The Preparation of Pd/Foam-Ni Electrode and Its Electrocatalytic Hydrodechlorination for Monochlorophenol Isomers
Abstract
1. Introduction
2. Results and Discussion
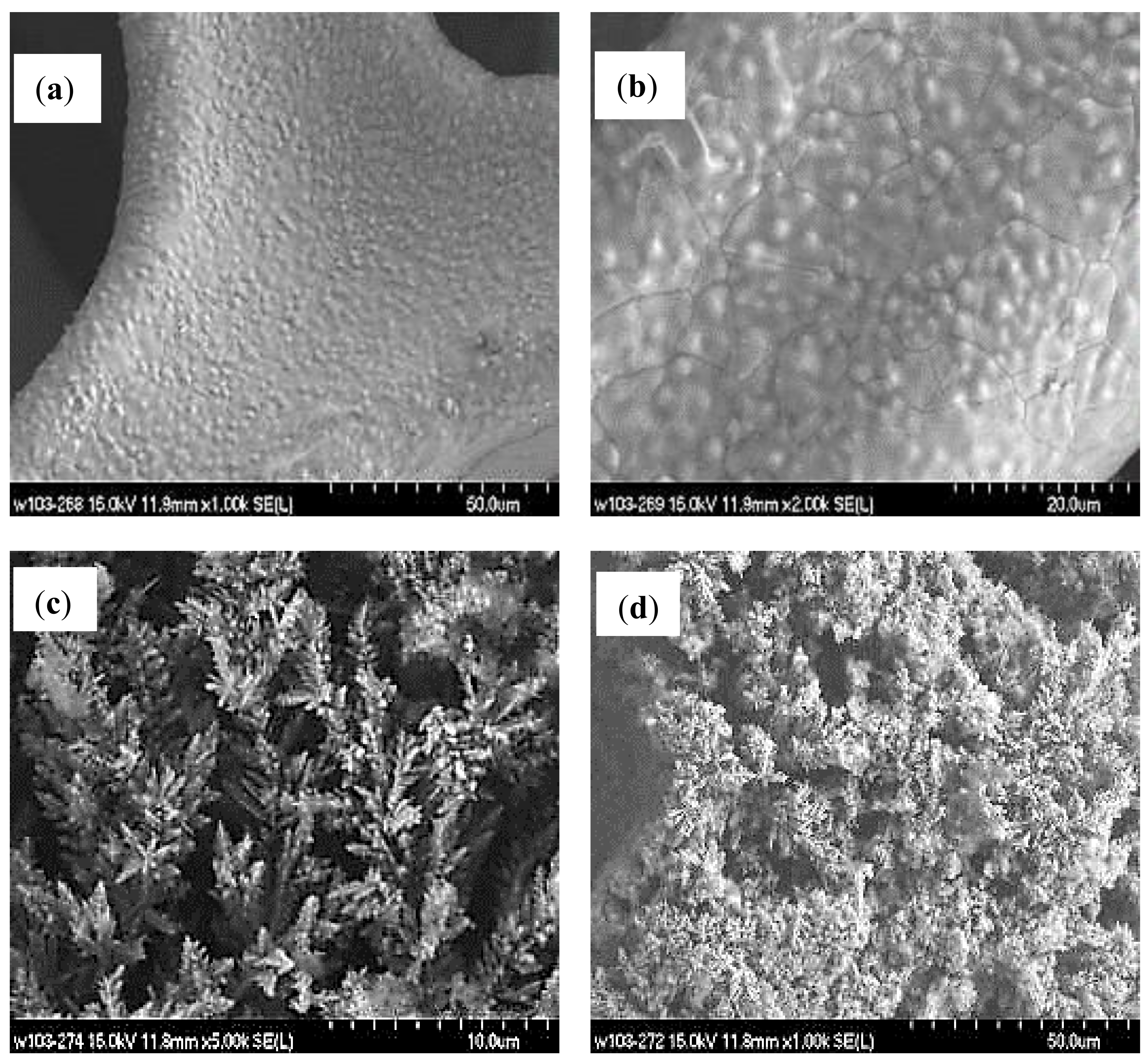
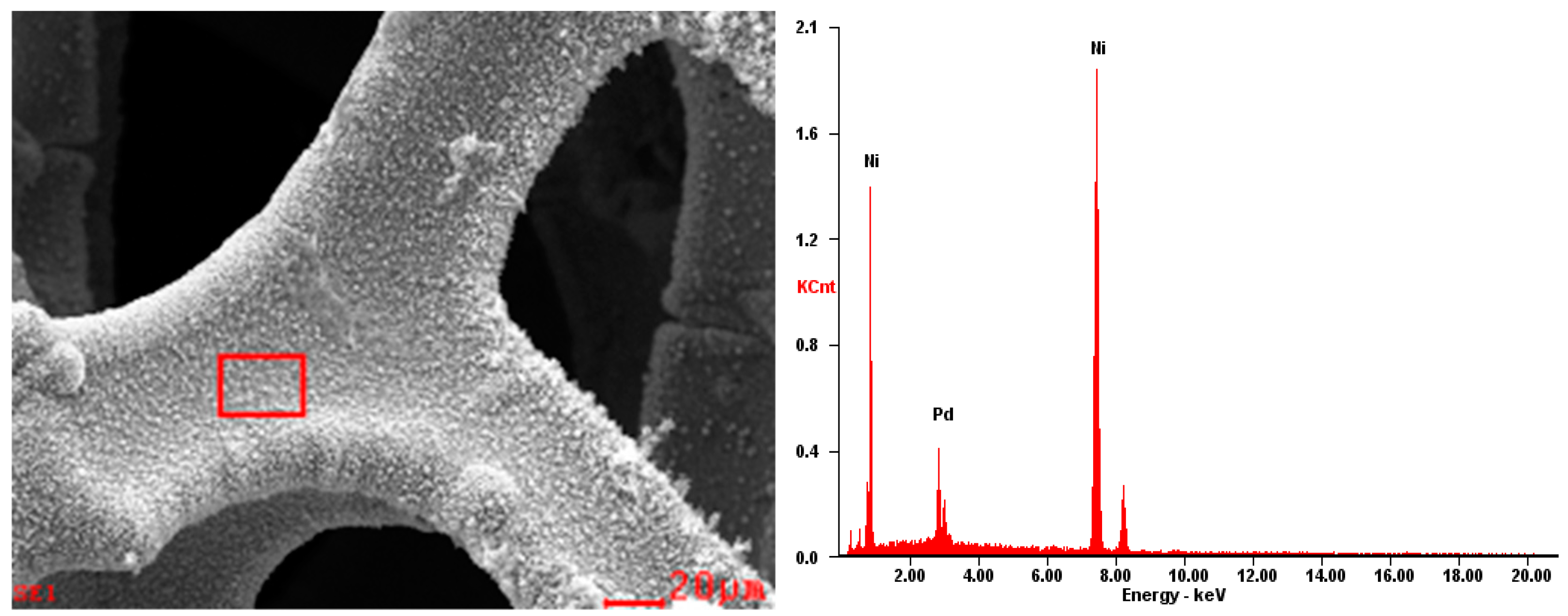
2.1. Morphology Analysis
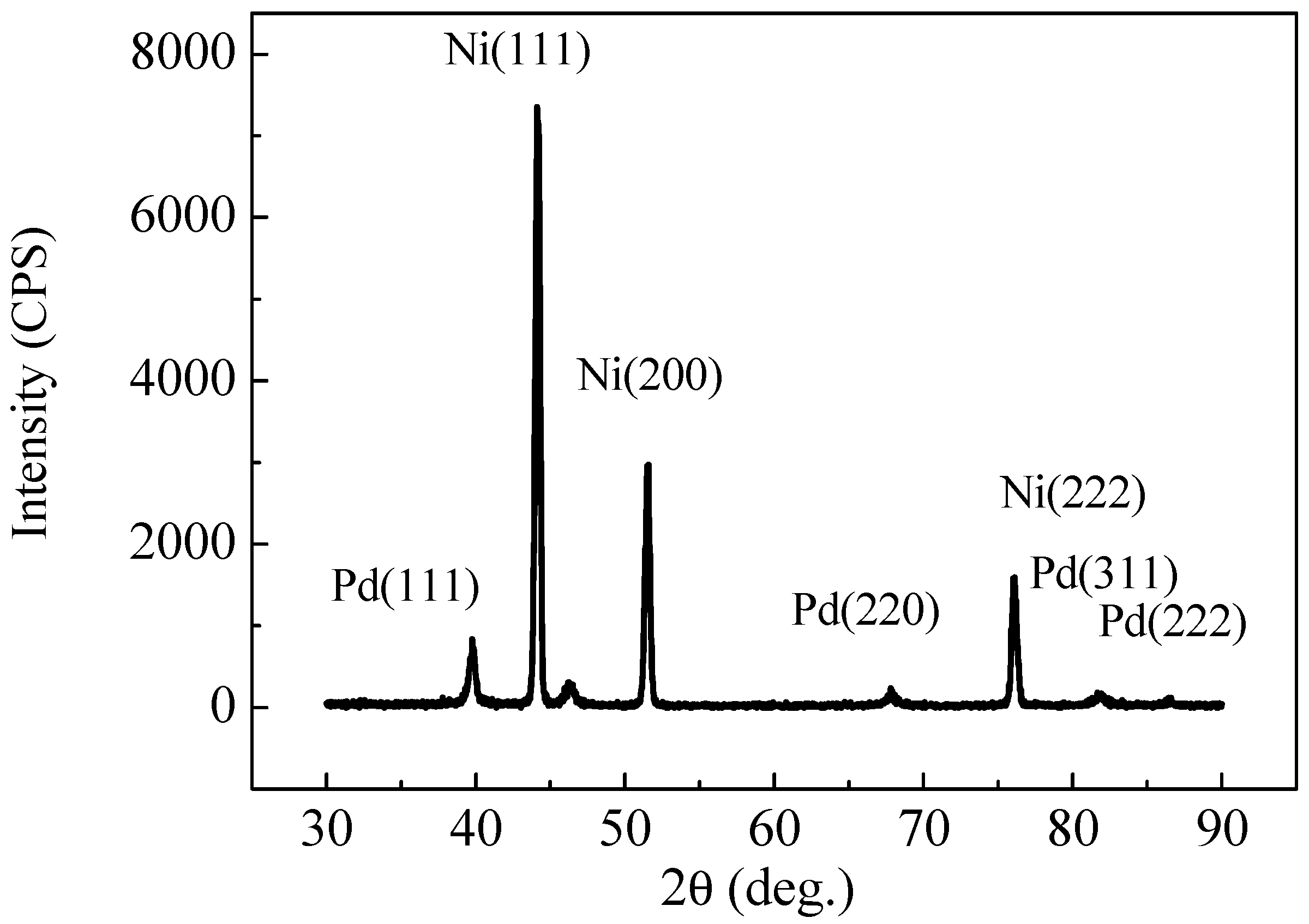
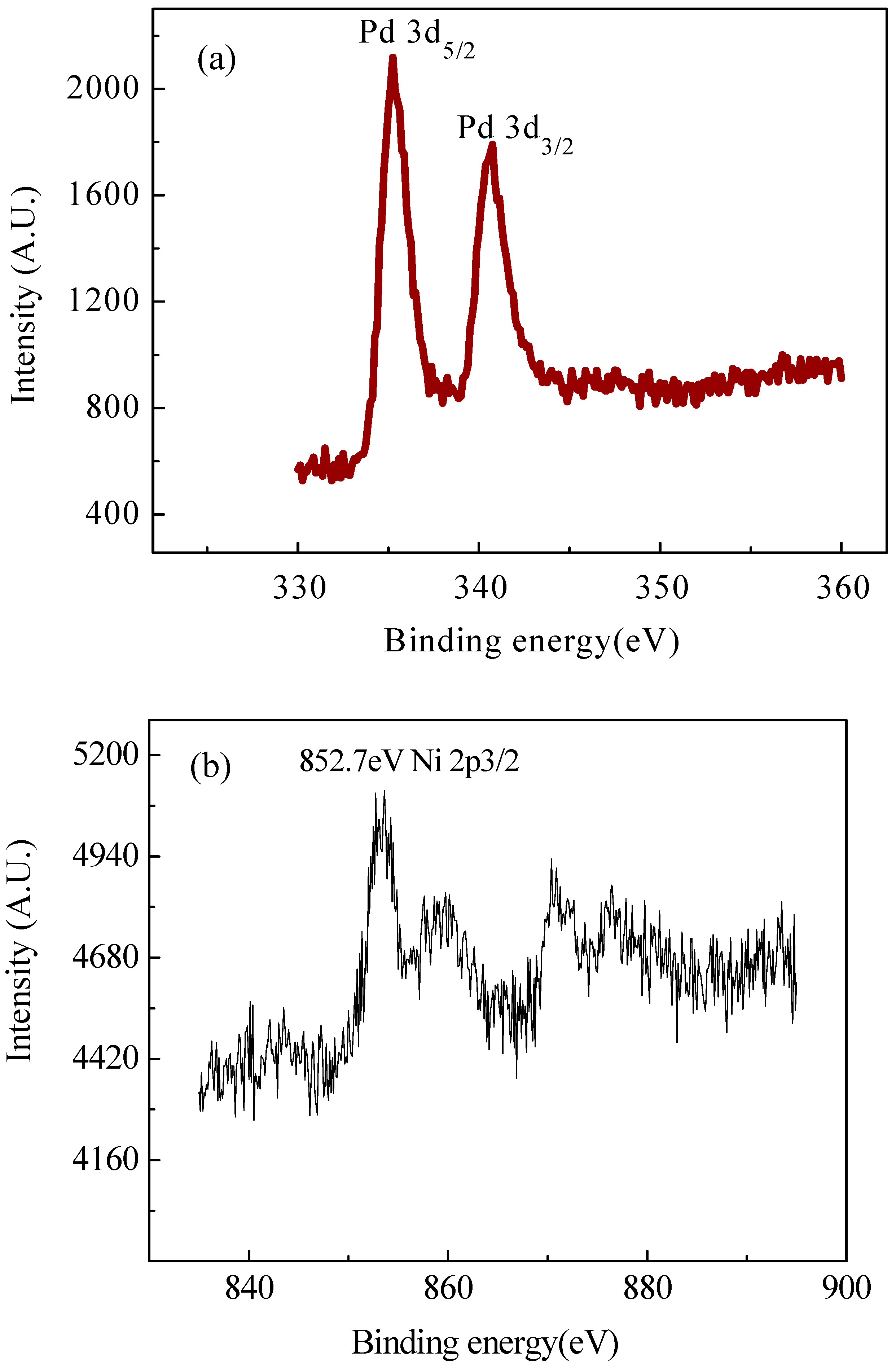
2.2. XRD and XPS Analysis
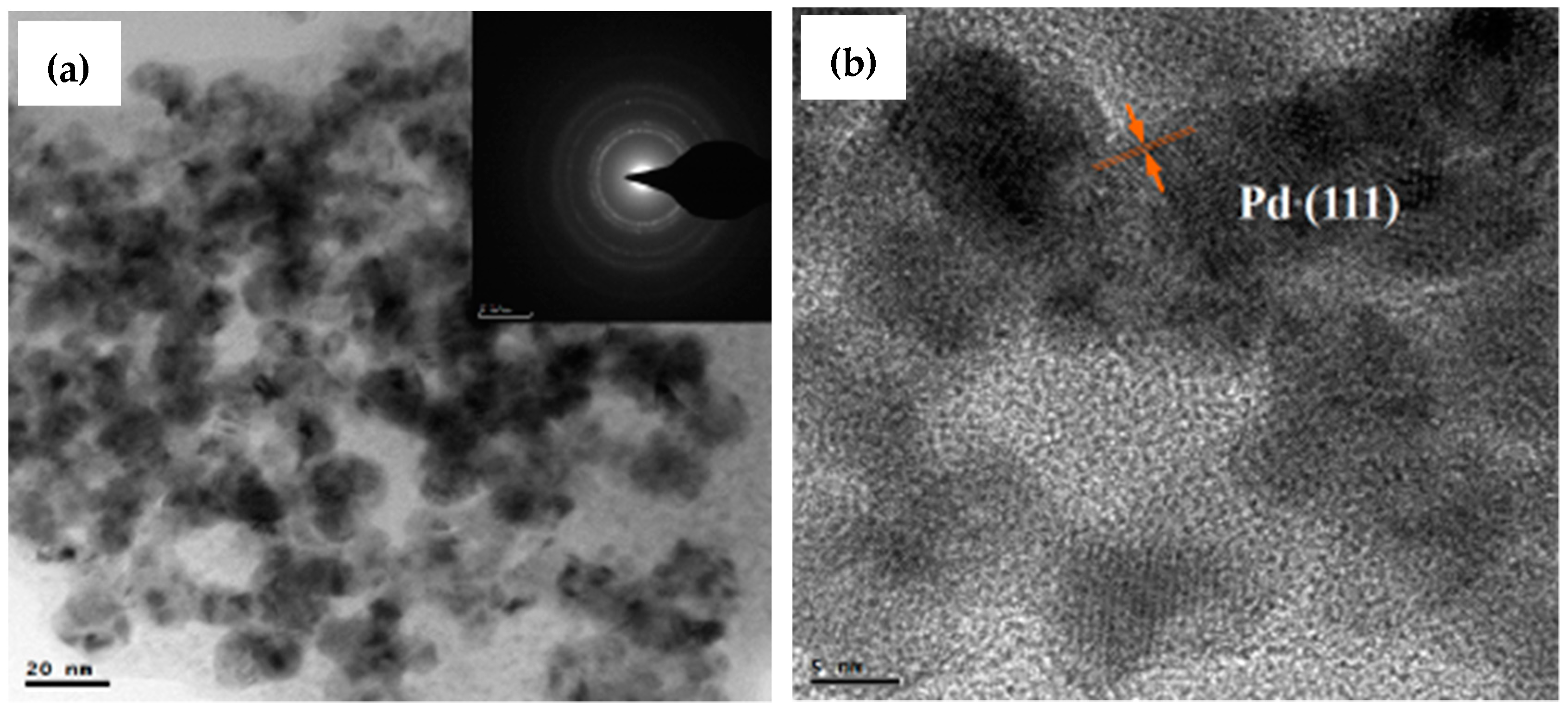
2.3. TEM Analysis
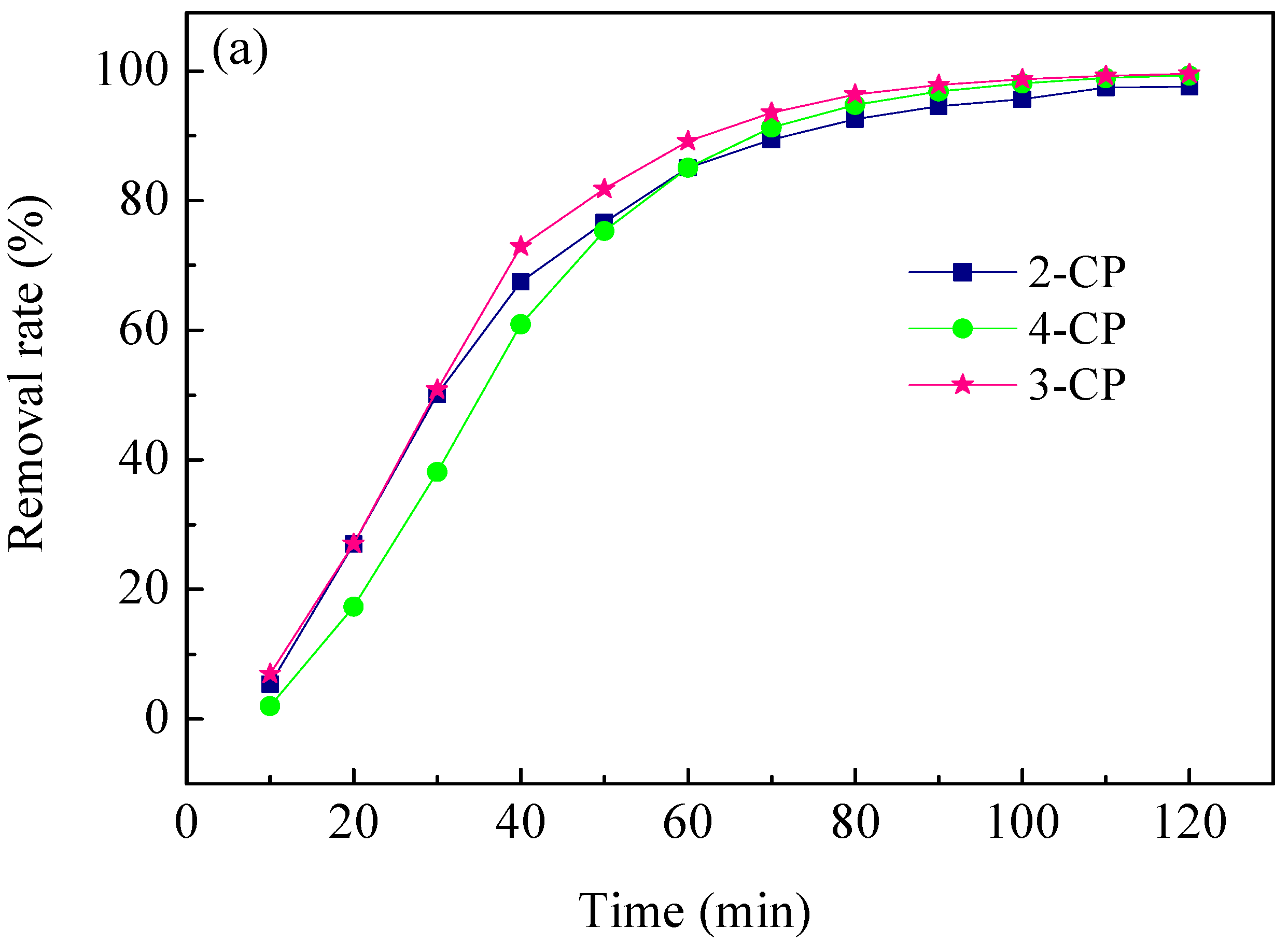
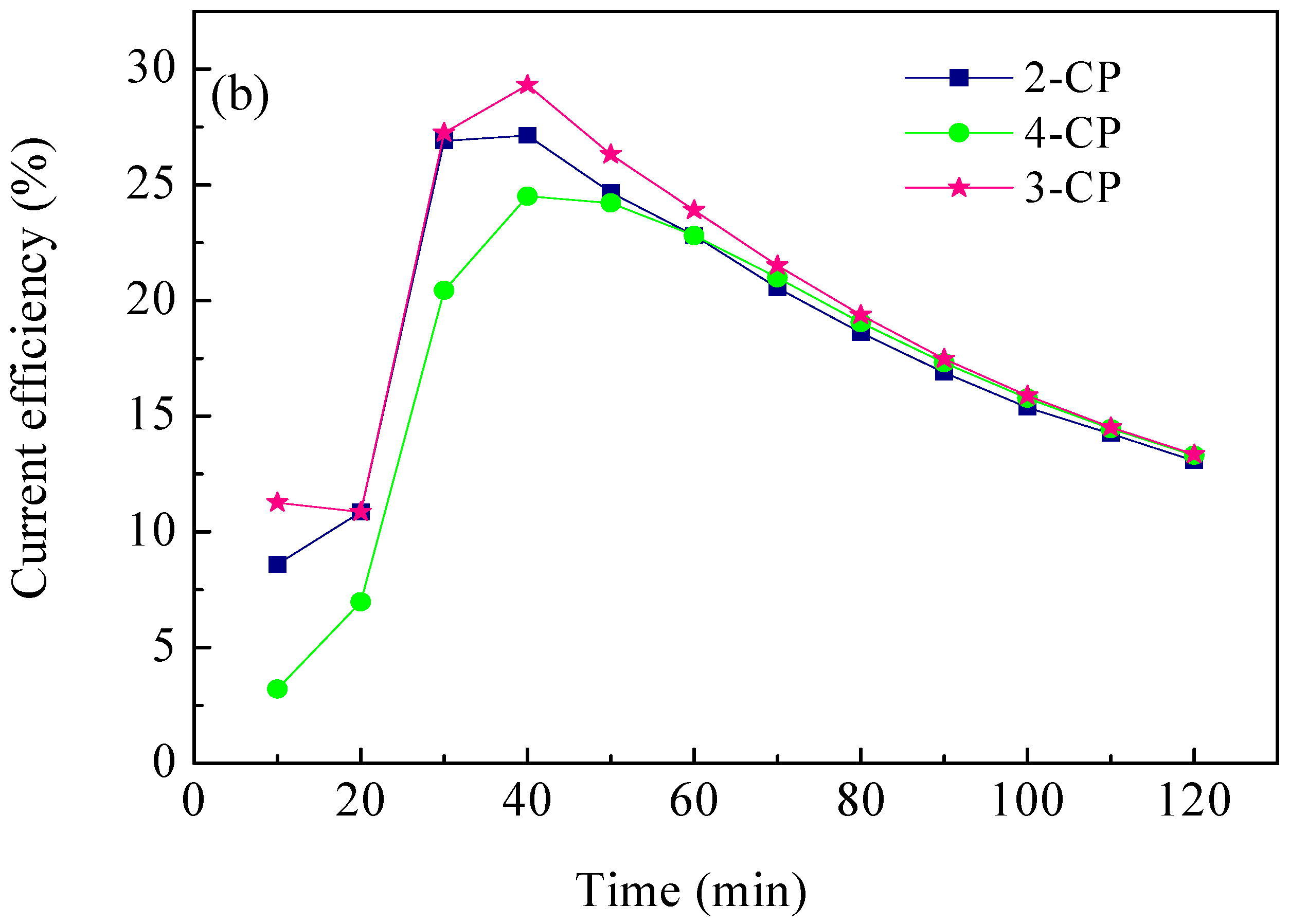
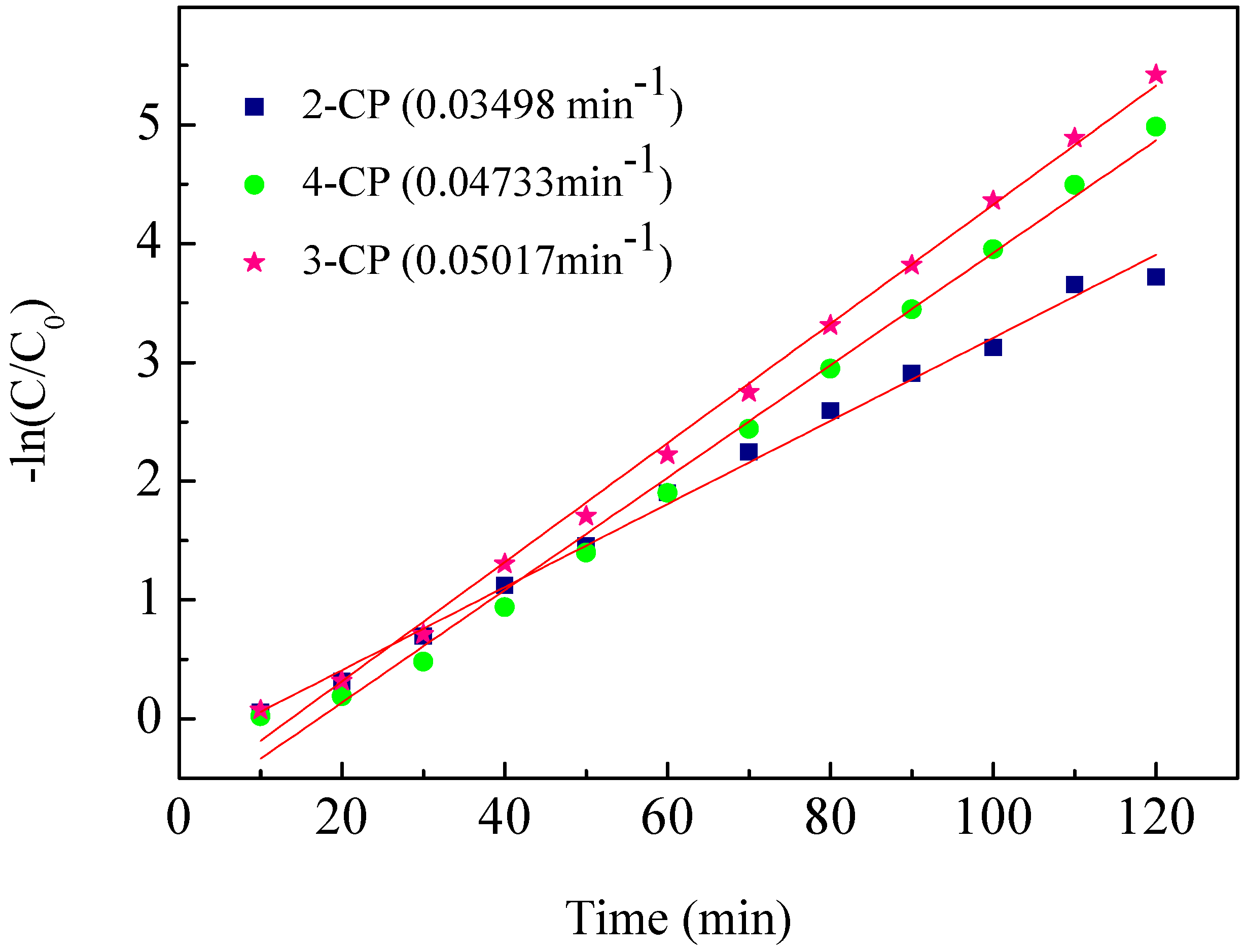
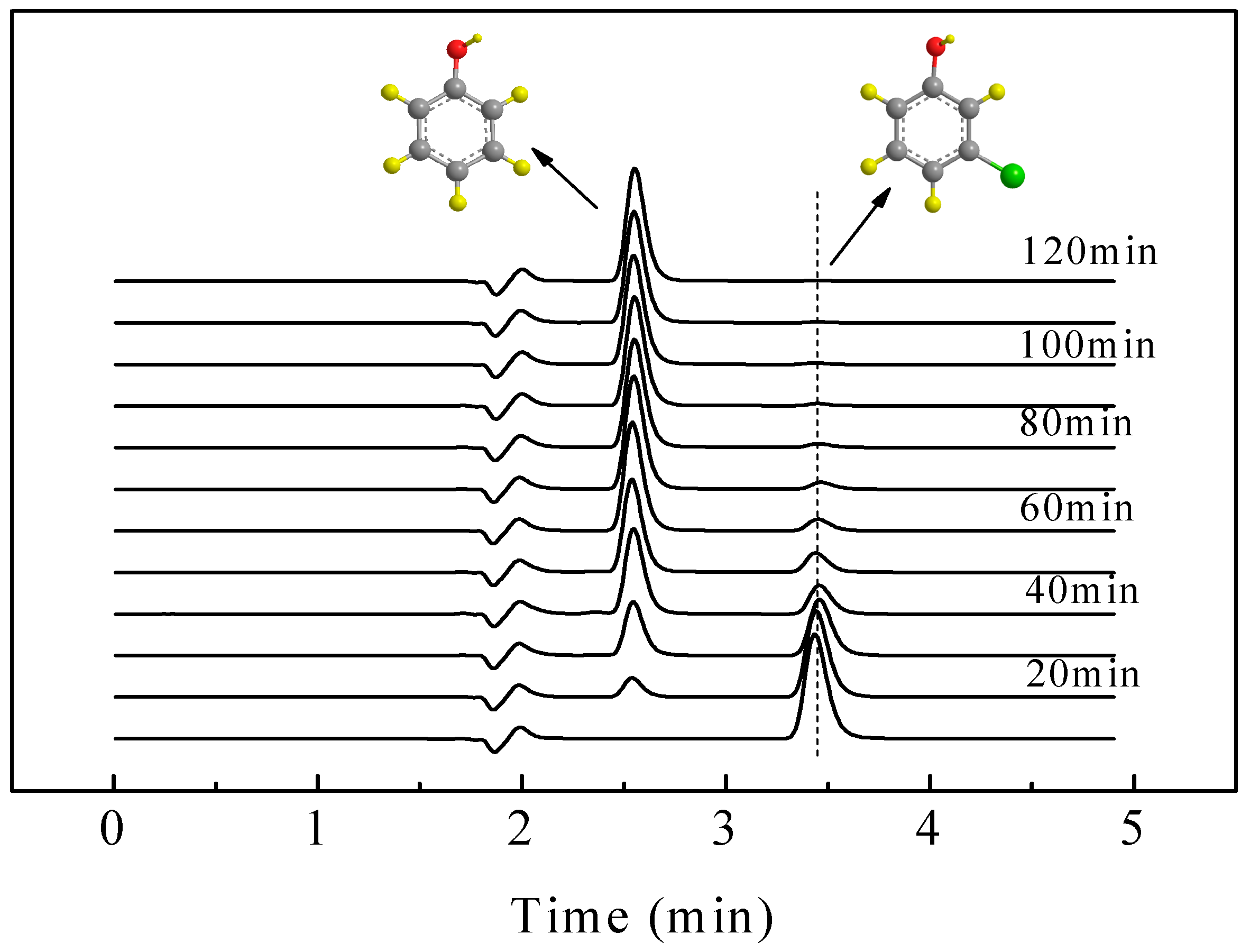
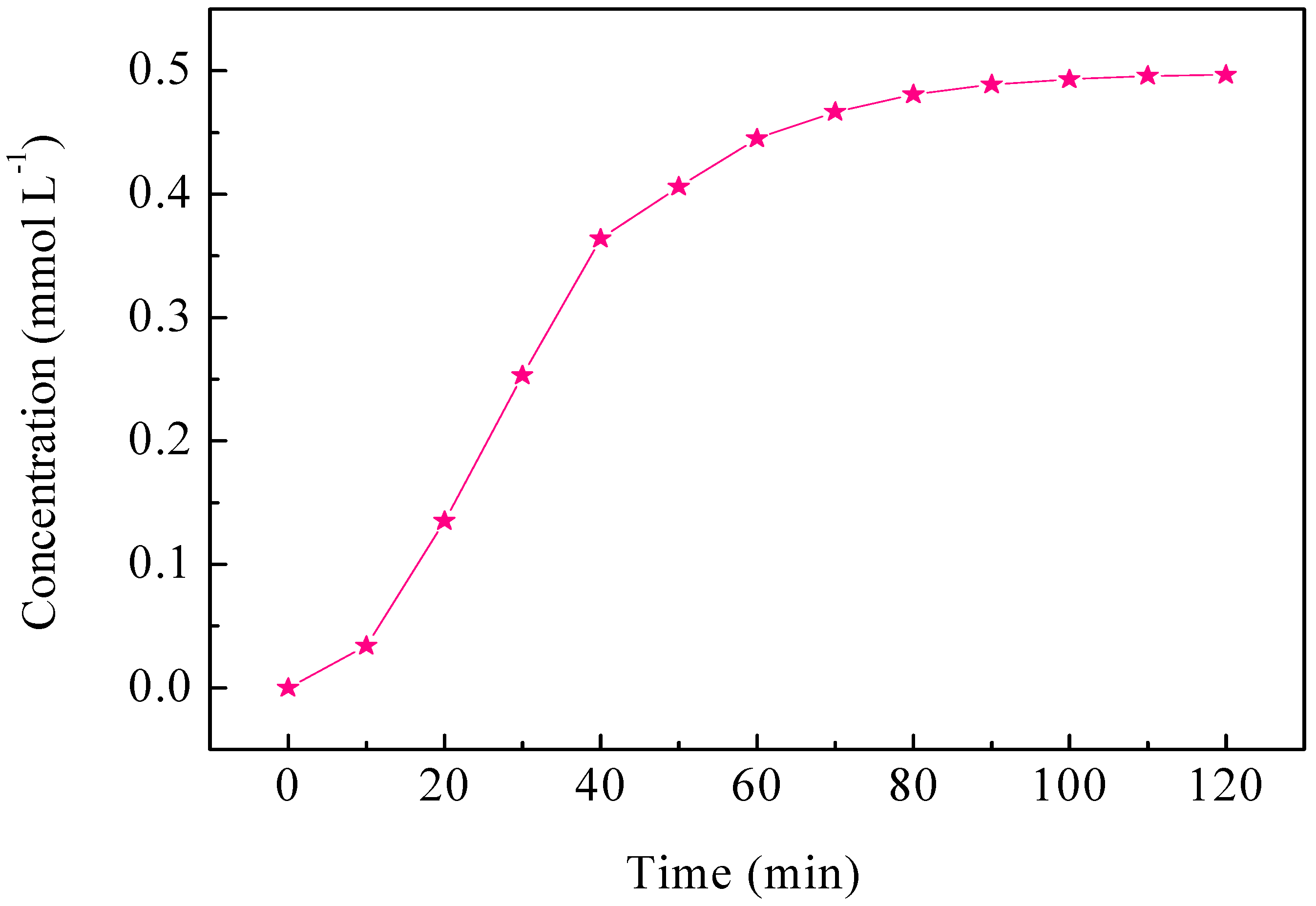
2.4. ECH of Monochlorophenol Isomers
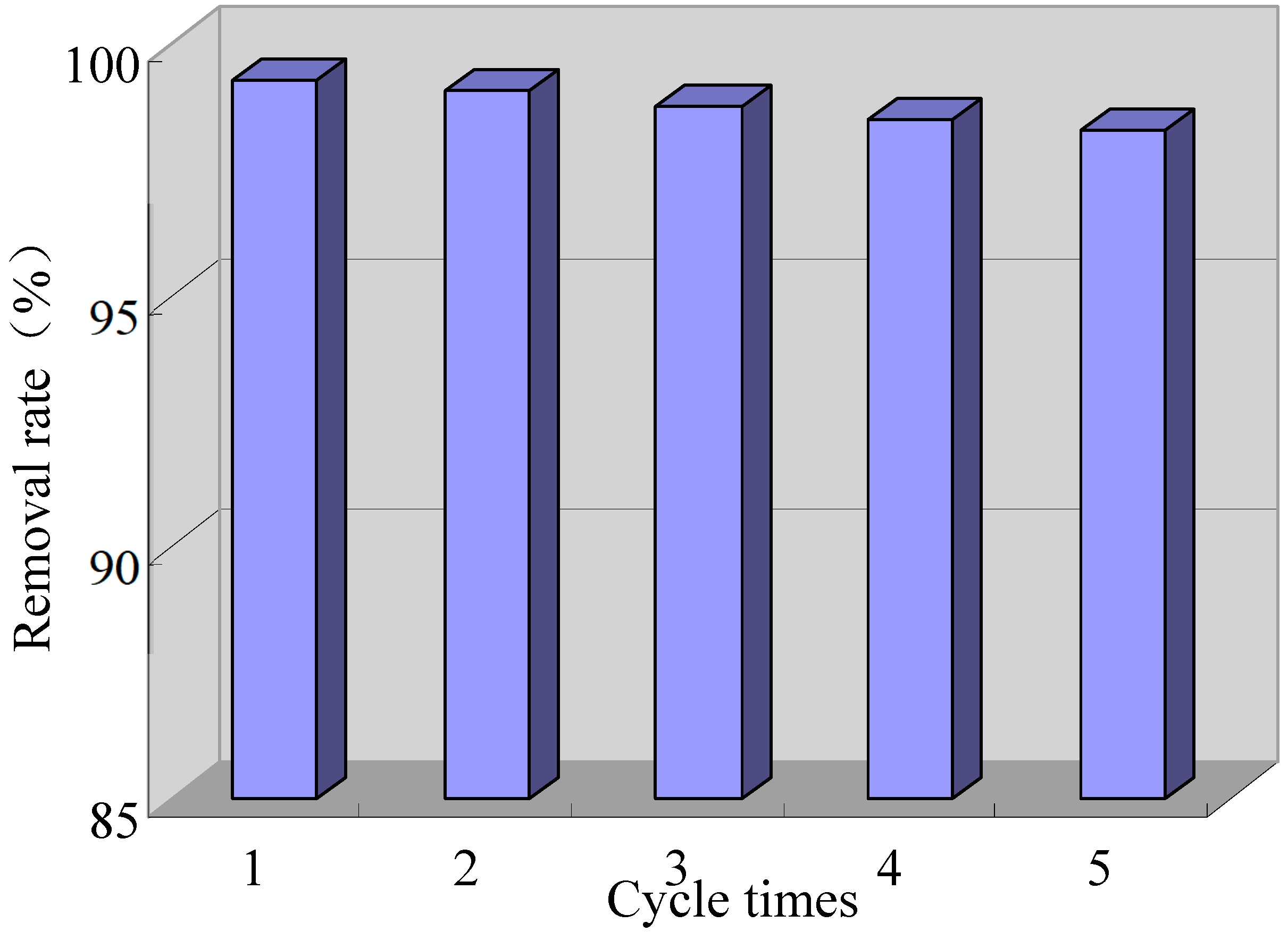
2.5. Stability of Composite Electrode
3. Materials and Methods
3.1. Reagents and Materials
3.2. Method
3.2.1. Preparation of Pd/Ni Cathode
3.2.2. Characterization of Pd/Ni Electrode
3.2.3. ECH of Monochlorophenols
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, G.; Keane, M.A. Catalyst deactivation during the liquid phase hydrodechlorination of 2,4-dichlorophenol over supported Pd: Influence of the support. Catal. Today 2003, 88, 27–36. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, X.; Shen, H.; Hu, X. Preparation of palladium–nickel loaded titanium electrode with surfactant assistance and its application in pentachlorophenol reductive dechlorination. Sep. Purif. Technol. 2014, 124, 224–230. [Google Scholar] [CrossRef]
- Sun, Z.; Song, G.; Du, R.; Hu, X. Modification of a Pd-loaded electrode with a carbon nanotubes–polypyrrole interlayer and its dechlorination performance for 2,3-dichlorophenol. RSC Adv. 2017, 7, 22054–22062. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Garcı́A-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chen, Y.C.; Chen, M.Y.; Tai, Y.T.; Tso, C.P. Dechlorination of hexachlorobenzene by using nanoscale Fe and nanoscale Pd/Fe bimetallic particles. Colloid Surf. A Physicochem. Eng. Asp. 2009, 332, 84–89. [Google Scholar] [CrossRef]
- Sun, C.; Baig, S.A.; Lou, Z.; Zhu, J.; Wang, Z.; Li, X.; Wu, J.; Zhang, Y.; Xu, X. Electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid using nanosized titanium nitride doped palladium/nickel foam electrodes in aqueous solutions. Appl. Catal. B Environ. 2014, 158–159, 38–47. [Google Scholar] [CrossRef]
- Christelle, B.; Shawn, R.; Dennis, T.; Abderrahmane, T.; Brian, K.G. Formation of polychlorinated dibenzo-p-dioxins and dibenzofurans from a mixture of chlorophenols over fly ash: Influence of water vapor. Environ. Sci. Technol. 2007, 41, 850–856. [Google Scholar]
- Sun, Z.; Shen, H.; Wei, X.; Hu, X. Electrocatalytic hydrogenolysis of chlorophenols in aqueous solution on Pd58Ni42 cathode modified with PPy and SDBS. Chem. Eng. J. 2014, 241, 433–442. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, K.; Wei, X.; Tong, S.; Hu, X. Electrocatalytic hydrodehalogenation of 2,4-dichlorophenol in aqueous solution on palladium–nickel bimetallic electrode synthesized with surfactant assistance. Int. J. Hydrogen Energy 2012, 37, 17862–17869. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Shan, J.; Zhang, J. Electrodeposition of palladium and reduced graphene oxide nanocomposites on foam-nickel electrode for electrocatalytic hydrodechlorination of 4-chlorophenol. J. Hazard. Mater. 2015, 290, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wei, X.; Han, Y.; Tong, S.; Hu, X. Complete dechlorination of 2,4-dichlorophenol in aqueous solution on palladium/polymeric pyrrole-cetyl trimethyl ammonium bromide/foam-nickel composite electrode. J. Hazard. Mater. 2013, 244–245, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, K.; Lv, X.; Fu, H.; Dong, X.; Chen, L.; Zhang, X.; Jiang, G. Palladium nanoparticles assembled on titanium nitride for enhanced electrochemical hydrodechlorination of 2,4-dichlorophenol in water. Chin. J. Catal. 2018, 39, 693–700. [Google Scholar] [CrossRef]
- Yang, B.; Deng, S.; Yu, G.; Lu, Y.; Zhang, H.; Xiao, J.; Chen, G.; Cheng, X.; Shi, L. Pd/Al bimetallic nanoparticles for complete hydrodechlorination of 3-chlorophenol in aqueous solution. Chem. Eng. J. 2013, 219, 492–498. [Google Scholar] [CrossRef]
- Xu, Y.H.; Cai, Q.Q.; Ma, H.X.; He, Y.; Zhang, H.; Ma, C.A. Optimisation of electrocatalytic dechlorination of 2,4-dichlorophenoxyacetic acid on a roughened silver–palladium cathode. Electrochim. Acta 2013, 96, 90–96. [Google Scholar] [CrossRef]
- Xu, F.; Deng, S.; Xu, J.; Zhang, W.; Wu, M.; Wang, B.; Huang, J.; Yu, G. Highly active and stable Ni-Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 4576–4582. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Xu, C.; Zhang, W.; Huang, L.Z. The important role of polyvinylpyrrolidone and Cu on enhancing dechlorination of 2,4-dichlorophenol by Cu/Fe nanoparticles: Performance and mechanism study. Appl. Surf. Sci. 2018, 435, 55–64. [Google Scholar] [CrossRef]
- Jiang, G.; Lan, M.; Zhang, Z.; Lv, X.; Lou, Z.; Xu, X.; Dong, F.; Zhang, S. Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenol in water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Xie, Q.; Yu, H.; Han, Y. Electrocatalytic hydrodehalogenation of pentachlorophenol at palladized multiwalled carbon nanotubes electrode. Appl. Catal. B Environ. 2008, 80, 122–128. [Google Scholar] [CrossRef]
- Song, C.; Cao, L.; Li, B.; Huang, X.; Ye, K.; Zhu, K.; Cao, D.; Cheng, K.; Wang, G. Highly efficient palladium nanoparticles decorated reduced graphene oxide sheets supported on nickel foam for hydrogen peroxide electroreduction. Appl. Surf. Sci. 2017, 426, 1046–1054. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, Z.; Wang, L.; Hou, Y.; Shi, Y.; Wu, L.; Li, Z. Facile synthesis of Pd–Fe nanoparticles modified Ni foam electrode and its behaviors in electrochemical reduction of tetrabromobisphenol A. Mater. Lett. 2016, 166, 300–303. [Google Scholar] [CrossRef]
- Guo, M.; Cheng, Y.; Yua, Y.; Hu, J. Ni-Co nanoparticles immobilized on a 3D Ni foam template as a highly efficient catalyst for borohydride electrooxidation in alkaline medium. Appl. Surf. Sci. 2017, 416, 439–445. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Xu, J.; Yu, J.; Qin, W.; Liang, X. Catalytic hydrodechlorination reactivity of monochlorophenols in aqueous solutions over palladium/carbon catalyst. Catal. Commun. 2009, 10, 456–458. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Zhou, S.; Yang, C.; Liu, S.; Guo, S.; Liu, Q.; Yu, J.; Chen, J. The influence of ion effects on the Pd-catalyzed hydrodechlorination of 4-chlorophenol in aqueous solutions. Catal. Commun. 2009, 10, 1443–1445. [Google Scholar] [CrossRef]
- Bera, D.; Kuiry, S.C.; Patil, S.; Seal, S. Palladium nanoparticle arrays using template-assisted electrodeposition. Appl. Phys. Lett. 2003, 82, 3089–3091. [Google Scholar] [CrossRef]
- Bera, D.; Kuiry, S.C.; Seal, S. Kinetics and growth mechanism of electrodeposited palladium nanocrystallites. J. Phys. Chem. B 2004, 108, 556–562. [Google Scholar] [CrossRef]
- Carrey, J.; Bouzehouane, K.; George, J.M.; Ceneray, C.; Blon, T.; Bibes, M.; Vaurès, A.; Fusil, S.; Kenane, S.; Vila, L. Electrical characterization of nanocontacts fabricated by nanoindentation and electrodeposition. Appl. Phys. Lett. 2002, 81, 760–762. [Google Scholar] [CrossRef]
- Mourato, A.; Wong, S.M.; Siegenthaler, H.; Abrantes, L.M. Polyaniline films containing palladium microparticles for electrocatalytic purposes. J. Solid State Electrochem. 2006, 10, 140–147. [Google Scholar] [CrossRef]
- Quayum, M.E.; Ye, S.; Uosaki, K. Mechanism for nucleation and growth of electrochemical palladium deposition on an Au(111) electrode. J. Electroanal. Chem. 2002, 520, 126–132. [Google Scholar] [CrossRef]
- Han, J.; Deming, R.L.; Tao, F.M. Theoretical study of molecular structures and properties of the complete series of chlorophenols. J. Phys. Chem. A 2004, 108, 7736–7743. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Cheng, X.; Xin, Y.; Xu, W.; Ma, Z.; Ma, J.; Ren, N.; Li, Q. Stability of palladium-polypyrrole-foam nickel electrode and its electrocatalytic hydrodechlorination for dichlorophenol isomers. Ind. Eng. Chem. Res. 2012, 51, 15557–15563. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Shuai, D. Electrocatalytic hydrodechlorination of 4-chlorobiphenyl in aqueous solution using palladized nickel foam cathode. Chemosphere 2007, 67, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, H.; Wang, L.; Ma, C.; Luan, C.; Zhao, B.; Zhang, Z.; Zhang, H.; Cheng, X.; Liu, J. The Preparation of Pd/Foam-Ni Electrode and Its Electrocatalytic Hydrodechlorination for Monochlorophenol Isomers. Catalysts 2018, 8, 378. https://doi.org/10.3390/catal8090378
Li J, Wang H, Wang L, Ma C, Luan C, Zhao B, Zhang Z, Zhang H, Cheng X, Liu J. The Preparation of Pd/Foam-Ni Electrode and Its Electrocatalytic Hydrodechlorination for Monochlorophenol Isomers. Catalysts. 2018; 8(9):378. https://doi.org/10.3390/catal8090378
Chicago/Turabian StyleLi, Junjing, Huan Wang, Liang Wang, Chang Ma, Cong Luan, Bin Zhao, Zhaohui Zhang, Hongwei Zhang, Xiuwen Cheng, and Junliang Liu. 2018. "The Preparation of Pd/Foam-Ni Electrode and Its Electrocatalytic Hydrodechlorination for Monochlorophenol Isomers" Catalysts 8, no. 9: 378. https://doi.org/10.3390/catal8090378
APA StyleLi, J., Wang, H., Wang, L., Ma, C., Luan, C., Zhao, B., Zhang, Z., Zhang, H., Cheng, X., & Liu, J. (2018). The Preparation of Pd/Foam-Ni Electrode and Its Electrocatalytic Hydrodechlorination for Monochlorophenol Isomers. Catalysts, 8(9), 378. https://doi.org/10.3390/catal8090378





