Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii
Abstract
1. Introduction
2. Results
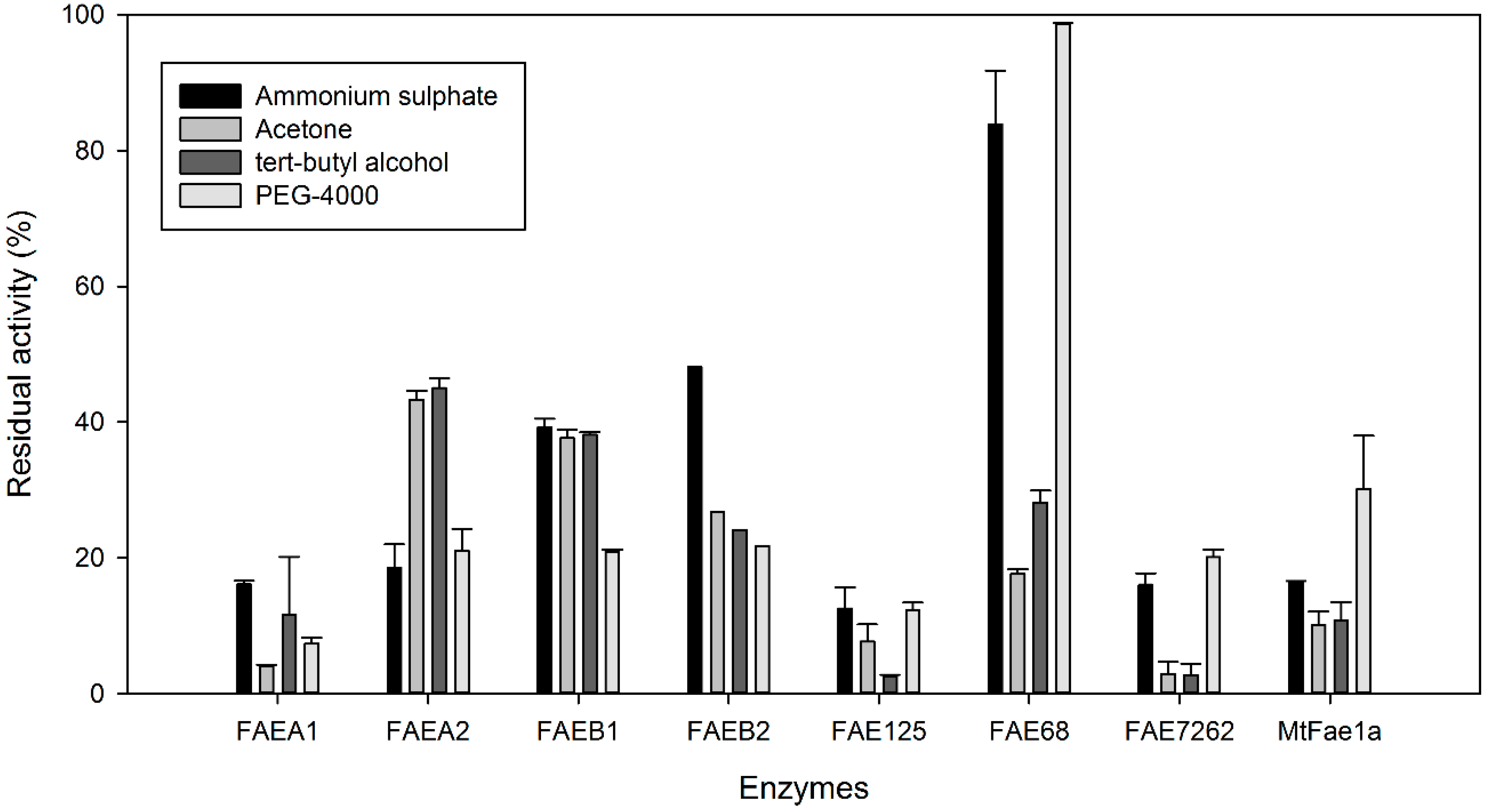
2.1. Effect of Precipitants on CLEA Preparation
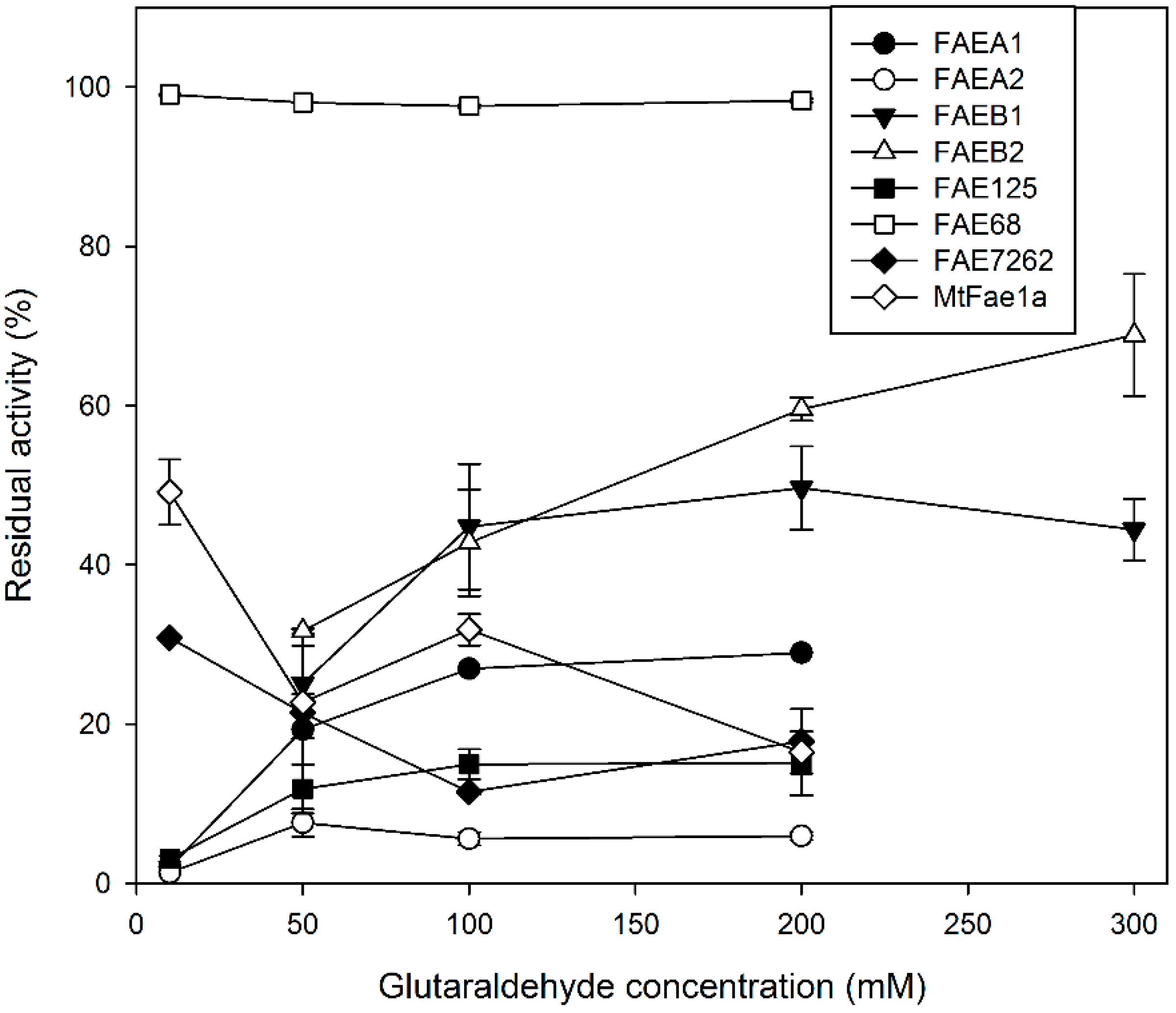
2.2. Effect of Glutaraldehyde Concentration on CLEA Activity
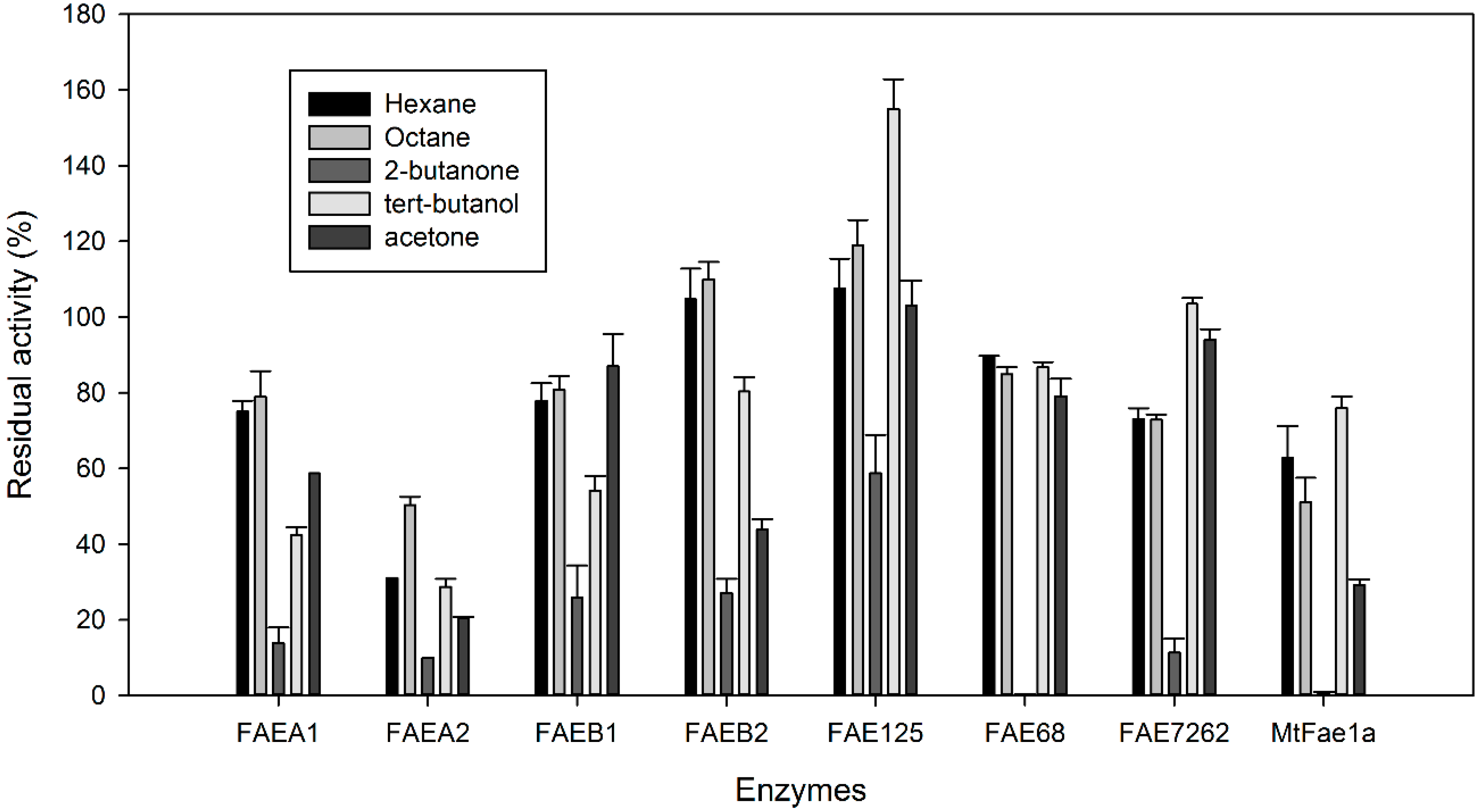
2.3. Stability of CLEAs in Organic Solvents
2.4. Operational Stability
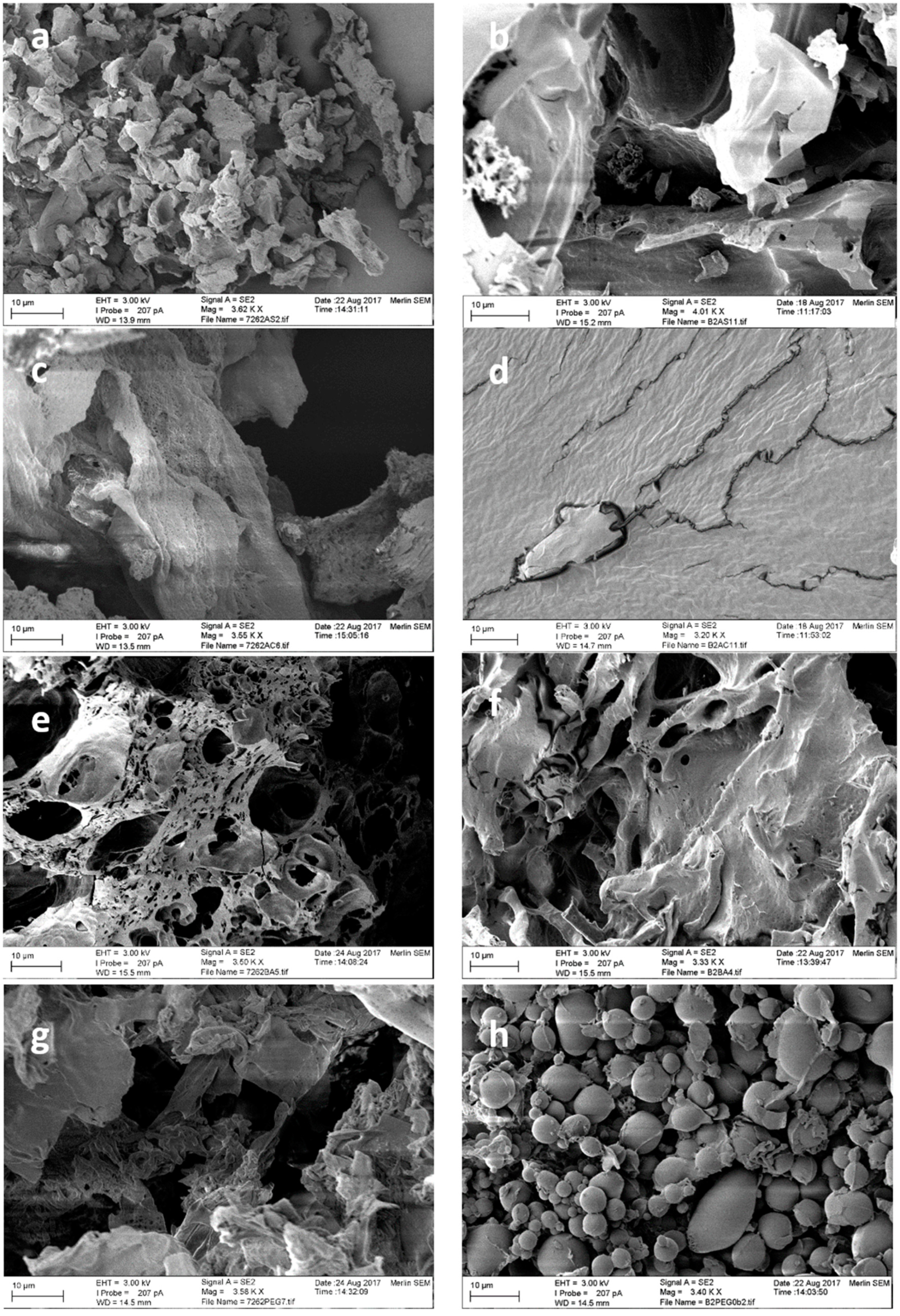
2.5. Structure of the Obtained CLEAs
3. Discussion
4. Materials and Methods
4.1. Materials and Enzymes
4.2. Enzyme Activities
4.3. Immobilization of Enzymes
4.4. Stability of CLEAS in Organic Solvents
4.5. Operational Stability
4.6. Structure of CLEAs
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Thörn, C.; Gustafsson, H.; Olsson, L. Immobilization of feruloyl esterases in mesoporous materials leads to improved transesterification yield. J. Mol. Catal. B Enzym. 2011, 72, 57–64. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Vafiadi, C.; Topakas, E.; Christakopoulos, P. Preparation of multipurpose cross-linked enzyme aggregates and their application to production of alkyl ferulates. J. Mol. Catal. B Enzym. 2008, 54, 35–41. [Google Scholar] [CrossRef]
- De Winter, K.; Soetaert, W.; Desmet, T. An imprinted cross-linked enzyme aggregate (iCLEA) of sucrose phosphorylase: Combining improved stability with altered specificity. Int. J. Mol. Sci. 2012, 13, 11333–11342. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gui, X.; Wang, G.; Yan, Y. Improving stability and activity of cross-linked enzyme aggregates based on polyethylenimine in hydrolysis of fish oil for enrichment of polyunsaturated fatty acids. Appl. Biochem. Biotechnol. 2012, 166, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Ba, S.; Jones, J.P.; Cabana, H. Hybrid bioreactor (HBR) of hollow fiber microfilter membrane and cross-linked laccase aggregates eliminate aromatic pharmaceuticals in wastewaters. J. Hazard. Mater. 2014, 280 (Suppl. C), 662–670. [Google Scholar] [CrossRef] [PubMed]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Utilization of cross-linked laccase aggregates in a perfusion basket reactor for the continuous elimination of endocrine-disrupting chemicals. Biotechnol. Bioeng. 2009, 102, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W. Feruloyl esterase: A key enzyme in biomass degradation. Appl. Biochem. Biotechnol. 2006, 133, 87–112. [Google Scholar] [CrossRef]
- Faulds, C.B.; Williamson, G. Release of ferulic acid from wheat bran by a ferulic acid esterase (FAE III) from Aspergillus niger. Appl. Microbiol. Biotechnol. 1995, 43, 1082–1807. [Google Scholar] [CrossRef] [PubMed]
- Benoit, I.; Navarro, D.; Marnet, N.; Rakotomanomana, N.; Lesage-Meessen, L.; Sigoillot, J.C.; Asther, M.; Asther, M. Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohydr. Res. 2006, 341, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, B.; Faulds, C.B.; Williamson, G. Enzymic release of ferulic acid from barley spent grain. J. Cereal Sci. 1997, 25, 285–288. [Google Scholar] [CrossRef]
- Okada, T.; Nakagawa, K.; Yamaguchi, N. Antioxidative activities of amino compounds on fats and oils: VIII. Antioxidative activity of ferulate and the synergistic effect of amino compounds. Nippon Shokuhin Kogyo Gakkaishi 1982, 29, 305–309. [Google Scholar] [CrossRef]
- Topakas, E.; Vafiadi, C.; Christakopoulos, P. Microbial production, characterization and applications of feruloyl esterases. Process Biochem. 2007, 42, 497–509. [Google Scholar] [CrossRef]
- Xu, Z.; He, H.; Zhang, S.; Guo, T.; Kong, J. Characterization of Feruloyl Esterases Produced by the Four Lactobacillus Species: L. amylovorus, L. acidophilus, L. farciminis and L. fermentum, Isolated from Ensiled Corn Stover. Front. Microbiol. 2017, 8, 941. [Google Scholar] [CrossRef] [PubMed]
- Kelle, S.; Nieter, A.; Krings, U.; Zelena, K.; Linke, D.; Berger, R.G. Heterologous production of a feruloyl esterase from Pleurotus sapidus synthesizing feruloyl-saccharide esters. Biotechnol. Appl. Biochem. 2016, 63, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yoshida, E.; Fukada, H.; Inoue, H.; Tokura, M.; Ishikawa, K. Characterization of a feruloyl esterase B from Talaromyces cellulolyticus. Biosci. Biotechnol. Biochem. 2015, 79, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.; van Rantwijk, F.; van der Wielen, L.A.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- De Rose, S.A.; Novak, H.; Dowd, A.; Singh, S.; Lang, D.A.; Littlechild, J. Stabilization of a Lipolytic Enzyme for Commercial Application. Catalysts 2017, 7, 91. [Google Scholar] [CrossRef]
- White, B.T. A Method for the Isolation of Bovine Liver Esterase. J. Dairy Sci. 1956, 39, 547–551. [Google Scholar] [CrossRef]
- Basaran, P.; Hang, Y.D. Purification and characterization of acetyl esterase from Candida guilliermondii. Lett. Appl. Microbiol. 2000, 30, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Raman Suri, C.; Sahoo, D.K. Molecular mechanism of polyethylene glycol mediated stabilization of protein. Biochem. Biophys. Res. Commun. 2010, 392, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, Y.; Yang, L.; Li, M.; Zhang, J. Preparation of Cross-Linked Enzyme Aggregates of Trehalose Synthase via Co-aggregation with Polyethyleneimine. Appl. Biochem. Biotechnol. 2014, 174, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Perzon, A.; Dicko, C.; Çobanoğlu, Ö.; Yükselen, O.; Eryilmaz, J.; Dey, E.S. Cellulase cross-linked enzyme aggregates (CLEA) activities can be modulated and enhanced by precipitant selection. J. Chem. Technol. Biotechnol. 2017, 92, 1645–1649. [Google Scholar] [CrossRef]
- López-Serrano, P.; Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates with enhanced activity: Application to lipases. Biotechnol. Lett. 2002, 24, 1379–1383. [Google Scholar] [CrossRef]
- Faulds, C.B.; Perez-Boada, M.; Martinez, A.T. Influence of organic co-solvents on the activity and substrate specificity of feruloyl esterases. Bioresour. Technol. 2011, 102, 4962–4967. [Google Scholar] [CrossRef] [PubMed]
- Takemori, S.; Furuya, E.; Suzuki, H.; Katagiri, M. Stabilization of Enzyme Activity by an Organic Solvent. Nature 1967, 215, 417. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Izquierdo, Á.; Picó, E.A.; López, C.; Serra, J.L.; Llama, M.J. Magnetic Cross-Linked Enzyme Aggregates (mCLEAs) of Candida antarctica Lipase: An Efficient and Stable Biocatalyst for Biodiesel Synthesis. PLoS ONE 2015, 9, e115202. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Du, X.; Jiang, W.; Tong, Y.; Zhao, Z.; Fang, R.; Feng, J.; Tang, L. Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1: Preparation, characterization and application as a biocatalyst. J. Biotechnol. 2018, 272–273, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Taboada-Puig, R.; Junghanns, C.; Demarche, P.; Moreira, M.T.; Feijoo, G.; Lema, J.M.; Agathos, S.N. Combined cross-linked enzyme aggregates from versatile peroxidase and glucose oxidase: Production, partial characterization and application for the elimination of endocrine disruptors. Bioresour. Technol. 2011, 102, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Leonov, L.; Jütten, P.; Cerullo, G.; Faraco, V.; Papadopoulou, A.; Kletsas, D.; Ralli, M.; Rova, U.; Christakopoulos, P. Optimized synthesis of novel prenyl ferulate performed by feruloyl esterases from Myceliophthora thermophila in microemulsions. Appl. Microbiol. Biotechnol. 2017, 101, 3213–3226. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Iancu, L.; Jutten, P.; Piechot, A.; Rova, U.; Christakopoulos, P. Optimization of enzymatic synthesis of antioxidants catalyzed by a novel feruloyl esterase from Talaromyces wortmanii (Fae125) in hexane via response surface methodology. 2018; submitted. [Google Scholar]
- Sangeetha, K.; Abraham, E.T. Preparation and characterization of cross-linked enzyme aggregates (CLEA) of Subtilisin for controlled release applications. Int. J. Biol. Macromol. 2008, 43, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Y.; Yang, Y.; Yang, Z. Activity and stability of cross-linked tyrosinase aggregates in aqueous and nonaqueous media. J. Biotechnol. 2011, 152, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tang, W.; Jiang, Y.; Ma, L.; He, Y.; Gao, J. Magnetic combined cross-linked enzyme aggregates of horseradish peroxidase and glucose oxidase: An efficient biocatalyst for dye decolourization. RSC Adv. 2016, 6, 90061–90068. [Google Scholar] [CrossRef]
- Tyagi, R.; Batra, R.; Gupta, M.N. Amorphous enzyme aggregates: Stability toward heat and aqueous-organic cosolvent mixtures. Enzyme Microb. Technol. 1999, 24, 348–354. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2016, 1, 166. [Google Scholar] [CrossRef]
- Aytar, B.S.; Bakir, U. Preparation of cross-linked tyrosinase aggregates. Process Biochem. 2008, 43, 125–131. [Google Scholar] [CrossRef]
- Kühnel, S.; Pouvreau, L.; Appeldoorn, M.M.; Hinz, S.W.; Schols, H.A.; Gruppen, H. The ferulic acid esterases of Chrysosporium lucknowense C1: Purification, characterization and their potential application in biorefinery. Enzyme Microb. Technol. 2012, 50, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.; Joosten, V.; Punt, P.J.; Gusakov, A.V.; Olson, P.T.; Joosten, R.; Bartels, J.; Visser, J.; Sinitsyn, A.P.; Emalfarb, M.A.; et al. Development of a mature fungal technology and production platform for industrial enzymes based on a Myceliophthora thermophila isolate, previously known as Chrysosporium lucknowense C1. Ind. Biotechnol. 2011, 7, 214–223. [Google Scholar] [CrossRef]
- Verdoes, J.C.; Punt, P.J.; Burlingame, R.; Bartels, J.; van Dijk, R.; Slump, E.; Meens, M.; Joosten, R.; Emalfarb, M. A dedicated vector for efficient library construction and high throughput screening in the hyphal fungus Chrysosporium lucknowense. Ind. Biotechnol. 2007, 3, 48–57. [Google Scholar] [CrossRef]
- Topakas, E.; Moukouli, M.; Dimarogona, M.; Christakopoulos, P. Expression, characterization and structural modelling of a feruloyl esterase from the thermophilic fungus Myceliophthora thermophila. Appl. Microbiol. Biotechnol. 2012, 94, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Topakas, E.; Stamatis, H.; Biely, P.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Purification and characterization of a feruloyl esterase from Fusarium oxysporum catalyzing esterification of phenolic acids in ternary water-organic solvent mixtures. J. Biotechnol. 2003, 102, 33–44. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerva, A.; Antonopoulou, I.; Enman, J.; Iancu, L.; Rova, U.; Christakopoulos, P. Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii. Catalysts 2018, 8, 208. https://doi.org/10.3390/catal8050208
Zerva A, Antonopoulou I, Enman J, Iancu L, Rova U, Christakopoulos P. Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii. Catalysts. 2018; 8(5):208. https://doi.org/10.3390/catal8050208
Chicago/Turabian StyleZerva, Anastasia, Io Antonopoulou, Josefine Enman, Laura Iancu, Ulrika Rova, and Paul Christakopoulos. 2018. "Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii" Catalysts 8, no. 5: 208. https://doi.org/10.3390/catal8050208
APA StyleZerva, A., Antonopoulou, I., Enman, J., Iancu, L., Rova, U., & Christakopoulos, P. (2018). Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii. Catalysts, 8(5), 208. https://doi.org/10.3390/catal8050208






