Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts
Abstract
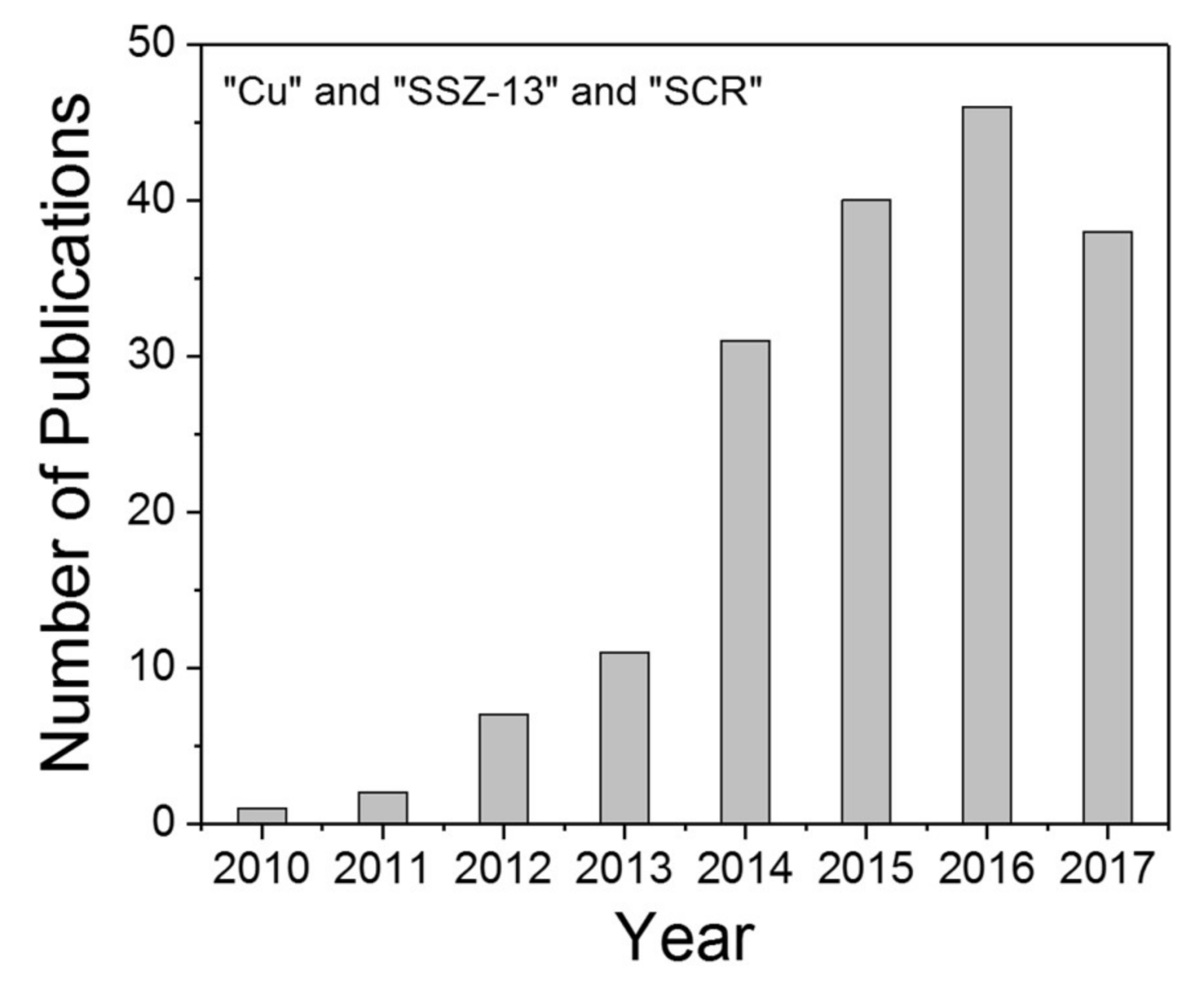
1. Introduction
2. Transformations of Cu Active Species
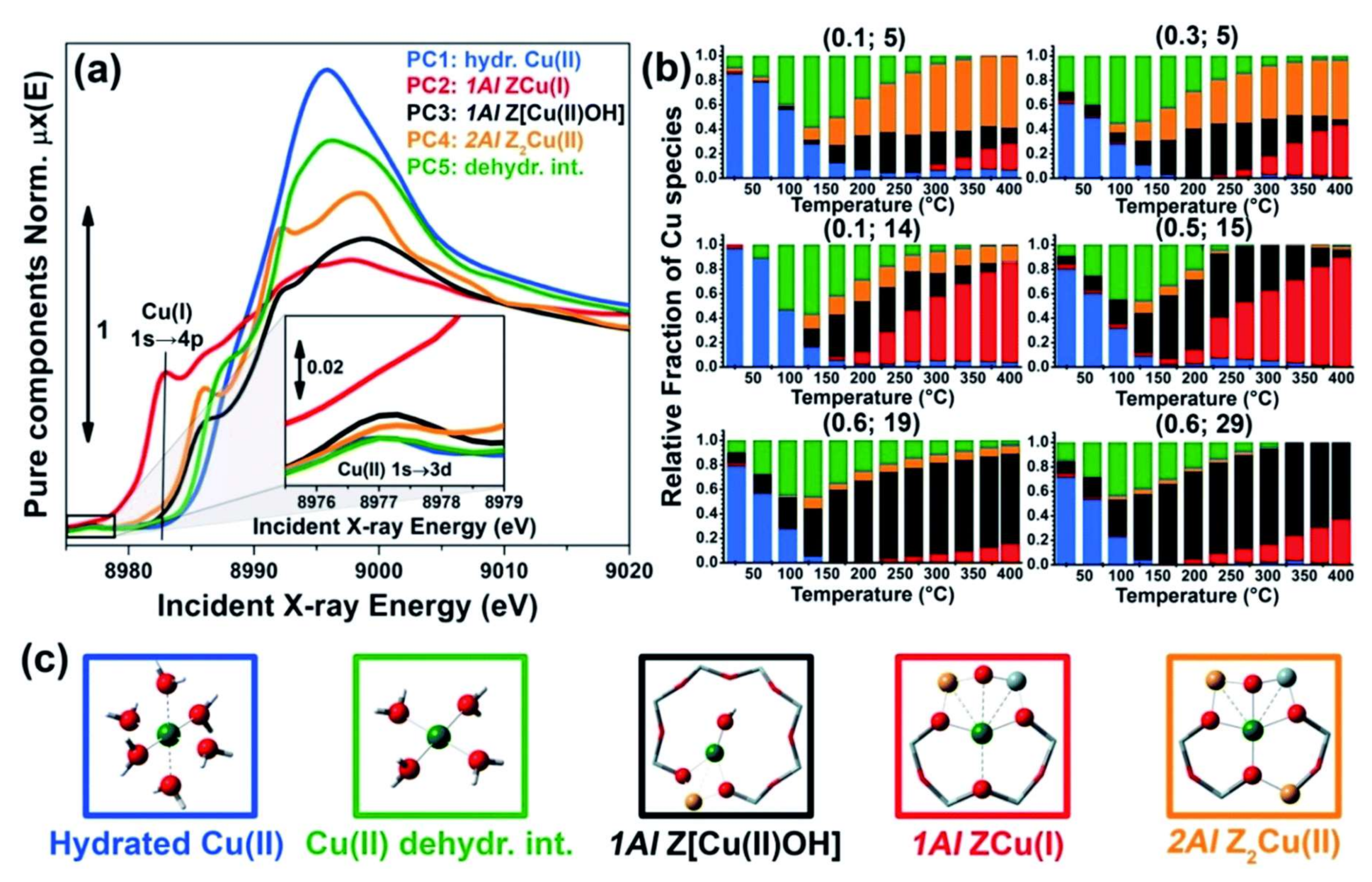
2.1. Dehydration
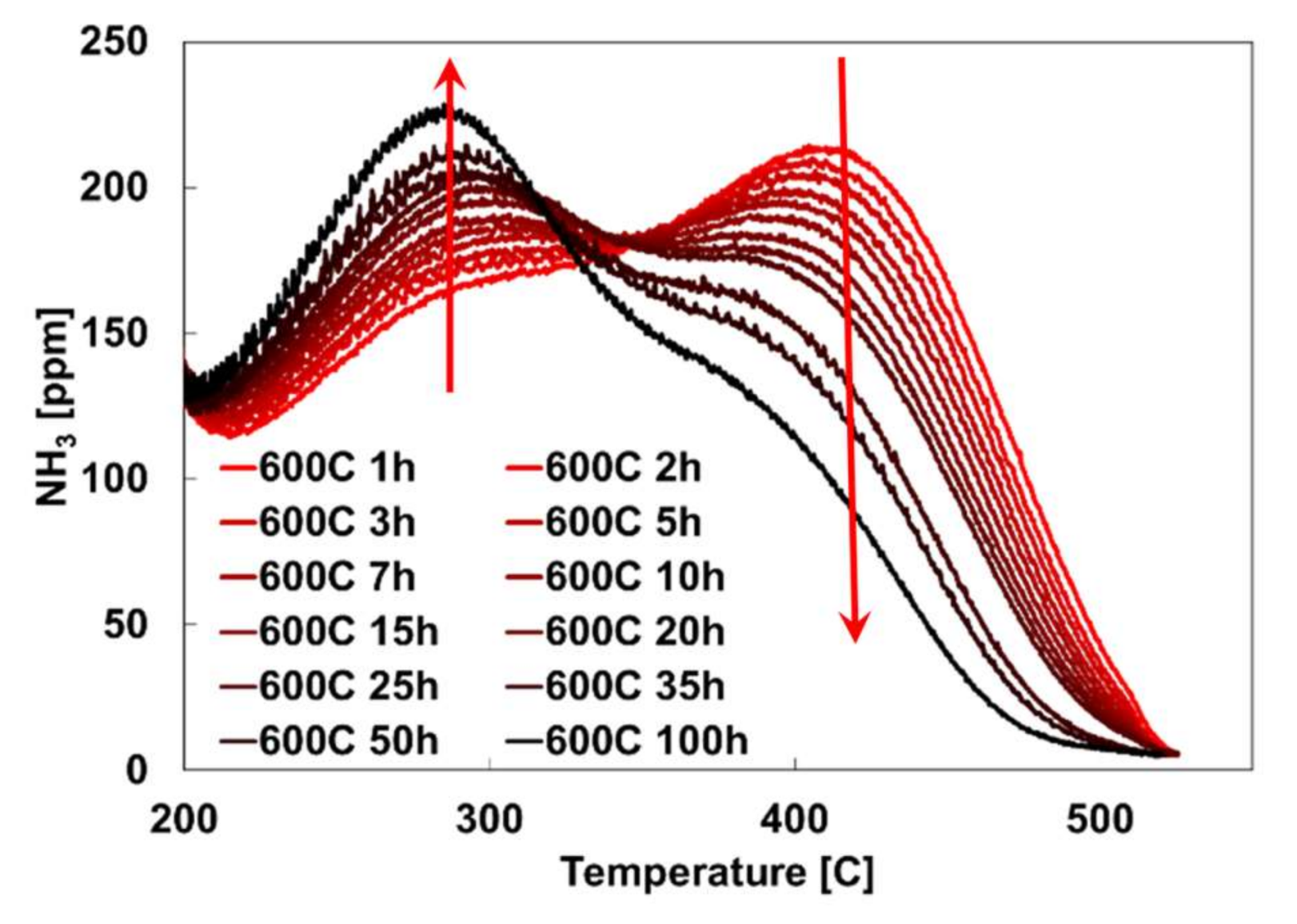
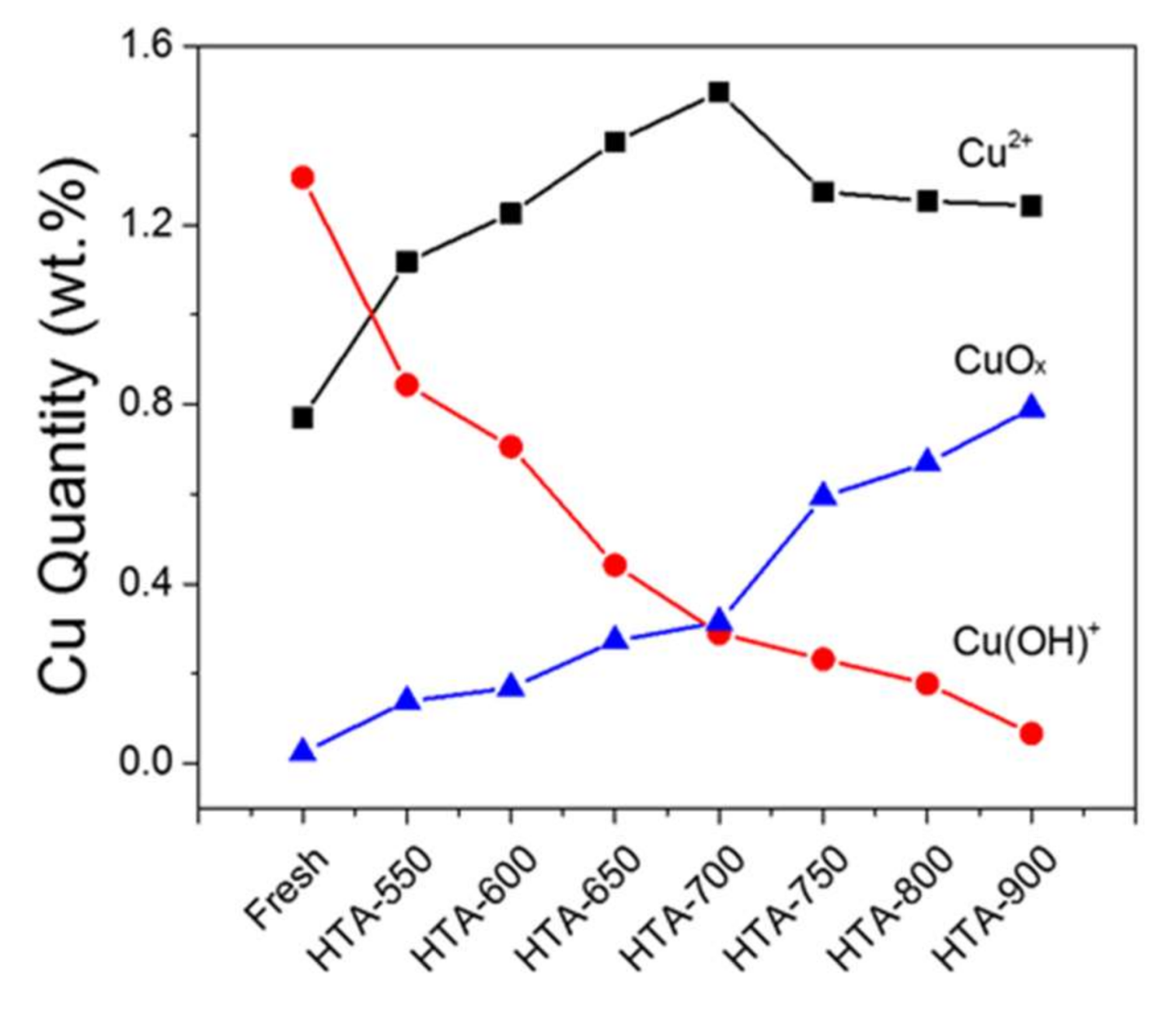
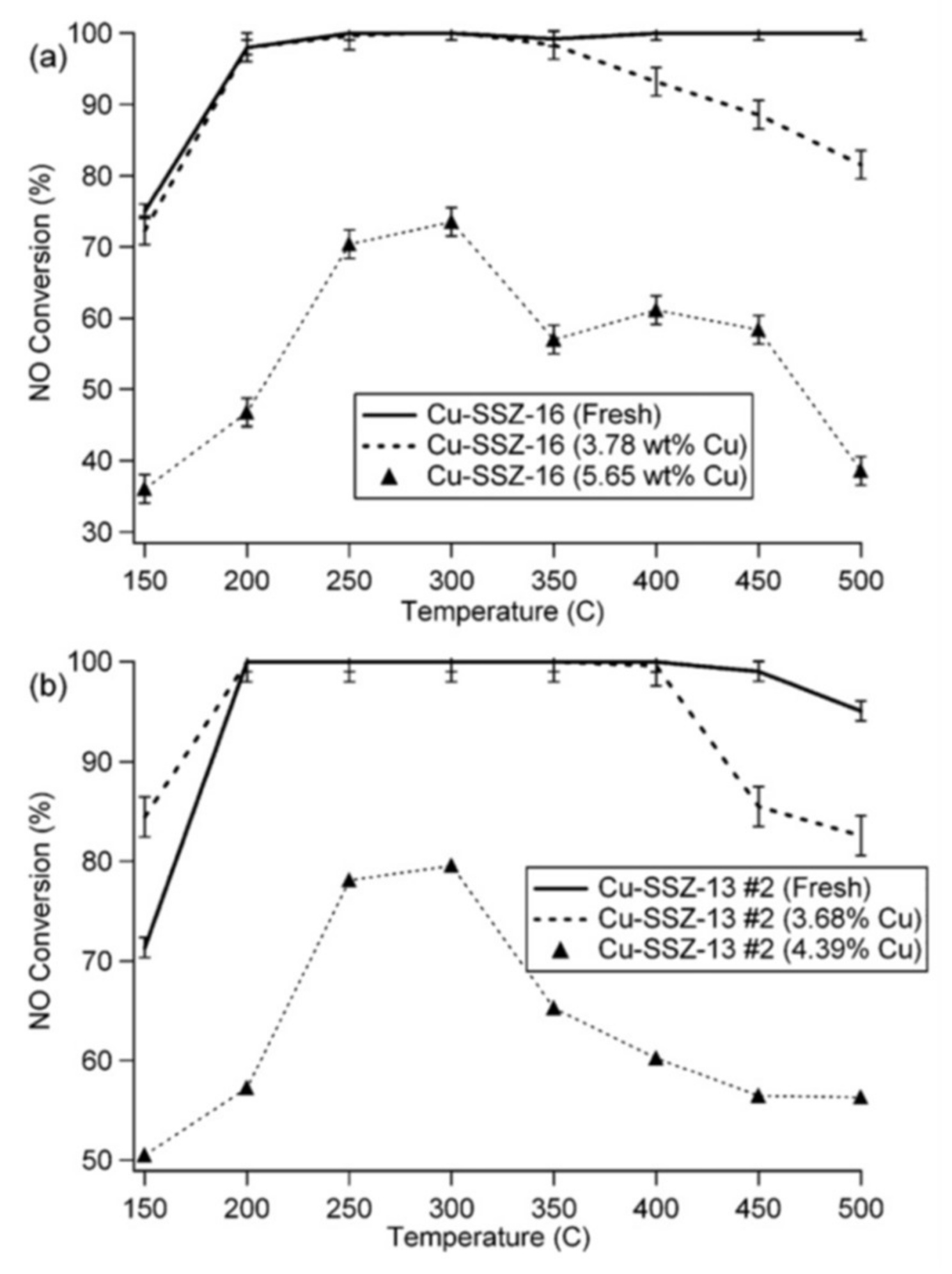
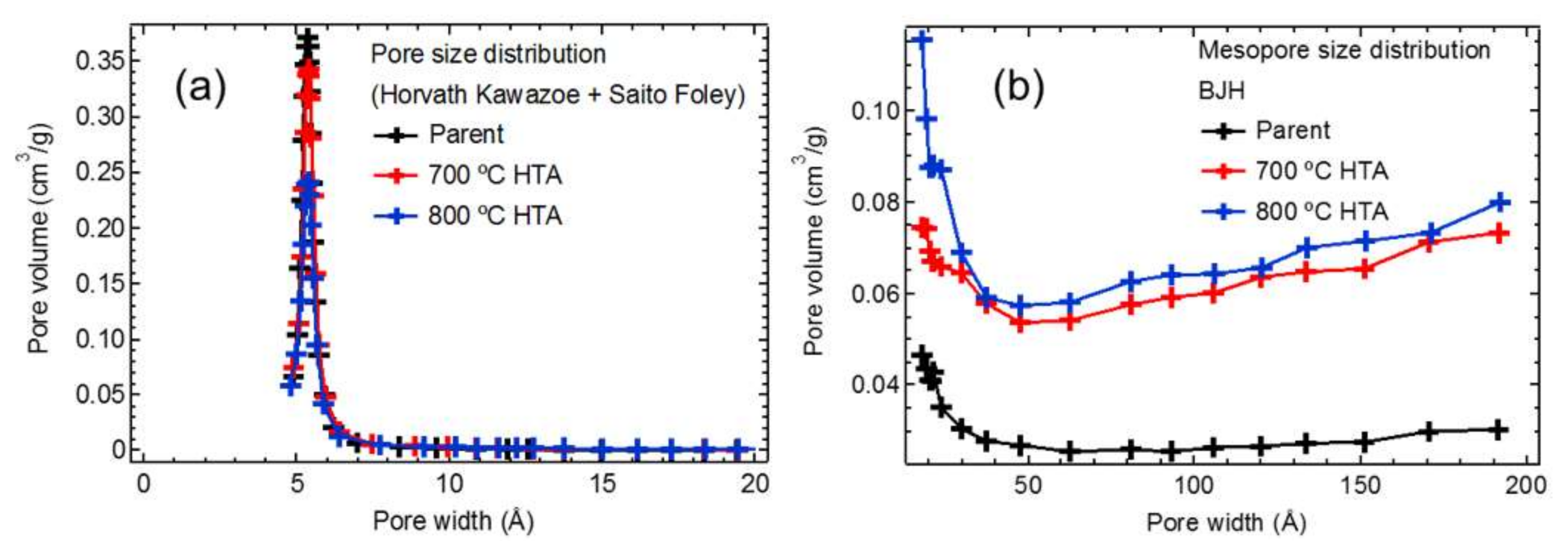
2.2. Hydrothermal Aging
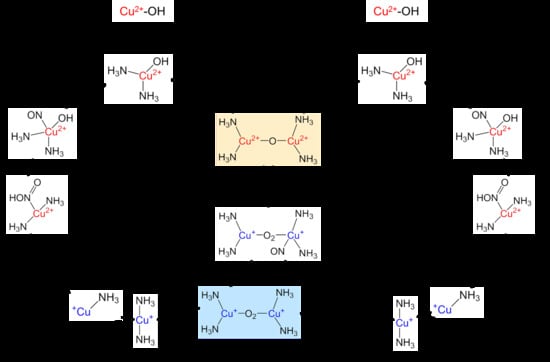
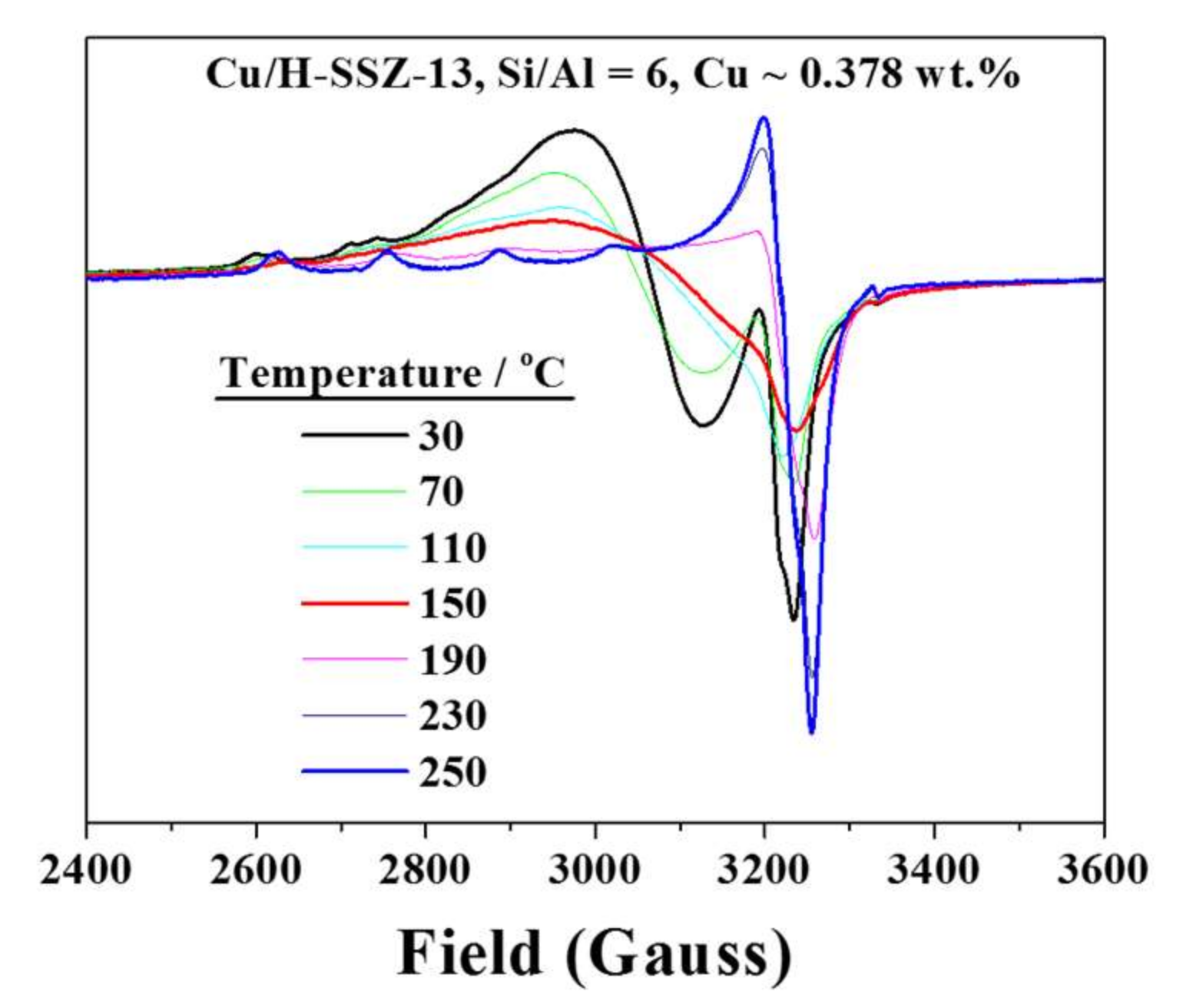
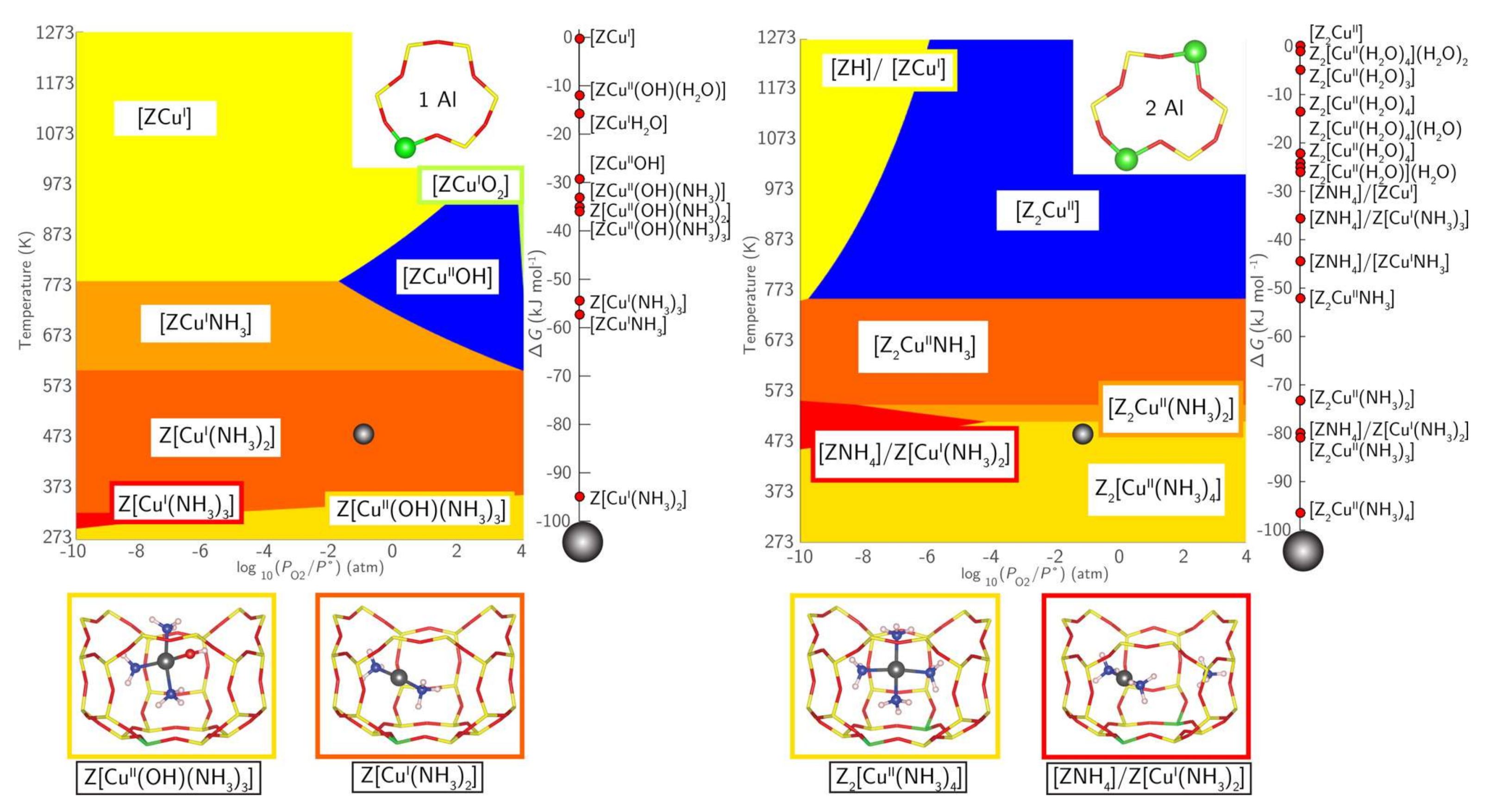
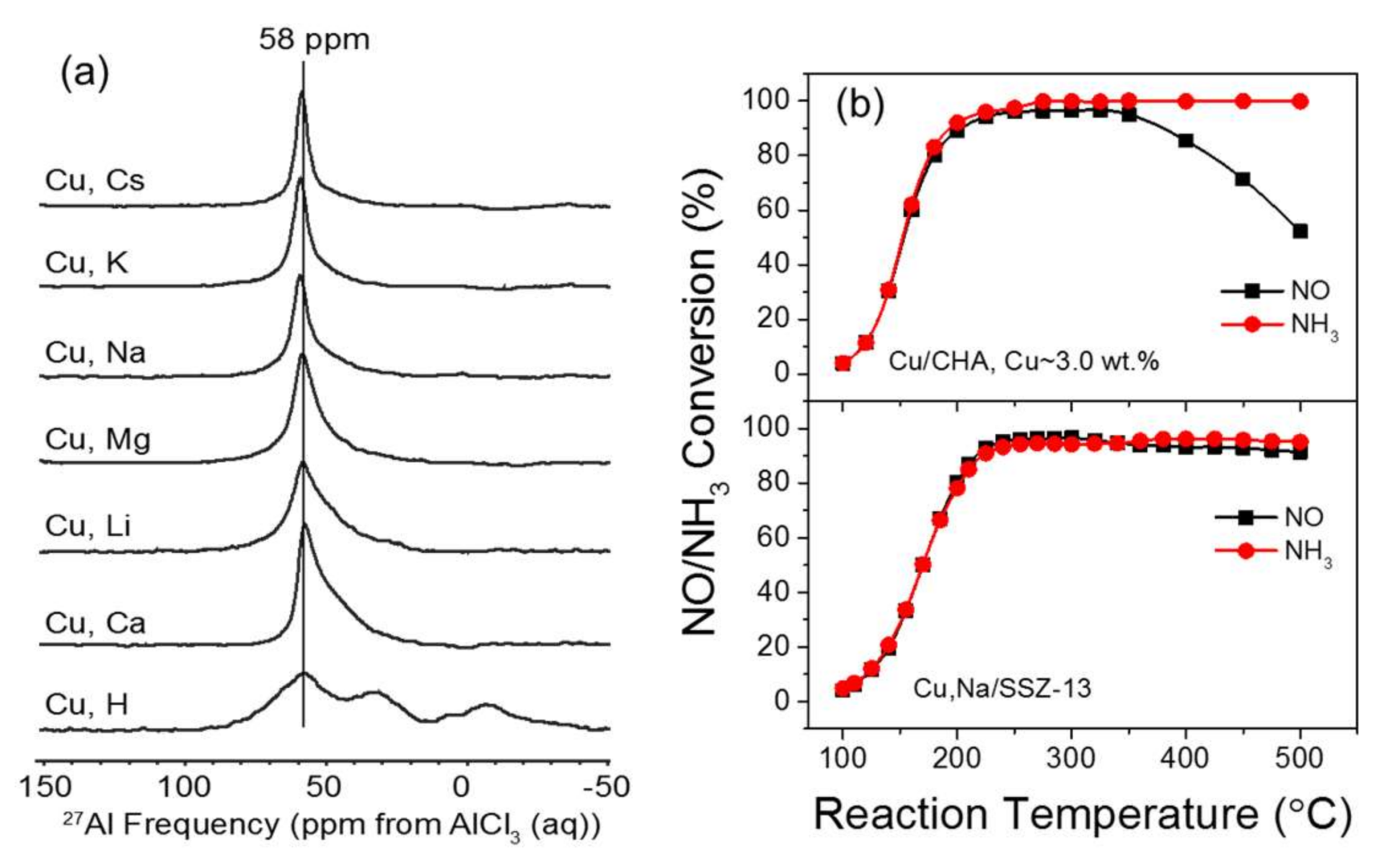
2.3. Interactions with NH3
3. NH3-SCR Mechanisms
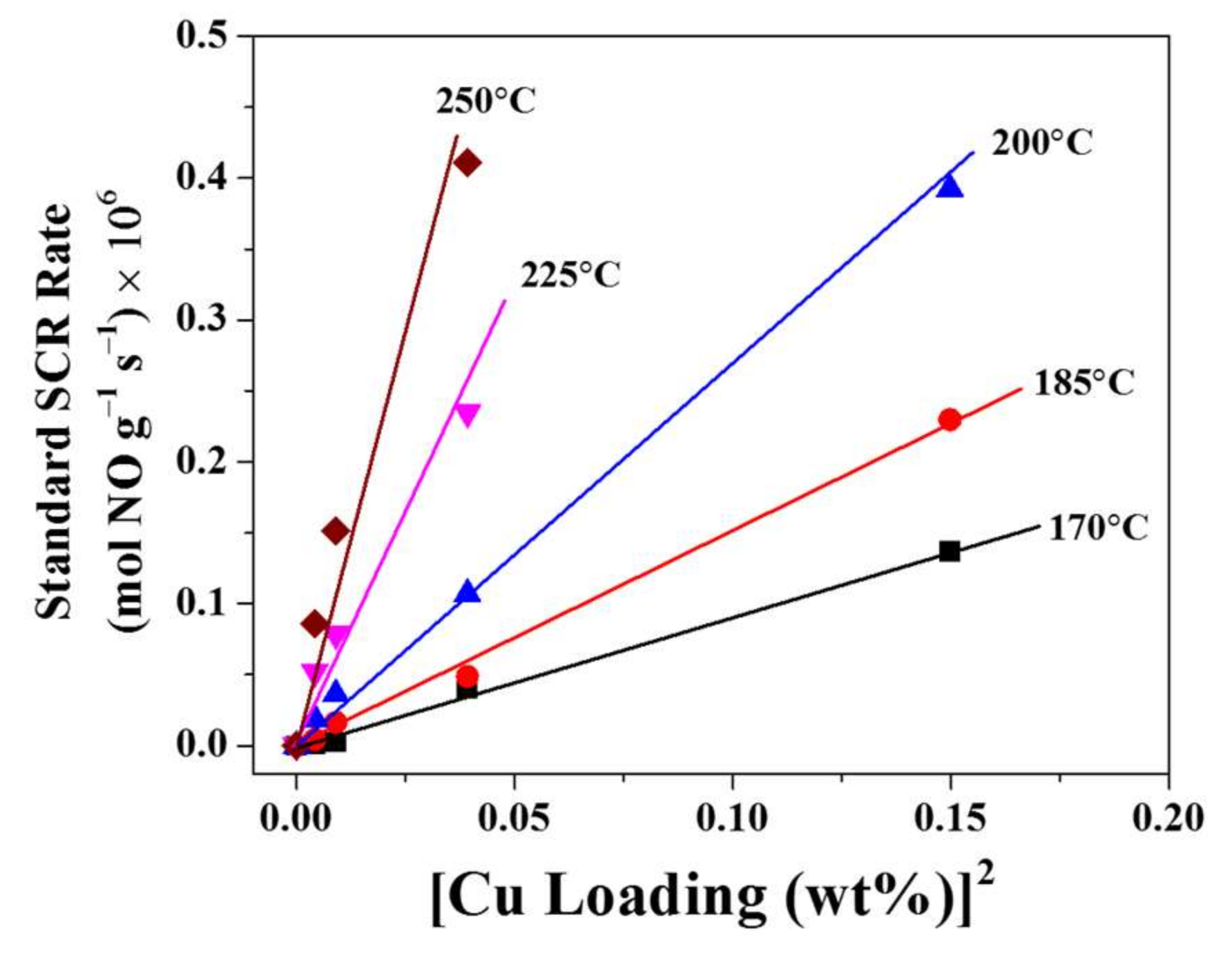
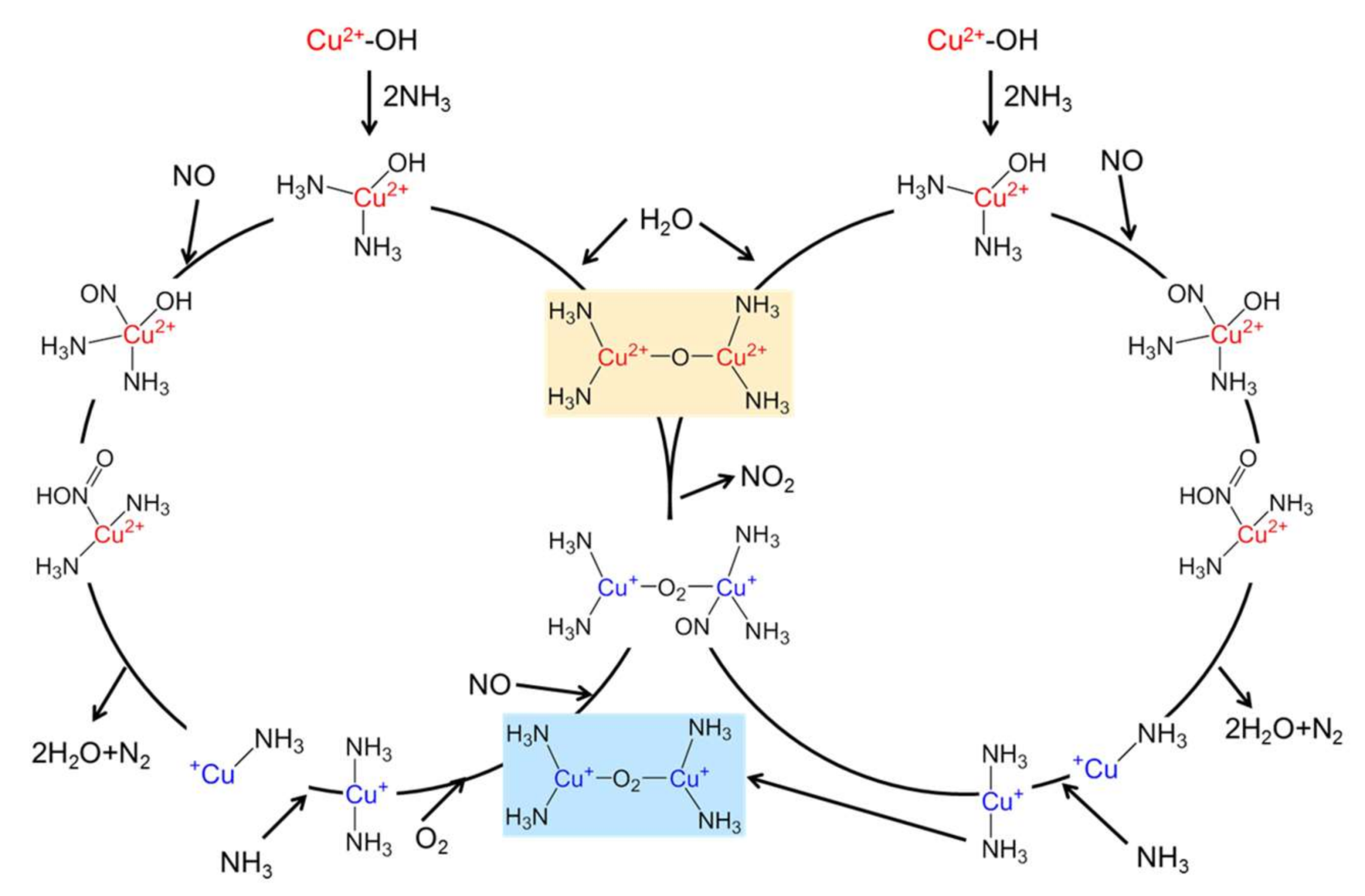
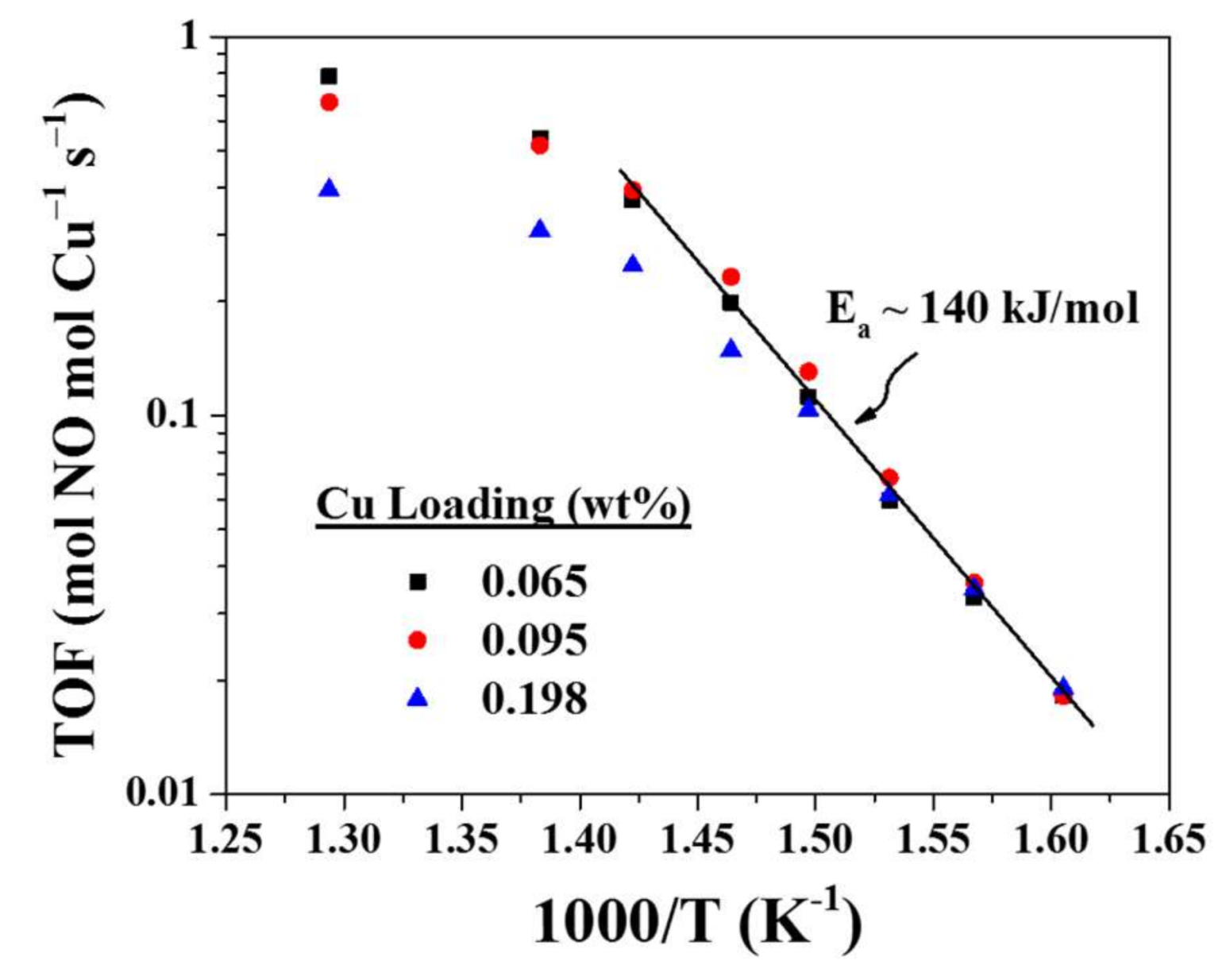
3.1. Standard NH3-SCR
3.2. Fast NH3-SCR
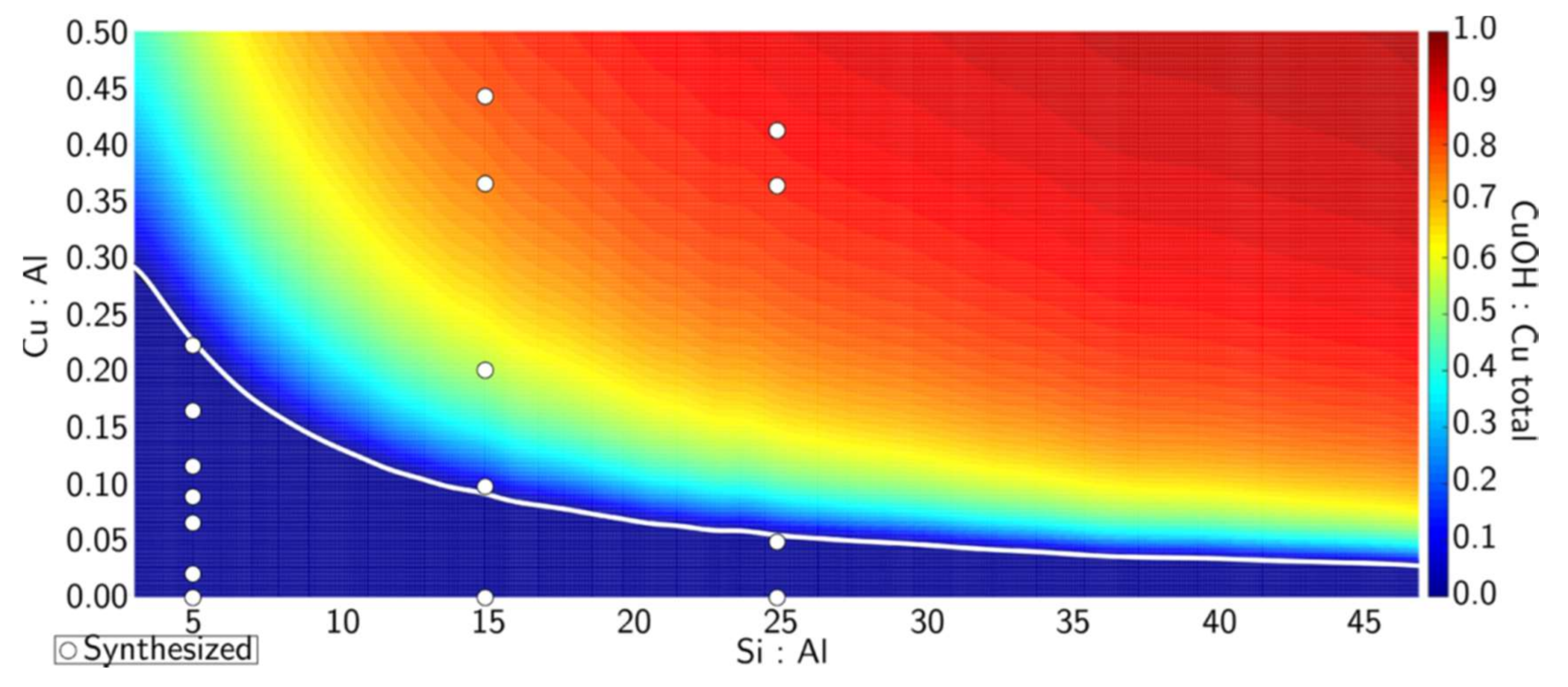
4. Towards Rational Design of Cu/SSZ-13
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andersen, P.J.; Collier, J.E.; Casci, J.L.; Chen, H.-Y.; Fedeyko, J.M.; Foo, R.K.S.; Rajaram, R.R. Transition metal/zeolite SCR catalysts. International Patent WO/2008/132452, 11 June 2008. [Google Scholar]
- Bull, I.; Xue, W.M.; Burk, P.; Boorse, R.S.; Jaglowski, W.M.; Koermer, G.S.; Moini, A.; Patchett, J.A.; Dettling, J.C.; Caudle, M.T. Copper CHA zeolite catalysts. U.S. Patent 7,601,662 B2, 13 October 2009. [Google Scholar]
- Bull, I.; Moini, A.; Koermer, G.S.; Patchett, J.A.; Jaglowski, W.M.; Roth, S. Zeolite catalyst with improved NOx reduction in SCR. U.S. Patent 7,704,475 B2, 27 April 2010. [Google Scholar]
- Andersen, P.J.; Chen, H.-Y.; Fedeyko, J.M.; Weigert, E. Small pore molecular sieve supported copper catalysts durable against lean/rich aging for the reduction of nitrogen oxides. U.S. Patent 7,998,443 B2, 16 August 2011. [Google Scholar]
- Gao, F.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Current understanding of Cu-exchanged Chabazite molecular sieves for use as commercial diesel engine DeNOx catalysts. Top. Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Catalysis science of NOx selective catalytic reduction with ammonia over Cu-SSZ-13 and Cu-SAPO-34. Adv. Catal. 2016, 59, 1–107. [Google Scholar]
- Wang, J.H.; Zhao, H.W.; Haller, G.; Li, Y.D. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Springer Science & Business Media: New York, NY, USA, 2014. [Google Scholar]
- Zones, S.I. Zeolite SSZ-13 and its method of preparation. U.S. Patent 4,544,538, 1 October 1985. [Google Scholar]
- Paolucci, C.; Verma, A.A.; Bates, S.A.; Kispersky, V.F.; Miller, J.T.; Gounder, R.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Isolation of the Copper Redox Steps in the Standard Selective Catalytic Reduction on Cu-SSZ-13. Angew. Chem. Int. Ed. 2014, 53, 11828–11833. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, J.R.; Bates, S.A.; Verma, A.A.; Delgass, W.N.; Ribeiro, F.H.; Miller, J.T.; Gounder, R. The dynamic nature of Brønsted acid sites in Cu–Zeolites during NOx selective catalytic reduction: Quantification by gas-phase ammonia titration. Top. Catal. 2015, 58, 424–434. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrom, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A consistent reaction scheme for the selective catalytic reduction of nitrogen oxides with ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, M.; Hueso, B.; Boronat, M.; Blasco, T.; Corma, A. Ammonia-containing species formed in Cu-Chabazite as per in situ EPR, solid-state NMR and DFT calculations. J. Phys. Chem. Lett. 2015, 6, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Lomachenko, K.A.; Borfecchia, E.; Negri, C.; Berlier, G.; Lamberti, C.; Beato, P.; Falsig, H.; Bordiga, S. The Cu-CHA deNOx Catalyst in Action: Temperature-Dependent NH3-Assisted Selective Catalytic Reduction Monitored by Operando XAS and XES. J. Am. Chem. Soc. 2016, 138, 12025–12028. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Wang, D.; Kumar, A.; Li, J.H.; Kamasamudram, K.; Currier, N.; Yezerets, A. Identification of two types of Cu sites in Cu/SSZ-13 and their unique responses to hydrothermal aging and sulfur poisoning. Catal. Today 2016, 267, 3–9. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin-Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T.; et al. Catalysis in a cage: Condition-dependent speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Gao, F.; Kamasamudram, K.; Currier, N.; Peden, C.H.F.; Yezerets, A. New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: Nature of acidic sites probed by NH3 titration. J. Catal. 2017, 348, 291–299. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.H.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. Selective catalytic reduction over Cu/SSZ-13: Linking homo-and heterogeneous catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Borfecchia, E.; Lomachenko, K.A.; Pankin, I.A.; Negri, C.; Berlier, G.; Beato, P.; Falsig, H.; Bordiga, S.; Lamberti, C. Composition-driven Cu-speciation and reducibility in Cu-CHA zeolite catalysts: A multivariate XAS/FTIR approach to complexity. Chem. Sci. 2017, 8, 6836–6851. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.C.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Oord, R.; Guo, W.; Poplawsky, J.D.; Weckhuysen, B.M. Nanoscale tomography reveals the deactivation of automotive copper-exchanged zeolite catalysts. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.T.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.H.; Grabow, L.C.; Epling, W.S. On the nature of Cu active centers in Cu-SSZ-13 and their responses to SO2 exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Godiksen, A.; Isaksen, O.L.; Rasmussen, S.B.; Vennestrom, P.N.R.; Mossin, S. Site-Specific Reactivity of Copper Chabazite Zeolites with Nitric Oxide, Ammonia and Oxygen. ChemCatChem 2018, 10, 366–370. [Google Scholar] [CrossRef]
- Andersen, C.W.; Borfecchia, E.; Bremholm, M.; Jorgensen, M.R.V.; Vennestrom, P.N.R.; Lamberti, C.; Lundegaard, L.F.; Iversen, B.B. Redox-Driven Migration of Copper Ions in the Cu-CHA Zeolite as Shown by the In Situ PXRD/XANES Technique. Angew. Chem. Int. Ed. 2017, 56, 10367–10372. [Google Scholar] [CrossRef] [PubMed]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Kröcher, O.; Nachtegaal, M.; Ferri, D. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Fickel, D.W.; Lobo, R.F. Copper coordination in Cu-SSZ-13 and Cu-SSZ-16 investigated by variable-temperature XRD. J. Phys. Chem. C 2010, 114, 1633–1640. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Ren, L.M.; Zhu, L.F.; Yang, C.G.; Chen, Y.M.; Sun, Q.; Zhang, H.Y.; Li, C.J.; Nawaz, F.; Meng, X.J.; Xiao, F.S. Designed copper–amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Washton, N.M.; Wang, Y.L.; Kollar, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Kwak, J.H.; Zhu, H.Y.; Lee, J.H.; Peden, C.H.F.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.W.; Bremholm, M.; Vennestrøm, P.N.R.; Blichfeld, A.B.; Lundegaard, L.F.; Iversen, B.B. Location of Cu2+ in CHA zeolite investigated by X-ray diffraction using the Rietveld/maximum entropy method. IUCrJ 2014, 1, 382–386. [Google Scholar] [CrossRef] [PubMed]
- McEwen, J.S.; Anggara, T.; Schneider, W.F.; Kispersky, V.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Integrated operando X-ray absorption and DFT characterization of Cu–SSZ-13 exchange sites during the selective catalytic reduction of NOx with NH3. Catal. Today 2012, 184, 129–144. [Google Scholar] [CrossRef]
- Kwak, J.H.; Varga, T.; Peden, C.H.F.; Gao, F.; Hanson, J.C.; Szanyi, J. Following the movement of Cu ions in a SSZ-13 zeolite during dehydration, reduction and adsorption: A combined in situ TP-XRD, XANES/DRIFTS study. J. Catal. 2014, 314, 83–93. [Google Scholar] [CrossRef]
- Godiksen, A.; Stappen, F.N.; Vennestrom, P.N.R.; Giordanino, F.; Rasmussen, S.B.; Lundegaard, L.F.; Mossin, S. Coordination environment of copper sites in Cu-CHA zeolite investigated by electron paramagnetic resonance. J. Phys. Chem. C 2014, 118, 23126–23138. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.L.; Walter, E.D.; Washton, N.M.; Mei, D.H.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; Gao, F. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Burton, S.D.; Szanyi, J.; Lee, J.H.; Peden, C.H.F. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203–209. [Google Scholar] [CrossRef]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Peden, C.H.F.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.S.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Vennestrom, P.N.R.; Janssens, T.V.W.; Kustov, A.; Grill, M.; Puig-Molina, A.; Lundegaard, L.F.; Tiruvalam, R.R.; Concepcion, P.; Corma, A. Influence of lattice stability on hydrothermal deactivation of Cu-ZSM-5 and Cu-IM-5 zeolites for selective catalytic reduction of NOx by NH3. J. Catal. 2014, 309, 477–490. [Google Scholar] [CrossRef]
- Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lazzarini, A.; Agostini, G.; Gallo, E.; Soldatov, A.V.; Beato, P.; Bordiga, S.; Lamberti, C. Interaction of NH3 with Cu-SSZ-13 catalyst: A complementary FTIR, XANES and XES study. J. Phys. Chem. Lett. 2014, 5, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Shwan, S.; Skoglundh, M.; Lundegaard, L.F.; Tiruvalam, R.R.; Janssens, T.V.W.; Carlsson, A.; Vennestrom, P.N.R. Solid-state ion-exchange of copper into zeolites facilitated by ammonia at low temperature. ACS Catal. 2015, 5, 16–19. [Google Scholar] [CrossRef]
- Clemens, A.K.S.; Shishkin, A.; Carlsson, P.A.; Skoglundh, M.; Martinez-Casado, F.J.; Matej, Z.; Balmes, O.; Harelind, H. Reaction-driven ion exchange of copper into zeolite SSZ-13. ACS Catal. 2015, 5, 6209–6218. [Google Scholar] [CrossRef]
- Verma, A.A.; Bates, S.A.; Anggara, T.; Paolucci, C.; Parekh, A.A.; Kamasamudram, K.; Yezerets, A.; Miller, J.T.; Delgass, W.N.; Schneider, W.F.; Ribeiro, F.H. NO oxidation: A probe reaction on Cu-SSZ-13. J. Catal. 2014, 312, 179–190. [Google Scholar] [CrossRef]
- Brandenberger, S.; Krocher, O.; Tissler, A.; Althoff, R. The state of the art in selective catalytic reduction of NOx by Ammonia using metal-exchanged zeolite catalysts. Catal. Rev. Sci. Eng. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Li, S.C.; Zheng, Y.; Gao, F.; Szanyi, J.; Schneider, W.F. Experimental and Computational Interrogation of Fast SCR Mechanism and Active Sites on H-Form SSZ-13. ACS Catal. 2017, 7, 5087–5096. [Google Scholar] [CrossRef]
- Hao, T.; Wang, J.; Yu, T.; Wang, J.Q.; Shen, M.Q. Effect of NO2 on the Selective Catalytic Reduction of NO with NH3 over Cu/SAPO-34 Molecular Sieve Catalyst. Acta Phys. Chim. Sin. 2014, 30, 1567–1574. [Google Scholar]
- Gao, F.; Wang, Y.L.; Kollar, M.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. A comparative kinetics study between Cu/SSZ-13 and Fe/SSZ-13 SCR catalysts. Catal. Today 2015, 258, 347–358. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wei, Z.H.; Kollar, M.; Gao, F.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. A comparative study of N2O formation during the selective catalytic reduction of NOx with NH3 on zeolite supported Cu catalysts. J. Catal. 2015, 329, 490–498. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.F.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged Chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A Gen. 2012, 427–428, 24–34. [Google Scholar] [CrossRef]
- Blakeman, P.G.; Burkholder, E.M.; Chen, H.Y.; Collier, J.E.; Fedeyko, J.M.; Jobson, H.; Rajaram, R.R. The role of pore size on the thermal stability of zeolite supported Cu SCR catalysts. Catal. Today 2014, 231, 56–63. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Deka, U.; van der Bij, H.E.; Paalanen, P.; Arstad, B.; Weckhuysen, B.M.; Beale, A.M. Chemical deactivation of Cu-SSZ-13 ammonia selective catalytic reduction (NH3-SCR) systems. Appl. Catal. B Environ. 2014, 154–155, 339–349. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.A.; Wang, Y.L.; Washton, N.M.; Walter, E.D.; Szanyi, J.; Gao, F.; Wang, Y.; Peden, C.H.F. Sub-micron Cu/SSZ-13: Synthesis and application as selective catalytic reduction (SCR) catalysts. Appl. Catal. B Environ. 2017, 201, 461–469. [Google Scholar] [CrossRef]
- Martinez-Franco, R.; Moliner, M.; Thogersen, J.R.; Corma, A. Efficient One-Pot Preparation of Cu-SSZ-13 Materials using Cooperative OSDAs for their Catalytic Application in the SCR of NOx. ChemCatChem 2013, 5, 3316–3323. [Google Scholar] [CrossRef]
- Martin, N.; Moliner, M.; Corma, A. High yield synthesis of high-silica Chabazite by combining the role of zeolite precursors and tetraethylammonium: SCR of NOx. Chem. Commun. 2015, 51, 9965–9968. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.M.; Chen, C.Y.; Pan, S.X.; Zhang, W.P.; Meng, X.J.; Maurer, S.; Feyen, M.; Muller, U.; Xiao, F.-S. Atom-economical synthesis of a high silica CHA zeolite using a solvent-free route. Chem. Commun. 2015, 51, 16920–16923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.C.; Yu, R.; Zhao, R.R.; Shi, C.; Gies, H.; Xiao, F.S.; de Vos, D.; Yokoi, T.; Bao, X.H.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Kovarik, L.; Washton, N.M.; Kukadapu, R.; Devaraj, A.; Wang, A.Y.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F.; Gao, F. Transformation of active sites in Fe/SSZ-13 SCR catalysts during hydrothermal aging: A spectroscopic, microscopic and kinetics study. ACS Catal. 2017, 7, 2458–2470. [Google Scholar] [CrossRef]
- Wang, D.; Jangjou, Y.; Liu, Y.; Sharma, M.K.; Luo, J.Y.; Li, J.H.; Kamasamudram, K.; Epling, W.S. A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2015, 165, 438–445. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.L.; Washton, N.M.; Kollar, M.; Szanyi, J.; Peden, C.H.F. Effects of alkali and alkaline earth cocations on the activity and hydrothermal stability of Cu/SSZ-13 NH3–SCR catalysts. ACS Catal. 2015, 5, 6780–6791. [Google Scholar] [CrossRef]
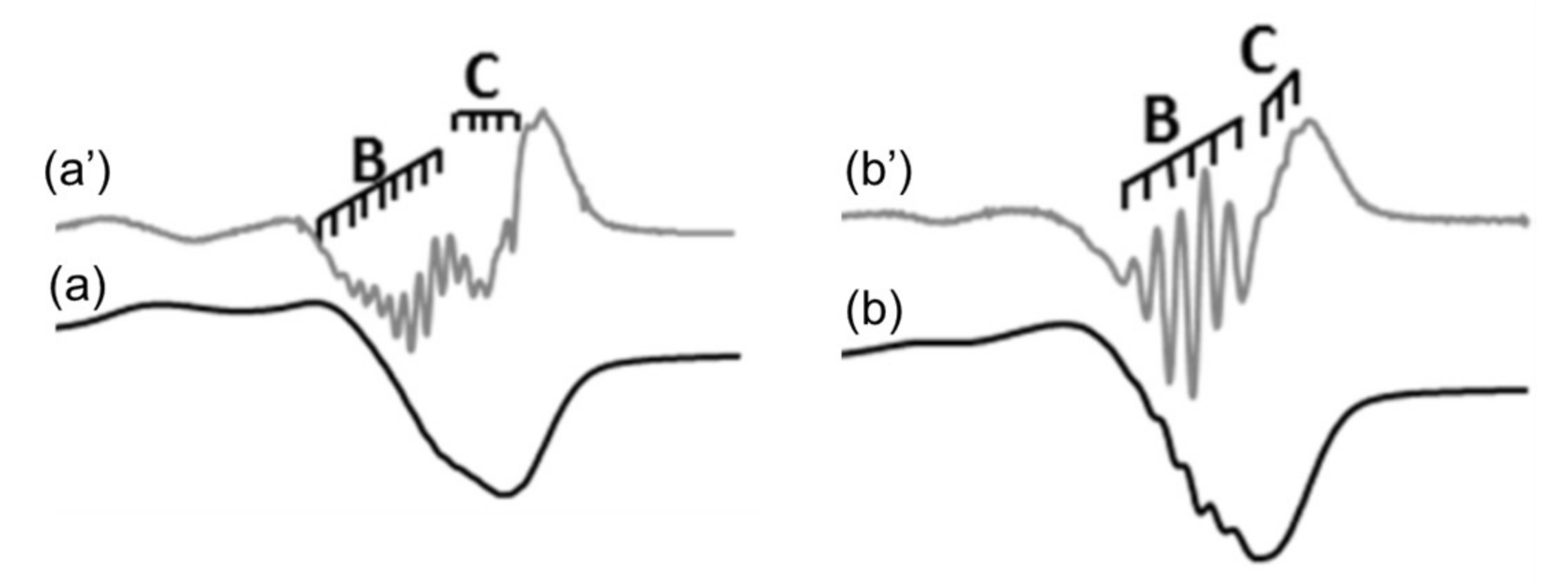
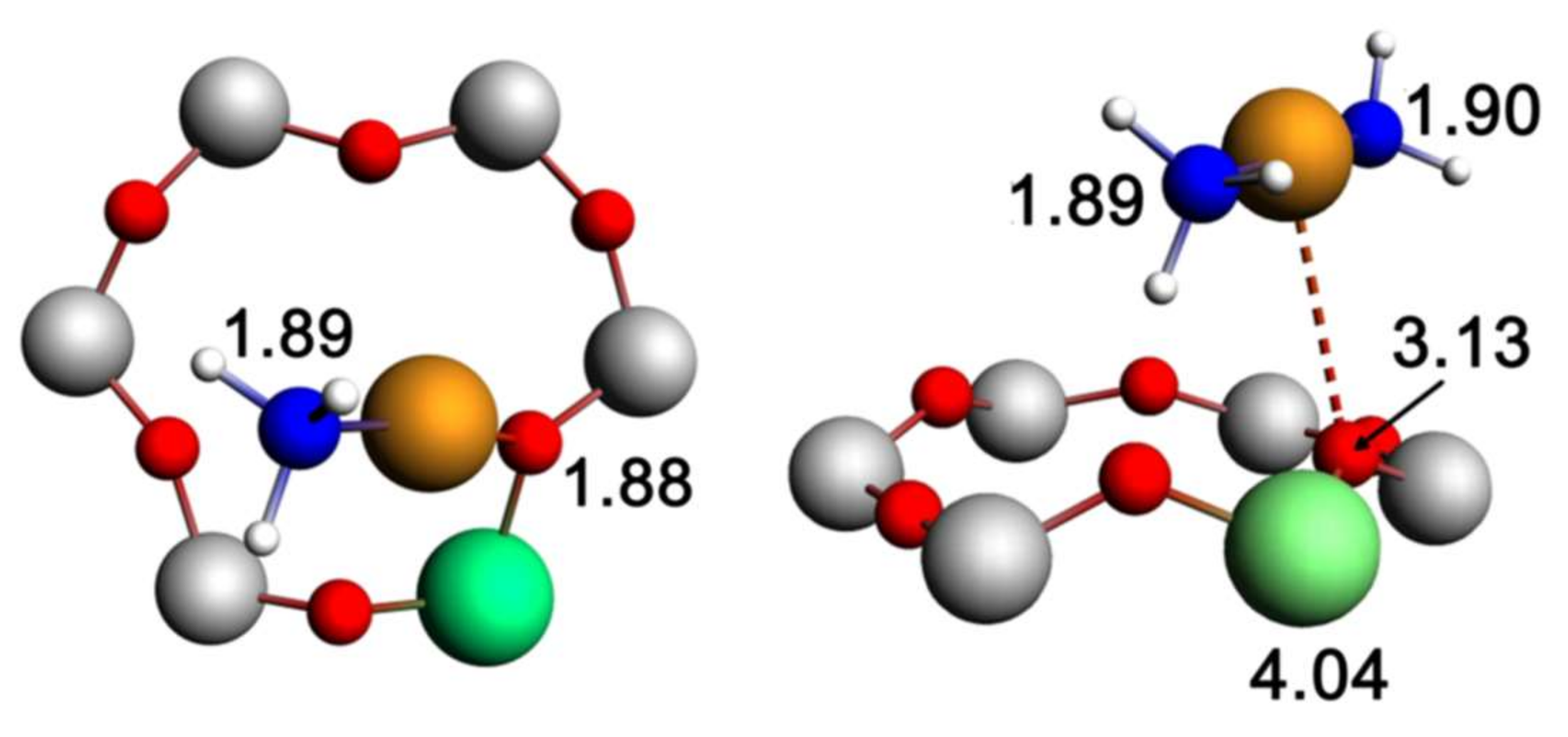
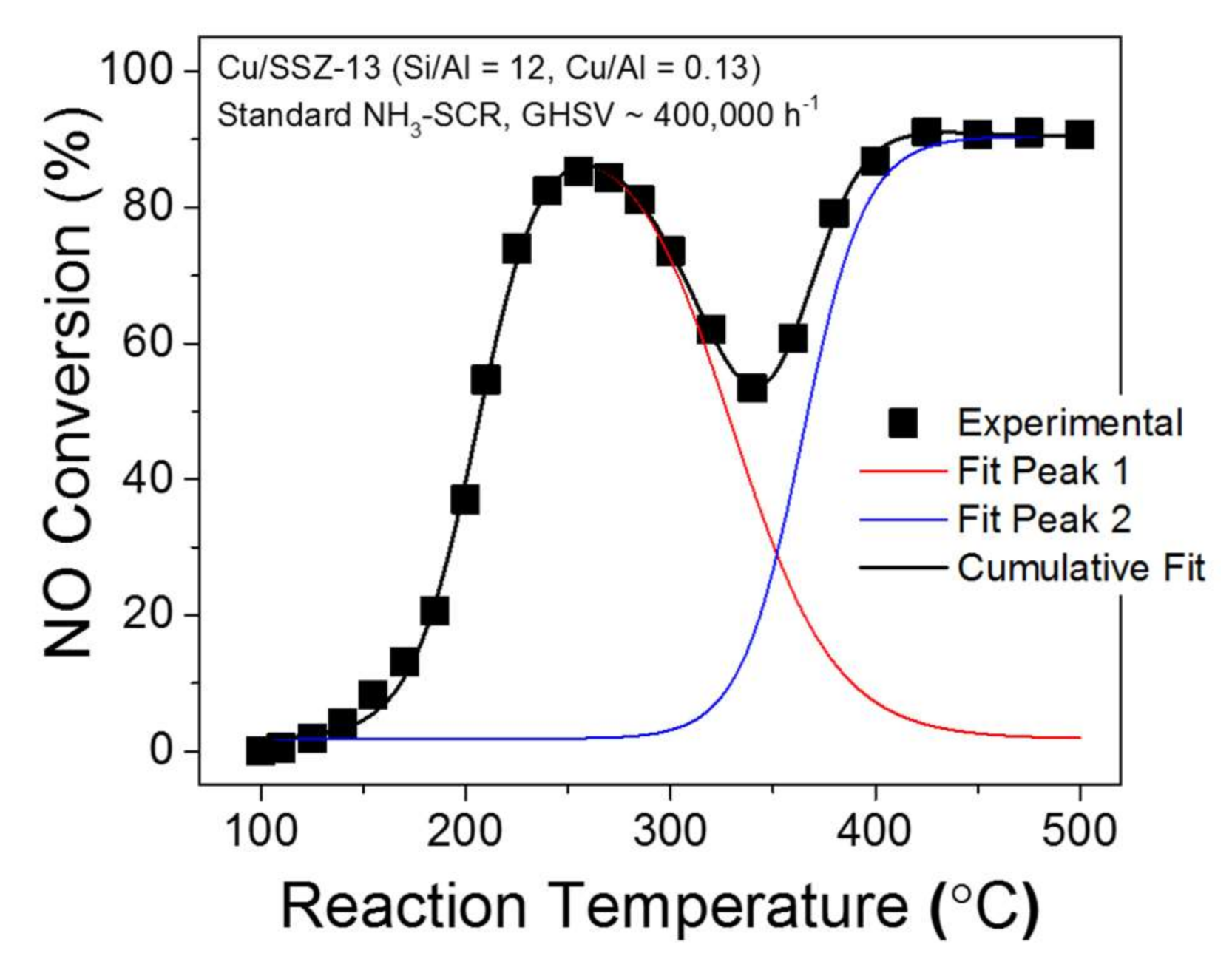
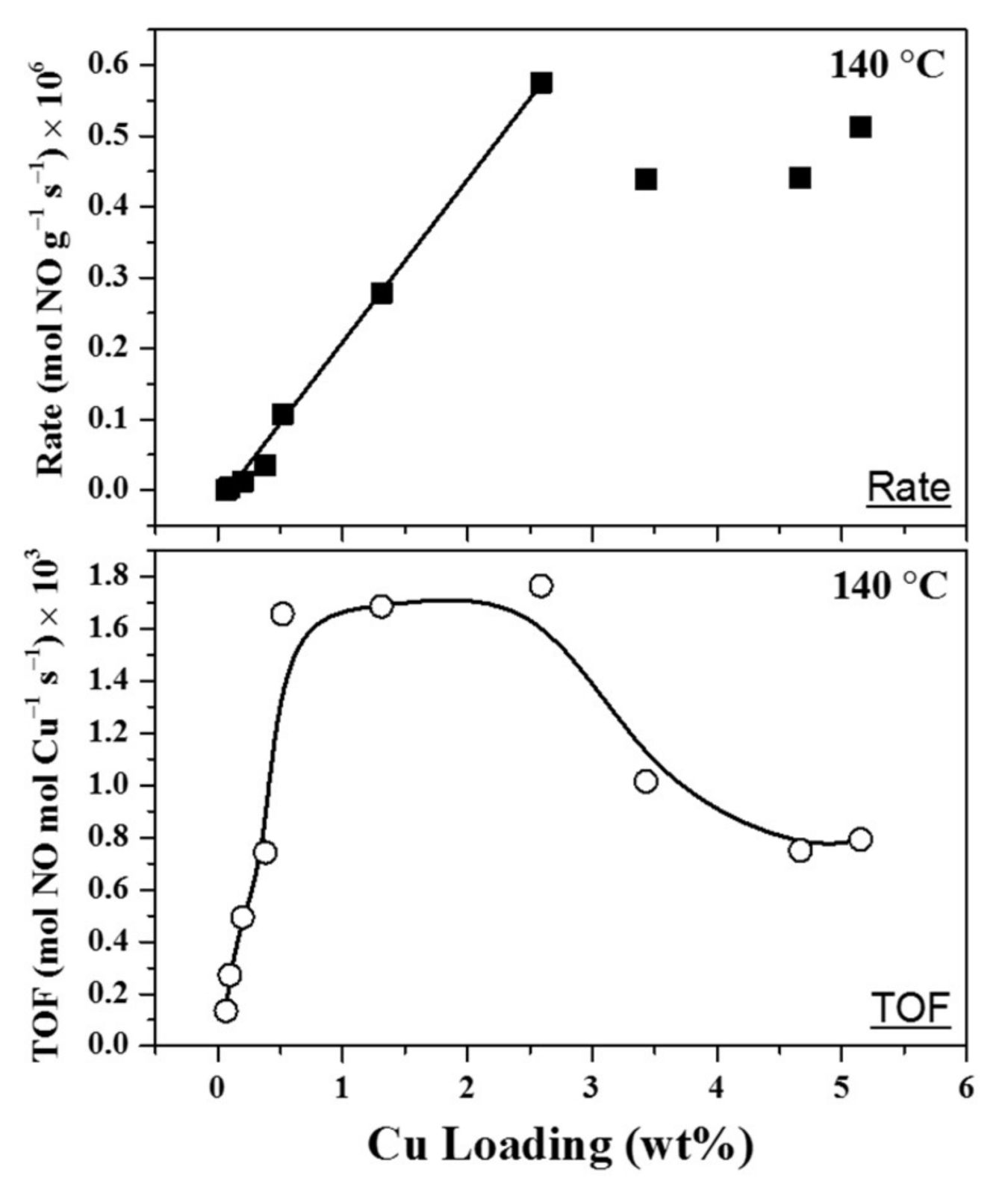
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Peden, C.H.F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. https://doi.org/10.3390/catal8040140
Gao F, Peden CHF. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts. 2018; 8(4):140. https://doi.org/10.3390/catal8040140
Chicago/Turabian StyleGao, Feng, and Charles H. F. Peden. 2018. "Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts" Catalysts 8, no. 4: 140. https://doi.org/10.3390/catal8040140
APA StyleGao, F., & Peden, C. H. F. (2018). Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts, 8(4), 140. https://doi.org/10.3390/catal8040140