Maximizing Anhydrosugar Production from Fast Pyrolysis of Eucalyptus Using Sulfuric Acid as an Ash Catalyst Inhibitor
Abstract
1. Introduction
2. Results and Discussion
2.1. X-Ray Fluorescence Spectroscopy (XRF) and XRD Analysis of Ash from Raw and H2SO4-Impregnated Eucalyptus
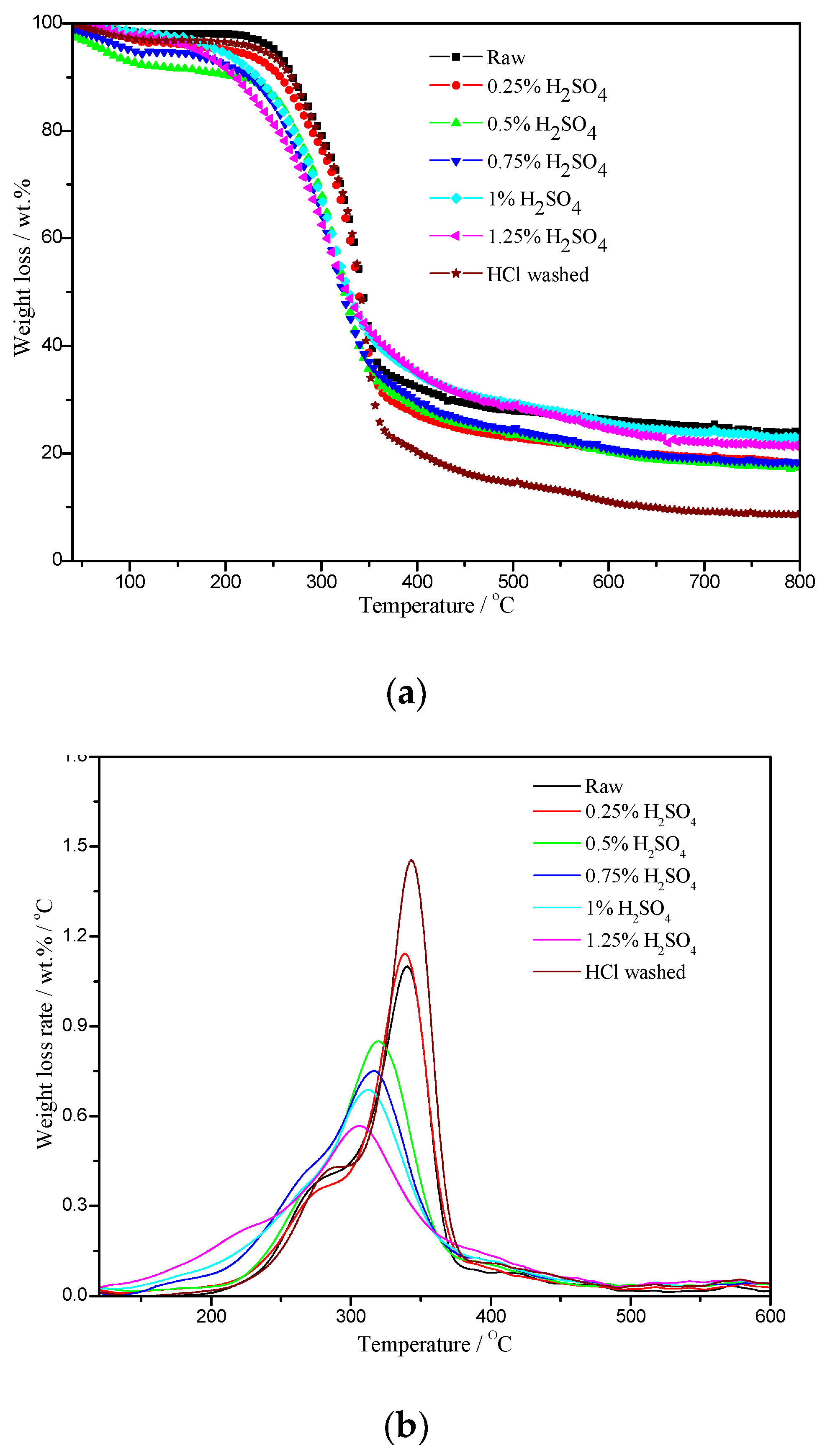
2.2. Thermogravimetric Analysis of Raw and H2SO4-Impregnated Eucalyptus
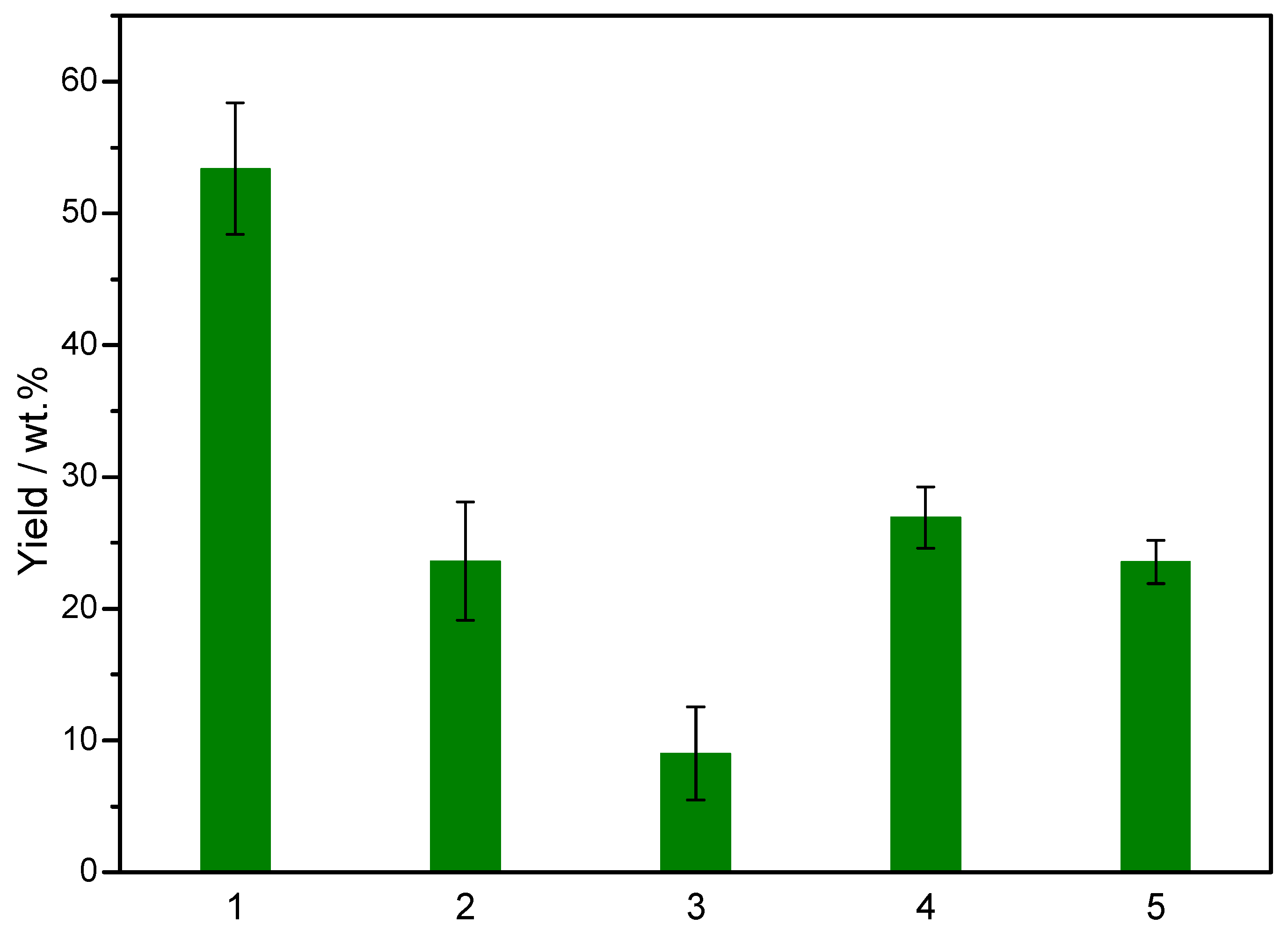
2.3. Product Distributions from Fast Pyrolysis of Raw and H2SO4-Impregnated Eucalyptus
2.4. The Possible Roles of H2SO4 during Fast Pyrolysis of H2SO4-Impregnated Eucalyptus
3. Experimental
3.1. Materials
3.2. H2SO4 Impregnation of Eucalyptus
3.3. Demineralization of Eucalyptus
3.4. Thermogravimetric Analysis
3.5. Fast Pyrolysis of Raw and H2SO4-Impregnated Eucalyptus
3.6. X-ray Diffractometer Analysis (XRD) and X-ray Fluorescence Analysis (XRF) of Ash
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Minh, D.P.; Gauthier, D.; Weiss-Hortala, E.; Nzihou, A.; Flamant, G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour. Technol. 2015, 182, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Jin, W.; Hu, J.; Xiao, R.; Luo, K.J.R.; Reviews, S.E. An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: Structures, pathways and interactions. Renew. Sustain. Energy Rev. 2015, 51, 761–774. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Wang, X.; He, F.; Li, H. Effect of torrefaction on structure and fast pyrolysis behavior of corncobs. Bioresour. Technol. 2013, 128, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G.J. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Cao, J.-P.; Li, L.-Y.; Morishita, K.; Xiao, X.-B.; Zhao, X.-Y.; Wei, X.-Y.; Takarada, T. Nitrogen transformations during fast pyrolysis of sewage sludge. Fuel 2013, 104, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, D.; Zhong, Z.; Song, M.; Pan, Q.; He, G. Fuels, Catalytic fast pyrolysis of biomass in a fluidized bed with fresh and spent fluidized catalytic cracking (FCC) catalysts. Energy Fuels 2009, 23, 6199–6206. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.-Z.; Zhu, X.-F. Overview of fuel properties of biomass fast pyrolysis oils. Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Liang, T.; Zhou, Y.; Luo, Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour. Technol. 2012, 104, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, X.-C.; Dong, C.-Q.; Zhang, Z.-F.; Zhang, X.-M.; Zhu, X.-F. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z.; Pyrolysis, A. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Li, R.; Zeng, K.; Soria, J.; Mazza, G.; Gauthier, D.; Rodriguez, R.; Flamant, G. Product distribution from solar pyrolysis of agricultural and forestry biomass residues. Renew. Energy 2016, 89, 27–35. [Google Scholar] [CrossRef]
- Eom, I.Y.; Kim, K.H.; Kim, J.Y.; Lee, S.M.; Yeo, H.M.; Choi, I.G.; Choi, J.W. Characterization of primary thermal degradation features of lignocellulosic biomass after removal of inorganic metals by diverse solvents. Bioresour. Technol. 2011, 102, 3437–3444. [Google Scholar] [CrossRef]
- Xing, S.; Yuan, H.; Huhetaoli; Qi, Y.; Lv, P.; Yuan, Z.; Chen, Y. Characterization of the decomposition behaviors of catalytic pyrolysis of wood using copper and potassium over thermogravimetric and Py-GC/MS analysis. Energy 2016, 114, 634–646. [Google Scholar] [CrossRef]
- Marathe, P.S.; Oudenhoven, S.R.G.; Heerspink, P.W.; Kersten, S.R.A.; Westerhof, R.J.M. Fast pyrolysis of cellulose in vacuum: The effect of potassium salts on the primary reactions. Chem. Eng. J. 2017, 329, 187–197. [Google Scholar] [CrossRef]
- Wiinikka, H.; Johansson, A.-C.; Sandström, L.; Öhrman, O.G. Fate of inorganic elements during fast pyrolysis of biomass in a cyclone reactor. Fuel 2017, 203, 537–547. [Google Scholar] [CrossRef]
- Keown, D.M.; Favas, G.; Hayashi, J.; Li, C.Z. Volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass: Differences between sugar cane bagasse and cane trash. Bioresour. Technol. 2005, 96, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, D.; Jones, J.; Brydson, R.; Ross, A. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- David, G.F.; Perez, V.H.; Rodriguez Justo, O.; Garcia-Perez, M. Effect of acid additives on sugarcane bagasse pyrolysis: Production of high yields of sugars. Bioresour. Technol. 2017, 223, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Zhao, Z.; Huang, Z.; Zhao, K.; Wei, G.; Jiang, L.; Wang, X.; He, F.; Li, H. Overcoming biomass recalcitrance for enhancing sugar production from fast pyrolysis of biomass by microwave pretreatment in glycerol. Green Chem. 2015, 17, 1167–1175. [Google Scholar] [CrossRef]
- Dalluge, D.L.; Daugaard, T.; Johnston, P.; Kuzhiyil, N.; Wright, M.M.; Brown, R.C. Continuous production of sugars from pyrolysis of acid-infused lignocellulosic biomass. Green Chem. 2014, 16, 4144–4155. [Google Scholar] [CrossRef]
- Zhou, S.; Mourant, D.; Lievens, C.; Wang, Y.; Li, C.-Z.; Garcia-Perez, M. Effect of sulfuric acid concentration on the yield and properties of the bio-oils obtained from the auger and fast pyrolysis of Douglas Fir. Fuel 2013, 104, 536–546. [Google Scholar] [CrossRef]
- Sui, X.W.; Wang, Z.; Liao, B.; Zhang, Y.; Guo, Q.X. Preparation of levoglucosenone through sulfuric acid promoted pyrolysis of bagasse at low temperature. Bioresour. Technol. 2012, 103, 466–469. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y. Effects of water washing and torrefaction on the pyrolysis behavior and kinetics of rice husk through TGA and Py-GC/MS. Bioresour. Technol. 2016, 199, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Z.; Liaw, S.-S.; Li, C.-Z.; Garcia-Perez, M. Effect of sulfuric acid on the pyrolysis of Douglas fir and hybrid poplar wood: Py-GC/MS and TG studies. J. Anal. Appl. Pyrolysis 2013, 104, 117–130. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Stevenson, T.T. Saccharification of douglas-fir wood by a combination of prehydrolysis and pyrolysis. J. Appl. Polym. Sci. 2010, 27, 4577–4585. [Google Scholar] [CrossRef]


| Element | Elemental Analysis (wt%) | |
|---|---|---|
| A | SA | |
| K | 21.20 | 18.60 |
| Ca | 13.64 | 9.50 |
| Na | 5.61 | 5.11 |
| Mg | 3.30 | 3.22 |
| Mn | 1.70 | 1.44 |
| Fe | 1.42 | 1.62 |
| P | 1.31 | 1.29 |
| S | 0.77 | 15.02 |
| Cl | 4.09 | 0.40 |
| Feedstocks | Char Yield (at 800 °C, wt%) | Tmax (°C) | Dmax (wt%/°C) |
|---|---|---|---|
| raw | 23.81 | 340.3 | 1.10 |
| 0.25% H2SO4 | 15.16 | 338.6 | 1.14 |
| 0.5% H2SO4 | 17.31 | 319.7 | 0.85 |
| 0.75% H2SO4 | 18.24 | 316.7 | 0.75 |
| 1% H2SO4 | 22.83 | 312.1 | 0.69 |
| 1.25% H2SO4 | 18.21 | 305.7 | 0.57 |
| Demineralized | 8.54 | 343.2 | 1.46 |
| Time (min) | Compounds | Content a/% | |||||
|---|---|---|---|---|---|---|---|
| Raw | H2SO4 Concentration (wt%) | ||||||
| 0.25 | 0.5 | 0.75 | 1 | 1.25 | |||
| 4.762 | Acetaldehyde | 2.11 | 1.49 | 1.16 | 1.38 | 1.32 | 1.12 |
| 8.306 | Acetaldehyde, hydroxy- | 2.82 | 0.84 | 0.98 | 0.55 | 0.59 | 0.3 |
| 37.615 | 4-Methyl-2,5-dimethoxybenzaldehyde | 2.85 | 0.93 | 1.1 | 0.74 | 0.85 | 0.48 |
| 41.216 | Benzaldehyde,4-hydroxy-3,5-dimethoxy- | 1.53 | 0.67 | 0.71 | 0.54 | 0.62 | 0.48 |
| 42.629 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | 0.89 | 0.7 | 0.8 | 0.69 | 0.68 | 0.72 |
| Total of aldehydes | 10.19 | 4.63 | 4.76 | 3.89 | 4.07 | 3.10 | |
| 5.657 | Acetone | 0.58 | 0.42 | 0.29 | 0.39 | 0.38 | 0.48 |
| 7.146 | Methyl vinyl ketone | 0.29 | 0.28 | 0.15 | 0.23 | 0.2 | 0.31 |
| 7.212 | 2,3-Butanedione | 0.75 | 0.44 | 0.34 | 0.38 | 0.35 | 0.32 |
| 10.568 | 2-Propanone, 1-hydroxy- | 3.72 | 0.94 | 0.74 | 0.61 | 0.68 | 0.58 |
| 13.819 | 1-Hydroxy-2-butanone | 0.41 | 0.55 | 0.07 | 0.35 | 0.5 | 0.15 |
| 19.587 | 2-Cyclopenten-1-one, 2-hydroxy- | 1.5 | 0.59 | 0.54 | 0.45 | 0.45 | 0.48 |
| 22.668 | 1,2-Cyclopentanedione, 3-methyl- | 1.28 | 0.58 | 0.55 | 0.51 | 0.51 | 0.52 |
| 26.953 | Levoglucosenone | 0.05 | 0.25 | 0.31 | 1.16 | 0.8 | 2.38 |
| Total of ketones | 8.58 | 4.04 | 2.99 | 4.07 | 3.87 | 5.22 | |
| 15.939 | Furfural | 1.9 | 2.83 | 2.21 | 3.07 | 2.74 | 6.83 |
| 17.334 | 2-Furanmethanol | 0.38 | 0.21 | 0.21 | 0.22 | 0.25 | 0.28 |
| 20.435 | 2-Furancarboxaldehyde, 5-methyl- | 0.27 | 0.36 | 0.36 | 0.33 | 0.29 | 0.55 |
| 21.406 | 2(5H)-Furanone | 0.6 | 0.21 | 0.19 | 0.16 | 0.18 | 0.18 |
| 31.857 | 5-Hydroxymethylfurfural | 0.46 | 0.81 | 0.76 | 0.59 | 0.63 | 0.56 |
| Total of furans | 3.61 | 4.43 | 3.73 | 4.36 | 4.10 | 8.40 | |
| 23.498 | Phenol | 0.46 | 0.35 | 0.26 | 0.32 | 0.35 | 0.38 |
| 24.129 | Phenol, 2-methoxy- | 0.78 | 0.5 | 0.64 | 0.49 | 0.6 | 0.7 |
| 27.07 | Creosol | 0.41 | 0.32 | 0.36 | 0.3 | 0.42 | 0.41 |
| 30.886 | 2-Methoxy-4-vinylphenol | 1.46 | 2.62 | 3.02 | 3.49 | 3.72 | 2.46 |
| 31.508 | Phenol, 2-methoxy-3-(2-propenyl)- | 0.27 | 0.12 | 0.11 | 0.11 | 0.1 | 0.2 |
| 32.253 | Phenol, 2,6-dimethoxy- | 2.31 | 1.23 | 1.36 | 1.16 | 1.43 | 1.6 |
| 34.204 | Phenol, 2-methoxy-4-(1-propenyl)- | 1.4 | 2.2 | 2.55 | 2.34 | 2.48 | 1.71 |
| 39.17 | (E)-2,6-Dimethoxy-4-(propenyl)phenol | 0.55 | 0.15 | 0.17 | 0.15 | 0.16 | 0.21 |
| Total of phenols | 7.65 | 7.59 | 8.47 | 8.36 | 9.27 | 7.77 | |
| 9.323 | Acetic acid | 7.42 | 4.17 | 3.44 | 3.38 | 3.57 | 4.07 |
| Total of acids | 7.42 | 4.17 | 3.44 | 3.38 | 3.57 | 4.07 | |
| 40.179 | levoglucosan | 9.88 | 40.14 | 43.2 | 41.33 | 38.93 | 27.95 |
| 43.45 | 1,6-Anhydro-β-d-glucofuranose (AGF) | 1.05 | 5.09 | 4.98 | 5.27 | 5.62 | 5.89 |
| Total of anhydrosugars | 11.85 | 49.31 | 53.57 | 53.58 | 50.02 | 37.63 | |
| Items | Elemental Analysis (wt%) a | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O b | Ash | H/C c | O/C d | |
| Raw | 48.10 | 5.96 | 0.13 | 0.06 | 45.37 | 0.38 | 0.12 | 0.94 |
| 0.5%H2SO4 | 47.30 | 5.74 | 0.09 | 0.17 | 46.26 | 0.44 | 0.12 | 0.98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Fan, Y.; Zheng, A.; Zhao, Z.; Wang, F.; Li, H. Maximizing Anhydrosugar Production from Fast Pyrolysis of Eucalyptus Using Sulfuric Acid as an Ash Catalyst Inhibitor. Catalysts 2018, 8, 609. https://doi.org/10.3390/catal8120609
Zhang D, Fan Y, Zheng A, Zhao Z, Wang F, Li H. Maximizing Anhydrosugar Production from Fast Pyrolysis of Eucalyptus Using Sulfuric Acid as an Ash Catalyst Inhibitor. Catalysts. 2018; 8(12):609. https://doi.org/10.3390/catal8120609
Chicago/Turabian StyleZhang, Dongyan, Yuyang Fan, Anqing Zheng, Zengli Zhao, Fengyun Wang, and Haibin Li. 2018. "Maximizing Anhydrosugar Production from Fast Pyrolysis of Eucalyptus Using Sulfuric Acid as an Ash Catalyst Inhibitor" Catalysts 8, no. 12: 609. https://doi.org/10.3390/catal8120609
APA StyleZhang, D., Fan, Y., Zheng, A., Zhao, Z., Wang, F., & Li, H. (2018). Maximizing Anhydrosugar Production from Fast Pyrolysis of Eucalyptus Using Sulfuric Acid as an Ash Catalyst Inhibitor. Catalysts, 8(12), 609. https://doi.org/10.3390/catal8120609





