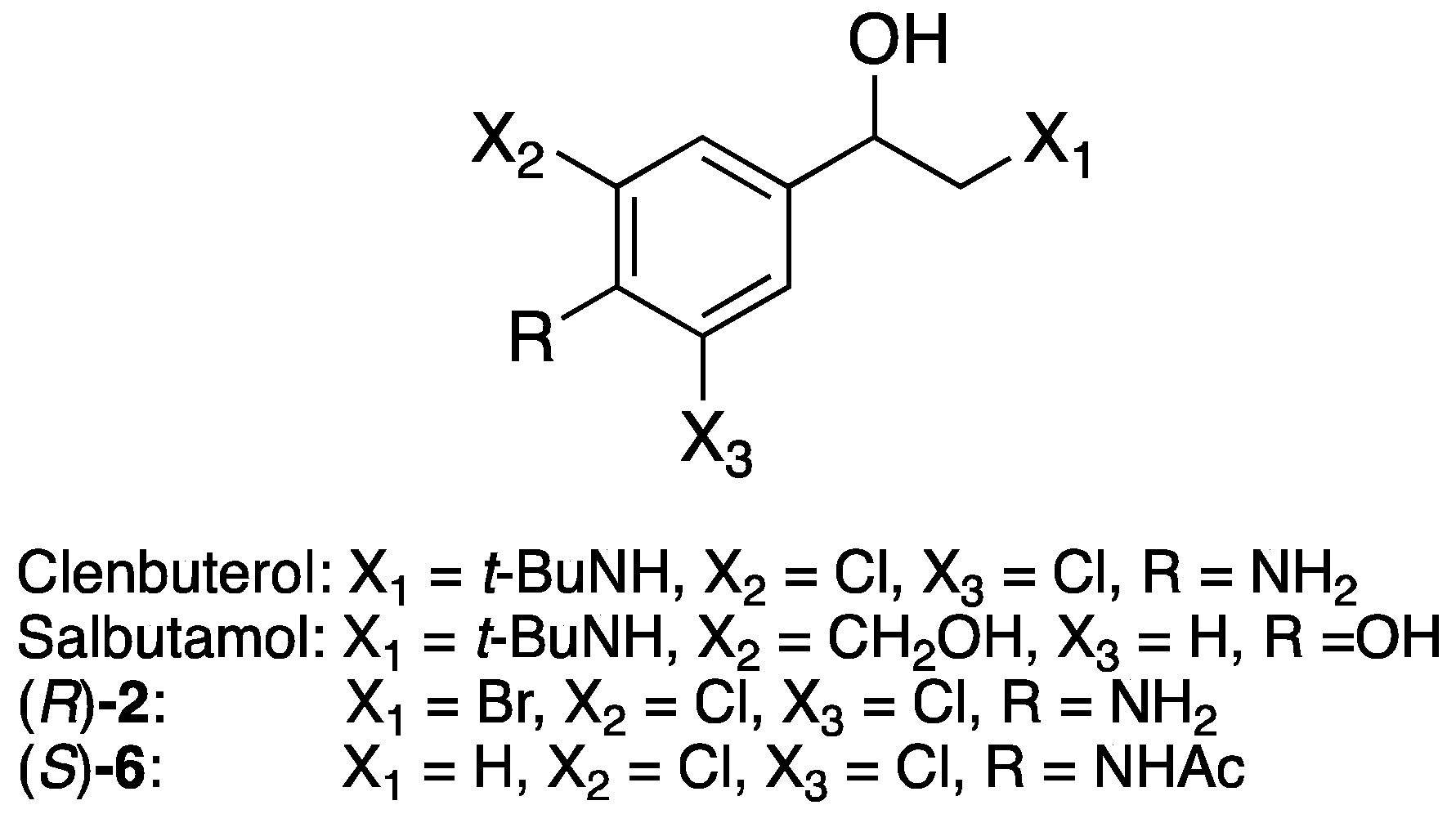
Analytical-grade chemicals and solvents were purchased from Sigma-Aldrich (Oslo, Norway). HPLC-grade solvents were purchased from Sigma-Aldrich. Dry solvents (THF and CH2Cl2) were acquired from a MBraun MB-SPS-800 solvent purification system (MBraun, München, Germany). Candida antarctica lipase A (activity 1612 U/g; lot no. BCBR2259V), lipase B (activity 1800 U/g, lot no BCBP3525V) immobilised on Immobead 150, recombinant from Aspergillus oryzae and glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides (G6P-DH, ≥550 U/mg, lot no. 107K7700V) were also purchased from Sigma-Aldrich. Crystalline KRED 228 (331.9 U/g, lot no. 20160509) was from Syncozymes Co., Ltd., (Shanghai, China). Flash chromatography was performed with silica gel from Sigma-Aldrich (pore size 60 Å, 230–400 mesh, 40–63 m particle size). Ketones 1, 4, 7 and clenbuterol were bought from Sigma-Aldrich (Oslo, Norway).
The enzyme-catalysed kinetic resolutions were performed in a New Brunswick G24 Environmental Incubator Shaker (New Brunswick Co. Inc., Edison, NJ, USA) at 30 °C and 200 or 300 rpm. Optical rotations ([α]D) were determined at 20 °C using a Perkin-Elmer 343 instrument (Perkin Elmer, Waltham, MA, USA) concentrations are given in g/100 mL. NMR spectra were recorded with a Bruker Avance DPX 400 instrument (Bruker, Rheinstetten, Germany) operating at 400 MHz for 1H and 100 MHz for 13C, respectively. Chemical shifts are in ppm rel. to tetramethylsilane (TMS) and coupling constants are in Hertz (Hz). High-resolution MS was performed on a WatersSynapt G2-S Q-TOF instrument (Waters MS Technologies, Manchester, UK) with WatersTM Software (Masslynx V4.1 SCN871, (Waters MS Technologies, Manchester, UK). Sample ionization was performed with an ASAP probe (APCI).
3.1. Achiral Chromatographic Analyses
Achiral GLC analyses were performed on an Agilent 7890A gas chromatograph, with an Agilent 7890B autosampler, a split injector (280 °C), a 4 mm ID tap GW liner (Agilent Technologies, Santa Clara, CA, USA), a flame ionisation detector (FID, 280 °C). All analyses were performed on a Restek Rtx®-1701 column (Crossbond® 14% cyanopropylphenyl–86% dimethylpolysiloxane, 30 m × 0.32 mm ID, df 0.25 μm, (Restek Corporation, Bellefonte, PA, USA), carrier gas He 5.0, temp. prog.: 100–280 °C/10 °C min−1.
3.2. Chiral Analyses
3.2.1. Chiral GLC-Analyses
The chiral GLC analyses of 3 and 6a were performed on an Agilent 7890B gas chromatograph (Agilent Technologies, Palo Alto, CA, USA), with an Agilent 7890B autosampler, an Agilent G4513A–7693A autoinjector, a split injector (225–230 °C) and a flame ionization detector (FID, 275 °C). The enantiomers of alcohol 3 were separated on a CP-Chirasil-Dex CB column (24.3 × 0.25 mm ID, df 0.25 μm) from Agilent technologies (Santa Clara, CA, USA) with He 5.0 as the carrier gas, flow 81 min−1, split flow 75 mL min−1 (25:1), temp. prog.: 100–150 °C/10 °C min−1, 150–160 °C/3 °C min−1, 160–170 °C/1 °C min−1. Retention times: tR (R)-3 = 25.363 min, tR (S)-3, RS = 1.82.
Separation of enantiomers of 6a was performed on the same system: flow 75 mL min−1, split flow 1:30, temp. prog.: 100–160 °C/10 °C min−1, 160–170 °C/1 °C min−1, 170–185 °C/0.5 °C min−1. Retention times tR (S)-6a = 44.467 min, tR (R)-6a = 45.255 min, RS = 2.05.
The chiral GLC analyses of 8 were- performed on a Varian 3380 gas chromatograph (Varian Inc., Mississauga, ON, USA), with a Varian CP-8410 autosampler, a split injector (200 °C), and a flame ionization detector (FID, 200 °C). The enantiomers of alcohol 8 and butanoic ester 9 were separated on a CP-Chirasil-Dex CB column (25 × 0.25 mm ID, df 0.25 μm) from Agilent technologies. Carrier gas H2 5.0, gas pressure 8 psi, split flow 60 mL/min, temp prog 100–140 °C/10 °C min−1 (5), 140–180 °C/10 °C min−1 (7). Retention times tR (S)-8 = 12.690 min, tR (R)-8 = 12.901 min, RS = 3.5, tR (S)-9 = 13.162 min, tR (R)-9 = 14.359 min, RS > 1.5.
3.2.2. Chiral HPLC Analyses
HPLC analyses were performed on an Agilent HPLC 1100 system (Nacalai tesque, Japan) with a manual injector (Rheodyne 77245i/Agilent 20 μL loop), and a variable wavelength detector (VWD) set to 254 nm. Alcohol 5a was separated on a Chiralcel OD-H column (250 mm L × 4.6 mm ID, 5 μm particle size, Daicel, Chiral Technologies Europe, Illkirch-Graffenstaden, France). Eluent: 7% propan-2-ol/93% n-hexane; 1 mL min−1, 1.5 μL injected. Retention times: tR (R)-5a = 84.474 min, tR (S)-5a = 91.765 min, RS = 1.56.
Alcohol 7a was separated on the same column, eluent: 17% propan-2-ol/83% n-hexane; 1 mL min−1, 2 μL injected. Retention times: tR (R)-7a = 14.037 min, tR (S)-7a = 17.035 min, RS = 2.55.
3.2.3. Capillary Zone Electrophoresis
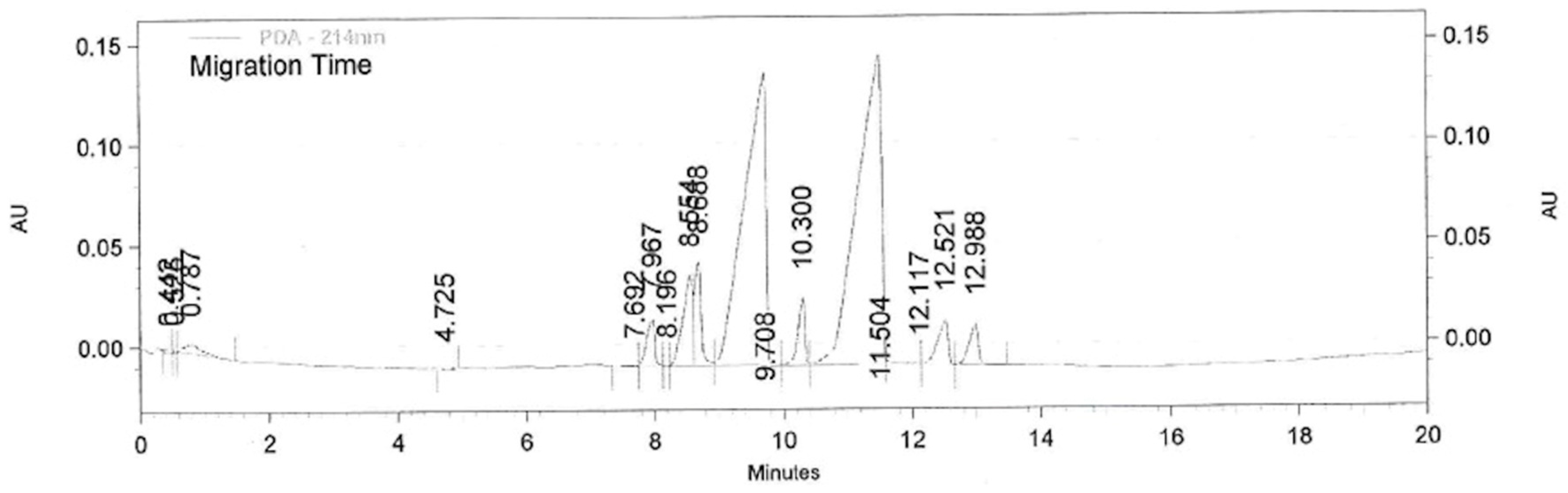
The chiral CZE analyses of clenbuterol and the corresponding butanoate were performed on a Beckman P/ACE MDQ capillary electrophoresis system (Beckman Coulter, Inc., Brea, CA, USA) in a fused silica capillary (31 cm × 50 μm, 21 cm to the detection window) with a diode array detector (DAD, 214 nm). Running buffer: pH 2.5 phosphate buffer (0.100 M), chiral selector: highly sulfonated β-cyclodextrin (5% w/v). Injection mode: hydrodynamic injection (0.5 psi for 5 s), separations voltage: −10 kV. Migration times: tM (R)-clenbuterol 9.708 min, tM (S)-clenbuterol at 11.504 min, tM butanoates: tM (R)-ester 12.521 min, tM (S)-ester 12.988 min.
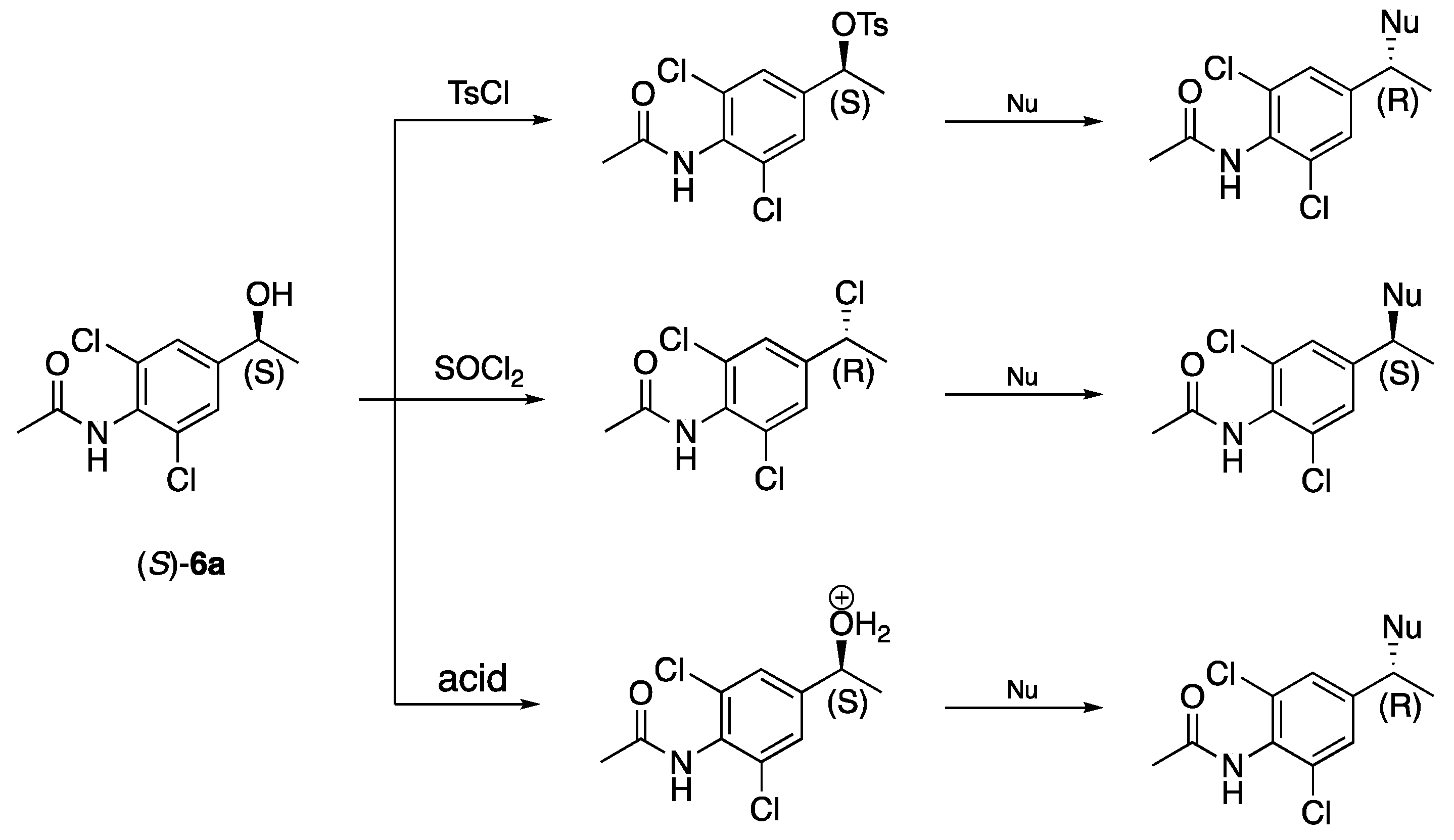
3.4. Absolute Configurations
The absolute configurations of (
R)-
2a, (
S)-
6a, and (
R)-
9 were determined by comparing the elution orders of the enantiomers with GLC elution orders of similar enantiopure compounds synthesised from (
S)-epichlorohydrin. Elution orders on Daicel Chiralcel OD-H of the faster reacting enantiomers were the same [
18,
24,
25]. Optical rotation values of (
R)-
2a, (
S)-
5, (
S)-
6a and (
R)-
7a have not been previously reported.
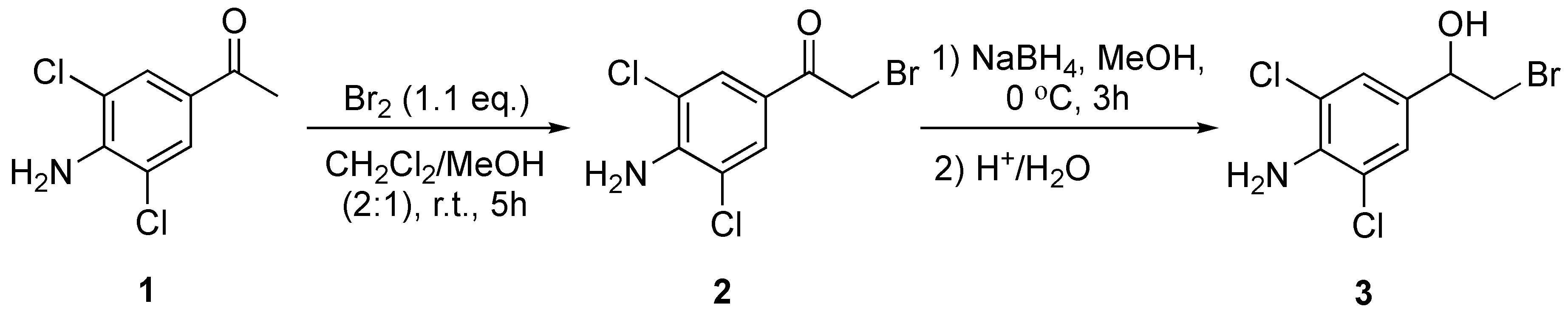
3.4.1. Synthesis of 1-(4-Amino-3,5-dichlorophenyl)-2-bromoethan-1-one (2)
To a solution of 1-(4-amino-3,5-dichlorophenyl)ethan-1-one (1) (1.10 g, 5.39 mmol) dissolved in CH2Cl2 (20 mL) and MeOH (10 mL), a solution of Br2 (0.167 mL, 3.23 mmol) in CH2Cl2 (6 mL) was added at a rate of 40 drops per minute under strong agitation at RT. After an observed color change from bromine red to pale yellow, two additional portions of Br2 (0.056 mL, 1.09 mmol) were added as described above. After the observed color change, the product was confirmed by TLC: Rf (2) = 0.58 (1:3 n-pentane: CH2Cl2). The reaction mixture was washed with satd. K2CO3 (2 × 40 mL) and brine (2 × 40 mL) before the organic layers were dried over MgSO4 and filtered before the solvents were removed under reduced pressure. The crude compound was stirred in EtOH (4.4 mL) at 50 °C for 30 min, and for an additional 60 min at RT. The solids were filtered and recrystallised from EtOAc. The crystals were dried under vacuum overnight to afford 2 in 86% yield (1.04 g, 4.22 mmol) with 98% purity (GLC). 1H NMR (400 MHz, CDCl3): δ 7.86 (s, 2H, Harom), 5.05 (s, 2H, NH2), 2.31 (s, 2H, CH2-Br). 13C NMR (600 MHz, CDCl3): δ 188.0, 144.9, 129.2, 124.0, 188.9, 29.8. MS (TOF-ASAP): [M + H]+ 281.9088 m/z.
3.4.2. Synthesis of 1-(4-Amino-3,5-dichlorophenyl)-2-bromoethan-1-ol (3)
1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-one (2) (0.50 g, 1.77 mmol) dissolved in EtOH (12 mL) at 0 °C was added NaBH4 (0.136 g, 3.60 mmol) in 4 portions over 20 min, under stirring. The reaction was slowly heated to RT, and the conversion was monitored by TLC: Rf (3) = 0.43 (1:3 n-pentane/CH2Cl2). After full conversion of the starting material, the reaction was quenched with dilute HCl until gas formation stopped. H2O and EtOH were removed through azeotropic distillation, and the solids were dissolved in EtOAc (50 mL), and extracted with satd. NaHCO3 (3 × 30 mL) and brine (3 × 30 mL). The aqueous layers were back extracted with EtOAc (2 × 50 mL), and the combined organic layers were dried over MgSO4. The solvent was removed under reduced pressure, and the crude product was purified by flash chromatography (1:3 n-pentane/CH2Cl2) to afford 3 as a light-yellow solid in 44% yield (0.22 g, 0.77 mmol) in 95% purity (GLC). 1H NMR (400 MHz, CDCl3): δ 7.22 (s, 2Harom), 4.80–4.76 (dd, 1H, CHOH, 3JHH = 3.4 Hz, 8.9 Hz), 4.49 (s, 2H, NH2), 3.59–3.55 (dd, 1H, CH-Br, 2JHH = 10.5 Hz, 3JHH = 3.4 Hz), 3.49–3.45 (dd, 1H, CH-Br, 3JHH = 8.9 Hz, 2JHH = 10.4 Hz). 13C NMR (400 MHz, CDCl3): δ 140.1, 130.4, 125.6, 119.6, 72.6, 39.8. MS (TOF-ASAP) [M + H]+ 283.9241 m/z.
3.4.3. Synthesis of N-(4-Acetyl-phenyl)acetamide (5)
To a stirred solution of 1-(4-aminophenyl)ethan-1-one (0.53 g, 3.92 mmol) in CH2Cl2 (15 mL), a solution of AcCl (0.56 mL, 7.84 mmol) in CH2Cl2 (2 mL) was added dropwise at RT. After 5 min, a white precipitate started to form, and a solution of Et3N (1.09 mL, 7.84 mmol) in CH2Cl2 (2 mL) was added dropwise, after which the solution cleared and turned a dark-yellow color. Full conversion was observed after 24 h by TLC: Rf (5) = 0.05 (1:4 EtOAc/n-pentane), and the solution was extracted with brine (3 × 15 mL). The aqueous layers were combined and extracted with EtOAc (2 × 15 mL), before the combined organic layers were dried over MgSO4, filtered, and the solvents were removed under reduced pressure. The crude product was recrystallised from EtOAc to afford 5 in 53% yield (0.37 g, 2.09 mmol) and 98% purity (GLC). 1H NMR (600 MHz, CDCl3): δ 7.95–7.93 (m, 2Harom, 2JHH, ortho = 8.7 Hz, 3JHH, meta = 2.5 Hz), 7.62–7.61 (d, 2H, Harom, 2JHH = 8.3 Hz), 7.42 (s, 1H, NHR), 2.58 (s, 3H, CO-CH3), 2.22 (s, 3H, NCO-CH3). 13C NMR (600 MHz, CDCl3): δ 196.9, 168.4, 142.2, 132.9, 129.8, 118.8, 26.4, 24.8. MS (TOF-ASAP) [M + H]+ 178.0867 m/z.
3.4.4. Synthesis of N-(4-Acetyl-2,6-dichlorophenyl)acetamide (6)
To a stirred solution of 1-(4-amino-3,5-dichlorophenyl)ethan-1-one (1) (6.00 g, 29.40 mmol) in CH2Cl2 (150 mL) AcCl (10.49 mL, 147.01 mmol) in CH2Cl2 (15 mL) was added. To the stirred solution Et3N (4.29 mL, 30.78 mmol) in CH2Cl2 (15 mL) was added dropwise. The reaction was monitored by TLC. Rf (6) = 0.64 (1:4 EtOAc/n-pentane). After 48 h, full conversion was observed. The reaction mixture was washed with satd. K2CO3 (2 × 100 mL) and brine (2 × 100 mL), before the organic layers were collected and dried over anhydrous MgSO4, filtered, and the solvent removed under reduced pressure. The crude product was recrystallized from EtOAc, and the purified compound dried in vacuo overnight to afford 6 as white crystals in 58% yield (4.20 g, 17.07 mmol) in 99% purity. 1H NMR (400 MHz, CDCl3): δ 7.86 (s, 2H, Harom), 7.42 (s, 1H, NHR), 2.56 (s, 3H, CO-CH3), 2.22 (s, 3H, NCO-CH3). 13C NMR (400 MHz, CDCl3): δ 195.0, 168.4, 136.7, 136.4, 133.9, 128.3, 26.6, 23.2. MS (TOF-ASAP) [M + H]+ 246.0089 m/z.
3.4.5. Synthesis of 1-(4-Chlorophenyl)prop-2-yn-1-ol (8)
A solution of ethynylmagnesium bromide (0.50 M in THF; 18 mL, 9.0 mmol) was cooled to −20 °C. A solution of 4-chlorobenzaldehyde (1.0105 g, 7.19 mmol) in THF (2.5 mL) was added, and the flask washed out with THF (0.5 mL), which was then added to the reaction mixture. The cooling bath was removed, and the reaction mixture was stirred at room temperature (RT) for 5 min. The reaction was quenched by addition of satd. aq. NH
4Cl (10 mL), and the mixture was extracted with Et
2O (2 × 20 mL). The combined organic extracts were dried with Na
2SO
4, filtered, and concentrated in vacuo. The residue was purified by flash chromatography (gradient 1:10–1:3 EtOAc/pentane) to give
8 in 90% yield (1.078 g, 6.47 mmol) as a pale yellow oil. R
f = 0.47 (1:3 EtOAc/pentane).
1H NMR (400 MHz, CDCl
3): δ = 7.51–7.48 (m, 2H, H
arom), 7.38–7.35 (m, 2H, H
arom), 5.45 (dd,
J = 6.1, 2.1 Hz, CHC≡), 2.68 (d,
J = 2.2 Hz, ≡CH), 2.21 (d,
J = 6.1 Hz, OH) ppm.
1H NMR spectroscopic data are in accordance with the literature data [
26].
3.5. Kinetic Resolution of Secondary Alcohols with Lipases
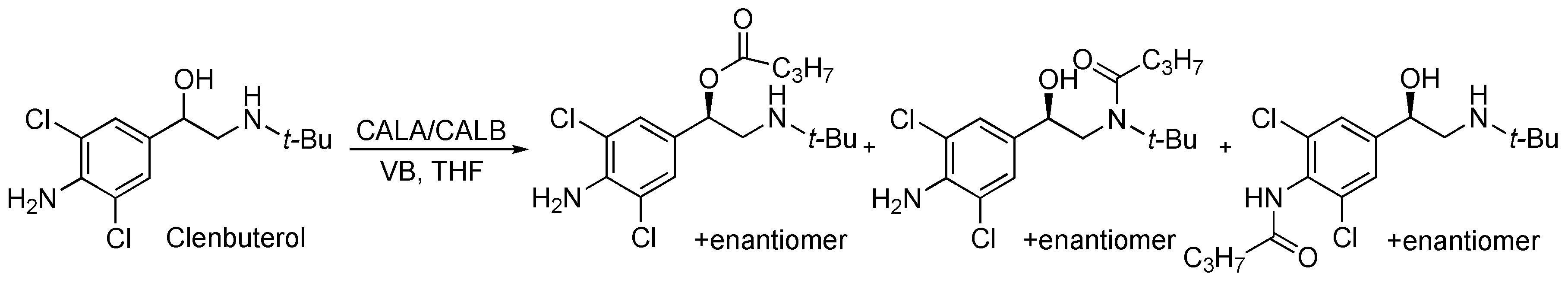
3.5.1. Kinetic Resolution of Clenbuterol
1-(4-Amino-3,5-dichlorophenyl)-2-(tert-butylamino)ethan-1-ol (clenbuterol, 31.2 mg, 0.11 mmol) dissolved in dry THF (4.0 mL) was added 3 pellets of molecular sieve, and vinyl butanoate (0.058 mL, 0.55 mmol) before the mixture was incubated at 30 °C and 200 rpm and the reaction was started by addition of lipase A from Candida antarctica (CALA, 55.4 mg). Aliquots of 100 L were collected every 30 min for the first 5 h, then every hour for 3 h, and every 24 h for 5 days. The solvent in the aliquots was removed with N2 before 0.100 M pH 2.5 phosphate buffer (0.5 mL) was added, and the samples were analysed by chiral CE, from which the enantiomeric excess of (R)-clenbuterol was calculated to 11% ee.
3.5.2. Kinetic Resolution of 1-(4-Amino-3,5-dichlorophenyl)-2-bromoethan-1-ol (3)
To a solution of racemic 1-(4-amino-3,5-dichlorophenyl)-2-bromoethan-1-ol (3, 55 mg, 0.19 mmol) and vinyl butanoate (0.1225 mL, 0.97 mmol) in CH2Cl2 (3 mL), lipase B from Candida antarctica (CALB, 55 mg) was added before the solution was placed in an incubator set to 30 °C and 200 rpm. Aliquots of 100 L were collected every 30 min for the first 4 h, then at every 12 h for 3 days, and finally every 24 h for 4 days. The aliquots were analysed by chiral GLC, and no conversion was observed. A new reaction was performed with CALA (60 mg), and aliquots were collected as described above, then analysed by chiral GLC. No conversion was observed.
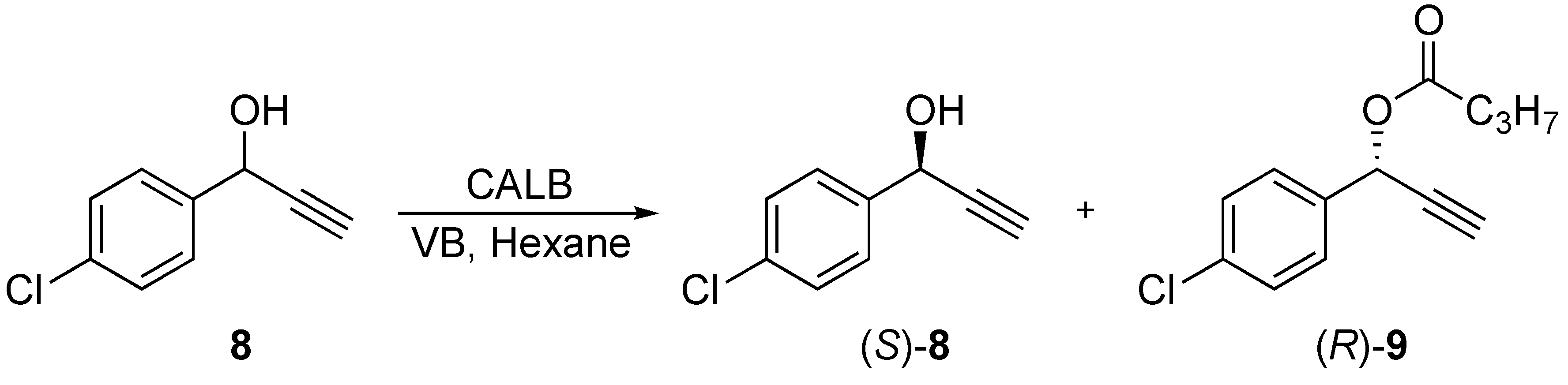
3.5.3. Kinetic Resolution of 1-(4-Chlorophenyl)prop-2-yn-1-ol (8)
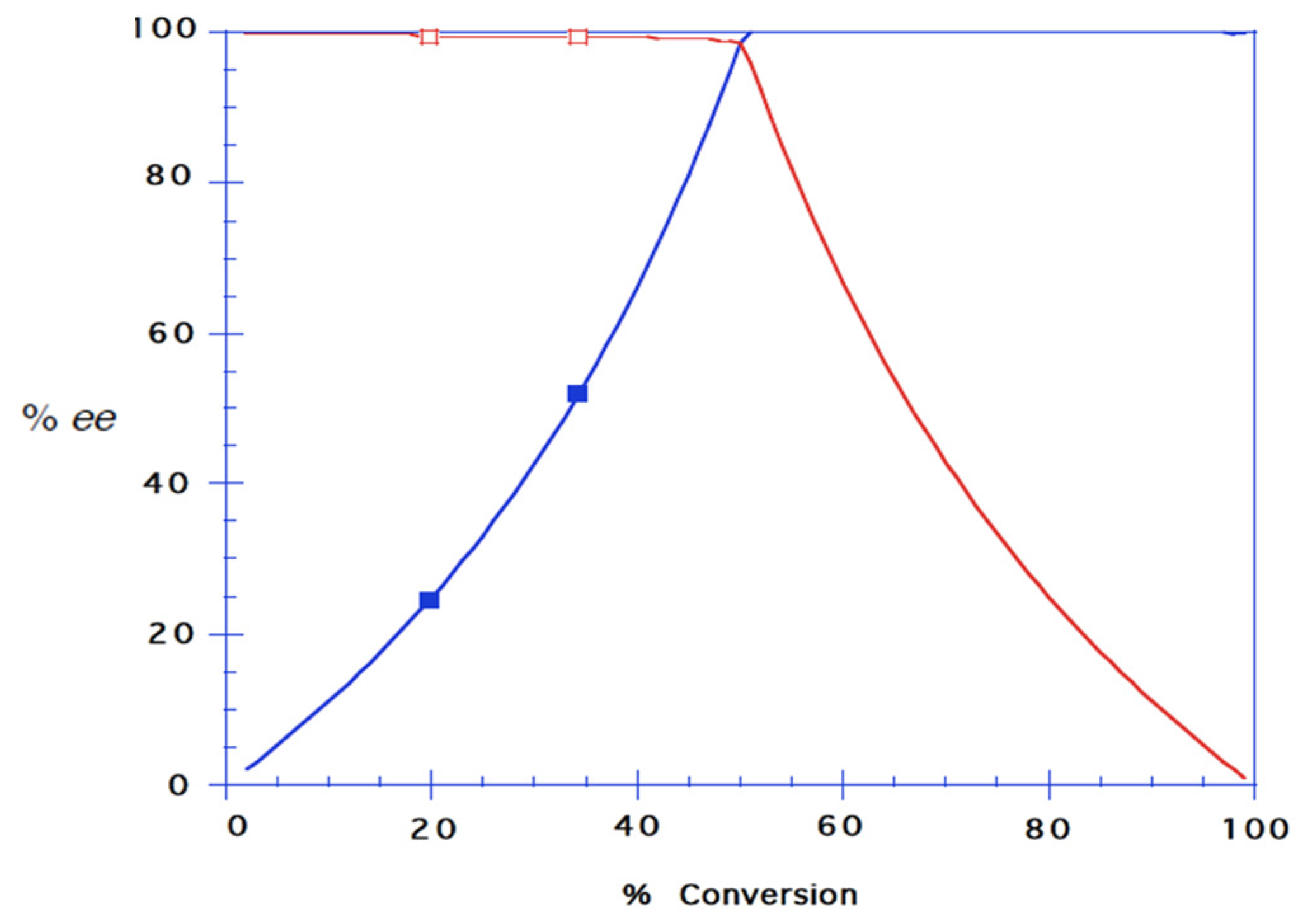
To a solution of 1-(4-chlorophenyl)prop-2-yn-1-ol (8) (20.0 mg, 0.12 mmol), and vinyl butanoate (0.069 mL, 0.60 mmol) in dry n-hexane (3.0 mL), 5 pellets of molecular sieve were added before the mixture was incubated at 40 °C and 200 rpm. CALB (22.1 mg) was added to the mixture, and aliquots (0.100 mL) were collected every 30 min for the first 3 h, and finally after 19.5 h. The samples were analysed by chiral GLC: tR (S)-8 = 12.880 min, tR (R)-8 = 13.087 min, RS = 3.5. After 2.5 h, (R)-8 was not observed by GLC. The ester (R)-9 was produced in 99% ee.
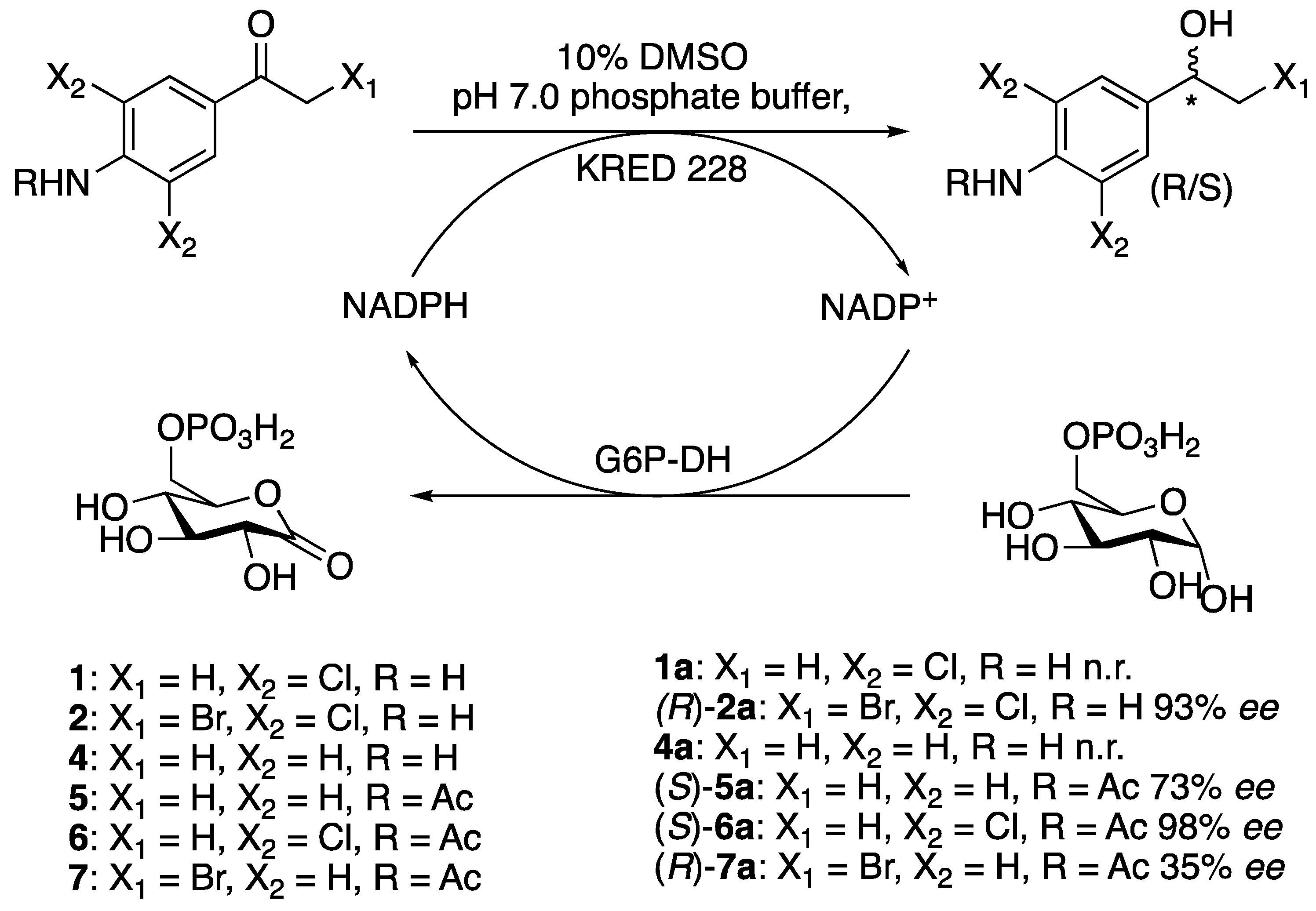
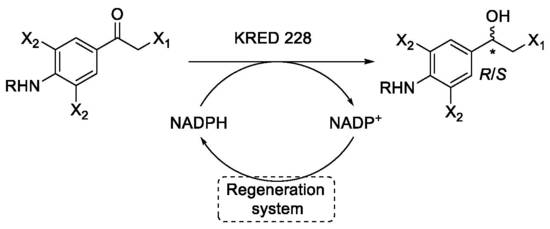
3.6. Asymmetrisations of Ketones by Ketoreductases
3.6.1. General Procedure
The ketones 1, 2 and 4–7 (2.5–60.8 mg, 0.01–0.16 mmol) were dissolved in DMSO (0.10 mL) and transferred to a solution of ketoreductase 228 (KRED 228), NADPH, glucose-6-phosphate (G6P) and glucose-6-phosphate dehydrogenase (G6P-DH) in pH 7.0 phosphate buffer (0.9 mL). The reactions were incubated at 30 °C and 300 rpm for 1–28 days, and monitored by TLC (1:2 n-pentane/EtOAc) every 24 h. After full conversion of the starting material, the mixtures were extracted with EtOAc (4 × 10 mL) and the organic phases were combined, washed with water (2 × 20 mL), dried over MgSO4, and filtered before the organic solvent was removed under reduced pressure. The crude products were either purified by flash chromatography (silica, EtOAc/n-pentane) to afford the pure alcohols, or the crude product was analysed by chiral GLC or chiral HPLC.
3.6.2. Asymmetric Synthesis of (R)-1-(4-Amino-3,5-dichlorophenyl)-2-bromoethan-1-ol, (R)-2a
1-(4-Amino-3,5-dichlorophenyl)-2-bromoethan-1-one (2) (28.6 mg, 0.10 mmol) in DMSO (0.10 mL), KRED 228 (10.4 mg), G6P (77.7 mg), G6P-DH (0.1 mL, 20 U) and NADPH (0.3 mg) in pH 7.0 phosphate buffer (0.90 mL). Reaction time: 4 weeks, (R)-2a was obtained in 93% ee. GLC of rac-2: tR (R) = 25.363 min, tR (S) = 25.636 min, RS = 1.9.
3.6.3. Asymmetric Synthesis of (S)-N-(4-Acetyl-phenyl)acetamide, (S)-5a
N-(4-Acetylphenyl)acetamide (5) (20.3 mg, 0.11 mmol) in DMSO (0.10 mL), KRED 228 (7.2 mg), G6P (94.1 mg), G6P-DH (0.1 mL, 20 U) and NADPH (0.3 mg) in pH 7.0 phosphate buffer (0.90 mL) was incubated for 2 weeks. After flash chromatography, (100% EtOAc,), (S)-5a was obtained in a 45% isolated yield, (8.8 mg, 0.05 mmol), 96% purity, 73% ee. Rf (S)-5 = 0.42 (EtOAc). HPLC of rac-5: tR (R) = 84.474 min, tR (S) = 91.765 min, RS =1.56. = −0.11 (c 0.88, EtOH). 1H NMR (600 MHz, CD3OD): δ 7.50–7.49 (d, 2H, Harom, 3JHH = 8.4 Hz), 7.31–7.30 (d, 2H, Harom, 3JHH = 8.4 Hz), 4.80–4.77 (q, 1H, HO-CH, 3JHH = 6.5 Hz), 2.11 (s, 3H, CO-CH3), 1.42–1.41 (d, 3H, CH-CH3, 3JHH = 6.5 Hz) 13C NMR (600 MHz, MeOD): δ 171.6 (CO), 143.4 (C), 138.8 (C), 126.9 (CHarom), 121.1 (CHarom), 70.5 (CH), 25.4 (CH3), 23.7 (CH3). MS (TOF-ASAP): [M + H]+ 180.1025 m/z.
3.6.4. Asymmetric Synthesis of (S)-N-(2,6-dichloro-4-(1-hydroxyethyl)phenyl)acetamide, (S)-6a
N-(4-Acetyl-2,6-dichlorophenyl)acetamide (6) (32.1 mg, 0.13 mmol) in DMSO (0.10 mL), KRED 228 (8.1 mg), G6P (74.4 mg, 0.286 mmol), G6P-DH (0.1 mL, 20 U) and NADPH (0.2 mg) in phosphate buffer (pH 7.0, 0.90 mL). Reaction time 48 h, flash chromatography (1:2 n-pentane/EtOAc), isolated yield (S)-6a: 31% yield (10.1 mg, 0.04 mmol), 99% purity, >98% ee. GLC of rac-6: tR (S)-6a = 44.46 min, tR (R)-6a = 45.25 min, RS = 2.05. (S)-6a = −0.21 (c 1.01, EtOH). 1H NMR (600 MHz, MeOD,): δ 7.45 (s, 2H, Harom), 4.82–4.79 (q, 1H, CH-OH, 3JHH = 6.3 Hz), 2.17 (s, 3H, CO-CH3), 1.42–1.41 (d, 3H, CH(OH)-CH3, 3JHH = 6.3 Hz). 13C NMR (100 MHz MeOD,): δ 172 (CO), 150 (C), 135.2 (C), 132.2 (C), 126.5 (CHAr), 69.4 (CH), 25.4 (CH3), 22.3 (CH3). MS: (ASAP-TOF) [M + H]+ 248.0245 m/z.
3.6.5. Asymmetric Synthesis of (R)-N-(4-(2-Bromo-1-hydroxyethyl)phenyl)acetamide, (R)-7a
N-(4-(2-Bromoacetyl)phenyl)acetamide (7) (27.1 mg, 0.11 mmol) in DMSO (0.10 mL), KRED 228 (8.7 mg), G6P (82.9 mg, 0,319 mmol), G6P-DH (0.1 mL, 20 U) and NADPH (0.4 mg) in phosphate buffer (pH 7.0, 0.90 mL). Two days reaction time, flash chromatography (100% EtOAc), Rf (R)-7a = 0.57, isolated yield: 27% (7.3 mg, 0.03 mmol), 98% purity, 35% ee. HPLC of rac-7: tR (R) = 14.037 min, tR (S) = 17.035 min, RS = 2.55. = +0.07 (c 0.73, EtOH). 1H NMR (600 MHz, CD3OD): δ 7.56–7.55 (m, 2H, Harom, 3JHH = 8.7 Hz), 7.36–7.35 (m, 2H, Harom, 3JHH = 8.6 Hz), 4.85–4.83 (m, 1H, HO-CH, 3JHH = 4.6 Hz, 3JHH = 7.7 Hz), 3.62–3.60 (dd, 1H, CH-Br, 3JHH = 4.4 Hz, 2JHH = 10.5 Hz), 3.56–3.53 (dd, 1H, CH-Br, 3JHH = 7.7 Hz, 2JHH = 10.5 Hz), 2.13 (s, 3H, CO-CH3). 13C NMR (600 MHz, CD3OD): δ 170.2, 138.2, 137.6, 126.4, 119.6, 73.1, 37.8, 22.4. MS (TOF-ASAP): [M + H]+ 258.0130 m/z.