Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl Hexanoate Synthesis
Abstract
1. Introduction
2. Results
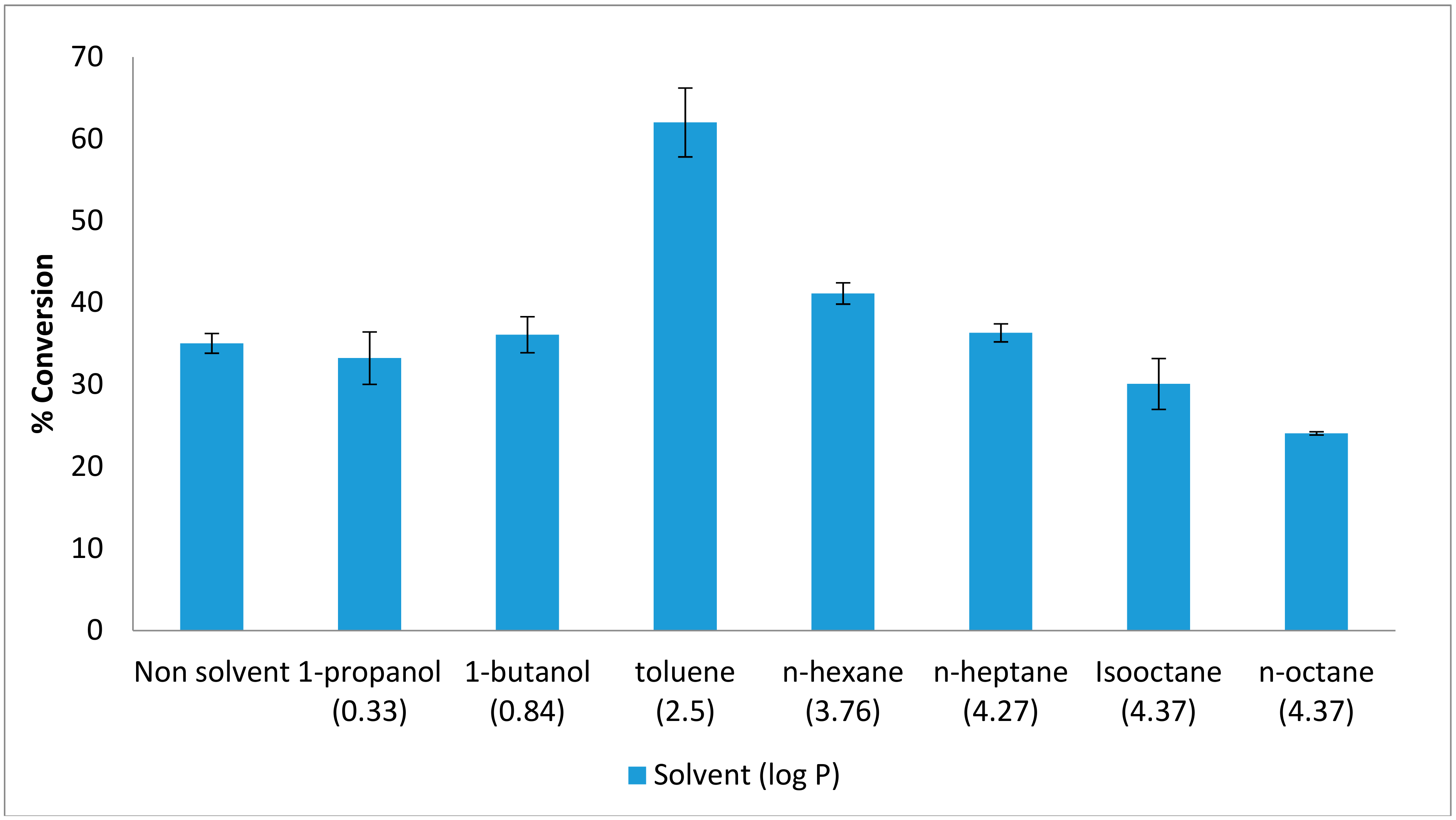
2.1. Effect of Organic Solvents on the Esterification Reaction
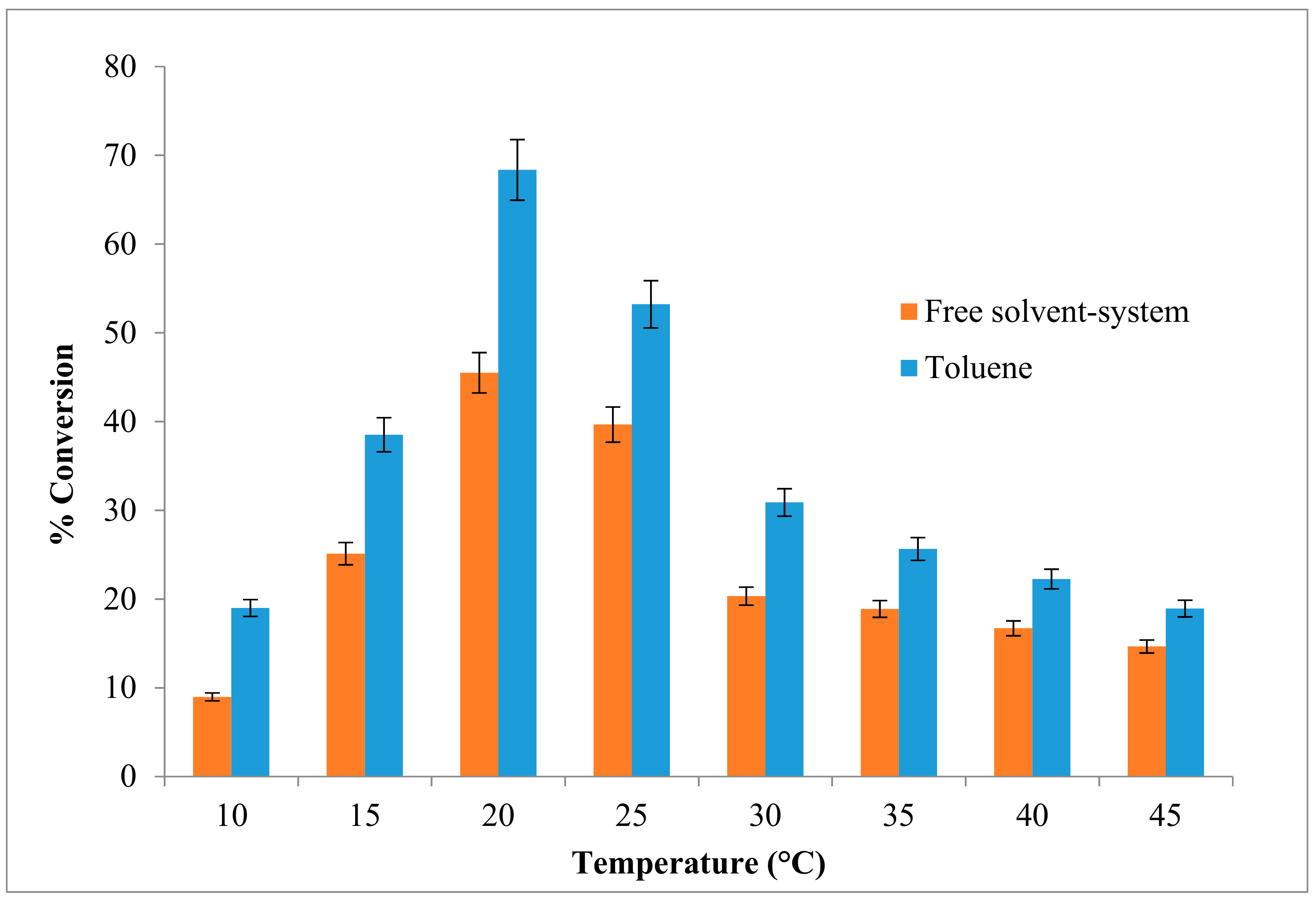
2.2. Effect of Temperature on the Esterification Reaction
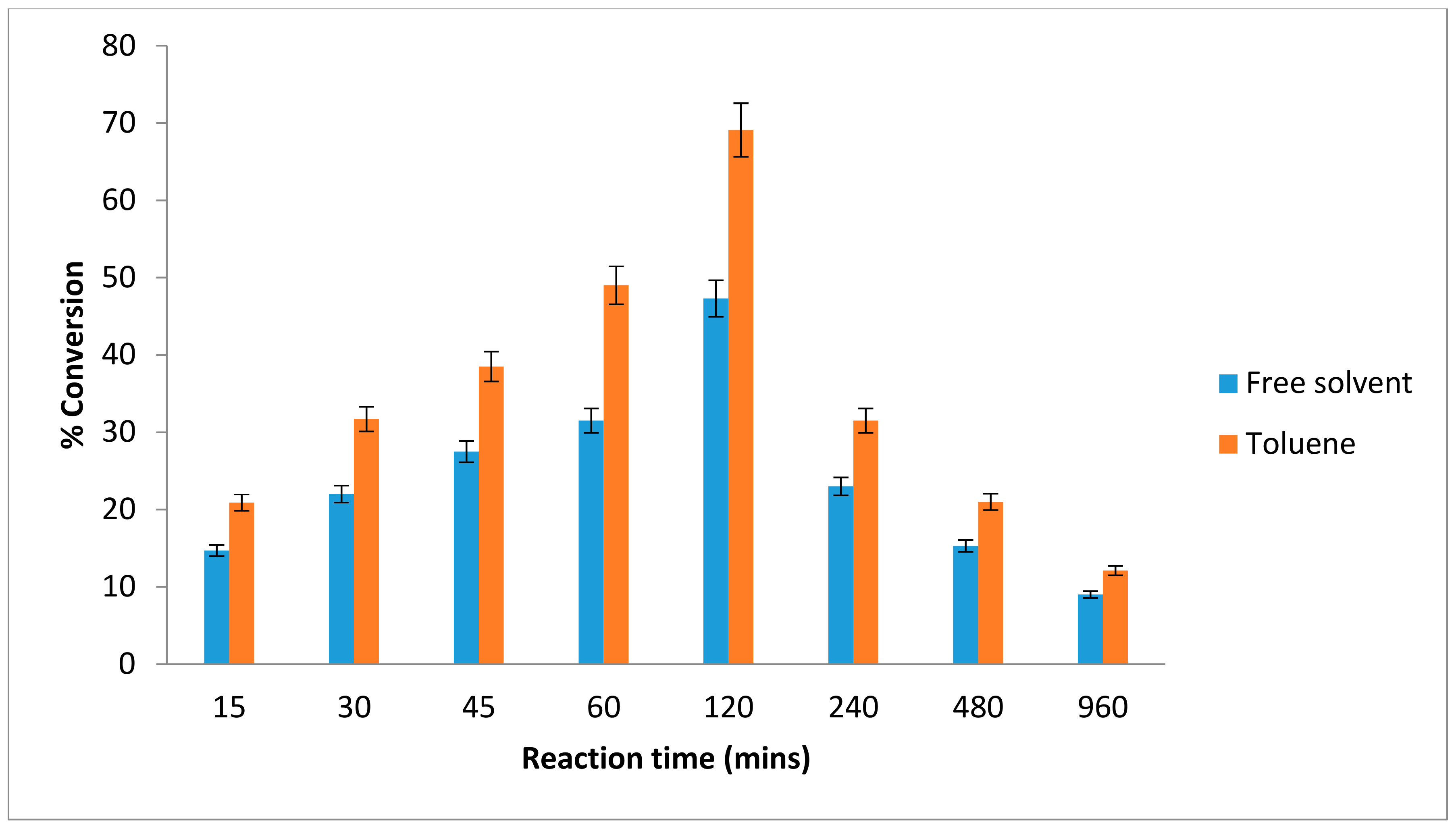
2.3. Effect of Time on the Esterification Reaction
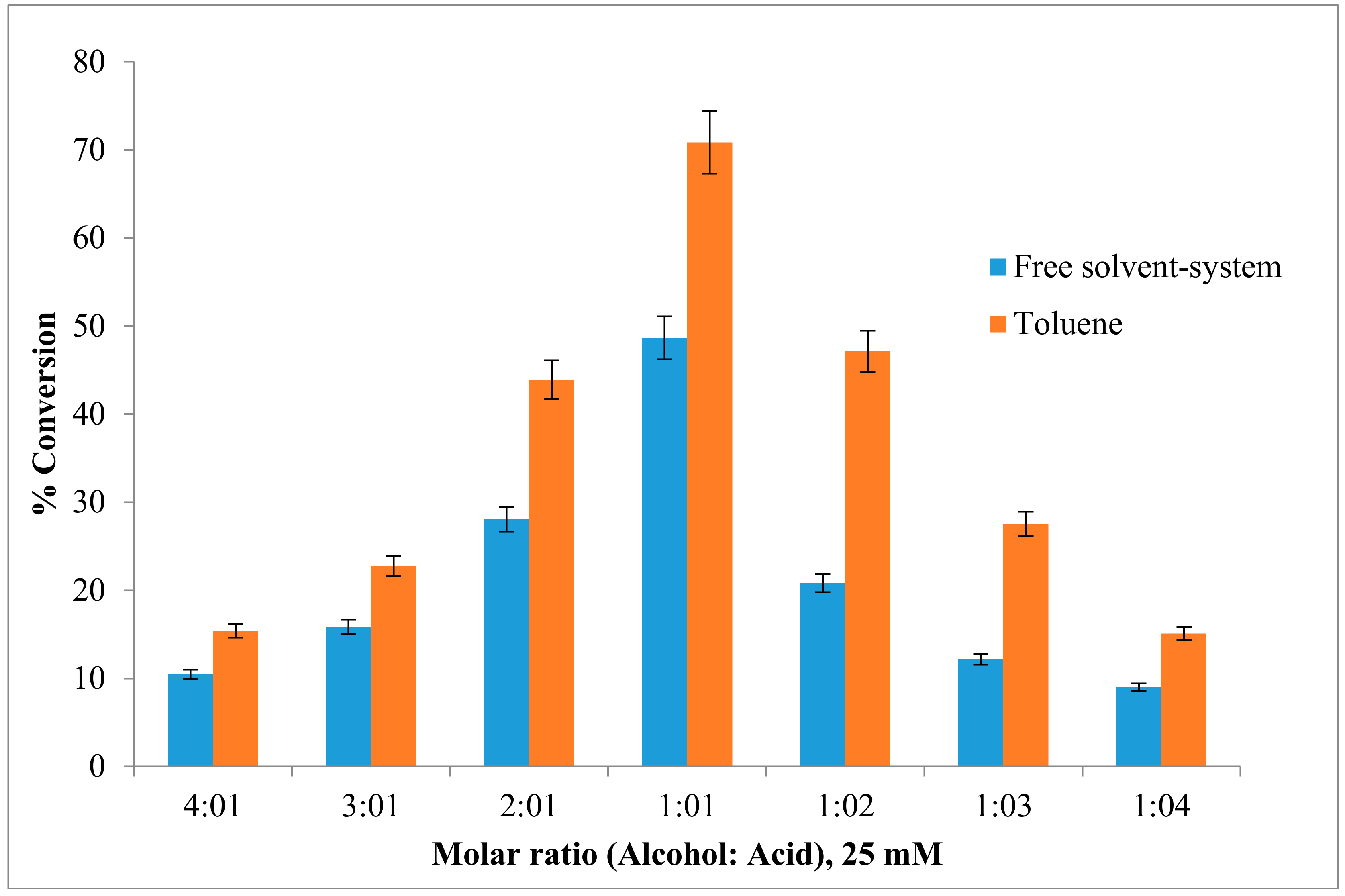
2.4. Effect of the Substrate Molar Ratio on the Esterification Reaction
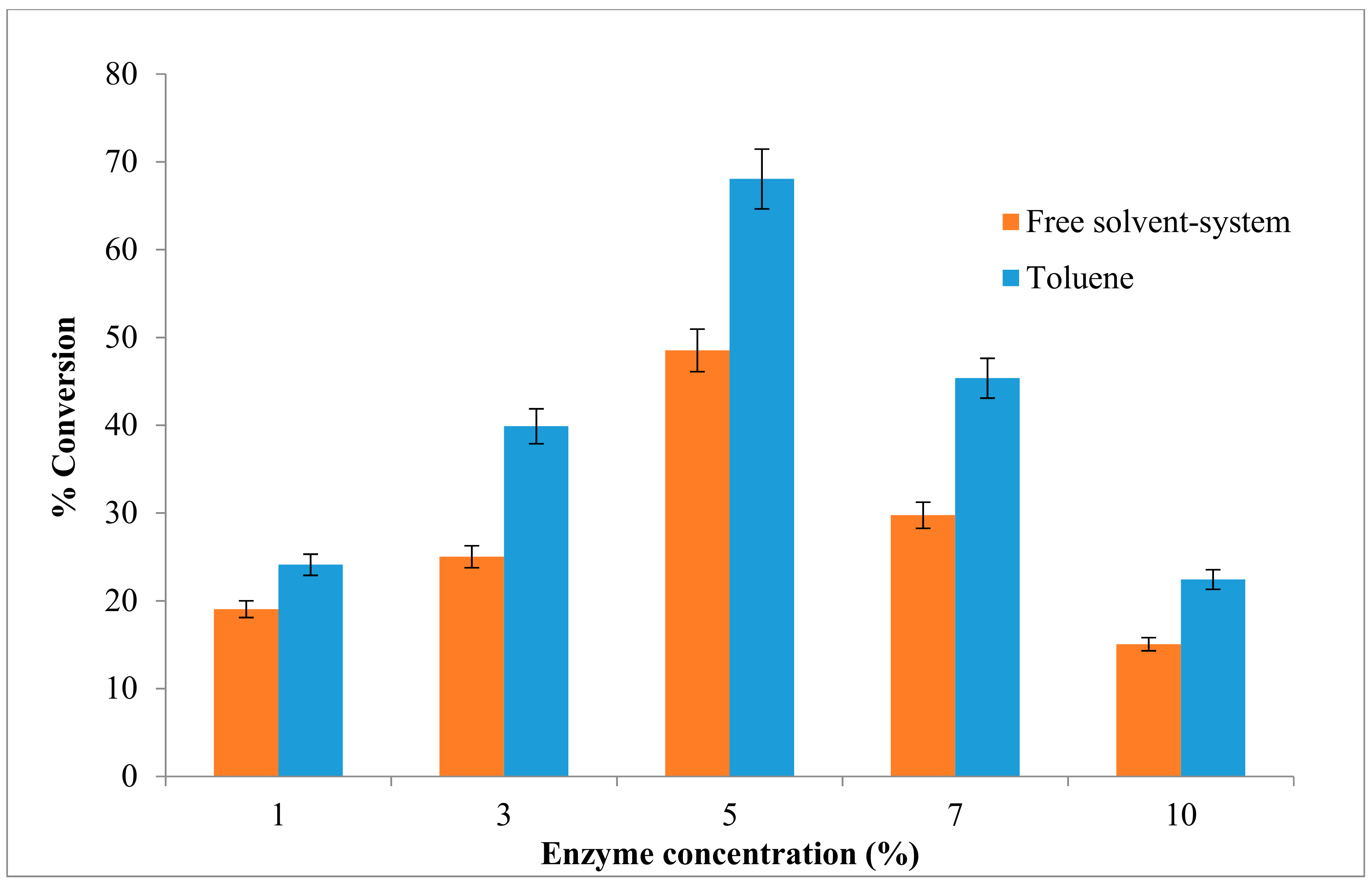
2.5. Effect of the Enzyme Concentration on the Esterification Reaction
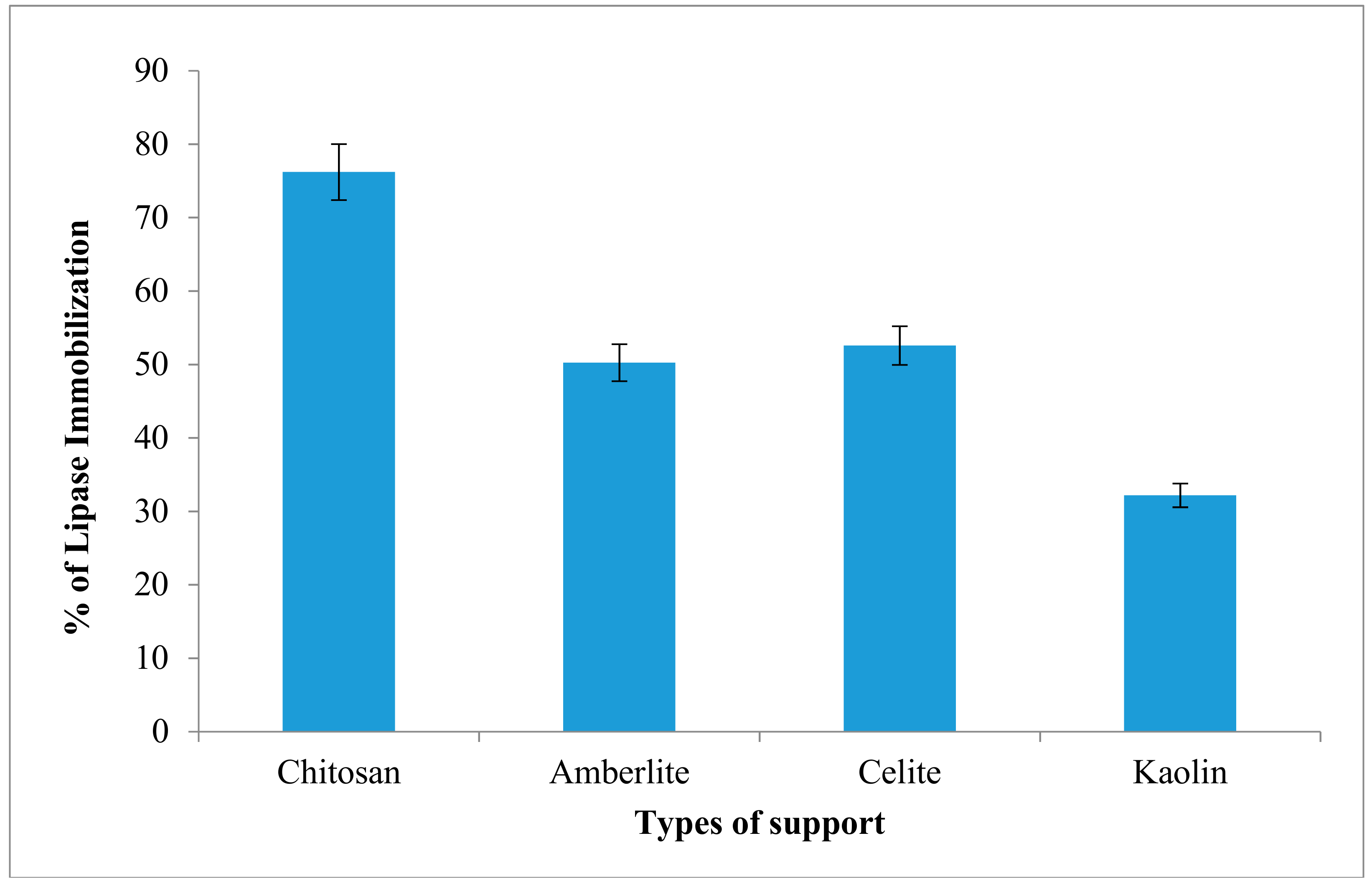
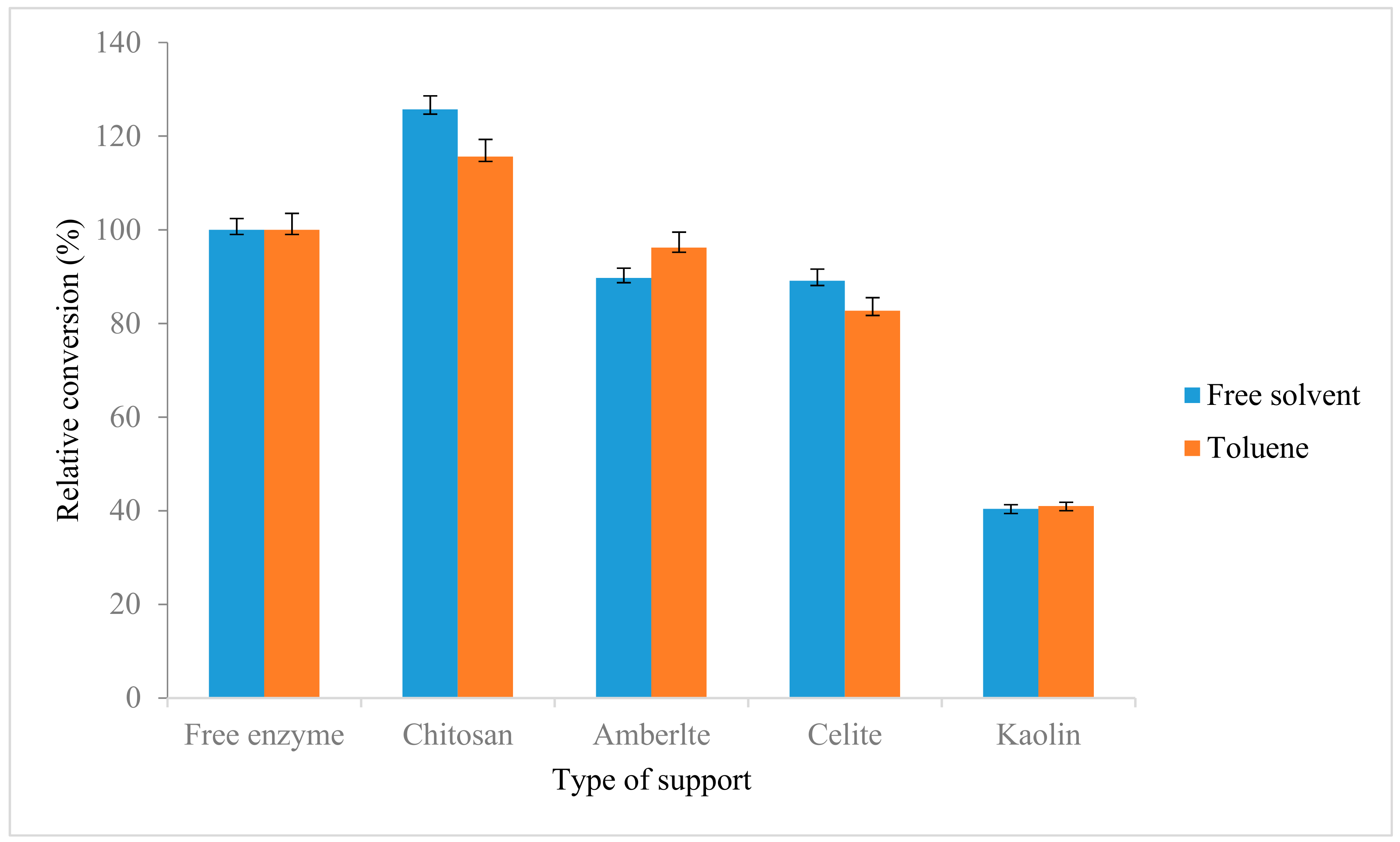
2.6. Immobilization of Lipase
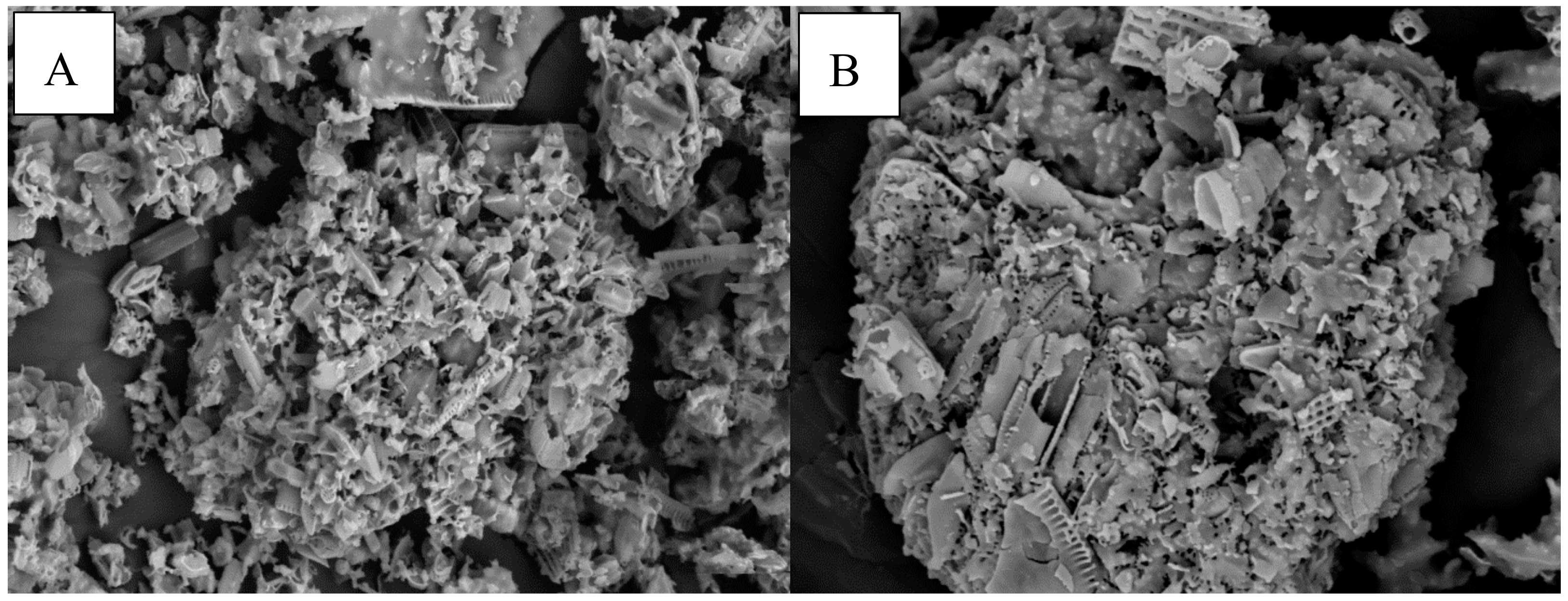
2.7. Morphology Analysis of Chitosan Using Scanning Electron Microscopy (SEM)
2.8. Fourier Transform Infrared (FTIR) and Gas Chromatography–Mass Spectrometry (GC–MS) Analyses of Flavor Ester
3. Materials and Methods
3.1. Materials
3.2. Preparation of Crude Freeze-Dried AMS8 Lipase
3.3. Lipase Hydrolytic Activity
3.4. Synthesis of Ethyl Hexanoate
3.4.1. In Solvent-Free System
3.4.2. In Toluene
3.5. Reaction Analysis
3.6. Effect of Reaction Temperature
3.7. Effect of Reaction Time
3.8. Effect of Substrate Concentration
3.9. Effect of Enzyme Concentration
3.10. Immobilization of AMS8 Using Commercial Supports
Esterification Reaction Using the Immobilized Lipase
3.11. Fourier Transform Infra-Red Spectroscopy (FTIR) and Gas Chromatography—Mass Spectrometry (GC–MS) Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Muhamad, S.K.; Radzi, S.M.; Othman, S.S.; Basyaruddin, M.; Rahman, A.; Noor, H.M. Optimization of lipase-catalyzed synthesis of flavor esters in solvent free system. J. Fundam. Sci. 2010, 6, 31–36. [Google Scholar]
- Joseph, B.; Ramteke, P.W.; Thomas, G. Cold active microbial lipases: Some hot issues and recent developments. Biotechnol. Adv. 2008, 26, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, P.; Kumari, A.; Kumar Garlapati, V.; Banerjee, R.; Nag, A. Enzymatic synthesis of fruit flavor esters by immobilized lipase from Rhizopus oligosporus optimized with response surface methodology. J. Mol. Catal. B Enzym. 2009, 60, 57–63. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-Chaâbouni, M.; Bezzine, S.; Miled, N.; Gargouri, Y. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzym. Microb. Technol. 2006, 38, 788–794. [Google Scholar] [CrossRef]
- Liaquat, M.; Apenten, R.K.O. Synthesis of Low Molecular Weight Flavor Esters Using Plant Seedling Lipases in Organic Media. J. Food Sci. 2000, 65, 295–299. [Google Scholar] [CrossRef]
- Abbas, H.; Hiol, A.; Deyris, V.; Comeau, L. Isolation and characterization of an extracellular lipase from Mucor sp strain isolated from palm fruit. Enzyme Microb. Technol. 2002, 31, 968–975. [Google Scholar] [CrossRef]
- Bisswanger, H. Practical Enzymology; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zhang, X.; Guan, R.-F.; Wu, D.-Q.; Chan, K.-Y. Enzyme immobilization on amino-functionalized mesostructured cellular foam surfaces, characterization and catalytic properties. J. Mol. Catal. B Enzym. 2005, 33, 43–50. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Reetz, M.T. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998, 16, 396–403. [Google Scholar] [CrossRef]
- Abdul Rahman, M.B.; Tajudin, S.M.; Hussein, M.Z.; Abdul Rahman, R.N.Z.R.; Salleh, A.B.; Basri, M. Application of natural kaolin as support for the immobilization of lipase from Candida rugosa as biocatalsyt for effective esterification. Appl. Clay Sci. 2005, 29, 111–116. [Google Scholar] [CrossRef]
- Reslow, M.; Adlercreutz, P.; Mattiasson, B. On the importance of the support material for bioorganic synthesis. Influence of water partition between solvent, enzyme and solid support in water-poor reaction media. Eur. J. Biochem. 1988, 172, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Ganasen, M.; Rahman, R.N.; Chor, A.L.; Salleh, A.B.; Basri, M. Cold-adapted RTX lipase from Antarctic Pseudomonas sp. strain AMS8: Isolation, molecular modeling and heterologous expression. Protein J. 2013, 32, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A. Improving Enzymes by Using Them in Organic Solvents. Nature 2001, 409, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ghamgui, H.; Karra-Chaâbouni, M.; Gargouri, Y. 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: A comparative study between n-hexane and solvent-free system. Enzym. Microb. Technol. 2004, 35, 355–363. [Google Scholar] [CrossRef]
- Torres, S.; Baigorí, M.D.; Swathy, S.L.; Pandey, A.; Castro, G.R. Enzymatic synthesis of banana flavour (isoamyl acetate) by Bacillus licheniformis S-86 esterase. Food Res. Int. 2009, 42, 454–460. [Google Scholar] [CrossRef]
- Latip, W.; Raja Abd Rahman, R.N.Z.; Leow, A.T.C.; Mohd Shariff, F.; Kamarudin, N.H.A.; Mohamad Ali, M.S. The Effect of N-Terminal Domain Removal towards the Biochemical and Structural Features of a Thermotolerant Lipase from an Antarctic Pseudomonas sp. strain AMS3. Int. J. Mol. Sci. 2018, 19, 560. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kanwar, S.S. Organic solvent tolerant lipases and applications. Sci. World J. 2014, 2014, 625258. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, R.Z.; Dordick, J.S.; Klibanov, A.M. Enzymatic analyses in organic solvents. Biotechnol. Bioeng. 1986, 28, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Pinyaphong, P.; Phutrakul, S. Synthesis of cocoa butter equivalent from palm oil by Carica papaya lipase-catalyzed interesterification. Chiang Mai J. Sci. 2009, 36, 359–368. [Google Scholar]
- Tan, S.; Apenten, R.K.O.; Knapp, J. Low temperature organic phase biocatalysis using cold-adapted lipase from psychrotrophic Pseudomonas P38. Food Chem. 1996, 57, 415–418. [Google Scholar] [CrossRef]
- Gupta, A.; Khare, S.K. Enzymes from solvent-tolerant microbes: Useful biocatalysts for non-aqueous enzymology. Crit. Rev. Biotechnol. 2009, 29, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Selvam, K.; Vishnupriya, B.; Maanvizhi, M. Enzymatic Synthesis of Fragrance Ester by Lipase from Marine Actinomycetes for Textile Industry. Int. J. Eng. Adv. Technol. 2013, 3, 91–96. [Google Scholar]
- Choo, D.; Kurihara, T.; Suzuki, T.; Soda, K. A Cold-Adapted Lipase of an Alaskan Gene Cloning and Enzyme Purification and Characterization A Cold-Adapted Lipase of an Alaskan Psychrotroph, Pseudomonas sp. Strain B11-1: Gene Cloning and Enzyme Purification and Characterization. Appl. Environ. Microbiol. 1998, 64, 486–491. [Google Scholar] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Feller, G.; Gerday, C.; Russell, N. Cold-adapted microorganisms: Adaptation strategies and biotechnological potential. In Encyclopedia of Environmental Microbiology; John Wiley & Sons, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Baba, T.; Ooguri, T.; Moriyama, S.; Terai, T.; Tominaga, Y. Ethyl esterification of docosahexaenoic acid in an organic solvent-free system with immobilized Candida antarctica lipase. J. Biosci. Bioeng. 2001, 92, 19–23. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Zhao, A.; Ma, X. Study of glucose ester synthesis by immobilized lipase from Candida sp. Catal. Commun. 2008, 9, 1369–1374. [Google Scholar] [CrossRef]
- Aracil, J.; Martinez, M.; Sa’nchez, N.; Corma, A. Formation of a jojoba oil analog by esterification of oleic acid using zeolites as catalyst. Zeolites 1992, 12, 233–236. [Google Scholar] [CrossRef]
- Gulati, R.; Arya, P.; Malhotra, B.; Prasad, A.K.; Saxena, R.K.; Kumar, J.; Watterson, A.C.; Parmar, V.S. Novel biocatalytic esterification reactions on fatty acids: Synthesis of sorbitol 1(6)-monostearate. Arch. Org. Chem. 2003, 2003, 159–170. [Google Scholar]
- Halling, P.J. Thermodynamic predictions for biocatalysis in nonconventional media: Theory, tests, and recommendations for experimental design and analysis. Enzyme Microb. Technol. 1994, 16, 178–206. [Google Scholar] [CrossRef]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Macarie, E.; Baratti, J. Short chain flavour ester synthesis by a new esterase from Bacillus licheniformis. J. Mol. Catal. B Enzym. 2000, 10, 377–383. [Google Scholar] [CrossRef]
- Gillies, B.; Yamazaki, H.; Armstrong, D.W. Production of flavor esters by immobilized lipase. Biotechnol. Lett. 1987, 9, 709–714. [Google Scholar] [CrossRef]
- Welsh, F.W.; Williams, R.E.; Dawson, K.H. Lipase Mediated Synthesis of Low Molecular Weight Flavor Esters. J. Food Sci. 1990, 55, 1679–1682. [Google Scholar] [CrossRef]
- Chowdary, G.V.; Ramesh, M.N.; Prapulla, S.G. Enzymic synthesis of isoamyl isovalerate using immobilized lipase from Rhizomucor miehei: A multivariate analysis. Process Biochem. 2000, 36, 331–339. [Google Scholar] [CrossRef]
- Marty, A.; Chulalaksananukul, W.; Willemot, R.M.; Condoret, J.S. Kinetics of lipase-catalyzed esterification in supercritical CO2. Biotechnol. Bioeng. 1992, 39, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Hari Krishna, S.; Divakar, S.; Prapulla, S.G.; Karanth, N.G. Enzymatic synthesis of isoamyl acetate using immobilized lipase from Rhizomucor miehei. J. Biotechnol. 2001, 87, 193–201. [Google Scholar] [CrossRef]
- Romero, M.D.; Calvo, L.; Alba, C.; Habulin, M.; Primožič, M.; Knez, Ž. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in supercritical carbon dioxide. J. Supercrit. Fluids 2005, 33, 77–84. [Google Scholar] [CrossRef]
- Bezbradica, D.; Mijin, D.; Šiler-Marinković, S.; Knežević, Z. The effect of substrate polarity on the lipase-catalyzed synthesis of aroma esters in solvent-free systems. J. Mol. Catal. B Enzym. 2007, 45, 97–101. [Google Scholar] [CrossRef]
- Sánchez, N.; Martinez, M.; Aracil, J.; Corma, A. Synthesis of oleyl oleate as a jojoba oil analog. J. Am. Oil Chem. Soc. 1992, 69, 1150–1153. [Google Scholar] [CrossRef]
- Xin, J.Y.; Li, S.B.; Xu, Y.; Wang, L.L. Enzymatic resolution of (S)-(+)-naproxen in a trapped aqueous-organic solvent biphase continuous reactor. Biotechnol. Bioeng. 2000, 68, 78–83. [Google Scholar] [CrossRef]
- Karra-Châabouni, M.; Ghamgui, H.; Bezzine, S.; Rekik, A.; Gargouri, Y. Production of flavour esters by immobilized Staphylococcus simulans lipase in a solvent-free system. Process Biochem. 2006, 41, 1692–1698. [Google Scholar] [CrossRef]
- Liaquat, M.; Khan, S.; Aslam, S.; Khan, A.; Khan, H.; Khan, S.M.; Ali, S.; Wahab, S.; Bhatti, H.N. Rape Seedling Lipase Catalyzed Synthesis of Flavor Esters Through Transesterification in Hexane. J. Chem. Soc. Pakistan 2012, 34, 144–150. [Google Scholar]
- Alves Macedo, G.; Soberón Lozano, M.M.; Pastore, G.M. Enzymatic synthesis of short chain citronellyl esters by a new lipase from Rhizopus sp. Electron. J. Biotechnol. 2003, 9, 72–75. [Google Scholar] [CrossRef]
- Zulkeflee, S.A.; Sata, S.A.; Aziz, N. Kinetic Model of Batch Lipase-Catalyzed Esterification Process with Function of Temperature and Water Content. Appl. Mech. Mater. 2013, 284–287, 423–428. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, P.K.; Sharma, N.; Pal, O.P.; Malviya, R. Applications of Chitosan and Chitosan Derivatives in Drug Delivery. Biol. Res. 2011, 5, 28–37. [Google Scholar]
- Othman, S.S.; Basri, M.; Hussein, M.Z.; Abdul Rahman, M.B.; Rahman, R.N.Z.A.; Salleh, A.B.; Jasmani, H. Production of highly enantioselective (−)-menthyl butyrate using Candida rugosa lipase immobilized on epoxy-activated supports. Food Chem. 2008, 106, 437–443. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, T.; Nie, K.; Wang, F. Immobilization of Lipase on Macroporous Resin and Its Application in Synthesis of Biodiesel in Low Aqueous Media. Chin. J. Biotechnol. 2006, 22, 114–118. [Google Scholar] [CrossRef]
- Rahman, M.B.A.; Basri, M.; Hussein, M.Z.; Rahman, R.N.Z.A.; Zainol, D.H.; Salleh, A.B. Immobilization of lipase from Candida rugosa on layered double hydroxides for esterification reaction. Appl. Biochem. Biotechnol. 2004, 118, 313–320. [Google Scholar] [CrossRef]
- Shu, C.; Cai, J.; Huang, L.; Zhu, X.; Xu, Z. Biocatalytic production of ethyl butyrate from butyric acid with immobilized Candida rugosa lipase on cotton cloth. J. Mol. Catal. B Enzym. 2011, 72, 139–144. [Google Scholar] [CrossRef]
- Dave, R.; Madamwar, D. Esterification in organic solvents by lipase immobilized in polymer of PVA–alginate–boric acid. Process Biochem. 2006, 41, 951–955. [Google Scholar] [CrossRef]
- Nawani, N.; Singh, R.; Kaur, J. Immobilization and stability studies of a lipase from thermophilic Bacillus sp: The effect of process parameters on immobilization of enzyme. Electron. J. Biotechnol. 2006, 9, 559–565. [Google Scholar] [CrossRef]
- Pereira, E.B.; Zanin, G.M.; Castro, H.F. Immobilization and catalytic properties of lipase on chitosan for hydrolysis and esterification reactions. Braz. J. Chem. Eng. 2003, 20, 343–355. [Google Scholar] [CrossRef]
- Lau, S.C.; Lim, H.N.; Basri, M.; Masoumi, H.R.F.; Tajudin, A.A.; Huang, N.M.; Andou, Y. Enhanced biocatalytic esterification with lipase-immobilized chitosan/graphene oxide beads. PLoS ONE 2014, 9, e104695. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Oliveira, J.V.; Pergher, S.; Oliveira, D.D. Screening of supports for immobilization of commercial porcine pancreatic lipase. Mater. Res. 2011, 14, 483–492. [Google Scholar] [CrossRef]
- Shindo, H.; Watanabe, D.; Onaga, T.; Urakawa, M.; Nakahara, O.; Huang, Q. Adsorption, activity, and kinetics of acid phosphatase as influenced by selected oxides and clay minerals. Soil Sci. Plant Nutr. 2002, 48, 763–767. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, W.; Cai, P. Adsorption, desorption and activities of acid phosphatase on various colloidal particles from an Ultisol. Colloids Surf. B Biointerfaces 2005, 45, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Bosley, J.A.; Clayton, J.C. Blueprint for a lipase support: Use of hydrophobic controlled-pore glasses as model systems. Biotechnol. Bioeng. 1994, 43, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Bosley, J. Turning lipases into industrial biocatalysts. Biochem. Soc. Trans. 1997, 25, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.B.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Kinetic studies of lipase from Candida rugosa. In Twenty-Second Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2001; pp. 739–752. [Google Scholar]
- Persson, M.; Wehtje, E.; Adlercreutz, P. Factors governing the activity of lyophilised and immobilised lipase preparations in organic solvents. ChemBioChem 2002, 3, 566–571. [Google Scholar] [CrossRef]
- Prabu, K.; Natarajan, E. Isolation and FTIR spectroscopy characterization of chitin from local sources. Adv. Appl. Sci. Res. 2012, 3, 1870–1875. [Google Scholar]
- Tai, H.-S.; Lee, J.-Y. Changes in the C-H stretching region of infrared spectra at the cholesteric phase transition. J. Phys. D Appl. Phys. 1990, 23, 940–944. [Google Scholar] [CrossRef]
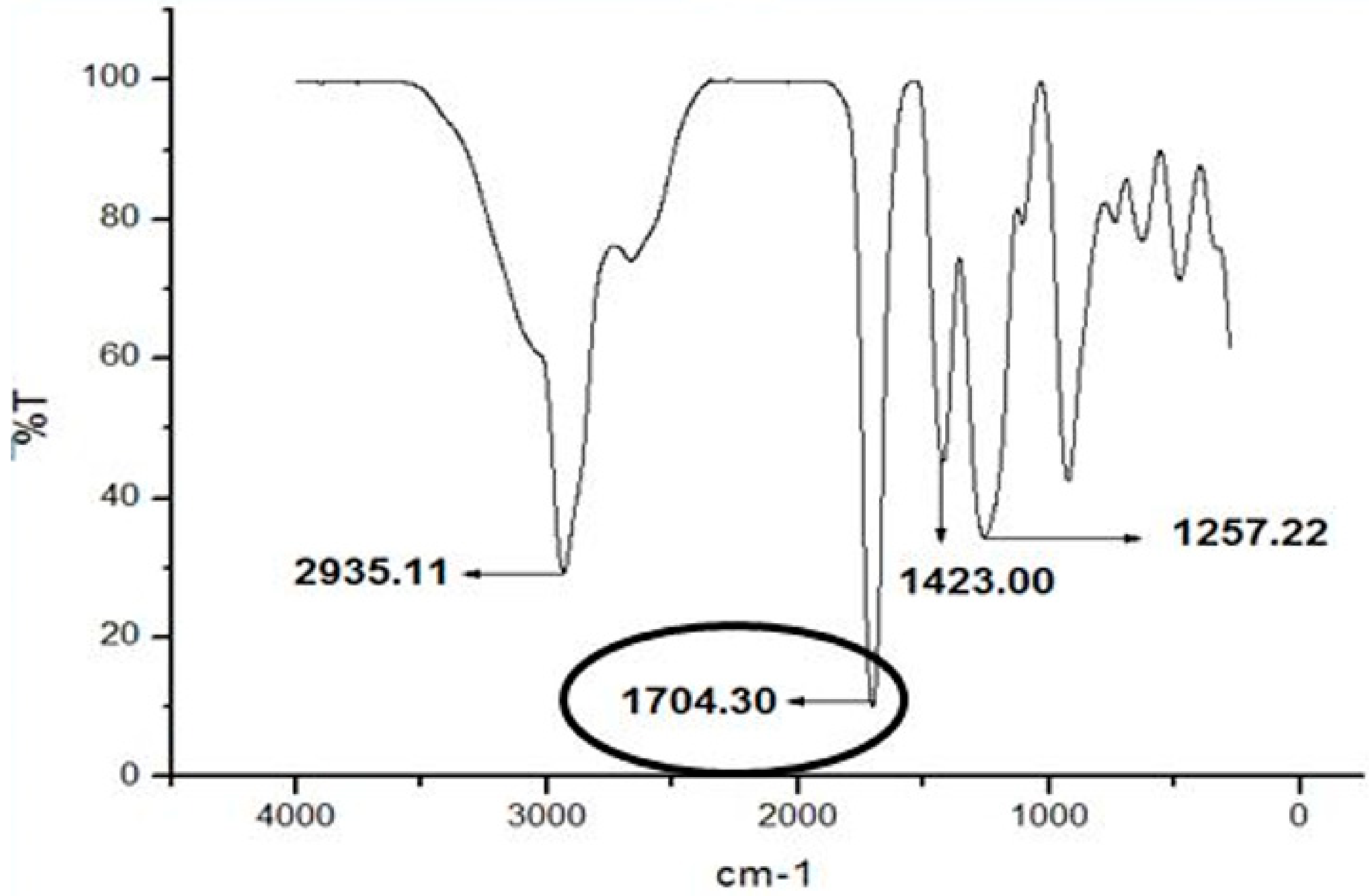
| Compound | Frequencies of Absorption (cm−1) | ||||
|---|---|---|---|---|---|
| v(C=O) | v(C-H) stretch | v(O-H) | v(C-O) | v(C-H) bend | |
| Ethyl hexanoate | 1704.30 | 2935.11 | - | 1257.22 | 1423.00 |
| m/z | Fragment Ion |
|---|---|
| 43 | |
| 60 | |
| 73 | |
| 88 | COO |
| 99 | |
| 115 | |
| 144 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, N.; Latip, W.; Abd Rahman, R.N.Z.; Salleh, A.B.; Mohamad Ali, M.S. Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl Hexanoate Synthesis. Catalysts 2018, 8, 234. https://doi.org/10.3390/catal8060234
Musa N, Latip W, Abd Rahman RNZ, Salleh AB, Mohamad Ali MS. Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl Hexanoate Synthesis. Catalysts. 2018; 8(6):234. https://doi.org/10.3390/catal8060234
Chicago/Turabian StyleMusa, Nurshakila, Wahhida Latip, Raja Noor Zaliha Abd Rahman, Abu Bakar Salleh, and Mohd Shukuri Mohamad Ali. 2018. "Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl Hexanoate Synthesis" Catalysts 8, no. 6: 234. https://doi.org/10.3390/catal8060234
APA StyleMusa, N., Latip, W., Abd Rahman, R. N. Z., Salleh, A. B., & Mohamad Ali, M. S. (2018). Immobilization of an Antarctic Pseudomonas AMS8 Lipase for Low Temperature Ethyl Hexanoate Synthesis. Catalysts, 8(6), 234. https://doi.org/10.3390/catal8060234




