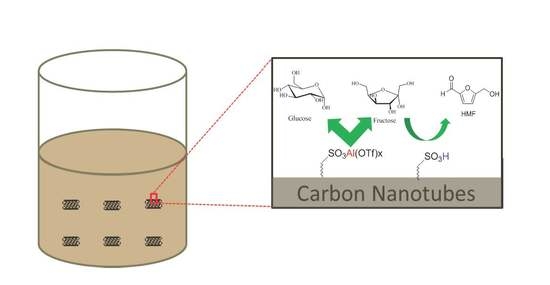
Pyrolyzing Renewable Sugar and Taurine on the Surface of Multi-Walled Carbon Nanotubes as Heterogeneous Catalysts for Hydroxymethylfurfural Production
Abstract
1. Introduction
2. Results and Discussion
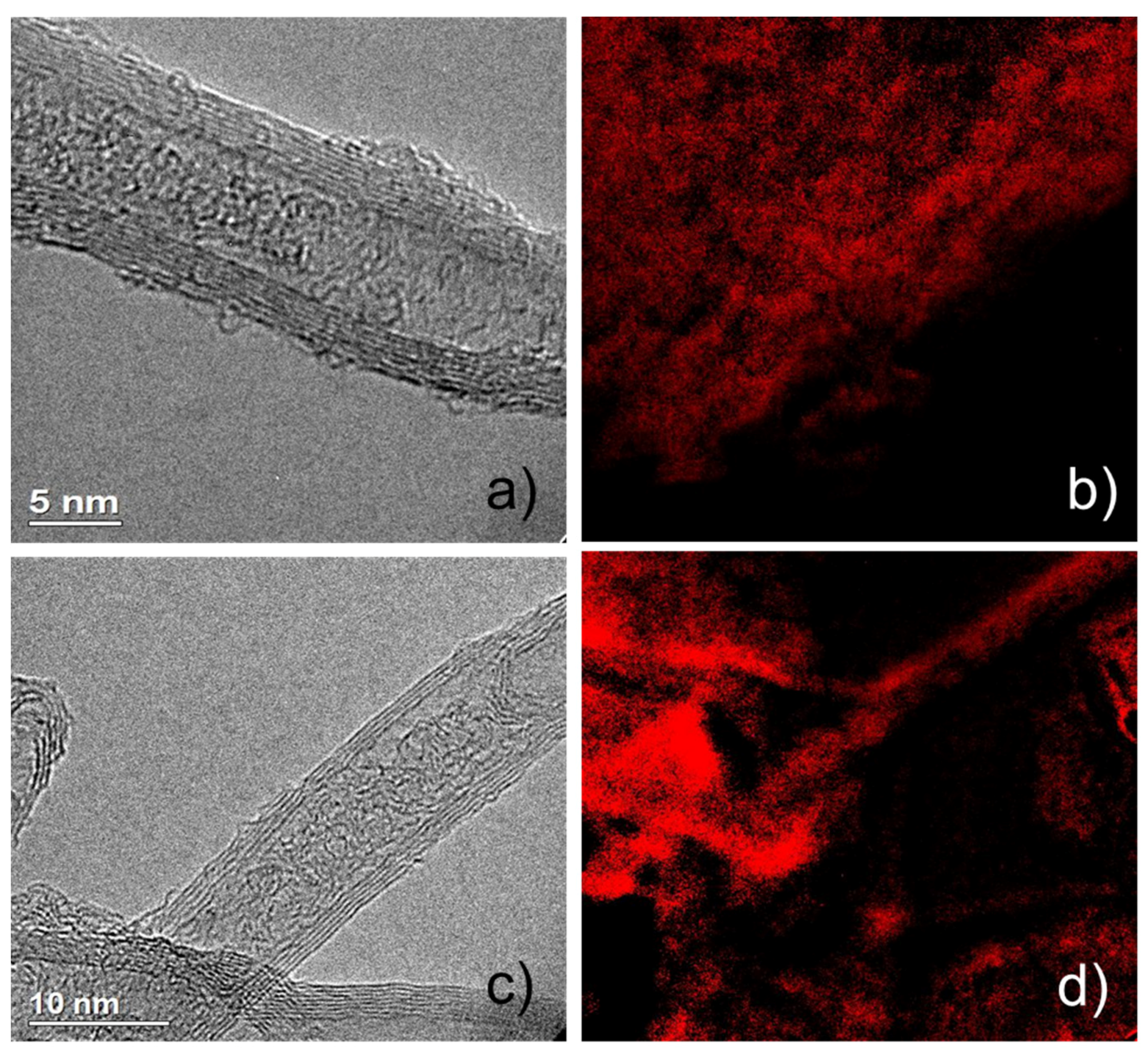
2.1. Catalyst Characterizations
2.2. Solid-State NMR
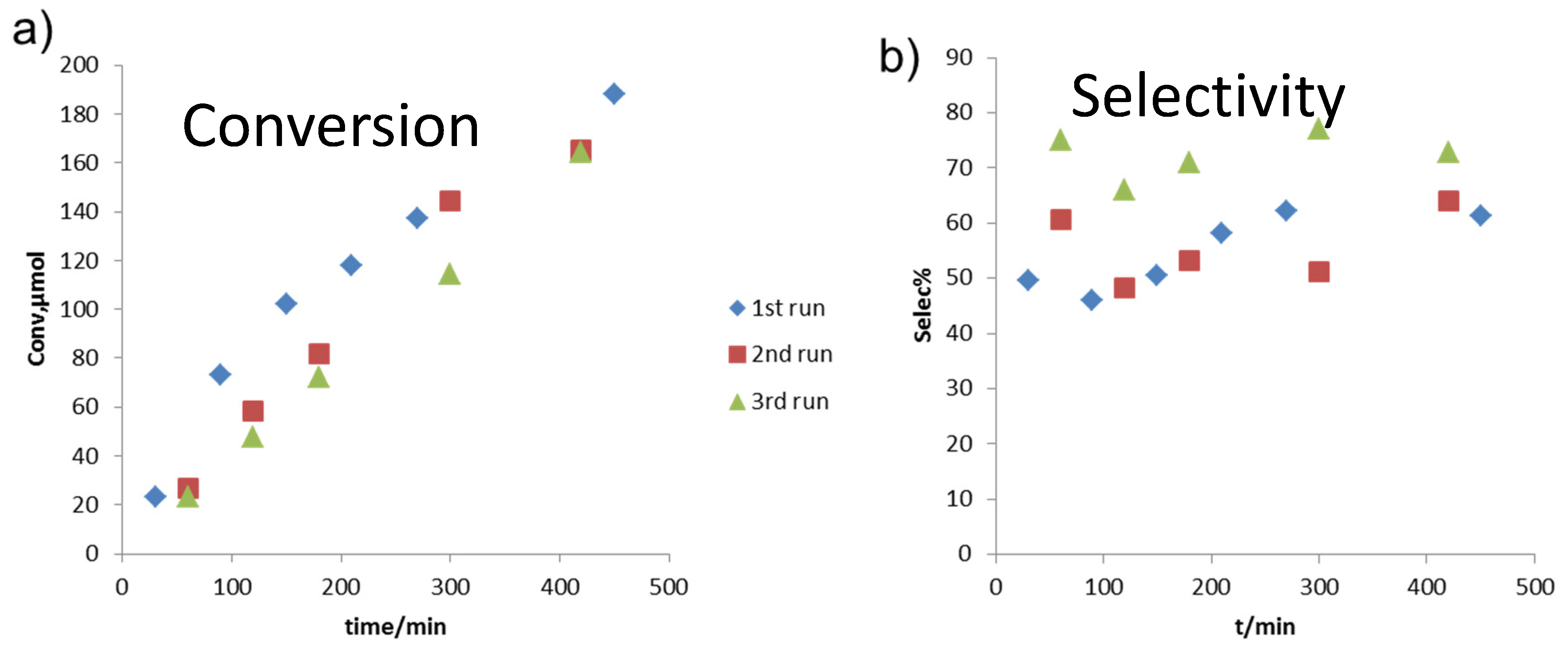
2.3. Reaction Testing
3. Experimental
3.1. Catalyst Synthesis
3.2. Materials Characterization
3.3. Solid-State NMR
3.4. Reaction Testing
3.5. Inductively Coupled Plasma Mass Spectrometer (ICP-MS) Analysis of Al Leaching
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Nikolau, B.J.; Perera, M.A.D.; Brachova, L.; Shanks, B. Platform biochemicals for a biorenewable chemical industry. Plant J. 2008, 54, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Shanks, B.H. Conversion of Biorenewable Feedstocks: New Challenges in Heterogeneous Catalysis. Ind. Eng. Chem. Res. 2010, 49, 10212–10217. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Schüβler, F.; D’Amico, A.; Danilina, N.; van Bokhoven, J.A.; Lercher, J.A.; Jones, C.W.; Sievers, C. Stability of Zeolites in Hot Liquid Water. J. Phys. Chem. C 2010, 114, 19582–19595. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Villa, A.; Majoulet, O.; Su, D.S.; Schlögl, R. Defect-Mediated Functionalization of Carbon Nanotubes as a Route to Design Single-Site Basic Heterogeneous Catalysts for Biomass Conversion. Angew. Chem. Int. Ed. 2009, 48, 6543–6546. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, A.; Dhepe, P.L. Sustainable green catalysis by supported metal nanoparticles. Chem. Rec. 2009, 9, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Serp, P.; Castillejos, E. Catalysis in Carbon Nanotubes. ChemCatChem 2010, 2, 41–47. [Google Scholar] [CrossRef]
- Anderson, J.M.; Johnson, R.L.; Schmidt-Rohr, K.; Shanks, B.H. Solid state NMR study of chemical structure and hydrothermal deactivation of moderate-temperature carbon materials with acidic SO3H sites. Carbon 2014, 74, 333–345. [Google Scholar] [CrossRef]
- Anderson, J.M.; Johnson, R.L.; Schmidt-Rohr, K.; Shanks, B.H. Hydrothermal degradation of model sulfonic acid compounds: Probing the relative sulfur-carbon bond strength in water. Catal. Commun. 2014, 51, 33–36. [Google Scholar] [CrossRef]
- Johnson, R.L.; Anderson, J.M.; Shanks, B.H.; Schmidt-Rohr, K. Simple One-Step Synthesis of Aromatic-Rich Materials with High Concentrations of Hydrothermally Stable Catalytic Sites, Validated by NMR. Chem. Mater. 2014, 26, 5523–5532. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, L.; Zhao, S.; Wang, X.; Wang, S. High selective production of 5-hydroymethylfurfural from fructose by a solid heteropolyacid catalyst. Fuel 2011, 90, 2289–2293. [Google Scholar] [CrossRef]
- Nikolla, E.; Roman-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, T.; Shanks, B.H.; Datye, A.K. Tuning the Location of Niobia/Carbon Composites in a Biphasic Reaction: Dehydration of D-Glucose to 5-Hydroxymethylfurfural. Catal. Lett. 2013, 143, 509–516. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Tao, Y.; Sun, W.; Wang, W. Preparation of 5-hydroxymethylfurfural from glucose catalyzed by silica-supported phosphotungstic acid heterogeneous catalyst. Fuel 2018, 226, 417–422. [Google Scholar] [CrossRef]
- Zuo, M.; Le, K.; Li, Z.; Jiang, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Green process for production of 5-hydroxymethylfurfural from carbohydrates with high purity in deep eutectic solvents. Ind. Crops Prod. 2017, 99, 1–6. [Google Scholar] [CrossRef]
- De Souza, R.L.; Yu, H.; Rataboul, F.; Essayem, N. 5-Hydroxymethylfurfural (5-HMF) Production from Hexoses: Limits of Heterogeneous Catalysis in Hydrothermal Conditions and Potential of Concentrated Aqueous Organic Acids as Reactive Solvent System. Challenges 2012, 3, 212–232. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-W.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Xie, H.; Liu, W.; Zhao, Z.K. Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Germanium(IV) Chloride in Ionic Liquids. ChemSusChem 2010, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pagan-Torres, Y.J.; Combs, E.J.; Dumesic, J.A.; Shanks, B.H. Water-Compatible Lewis Acid-Catalyzed Conversion of Carbohydrates to 5-Hydroxymethylfurfural in a Biphasic Solvent System. Top. Catal. 2012, 55, 657–662. [Google Scholar] [CrossRef]
- Johnson, R.L.; Schmidt-Rohr, K. Quantitative solid-state 13C NMR with signal enhancement by multiple cross polarization. J. Magn. Reson. 2014, 239, 44–49. [Google Scholar] [CrossRef] [PubMed]





| Sample Code | Isotopic Labeling | Heating Conditions |
|---|---|---|
| GT250/10-CNT-GC | Uniform 13C: glucose | Pyrolysis temperature of 250 °C Ramp of 1 °C from room temp to 120 °C,10 °C from 120 °C to 250 °C |
| GT250/1-GC | Uniform 13C: glucose | Pyrolysis temperature of 250 °C Ramp of 1 °C throughout the temperature range |
| GT250/10-CNT-GC/TN | Uniform 13C: glucose Uniform 15N: taurine | Pyrolysis temperature of 250 °C Ramp of 1 °C from r.t. to 120 °C,10 °C from 120 °C to 250 °C |
| GT250/1-GC/TN | Uniform 13C: glucose Uniform 15N: taurine | Pyrolysis temperature of 250 °C Ramp of 1 °C throughout the temperature range |
| GT250/10-CNT-TC | Uniform 13C: taurine | Pyrolysis temperature of 250 °C Ramp of 1 °C from r.t. to 120 °C,10 °C from 120 °C to 250 °C |
| GT250/1-TC | Uniform 13C: taurine | Pyrolysis temperature of 250 °C Ramp of 1 °C throughout the temperature range |
| GT200/1-CNT-TC | Uniform 13C: taurine | Pyrolysis temperature of 200 °C Ramp of 1 °C throughout the temperature range |
| GT250/1-CNT-TC | Uniform 13C: taurine | Pyrolysis temperature of 250 °C Ramp of 1 °C throughout the temperature range |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Fu, J.; Wang, T. Pyrolyzing Renewable Sugar and Taurine on the Surface of Multi-Walled Carbon Nanotubes as Heterogeneous Catalysts for Hydroxymethylfurfural Production. Catalysts 2018, 8, 517. https://doi.org/10.3390/catal8110517
Ji H, Fu J, Wang T. Pyrolyzing Renewable Sugar and Taurine on the Surface of Multi-Walled Carbon Nanotubes as Heterogeneous Catalysts for Hydroxymethylfurfural Production. Catalysts. 2018; 8(11):517. https://doi.org/10.3390/catal8110517
Chicago/Turabian StyleJi, Huiping, Jie Fu, and Tianfu Wang. 2018. "Pyrolyzing Renewable Sugar and Taurine on the Surface of Multi-Walled Carbon Nanotubes as Heterogeneous Catalysts for Hydroxymethylfurfural Production" Catalysts 8, no. 11: 517. https://doi.org/10.3390/catal8110517
APA StyleJi, H., Fu, J., & Wang, T. (2018). Pyrolyzing Renewable Sugar and Taurine on the Surface of Multi-Walled Carbon Nanotubes as Heterogeneous Catalysts for Hydroxymethylfurfural Production. Catalysts, 8(11), 517. https://doi.org/10.3390/catal8110517