MPV Reduction of Furfural to Furfuryl Alcohol on Mg, Zr, Ti, Zr–Ti, and Mg–Ti Solids: Influence of Acid–Base Properties
Abstract
1. Introduction
2. Results and Discussion
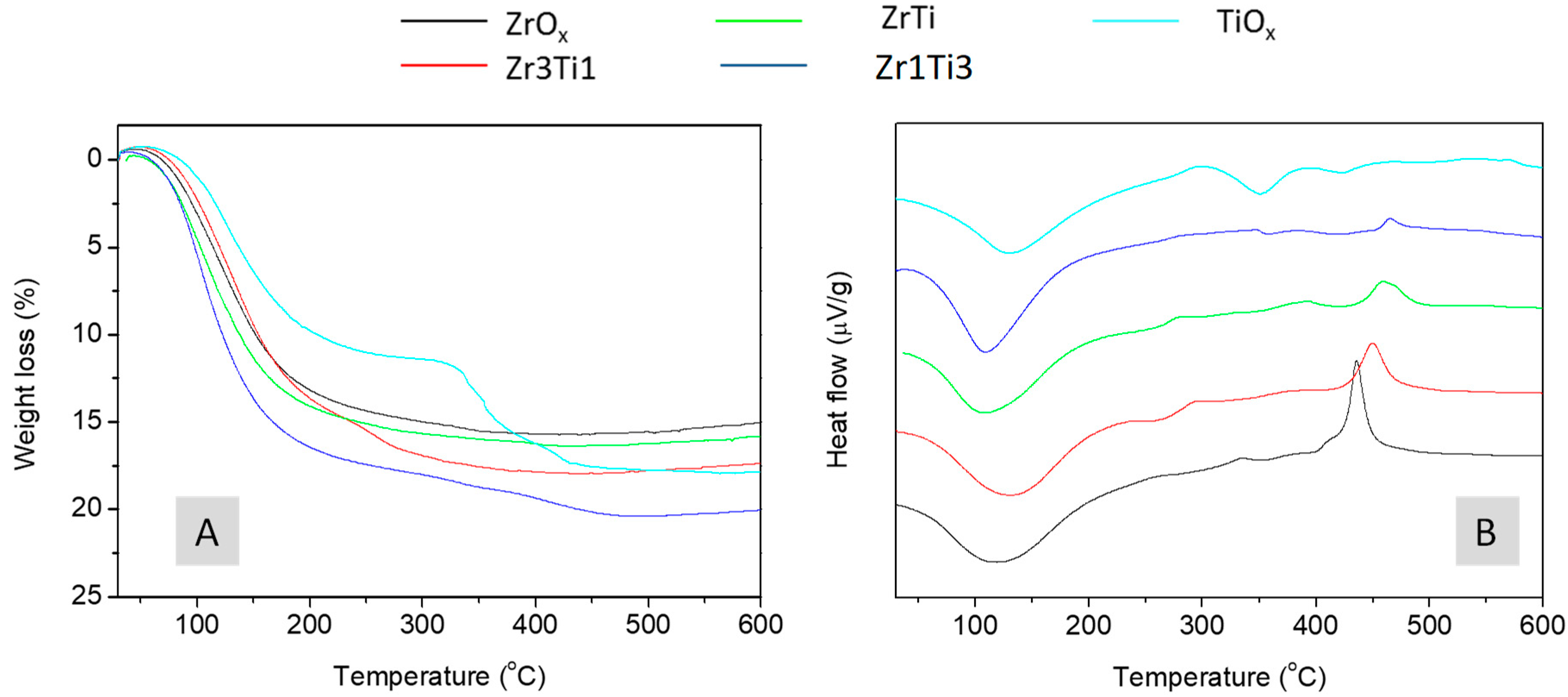
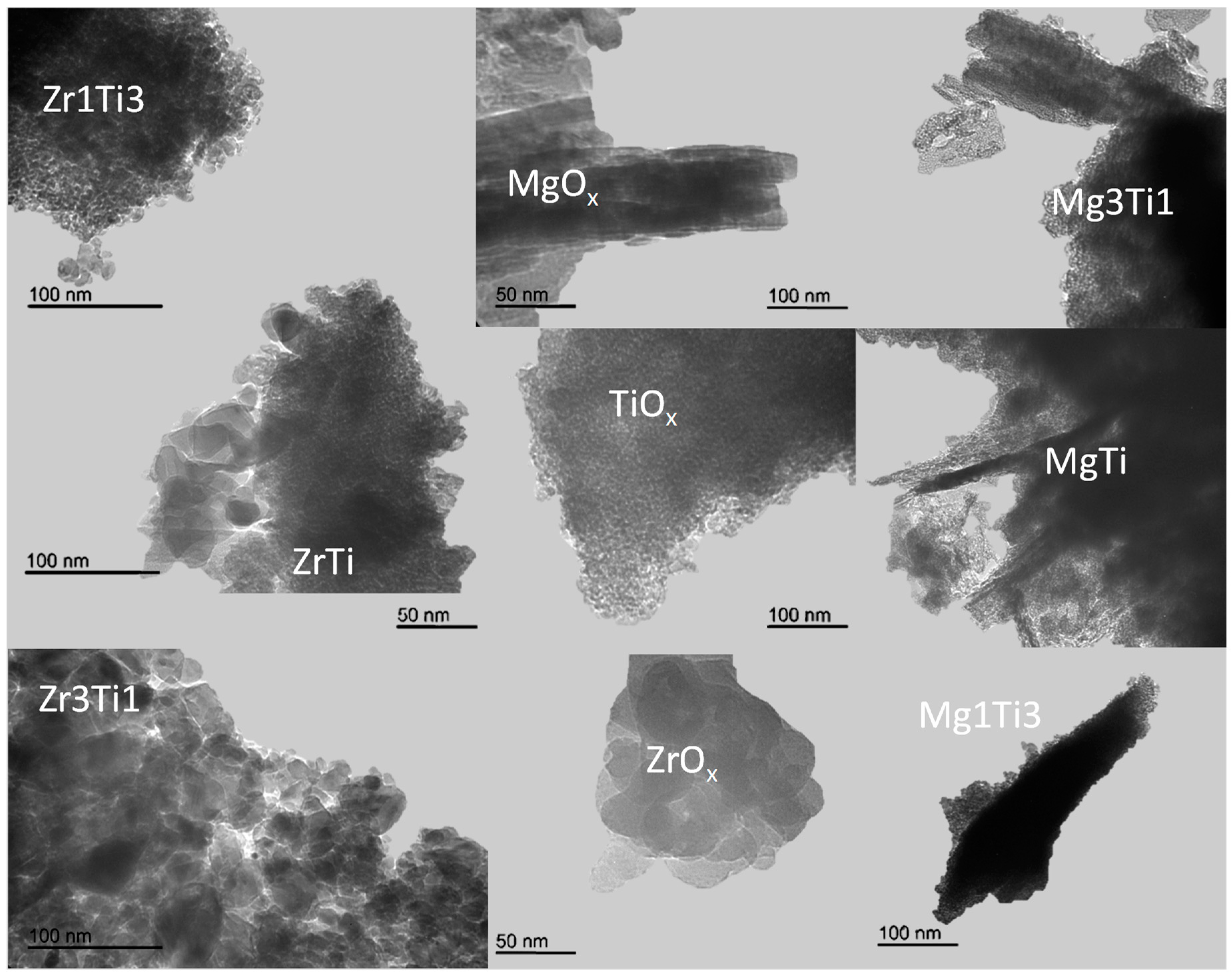
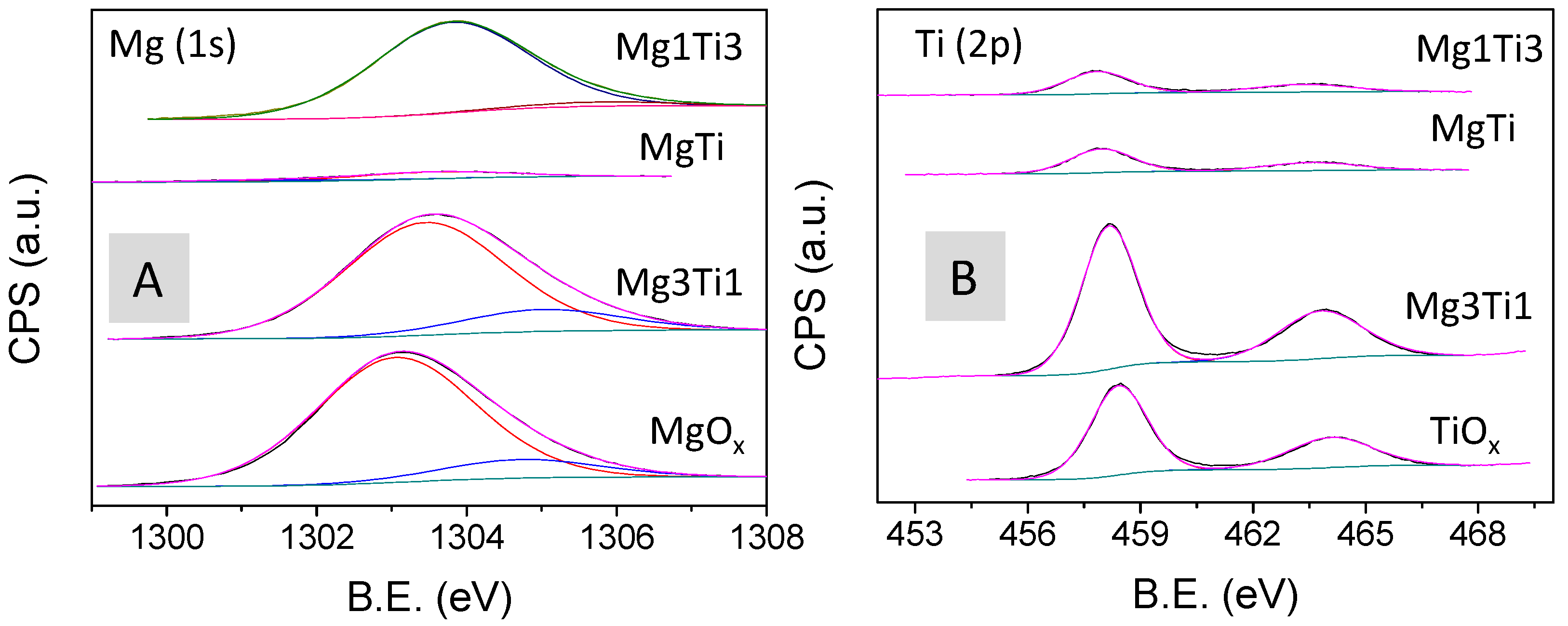
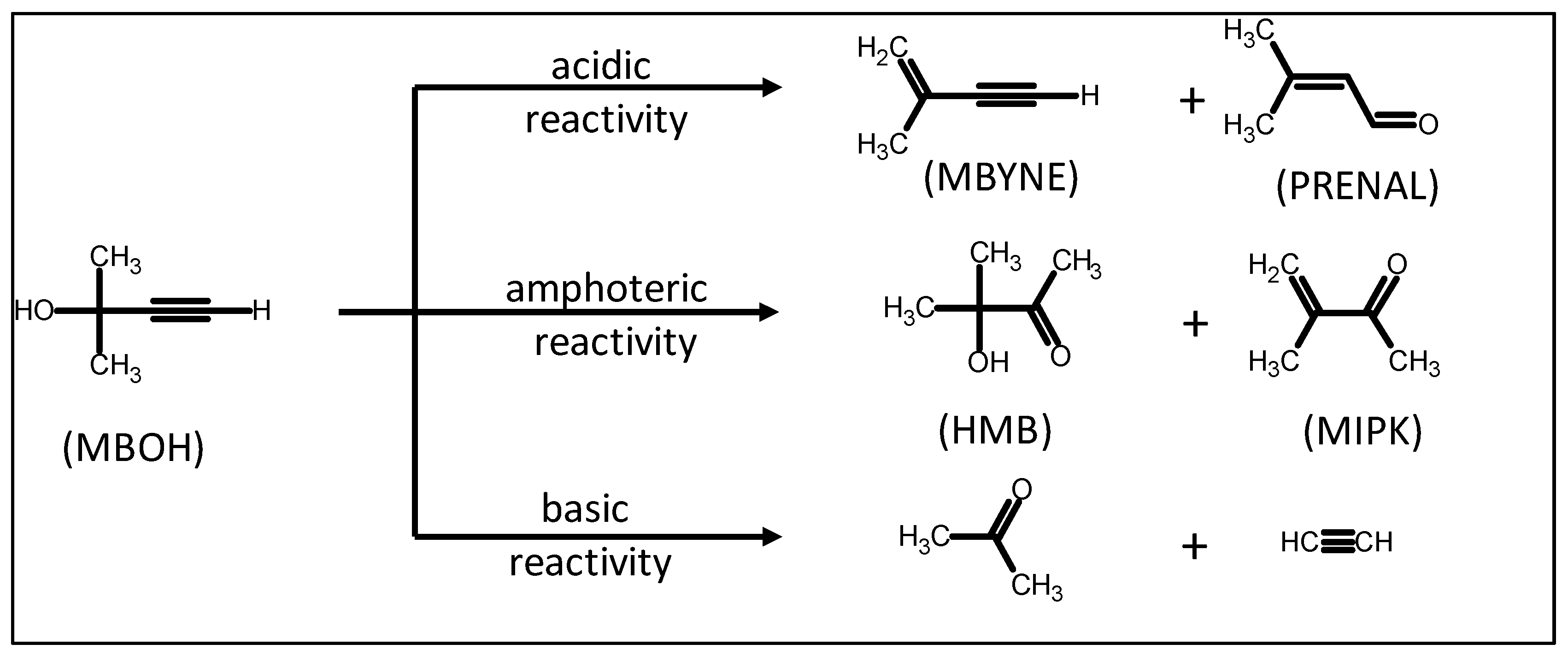
2.1. Textural, Structural, and Acid–Base Characterization of the Solids
2.2. Catalytic Activity in Furfural Hydrogenation into Furfuryl Alcohol
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Turhollow, A.; Perlack, R.; Eaton, L.; Langholtz, M.; Brandt, C.; Downing, M.; Wright, L.; Skog, K.; Hellwinckel, C.; Stokes, B.; et al. The updated billion-ton resource assessment. Biomass Bioenergy 2014, 70, 149–164. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Bauer, K.; Garbe, D. Common Fragrance and Flavour Materials. Preparation, Properties and Uses; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1985. [Google Scholar]
- Liu, H.; Huang, Z.; Zhao, F.; Cui, F.; Li, X.; Xia, C.; Chen, J. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2-and 1,5-pentanediols over a non-precious Cu-Mg3AlO4.5 bifunctional catalyst. Catal. Sci. Technol. 2016, 6, 668–671. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Zhu, W.; Xiao, G. Selective hydrogenation of furfuryl alcohol to tetrahydrofurfuryl alcohol over Ni/γ-Al2O3 catalysts. Res. Chem. Intermed. 2017, 43, 1179–1195. [Google Scholar] [CrossRef]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Thompson, S.T.; Lamb, H.H. Palladium–Rhenium Catalysts for Selective Hydrogenation of Furfural: Evidence for an Optimum Surface Composition. ACS Catal. 2016, 6, 7438–7447. [Google Scholar] [CrossRef]
- Meerwein, H.; Schmidt, R. Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen. Justus Liebigs Annalen der Chemie 1925, 444, 221–238. [Google Scholar] [CrossRef]
- Ooi, T.; Ichikawa, H.; Maruoka, K. Practical Approach to the Meerwein–Ponndorf–Verley Reduction of Carbonyl Substrates with New Aluminum Catalysts. Angew. Chem. 2001, 113, 3722–3724. [Google Scholar] [CrossRef]
- Ivanov, V.; Bachelier, J.; Audry, F.; Lavalley, J.C. Study of the Meerwein-Pondorff-Verley Reaction Between Ethanol and Acetone on Various Metal-Oxides. J. Mol. Catal. 1994, 91, 45–59. [Google Scholar] [CrossRef]
- Montes, V.; Miñambres, J.F.; Khalilov, A.N.; Boutonnet, M.; Marinas, J.M.; Urbano, F.J.; Maharramov, A.M.; Marinas, A. Chemoselective hydrogenation of furfural to furfuryl alcohol on ZrO2 systems synthesized through the microemulsion method. Catal. Today 2018, 306, 89–95. [Google Scholar] [CrossRef]
- Axpuac, S.; Aramendía, M.A.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Montes-Jiménez, V.; Urbano, F.J.; Borau, V. Study of structure-performance relationships in Meerwein-Ponndorf-Verley reduction of crotonaldehyde on several magnesium and zirconium-based systems. Catal. Today 2012, 187, 183–190. [Google Scholar] [CrossRef]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated reduction and acid-catalysed conversion of furfural in alcohol medium using Zr,Al-containing ordered micro/mesoporous silicates. Appl. Catal. B Environ 2016, 182, 485–503. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernandez, B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911–1927. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B. Dehydration of Xylose to Furfural in Alcohol Media in the Presence of Solid Acid Catalysts. ChemCatChem 2016, 8, 2089–2099. [Google Scholar] [CrossRef]
- Kim, M.S.; Simanjuntak, F.S.H.; Lim, S.; Jae, J.; Ha, J.-M.; Lee, H. Synthesis of alumina–carbon composite material for the catalytic conversion of furfural to furfuryl alcohol. J. Ind. Eng. Chem. 2017, 52, 59–65. [Google Scholar] [CrossRef]
- Lopez-Asensio, R.; Cecilia, J.A.; Jimenez-Gomez, C.P.; Garcia-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Selective production of furfuryl alcohol from furfural by catalytic transfer hydrogenation over commercial aluminas. Appl. Catal. A Gen. 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Stefanic, G.; Music, S.; Popovic, S.; Sekulic, A. FT-IR and laser Raman spectroscopic investigation of the formation and stability of low temperature t-ZrO2. J. Mol. Struct. 1997, 408–409, 391–394. [Google Scholar] [CrossRef]
- Formosa, J.; Chimenos, J.M.; Lacasta, A.M.; Haurie, L. Thermal study of low-grade magnesium hydroxide used as fire retardant and in passive fire protection. Thermochim. Acta 2011, 515, 43–50. [Google Scholar] [CrossRef]
- Lauron-Pernot, H.; Luck, F.; Popa, J.M. Methylbutynol: A new and simple diagnostic tool for acidic and basic sites of solids. Appl. Catal. 1991, 78, 213. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Halawy, S.A.; Mohamed, M.A.; Abdelkader, A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2012, 127, 307–315. [Google Scholar] [CrossRef]
- Lu, J.; Kosuda, K.M.; Van Duyne, R.P.; Stair, P.C. Surface Acidity and Properties of TiO2/SiO2 Catalysts Prepared by Atomic Layer Deposition: UV-visible Diffuse Reflectance, DRIFTS, and Visible Raman Spectroscopy Studies. J. Phys. Chem. C 2009, 113, 12412–12418. [Google Scholar] [CrossRef][Green Version]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Komanoya, T.; Nakajima, K.; Kitano, M.; Hara, M. Synergistic Catalysis by Lewis Acid and Base Sites on ZrO2 for Meerwein-Ponndorf-Verley Reduction. J. Phys. Chem. C 2015, 119, 26540–26546. [Google Scholar] [CrossRef]
- Guo, Z.-K.; Hong, Y.-C.; Xu, B.-Q. Transfer hydrogenation of cinnamaldehyde with 2-propanol on Al2O3 and SiO2-Al2O3 catalysts: Role of Lewis and Brönsted acidic sites. Catal. Sci. Technol. 2017, 7, 4511–4519. [Google Scholar] [CrossRef]
- Miñambres, J.F.; Aramendía, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Liquid and gas-phase Meerwein–Ponndorf–Verley reduction of crotonaldehyde on ZrO2 catalysts modified with Al2O3, Ga2O3 and In2O3. J. Mol. Catal. A Chem. 2011, 338, 121–129. [Google Scholar] [CrossRef]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol-gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef] [PubMed]
- Minambres, J.F.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Activity and deactivation of catalysts based on zirconium oxide modified with metal chlorides in the MPV reduction of crotonaldehyde. Appl. Catal. B Environ. 2013, 140, 386–395. [Google Scholar] [CrossRef]
- Minambres, J.F.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Chemoselective crotonaldehyde hydrogen transfer reduction over pure and supported metal nitrates. J. Catal. 2012, 295, 242–253. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Influence of the preparation method on the structural and surface properties of various magnesium oxides and their catalytic activity in the Meerwein-Ponndorf-Verley reaction. Appl. Catal. A Gen. 2003, 244, 207–215. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Garcia, I.M.; Jimenez, C.; Marinas, A.; Marinas, J.M.; Porras, A.; Urbano, F.J. Comparison of Different Organic Test Reaction over Acid-Base Catalysts. Appl. Catal. A Gen. 1999, 184, 115–125. [Google Scholar] [CrossRef]



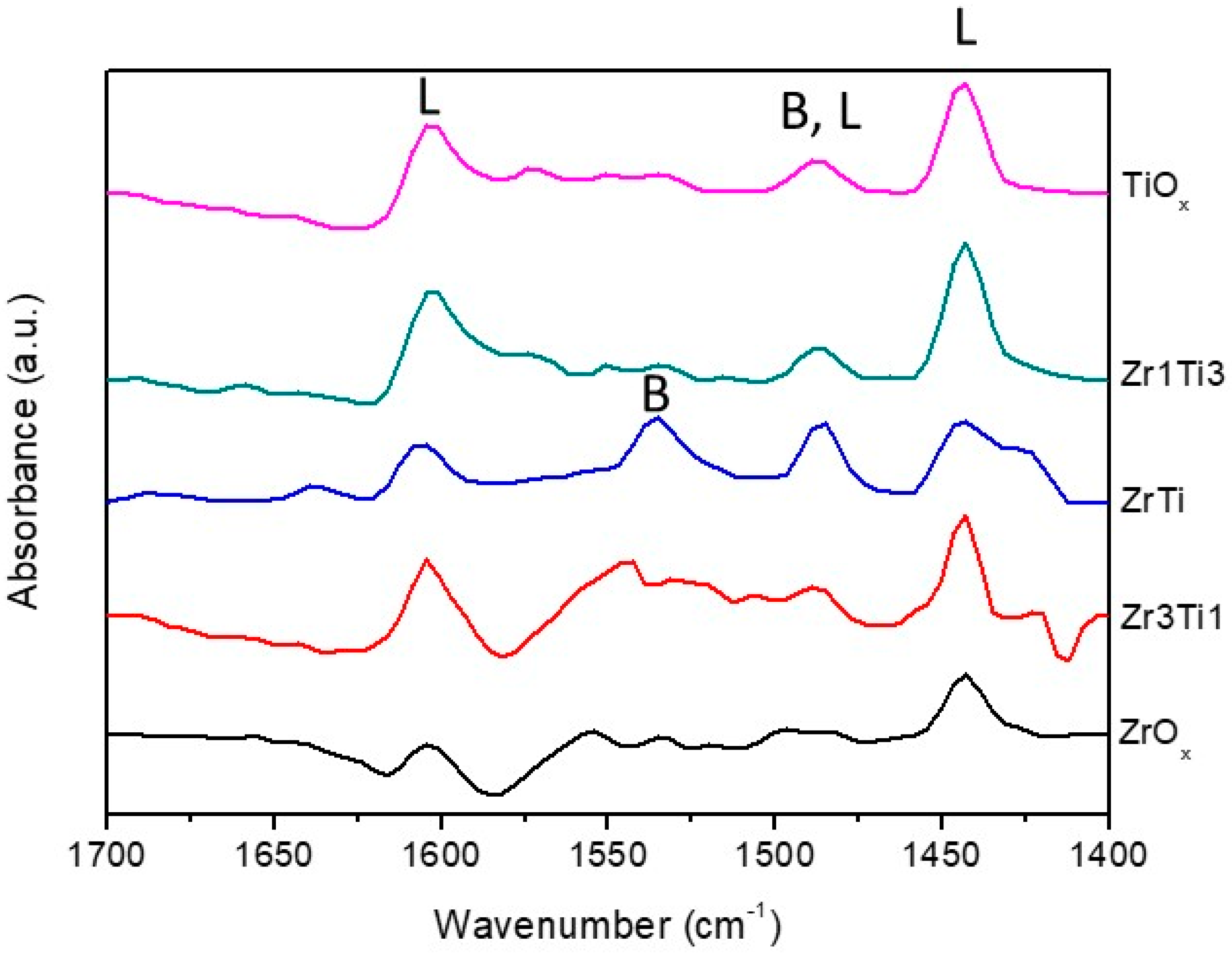
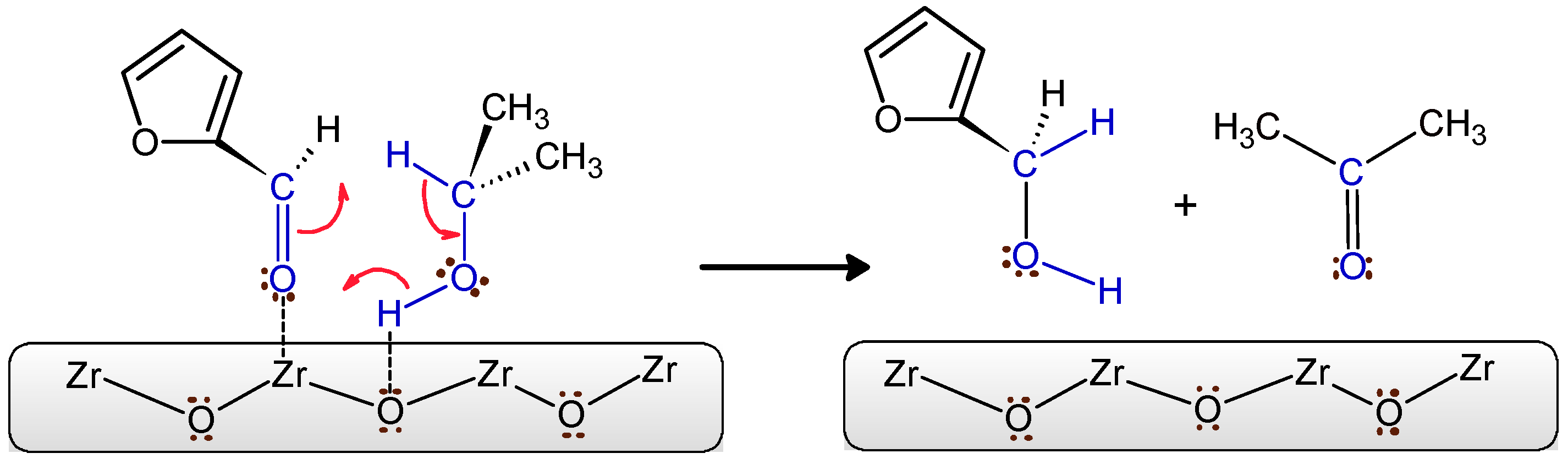
| Catalyst | BET Surface Area (m2/g) | M/Ti Ratio (M = Zr or Mg) | ||
|---|---|---|---|---|
| Nominal | ICP-MS | XPS | ||
| ZrOx | 221 | - | - | - |
| Zr3Ti1 | 251 | 3.0 | 2.43 | 2.29 |
| ZrTi | 263 | 1.0 | 0.78 | 1.03 |
| Zr1Ti3 | 219 | 0.33 | 0.34 | 0.45 |
| TiOx | 232 | - | - | - |
| Mg1Ti3 | 42 | 0.33 | 0.45 | 0.39 |
| MgTi | 81 | 1.0 | 1.06 | 0.62 |
| Mg3Ti1 | 68 | 3.0 | 3.02 | 3.5 |
| MgOx | 66 | - | - | - |
| Catalyst | µmol CO2/g | µmol Py/g | CO2/Py |
|---|---|---|---|
| ZrOx | 774 | 707 | 1.09 |
| Zr3Ti1 | 728 | 753 | 0.97 |
| ZrTi | 658 | 921 | 0.71 |
| Zr1Ti3 | 460 | 635 | 0.72 |
| TiOx | 371 | 650 | 0.57 |
| Mg1Ti3 | 508 | 622 | 0.82 |
| MgTi | 1126 | 616 | 1.83 |
| Mg3Ti1 | 1142 | 354 | 3.22 |
| MgOx | 1096 | 323 | 3.39 |
| Catalyst | Conversion (%) | Sbasic (%) | Sacid (%) | Samphoteric (%) |
|---|---|---|---|---|
| MgOx | 5.6 | 96.2 | 3.8 | 0 |
| TiOx | 1.0 | 12.3 | 73.1 | 14.6 |
| ZrOx | 1.0 | 26.3 | 20.3 | 53.4 |
| Catalyst | Conventional Heating | Microwave Heating | ||||
|---|---|---|---|---|---|---|
| Conversion (%) | Selectivity FUOL (%) | Yield FUOL (%) | Conversion (%) | Selectivity FUOL (%) | Yield FUOL (%) | |
| ZrOx | 50.1 | 90.4 | 45.3 | 27.6 | 96.8 | 26.7 |
| Zr3Ti1 | 42.9 | 88.2 | 37.8 | 19.8 | 97.9 | 19.4 |
| ZrTi | 30.2 | 79.0 | 23.9 | 20.9 | 75.2 | 15.7 |
| Zr1Ti3 | 22.3 | 80.5 | 18.0 | 17.1 | 79.5 | 13.6 |
| TiOx | 16.2 | 68.7 | 11.1 | 7.4 | 53.5 | 4.0 |
| Mg1Ti3 | 11.6 | 35.4 | 4.1 | 7.2 | 31.5 | 2.3 |
| MgTi | 13.4 | 46.6 | 6.3 | 8.6 | 47.8 | 3.1 |
| Mg3Ti1 | 15.8 | 48.0 | 7.6 | 7.4 | 46.0 | 3.4 |
| MgOx | 15.2 | 56.0 | 8.5 | 7.9 | 57.8 | 4.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Carrillo, J.; Parejas, A.; Cuesta-Rioboo, M.J.; Marinas, A.; Urbano, F.J. MPV Reduction of Furfural to Furfuryl Alcohol on Mg, Zr, Ti, Zr–Ti, and Mg–Ti Solids: Influence of Acid–Base Properties. Catalysts 2018, 8, 539. https://doi.org/10.3390/catal8110539
Hidalgo-Carrillo J, Parejas A, Cuesta-Rioboo MJ, Marinas A, Urbano FJ. MPV Reduction of Furfural to Furfuryl Alcohol on Mg, Zr, Ti, Zr–Ti, and Mg–Ti Solids: Influence of Acid–Base Properties. Catalysts. 2018; 8(11):539. https://doi.org/10.3390/catal8110539
Chicago/Turabian StyleHidalgo-Carrillo, Jesús, Almudena Parejas, Manuel Jorge Cuesta-Rioboo, Alberto Marinas, and Francisco José Urbano. 2018. "MPV Reduction of Furfural to Furfuryl Alcohol on Mg, Zr, Ti, Zr–Ti, and Mg–Ti Solids: Influence of Acid–Base Properties" Catalysts 8, no. 11: 539. https://doi.org/10.3390/catal8110539
APA StyleHidalgo-Carrillo, J., Parejas, A., Cuesta-Rioboo, M. J., Marinas, A., & Urbano, F. J. (2018). MPV Reduction of Furfural to Furfuryl Alcohol on Mg, Zr, Ti, Zr–Ti, and Mg–Ti Solids: Influence of Acid–Base Properties. Catalysts, 8(11), 539. https://doi.org/10.3390/catal8110539








