DBD Plasma-ZrO2 Catalytic Decomposition of CO2 at Low Temperatures
Abstract
1. Introduction
2. Results and Discussion
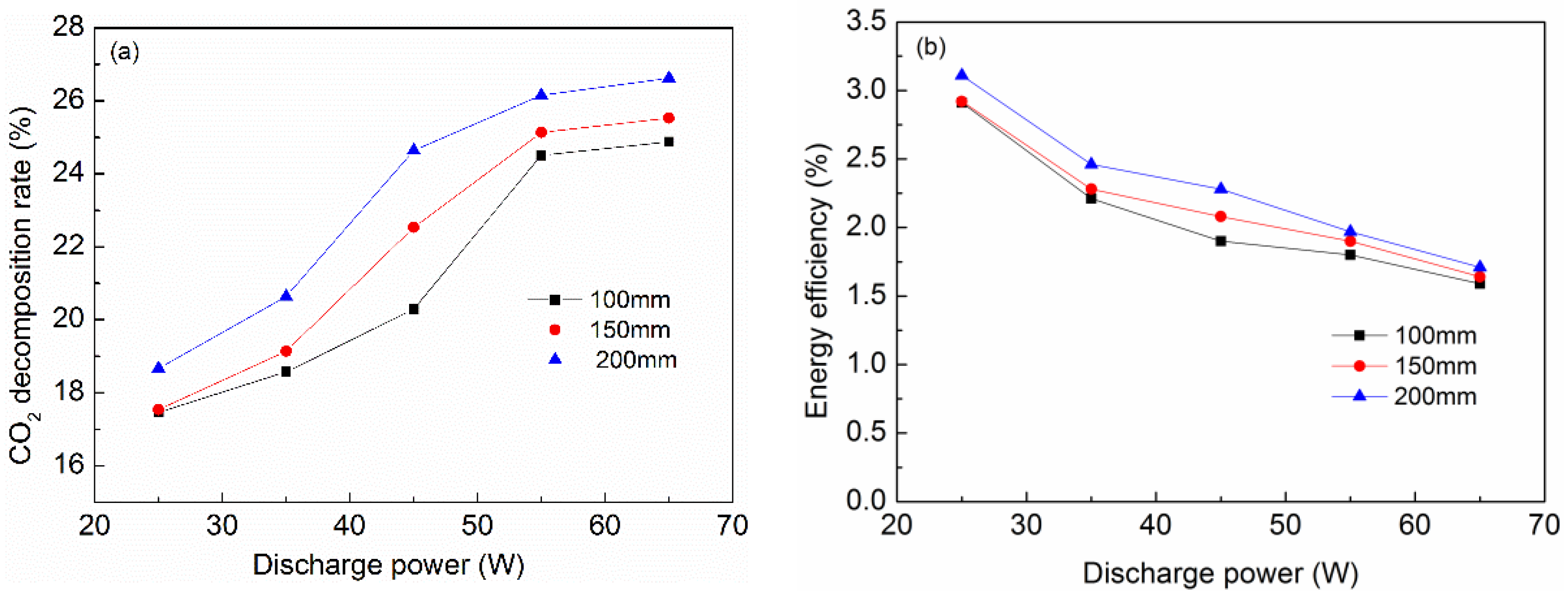
2.1. Effect of Discharge Length on CO2 Decomposition Rate and Energy Efficiency
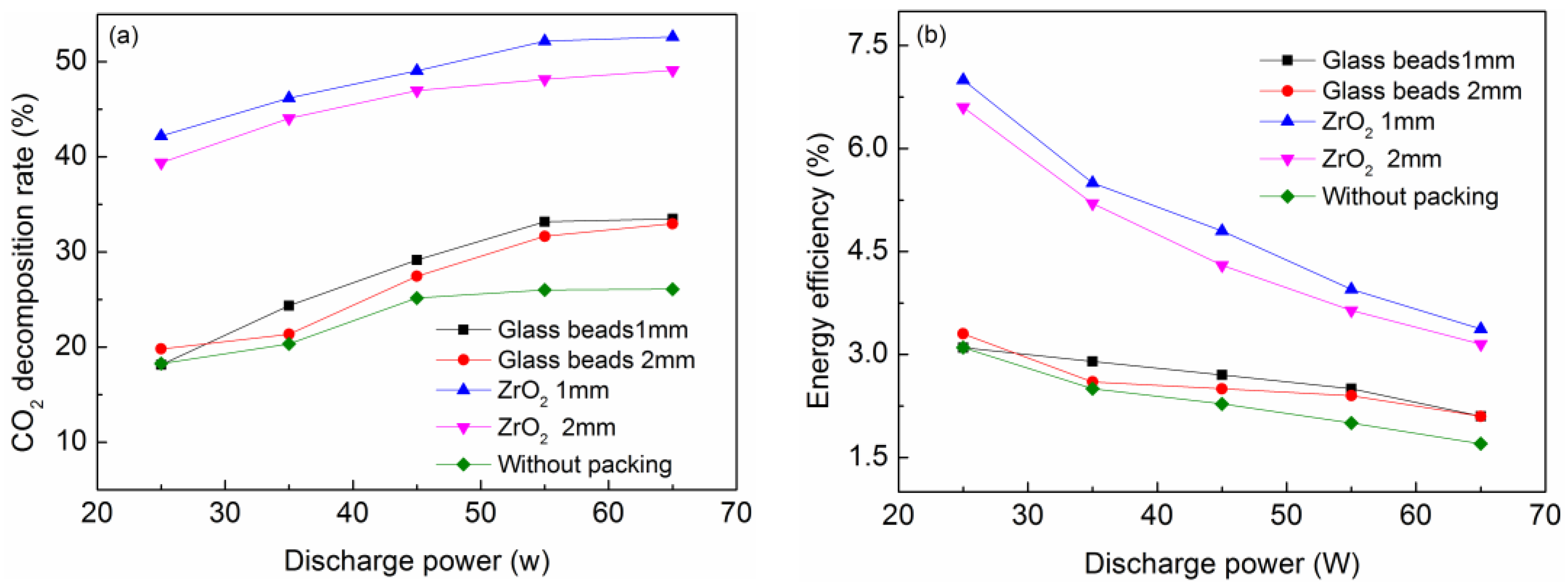
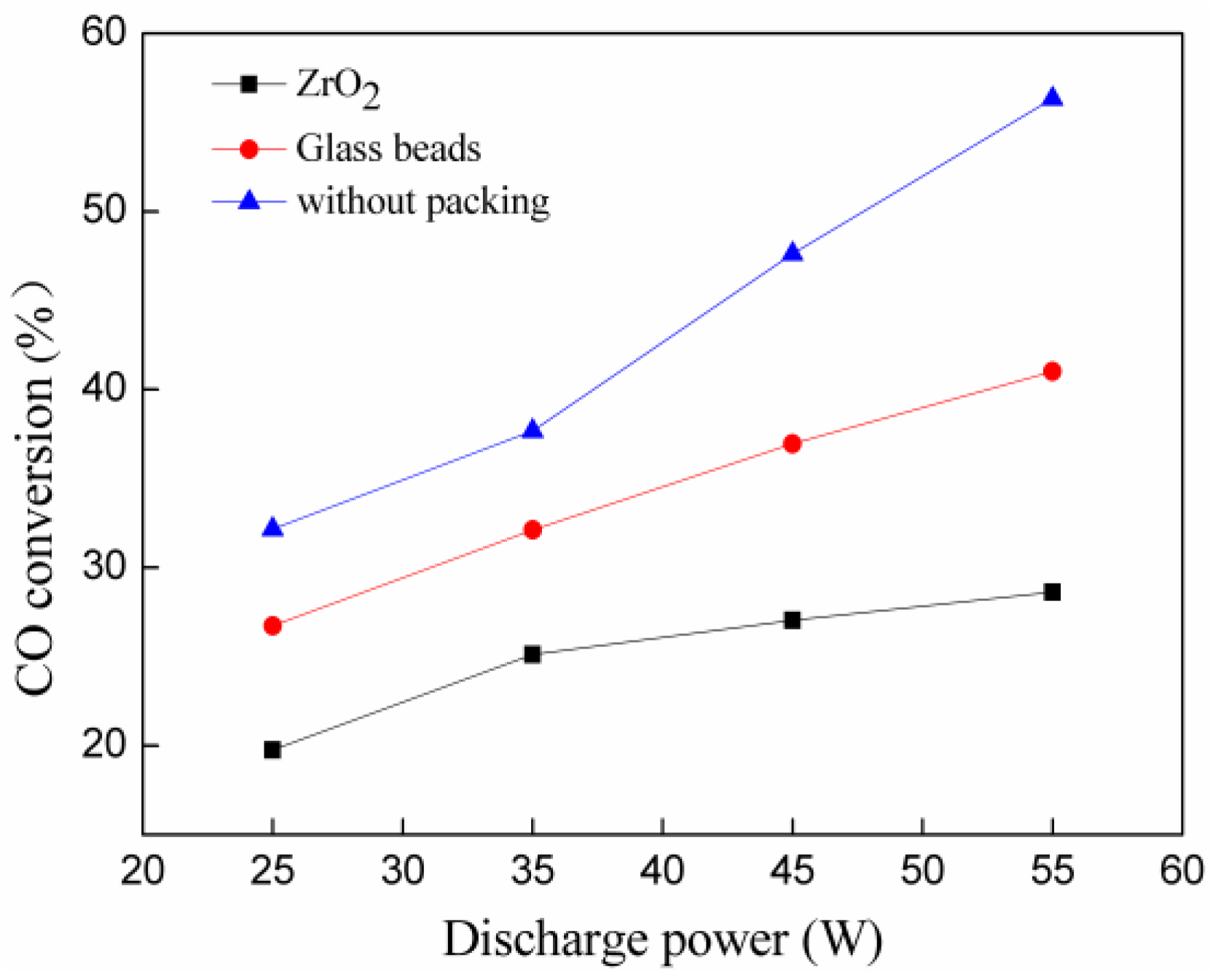
2.2. Effect of Discharge Power and Beads Size on CO2 Decomposition and Energy Efficiency
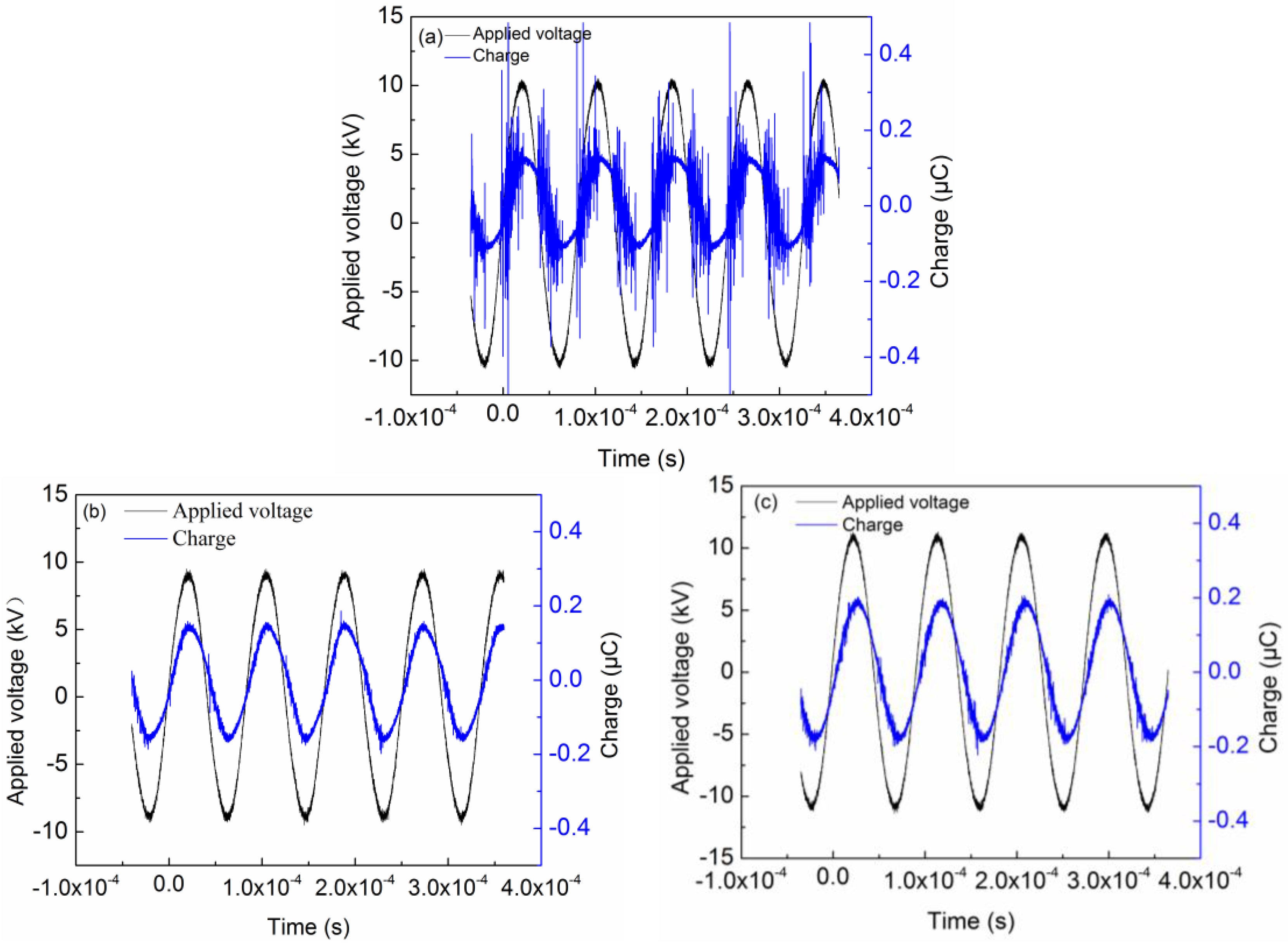
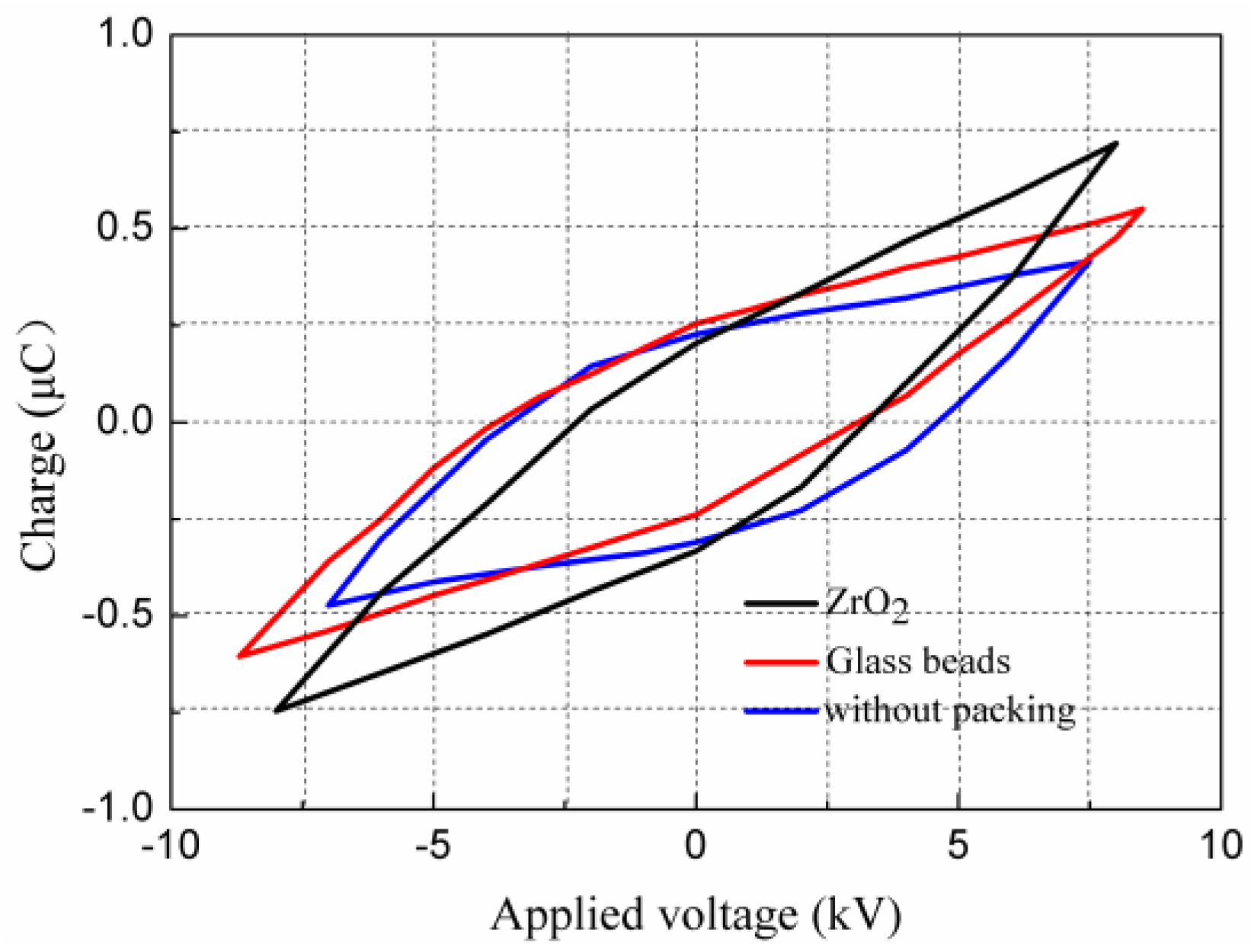
2.3. Effect of Packing Materials on Discharge Characteristics
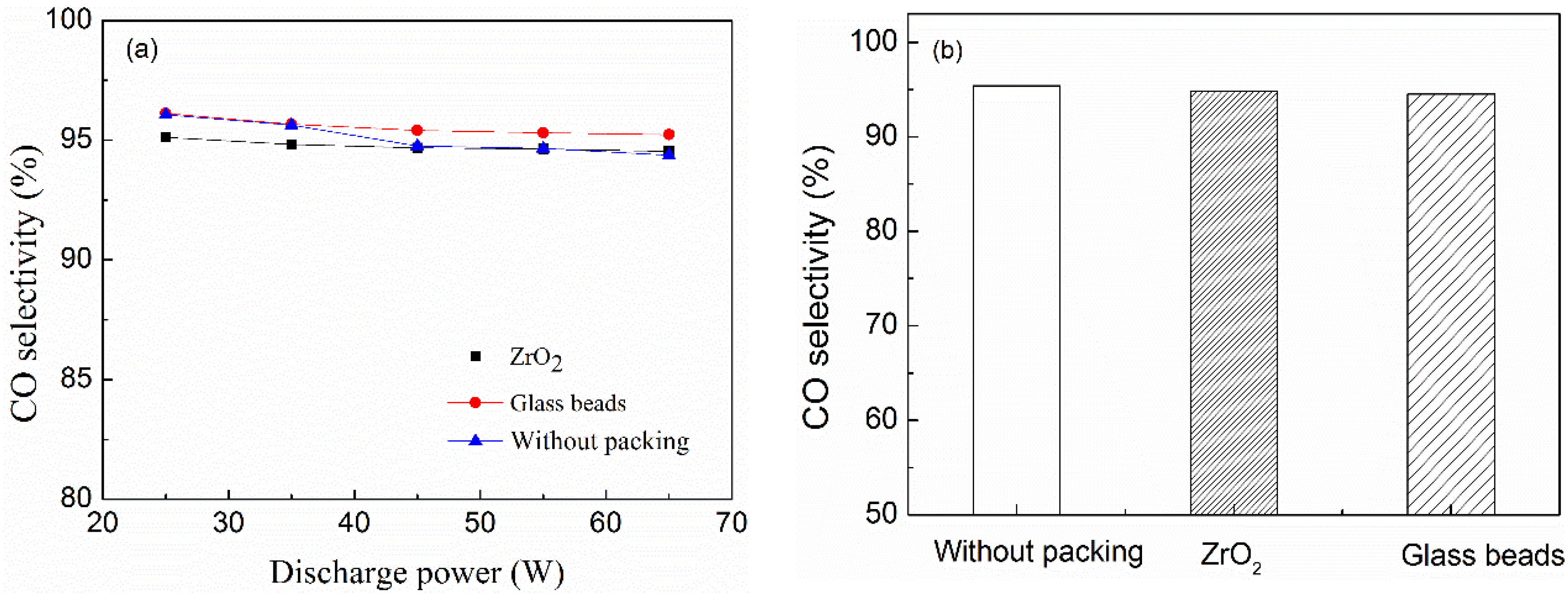
2.4. Effect of Packing Materials on CO Selectivity
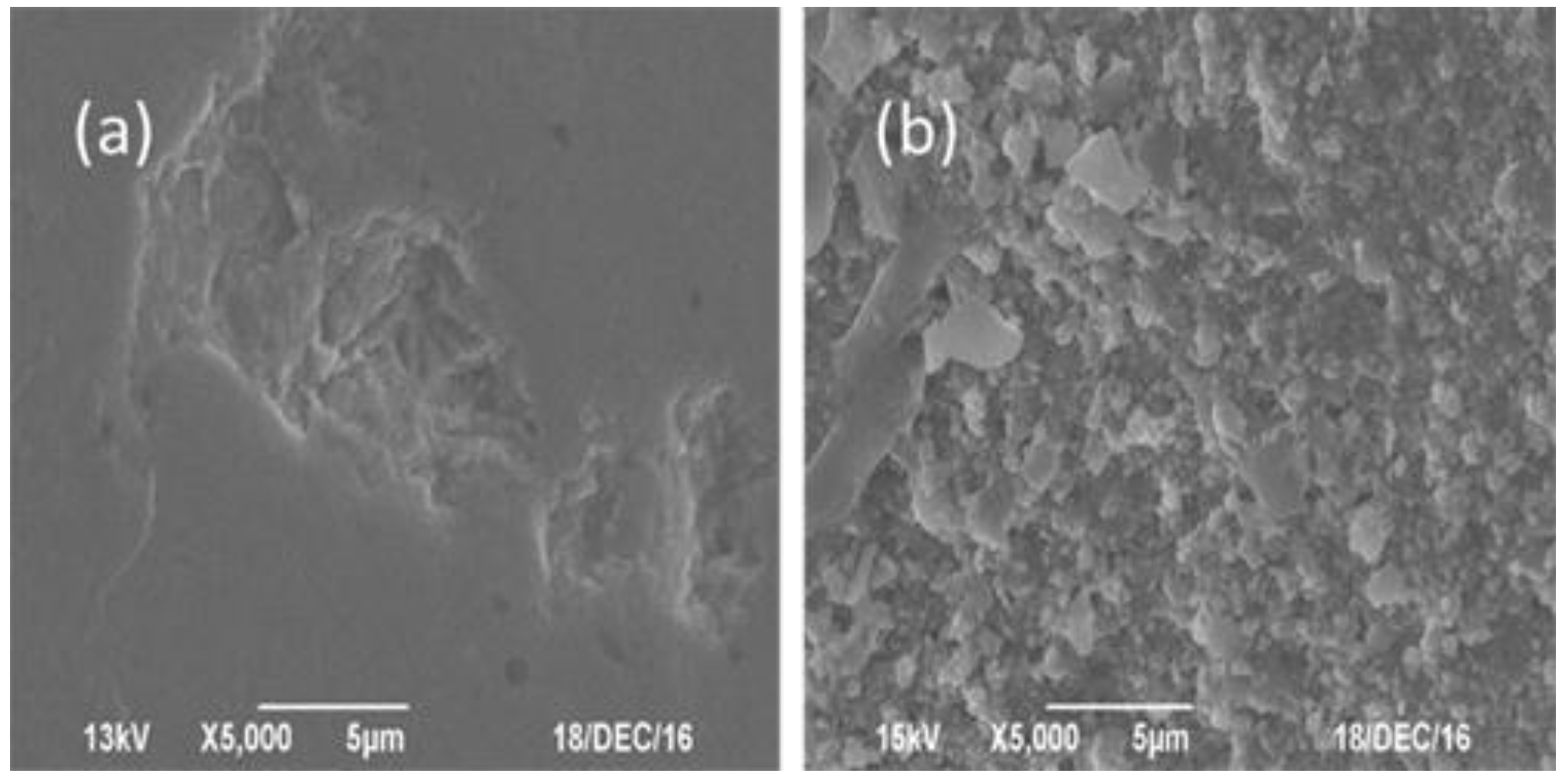
2.5. The Investigation of Carbon Deposition
2.6. Reaction of CO and O2
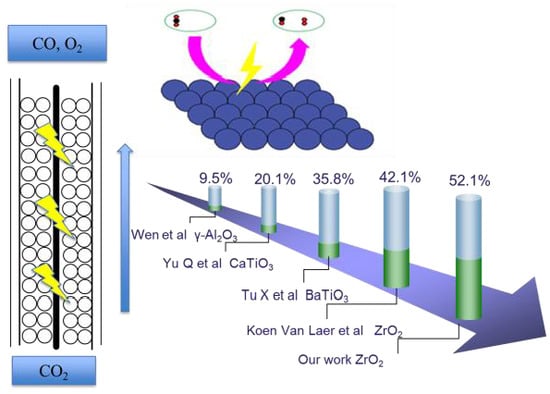
2.7. Comparison of Obtained Values in Different Packed DBD Reactors
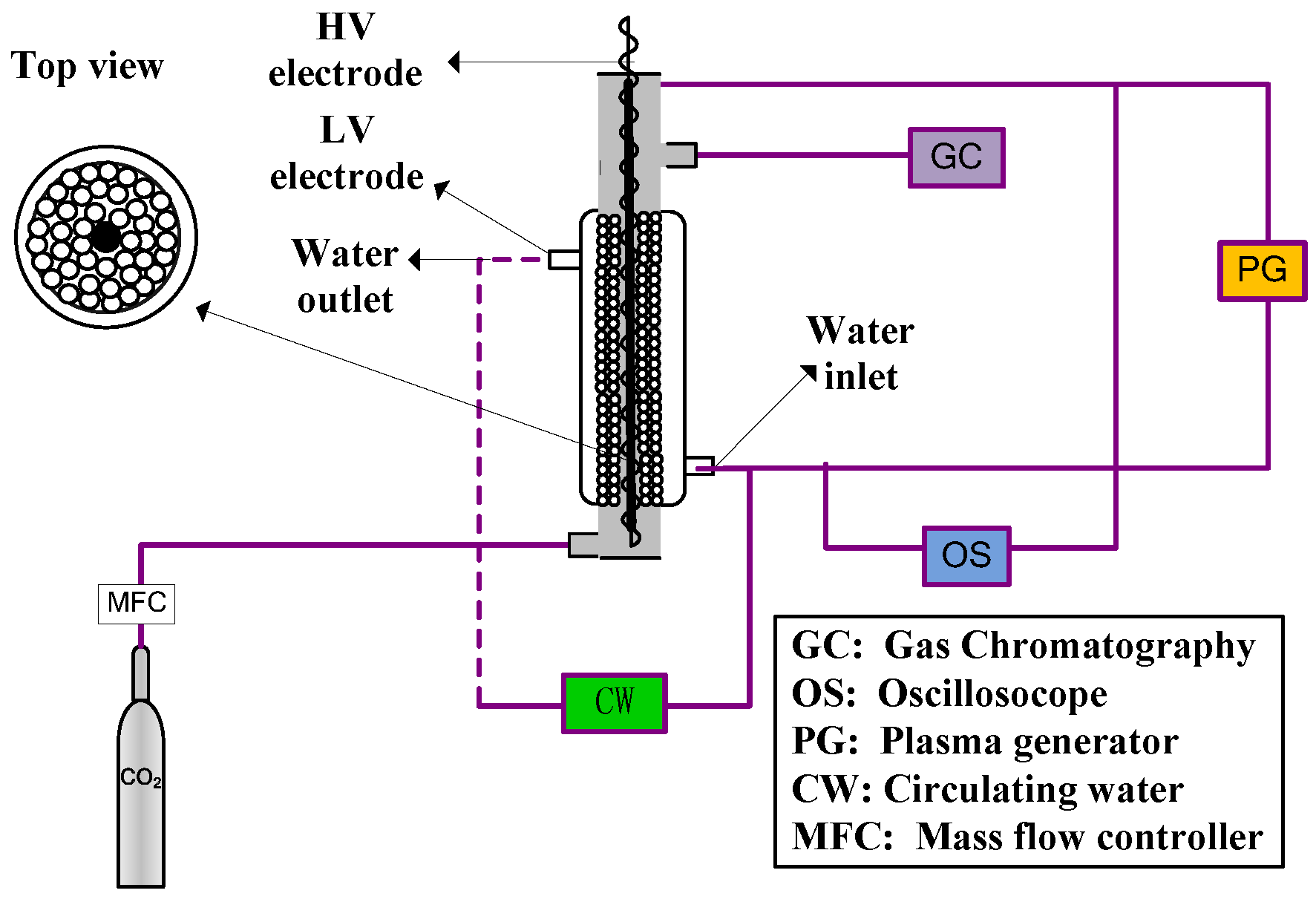
3. Experimental Setup
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Razali, N.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Heterogeneous catalysts for production of chemicals using carbon dioxide as raw material: A review. Renew. Sustain. Energy Rev. 2012, 16, 4951–4964. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Lebouvier, A.; Iwarere, S.A.; Argenlieu, P.D.; Ramjugernath, D.; Fulcheri, L. Assessment of carbon dioxide dissociation as a new route for syngas production: A Comparative review and potential of plasma-Based technologies. Energy Fuels 2013, 27, 2712–2722. [Google Scholar] [CrossRef]
- Ayzner, A.L.; Wanger, D.D.; Tassone, C.J.; Tolbert, S.H.; Schwartz, B.J. Room to improve conjugated polymer-based solar cells: Understanding how thermal annealing affects the fullerene component of a bulk heterojunction photovoltaic device. J. Phys. Chem. C 2008, 112, 18711–18716. [Google Scholar] [CrossRef]
- Kim, S.S.; Sang, M.L.; Hong, S.C. A study on the reaction characteristics of CO2 decomposition using iron oxides. J. Ind. Eng. Chem. 2012, 18, 860–864. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sour. Sci. Technol. 2015, 24, 015011. [Google Scholar] [CrossRef]
- Aerts, R.; Tu, X.; De Bie, C.; Whitehead, J.C.; Bogaerts, A. An Investigation into the dominant reactions for ethylene destruction in non-thermal atmospheric plasmas. Plasma Process. Polym. 2012, 9, 994–1000. [Google Scholar] [CrossRef]
- Harling, A.M.; Glover, D.J.; Whitehead, J.C.; Zhang, K. Novel method for enhancing the destruction of environmental pollutants by the combination of multiple plasma discharges. Environ. Sci. Technol. 2008, 42, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mei, D.; Shen, Z.; Tu, X. Nono-xidative conversion of methane in a dielectric barrier discharge reactor: Prediction of reaction performance based on neural model. J. Phys. Chem. C 2014, 118, 10686–10693. [Google Scholar] [CrossRef]
- Snoeckx, R.; Aerts, R.; Tu, X.; Bogaerts, A. Plasma-based dry reforming: A computational study ranging from nanoseconds to seconds timescale. J. Phys. Chem. A 2013, 117, 4857–4970. [Google Scholar] [CrossRef]
- Xin, T.; Whitehead, J.C. Plasma dry reforming of methane in an atmospheric pressure AC gliding arc discharge: Co-generation of Syngas and carbon nanomaterials. Int. J. Hydrog. Energy 2014, 39, 9658–9669. [Google Scholar]
- Yu, L.; Tu, X.; Li, X.; Wang, Y.; Chi, Y.; Yan, J. Destruction of acenaphthene, fluorene, anthracene and pyrene by a dc gliding arc plasma reactor. J. Hazard. Mater. 2010, 180, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Gallon, H.J.; Twigg, M.V.; Gorry, P.A.; Whitehead, J.C. Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2011, 44, 274007. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]
- Wang, B.; Yan, W.; Ge, W.; Duan, X. kinetic model of the methane conversion into higher hydrocarbons with a dielectric barrier discharge microplasma reactor. Chem. Eng. J. 2013, 234, 354–360. [Google Scholar] [CrossRef]
- Horvath, G.; Skalny, J.D.; Mason, N.J. FTIR study of decomposition of carbon doxide in DC corona discharges. J. Phys. D Appl. Phys. 2008, 41, 207–225. [Google Scholar] [CrossRef]
- Mikoviny, T.; Kocan, M.; Matejcik, S.; Mason, N.J.; Skalny, J.D. Experimental study of negative corona discharge in pure carbon dioxide and its mixtures with oxygen. J. Phys. D Appl. Phys. 2003, 37, 64–73. [Google Scholar] [CrossRef]
- Wen, Y.; Jiang, X. Decomposition of CO2 Using Pulsed Corona Discharges Combined with Catalyst. Plasma Chem. Plasma Process. 2001, 21, 665–678. [Google Scholar] [CrossRef]
- Kozak, T.; Bogaerts, A. Splitting of CO2 by vibrational excitation in non-equilibrium plasmas: A reaction kinetics model. Plasma Sour. Sci. Technol. 2014, 23, 045004. [Google Scholar] [CrossRef]
- Nunnally, T.; Gustol, K.; Rabinovich, A.; Fridman, A.; Gutsol, A.; Kemoun, A. Dissociation of CO2 conversion in a low current gliding arc plasmatron. J. Phys. D Appl. Phys. 2011, 44, 274009. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, F.; Zhou, A.; Ma, C.; Dai, B. High-efficiency removal of NOx using dielectric barrier discharge nonthermal plasma with water as an outer electrode. Plasma Sci. Technol. 2018, 20, 014020. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Zhu, M.; Tian, Z.; Dan, J.; Li, J.; Wang, Q. Ultralow-weight loading Ni catalyst supported on two-dimensional vermiculite for carbon monoxide methanation. Chin. J. Chem. Eng. 2017. [CrossRef]
- Wang, Y.; Yu, F.; Zhu, M.; Ma, C.; Zhao, D.; Wang, C.; Zhou, A.; Guo, X. N-doping of plasma exfoliated graphene oxide via dielectric barrier discharge plasma treatment for oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2011–2017. [Google Scholar] [CrossRef]
- Indarto, A.; Yang, D.R.; Choi, J.W.; Lee, H.; Song, H.K. Gliding arc plasma processing of CO2 conversion. J. Hazard. Mater. 2007, 146, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Okazaki, K. Non-thermal plasma plasma catalysis of methane: Principles, energy efficiency, and applications. Catal. Today 2013, 211, 29–38. [Google Scholar] [CrossRef]
- Ozkan, A.; Dufour, T.; Arnoult, G.; Keyzer, P.D.; Bogaerts, A.; Reniers, F. CO2-CH4 conversion and syngas formation at atmospheric pressure using a multi-electrode dielectric barrier discharge. J. CO2 Util. 2015, 9, 74–81. [Google Scholar] [CrossRef]
- Yu, Q.; Kong, M.; Liu, T.; Fei, J.; Zheng, X. Characterstics of the Decomposition of CO2 in a Dielectric Packed-Bed Plasma Reactor. Plasma Chem. Plasma Process. 2012, 32, 153–163. [Google Scholar] [CrossRef]
- Li, R.; Tang, Q.; Shu, Y.; Sato, T. Investigation of dielectric barrier discharge dependence on permittivity of barrier materials. Appl. Phys. Lett. 2007, 90, 131502. [Google Scholar] [CrossRef]
- Li, R.; Tang, Q.; Yin, S.; Yamaguchi, Y.; Sato, T. Decomposition of carbon dioxide by the dielectric barrier discharge (DBD) Plasma Using Ca0.7Sr0.3TiO3 barrier. Chem. Lett. 2004, 33, 412–413. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Liu, X.; Wang, X. Enhancement of CO2 conversion rate and conversion efficiency by homogeneous discharges. Plasma Chem. Plasma Process. 2012, 32, 979–989. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouet, J.M.; Batiot-Dupeyrat, C. Carbon dioxide dissociation to carbon monoxide by non-thermal plasma. J CO2 Util. 2015, 12, 54–61. [Google Scholar] [CrossRef]
- Ray, D.; Subrahmanyam, C. CO2 decomposition in a packed dbd plasma reactor: Influence of packing materials. RSC Adv. 2016, 6, 39492–39499. [Google Scholar] [CrossRef]
- Duan, X.; Hu, Z.; Li, Y.; Wang, B. Effect of dielectric packing materials on the decomposition of carbon dioxide using dbd microplasma reactor. AIChE J. 2015, 61, 898–903. [Google Scholar] [CrossRef]
- Mei, D.; He, Y.; Liu, S.; Yan, J.; Tu, X. Optimization of CO2 conversion in a cylindrical dielectric barrier discharge reactor using design of experiments. Plasma Process. Polym. 2016, 13, 544–556. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; Wu, C.; Ashford, B.; Williams, P.T.; Tu, X. Plasma-photocatalytic conversion of CO2 at low temperatures: Understanding the synergistic effect of plasma-catalysis. Appl. Catal. B Environ. 2016, 182, 525–532. [Google Scholar] [CrossRef]
- Mei, D.; Tu, X. Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design. J. CO2 Util. 2017, 19, 68–78. [Google Scholar] [CrossRef]
- Zhou, A.; Chen, D.; Dai, B.; Ma, C.; Li, P.; Yu, F. Direct decomposition of CO2 using self-cooling dielectric barrier discharge plasma. Greenh. Gases Sci. Technol. 2017, 7, 721–730. [Google Scholar] [CrossRef]
- Van Laer, K.; Bogaerts, A. Improving the conversion and energy efficiency of carbon dioxide splitting in a zirconia-packed dielectric barrier discharge reactor. Energy Technol. 2015, 3, 1038–1044. [Google Scholar] [CrossRef]
- Ozkan, A.; Dufour, T.; Silva, T.; Britun, N.; Snyders, R.; Bogaerts, A.; Reniers, F. The influence of power and frequency on the filamentary behavior of a flowing DBD-application to the splitting of CO2. Plasma Sour. Sci. Technol. 2016, 25, 025013. [Google Scholar] [CrossRef]
- Dou, B.; Feng, B.; Wang, C.; Jia, Q.; Li, J. Discharge characteristics and abatement of volatile organic compounds using plasma reactor packed with ceramic Raschig rings. J. Electrost. 2013, 71, 939–944. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Z.; Tong, J.; Yang, M.; Jiang, Z.; Li, C. Direct thermolysis of CO2 into CO and O2. Chem. Commun. 2017, 53, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Liu, X.; Phan, A.N.; Luo, K. A study on CO2 decomposition to CO and O2 by the combination of catalysis and dielectric barrier discharges at low temperatures and ambient pressure. Ind. Eng. Chem. Res. 2017, 56, 3204–3216. [Google Scholar] [CrossRef]
- Ozkan, A.; Bogaerts, A.; Reniers, F. Routes to increase the conversion and the energy efficiency in the splitting of CO2 by a dielectric barrier discharge. J. Phys. D Appl. Phys. 2017, 50, 084004. [Google Scholar] [CrossRef]
| Packing Materials | ZrO2 | ZrO2 | BaTiO3 | BaTiO3 | CaTiO3 |
|---|---|---|---|---|---|
| Reactor | Quartz | Glass | Quartz | Quartz | Quartz |
| Decomposition rate (%) | 42 | 52.1 | 28 | 38.3 | 20.5 |
| Energy efficiency (%) | 9.6 | 7.0 | 7.1 | 17 | 4.8 |
| CO selectivity (%) | 50 | 94–96 | — | — | — |
| Power (w) | 60 | 55 | 50 | — | 35.3 |
| Reference | 38 | This work | 6 | 35 | 27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Chen, D.; Ma, C.; Yu, F.; Dai, B. DBD Plasma-ZrO2 Catalytic Decomposition of CO2 at Low Temperatures. Catalysts 2018, 8, 256. https://doi.org/10.3390/catal8070256
Zhou A, Chen D, Ma C, Yu F, Dai B. DBD Plasma-ZrO2 Catalytic Decomposition of CO2 at Low Temperatures. Catalysts. 2018; 8(7):256. https://doi.org/10.3390/catal8070256
Chicago/Turabian StyleZhou, Amin, Dong Chen, Cunhua Ma, Feng Yu, and Bin Dai. 2018. "DBD Plasma-ZrO2 Catalytic Decomposition of CO2 at Low Temperatures" Catalysts 8, no. 7: 256. https://doi.org/10.3390/catal8070256
APA StyleZhou, A., Chen, D., Ma, C., Yu, F., & Dai, B. (2018). DBD Plasma-ZrO2 Catalytic Decomposition of CO2 at Low Temperatures. Catalysts, 8(7), 256. https://doi.org/10.3390/catal8070256