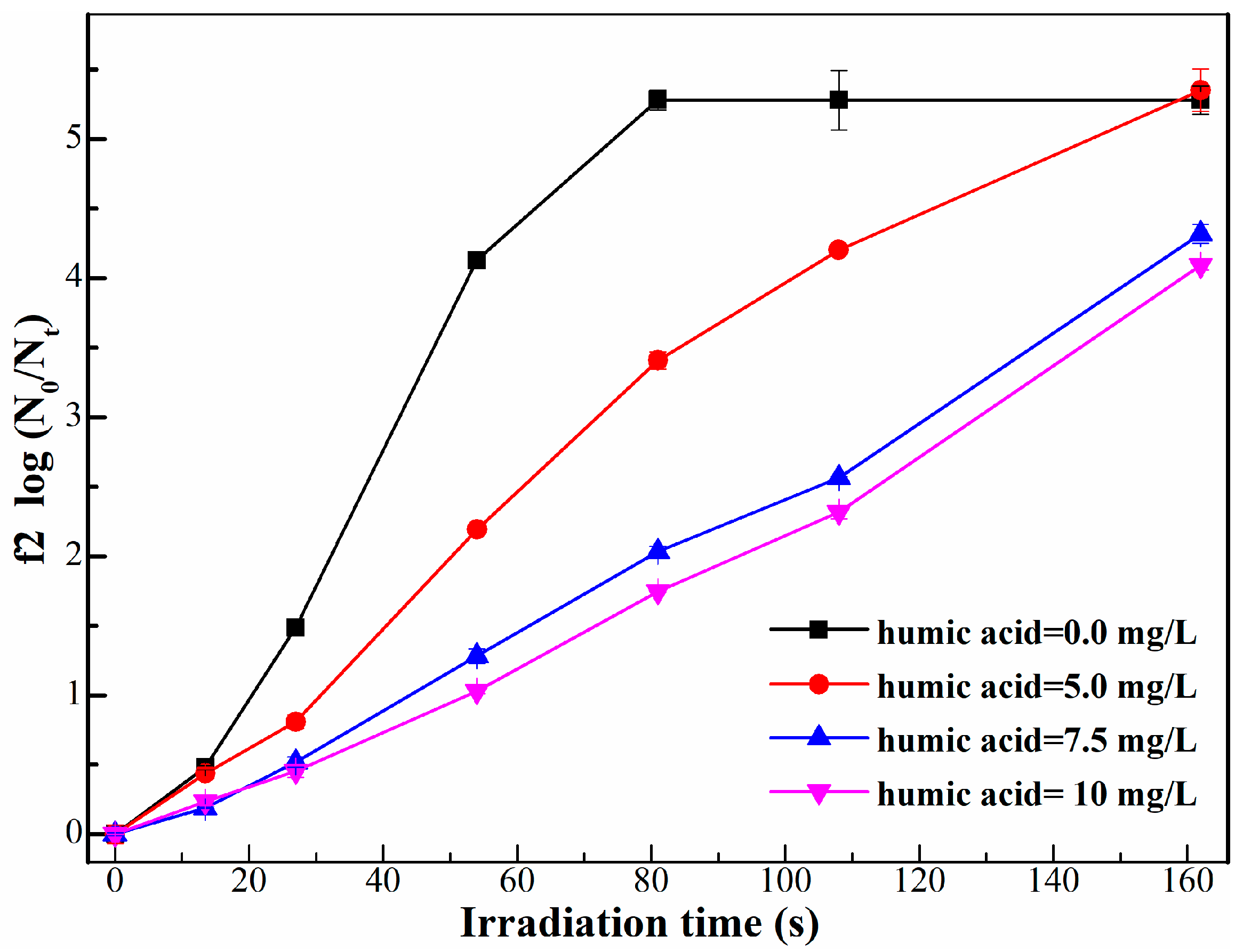
2.1.3. Effect on Photocatalysis
2.2. Influence of Humic Acid on PMR under Different Parameters
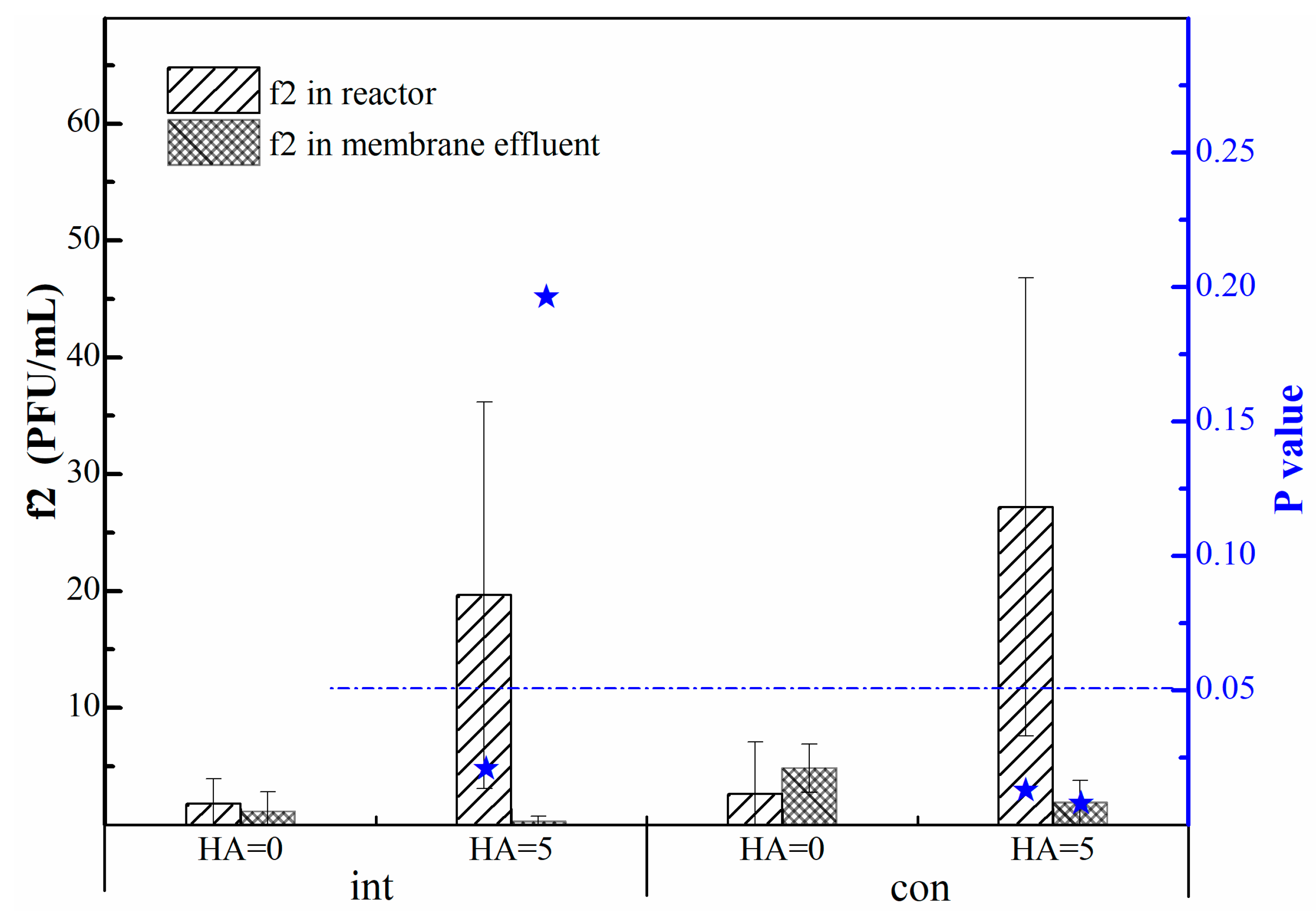
2.2.1. Operation Mode
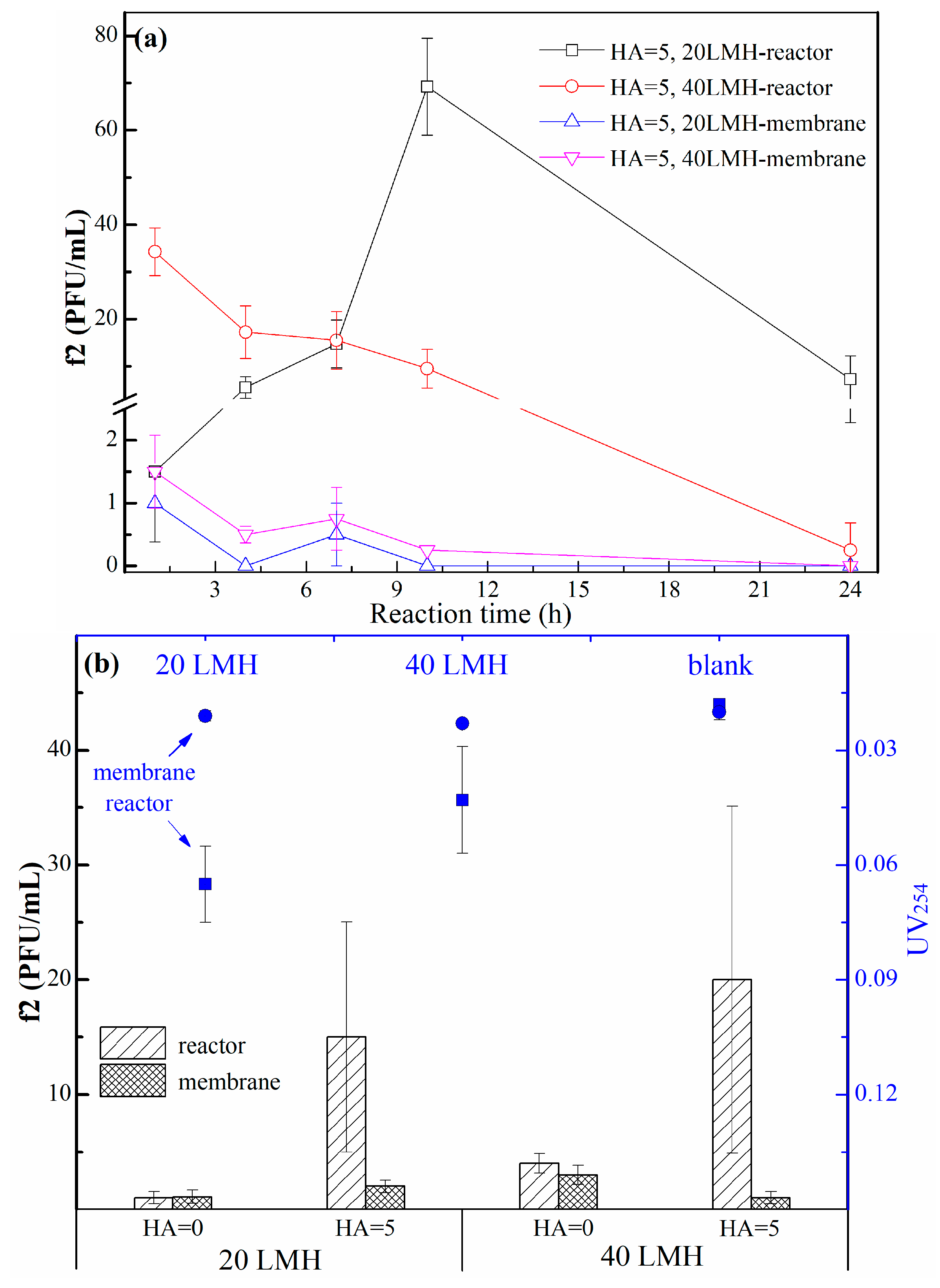
2.2.2. Membrane Flux
3. Materials and Methods
3.1. Model Virus and Chemicals
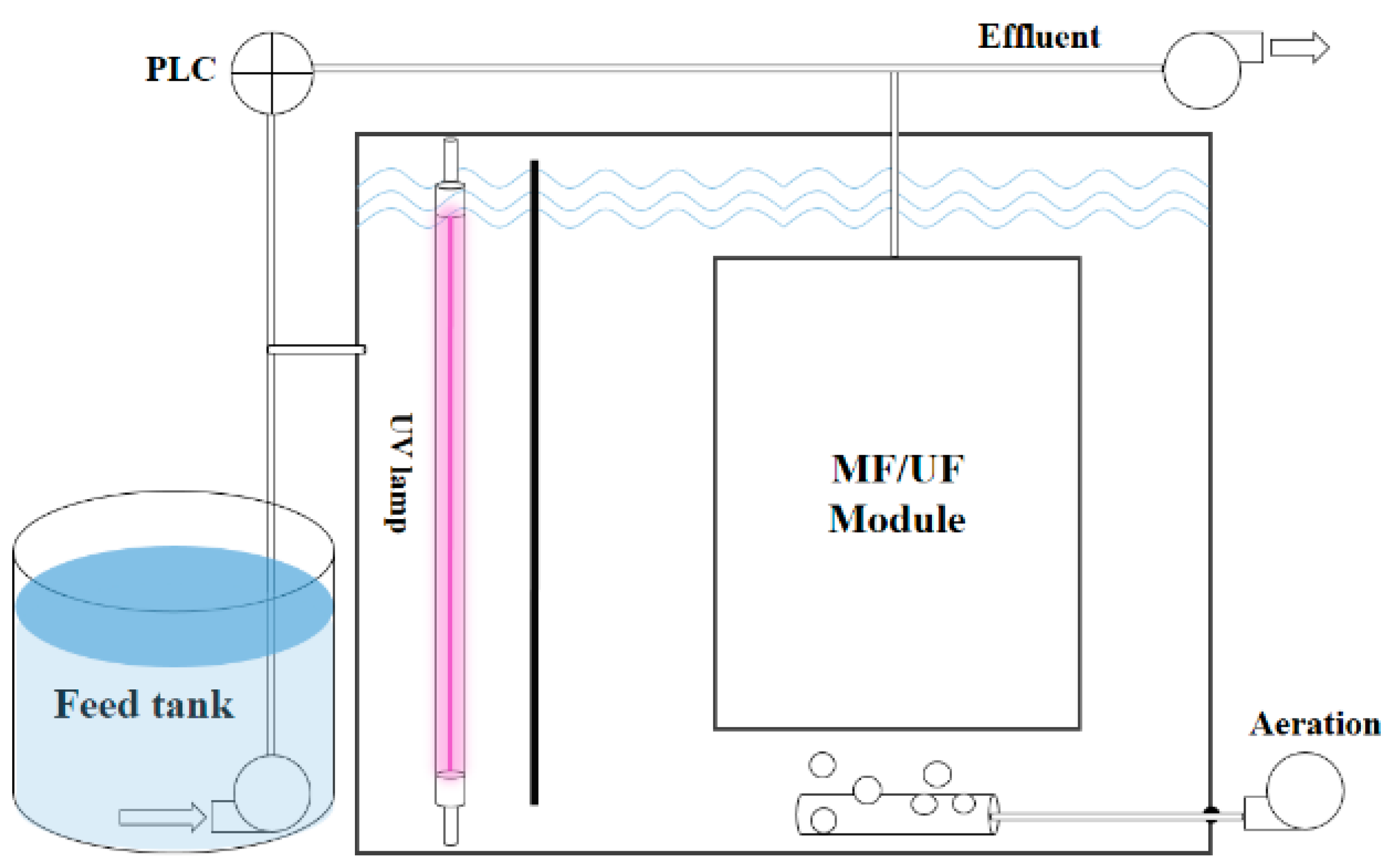
3.2. Experiment Setup
3.3. Methods of Data Analysis and Virus Culture
4. Conclusions
- (1)
- The adsorption efficiency of TiO2-P25 on phage f2 decreased in the presence of humic acid, possibly due to the competition of adsorption sites between humic acid and phage f2 in the PMR system in the dark.
- (2)
- The benzene ring structure of humic acid molecules, which could adsorb ultraviolet with the wavelength of 254 nm, reduced the effective dose of UV in the reaction system, indirectly weakening the photocatalytic disinfection efficiency of phage f2.
- (3)
- When humic acid exists in the actual water environment, in addition to the influence of humic acid on UV disinfection, the effect of humic acid on photocatalytic disinfection mainly involves two parts: the adsorption of phage f2 by TiO2-P25 is relatively blocked, which further reduces the photocatalytic disinfection efficiency of phage f2; On the other side, the hydroxyl radicals produced by TiO2-P25 in UV light are consumed by humic acid, resulting in the decrease of photocatalytic disinfection efficiency of phage f2.
- (4)
- In the intermittent operation mode, humic acid had no significant effect on the concentration of phage f2 in membrane effluent, which was less than 2 PFU/mL. While the concentration of phage f2 in the reactor was significantly affected by humic acid, which was more than 20 PFU/mL.
- (5)
- When there is a certain amount of humic acid in the water environment, intermittent operation mode or higher membrane flux (40 L/(m2·h)) was selected to partly alleviate the adverse effects of humic acid.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vital, M.; Füchslin, H.P.; Hammes, F.; Egli, T. Growth of Vibrio cholerae O1 Ogawa Eltor in freshwater. Microbiology 2007, 153, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Fisher, M.B.; Keane, D.A.; Fernández-Ibáñez, P.; Colreavy, J.; Hinder, S.J.; McGuigan, K.G.; Pillai, S.C. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B Environ. 2013, 130–131, 8–13. [Google Scholar] [CrossRef]
- Gibson, K.E. Viral pathogens in water: Occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014, 4, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Cedergren, M.I.; Selbing, A.J.; Fman, O.L.; Llen, B.K. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environ. Res. 2002, 89, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.A.; Bounty, S.; Beck, S.; Chan, C.; Mcguire, C.; Linden, K.G. Photoreactivation of bacteriophages after UV disinfection: Role of genome structure and impacts of UV source. Water Res. 2014, 55, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.Z.; Sillanpää, M. Virus Sensitivity Index of UV disinfection. Environ. Technol. 2015, 36, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Ller, M.O. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Marcó, M.B.; Quiberoni, A.; Negro, A.C.; Reinheimer, J.A.; Alfano, O.M. Evaluation of the photocatalytic inactivation efficiency of dairy bacteriophages. Chem. Eng. J. 2011, 172, 987–993. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Sordo, C.; Cruz, C. Kinetics of the photocatalytic disinfection of Escherichia coli suspensions. Appl. Catal. B Environ. 2008, 82, 27–36. [Google Scholar] [CrossRef]
- Misstear, D.B.; Gill, L.W. The inactivation of phages MS2, ΦX174 and PR772 using UV and solar photocatalysis. J. Photochem. Photobiol. B Biol. 2012, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, J.C.; Sierka, R.A. Inactivation of Phage MS2 by Iron-Aided Titanium Dioxide Photocatalysis. Appl. Environ. Microbiol. 1994, 60, 344–347. [Google Scholar] [PubMed]
- Moslehyani, A.; Ismail, A.F.; Othman, M.H.D.; Matsuura, T. Hydrocarbon degradation and separation of bilge water via a novel TiO2-HNTs/PVDF-based photocatalytic membrane reactor (PMR). RSC Adv. 2015, 5, 14147–14155. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Q.; Chen, L.; Wang, J.; Cheng, R. Photocatalytic membrane reactor (PMR) for virus removal in water: Performance and mechanisms. Chem. Eng. J. 2015, 277, 124–129. [Google Scholar] [CrossRef]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Navío, J.A.; Fernández-Hevia, D.; Doña-Rodríguez, J.M. Enhancement of stability and photoactivity of TiO2 coatings on annular glass reactors to remove emerging pollutants from waters. Chem. Eng. J. 2015, 279, 488–497. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Zhang, Y.; Zhao, Y.; Quan, X. Effects of Cu (II) and humic acid on atrazine photodegradation. J. Environ. Sci. 2011, 23, 773–777. [Google Scholar] [CrossRef]
- Le-Clech, P.; Lee, E.K.; Chen, V. Hybrid photocatalysis/membrane treatment for surface waters containing low concentrations of natural organic matters. Water Res. 2006, 40, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lei, Y.; Chen, D.; Cheng, R. Adsorption characteristics of f2 bacteriophage onto nanometer titanium dioxide. Sci. Sin. Chim. 2013, 43, 610–617. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Talley, J.W.; Neal, C.R.; Wang, H. Effect of NOM on arsenic adsorption by TiO2 in simulated As (III)-contaminated raw waters. Water Res. 2008, 42, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Lunell, S. Binding of bi-isonicotinic acid to anatase TiO2 (101). Sol. Energy Mater. Sol. Cells 2000, 63, 139–148. [Google Scholar] [CrossRef]
- Wei, D.; Li, S.; Fang, L.; Zhang, Y. Effect of environmental factors on enhanced adsorption and photocatalytic regeneration of molecular imprinted TiO2 polymers for fluoroquinolones. Environ. Sci. Pollut. Res. 2018, 25, 6729–6738. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.E. A Note on the Variation of the Rate of Disinfection with Change in the Concentration of the Disinfectant. Epidemiol. Infect. 1908, 8, 536–542. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2–ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Birben, N.C.; Uyguner-Demirel, C.S.; Kavurmaci, S.S.; Gürkan, Y.Y.; Turkten, N.; Cinar, Z.; Bekbolet, M. Application of Fe-doped TiO2 specimens for the solar photocatalytic degradation of humic acid. Catal. Today 2017, 281, 78–84. [Google Scholar] [CrossRef]
- Jayalath, S.; Wu, H.; Larsen, S.C.; Grassian, V.H. Surface adsorption of Suwannee river humic acid on TiO2 Nanoparticles: A Study of pH and Particle Size. Langmuir 2018, 34, 3136–3145. [Google Scholar] [CrossRef] [PubMed]
- Schijven, J.F.; Hassanizadeh, S.M. Removal of Viruses by Soil Passage: Overview of Modeling, Processes, and Parameters. Crit. Rev. Environ. Sci. Technol. 2000, 30, 49–127. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Intercience Publishers Inc.: New York, NY, USA, 1959; pp. 443–451. [Google Scholar]
- Chick, H. An Investigation of the Laws of Disinfection. J. Hyg. 1908, 8, 92–158. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Shen, L.; Wang, Q.; Xiang, S.; Shi, L.; Zheng, X.; Lv, W. Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid. Catalysts 2018, 8, 284. https://doi.org/10.3390/catal8070284
Cheng R, Shen L, Wang Q, Xiang S, Shi L, Zheng X, Lv W. Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid. Catalysts. 2018; 8(7):284. https://doi.org/10.3390/catal8070284
Chicago/Turabian StyleCheng, Rong, Liangjie Shen, Qi Wang, Shaoyu Xiang, Lei Shi, Xiang Zheng, and Wenzhou Lv. 2018. "Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid" Catalysts 8, no. 7: 284. https://doi.org/10.3390/catal8070284
APA StyleCheng, R., Shen, L., Wang, Q., Xiang, S., Shi, L., Zheng, X., & Lv, W. (2018). Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid. Catalysts, 8(7), 284. https://doi.org/10.3390/catal8070284