Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by Entrapment in Polyvinyl Alcohol and Its Application to Neo-Fructooligosaccharides Production
Abstract
1. Introduction
2. Results and Discussion
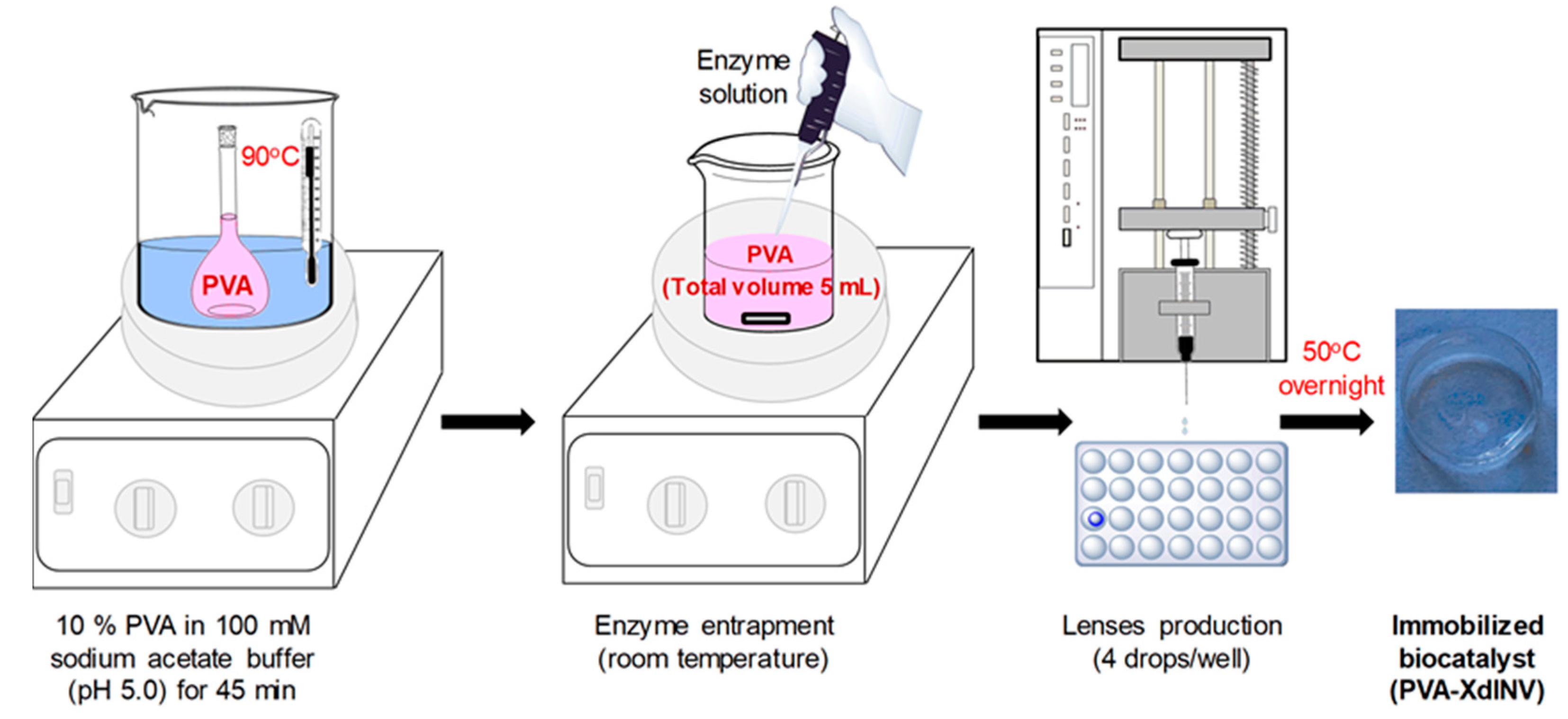
2.1. Immobilization of pXd-INV in PVA Hydrogels
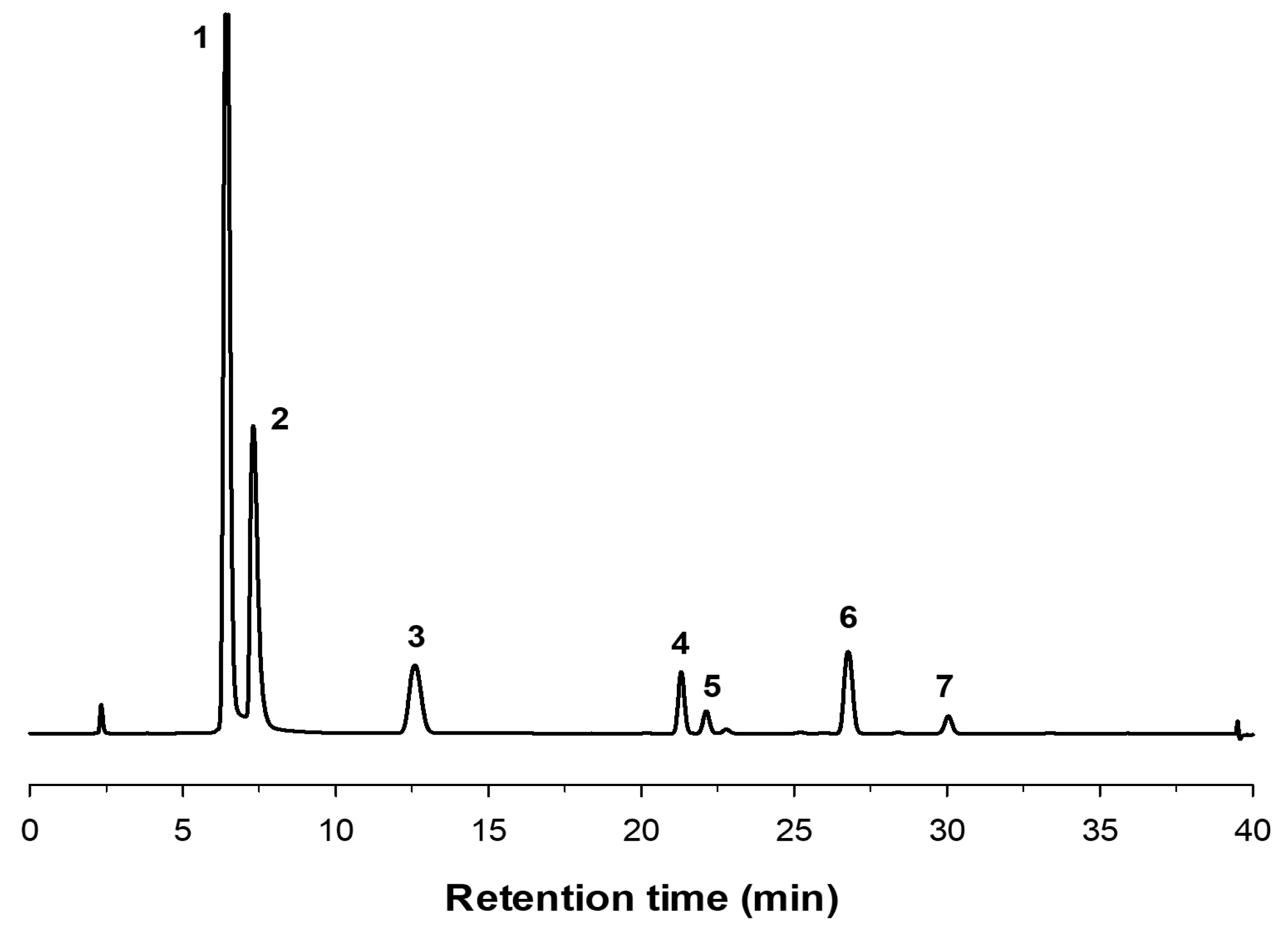
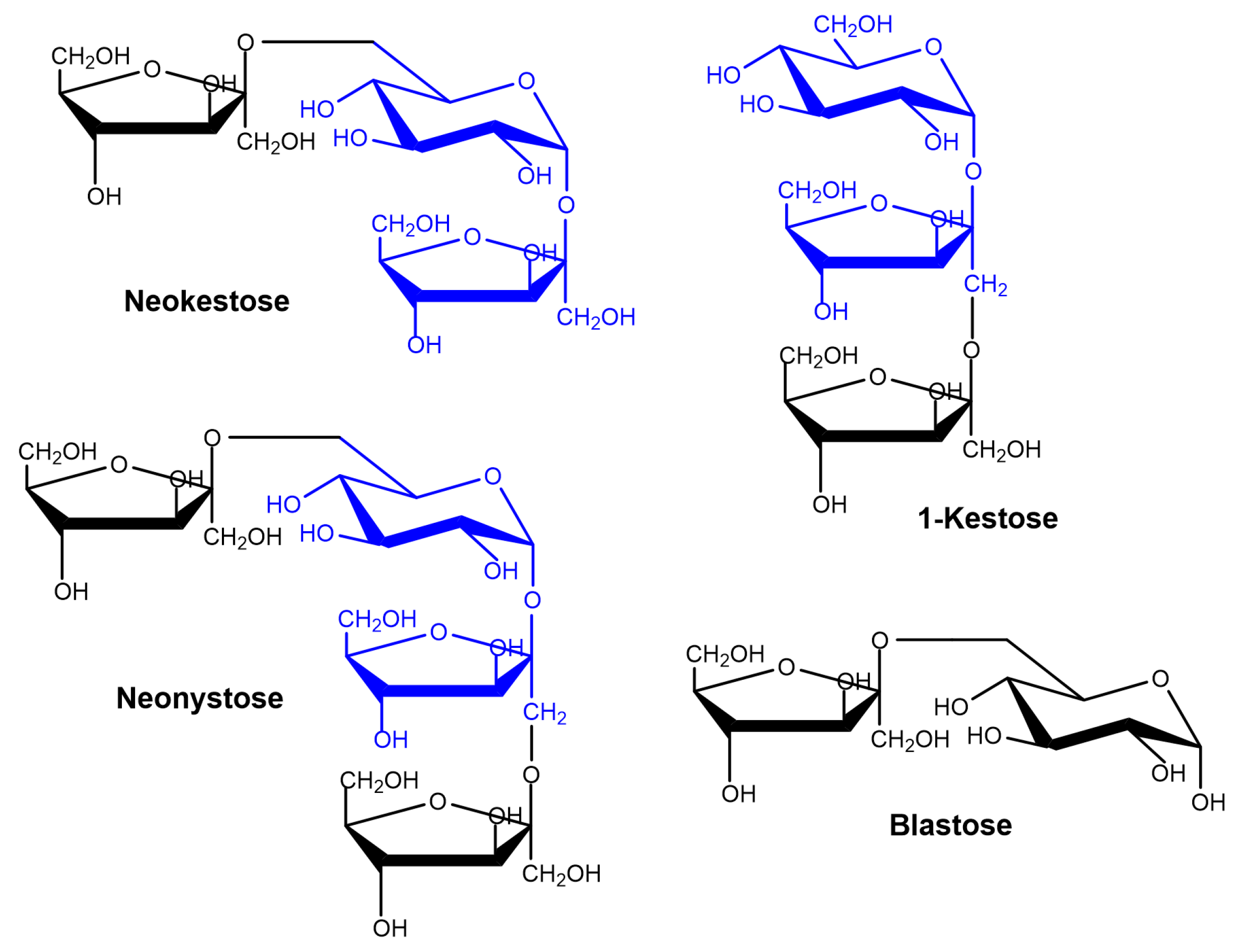
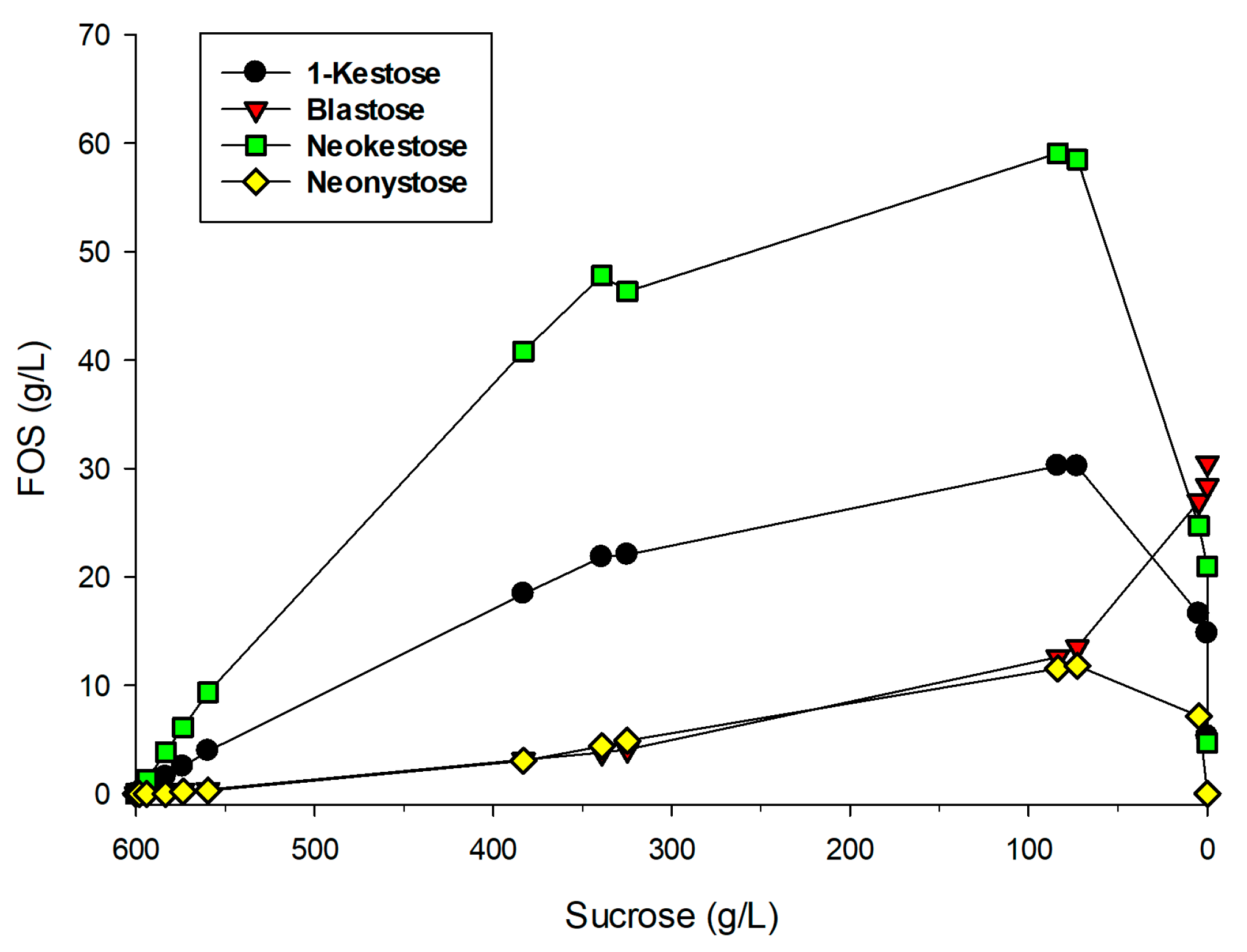
2.2. Production of FOS by Entrapped pXd-INV
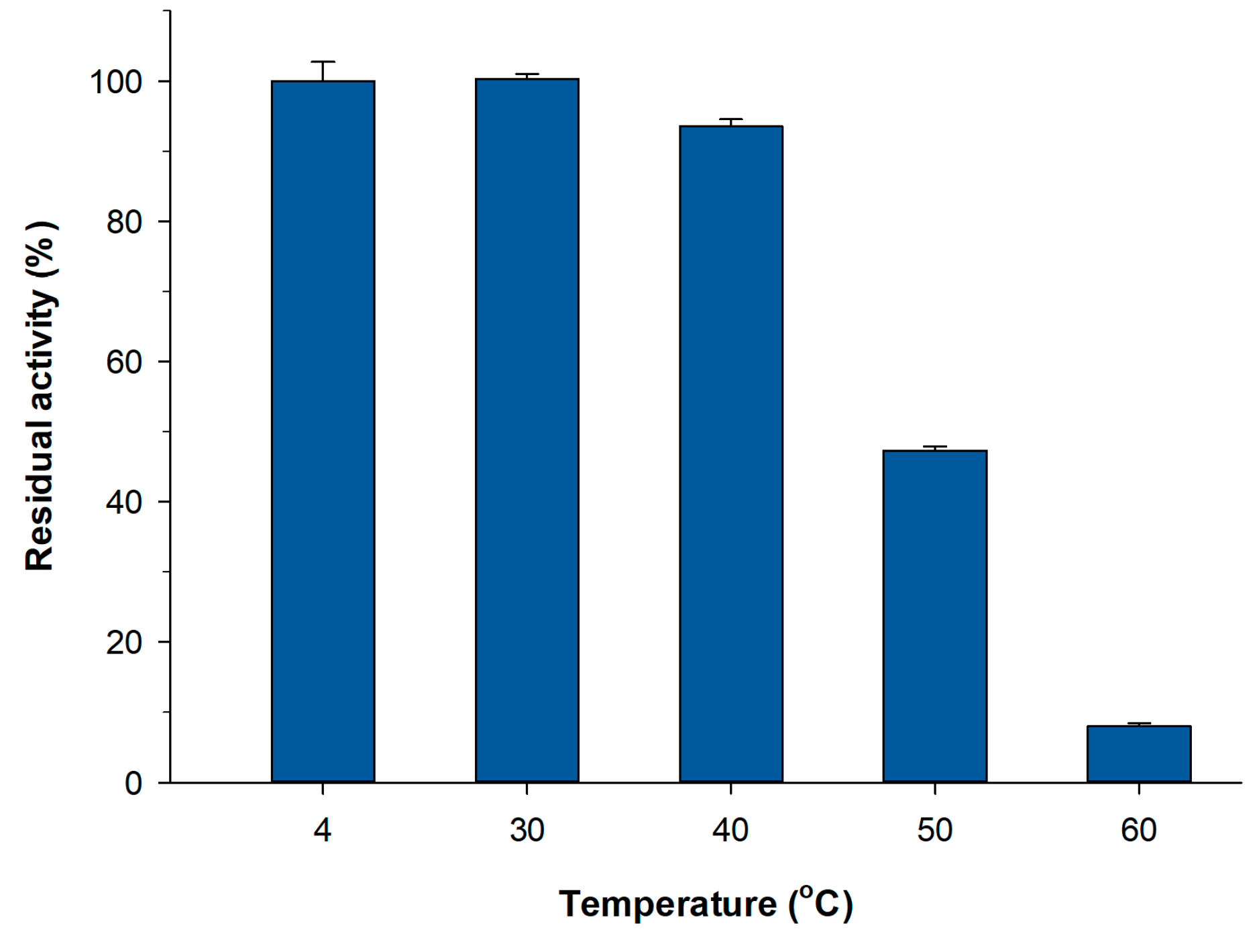
2.3. Thermal Stability of PVA Lens-Shaped Particles
2.4. Kinetics of Neo-FOS Formation by Entrapped pXd-INV
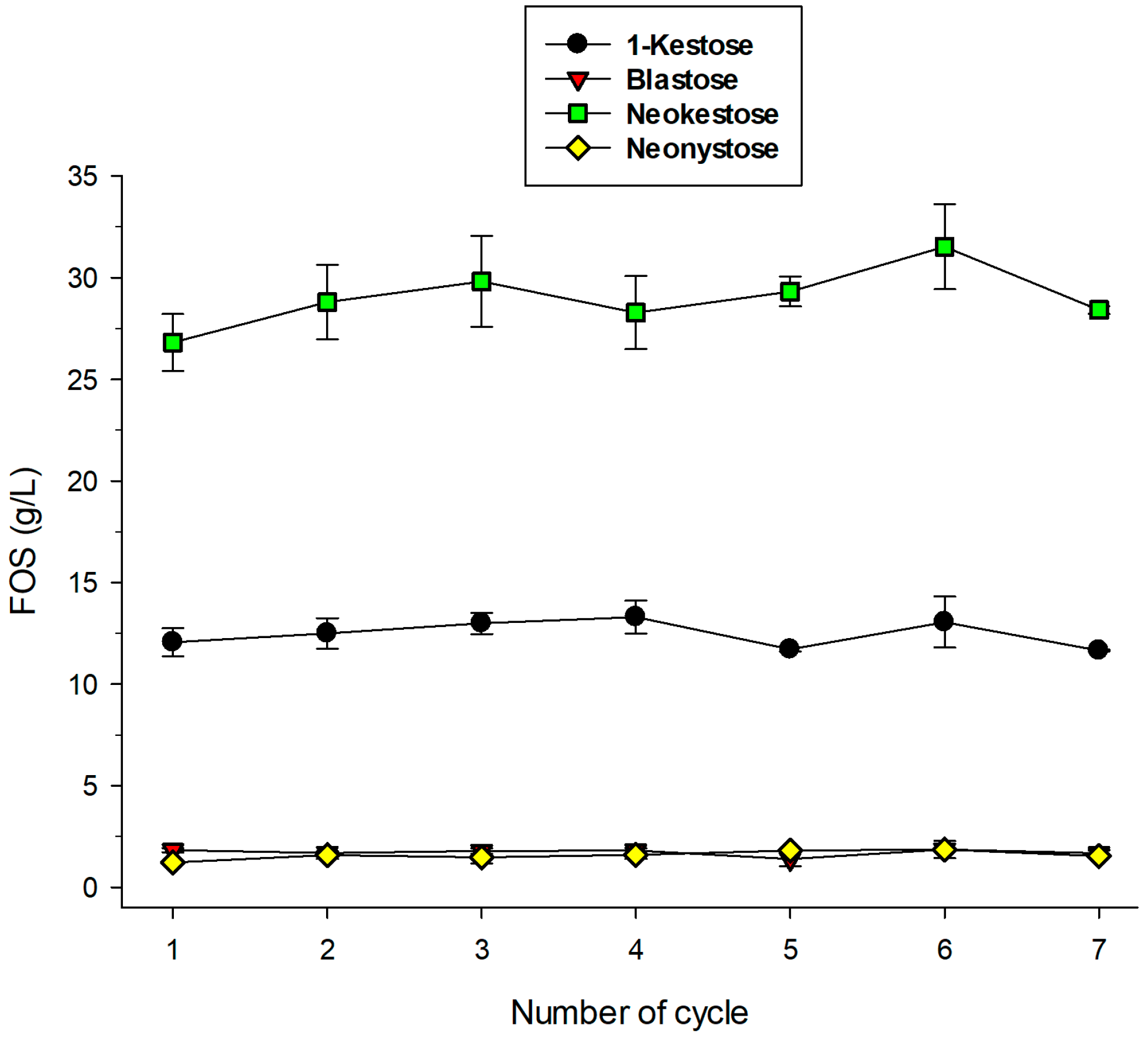
2.5. Operational Stability of Immobilized pXd-INV for Neo-FOS Production
3. Materials and Methods
3.1. Materials
3.2. β-Fructofuranosidase Activity Source
3.3. Entrapment of β-Fructofuranosidase in PVA Lenses
3.4. Enzyme Activity Assay
3.5. Microscale Assay for the Operational Stability of Immobilized pXd-INV
3.6. Thermostability of the Immobilized PVA Particles
3.7. Analysis of Fructooligosacharides by HPAEC-PAD
3.8. Production of Fructooligosacharides by Immobilized pXd-INV
3.9. Operational Stability of Immobilized pXd-INV for Neo-FOS Production
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Plou, F.J.; Gómez de Segura, A.; Ballesteros, A. Application of glycosidases and transglycosidases for the synthesis of oligosaccharides. In Industrial Enzymes: Structure, Function and Application; Polaina, J., MacCabe, A.P., Eds.; Springer: New York, NY, USA, 2007; pp. 141–157. [Google Scholar]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotech. 2017, 183, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Eida, T.; Takizawa, T.; Tokunaga, T.; Tashiro, Y. Effects of fructooligosaccharides on intestinal flora and human health. Bifidobact. Microflora 1986, 5, 37–50. [Google Scholar] [CrossRef]
- Cruz, R.; Cruz, V.D.; Belini, M.Z.; Belote, J.G.; Vieira, C.R. Production of fructooligosaccharides by the mycelia of Aspergillus japonicus immobilized in calcium alginate. Biores. Technol. 1998, 65, 139–143. [Google Scholar] [CrossRef]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Teixeira, J.A.; Aguilar, C.N. Biotechnological production and application of fructooligosaccharides. Crit. Rev. Biotechnol. 2016, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kilian, S.; Kritzinger, S.; Rycroft, C.; Gibson, G.; du Preez, J. The effects of the novel bifidogenic trisaccharide, neokestose, on the human colonic microbiota. World J. Microb. Biot. 2002, 18, 637–644. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, J.H.; Kang, S.W.; Park, S.W.; Kim, S.W. Studies on production and physical properties of neo-FOS produced by co-immobilized Penicillium citrinum and neo-fructosyltransferase. Eur. Food Res. Technol. 2007, 225, 457–462. [Google Scholar] [CrossRef]
- Linde, D.; Macias, I.; Fernández-Arrojo, L.; Plou, F.J.; Jiménez, A.; Fernández-Lobato, M. Molecular and biochemical characterization of a β-fructofuranosidase from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2009, 75, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Escudero, M.; Gimeno-Perez, M.; Gonzalez, B.; Linde, D.; Merzdo, Z.; Fernandez-Lobato, M.; Sanz-Aparicio, J. Structural analysis of β-fructofuranosidase from Xanthophyllomyces dendrorhous reveals unique features and the crucial role of n-glycosylation in oligomerization and activity. J. Biol. Chem. 2016, 291, 6843–6857. [Google Scholar] [CrossRef] [PubMed]
- Linde, D.; Rodríguez-Colinas, B.; Estévez, M.; Poveda, A.; Plou, F.J.; Fernández Lobato, M. Analysis of neofructooligosaccharides production mediated by the extracellular β-fructofuranosidase from Xanthophyllomyces dendrorhous. Biores. Technol. 2012, 109, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Pérez, M.; Linde, D.; Fernández-Arrojo, L.; Plou, F.J.; Fernández-Lobato, M. Heterologous overproduction of β-fructofuranosidase from yeast Xanthophyllomyces dendrorhous, an enzyme producing prebiotic sugars. Appl. Microbiol. Biotechnol. 2015, 99, 3459–3467. [Google Scholar] [CrossRef] [PubMed]
- Gimeno-Perez, M.; Santos-Moriano, P.; Fernandez-Arrojo, L.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.O.; Fernandez-Lobato, M.; Plou, F.J. Regioselective synthesis of neo-erlose by the β-fructofuranosidase from Xanthophyllomyces dendrorhous. Process Biochem. 2014, 49, 423–429. [Google Scholar] [CrossRef]
- Madhavan, A.; Sindhu, R.; Binod, P.; Sukumaran, R.K.; Pandey, A. Strategies for design of improved biocatalysts for industrial applications. Biores. Technol. 2017, 245, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Torres-Salas, P.; Del Monte-Martinez, A.; Cutiño-Avila, B.; Rodriguez-Colinas, B.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Immobilized biocatalysts: Novel approaches and tools for binding enzymes to supports. Adv. Mater. 2011, 23, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Reyes-Duarte, D.; Lopez-Cortes, N.; Ferrer, M.; Ballesteros, A.; Plou, F.J. Acetylation of vitamin E by Candida antarctica lipase B immobilized on different carriers. Process Biochem. 2008, 43, 145–153. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Monsalve-Ledesma, L.; Ortega-Munoz, M.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Santoyo-Gonzalez, F.; Plou, F.J. Vinyl sulfone-activated silica for efficient covalent immobilization of alkaline unstable enzymes: Application to levansucrase for fructooligosaccharide synthesis. RSC Adv. 2016, 6, 64175–64181. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Woodley, J.M.; Plou, F.J. Continuous production of chitooligosaccharides by an immobilized enzyme in a dual-reactor system. J. Mol. Catal. B Enzym. 2016, 133, 211–217. [Google Scholar] [CrossRef]
- Kahar, U.M.; Sani, M.H.; Chan, K.G.; Goh, K.M. Immobilization of α-amylase from Anoxybacillus sp. SK3-4 on ReliZyme and Immobead supports. Molecules 2016, 21, 1196. [Google Scholar] [CrossRef] [PubMed]
- Carević, M.; Ćorović, M.; Mihailović, M.; Banjanac, K.; Milisavljević, A.; Veličković, D.; Bezbradica, D. Galacto-oligosaccharide synthesis using chemically modified β-galactosidase from Aspergillus oryzae immobilised onto macroporous amino resin. Int. Dairy J. 2016, 54, 50–57. [Google Scholar] [CrossRef]
- Gomez de Segura, A.; Alcalde, M.; Bernabe, M.; Ballesteros, A.; Plou, F.J. Synthesis of methyl α-d-glucooligosaccharides by entrapped dextransucrase from Leuconostoc mesenteroides B-1299. J. Biotechnol. 2006, 124, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, M.; Plou, F.J.; de Segura, A.G.; Remaud-Simeon, M.; Willemot, R.M.; Monsan, P.; Ballesteros, A. Immobilization of native and dextran-free dextransucrases from Leuconostoc mesenteroides NRRL B-512F for the synthesis of glucooligosaccharides. Biotechnol. Tech. 1999, 13, 749–755. [Google Scholar] [CrossRef]
- Gadea, J.L.; Cesteros, L.C.; Katime, I. Chemical-physical behavior of hydrogels of poly(vinyl alcohol) and poly(ethylene glycol). Eur. Polym. J. 2013, 49, 3582–3589. [Google Scholar] [CrossRef]
- Nuneslentik, M.A.P.; Gois, P.M.P.; Rosa, M.E.; Martins, S.; Fernandes, P.C.B.; Ribeiro, M.H.L. Boronic acids as efficient cross linkers for PVA: Synthesis and application of tunable hollow microspheres in biocatalysis. Tetrahedron 2016, 72, 7293–7305. [Google Scholar]
- Durieux, A.; Nicolay, X.; Simon, J.P. Continuous malolactic fermentation by Oenococcus oeni entrapped in LentiKats. Biotechnol. Lett. 2000, 22, 1679–1684. [Google Scholar] [CrossRef]
- Schlieker, M.; Vorlop, K.-D. A novel immobilization method for entrapment: LentiKats. In Immobilization of Enzymes and Cells; Guisan, J.M., Ed.; Springer: Heidelberg, Germany, 2006; pp. 333–343. [Google Scholar]
- Ariga, O.; Kubo, T.; Sano, Y. Effective diffusivity of glucose in PVA hydrogel. J. Ferment. Bioeng. 1994, 78, 200–201. [Google Scholar] [CrossRef]
- Gómez de Segura, A.; Alcalde, M.; Plou, F.J.; Remaud-Simeon, M.; Monsan, P.; Ballesteros, A. Encapsulation in LentiKats of dextransucrase from Leuconostoc mesenteroides NRRL B-1299, and its effect on product selectivity. Biocatal. Biotransform. 2003, 21, 325–331. [Google Scholar] [CrossRef]
- Imai, K.; Shiomi, T.; Uchida, K.; Miya, M. Immobilization of enzyme into poly(vinyl alcohol) membrane. Biotechnol. Bioeng. 1986, 28, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.P.; Rosa, M.E.; Fernandes, P.C.B.; Ribeiro, M.H.L. Operational stability of naringinase PVA lens-shaped microparticles in batch stirred reactors and mini packed bed reactors-one step closer to industry. Biores. Technol. 2014, 164, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzyme Microb. Tech. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Luo, M.; Wang, W.; Zhao, Q.; Li, M.; Chen, Y.; Lu, Z.; Liu, K.; Wang, D. Chemiluminescence biosensor for hydrogen peroxide determination by immobilizing horseradish peroxidase onto PVA-CO-PE nanofiber membrane. Eur. Polym. J. 2017, 91, 307–314. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Li, P.; Wang, Z.; Ran, H. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci. Rep. 2017, 7, 11913. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Arrojo, L.; Santos-Moriano, P.; Rodriguez-Colinas, B.; Ballesteros, A.O.; Plou, F.J. Micro-scale procedure for enzyme immobilization screening and operational stability assays. Biotechnol. Lett. 2015, 37, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, I.; Fernandez-Arrojo, L.; Garcia-Arellano, H.; Ferrer, M.; Ballesteros, A.; Plou, F.J. Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J. Biotechnol. 2007, 128, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, P.; Fernandez-Arrojo, L.; Romano, D.; Santos-Moriano, P.; Gimeno-Perez, M.; Poveda, A.; Gandolfi, R.; Fernandez-Lobato, M.; Molinari, F.; Plou, F.J. Production of fructooligosaccharides by mycelium-bound transfructosylation activity present in Cladosporium cladosporioides and Penicilium sizovae. Process Biochem. 2014, 49, 2174–2180. [Google Scholar] [CrossRef][Green Version]
- Alves, M.H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Rescignano, N.; Fortunati, E.; Montesano, S.; Emiliani, C.; Kenny, J.M.; Martino, S.; Armentano, I. PVA bio-nanocomposites: A new take-off using cellulose nanocrystals and PLGA nanoparticles. Carbohydr. Polym. 2014, 99, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Alonso, P.; Fernandez-Arrojo, L.; Plou, F.J.; Fernandez-Lobato, M. Biochemical characterization of a β-fructofuranosidase from Rhodotorula dairenensis with transfructosylating activity. FEMS Yeast Res. 2009, 9, 768–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gutierrez-Alonso, P.; Gimeno-Perez, M.; Ramirez-Escudero, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernandez-Lobato, M. Molecular characterization and heterologous expression of a Xanthophyllomyces dendrorhous α-glucosidase with potential for prebiotics production. Appl. Microbiol. Biot. 2016, 100, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, I.; de Segura, A.G.; Fernandez-Arrojo, L.; Alcalde, M.; Yates, M.; Rojas-Cervantes, M.L.; Plou, F.J.; Ballesteros, A. Immobilisation of fructosyltransferase from Aspergillus aculeatus on epoxy-activated Sepabeads EC for the synthesis of fructo-oligosaccharides. J. Mol. Catal. B Enzym. 2005, 35, 19–27. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Karboune, S.; Mateo, C. Immobilization and stabilization of levansucrase biocatalyst of high interest for the production of fructooligosaccharides and levan. J. Chem. Technol. Biot. 2016, 91, 2440–2448. [Google Scholar] [CrossRef]
- Palomo, J.M. Modulation of enzymes selectivity via immobilization. Curr. Org. Synth. 2009, 6, 1–14. [Google Scholar] [CrossRef]
- Plou, F.J.; Fernandez-Arrojo, L.; Santos-Moriano, P.; Ballesteros, A.O. Application of immobilized enzymes for the synthesis of bioactive fructooligosaccharides. In Food Oligosaccharides: Production, Analysis and Bioactivity; Moreno, F.J., Sanz, M.L., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; pp. 200–216. [Google Scholar]
- Fernandez-Arrojo, L.; Rodriguez-Colinas, B.; Gutierrez-Alonso, P.; Fernandez-Lobato, M.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Dried alginate-entrapped enzymes (DALGEEs) and their application to the production of fructooligosaccharides. Process Biochem. 2013, 48, 677–682. [Google Scholar] [CrossRef]
- Mouelhi, R.; Abidi, F.; Marzouki, M.N. An improved method for the production of fructooligosaccharides by immobilized β-fructofuranosidase from Sclerotinia sclerotiorum. Biotechnol. Appl. Biochem. 2016, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, A.S.G.; Aydos, L.F.; Klein, M.P.; Rodrigues, R.C.; Hertz, P.F. Fructooligosaccharides synthesis by highly stable immobilized β-fructofuranosidase from Aspergillus aculeatus. Carbohydr. Polym. 2014, 103, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Oliveira, E.; Maugeri, F. Characterization of the immobilized fructosyltranferase from Rhodotorula sp. Int. J. Food Eng. 2010, 6. [Google Scholar] [CrossRef]
- Alvaro-Benito, M.; de Abreu, M.; Fernandez-Arrojo, L.; Plou, F.J.; Jimenez-Barbero, J.; Ballesteros, A.; Polaina, J.; Fernandez-Lobato, M. Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J. Biotechnol. 2007, 132, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.P.; Vila-Real, H.; Fernandes, P.C.B.; Ribeiro, M.H.L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotech. 2010, 160, 2129–2147. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bauer, L.L.; Fahey, G.C.; Hogarth, A.J.C.L.; Wolf, B.W.; Hunter, D.E. Selected fructooligosaccharide (1-kestose, nystose, and 1F-β-fructofuranosylnystose) composition of foods and feeds. J. Agric. Food Chem. 1997, 45, 3076–3082. [Google Scholar] [CrossRef]
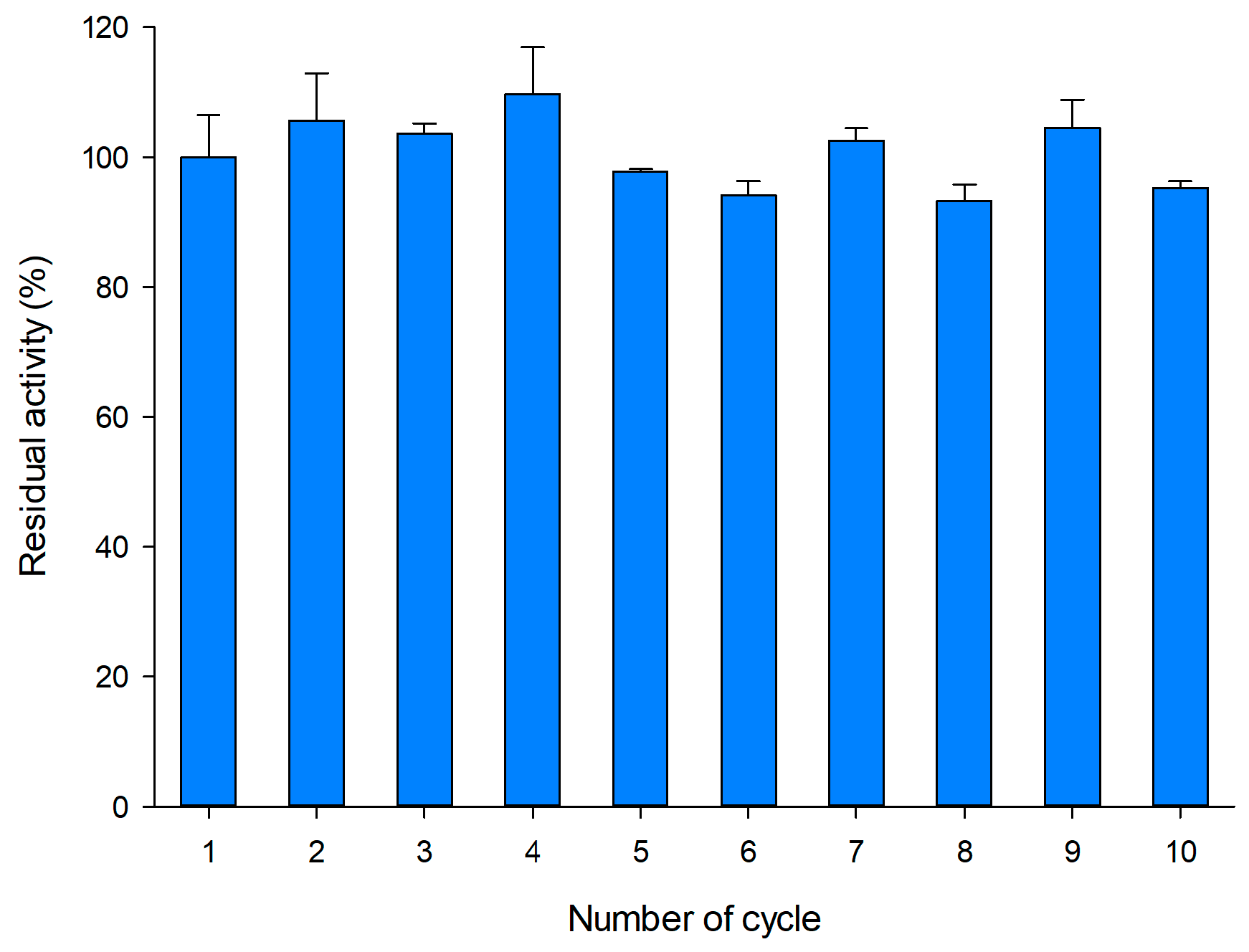
| Total Initial Activity (U) a | Volume of Biocatalyst (mL) | Lens Volume (µL) b | Activity of the Biocatalyst (U/lens) a | Activity of the Biocatalyst (U/ml) a | Recovered Activity (%) c |
|---|---|---|---|---|---|
| 35.5 | 4.8 | 56.3 | 0.34 | 5.96 | 80.6 |
| 84.5 | 4.7 | 52.6 | 0.33 | 6.20 | 34.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Míguez, N.; Gimeno-Pérez, M.; Fernández-Polo, D.; Cervantes, F.V.; Ballesteros, A.O.; Fernández-Lobato, M.; Ribeiro, M.H.; Plou, F.J. Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by Entrapment in Polyvinyl Alcohol and Its Application to Neo-Fructooligosaccharides Production. Catalysts 2018, 8, 201. https://doi.org/10.3390/catal8050201
Míguez N, Gimeno-Pérez M, Fernández-Polo D, Cervantes FV, Ballesteros AO, Fernández-Lobato M, Ribeiro MH, Plou FJ. Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by Entrapment in Polyvinyl Alcohol and Its Application to Neo-Fructooligosaccharides Production. Catalysts. 2018; 8(5):201. https://doi.org/10.3390/catal8050201
Chicago/Turabian StyleMíguez, Noa, María Gimeno-Pérez, David Fernández-Polo, Fadia V. Cervantes, Antonio O. Ballesteros, María Fernández-Lobato, María H. Ribeiro, and Francisco J. Plou. 2018. "Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by Entrapment in Polyvinyl Alcohol and Its Application to Neo-Fructooligosaccharides Production" Catalysts 8, no. 5: 201. https://doi.org/10.3390/catal8050201
APA StyleMíguez, N., Gimeno-Pérez, M., Fernández-Polo, D., Cervantes, F. V., Ballesteros, A. O., Fernández-Lobato, M., Ribeiro, M. H., & Plou, F. J. (2018). Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by Entrapment in Polyvinyl Alcohol and Its Application to Neo-Fructooligosaccharides Production. Catalysts, 8(5), 201. https://doi.org/10.3390/catal8050201








