Influence of Sulfur-Containing Sodium Salt Poisoned V2O5–WO3/TiO2 Catalysts on SO2–SO3 Conversion and NO Removal
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Different Catalysts on SO3 Generation
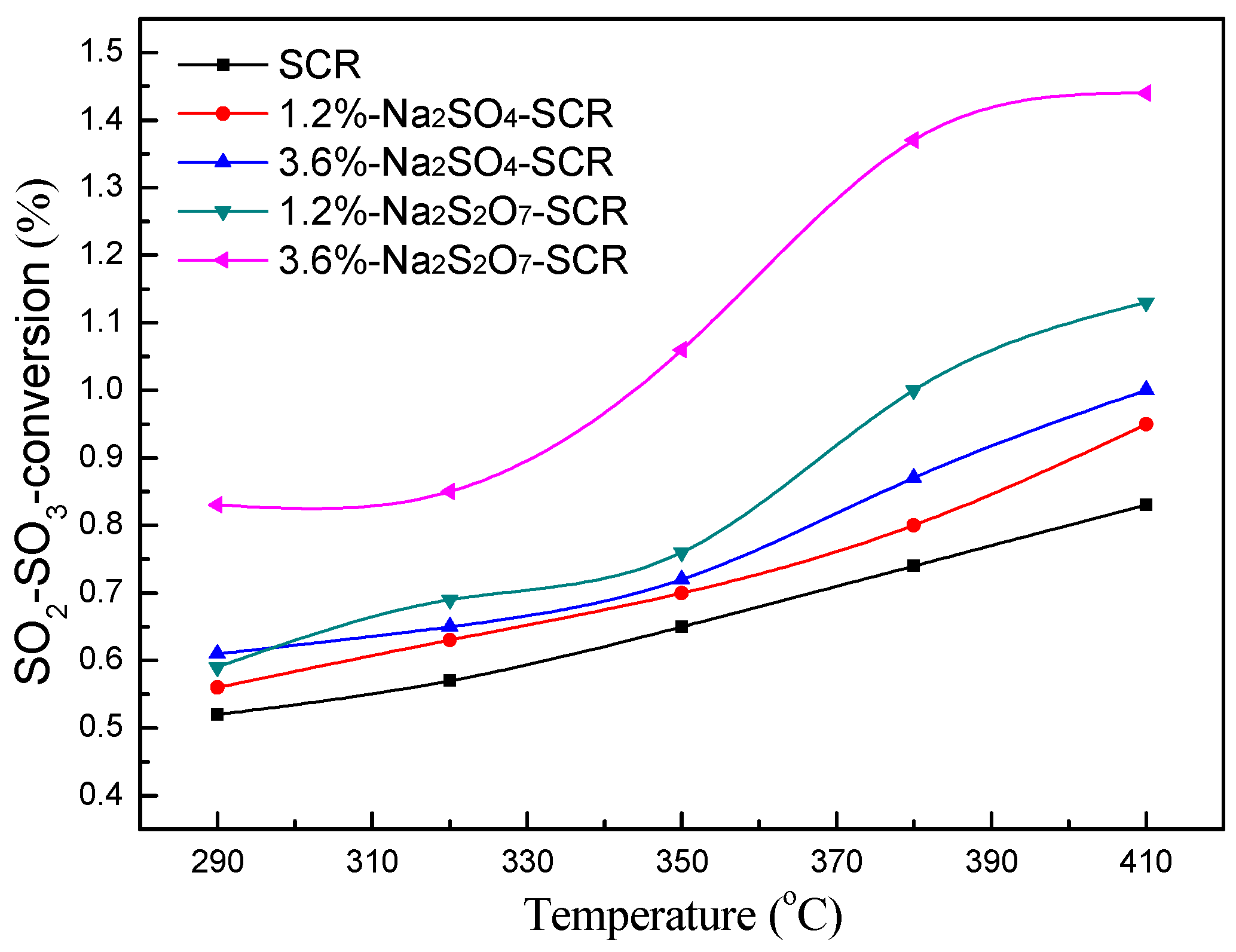
2.1.1. Effect of Temperature on SO3 Generation
2.1.2. Effect of SO2 on SO3 Generation
2.2. Performance of Different Catalysts
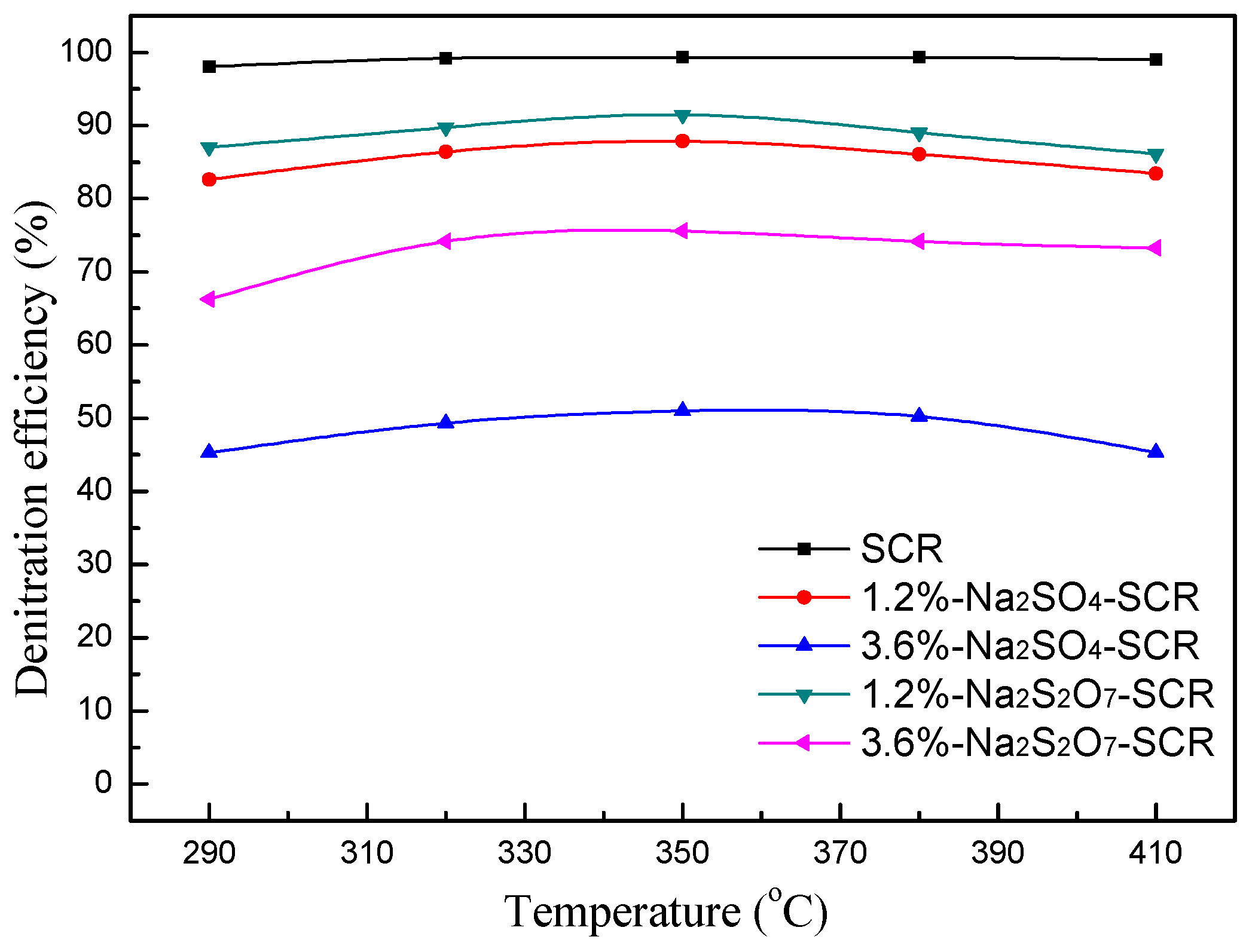
2.2.1. Effect of Temperature on NO Conversion
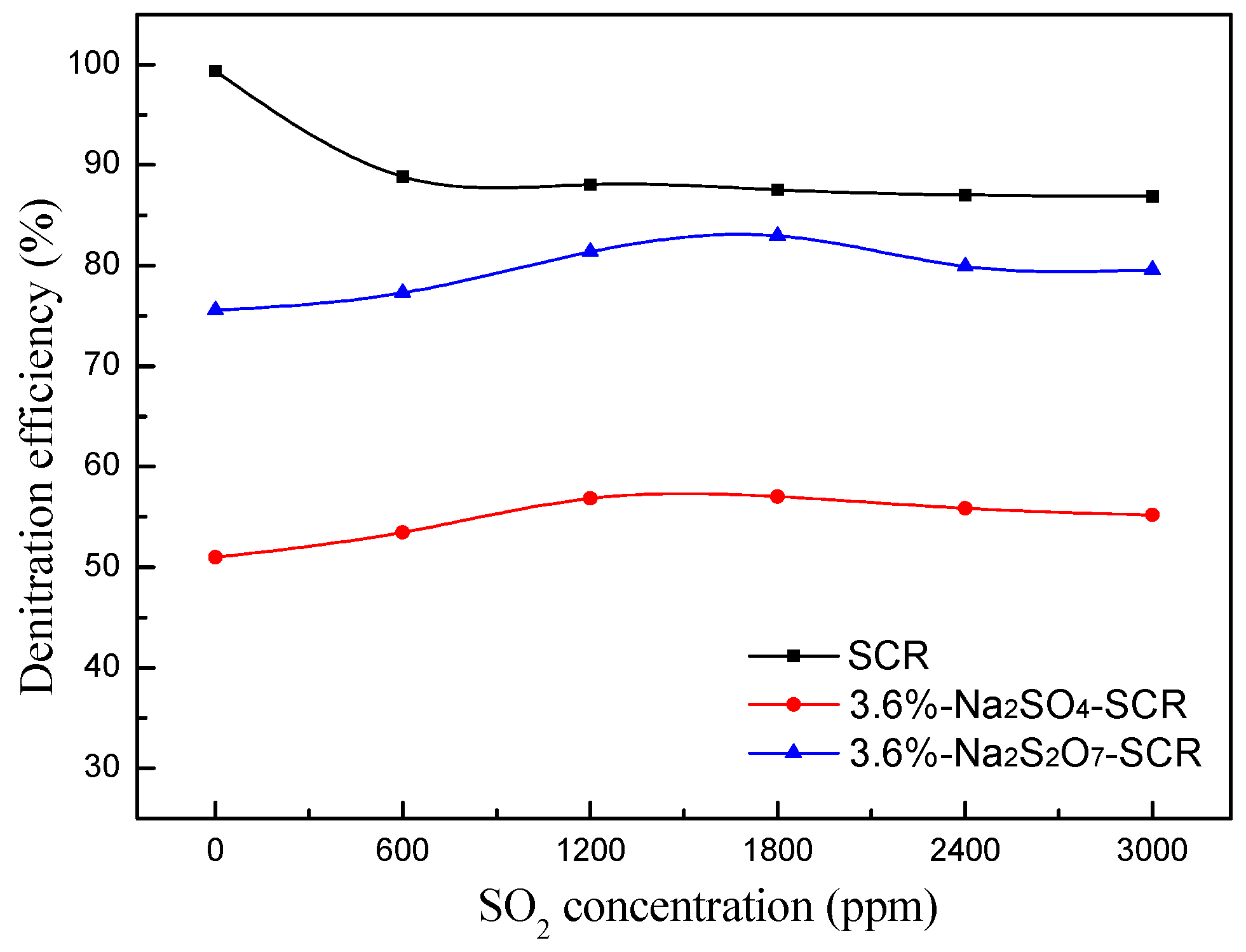
2.2.2. Effect of SO2 on NO Conversion
2.3. Catalyst Characterization
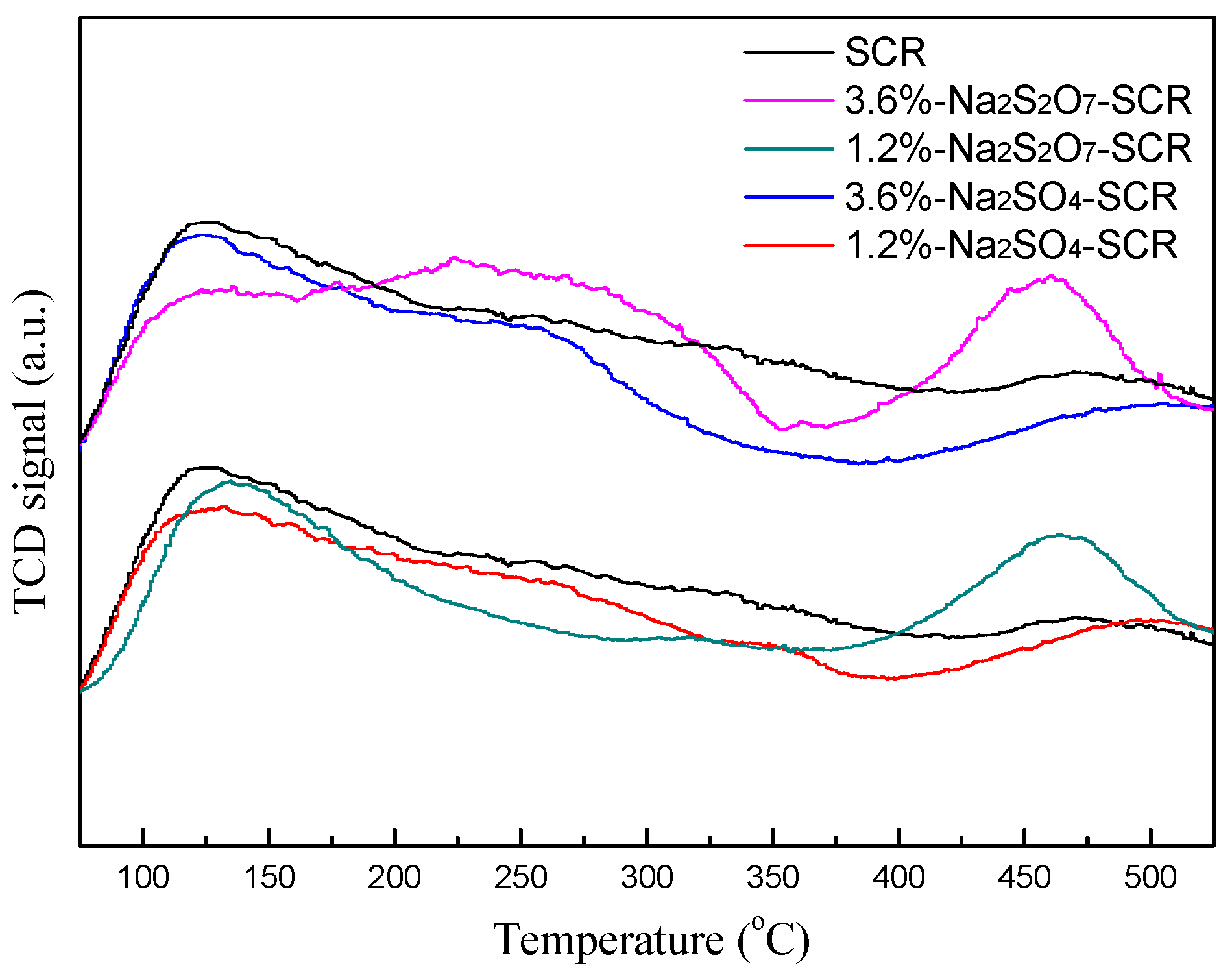
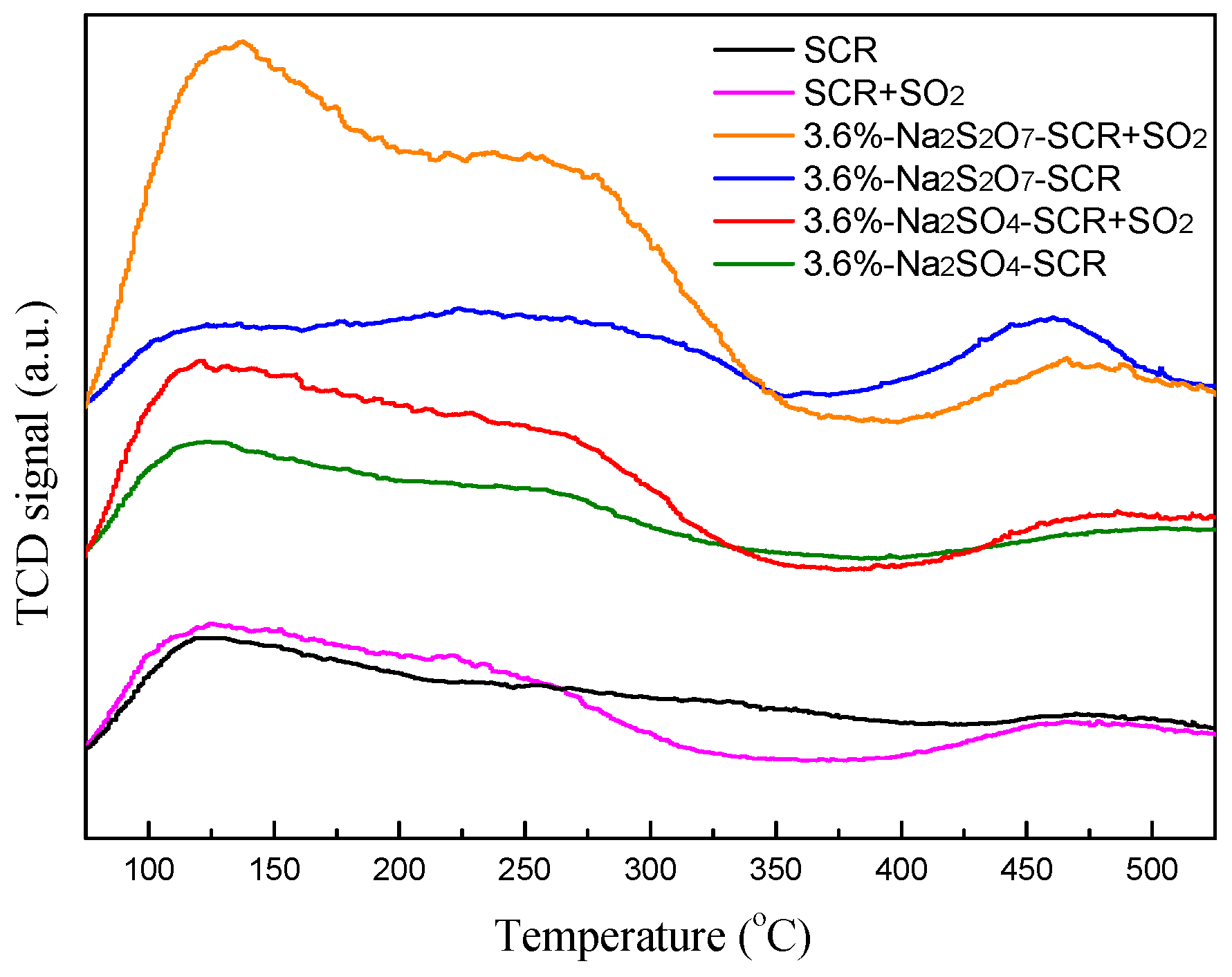
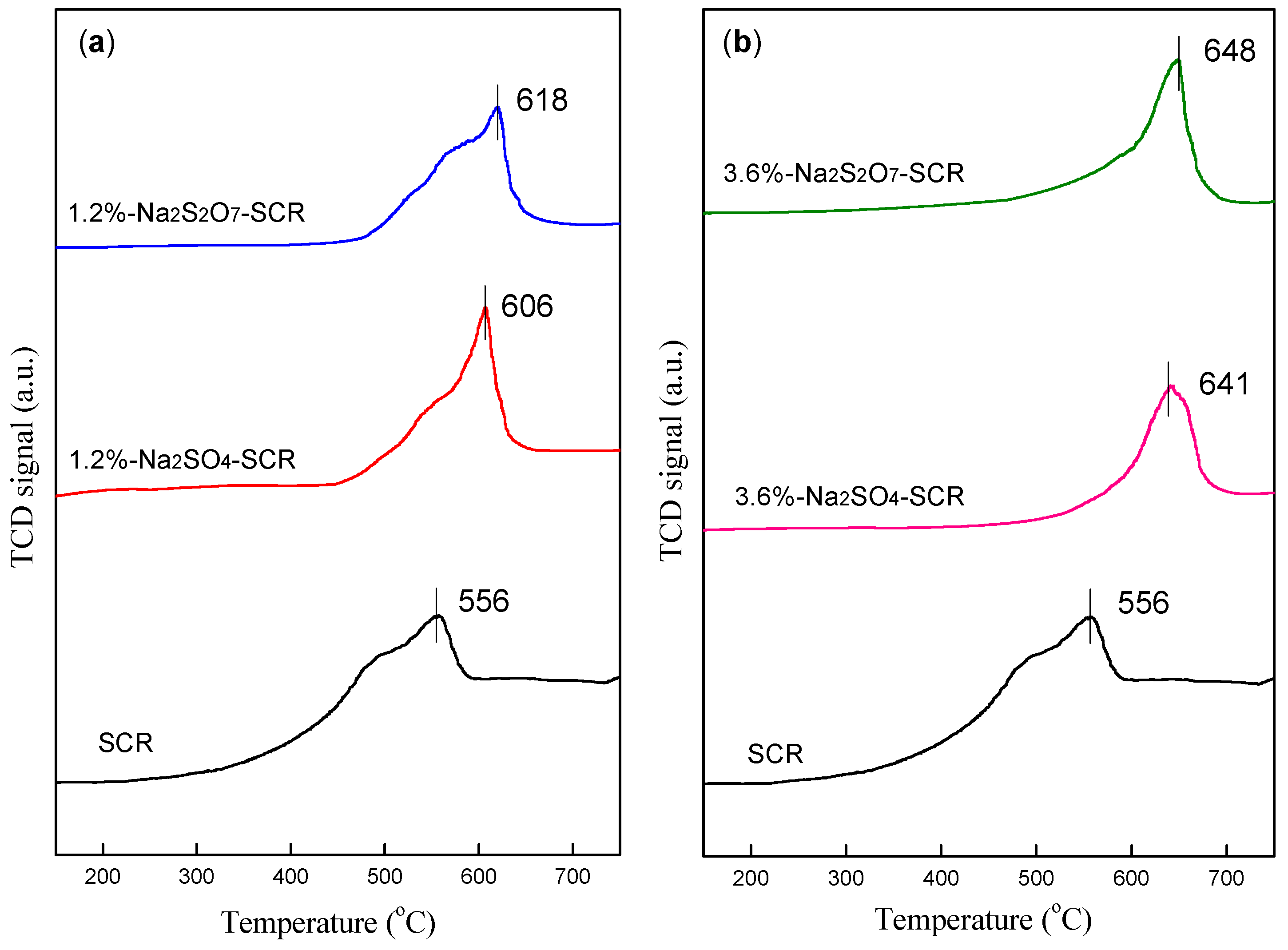
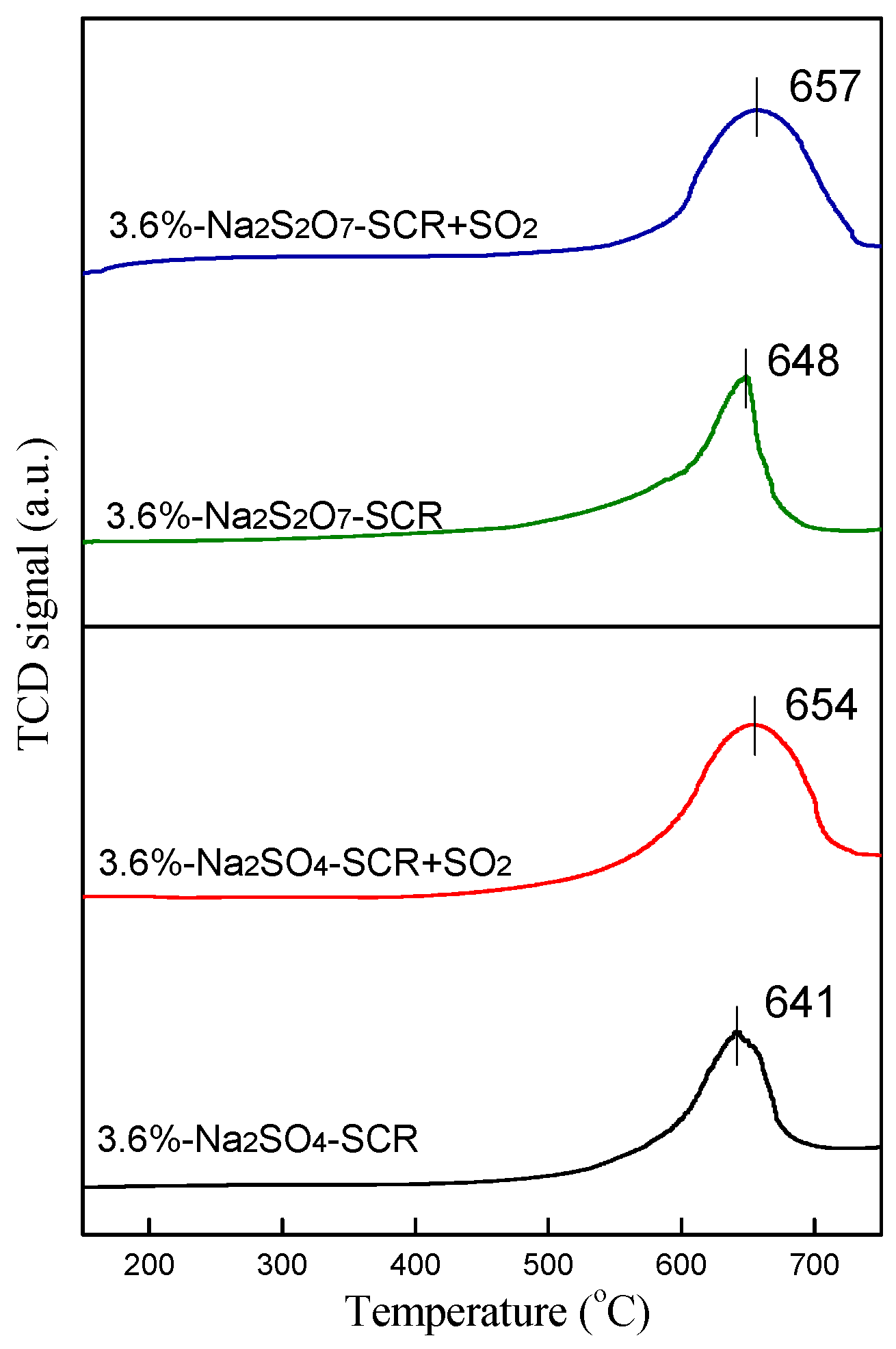
2.3.1. NH3-TPD and H2-TPR Analysis
2.3.2. XPS Analysis
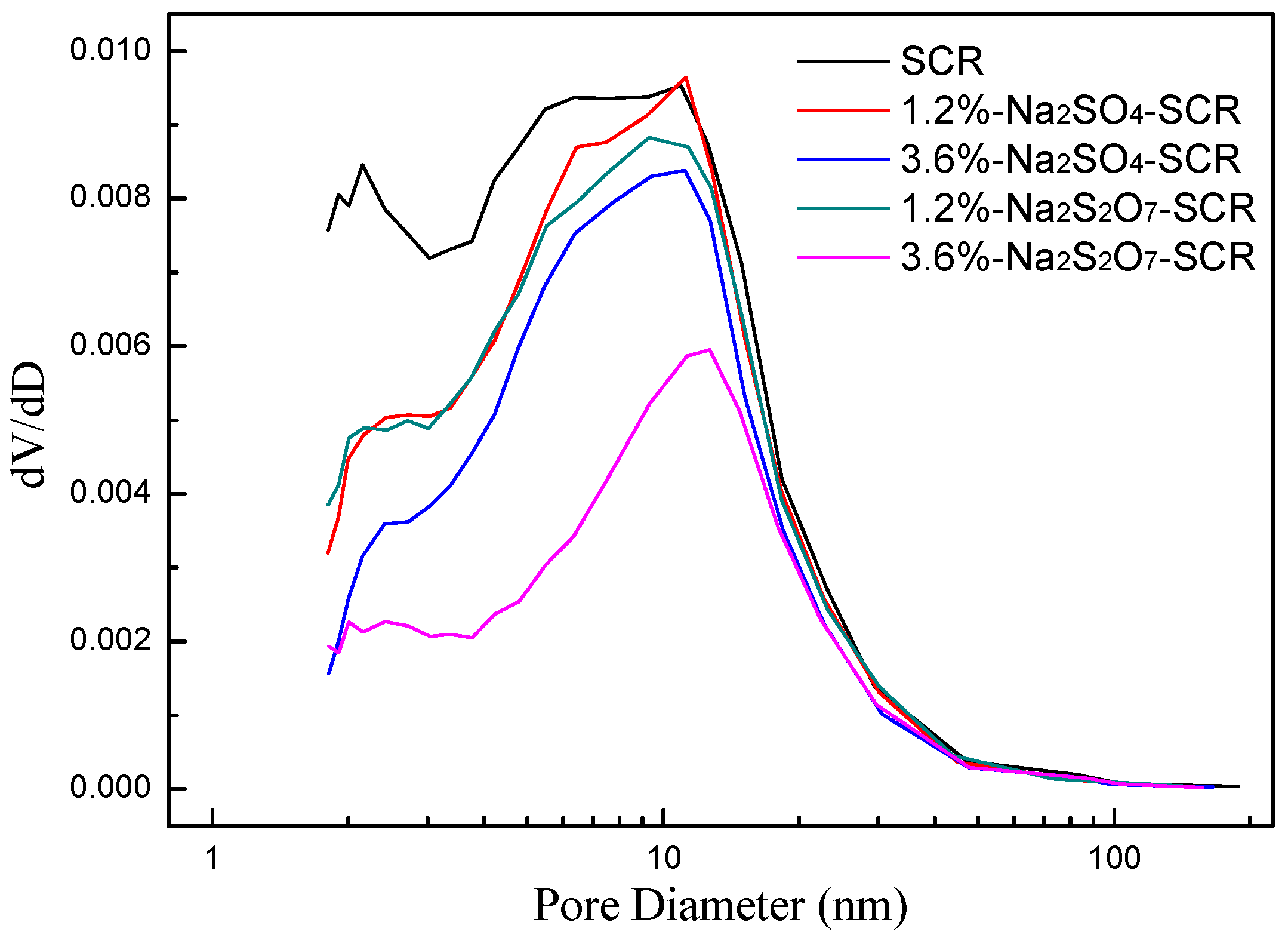
2.3.3. BET and XRD Analysis
2.3.4. FT-IR Analysis
3. Experimental
3.1. Sample Preparation
3.2. Catalyst Characterization
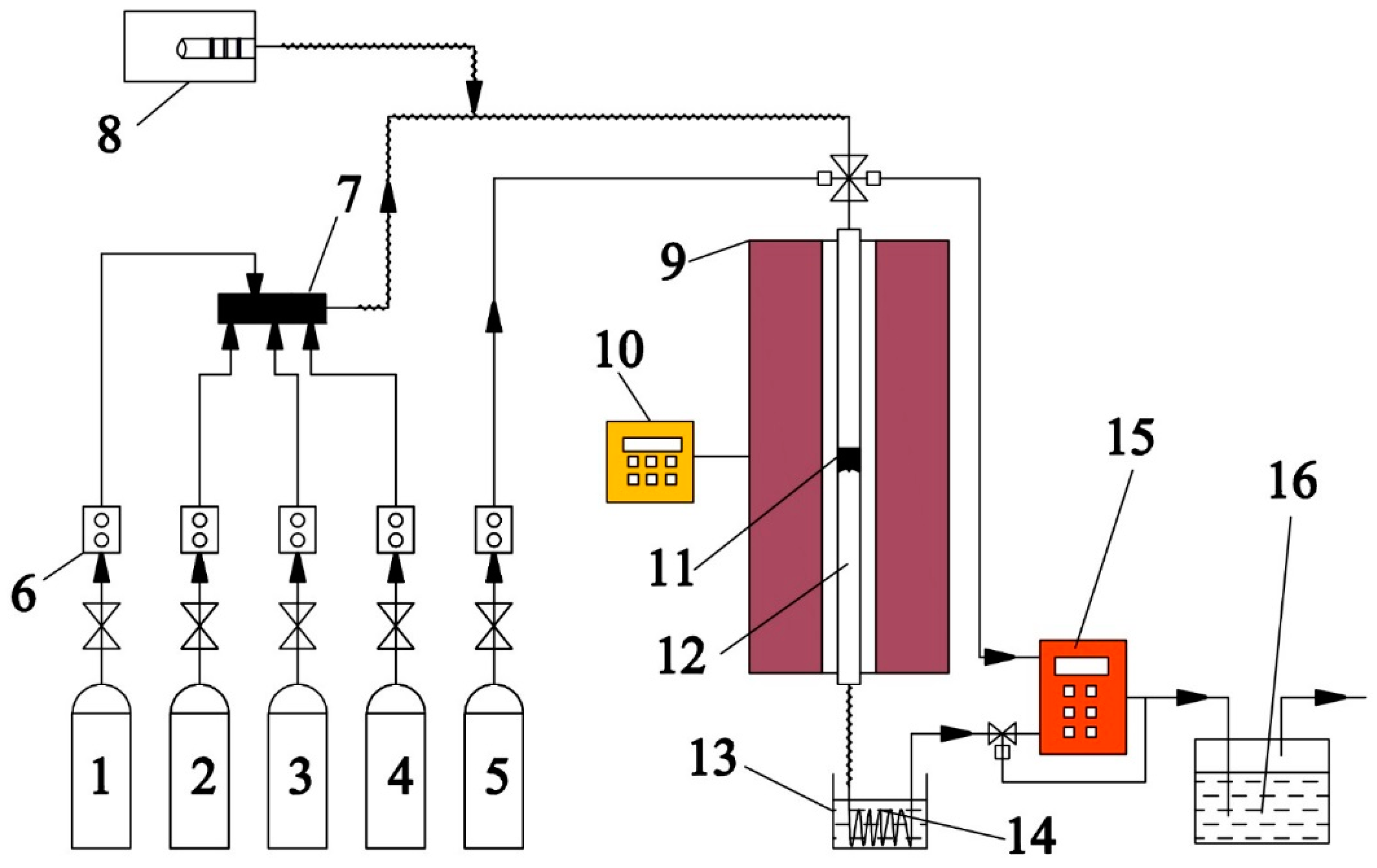
3.3. Test. Setup
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Qiu, Y.; He, S.; Liu, N.; Xiao, C.; Liu, L. Investigating the driving forces of NOx generation from energy consumption in China. J. Clean. Prod. 2018, 184, 836–846. [Google Scholar] [CrossRef]
- Yang, J.; Sun, R.; Sun, S.; Zhao, N.; Hao, N.; Chen, H.; Wang, Y.; Guo, H.; Meng, J. Experimental study on NOx reduction from staging combustion of high volatile pulverized coals. Part 1. Air staging. Fuel Process. Technol. 2014, 126, 266–275. [Google Scholar] [CrossRef]
- Kim, M.H.; Yang, K.H. The role of Fe2O3 species in depressing the formation of N2O in the selective reduction of NO by NH3 over V2O5/TiO2-based catalysts. Catalysts 2018, 8, 134. [Google Scholar] [CrossRef]
- Qi, X.; Song, G.; Song, W.; Lu, Q. Influence of sodium-based materials on the slagging characteristics of Zhundong coal. J. Energy Inst. 2017, 90, 914–922. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Zhao, D.; Cao, Q.; Gao, J.; Wu, S. Transformation mechanism of sodium during pyrolysis of Zhundong coal. Fuel 2018, 233, 29–36. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Hu, W.; Zhu, X.; Qu, W.; Wu, W.; Zheng, C.; Gao, X. New insight into alkali resistance and low temperature activation on vanadia-titania catalysts for selective catalytic reduction of NO. Appl. Surf. Sci. 2019, 466, 99–109. [Google Scholar] [CrossRef]
- Lisi, L.; Lasorella, G.; Malloggi, S.; Russo, G. Single and combined deactivating effect of alkali metals and HCl on commercial SCR catalysts. Appl. Catal. B Environ. 2004, 50, 251–258. [Google Scholar] [CrossRef]
- Lei, T.; Li, Q.; Chen, S.; Liu, Z.; Liu, Q. KCl-induced deactivation of V2O5–WO3/TiO2 catalyst during selective catalytic reduction of NO by NH3: Comparison of poisoning methods. Chem. Eng. J. 2016, 296, 1–10. [Google Scholar] [CrossRef]
- Klimczak, M.; Kern, P.; Heinzelmann, T.; Lucas, M.; Claus, P. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts—Part I: V2O5–WO3/TiO2 catalysts. Appl. Catal. B Environ. 2010, 95, 39–47. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Mnichowicz, B.; Harinath, A.V.; Li, H.; Bahrami, B. Chemical deactivation of commercial vanadium SCR catalysts in diesel emission control application. Chem. Eng. J. 2016, 287, 680–690. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Huang, X.; Li, X.; Su, W.; Sun, X.; Wang, D.; Hao, J. Deactivation Mechanism of Potassium on the V2O5/CeO2 Catalysts for SCR Reaction: Acidity, Reducibility and Adsorbed-NOx. Environ. Sci. Technol. 2014, 48, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, J.; Shi, W.; Xu, J.; Hao, J. Design strategies for development of SCR catalyst: Improvement of alkali poisoning resistance and novel regeneration method. Environ. Sci. Technol. 2012, 46, 12623–12629. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Shi, C.; Li, M.; Zhang, T.; Wang, C.; Jiang, L.; Wang, X. The effect of cations (NH4+, Na+, K+, and Ca2+) on chemical deactivation of commercial SCR catalyst by bromides. Chin. J. Catal. 2018, 39, 710–717. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.A.; Wang, P.; Du, Y.; Liu, X.; Chen, W.; Che, D. Ash deposition and alkali metal migration during Zhundong high-alkali coal gasification. Energy Procedia 2017, 105, 1350–1355. [Google Scholar] [CrossRef]
- Yang, S.; Song, G.; Na, Y.; Yang, Z. Alkali metal transformation and ash deposition performance of high alkali content Zhundong coal and its gasification fly ash under circulating fluidized bed combustion. Appl. Therm. Eng. 2018, 141, 29–41. [Google Scholar] [CrossRef]
- Niu, Y.; Gong, Y.; Zhang, X.; Liang, Y.; Wang, D.; Hui, S.E. Effects of leaching and additives on the ash fusion characteristics of high-Na/Ca Zhundong coal. J. Energy Inst. 2018. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.; Liu, Z.; Liu, Q. Combined effect of KCl and SO2 on the selective catalytic reduction of NO by NH3 over V2O5/TiO2 catalyst. Appl. Catal. B Environ. 2015, 164, 475–482. [Google Scholar] [CrossRef]
- Alvarez, E.; Blanco, J.; Avila, P.; Knapp, C. Activation of monolithic catalysts based on diatomaceous earth for sulfur dioxide oxidation. Catal. Today 1999, 53, 557–563. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, J.; Yang, C.; Lin, F.; Guang, H.; Kong, M.; Liu, Q. Comparative study of the poisoning effect of NaCl and Na2O on selective catalytic reduction of NO with NH3 over V2O5–WO3/TiO2 catalyst. J. Energy Inst. 2018, 1–8. [Google Scholar] [CrossRef]
- Hu, W.; Gao, X.; Deng, Y.; Qu, R.; Zheng, C.; Zhu, X.; Cen, K. Deactivation mechanism of arsenic and resistance effect of SO42− on commercial catalysts for selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2016, 293, 118–128. [Google Scholar] [CrossRef]
- Chen, J.P.; Buzanowski, M.A.; Yang, R.T.; Cichanowicz, J.E. Deactivation of the vanadia catalyst in the selective catalytic reduction process. J. Air Waste Manag. Assoc. 2012, 40, 1403–1409. [Google Scholar] [CrossRef]
- Dahlin, S.; Nilsson, M.; Bäckström, D.; Bergman, S.L.; Bengtsson, E.; Bernasek, S.L.; Pettersson, L.J. Multivariate analysis of the effect of biodiesel-derived contaminants on V2O5–WO3/TiO2 SCR catalysts. Appl. Catal. B Environ. 2016, 183, 377–385. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, R.; Mo, J.; Li, J.; Hao, J. The poisoning effects of calcium on V2O5–WO3/TiO2 catalyst for the SCR reaction: Comparison of different forms of calcium. Mol. Catal. 2017, 434, 16–24. [Google Scholar] [CrossRef]
- Nicosia, D.; Czekaj, I.; Kröcher, O. Chemical deactivation of V2O5/WO3–TiO2 SCR catalysts by additives and impurities from fuels, lubrication oils and urea solution. Part II. Characterization study of the effect of alkali and alkaline earth metals. Appl. Catal. B Environ 2008, 77, 228–236. [Google Scholar] [CrossRef]
- Dunn, J.P.; Koppula, P.R.; Stenger, H.G.; Wachs, I.E. Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts. Appl. Catal. B Environ. 1998, 19, 103–117. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Cao, Y.; Yao, X.; Ge, C.; Gao, F.; Deng, Y.; Tang, C.; Dong, L. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2015, 165, 589–598. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Liu, Q.; Guo, S.; Huang, Z.; Xiao, Y. SO2 and NO removal from flue gas over V2O5/AC at lower temperatures—Role of V2O5 on SO2 removal. Fuel Process. Technol. 2008, 89, 242–248. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Liang, D.; Zhou, M.; Li, F.; Li, R. Lewis and Brønsted acids in super-acid catalyst SO42−/ZrO2–SiO2. J. Mater. Sci. 2009, 44, 6736–6740. [Google Scholar] [CrossRef]
- Xiao, H.; Ru, Y.; Cheng, Q.; Zhai, G.; Dou, C.; Qi, C.; Chen, Y. Effect of sodium sulfate in ash on sulfur trioxide formation in the postflame region. Energy Fuels 2018, 32, 8668–8675. [Google Scholar] [CrossRef]
- Schwaemmle, T.; Heidel, B.; Brechtel, K.; Scheffknecht, G. Study of the effect of newly developed mercury oxidation catalysts on the DeNOx-activity and SO2–SO3-conversion. Fuel 2012, 101, 179–186. [Google Scholar] [CrossRef]
- Guo, X.; Bartholomew, C.; Hecker, W.; Baxter, L. Effects of sulfate species on V2O5/TiO2 SCR catalysts in coal and biomass-fired systems. Appl. Catal. B Environ. 2009, 92, 30–40. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Wang, H.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Anstrom, M.; Topsøe, N.; Dumesic, J.A. Density functional theory studies of mechanistic aspects of the SCR reaction on vanadium oxide catalysts. J. Catal. 2003, 213, 115–125. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, Z.; Liu, Z.; Liu, Q. Formation and reaction of ammonium sulfate salts on V2O5/AC catalyst during selective catalytic reduction of nitric oxide by ammonia at low temperatures. J. Catal. 2003, 214, 213–219. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Si, W.; Luo, J.; Dai, Q.; Luo, X.; Liu, X.; Hao, J. Insight into deactivation of commercial SCR catalyst by arsenic: An experiment and DFT study. Environ. Sci. Technol. 2014, 48, 13895–13900. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gao, X.; Qiu, K.; Luo, Z.; Cen, K. The Reaction of poisonous alkali oxides with vanadia SCR catalyst and the afterward influence: A DFT and experimental study. J. Phys. Chem. C 2015, 119, 1905–1912. [Google Scholar] [CrossRef]
- Zheng, Y.; Jensen, A.D.; Johnsson, J.E.; Thøgersen, J.R. Deactivation of V2O5–WO3/TiO2 SCR catalyst at biomass fired power plants: Elucidation of mechanisms by lab- and pilot-scale experiments. Appl. Catal. B Environ. 2008, 83, 186–194. [Google Scholar] [CrossRef]
- Giakoumelou, I.; Fountzoula, C.; Kordulis, C.; Boghosian, S. Molecular structure and catalytic activity of V2O5/TiO2 catalysts for the SCR of NO by NH3: In situ Raman spectra in the presence of O2, NH3, NO, H2, H2O, and SO2. J. Catal. 2006, 239, 1–12. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, Z.; Liu, Z. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures. Appl. Catal. B Environ. 2002, 39, 361–368. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. The poisoning effect of alkali metals doping over nano V2O5–WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Yu, Y.; Miao, J.; He, C.; Chen, J.; Li, C.; Douthwaite, M. The remarkable promotional effect of SO2 on Pb-poisoned V2O5–WO3/TiO2 catalysts: An in-depth experimental and theoretical study. Chem. Eng. J. 2018, 338, 191–201. [Google Scholar] [CrossRef]
- Gan, L.; Guo, F.; Yu, J.; Xu, G. Improved low-temperature activity of V2O5–WO3/TiO2 for denitration using different vanadium precursors. Catalysts 2016, 6, 25. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Liu, J.; Liu, H.; Guo, Y.; Sun, B. Plasma-assisted catalytic conversion of NO over Cu-Fe catalysts supported on ZSM-5 and carbon nanotubes at low temperature. Fuel Process. Technol. 2018, 178, 53–61. [Google Scholar] [CrossRef]
- Wan, Q.; Duan, L.; Li, J.; Chen, L.; He, K.; Hao, J. Deactivation performance and mechanism of alkali (earth) metals on V2O5–WO3/TiO2 catalyst for oxidation of gaseous elemental mercury in simulated coal-fired flue gas. Catal. Today 2011, 175, 189–195. [Google Scholar] [CrossRef]
- Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5-WO3/TiO2 catalyst synthesized from waste catalyst on selective catalytic reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef]
- Economidis, N.V.; Peña, D.A.; Smirniotis, P.G. Comparison of TiO2-based oxide catalysts for the selective catalytic reduction of NO: Effect of aging the vanadium precursor solution. Appl. Catal. B Environ. 1999, 23, 123–134. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, Y.; Qi, C.; Ru, Y. Effect of Na poisoning catalyst (V2O5–WO3/TiO2) on denitration process and SO3 formation. Appl. Surf. Sci. 2018, 433, 341–348. [Google Scholar] [CrossRef]
- Shpanchenko, R.V.; Chernaya, V.V.; Antipov, E.V.; Hadermann, J.; Kaul, E.E.; Geibel, C. Synthesis, structure and magnetic properties of the new mixed-valence vanadate Na2SrV3O9. J. Solid State Chem. 2003, 173, 244–250. [Google Scholar] [CrossRef]
- Lewandowska, A.E.; Calatayud, M.; Lozano-Diz, E.; Minot, C.; Bañares, M.A. Combining theoretical description with experimental in situ studies on the effect of alkali additives on the structure and reactivity of vanadium oxide supported catalysts. Catal. Today 2008, 139, 209–213. [Google Scholar] [CrossRef]
- Busca, G.; Saussey, H.; Saur, O.; Lavalley, J.C.; Lorenzelli, V. FT-IR characterization of the surface acidity of different titanium dioxide anatase preparations. Appl. Catal. 1985, 14, 245–260. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, H.; Xu, Q.; Zhang, Y.; Yang, L. FT-IR study of the SO2 oxidation behavior in the selective catalytic reduction of NO with NH3 over commercial catalysts. J. Fuel Chem. Technol. 2015, 43, 1018–1024. [Google Scholar] [CrossRef]



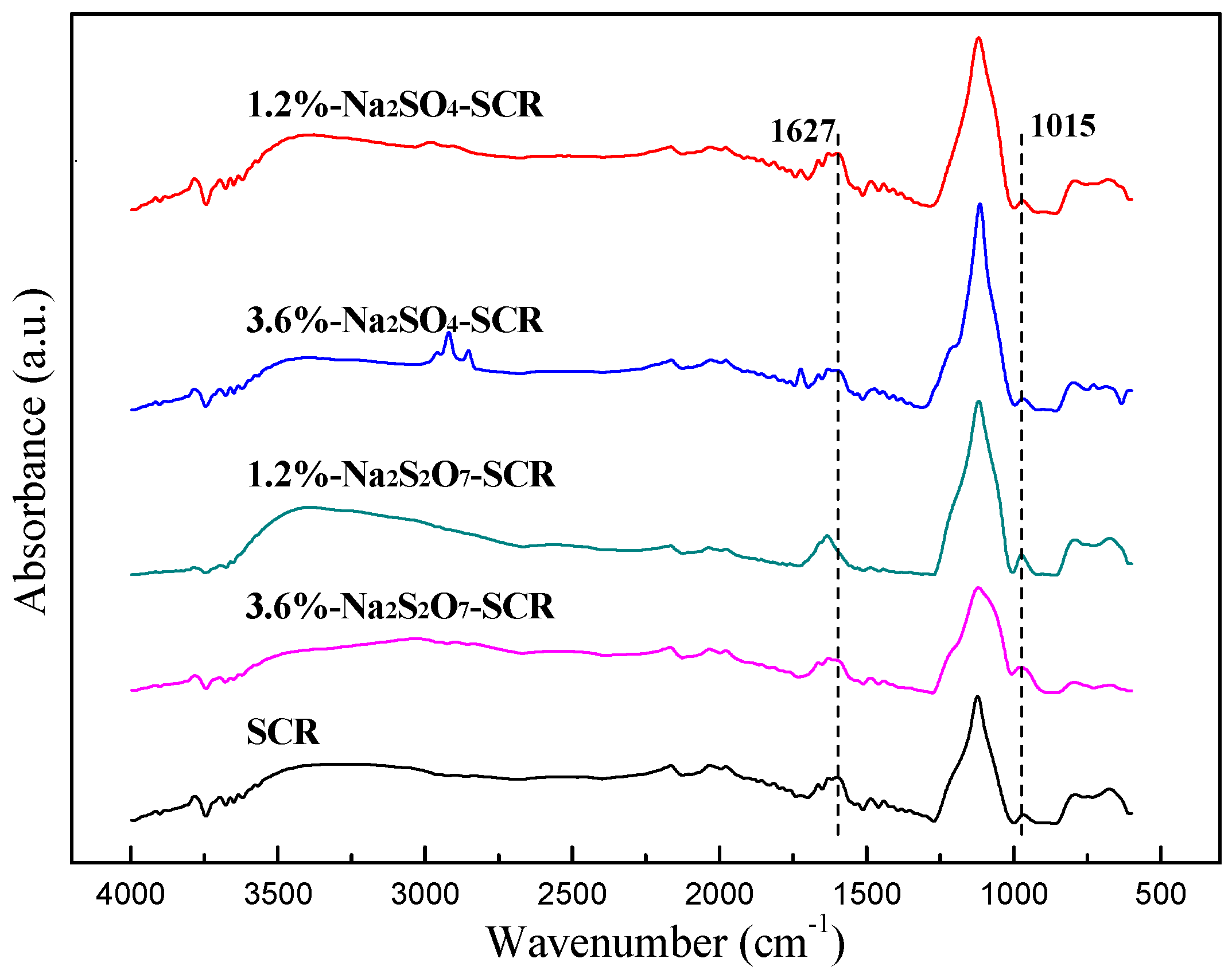
| Samples | Surface Atomic Concentration (%) | |||||
|---|---|---|---|---|---|---|
| O | Na | Ti | V | W | S | |
| Pure catalyst | 65.04 | 0.07 | 16.27 | 0.60 | 3.50 | 0.05 |
| 1.2%-Na2SO4-SCR | 60.24 | 4.42 | 14.32 | 0.40 | 2.61 | 1.13 |
| 3.6%-Na2SO4-SCR | 59.88 | 5.58 | 13.01 | 0.35 | 2.14 | 3.54 |
| 1.2%-Na2S2O7-SCR | 61.93 | 4.17 | 15.19 | 0.44 | 2.77 | 2.32 |
| 3.6%-Na2S2O7-SCR | 61.08 | 5.25 | 14.77 | 0.38 | 2.69 | 5.12 |
| Samples | Surface Atomic Concentration (%) | Surface Atomic Ratio | |||
|---|---|---|---|---|---|
| Oα/(Oα+Oβ) | Oβ/(Oα+Oβ) | V4+ | V5+ | V4+/V5+ | |
| Pure catalyst | 44.3 | 55.7 | 43.6 | 56.4 | 0.77 |
| 1.2%-Na2SO4-SCR | 51.0 | 49.0 | 41.4 | 58.6 | 0.71 |
| 3.6%-Na2SO4-SCR | 53.1 | 46.9 | 40.2 | 59.8 | 0.67 |
| 1.2%-Na2S2O7-SCR | 51.8 | 48.2 | 40.6 | 59.4 | 0.69 |
| 3.6%-Na2S2O7-SCR | 55.3 | 44.7 | 39.8 | 60.2 | 0.66 |
| Samples | SBET (m2·g−1) | Vtotal (cm3·g−1) | Dp (nm) |
|---|---|---|---|
| Pure catalyst | 95 | 0.21 | 9.3 |
| 1.2%-Na2SO4-SCR | 75 | 0.18 | 10.5 |
| 1.2%-Na2S2O7-SCR | 69 | 0.17 | 10.5 |
| 3.6%-Na2SO4-SCR | 63 | 0.16 | 11.3 |
| 3.6%-Na2S2O7-SCR | 44 | 0.12 | 11.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Dou, C.; Shi, H.; Ge, J.; Cai, L. Influence of Sulfur-Containing Sodium Salt Poisoned V2O5–WO3/TiO2 Catalysts on SO2–SO3 Conversion and NO Removal. Catalysts 2018, 8, 541. https://doi.org/10.3390/catal8110541
Xiao H, Dou C, Shi H, Ge J, Cai L. Influence of Sulfur-Containing Sodium Salt Poisoned V2O5–WO3/TiO2 Catalysts on SO2–SO3 Conversion and NO Removal. Catalysts. 2018; 8(11):541. https://doi.org/10.3390/catal8110541
Chicago/Turabian StyleXiao, Haiping, Chaozong Dou, Hao Shi, Jinlin Ge, and Li Cai. 2018. "Influence of Sulfur-Containing Sodium Salt Poisoned V2O5–WO3/TiO2 Catalysts on SO2–SO3 Conversion and NO Removal" Catalysts 8, no. 11: 541. https://doi.org/10.3390/catal8110541
APA StyleXiao, H., Dou, C., Shi, H., Ge, J., & Cai, L. (2018). Influence of Sulfur-Containing Sodium Salt Poisoned V2O5–WO3/TiO2 Catalysts on SO2–SO3 Conversion and NO Removal. Catalysts, 8(11), 541. https://doi.org/10.3390/catal8110541





