Development of Two Novel Processes for Hydrogenation of CO2 to Methanol over Cu/ZnO/Al2O3 Catalyst to Improve the Performance of Conventional Dual Type Methanol Synthesis Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reaction Scheme and Kinetics
2.2. Process Description
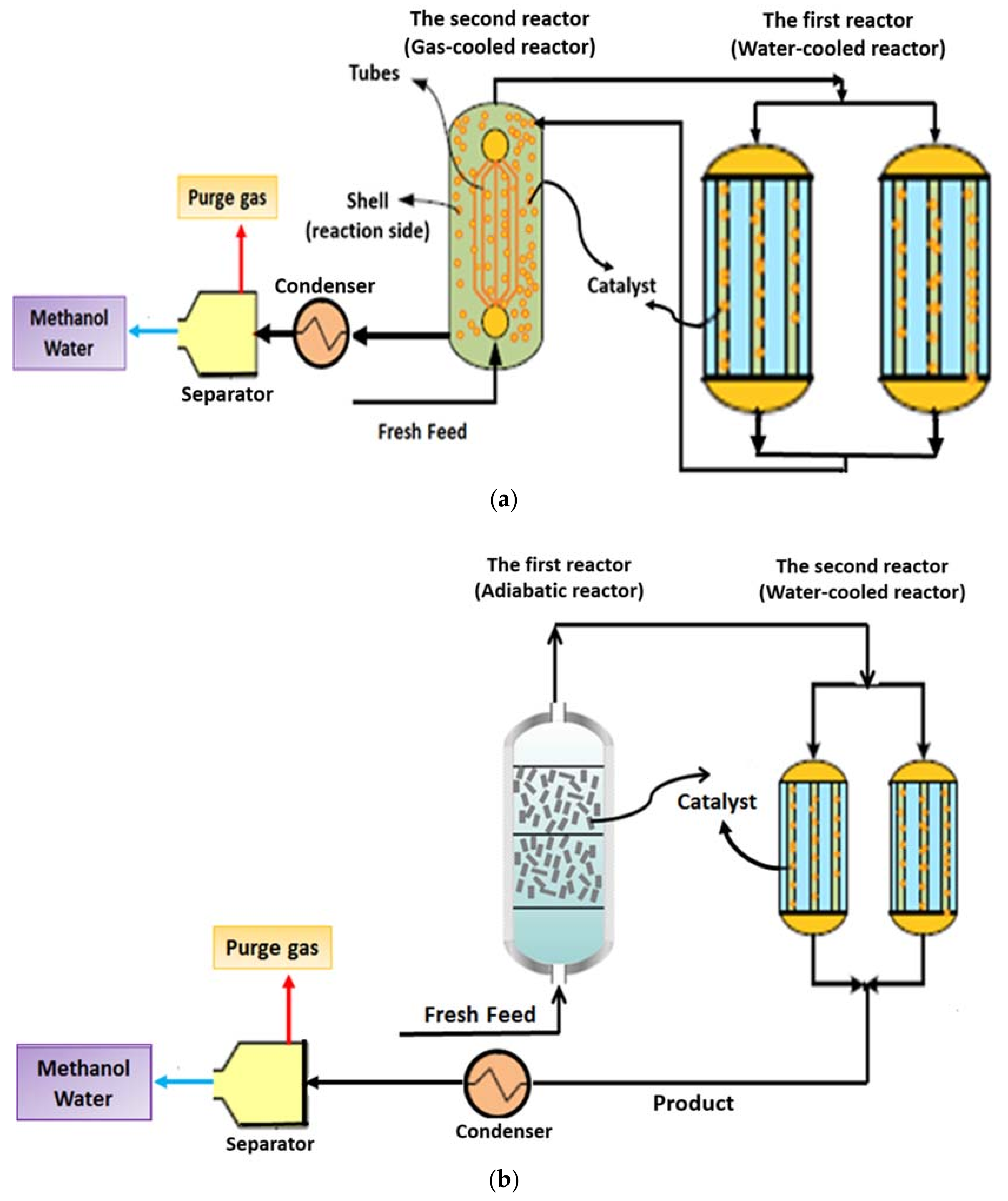
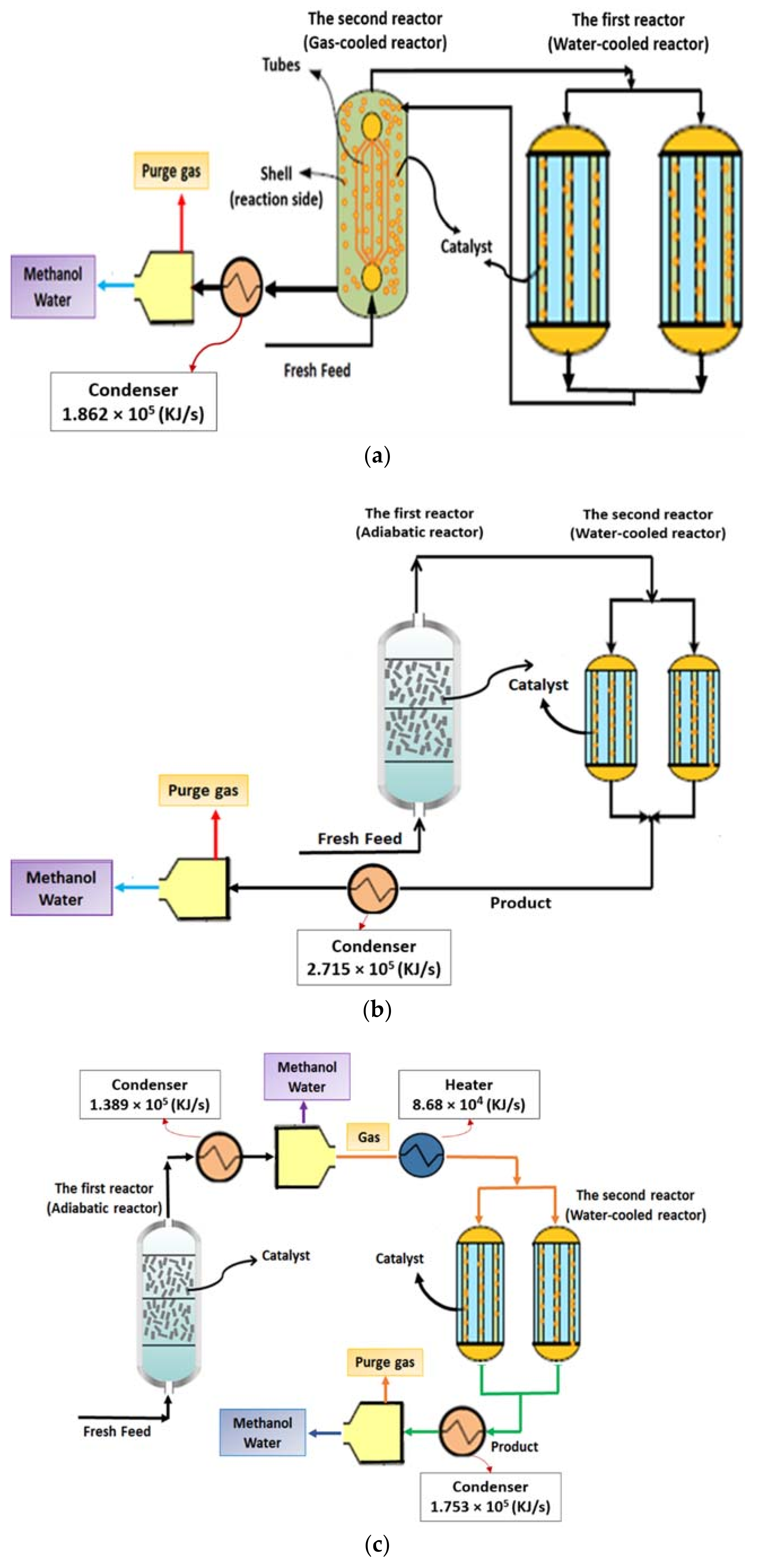
2.2.1. Conventional Methanol Synthesis Process with Two Reactors (CR Configuration)
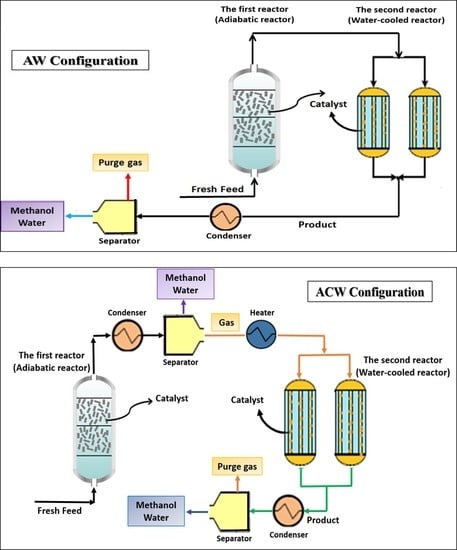
2.2.2. AW Configuration
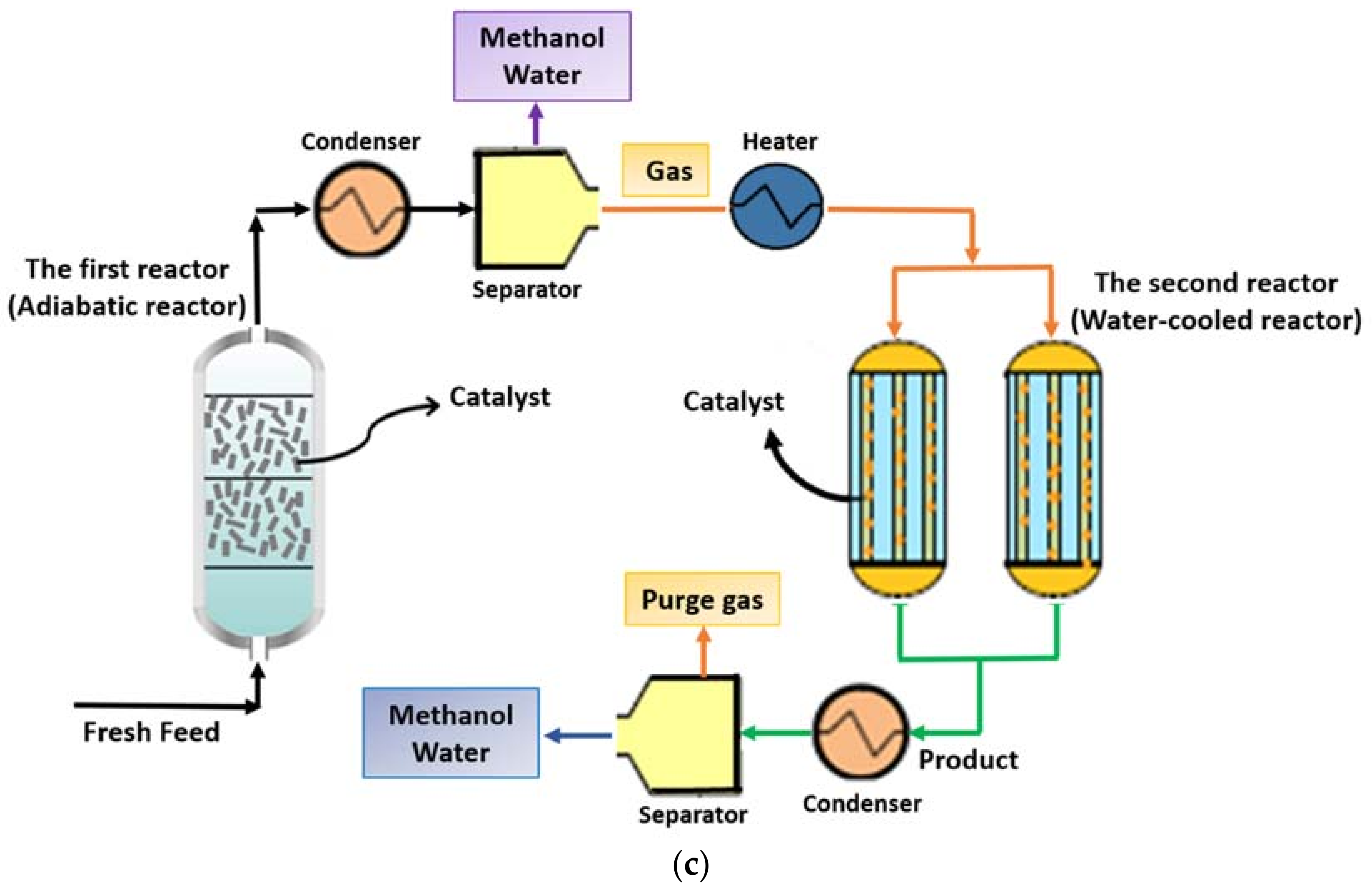
2.2.3. ACW Configuration
2.3. Mathematical Model
2.3.1. Reactor Model
2.3.2. Thermodynamic Model
Dew Point Calculation
Flash Calculation
2.4. Model Validation
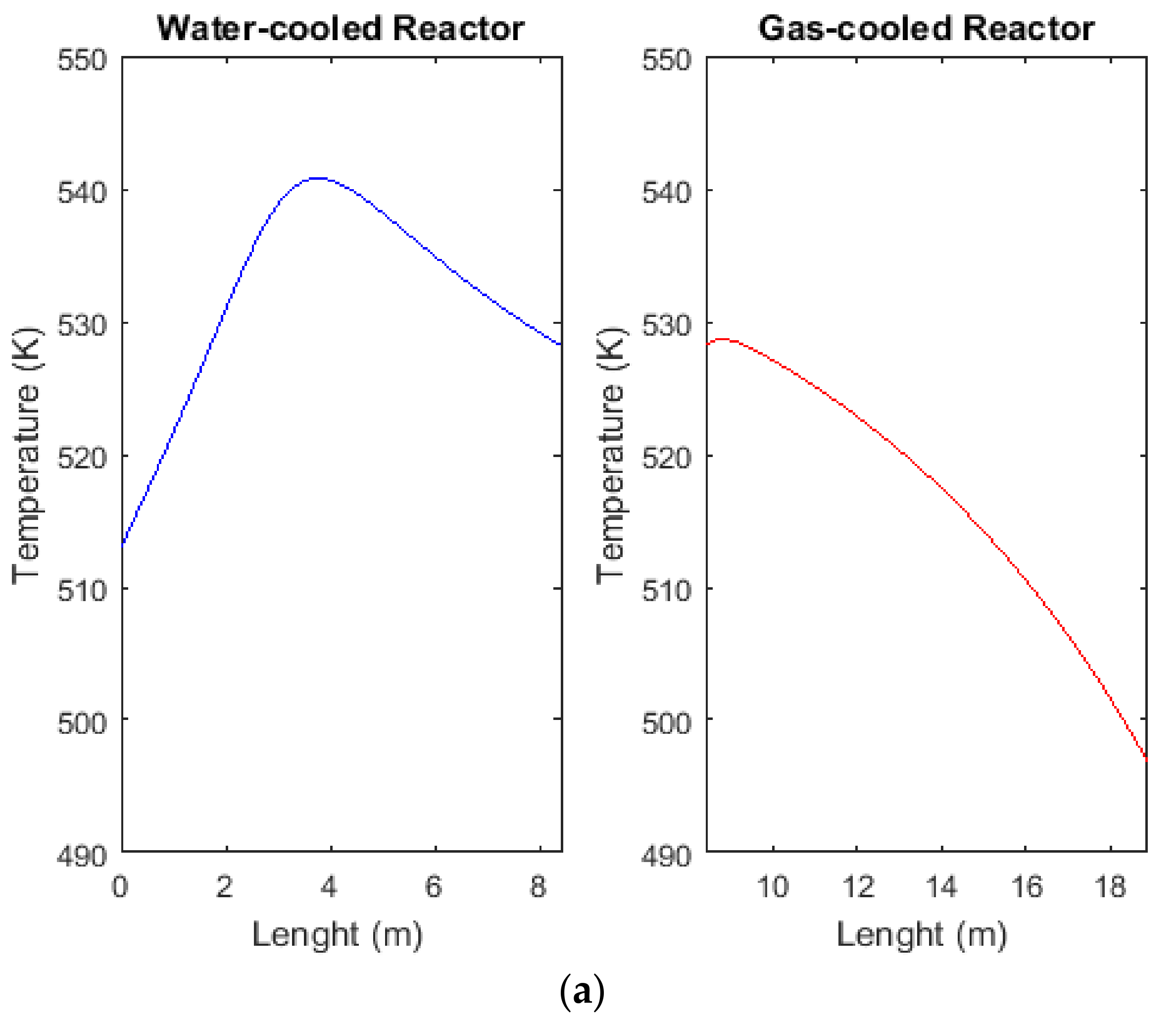
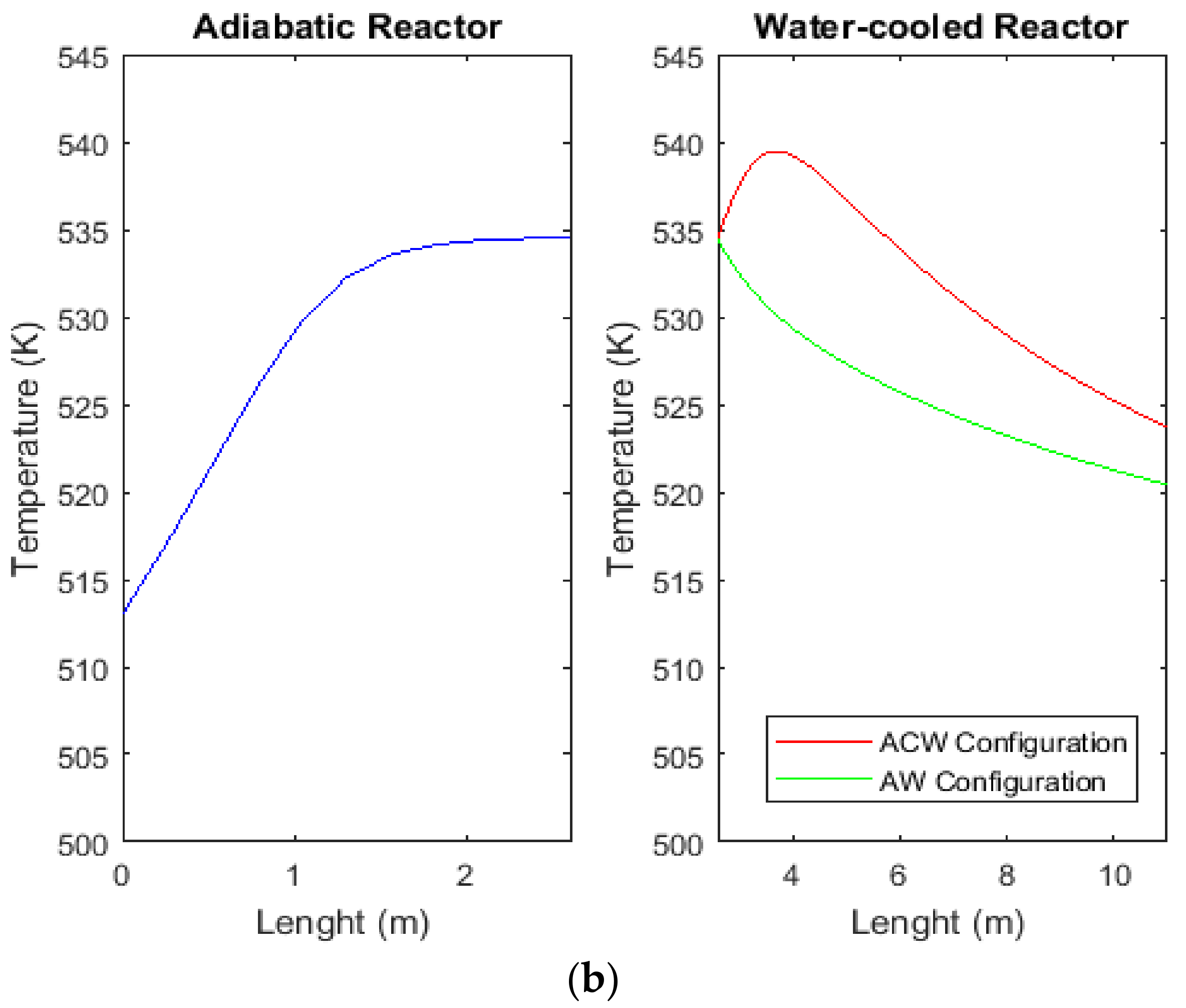
2.5. Comparison between Temperature Profiles of CR, AW, and ACW Configurations
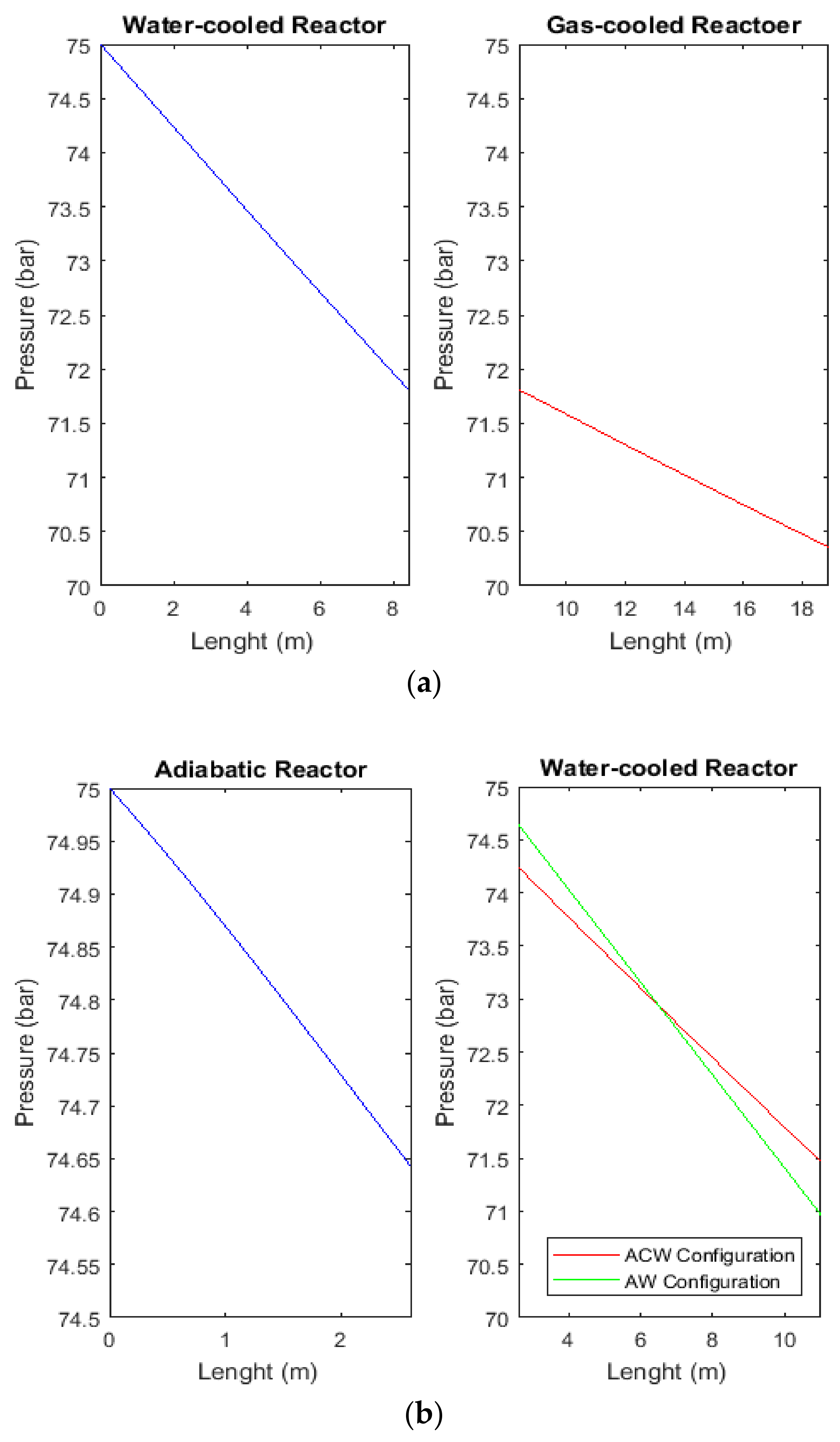
2.6. Comparison between Pressure Profiles of CR, AW, and ACW Configurations
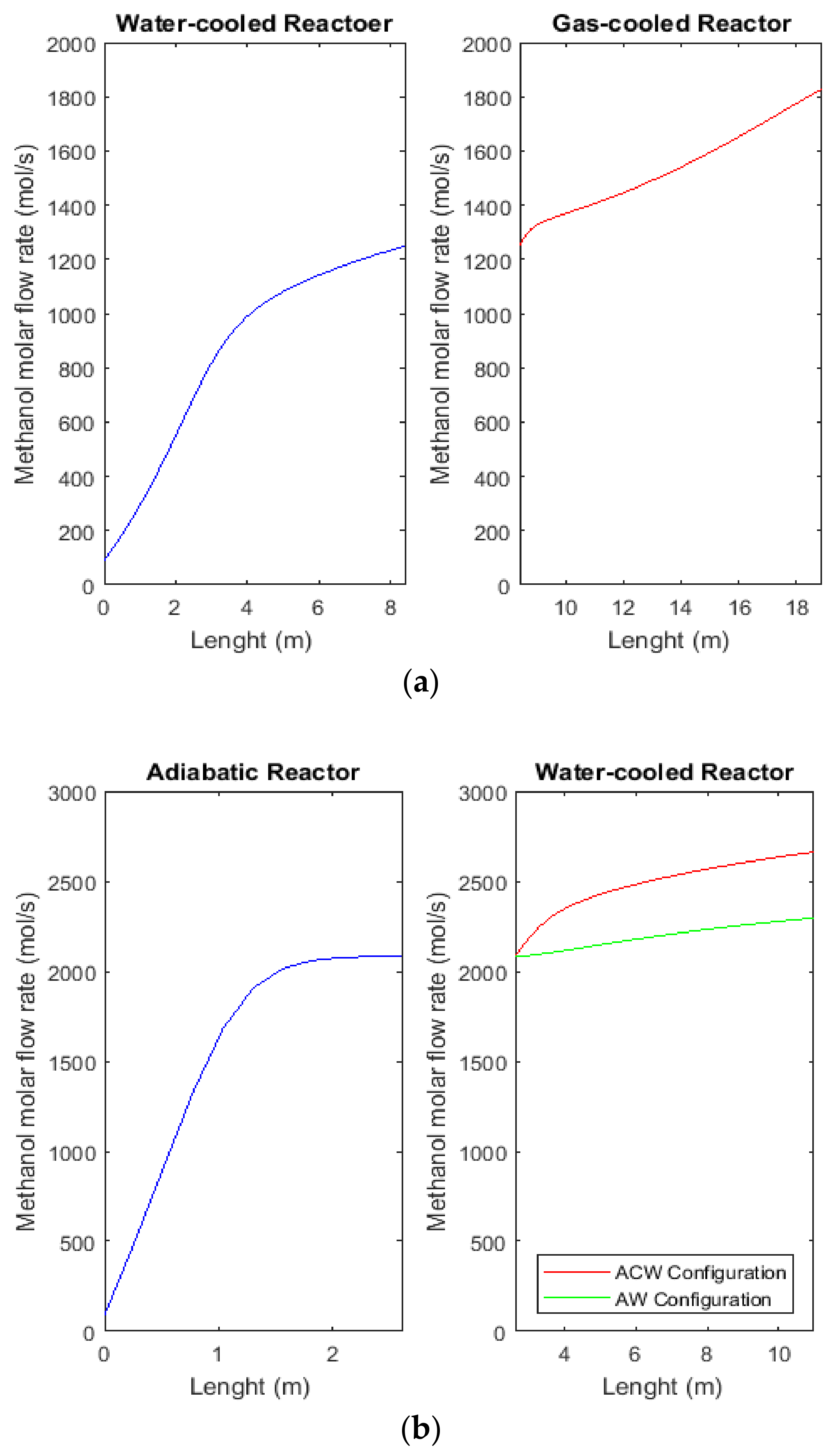
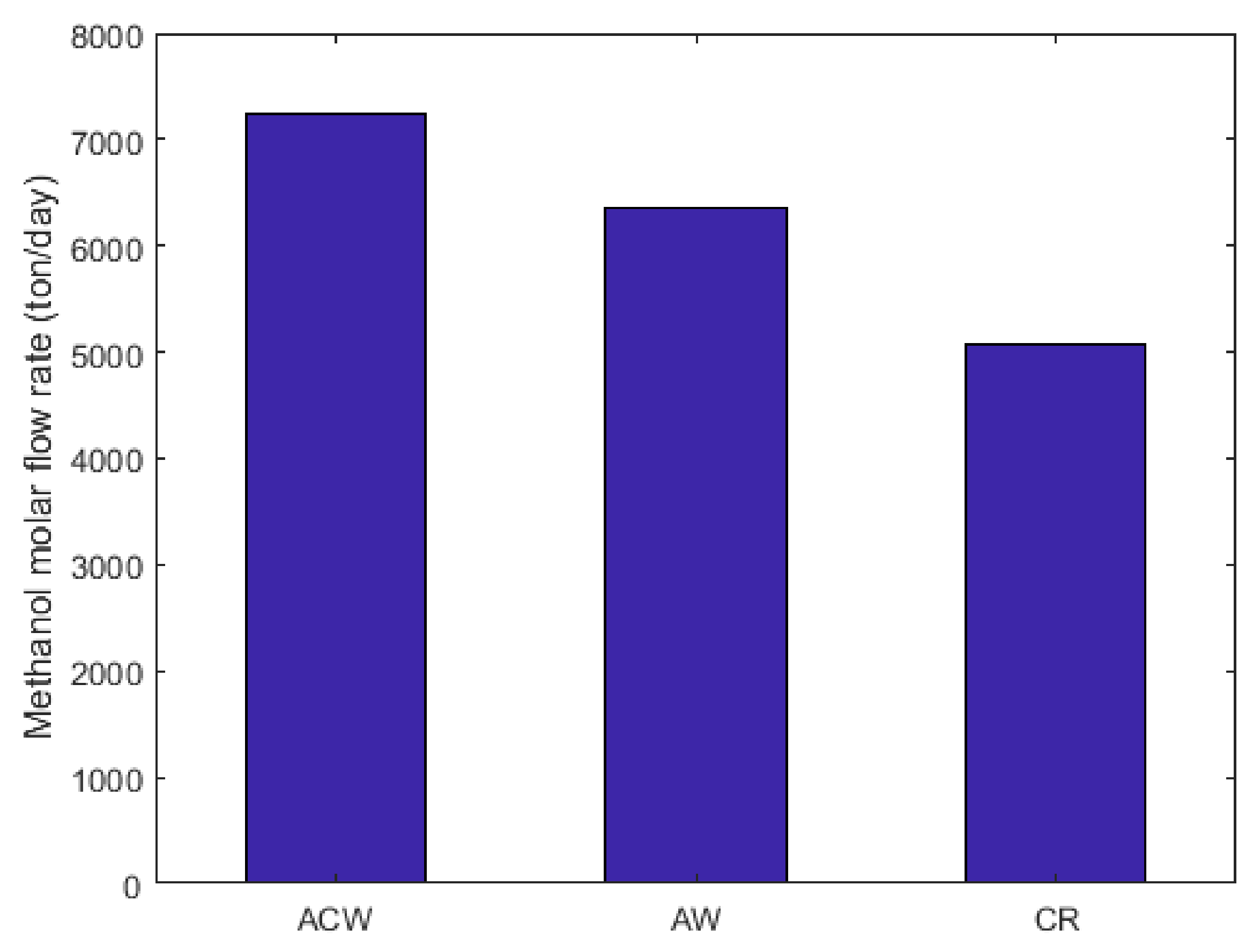
2.7. Comparison between Molar Flow Rate of Methanol in CR, AW, and ACW Configurations
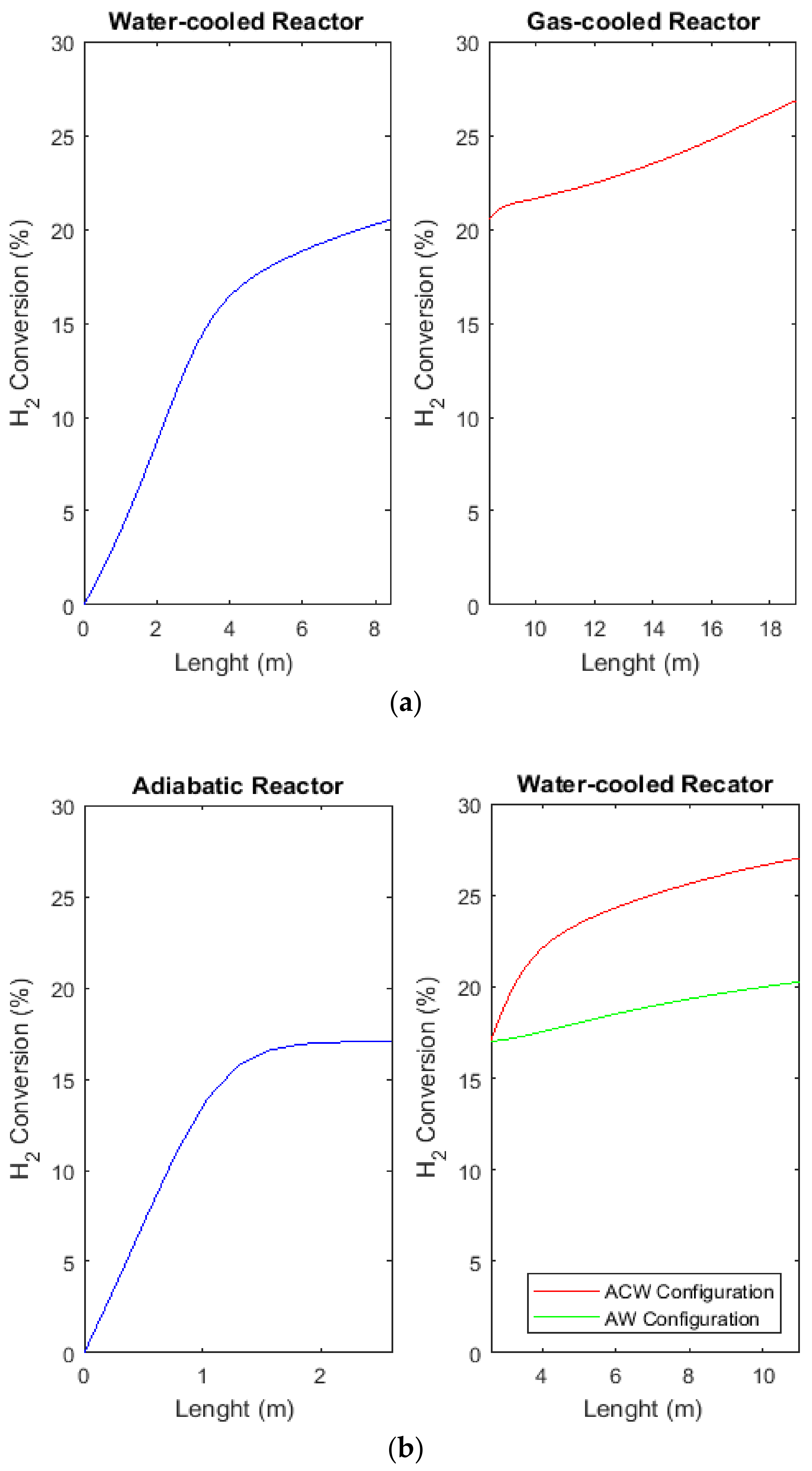
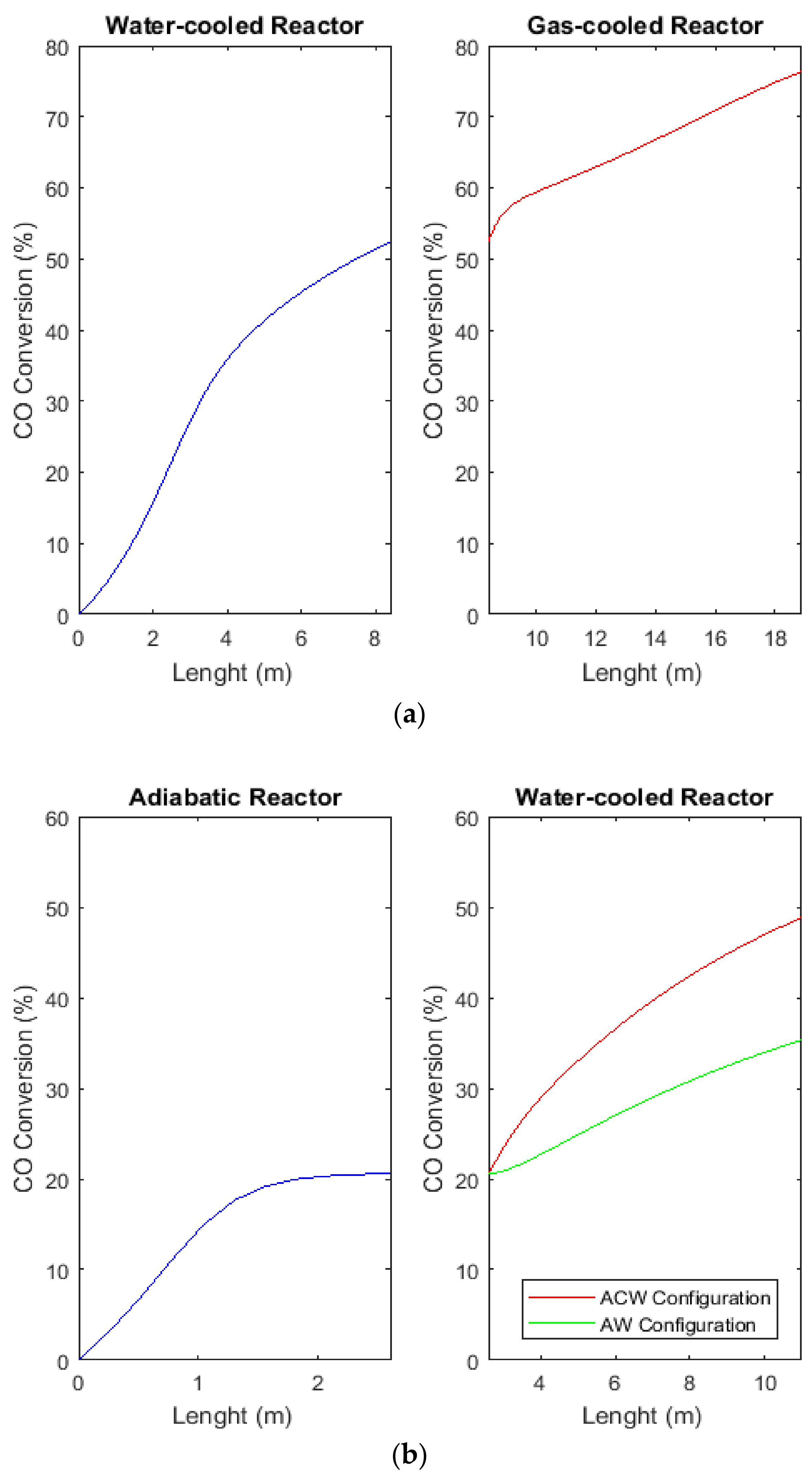
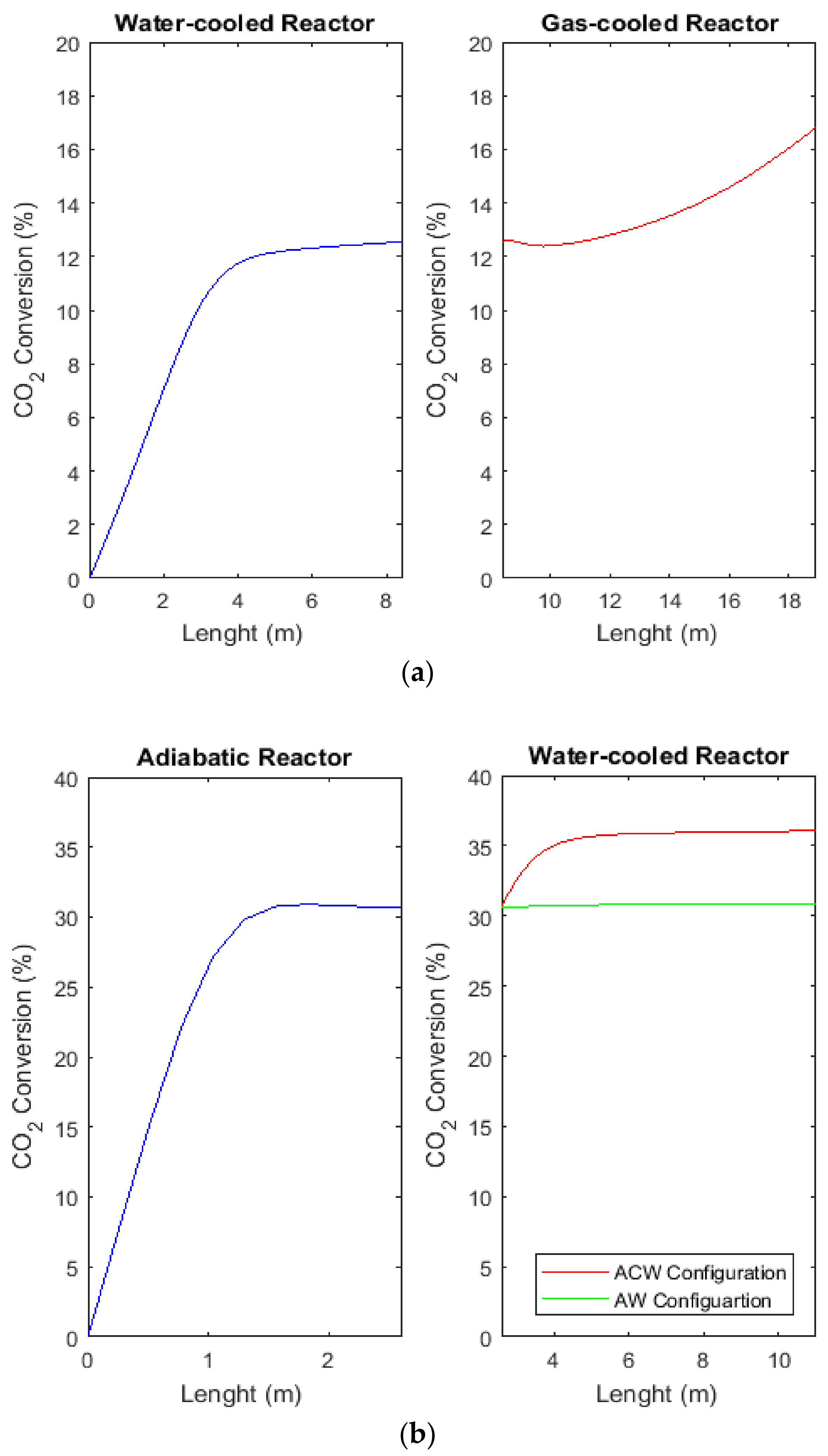
2.8. Comparison of H2, CO, and CO2 Conversions between CR, AW, and ACW Configurations
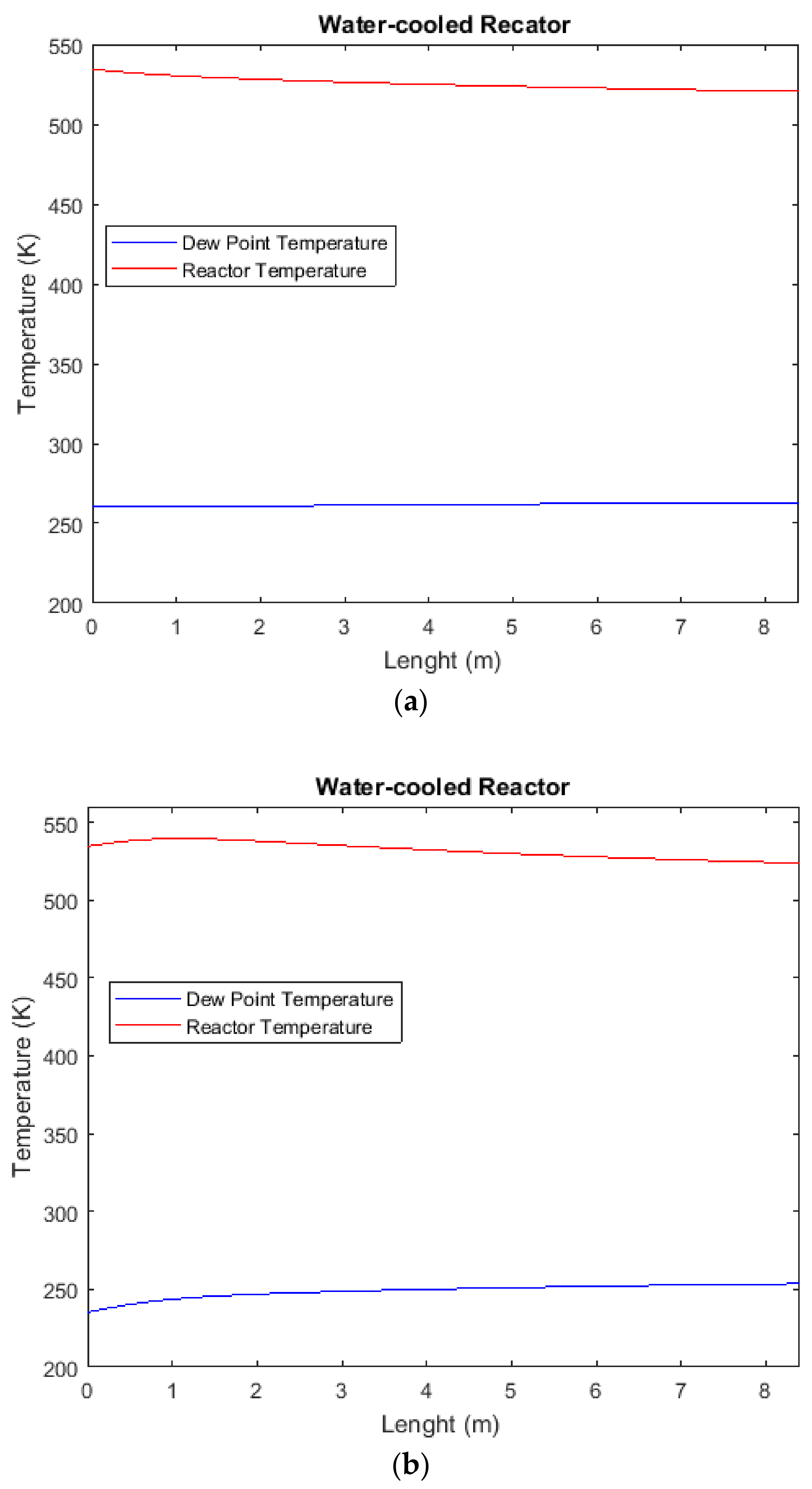
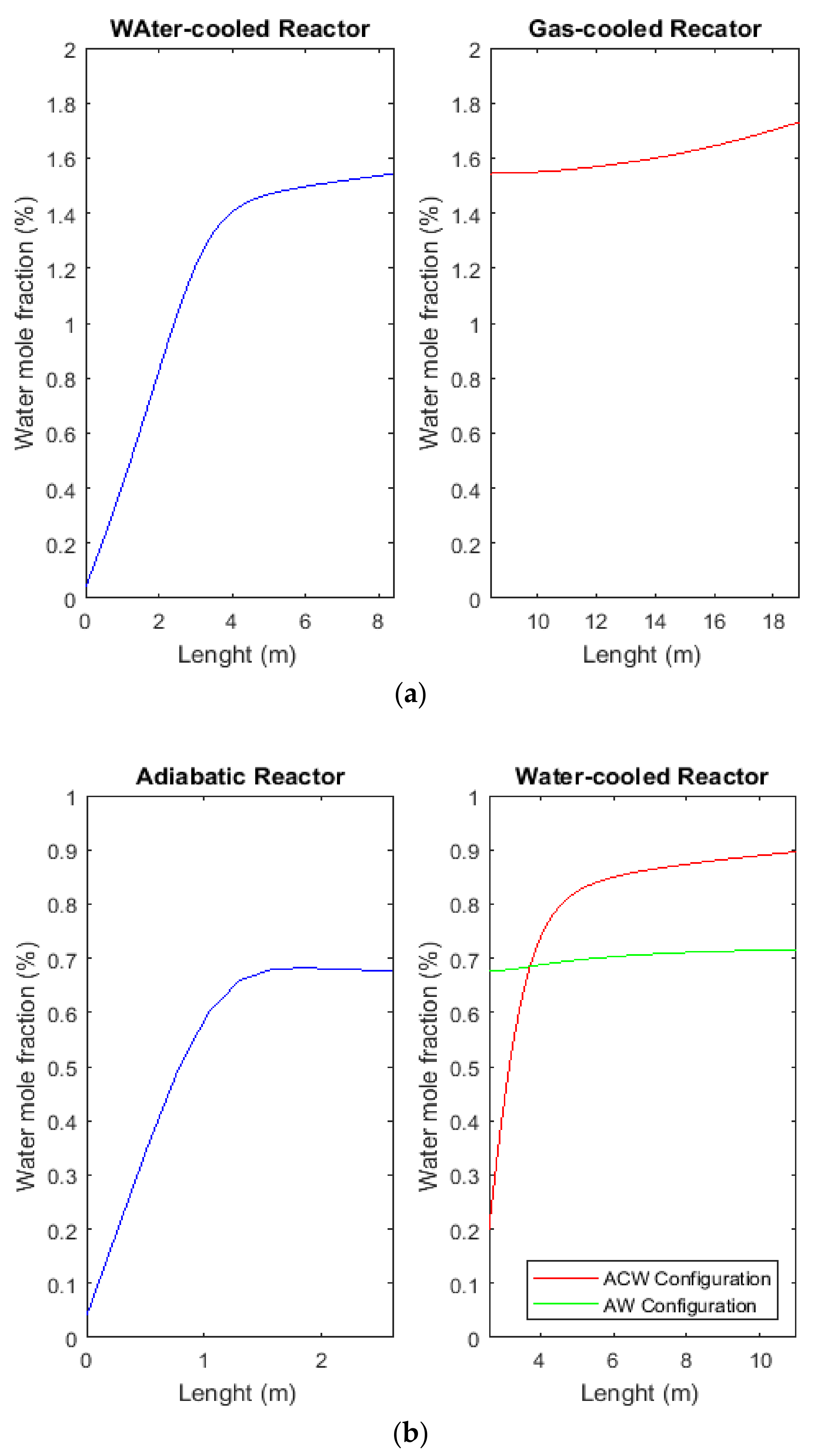
2.9. Comparison of H2O Mole Fraction between CR, AW, and ACW Configurations
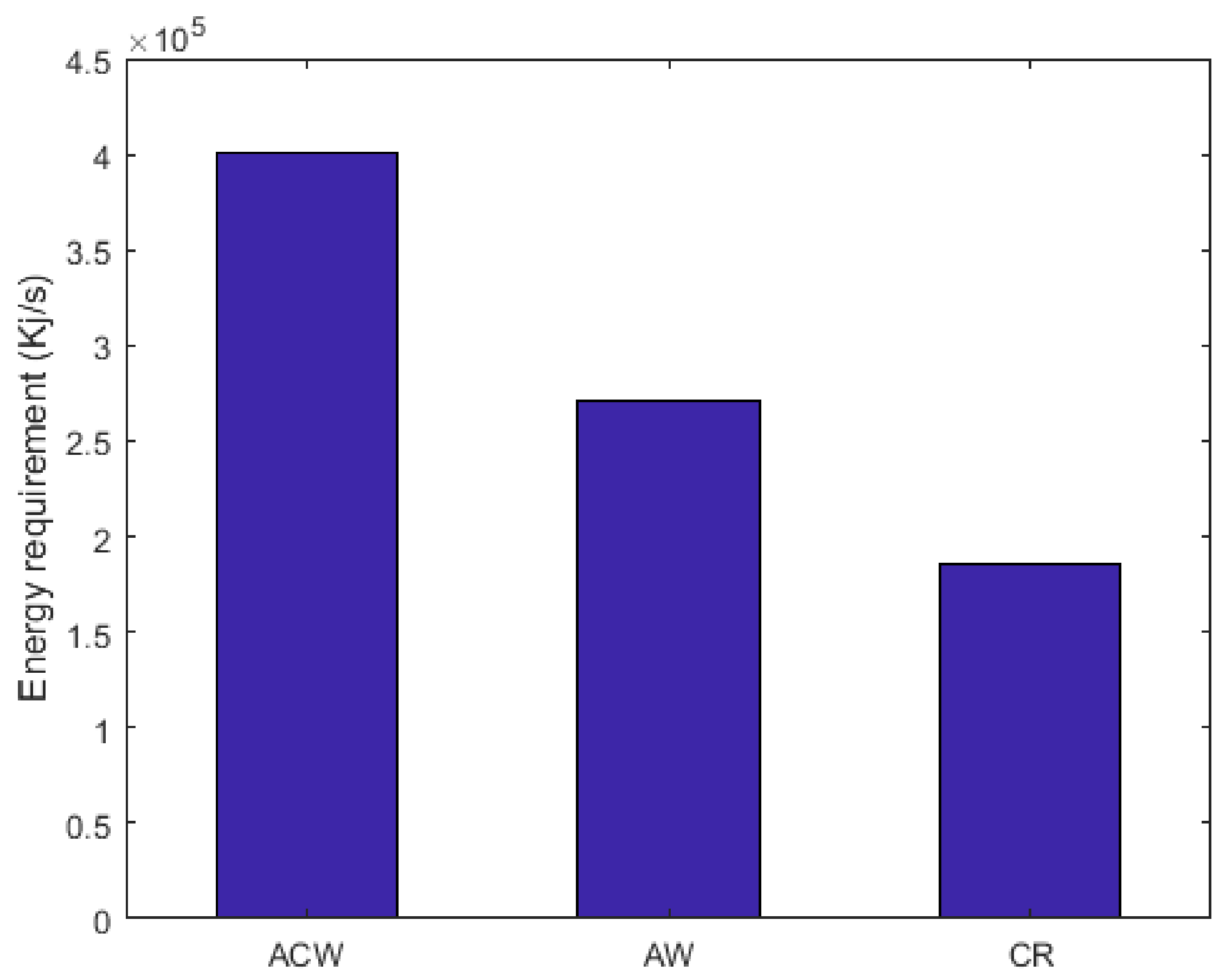
2.10. Energy Requirement and Methanol Molar Flow Rate Comparison between CR, AW, and ACW Configurations
3. Conclusions
Author Contributions
Conflicts of Interest
Nomenclatures
| Ac | reaction side cross-section (m2) |
| Ei | activation energy of reaction (kJ kmol−1) |
| Cp | specific heat of gas (J mol−1 K−1) |
| dp | diameter of particle (m) |
| fi | fugacity (Pa) |
| Ft | total molar flow rate (mol s−1) |
| Fi | the component’s molar flow rate (mol s−1) |
| ∆Hf,i | component (i) formation enthalpy (J mol−1) |
| ki | reaction rate coefficient (mol kg-1 s−1 bar−1/2) |
| Ki | adsorption equilibrium constant (bar−1) |
| Ki | k value |
| Kpi | constant of equilibrium |
| L | length of reactor (m) |
| P | total pressure (Pa) |
| R | universal gas constant (J mol−1 K−1) |
| ri | rate of reaction (mol kg−1 s−1) |
| U | overall heat transfer coefficient between two sides (W m−2 K−1) |
| Vfrac | vapor fraction |
| T | temperature (K) |
| ug | gas velocity (m s−1) |
| xi | component mole fraction in the liquid phase |
| yi | component mole fraction in the gas phase |
| z | axial reactor coordinate (m) |
| Z | compressibility factor |
| Greek letter | |
| ∆H | heat of reaction (J mol−1) |
| 𝜈i,j | component stoichiometric coefficient in each reaction |
| 𝜀 | void fraction of bed |
| φi | fugacity coefficient |
| 𝜇 | viscosity (Pa s) |
| 𝜌 | gas phase density (kg m−3) |
| 𝜌b | catalyst bed density (kg m−3) |
| 𝜂 | effectiveness factor of catalyst |
| Subscript and Superscripts | |
| i | indicator of component |
| j | indicator of reaction |
| 0 | inlet condition |
| v | vapor phase |
| l | liquid phase |
| g | in bulk of gas phase |
| Abbreviations | |
| CFD | computational fluid dynamics |
| CR | conventional methanol synthesis reactor |
| AW | name of the proposed configuration |
| ACW | name of the proposed configuration |
| RWGS | reverse water gas shift reaction |
| DAE | differential-algebraic equations |
References
- Bayat, M.; Heravi, M.; Rahimpour, M.R. Sorption enhanced process by integrated heat-exchanger reactor assisted by fluidization concept for methanol synthesis. Chem. Eng. Process. Process Intensif. 2016, 110, 30–43. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Haghbakhsh, R.; Rahimpour, M.R. Investigation of thermally double coupled double membrane heat exchanger reactor to produce dimethyl ether and methyl formate. J. Nat. Gas Sci. Eng. 2016, 32, 185–197. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Mohammadi, M.; Rahimpour, M.R. Simultaneous production of dimethyl ether (DME), methyl formate (MF) and hydrogen from methanol in an integrated thermally coupled membrane reactor. J. Nat. Gas Sci. Eng. 2015, 26, 595–607. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Parhoudeh, M.; Rahimpour, M.R. Optimal conditions in converting methanol to dimethyl ether, methyl formate, and hydrogen utilizing a double membrane heat exchanger reactor. J. Nat. Gas Sci. Eng. 2016, 28, 31–45. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Darvishi, A.; Rahimpour, M.R. A heat exchanger reactor equipped with membranes to produce dimethyl ether from syngas and methyl formate and hydrogen from methanol. Int. J. 2016, 3, 65. [Google Scholar]
- Khanipour, M.; Mirvakili, A.; Bakhtyari, A.; Farniaei, M.; Rahimpour, M.R. Enhancement of synthesis gas and methanol production by flare gas recovery utilizing a membrane based separation process. Fuel Process. Technol. 2017, 166, 186–201. [Google Scholar] [CrossRef]
- Mirvakili, A.; Bakhtyari, A.; Rahimpour, M.R. A CFD modeling to investigate the impact of flow mal-distribution on the performance of industrial methanol synthesis reactor. Appl. Therm. Eng. 2018, 128, 64–78. [Google Scholar] [CrossRef]
- Agahzamin, S.; Mirvakili, A.; Rahimpour, M.R. Investigation and recovery of purge gas streams to enhance synthesis gas production in a mega methanol complex. J. CO2 Util. 2016, 16, 157–168. [Google Scholar] [CrossRef]
- Mirvakili, A.; Rahimpour, M.R. Mal-distribution of temperature in an industrial dual-bed reactor for conversion of CO2 to methanol. Appl. Therm. Eng. 2015, 91, 1059–1070. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Askari, F.; Rahimpour, M.R.; Jahanmiri, A.; Mostafazadeh, A.K. Dynamic Simulation and Optimization of a Dual-Type Methanol Reactor Using Genetic Algorithms. Chem. Eng. Technol. 2008, 31, 513–524. [Google Scholar] [CrossRef]
- Santangelo, D.L.; Ahón, V.R.; Costa, A.L. Optimization of methanol synthesis loops with quench reactors. Chem. Eng. Technol. 2008, 31, 1767–1774. [Google Scholar] [CrossRef]
- Parvasi, P.; Mostafazadeh, A.K.; Rahimpour, M.R. Dynamic modeling and optimization of a novel methanol synthesis loop with hydrogen-permselective membrane reactor. Int. J. Hydrog. Energy 2009, 34, 3717–3733. [Google Scholar] [CrossRef]
- Parvasi, P.; Rahimpour, M.R.; Jahanmiri, A. Incorporation of dynamic flexibility in the design of a methanol synthesis loop in the presence of catalyst deactivation. Chem. Eng. Technol. 2008, 31, 116–132. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Moghtaderi, B.; Jahanmiri, A.; Rezaie, N. Operability of an industrial methanol synthesis reactor with mixtures of fresh and partially deactivated catalyst. Chem. Eng. Technol. 2005, 28, 226–234. [Google Scholar] [CrossRef]
- Sadeghi, S.; Vafajoo, L.; Kazemeini, M.; Fattahi, M. Modeling of the methanol synthesis catalyst deactivation in a spherical bed reactor: An environmental challenge. APCBEE Procedia 2014, 10, 84–90. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Fathikalajahi, J.; Jahanmiri, A. Selective kinetic deactivation model for methanol synthesis from simultaneous reaction of CO2 and CO with H2 on a commercial copper/zinc oxide catalyst. Can. J. Chem. Eng. 1998, 76, 753–761. [Google Scholar] [CrossRef]
- Wagialla, K.; Elnashaie, S. Fluidized-bed reactor for methanol synthesis. A theoretical investigation. Ind. Eng. Chem. Res. 1991, 30, 2298–2308. [Google Scholar] [CrossRef]
- Graaf, G.H.; Scholtens, H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Intra-particle diffusion limitations in low-pressure methanol synthesis. Chem. Eng. Sci. 1990, 45, 773–783. [Google Scholar] [CrossRef]
- Løvik, I. Modelling, Estimation and Optimization of the Methanol Synthesis with Catalyst Deactivation. Ph.D. Thesis, Department of Chemical Engineering (NTNU), Trondheim, Norway, 2001. [Google Scholar]
- Velardi, S.A.; Barresi, A.A. Methanol synthesis in a forced unsteady-state reactor network. Chem. Eng. Sci. 2002, 57, 2995–3004. [Google Scholar] [CrossRef]
- Rahimpour, M.R. A two-stage catalyst bed concept for conversion of carbon dioxide into methanol. Fuel Process. Technol. 2008, 89, 556–566. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Bayat, M. A novel cascade membrane reactor concept for methanol synthesis in the presence of long-term catalyst deactivation. Int. J. Energy Res. 2010, 34, 1356–1371. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mostafazadeh, A.K.; Barmaki, M. Application of hydrogen-permselective Pd-based membrane in an industrial single-type methanol reactor in the presence of catalyst deactivation. Fuel Process. Technol. 2008, 89, 1396–1408. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Ghader, S. Enhancement of CO conversion in a novel Pd–Ag membrane reactor for methanol synthesis. Chem. Eng. Process. Process Intensif. 2004, 43, 1181–1188. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Lotfinejad, M. Enhancement of Methanol Production in a Membrane Dual-Type Reactor. Chem. Eng. Technol. 2007, 30, 1062–1076. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Lotfinejad, M. Co-current and Countercurrent Configurations for a Membrane Dual Type Methanol Reactor. Chem. Eng. Technol. 2008, 31, 38–57. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Alizadehhesari, K. Enhancement of carbon dioxide removal in a hydrogen-permselective methanol synthesis reactor. Int. J. Hydrog. Energy 2009, 34, 1349–1362. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Alizadehhesari, K. A Novel Fluidized-Bed Membrane Dual-Type Reactor Concept for Methanol Synthesis. Chem. Eng. Technol. 2008, 31, 1775–1789. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Bayat, M.; Rahmani, F. Enhancement of methanol production in a novel cascading fluidized-bed hydrogen permselective membrane methanol reactor. Chem. Eng. J. 2010, 157, 520–529. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Bayat, M.; Rahmani, F. Dynamic simulation of a cascade fluidized-bed membrane reactor in the presence of long-term catalyst deactivation for methanol synthesis. Chem. Eng. Sci. 2010, 65, 4239–4249. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Elekaei, H. Enhancement of methanol production in a novel fluidized-bed hydrogen-permselective membrane reactor in the presence of catalyst deactivation. Int. J. Hydrog. Energy 2009, 34, 2208–2223. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Behjati, H.E. Dynamic optimization of membrane dual-type methanol reactor in the presence of catalyst deactivation using genetic algorithm. Fuel Process. Technol. 2009, 90, 279–291. [Google Scholar] [CrossRef]
- Manenti, F.; Cieri, S.; Restelli, M.; Bozzano, G. Dynamic modeling of the methanol synthesis fixed-bed reactor. Comput. Chem. Eng. 2013, 48, 325–334. [Google Scholar] [CrossRef]
- Flavio, M.; Garcon-Leon, A.R.; Giulia, B. Energy-process integration of the gas-cooled/water-cooled fixed-bed reactor network for methanol synthesis. Chem. Eng. Trans. 2013, 1243–1248. [Google Scholar]
- Bayat, M.; Rahimpour, M.R. Dynamic optimal analysis of a novel cascade membrane methanol reactor by using genetic algorithm (GA) method. Energy Syst. 2013, 4, 137–164. [Google Scholar] [CrossRef]
- Pask, J.A. Effect of Water Vapor on Sintering of Ceramic Oxides. In Sintering Key Papers; Springer: Dordrecht, The Netherlands, 1990; pp. 579–592. [Google Scholar]
- Wu, J.; Saito, M.; Takeuchi, M.; Watanabe, T. The stability of Cu/ZnO-based catalysts in methanol synthesis from a CO2-rich feed and from a CO-rich feed. Appl. Catal. A Gen. 2001, 218, 235–240. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R.; Shariati, A. Development of an Efficient Methanol Production Process for Direct CO2 Hydrogenation over a Cu/ZnO/Al2O3 Catalyst. Catalysts 2017, 7, 332. [Google Scholar] [CrossRef]
- Umegaki, T.; Kojima, Y.; Omata, K. Effect of oxide coating on performance of copper-zinc oxide-based catalyst for methanol synthesis via hydrogenation of carbon dioxide. Materials 2015, 8, 7738–7744. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.V.; Spencer, M.S. Deactivation of supported copper metal catalysts for hydrogenation reactions. Appl. Catal. A Gen. 2001, 212, 161–174. [Google Scholar] [CrossRef]
- Twigg, M.V.; Spencer, M.S. Deactivation of copper metal catalysts for methanol decomposition, methanol steam reforming and methanol synthesis. Top. Catal. 2003, 22, 191–203. [Google Scholar] [CrossRef]
- Hughes, R. Deactivation of Catalysts; Academic Press: London, UK, 1984. [Google Scholar]
- Spencer, M. Stable and metastable metal surfaces in heterogeneous catalysis. Nature 1986, 323, 685. [Google Scholar] [CrossRef]
- Kung, H.H. Deactivation of methanol synthesis catalysts—A review. Catal. Today 1992, 11, 443–453. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. How oxide carriers control the catalytic functionality of the Cu–ZnO system in the hydrogenation of CO2 to methanol. Catal. Today 2013, 210, 39–46. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. Effects of oxide carriers on surface functionality and process performance of the Cu–ZnO system in the synthesis of methanol via CO2 hydrogenation. J. Catal. 2013, 300, 141–151. [Google Scholar] [CrossRef]
- Saito, M.; Fujitani, T.; Takeuchi, M.; Watanabe, T. Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl. Catal. A Gen. 1996, 138, 311–318. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bunora, G.; Spadaro, L.; Frusteri, F. Synthesis, characterization and activity pattern of Cu–ZnO/ZrO2 catalysts in the hydrogenation of carbon dioxide to methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Rahmatmand, B.; Keshavarz, P.; Ayatollahi, S. Study of absorption enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 nanoparticles in water and amine solutions. J. Chem. Eng. Data 2016, 61, 1378–1387. [Google Scholar] [CrossRef]
- Graaf, G.; Stamhuis, E.; Beenackers, A. Kinetics of low-pressure methanol synthesis. Chem. Eng. Sci. 1988, 43, 3185–3195. [Google Scholar]
- Eastland, P.H.D. A Reactor System, Plant and a Process for the Production of Methanol from Synthesis Gas; Johnson Matthey Davy Technologies Ltd.: London, UK, 2012. [Google Scholar]
- Rezaie, N.; Jahanmiri, A.; Moghtaderi, B.; Rahimpour, M.R. A comparison of homogeneous and heterogeneous dynamic models for industrial methanol reactors in the presence of catalyst deactivation. Chem. Eng. Process. Process Intensif. 2005, 44, 911–921. [Google Scholar] [CrossRef]
- Bertau, M.; Offermanns, H.; Plass, L.; Schmidt, F. Methanol: The Basic Chemical and Energy Feedstock of the Future; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2014. [Google Scholar]
- Hartig, F.; Keil, F.J. Large-scale spherical fixed bed reactors: Modeling and optimization. Ind. Eng. Chem. Res. 1993, 32, 424–437. [Google Scholar] [CrossRef]
- Bennekom, J.G.V.; Winkelman, J.G.M.; Venderbosch, R.H.; Nieland, S.D.G.B.; Heeres, H.J. Modeling and experimental studies on phase and chemical equilibria in high-pressure methanol synthesis. Ind. Eng. Chem. Res. 2012, 51, 12233–12243. [Google Scholar] [CrossRef]
- Mathias, P.M. A versatile phase equilibrium equation of state. Ind. Eng. Chem. Process Des. Dev. 1983, 22, 385–391. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2015; ISBN 0073104450. [Google Scholar]
| Adsorption Equilibrium Constants | ||
|---|---|---|
| A | B | |
| KCO | ||
| KCO2 | ||
| Rate Constants | ||
| A | B | |
| Equilibrium Constants | ||
| A | B | |
| 5139 | 12.621 | |
| 3066 | 10.592 | |
| −2073 | −2.029 | |
| Parameters | Water-Cooled Reactor | Gas-Cooled Reactor |
|---|---|---|
| Values | Values | |
| Shell diameter (m) | 4.5 | 5.5 |
| Tube diameter (m) | 0.038 | 0.0254 |
| Reactor length (m) | 8.4 | 10.5 |
| Particle diameter (m) | 0.0057 | 0.0057 |
| Catalyst bed density (kg/m3) | 1140 | 1140 |
| Bed void fraction | 0.39 | 0.39 |
| Number of tubes | 5955 | 3026 |
| Molar flow of feed in each tube (mol/s) | 1.5 | - |
| Input shell side pressure (bar) | - | 71.8 |
| Input tube side pressure (bar) | 75 | 76.98 |
| Temperature of the shell side (K) | 513 | - |
| Mass flow rate of the shell side (kg/h) | 151,437 | - |
| Feed Composition (Mole Basis) | Values | |
| H2 | 0.6334 | |
| CH4 | 0.1006 | |
| CO2 | 0.0832 | |
| H2O | 0.00039 | |
| N2 | 0.093 | |
| CH3OH | 0.0049 | |
| CO | 0.085 | |
| Feed Conditions | Values |
|---|---|
| Feed mole fraction | |
| CO | 0.0868 |
| CO2 | 0.0849 |
| H2 | 0.6461 |
| CH4 | 0.0947 |
| N2 | 0.0847 |
| H2O | 0.001 |
| CH3OH | 0.0037 |
| Input temperature (K) | 401 |
| Molar flow of feed in each tube (mol/s) | 1.8 |
| Feed input pressure (bar) | 76 |
| Parameters | Adiabatic Reactor | Water-Cooled Reactor |
|---|---|---|
| Values | Values | |
| Shell diameter (m) | - | 4.5 |
| Tube diameter (m) | - | 0.038 |
| Reactor diameter (m) | 5.5 | - |
| Reactor length (m) | 2.6 | 8.4 |
| Particle diameter (m) | 0.0057 | 0.0057 |
| Catalyst bed density (kg/m3) | 1140 | 1140 |
| Bed void fraction | 0.39 | 0.39 |
| Number of tubes | - | 5955 |
| Rate of feed stream (mol/s) | 17,865 | - |
| Input pressure (bar) | 75 | - |
| Input temperature (K) | 513 | - |
| Input tube side pressure (bar) | - | 74.64 |
| Input shell side temperature (K) | - | 513 |
| Mass flow rate of the shell side (kg/h) | - | 151,437 |
| Feed Composition (Mole Basis) | Values | |
| H2 | 0.6334 | |
| CH4 | 0.1006 | |
| CO2 | 0.0832 | |
| H2O | 0.00039 | |
| N2 | 0.093 | |
| CH3OH | 0.0049 | |
| CO | 0.085 | |
| Parameters | Values |
|---|---|
| Shell diameter (m) | 4.5 |
| Tube diameter (m) | 0.038 |
| Reactor length (m) | 8.4 |
| Number of tubes | 5955 |
| Molar flow of feed in each tube (mol/s) | 1.34 |
| Input tube side pressure (bar) | 74.24 |
| Input tube side temperature (K) | 534.4 |
| Temperature of the shell side (K) | 513 |
| Mass flow rate of the shell side (kg/h) | 151,437 |
| Feed Mole Fraction | Values |
| H2 | 0.5871 |
| CH4 | 0.1119 |
| CO2 | 0.0632 |
| H2O | 0.0020 |
| N2 | 0.1036 |
| CH3OH | 0.0568 |
| CO | 0.0754 |
| Parameters | Values |
|---|---|
| Reactor diamaeter (m) | 5.5 |
| Reactor length (m) | 2.6 |
| Rate of feed stream (mol/s) | 17,865 |
| Input pressure (bar) | 75 |
| Input temperature (K) | 513 |
| Feed Mole Fraction | Values |
| H2 | 0.6334 |
| CH4 | 0.1006 |
| CO2 | 0.0832 |
| H2O | 0.00039 |
| N2 | 0.093 |
| CH3OH | 0.0049 |
| CO | 0.085 |
| Parameters | Inlet Flow | Outlet Flow (Vapor Phase) | Outlet Flow (Liquid Phase) |
|---|---|---|---|
| Molar flow (mol/s) | 17,217 | 15,955 | 1260 |
| Component | Mole Fraction | Mole Fraction | Mole Fraction |
| CH3OH | 0.1185 | 0.0568 | 0.8988 |
| CO2 | 0.0598 | 0.0632 | 0.0173 |
| CO | 0.0700 | 0.0754 | 0.0016 |
| H2O | 0.0067 | 0.0020 | 0.0652 |
| H2 | 0.5445 | 0.5871 | 0.0054 |
| N2 | 0.0962 | 0.1036 | 0.0037 |
| CH4 | 0.1043 | 0.1119 | 0.0080 |
| Condenser Duty (KJ/s) | 138,861 | ||
| Parameters | Model | Industrial Data | Error % | |
|---|---|---|---|---|
| Reactor Outlet | Reactor Inlet | Reactor Outlet | ||
| Temperature (K) | 493.43 | 401 | 495 | 0.31 |
| Mole Fraction | ||||
| CH3OH | 0.1062 | 0.0037 | 0.104 | 2.14 |
| CO2 | 0.0815 | 0.0849 | 0.0709 | 14.89 |
| CO | 0.0227 | 0.0868 | 0.0251 | −9.42 |
| H2O | 0.0204 | 0.001 | 0.0234 | −12.70 |
| H2 | 0.5572 | 0.6461 | 0.5519 | 0.96 |
| N2 | 0.1009 | 0.0828 | 0.1107 | −8.81 |
| CH4 | 0.1133 | 0.0947 | 0.114 | −0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmatmand, B.; Rahimpour, M.R.; Keshavarz, P. Development of Two Novel Processes for Hydrogenation of CO2 to Methanol over Cu/ZnO/Al2O3 Catalyst to Improve the Performance of Conventional Dual Type Methanol Synthesis Reactor. Catalysts 2018, 8, 255. https://doi.org/10.3390/catal8070255
Rahmatmand B, Rahimpour MR, Keshavarz P. Development of Two Novel Processes for Hydrogenation of CO2 to Methanol over Cu/ZnO/Al2O3 Catalyst to Improve the Performance of Conventional Dual Type Methanol Synthesis Reactor. Catalysts. 2018; 8(7):255. https://doi.org/10.3390/catal8070255
Chicago/Turabian StyleRahmatmand, Behnaz, Mohammad Reza Rahimpour, and Peyman Keshavarz. 2018. "Development of Two Novel Processes for Hydrogenation of CO2 to Methanol over Cu/ZnO/Al2O3 Catalyst to Improve the Performance of Conventional Dual Type Methanol Synthesis Reactor" Catalysts 8, no. 7: 255. https://doi.org/10.3390/catal8070255
APA StyleRahmatmand, B., Rahimpour, M. R., & Keshavarz, P. (2018). Development of Two Novel Processes for Hydrogenation of CO2 to Methanol over Cu/ZnO/Al2O3 Catalyst to Improve the Performance of Conventional Dual Type Methanol Synthesis Reactor. Catalysts, 8(7), 255. https://doi.org/10.3390/catal8070255