Waste into Fuel—Catalyst and Process Development for MSW Valorisation
Abstract
:1. Introduction
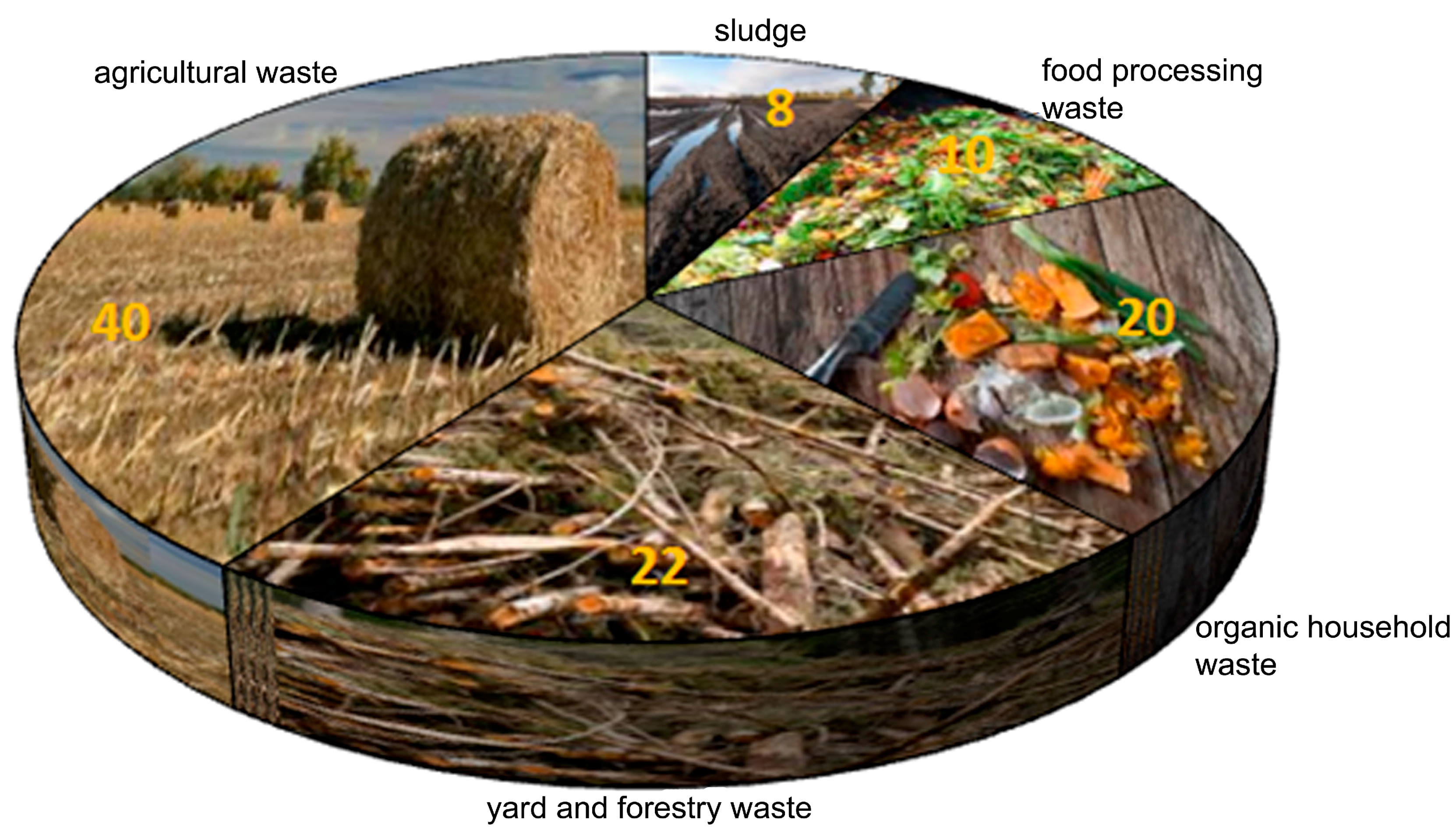
2. Waste: Problems and Opportunities
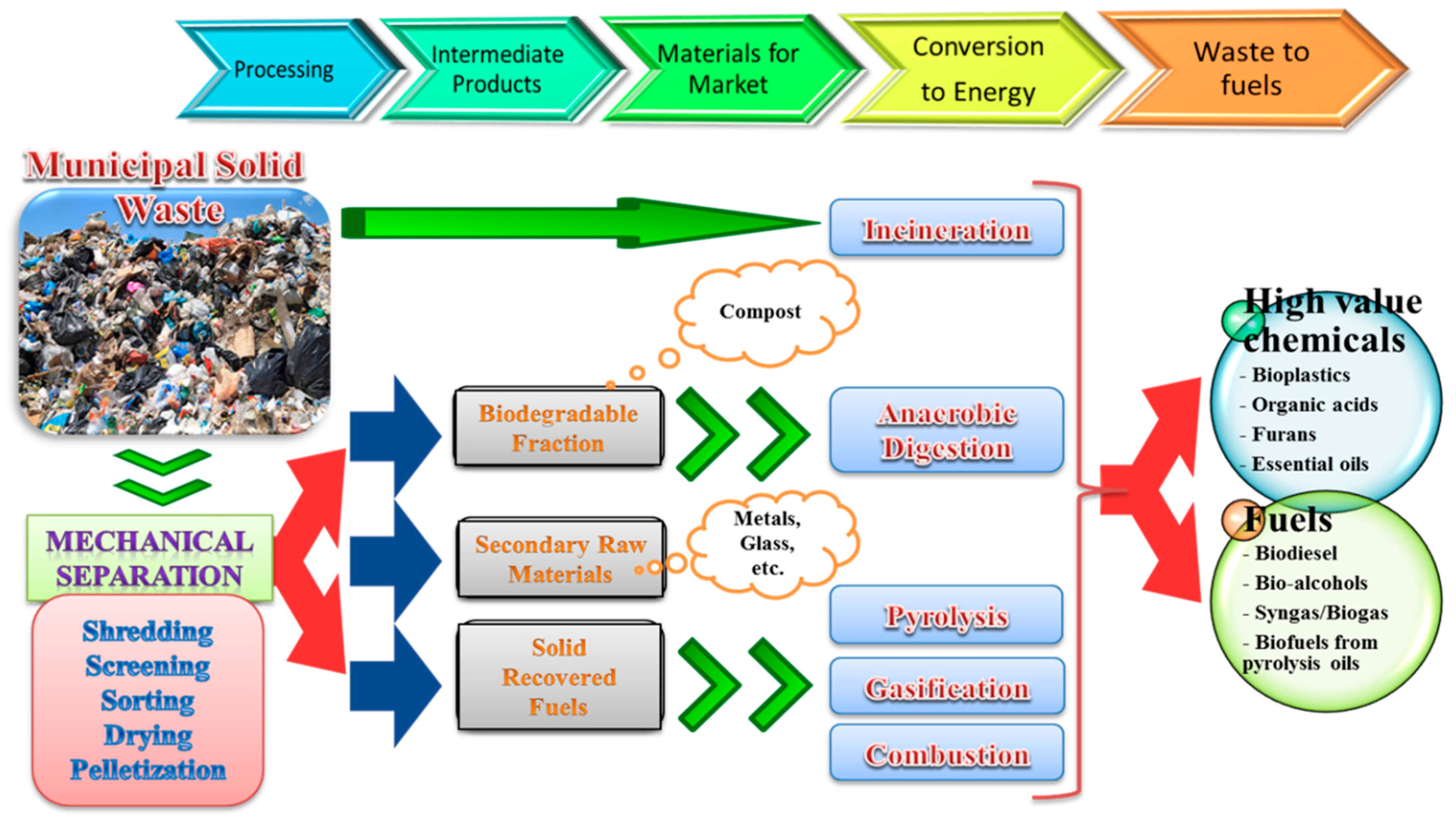
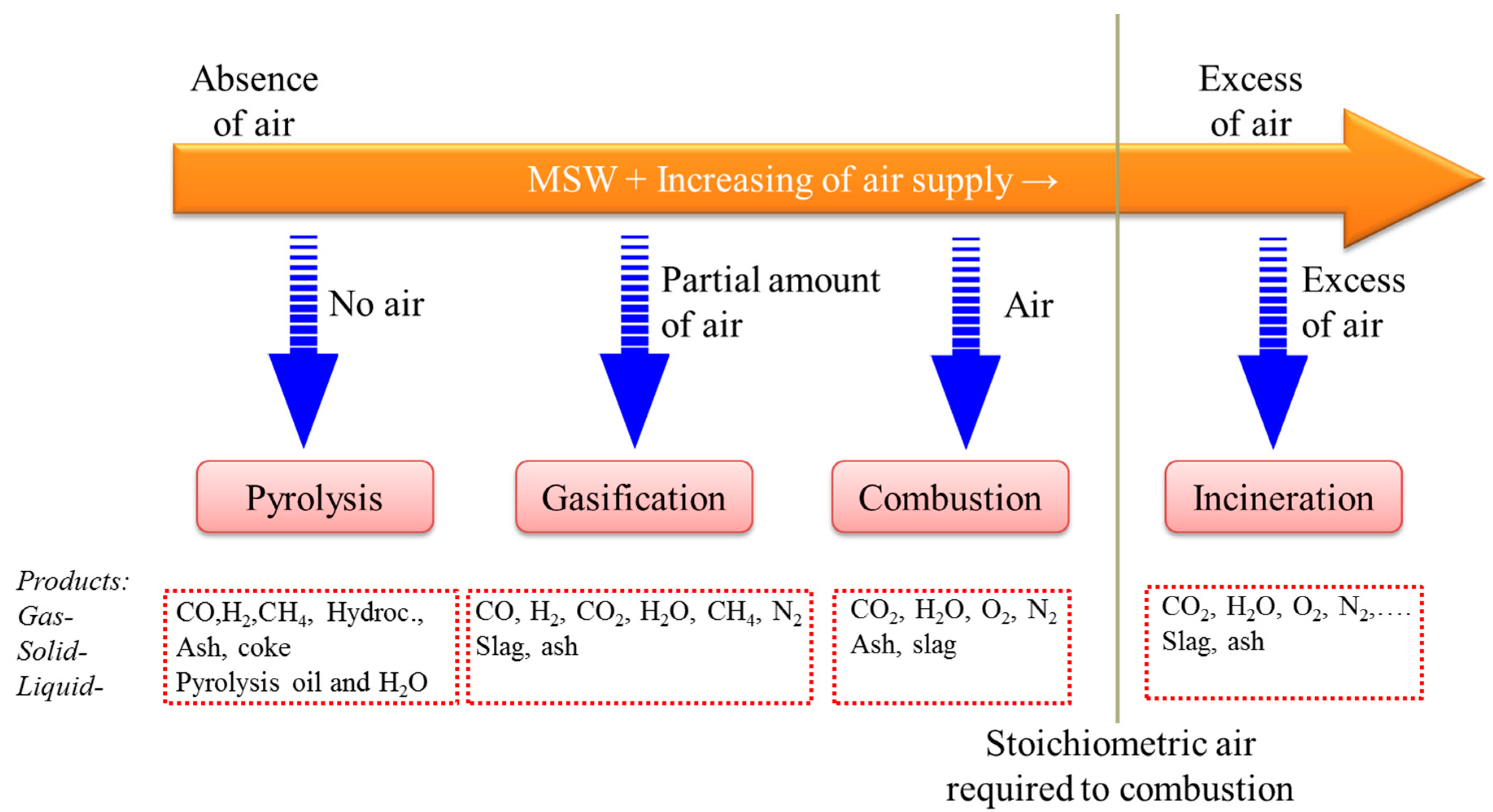
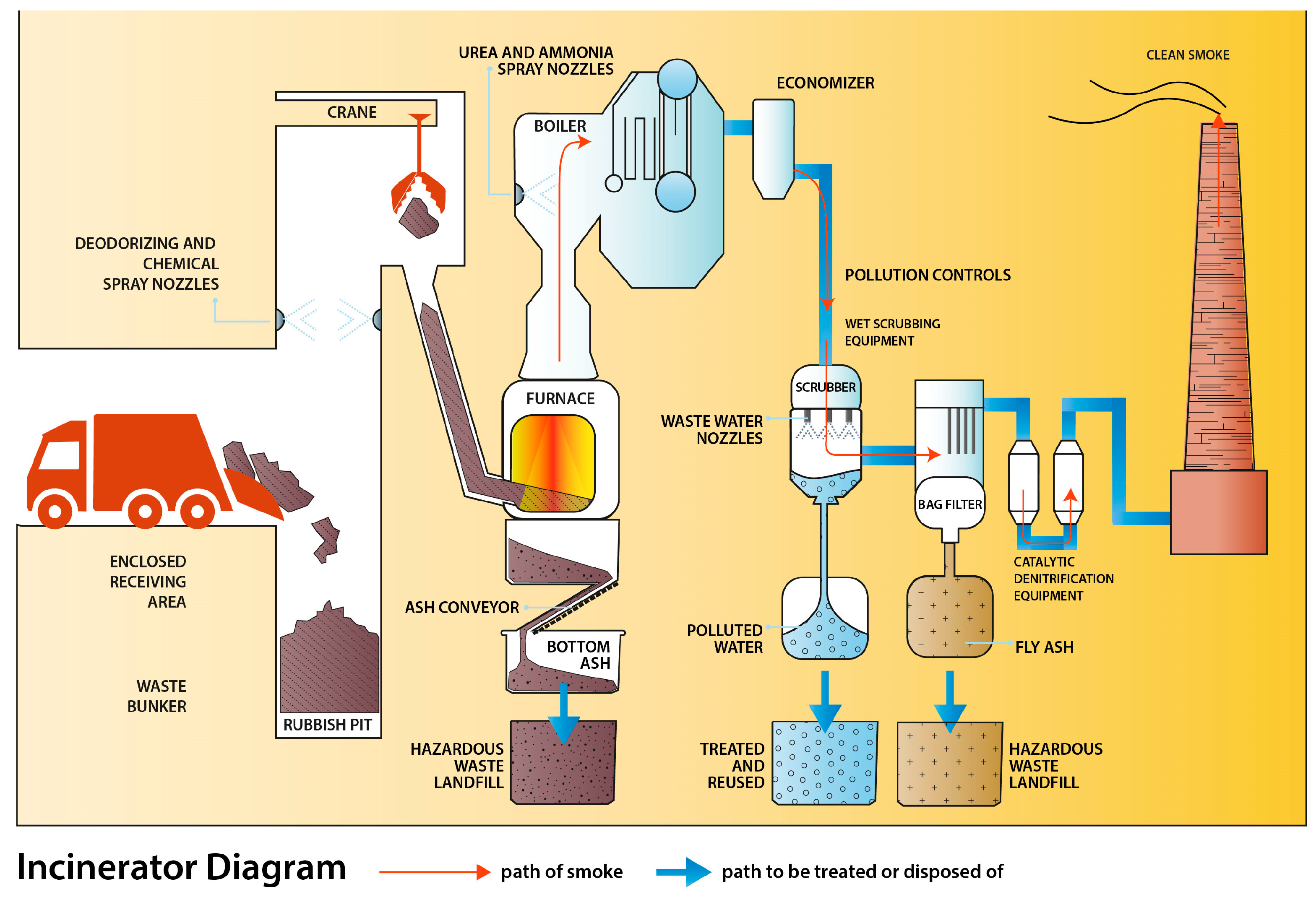
3. Waste Processing
4. Catalyst and Sorbents Screening for Chlorine Removal and Other Pollutant Considerations
5. Future Prospects and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malins, C.; Searle, S.; Malins, C.; Baral, A.; Turley, D.; Hopwood, L. Wasted: Europe’s Untrapped Resource: An Assessment of Advanced Biofuels from Wastes & Residues; International Council on Clean Transportation: Berlin, Germany, 2014. [Google Scholar]
- Uslu, A.; Pedersen, J.; Resch, G.; Fritsche, U. Maximising the Environmental Benefits of Europe’s Bioenergy Potential; European Environment Agency (EEA): Copenhagen, Denmark, 2008; ISSN 1725-2237. [Google Scholar]
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar]
- Ferrer-i-Carbonell, A.; Gowdy, J.M. Environmental degradation and happiness. Ecol. Econ. 2007, 60, 509–516. [Google Scholar] [CrossRef]
- Williams, R.B.; Zhang, T. Survey of MSW Conversion Options; CEC-500-11-020; California Biomass Collaborative, University of California: Davis, CA, USA, 2013. [Google Scholar]
- Jenkins, S.D.; Legrand, R. Conversion technologies: A new option for MSW management. In Proceedings of the 13th Annual North American Waste to Energy Conference (NAWTEC13-3153), Orlando, FL, USA, 23–25 May 2005; pp. 55–61. [Google Scholar]
- Persson, T.; Murphy, J.D.; Jannasch, A.-K.; Ahern, E.; Liebetrau, J.; Trommler, M.; Toyama, J.; Baxter, D.E. A Perspective on the Potential Role of Biogas in Smart Energy Grids; Technical Brochure; IEA Bioenergy: Paris, France, 2014. [Google Scholar]
- Omari, A.M.; Kichonge, B.N.; John, G.R.; Njau, K.N.; Mtui, P.L. Potential of Municipal solid waste, as renewable energy source- A case study of Arusha, Tanzania. Int. J. Renew. Energy Technol. Res. 2014, 3, 31–39. [Google Scholar]
- Eddine, B.T.; Salah, M.M. Solid waste as renewable source of energy: Current and future possibility in Algeria. Int. J. Energy Environ. Eng. 2012, 3, 1–12. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Nicolini, A.; Coccia, V.; Petrozzi, A. Production of bioethanol in a second generation prototype from pine wood chips. Energy Procedia 2014, 45, 42–51. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Quintero, R.F.C.; Pieta, I.S. Catalytic Dry Reforming for Biomass-Based Fuels Processing: Progress and Future Perspectives. Energy Technol. 2016, 4, 881–890. [Google Scholar] [CrossRef]
- Bosmans, A.; Vanderreydt, I.; Geysen, D.; Helsen, L. The crucial role of Waste-to-Energy technologies in enhanced landfill mining: A technology review. J. Clean. Prod. 2013, 55, 10–23. [Google Scholar] [CrossRef]
- Quaghebeur, M.; Laenen, B.; Geysen, D.; Nielsen, P.; Pontikes, Y.; van Gerven, T.; Spooren, J. Characterization of landfilled materials: Screening of the enhanced landfill mining potential. J. Clean. Prod. 2013, 55, 72–83. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Saha, U.K.; Sahoo, N. Theoretical performance limits of a syngas-diesel fueled compression ignition engine from second law analysis. Energy 2011, 36, 760–769. [Google Scholar] [CrossRef]
- Zinoviev, S.; Muller-Langer, F.; Das, P.; Bertero, N.; Fornasiero, P.; Kaltschmitt, M.; Centi, G.; Miertus, S. Next-Generation Biofuels: Survey of Emerging Technologies and Sustainability Issues. Chemsuschem 2010, 3, 1106–1133. [Google Scholar] [CrossRef] [PubMed]
- Pieta, I.S.; Resini, C.; Donazzi, A. Fuel processing for solid oxide fuel cells. In Modeling, Design, Construction, and Operation of Power Generators with Solid Oxide Fuel Cells: From Single Cell to Complete Power System; Springer: New York, NY, USA, 2017; Chapter 4; ISBN 978-973-319-75602-75608. [Google Scholar]
- Borgwardt, R.H. Transportation fuel from cellulosic biomass: A comparative assessment of ethanol and methanol options. J. Power Energy 1999, 213, 399–407. [Google Scholar] [CrossRef]
- Ptasinski, K.J. Thermodynamic efficiency of biomass gasification and biofuels conversion. Biofuels Bioprod. Biorefin. 2008, 2, 239–253. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. 2012, 38, 522–550. [Google Scholar]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustan. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Keasling, J.D. Advanced biofuel production in microbes. Biotechnol. J. 2010, 5, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, U982–U985. [Google Scholar] [CrossRef] [PubMed]
- Abdollahifar, M.; Haghighi, M.; Babaluo, A.A. Syngas production via dry reforming of methane over Ni/Al2O3-MgO nanocatalyst synthesized using ultrasound energy. J. Ind. Energy Chem. 2014, 20, 1845–1851. [Google Scholar] [CrossRef]
- Garcia-Dieguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Nanostructured Pt- and Ni-based catalysts for CO2-reforming of methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- Huber, G.W.; Dumesic, J.A. An overview of aqueous-phase catalytic processes for production of hydrogen and alkanes in a biorefinery. Catal. Today 2006, 111, 119–132. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Andersen, V.F.; Anderson, J.E.; Wallington, T.J.; Mueller, S.A.; Nielsen, O.J. Distillation Curves for Alcohol-Gasoline Blends. Energy Fuel 2010, 24, 2683–2691. [Google Scholar] [CrossRef]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Xu, C.B.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Liu, S.Y.; Mei, D.H.; Nahil, M.A.; Gadkari, S.; Gu, S.; Williams, P.T.; Tu, X. Hybrid plasma-catalytic steam reforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts. Fuel Process. Technol. 2017, 166, 269–275. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Bain, R.L.; Davis, R.E.; Dutta, A.; Feik, C.J.; Gaston, K.R.; Jablonski, W.; Phillips, S.D.; Nimlos, M.R. Pilot-Scale Gasification of Corn Stover, Switchgrass, Wheat Straw, and Wood: 1. Parametric Study and Comparison with Literature. Ind. Eng. Chem. Res. 2010, 49, 1859–1871. [Google Scholar] [CrossRef]
- Courson, C.; Makaga, E.; Petit, C.; Kiennemann, A. Development of Ni catalysts for gas production from biomass gasification. Reactivity in steam- and dry-reforming. Catal. Today 2000, 63, 427–437. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Dou, B.L.; Wang, C.; Chen, H.S.; Song, Y.C.; Xie, B.Z.; Xu, Y.J.; Tan, C.Q. Research progress of hot gas filtration, desulphurization and HCl removal in coal-derived fuel gas: A review. Chem. Eng. Res. Des. 2012, 90, 1901–1917. [Google Scholar] [CrossRef]
- Kowalik, P.; Antoniak-Jurak, K.; Blesznowski, M.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J.; Pieta, I.S. Biofuel steam reforming catalyst for fuel cell application. Catal. Today 2015, 254, 129–134. [Google Scholar] [CrossRef]
- Ates, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Islam, M.N.; Beg, M.R.A.; Islam, M.R. Pyrolytic oil from fixed bed pyrolysis of municipal solid waste and its characterization. Renew. Energy 2005, 30, 413–420. [Google Scholar] [CrossRef]
- Grieco, E.M.; Baldi, G. Pyrolysis of polyethylene mixed with paper and wood: Interaction effects on tar, char and gas yields. Waste Manag. 2012, 32, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Sorum, L.; Gronli, M.G.; Hustad, J.E. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Wang, N.; Chen, D.Z.; Arena, U.; He, P.J. Hot char-catalytic reforming of volatiles from MSW pyrolysis. Appl. Energy 2017, 191, 111–124. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Rouboa, A. Municipal solid waste gasification in semi-industrial conditions using air-CO2 mixtures. Energy 2016, 104, 42–52. [Google Scholar] [CrossRef]
- Luo, S.Y.; Zhou, Y.M.; Yi, C.J. Syngas production by catalytic steam gasification of municipal solid waste in fixed-bed reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- Li, J.F.; Liao, S.Y.; Dan, W.Y.; Jia, K.L.; Zhou, X.R. Experimental study on catalytic steam gasification of municipal solid waste for bioenergy production in a combined fixed bed reactor. Biomass Bioenergy 2012, 46, 174–180. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Catalytic dry reforming of waste plastics from different waste treatment plants for production of synthesis gases. Waste Manag. 2016, 58, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.M.; Williams, P.T. Pyrolysis-catalytic dry (CO2) reforming of waste plastics for syngas production: Influence of process parameters. Fuel 2017, 193, 7–14. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Manipulating the H-2/CO ratio from dry reforming of simulated mixed waste plastics by the addition of steam. Fuel Process. Technol. 2017, 156, 331–338. [Google Scholar] [CrossRef]
- Saad, J.M.; Nahil, M.A.; Wu, C.F.; Williams, P.T. Influence of nickel-based catalysts on syngas production from carbon dioxide reforming of waste high density polyethylene. Fuel Process. Technol. 2015, 138, 156–163. [Google Scholar] [CrossRef]
- Fuentes-Cano, D.; Gomez-Barea, A.; Nilsson, S.; Ollero, P. Decomposition kinetics of model tar compounds over chars with different internal structure to model hot tar removal in biomass gasification. Chem. Eng. J. 2013, 228, 1223–1233. [Google Scholar] [CrossRef]
- Matsuhara, T.; Hosokai, S.; Norinaga, K.; Matsuoka, K.; Li, C.Z.; Hayashi, J. In-Situ Reforming of Tar from the Rapid Pyrolysis of a Brown Coal over Char. Energy Fuel 2010, 24, 76–83. [Google Scholar] [CrossRef]
- Min, Z.H.; Yimsiri, P.; Asadullah, M.; Zhang, S.; Li, C.Z. Catalytic reforming of tar during gasification. Part II. Char as a catalyst or as a catalyst support for tar reforming. Fuel 2011, 90, 2545–2552. [Google Scholar] [CrossRef]
- Li, C.Z. Importance of volatile-char interactions during the pyrolysis and gasification of low-rank fuels—A review. Fuel 2013, 112, 609–623. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, W.Q.; Ji, W. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl. Energy 2011, 88, 1656–1663. [Google Scholar] [CrossRef]
- Zhang, S.; Asadullah, M.; Dong, L.; Tay, H.L.; Li, C.Z. An advanced biomass gasification technology with integrated catalytic hot gas cleaning. Part II: Tar reforming using char as a catalyst or as a catalyst support. Fuel 2013, 112, 646–653. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zhao, P.T.; Shao, Q.F.; Takahashi, F.; Yoshikawa, K. In situ catalytic conversion of tar using rice husk char/ash supported nickel-iron catalysts for biomass pyrolytic gasification combined with the mixing-simulation in fluidized-bed gasifier. Appl. Energy 2015, 160, 808–819. [Google Scholar] [CrossRef]
- Gilbert, P.; Ryu, C.; Sharifi, V.; Swithenbank, J. Tar reduction in pyrolysis vapours from biomass over a hot char bed. Bioresour. Technol. 2009, 100, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Gallastegi-Villa, M.; Aranzabal, A.; Gonzalez-Marcos, J.A.; Gonzalez-Velasco, J.R. Tailoring dual redox-acid functionalities in VOx/TiO2/ZSM5 catalyst for simultaneous abatement of PCDD/Fs and NOx from municipal solid waste incineration. Appl. Catal. B 2017, 205, 310–318. [Google Scholar] [CrossRef]
- Yan, Q.H.; Nie, Y.; Yang, R.Y.; Cui, Y.H.; O’Hare, D.; Wang, Q. Highly dispersed CuyAlOx, mixed oxides as superior low-temperature alkali metal and SO2 resistant NH3-SCR catalysts. Appl. Catal. A 2017, 538, 37–50. [Google Scholar] [CrossRef]
- Krishnan, G.N.; Wood, B.J.; Brittain, R.D.; Lau, K.H. Vaporization of Alkali and Trace Metal Impurities in Coal Gasification and Combustion Systems; Universität Karlsruhe: Karlsruhe, Germany, 1996; pp. 651–663. [Google Scholar]
- Cao, J.; Zhong, W.Q.; Jin, B.S.; Wang, Z.F.; Wang, K. Treatment of Hydrochloric Acid in Flue Gas from Municipal Solid Waste Incineration with Ca-Mg-Al Mixed Oxides at Medium-High Temperatures. Energy Fuel 2014, 28, 4112–4117. [Google Scholar] [CrossRef]
- Chyang, C.; Han, Y.; Zhong, Z. Study of HCl Absorption by CaO at High Temperature. Energy Fuel 2009, 23, 3948–3953. [Google Scholar] [CrossRef]
- Blazso, M.; Jakab, E. Effect of metals, metal oxides, and carboxylates on the thermal decomposition processes of poly (vinyl chloride). J. Anal. Appl. Pyrolysis 1999, 49, 125–143. [Google Scholar] [CrossRef]
- Horikawa, S.; Takai, Y.; Ukei, H.; Azuma, N.; Ueno, A. Chlorine gas recovery from polyvinyl chloride. J. Anal. Appl. Pyrolysis 1999, 51, 167–179. [Google Scholar] [CrossRef]
- Miranda, R.; Pakdel, H.; Roy, C.; Vasile, C. Vacuum pyrolysis of commingled plastics containing PVC II. Product analysis. Polym. Degrad. Stab. 2001, 73, 47–67. [Google Scholar] [CrossRef]
- Weinell, C.E.; Jensen, P.I.; Damjohansen, K.; Livbjerg, H. Hydrogen-Chloride Reaction with Lime and Limestone—Kinetics and Sorption Capacity. Ind. Eng. Chem. Res. 1992, 31, 164–171. [Google Scholar] [CrossRef]
- Hofbauer, H.; Rauch, R.; Ripfel-Nitsche, K. Gas Cleaning for Synthesis Applications; Vienna University of Technology: Vienna, Austria, 2007. [Google Scholar]
- Wang, W.Y.; Ye, Z.C.; Bjerle, I. The kinetics of the reaction of hydrogen chloride with fresh and spent Ca-based desulfurization sorbents. Fuel 1996, 75, 207–212. [Google Scholar] [CrossRef]
- Fusch, Y.; Schwerdtfeger, K. Hot Dechlorination and Hot Desulfurization of Reducing Gases with Lime Pellets; Universität Karlsruhe: Karlsruhe, Germany, 1996; pp. 426–438. [Google Scholar]
- Mitchell, S.C. Hot Gas Cleanup of Sulphur, Nitrogen, Minor, and Trace Elements; Technical Report; IEA Coal Research: London, UK, 1998. [Google Scholar]
- Bartoňová, L. Effect of CaO, Al2O3 and Fe2O3 in coal ash on the retention of acidforming elements during coal combustion. WSEAS Trans. Power Syst. 2014, 9, 486–494. [Google Scholar]
- Fujita, S.; Suzuki, K.; Ohkawa, M.; Shibasaki, Y.; Mori, T. Reaction of hydrogrossular with hydrogen chloride gas at high temperature. Chem. Mater. 2001, 13, 2523–2527. [Google Scholar] [CrossRef]
- Dou, B.L.; Gao, J.S.; Sha, X.Z. A study on the reaction kinetics of HCl removal from high-temperature coal gas. Fuel Process. Technol. 2001, 72, 23–33. [Google Scholar] [CrossRef]
- Nunokawa, M.; Kobayashi, M.; Shirai, H. Halide compound removal from hot coal-derived gas with reusable sodium-based sorbent. Powder Technol. 2008, 180, 216–221. [Google Scholar] [CrossRef]
- Lee, M.T.; Wang, Z.Q.; Chang, J.R. Activated-carbon-supported NaOH for removal of HCl from reformer process streams. Ind. Eng. Chem. Res. 2003, 42, 6166–6170. [Google Scholar] [CrossRef]
- Cheng, L.S. Process for Removing HCl from Hydrocarbon Streams. U.S. Patent 6060033A, 22 April 1998. [Google Scholar]
- Kameda, T.; Uchiyama, N.; Yoshioka, T. Treatment of gaseous hydrogen chloride using Mg-Al layered double hydroxide intercalated with carbonate ion. Chemosphere 2010, 81, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Uchiyama, N.; Yoshioka, T. Removal of HCl, SO2, and NO by treatment of acid gas with Mg-Al oxide slurry. Chemosphere 2011, 82, 587–591. [Google Scholar] [CrossRef] [PubMed]
| Process | bioW or MSW | Catalyst | Catalyst Location | Temperature, K | Refs. |
|---|---|---|---|---|---|
| SR | Model tar | Commercial coconut and coal chars, sewage sludge char | Fixed-bed reactor | 1023–1223 | [50] |
| Brown coal | Brown Coal char | Fixed-bed reactor | 1023–1173 | [51] | |
| Mallee wood | Iron-loaded char, nickel-loaded char | Separate fixed-bed quartz reforming reactor | 773–1123 | [52] | |
| SR/DR | Brown coal | Brown coal char | Separate tar cracking reactor after the gasifier | 773–1173 | [53] |
| DR | Sawdust | Ni/char, wood char and coal char without Ni | Updraft biomass gasifier together with biomass | 923–1123 | [54] |
| Mallee wood | Biomass char, iron-loaded biomass char, iron-loaded brown coal char | Catalytic reactor at the top of the gasifier | 1073 | [55] | |
| Rice husk | Nickel-iron supported on rice husk char and rice husk ash | Pyrolysis reactor mixed with the raw feedstock | 1073 | [56] | |
| Pinewood | Pinewood char | Separate tar cracking zone after the pyrolysis unit | 773–1073 | [57] | |
| Municipal solid waste plastic | Ni/Al2O3 and Ni-Co/Al2O3 | Two-stage fixed bed reactor | 1073 | [46] | |
| Ni-Co/Al2O3 and Ni-Mg/Al2O3 | [48] | ||||
| Ni-Co-Al2O3 | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieta, I.S.; Epling, W.S.; Kazmierczuk, A.; Lisowski, P.; Nowakowski, R.; Serwicka, E.M. Waste into Fuel—Catalyst and Process Development for MSW Valorisation. Catalysts 2018, 8, 113. https://doi.org/10.3390/catal8030113
Pieta IS, Epling WS, Kazmierczuk A, Lisowski P, Nowakowski R, Serwicka EM. Waste into Fuel—Catalyst and Process Development for MSW Valorisation. Catalysts. 2018; 8(3):113. https://doi.org/10.3390/catal8030113
Chicago/Turabian StylePieta, Izabela S., William S. Epling, Alicja Kazmierczuk, Pawel Lisowski, Robert Nowakowski, and Ewa M. Serwicka. 2018. "Waste into Fuel—Catalyst and Process Development for MSW Valorisation" Catalysts 8, no. 3: 113. https://doi.org/10.3390/catal8030113
APA StylePieta, I. S., Epling, W. S., Kazmierczuk, A., Lisowski, P., Nowakowski, R., & Serwicka, E. M. (2018). Waste into Fuel—Catalyst and Process Development for MSW Valorisation. Catalysts, 8(3), 113. https://doi.org/10.3390/catal8030113