Hydrogen Production from Chemical Looping Reforming of Ethanol Using Ni/CeO2 Nanorod Oxygen Carrier
Abstract
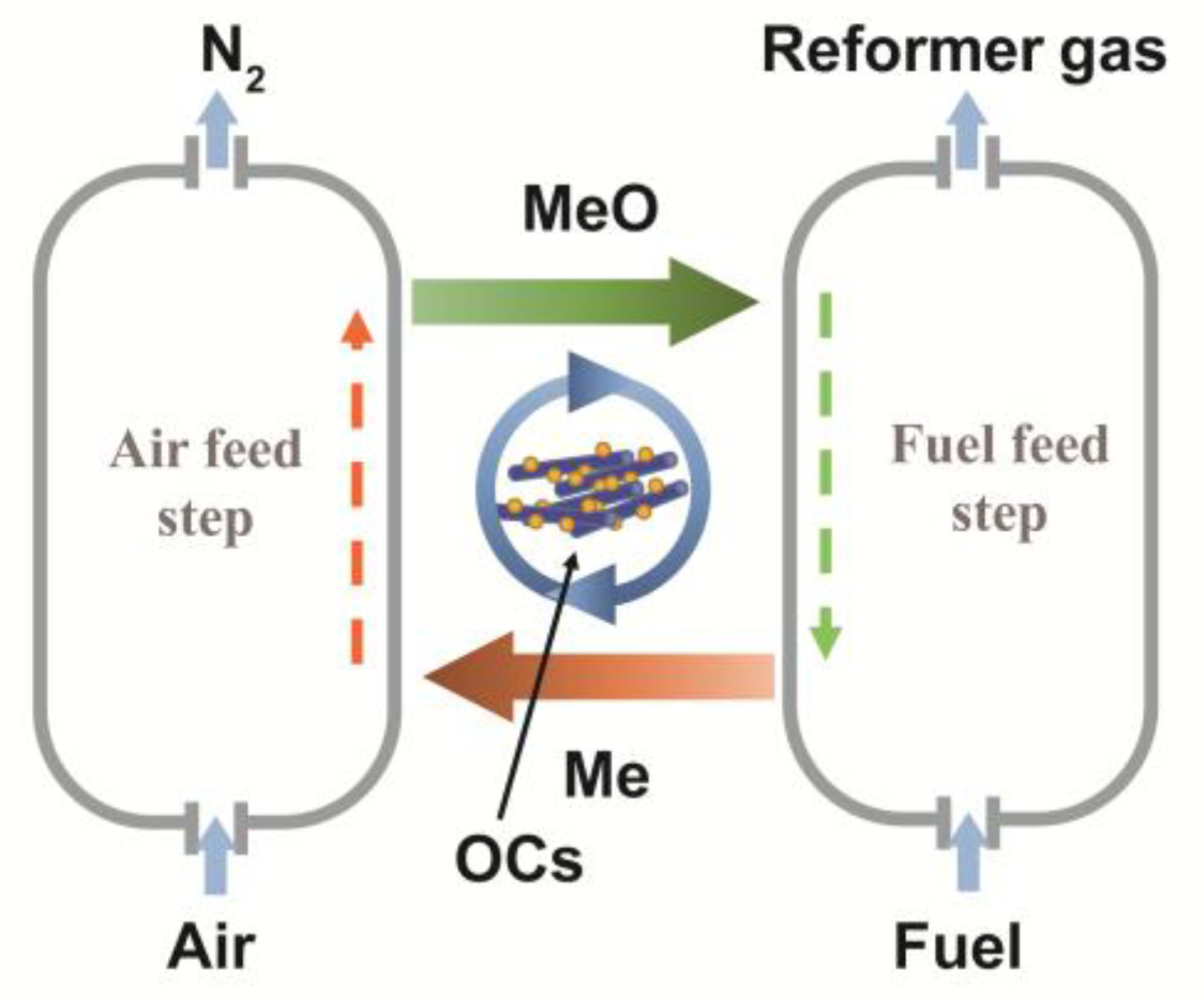
1. Introduction
2. Results and discussion
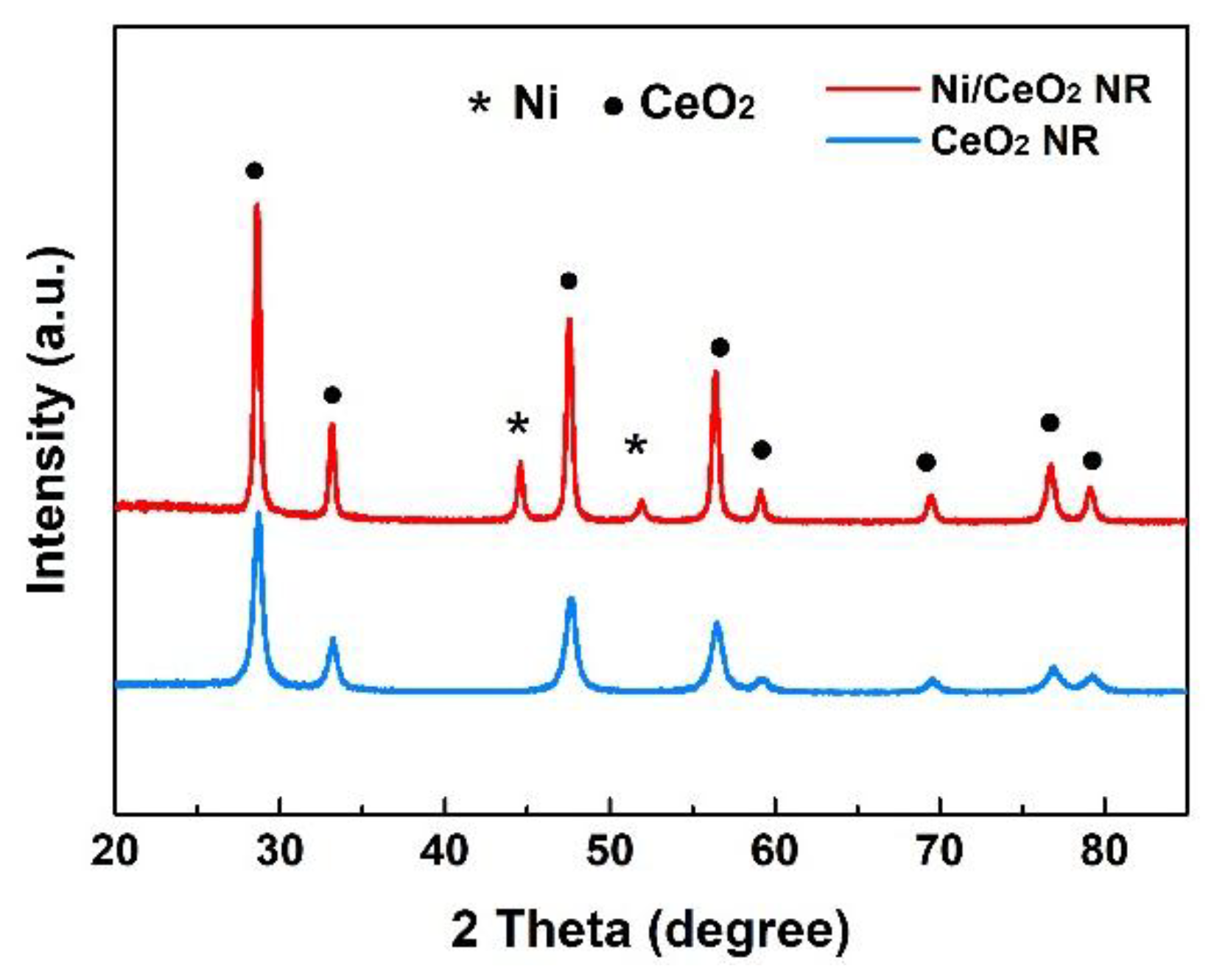
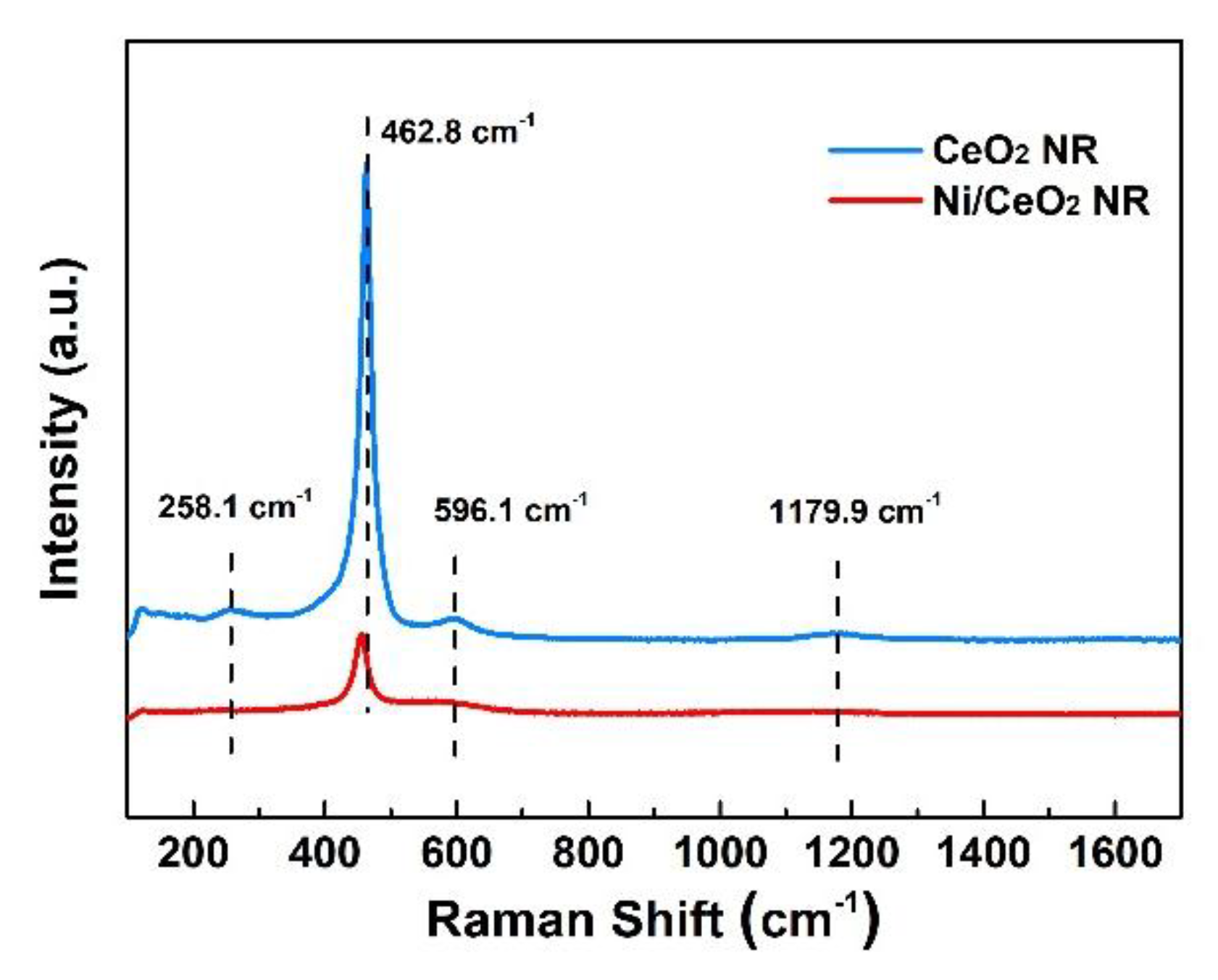
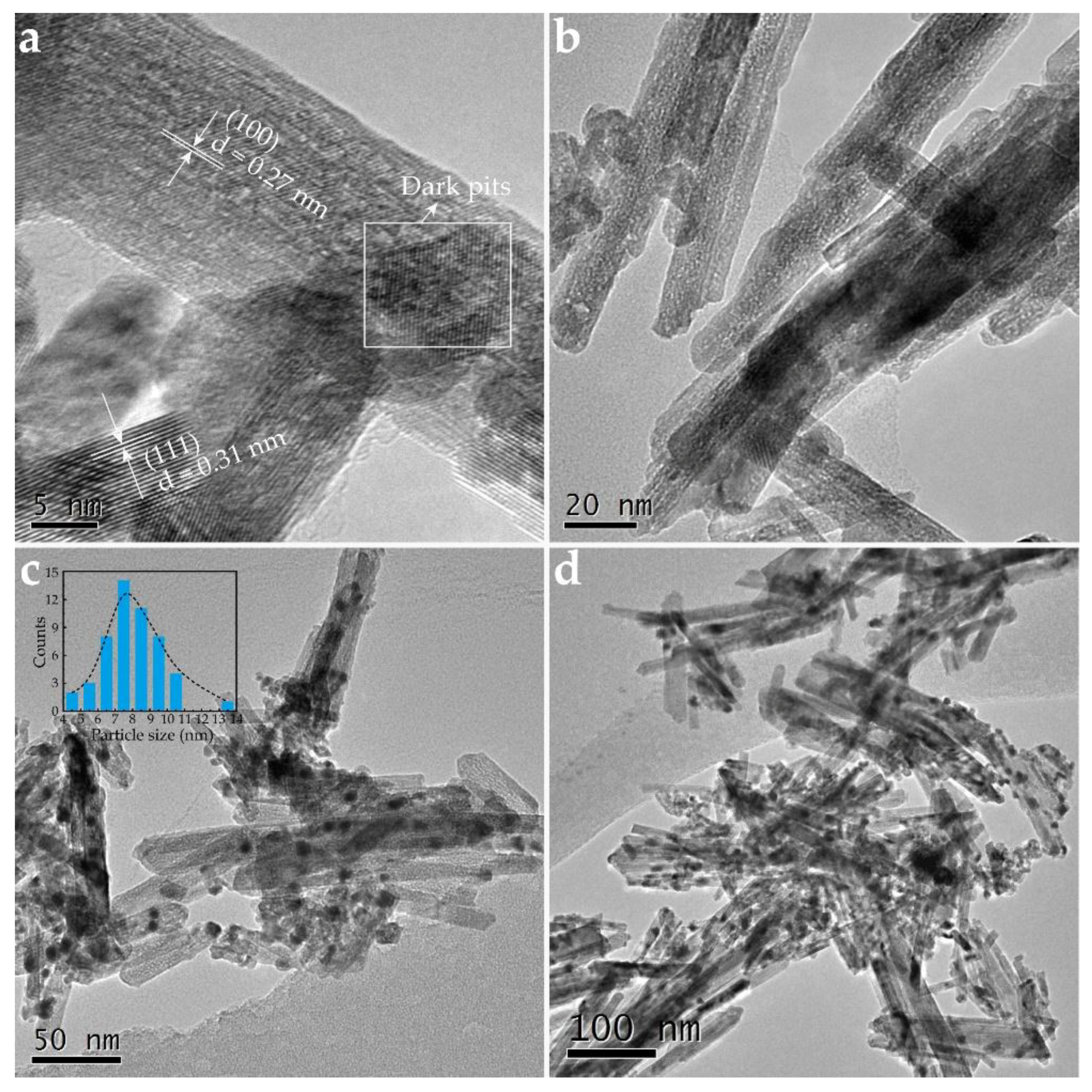
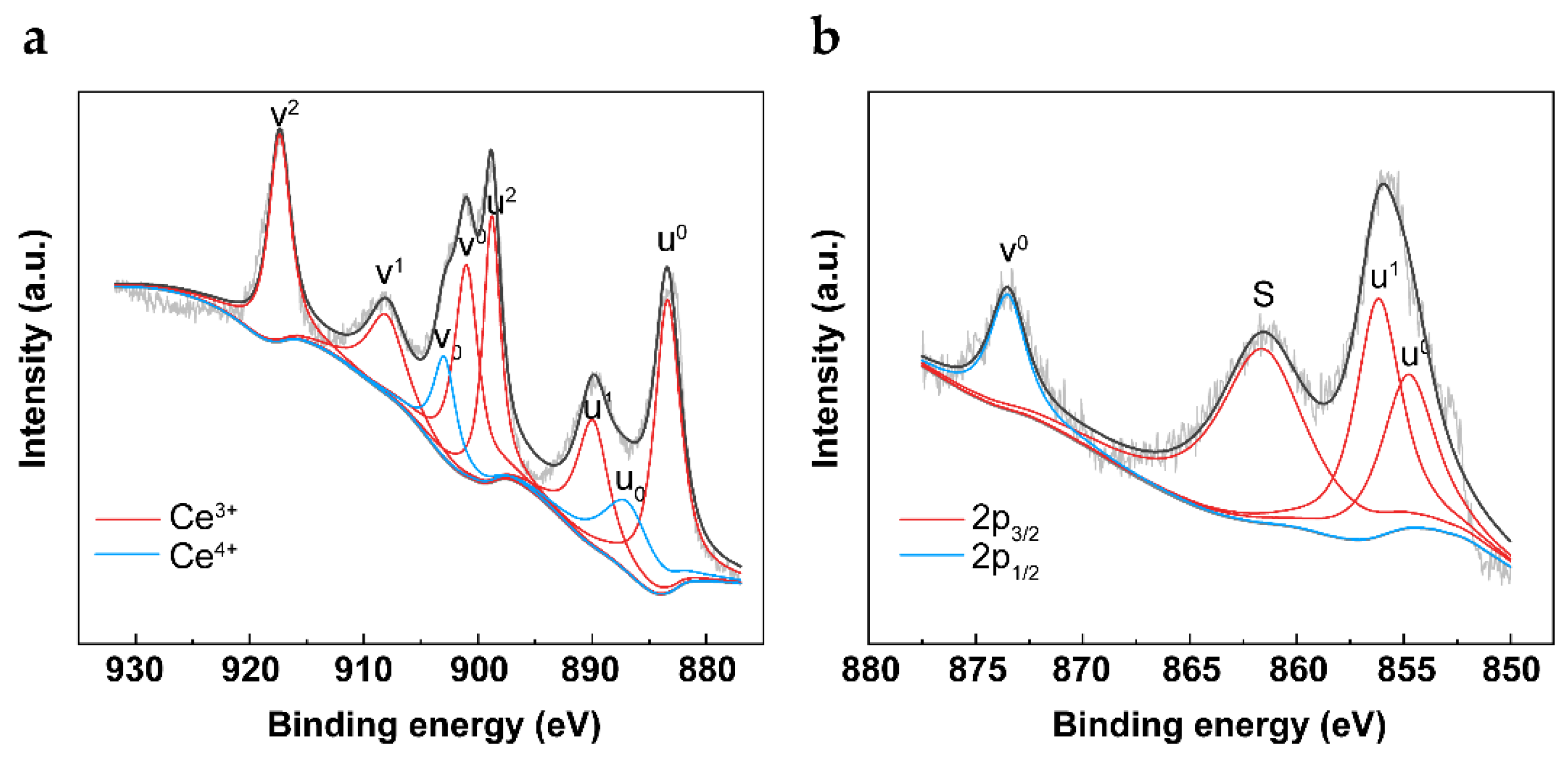
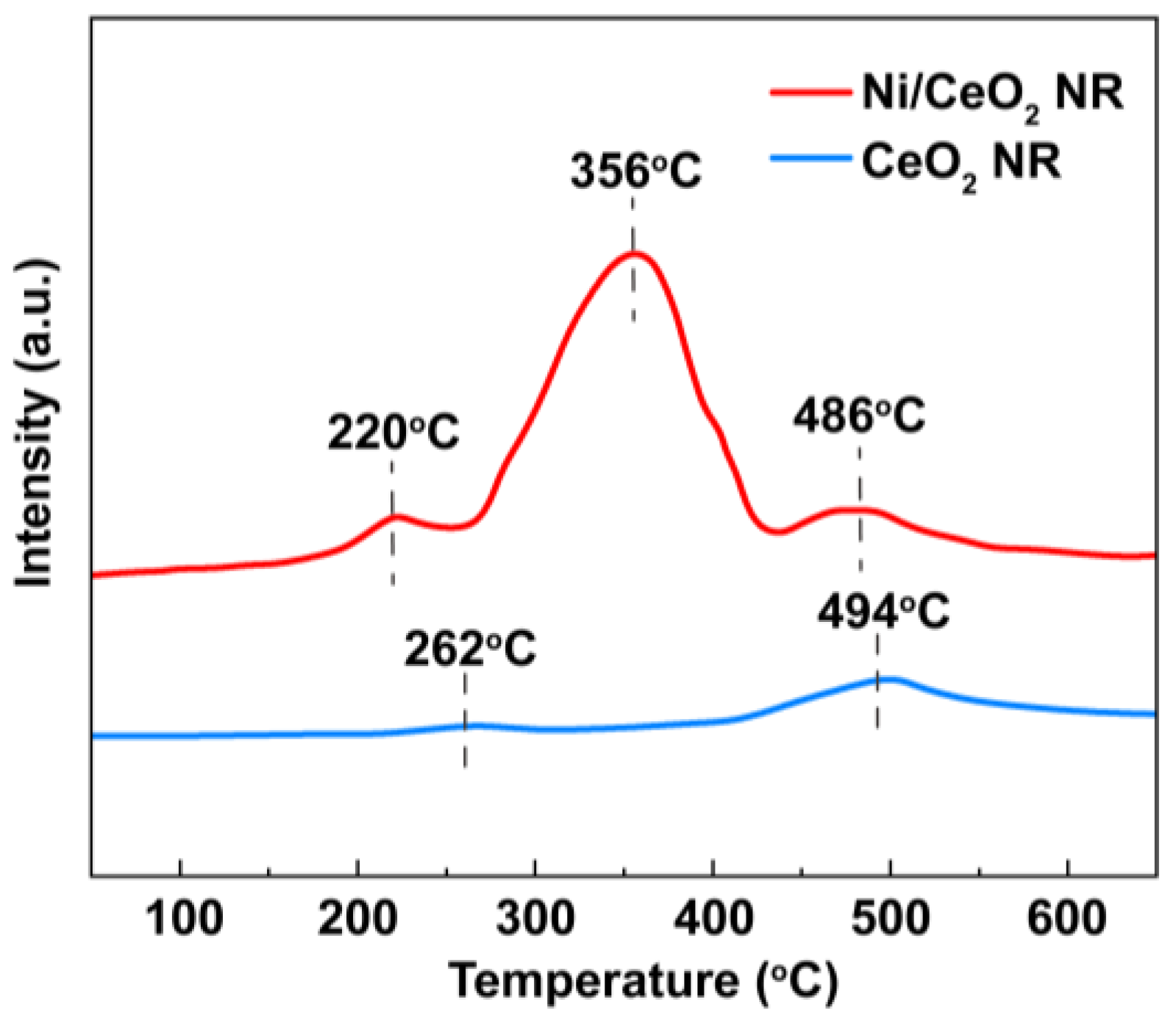
2.1. Characterization of OCs
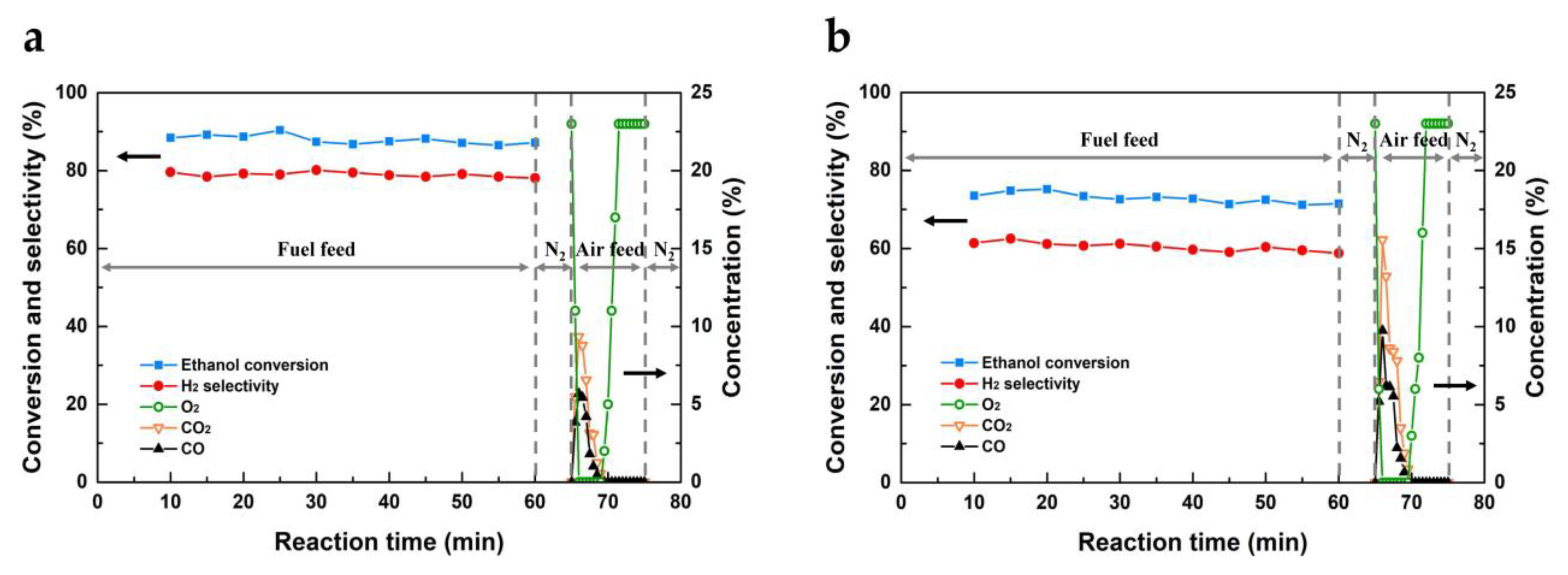
2.2 Activity Tests of OCs
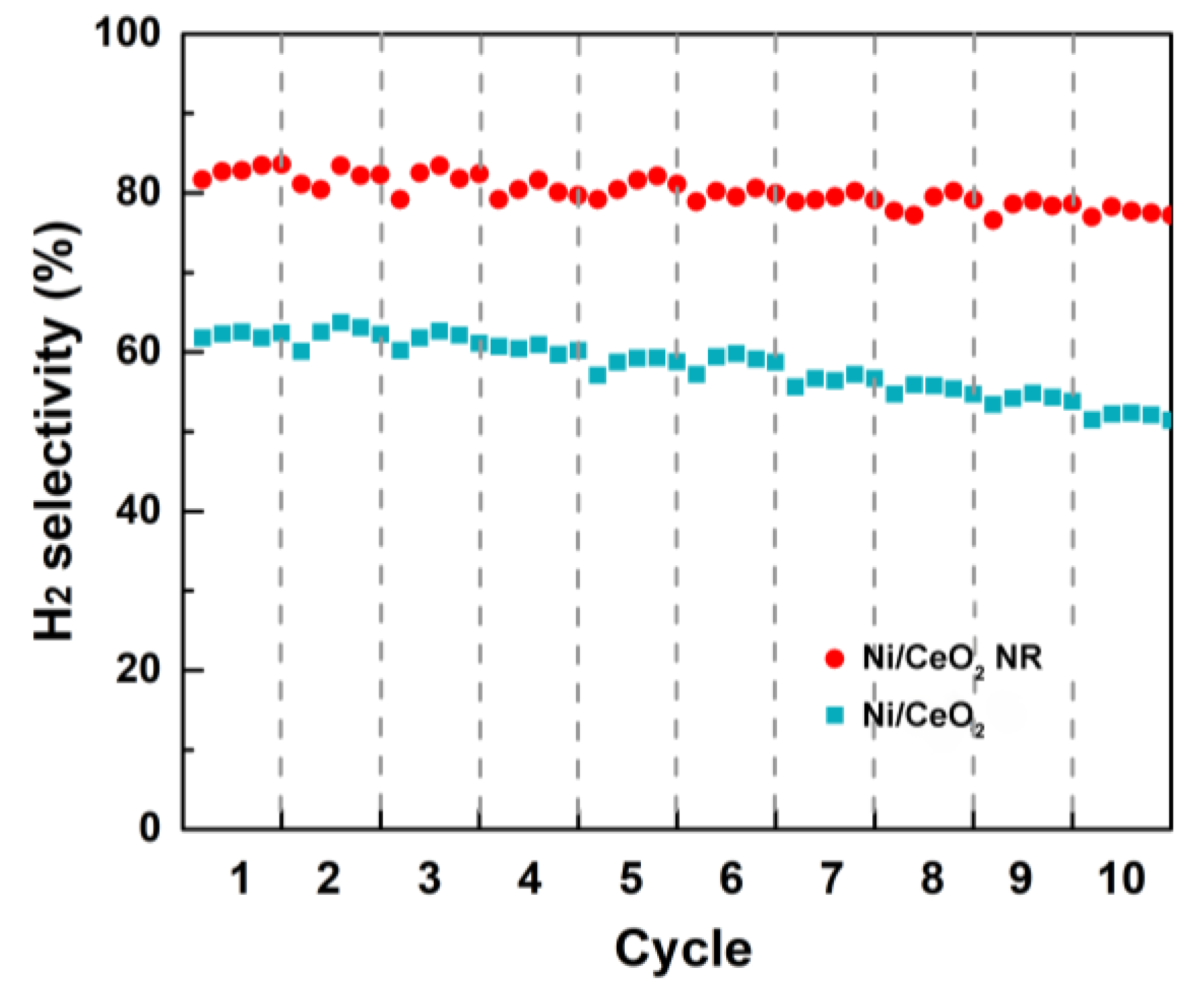
2.3. Stability Tests
3. Materials and Methods
3.1. Preparation of OCs
3.2. Characterization of OCs
3.3. Activity and Stability Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Chen, Y.; Cheng, Z.; Luo, X.; Jia, L.; Song, M.; Jiang, B.; Dou, B. Sorption-Enhanced Steam Reforming of Glycerol for Hydrogen Production over a NiO/NiAl2O4 Catalyst and Li2ZrO3-Based sorbent. Energy Fuels 2015, 29, 7408–7418. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Li, L.; Song, Y.; Jiang, B.; Wang, K.; Zhang, Q. A novel oxygen carrier for chemical looping reforming: LaNiO3 perovskite supported on montmorillonite. Energy 2017, 131, 58–66. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K. Hydrogen production by sorption-enhanced chemical looping steam reforming of ethanol in an alternating fixed-bed reactor: Sorbent to catalyst ratio dependencies. Energy Convers. Manage. 2018, 155, 243–252. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production and reduction of Ni-based oxygen carriers during chemical looping steam reforming of ethanol in a fixed-bed reactor. Int. J. Hydrogen Energy 2017, 42, 26217–26230. [Google Scholar] [CrossRef]
- de Diego, L.F.; Ortiz, M.; García-Labiano, F.; Adánez, J.; Abad, A.; Gayán, P. Hydrogen production by chemical-looping reforming in a circulating fluidized bed reactor using Ni-based oxygen carriers. J. Power Sources 2009, 192, 27–34. [Google Scholar] [CrossRef]
- Johansson, M.; Mattisson, T.; Lyngfelt, A.; Abad, A. Using continuous and pulse experiments to compare two promising nickel-based oxygen carriers for use in chemical-looping technologies. Fuel 2008, 87, 988–1001. [Google Scholar] [CrossRef]
- Zafar, Q.; Mattisson, T.; Gevert, B. Redox investigation of some oxides of transition-state metals Ni, Cu, Fe, and Mn supported on SiO2 and MgAl2O4. Energy Fuels 2006, 20, 34–44. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B-Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Dharanipragada, N.V.R.A.; Galvita, V.V.; Poelman, H.; Buelens, L.C.; Detavernier, C.; Marin, G.B. Bifunctional Co- and Ni- ferrites for catalyst-assisted chemical looping with alcohols. Appl. Catal. B-Environ. 2018, 222, 59–72. [Google Scholar] [CrossRef]
- García-Labiano, F.; de Diego, L.F.; Adánez, J.; Abad, A.; Gayán, P. Temperature variations in the oxygen carrier particles during their reduction and oxidation in a chemical-looping combustion system. Chem. Eng. Sci. 2005, 60, 851–862. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.Q.; Zhang, C.; Song, Y.C.; Chen, H.; Xu, Y. Hydrogen production by chemical looping steam reforming of ethanol using NiO/montmorillonite oxygen carriers in a fixed-bed reactor. Chem. Eng. J. 2016, 298, 96–106. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, X. Study of reaction mechanism of methane conversion over Ni-based oxygen carrier in chemical looping reforming. Fuel 2017, 210, 866–872. [Google Scholar] [CrossRef]
- Li, L.; Tang, D.; Song, Y.C.; Jiang, B.; Zhang, Q. Hydrogen production from ethanol steam reforming on Ni-Ce/MMT catalysts. Energy 2018, 149, 937–943. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B-Environ. 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H.F. Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, S.D.; Rodriguez, J.A.; Stacchiola, D. Electronic metal–support interactions and the production of hydrogen through the water-gas shift reaction and ethanol steam reforming: Fundamental studies with well-defined model catalysts. Top. Catal. 2013, 56, 1488–1498. [Google Scholar] [CrossRef]
- Jiang, B.; Li, L.; Bian, Z.F.; Li, Z.; Othman, M.; Sun, Z.; Tang, D.; Kawi, S.; Dou, B. Hydrogen generation from chemical looping reforming of glycerol by Ce-doped nickel phyllosilicate nanotube oxygen carriers. Fuel 2018, 222, 185–192. [Google Scholar] [CrossRef]
- Wang, K.; Dou, B.; Jiang, B.; Song, Y.C.; Zhang, C.; Zhang, Q.; Chen, H.; Xu, H. Renewable hydrogen production from chemical looping steam reforming of ethanol using xCeNi/SBA-15 oxygen carriers in a fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12899–12909. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Morphology dependence of catalytic properties of Ni/CeO2 nanostructures for carbon dioxide reforming of methane. J. Phys. Chem. C 2012, 116, 10009–10016. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B-Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Yi, G.; Xu, Z.; Guo, G.; Tanaka, K.I.; Yuan, Y. Morphology effects of nanocrystalline CeO2 on the preferential CO oxidation in H2-rich gas over Au/CeO2 catalyst. Chem. Phys. Lett. 2009, 479, 128–132. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L. CTAB assisted hydrothermal synthesis, controlled conversion and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J. Solid State Chem. 2008, 181, 1298–1306. [Google Scholar] [CrossRef]
- Mamontov, E.; Egami, T.; Brezny, R.; Koranne, M.; Tyagi, S. Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J. Phys. Chem. B 2000, 104, 11110–11116. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure–activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B-Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman analysis of mode softening in nanoparticle CeO2−δ and Au-CeO2−δ during CO oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wu, F.; Zhu, B.; Gao, X.; Zhu, H.; Yan, T.; Huang, W.; Wu, S.; Song, D. CeO2 nanorods and gold nanocrystals supported on CeO2 nanorods as catalyst. J. Phys. Chem. B 2005, 109, 19169–19174. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, K.; Wang, L.; Wang, B.; Li, Y. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods. J. Am. Chem. Soc. 2009, 131, 3140–3141. [Google Scholar] [CrossRef] [PubMed]
- Sayle, D.C.; Feng, X.; Ding, Y.; Wang, Z.L.; Sayle, T.X. “Simulating synthesis”: Ceria nanosphere self-assembly into nanorods and framework architectures. J. Am. Chem. Soc. 2007, 129, 7924–7935. [Google Scholar] [CrossRef] [PubMed]
- Ta, N.; Liu, J.; Chenna, S.; Crozier, P.A.; Li, Y.; Chen, A.; Shen, W. Stabilized gold nanoparticles on ceria nanorods by strong interfacial anchoring. J. Am. Chem. Soc. 2012, 134, 20585–20588. [Google Scholar] [CrossRef] [PubMed]
- Lemonidou, A.; Goula, M.; Vasalos, I. Carbon dioxide reforming of methane over 5 wt % nickel calcium aluminate catalysts–Effect of preparation method. Catal. Today 1998, 46, 175–183. [Google Scholar] [CrossRef]
- Tang, S.; Lin, J.; Tan, K.L. Partial oxidation of methane to syngas over Ni/MgO, Ni/CaO and Ni/CeO2. Catal. Lett. 1998, 51, 169–175. [Google Scholar] [CrossRef]
- Maitarad, P.; Han, J.; Zhang, D.; Shi, L.; Namuangruk, S.; Rungrotmongkol, T. Structure–activity relationships of NiO on CeO2 nanorods for the selective catalytic reduction of NO with NH3: Experimental and DFT studies. J. Phys. Chem. C 2014, 118, 9612–9620. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Van Veen, A.; Provendier, H.; Mirodatos, C.; Shen, W. Autothermal reforming of ethanol for hydrogen production over a Rh/CeO2 catalyst. Catal. Today 2008, 138, 152–156. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.Q.; Song, Y.C.; Chen, H.; Zhang, C.; Xu, Y.; Li, M. Hydrogen production from chemical looping steam reforming of glycerol by Ni based Al-MCM-41 oxygen carriers in a fixed-bed reactor. Fuel 2016, 183, 170–176. [Google Scholar] [CrossRef]
- Li, L.; Tang, D.; Song, Y.C.; Jiang, B. Dual-film optofluidic microreactor with enhanced light-harvesting for photocatalytic applications. Chem. Eng. J. 2018, 339, 71–77. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Zhou, G.; Stacchiola, D.; Stach, E.; Rodriguez, J. Steam reforming of ethanol on Ni/CeO2: Reaction Pathway and interaction between Ni and the CeO2 support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, C.; Wang, K.Q.; Dou, B.; Song, Y.C.; Chen, H.; Xu, Y. Highly dispersed Ni/montmorillonite catalyst for glycerol steam reforming: Effect of Ni loading and calcination temperature. Appl. Therm. Eng. 2016, 109, 99–108. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Song, Y.C.; Zhang, C.; Du, B.; Chen, H.; Xu, Y. Hydrogen production from chemical looping steam reforming of glycerol by Ni-based oxygen carrier in a fixed-bed reactor. Chem. Eng. J. 2015, 280, 459–467. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.Q.; Zhang, C.; Li, M.; Chen, H.; Xu, Y. Sorption enhanced steam reforming of biodiesel by-product glycerol on Ni-CaO-MMT multifunctional catalysts. Chem. Eng. J. 2017, 313, 207–216. [Google Scholar] [CrossRef]
| Sample | Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) | Ni Content (wt %) | Ni Dispersion (m2/gNi) | Ni Crystal Size/Ni Average Particle Size (nm) | CeO2 Crystal Size (nm) |
|---|---|---|---|---|---|---|---|
| Ni/CeO2-NR | 75.1 | 18.2 | 0.37 | 9.7 1 | 6.5 2 | 8.9 3/8.1 4 | 16.1 3 |
| CeO2-NR | 90.3 | 26.5 | 0.80 | N/A | N/A | N/A | 14.8 |
| OCs | Ni Loading (wt %) | T (°C) | S/C | Tested Cycles | Reactant | Average Conversion (%) | Average H2 Yield (%) | References |
|---|---|---|---|---|---|---|---|---|
| CeNi/MCM-41 | 6 | 650 | 1.5 | 10 | Glycerol | ~90 | 6.2 | [37] |
| LaNiO3/MMT | 13 | 650 | 3 | 10 | Ethanol | ~90 | - | [3] |
| Ni/MMT | 19.9 | 650 | 4 | 20 | Ethanol | ~78 | - | [13] |
| CeNi/PSNT | 24.7 | 650 | 3 | 10 | Glycerol | ~100 | 12.5 | [19] |
| CeNi/SBA-15 | 12 | 650 | 3 | 14 | Ethanol | ~84 | - | [20] |
| Ni/CeO2-NR | 9.7 | 650 | 4 | 10 | Ethanol | ~90 | - | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jiang, B.; Tang, D.; Zheng, Z.; Zhao, C. Hydrogen Production from Chemical Looping Reforming of Ethanol Using Ni/CeO2 Nanorod Oxygen Carrier. Catalysts 2018, 8, 257. https://doi.org/10.3390/catal8070257
Li L, Jiang B, Tang D, Zheng Z, Zhao C. Hydrogen Production from Chemical Looping Reforming of Ethanol Using Ni/CeO2 Nanorod Oxygen Carrier. Catalysts. 2018; 8(7):257. https://doi.org/10.3390/catal8070257
Chicago/Turabian StyleLi, Lin, Bo Jiang, Dawei Tang, Zhouwei Zheng, and Cong Zhao. 2018. "Hydrogen Production from Chemical Looping Reforming of Ethanol Using Ni/CeO2 Nanorod Oxygen Carrier" Catalysts 8, no. 7: 257. https://doi.org/10.3390/catal8070257
APA StyleLi, L., Jiang, B., Tang, D., Zheng, Z., & Zhao, C. (2018). Hydrogen Production from Chemical Looping Reforming of Ethanol Using Ni/CeO2 Nanorod Oxygen Carrier. Catalysts, 8(7), 257. https://doi.org/10.3390/catal8070257






