Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural Properties
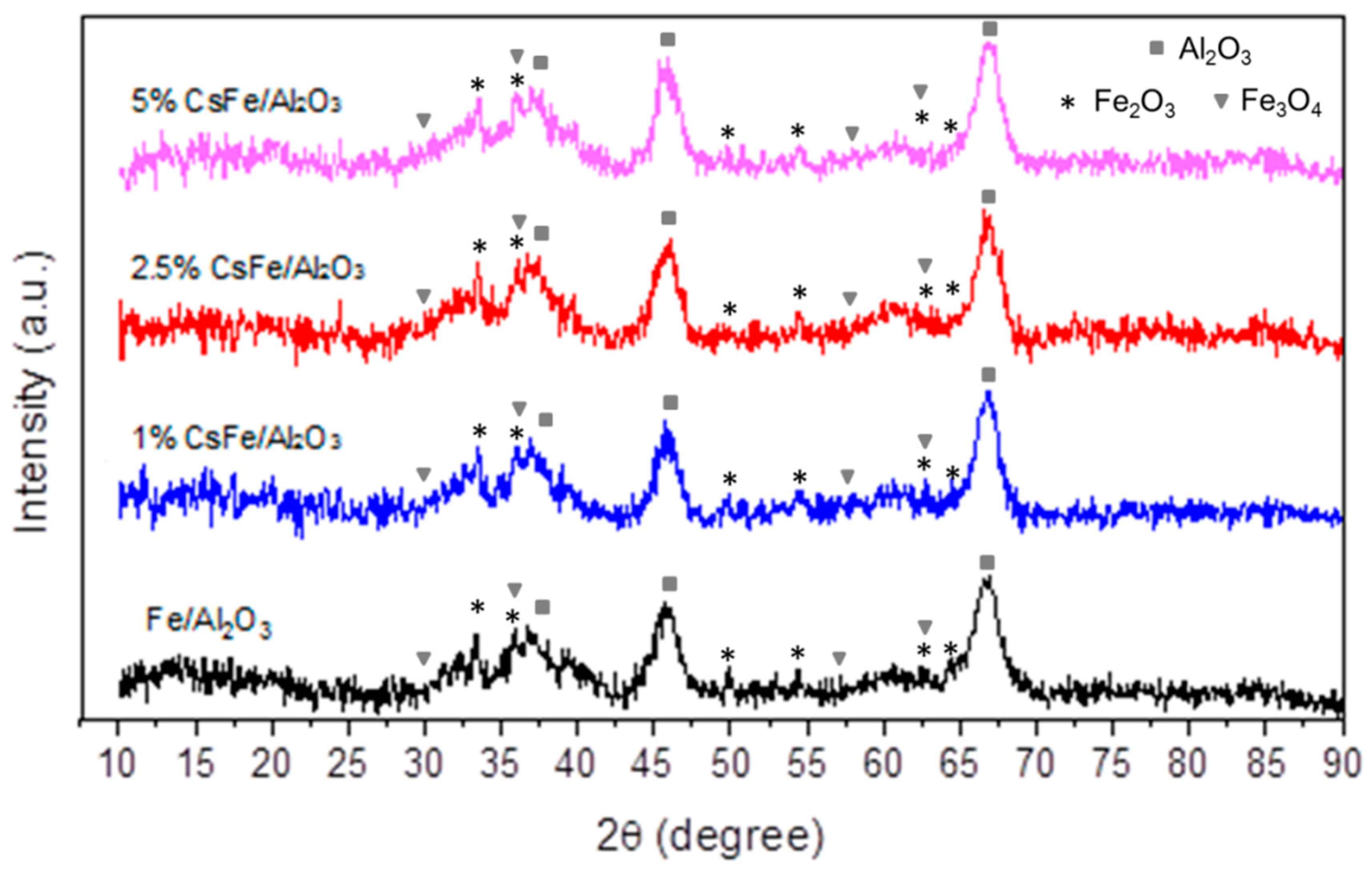
2.2. XRD
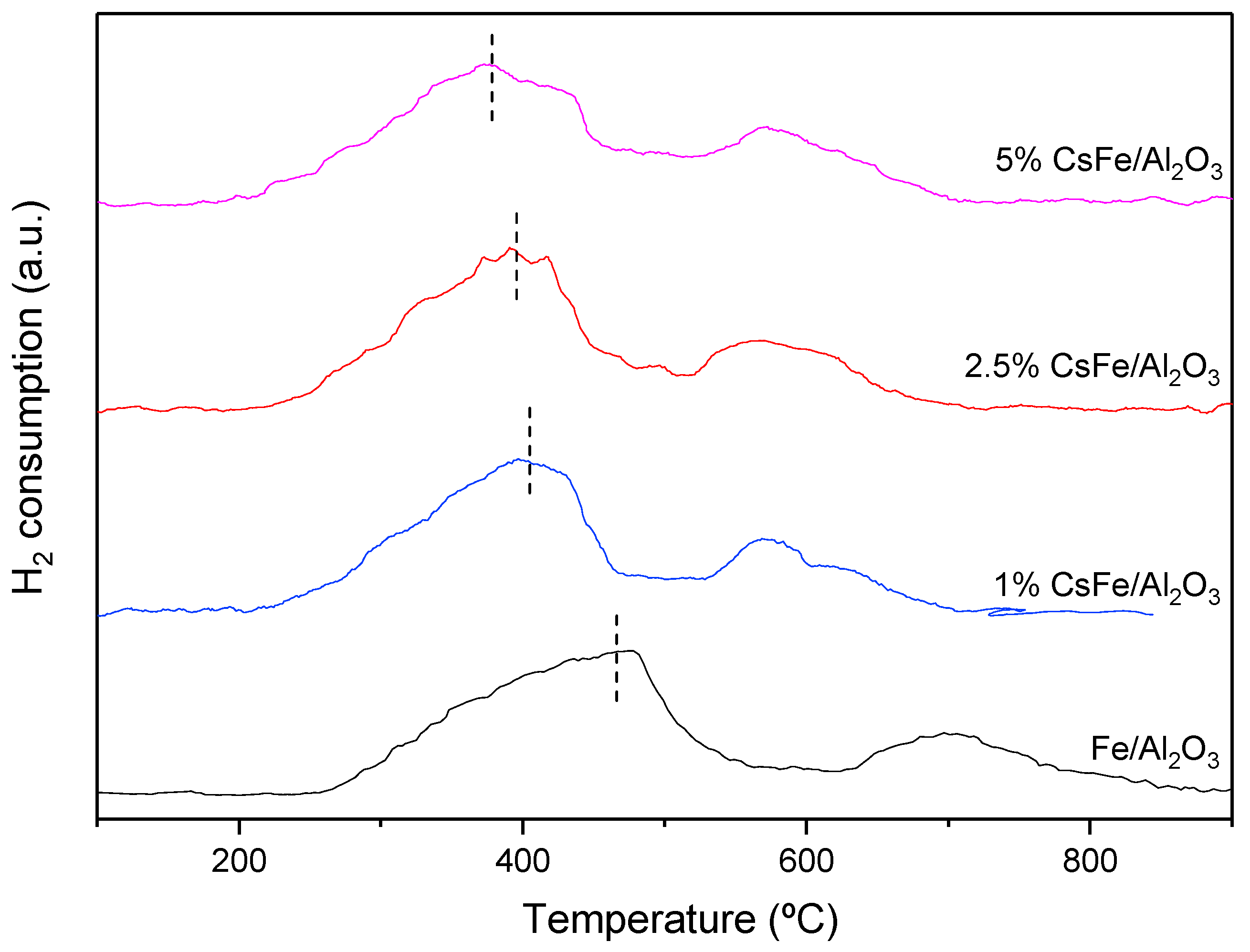
2.3. H2-TPR
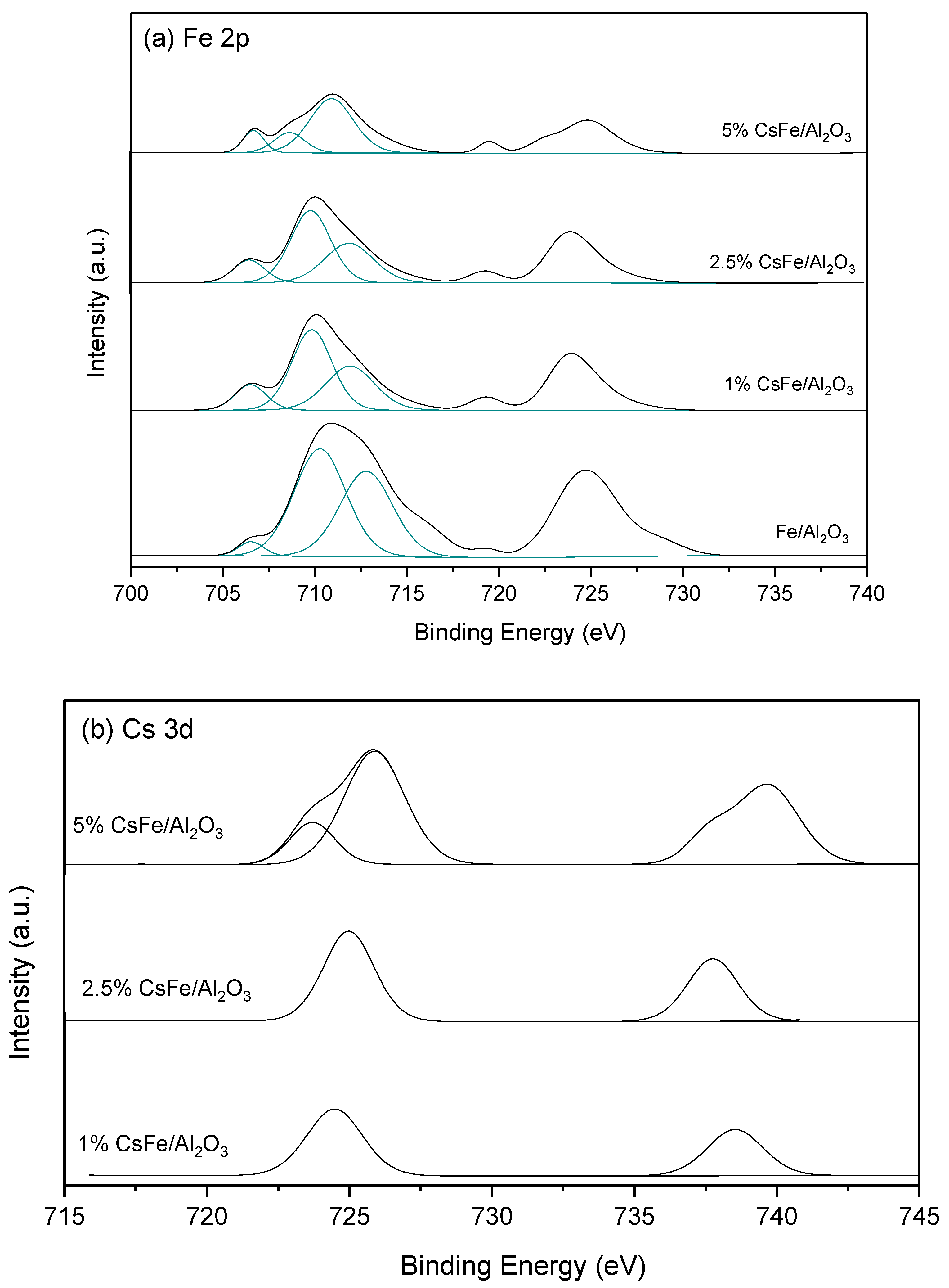
2.4. XPS
2.5. Catalytic Behaviour
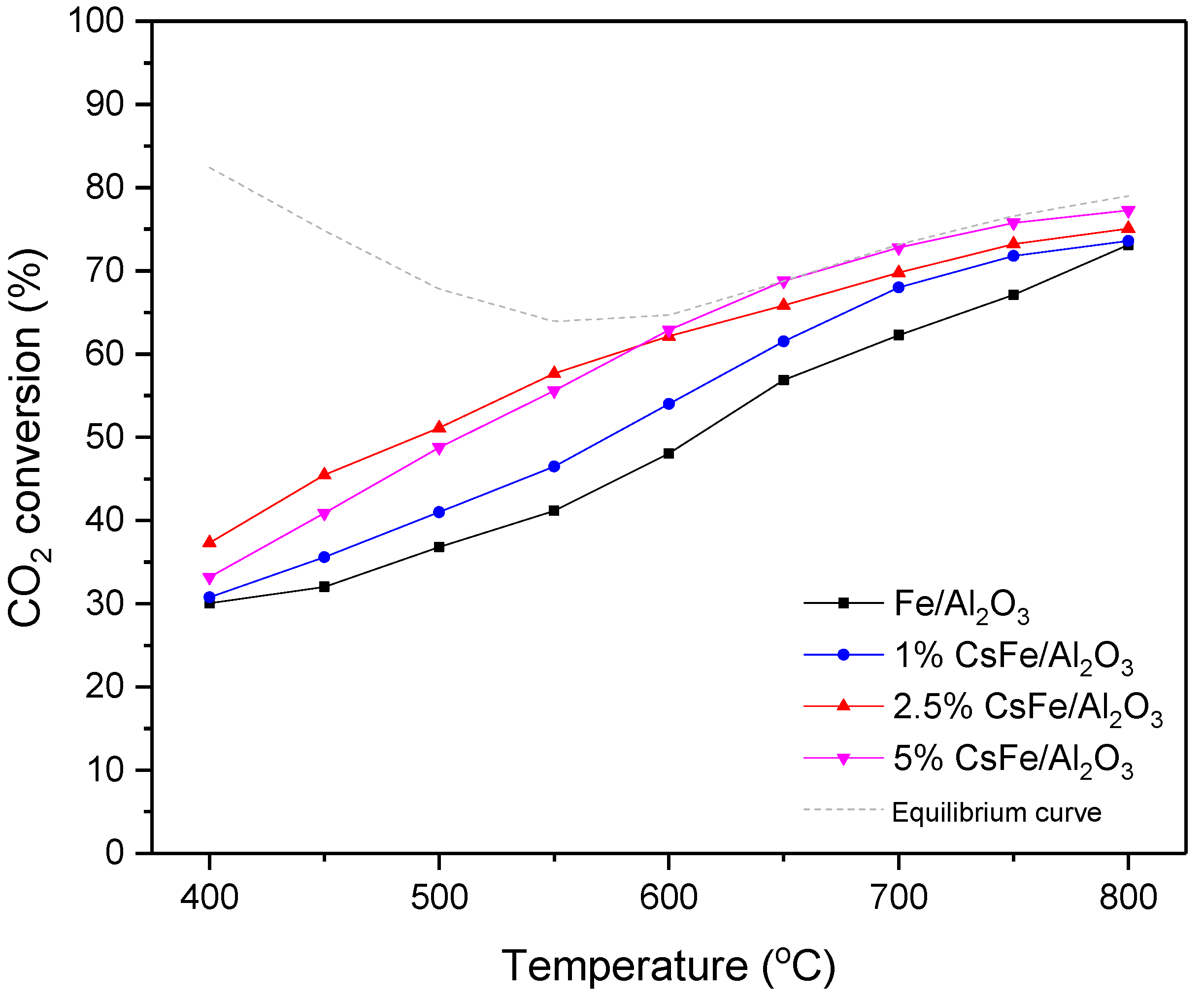
2.5.1. Effect of the Promoter on Catalytic Performance
2.5.2. Effect of H2:CO2 Ratio on Catalytic Performance
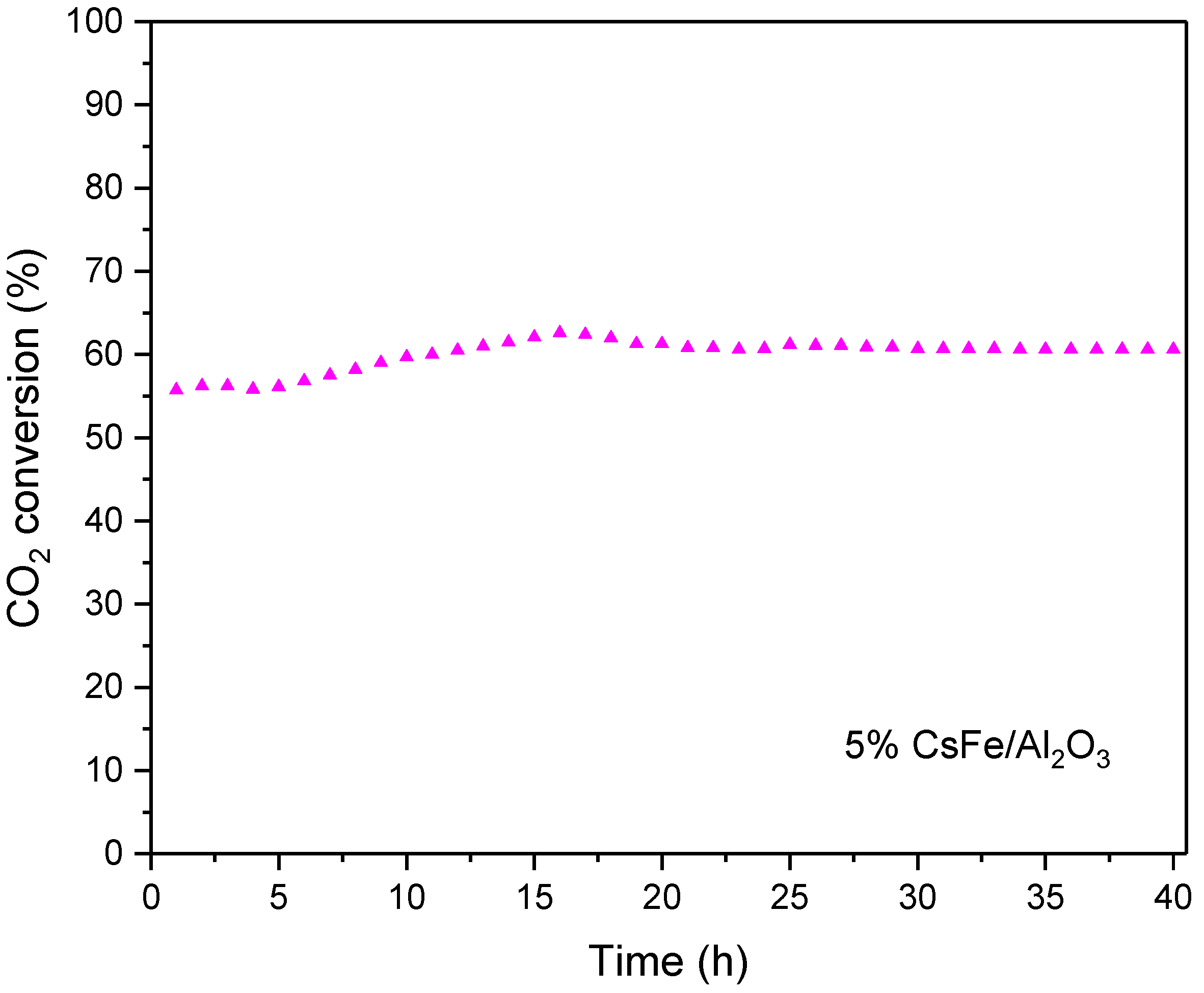
2.5.3. Stability Test
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterisation
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nooa Research. Available online: https://research.noaa.gov/article/ArtMID/587/ArticleID/2362/Another-climate-milestone-falls-at-NOAA%E2%80%99s-Mauna-Loa-observatory (accessed on 7 November 2018).
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Schure, K.M.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions: 2017 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for fischer-tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Joo, O.-S.; Jung, K.-D.; Yonsoo, J. Camere process for methanol synthesis from CO2 hydrogenation. In Studies in Surface Science and Catalysis; Park, S.-E., Chang, J.-S., Lee, K.-W., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 153, pp. 67–72. [Google Scholar]
- Samimi, F.; Rahimpour, M.R.; Shariati, A. Development of an Efficient Methanol Production Process for Direct CO2 Hydrogenation over a Cu/ZnO/Al2O3 Catalyst. Catalysts 2017, 7, 332. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 conversion by reverse water gas shift catalysis: Comparison of catalysts, mechanisms and their consequences for CO2 conversion to liquid fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xia, S.; Wang, C.; Sun, F. Entropy generation minimization for the reverse water gas shift (RWGS) reactors. Entropy 2018, 20, 415. [Google Scholar] [CrossRef]
- Liang, B.; Duan, H.; Su, X.; Chen, X.; Huang, Y.; Chen, X.; Delgado, J.J.; Zhang, T. Promoting role of potassium in the reverse water gas shift reaction on Pt/mullite catalyst. Catal. Today 2017, 281, 319–326. [Google Scholar] [CrossRef]
- Álvarez Galván, C.; Schumann, J.; Behrens, M.; Fierro, J.L.G.; Schlögl, R.; Frei, E. Reverse water-gas shift reaction at the Cu/ZnO interface: Influence of the cu/zn ratio on structure-activity correlations. Appl. Catal. B 2016, 195, 104–111. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D. Study of bimetallic Cu–Ni/γ-Al2O3 catalysts for carbon dioxide hydrogenation. Int. J. Hydrogen Energy 1999, 24, 351–354. [Google Scholar] [CrossRef]
- Nagai, M.; Kurakami, T. Reverse water gas shift reaction over molybdenum carbide. J. Chem. Eng. Jpn. 2005, 38, 807–812. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The brønsted–evans–polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Peña, D.; Cognigni, A.; Neumayer, T.; van Beek, W.; Jones, D.S.; Quijada, M.; Rønning, M. Identification of carbon species on iron-based catalysts during fischer-tropsch synthesis. Appl. Catal. A 2018, 554, 10–23. [Google Scholar] [CrossRef]
- Wenzel, M.; Aditya Dharanipragada, N.V.R.; Galvita, V.V.; Poelman, H.; Marin, G.B.; Rihko-Struckmann, L.; Sundmacher, K. Co production from CO2 via reverse water–gas shift reaction performed in a chemical looping mode: Kinetics on modified iron oxide. J. CO2 Util. 2017, 17, 60–68. [Google Scholar] [CrossRef]
- Chen, X.; Su, X.; Duan, H.; Liang, B.; Huang, Y.; Zhang, T. Catalytic performance of the Pt/TiO2 catalysts in reverse water gas shift reaction: Controlled product selectivity and a mechanism study. Catal. Today 2017, 281, 312–318. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Liu, Y. Reverse water gas shift reaction over co-precipitated Ni-CeO2 catalysts. J. Rare Earths 2008, 26, 66–70. [Google Scholar] [CrossRef]
- Kharaji, A.G.; Shariati, A.; Takassi, M.A. A novel γ-Alumina supported Fe-Mo bimetallic catalyst for reverse water gas shift reaction. Chin. J. Chem. Eng. 2013, 21, 1007–1014. [Google Scholar] [CrossRef]
- Jurković, D.L.; Pohar, A.; Dasireddy, V.D.B.C.; Likozar, B. Effect of copper-based catalyst support on reverse water-gas shift reaction (RWGS) activity for CO2 reduction. Chem. Eng. Technol. 2017, 40, 973–980. [Google Scholar] [CrossRef]
- Santos, J.L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na. C 2018, 4, 47. [Google Scholar] [CrossRef]
- Sai Prasad, P.S.; Bae, J.W.; Jun, K.-W.; Lee, K.-W. Fischer–tropsch synthesis by carbon dioxide hydrogenation on fe-based catalysts. Catal. Surv. Asia 2008, 12, 170–183. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Infantes-Molina, A.; Lietti, L.; Forzatti, P.; Iaquaniello, G.; Palo, E.; Picutti, B.; Brignoli, F. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk fe-catalyst. Appl. Catal. B 2017, 200, 530–542. [Google Scholar] [CrossRef]
- Park, J.C.; Yeo, S.C.; Chun, D.H.; Lim, J.T.; Yang, J.-I.; Lee, H.-T.; Hong, S.; Lee, H.M.; Kim, C.S.; Jung, H. Highly activated K-doped iron carbide nanocatalysts designed by computational simulation for fischer–tropsch synthesis. J. Mater. Chem. A 2014, 2, 14371–14379. [Google Scholar] [CrossRef]
- Loiland, J.A.; Wulfers, M.J.; Marinkovic, N.S.; Lobo, R.F. Fe/γ-Al2O3 and Fe-K/γ-Al2O3 as reverse water-gas shift catalysts. Catal. Sci. Technol. 2016, 6, 5267–5279. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Riesco-García, J.M.; Penelás-Pérez, G.; Urakawa, A. Enabling continuous capture and catalytic conversion of flue gas CO2 to syngas in one process. J. CO2 Util. 2016, 14, 106–111. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Baibars, F.; le Saché, E.; Arellano-García, H.; Gu, S.; Reina, T.R. CO2 valorisation via reverse water-gas shift reaction using advanced Cs doped Fe-Cu/Al2O3 catalysts. J. CO2 Util. 2017, 21, 423–428. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Chen, X.; Duan, H.; Liang, B.; Liu, Q.; Liu, X.; Ren, Y.; Huang, Y.; Zhang, T. Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl. Catal. B 2017, 216, 95–105. [Google Scholar] [CrossRef]
- Ahlers, S.J.; Pohl, M.-M.; Radnik, J.; Linke, D.; Kondratenko, E.V. Catalytic role and location of cs promoter in Cs–Au/TiO2 catalysts for propanol synthesis from CO2, C2H4 and H2. Appl. Catal. B 2015, 176–177, 570–577. [Google Scholar] [CrossRef]
- Ali, S.; Mohd Zabidi, N.A.; Subbarao, D. Correlation between fischer-tropsch catalytic activity and composition of catalysts. Chem. Cent. J. 2011, 5, 68. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Nist X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 8 November 2018).
- Park, S.-W.; Joo, O.-S.; Jung, K.-D.; Kim, H.; Han, S.-H. Development of ZnO/Al2O3 catalyst for reverse-water-gas-shift reaction of camere (carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction) process. Appl. Catal. A 2001, 211, 81–90. [Google Scholar] [CrossRef]
- Aljishi, A.; Veilleux, G.; Lalinde, J.A.H.; Kopyscinski, J. The effect of synthesis parameters on ordered mesoporous nickel alumina catalyst for CO2 methanation. Appl. Catal. A 2018, 549, 263–272. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, G.; Liu, K.; Yang, Y.; Xiang, H.; Li, Y. Adsorption and reaction of CO and hydrogen on iron-based fischer–tropsch synthesis catalysts. J. Mol. Catal. A Chem. 2010, 328, 35–43. [Google Scholar] [CrossRef]
- Ghodoosi, F.; Khosravi-Nikou, M.R.; Shariati, A. Mathematical modeling of reverse water-gas shift reaction in a fixed-bed reactor. Chem. Eng. Technol. 2017, 40, 598–607. [Google Scholar] [CrossRef]



| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Fe/Al2O3 | 174 | 0.470 | 8.73 |
| 1% CsFe/Al2O3 | 168 | 0.467 | 8.83 |
| 2.5% CsFe/Al2O3 | 153 | 0.421 | 8.75 |
| 5% CsFe/Al2O3 | 149 | 0.401 | 8.83 |
| Catalysts | Fe 2p3/2 (eV) | Cs 3d5/2 (eV) | Fetotal/Al (at/at) | Cs/Fe | ||
|---|---|---|---|---|---|---|
| Fe3+ | Fe2+ | Fe | Cs1+ | |||
| 5% CsFe/Al2O3 | 710.9 (62%) | 708.6 (18%) | 706.7 (20%) | 723.7–725.9 | 0.077 | 0.708 |
| 2.5% CsFe/Al2O3 | 711.5 (25%) | 709.5 (63%) | 706.7 (14%) | 725.5 | 0.048 | 0.460 |
| 1% CsFe/Al2O3 | 711.9 (34%) | 709.8 (52%) | 706.5 (13%) | 724.5 | 0.059 | 0.202 |
| Fe-Al2O3 | 712.8 (43%) | 710.3 (53%) | 706.5 (4%) | - | 0.077 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor-Pérez, L.; Shah, M.; Le Saché, E.; Ramirez Reina, T. Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts 2018, 8, 608. https://doi.org/10.3390/catal8120608
Pastor-Pérez L, Shah M, Le Saché E, Ramirez Reina T. Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts. 2018; 8(12):608. https://doi.org/10.3390/catal8120608
Chicago/Turabian StylePastor-Pérez, Laura, Mihir Shah, Estelle Le Saché, and Tomas Ramirez Reina. 2018. "Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter" Catalysts 8, no. 12: 608. https://doi.org/10.3390/catal8120608
APA StylePastor-Pérez, L., Shah, M., Le Saché, E., & Ramirez Reina, T. (2018). Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts, 8(12), 608. https://doi.org/10.3390/catal8120608







