Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Investigations on the Effect of Zn Content and ZrO2 as the Support and Dispersant
Abstract
1. Introduction
2. Results
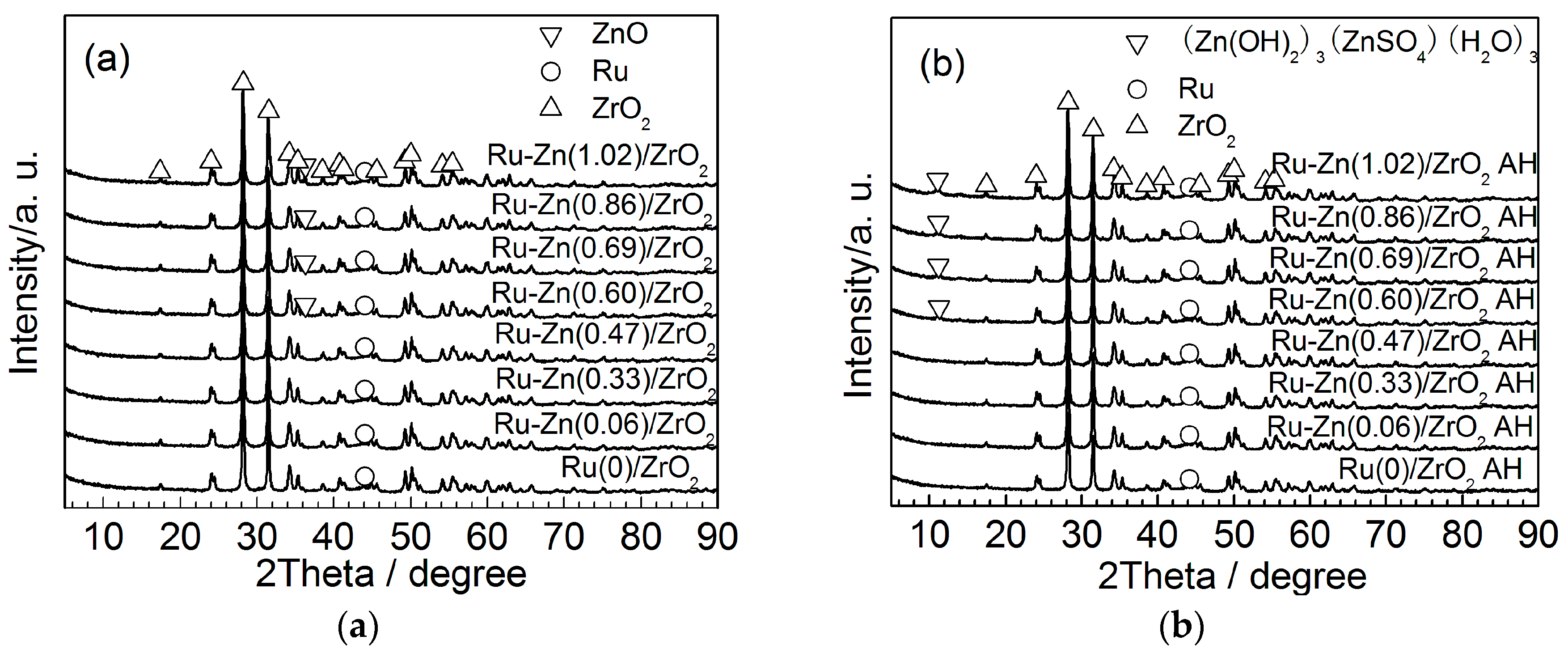
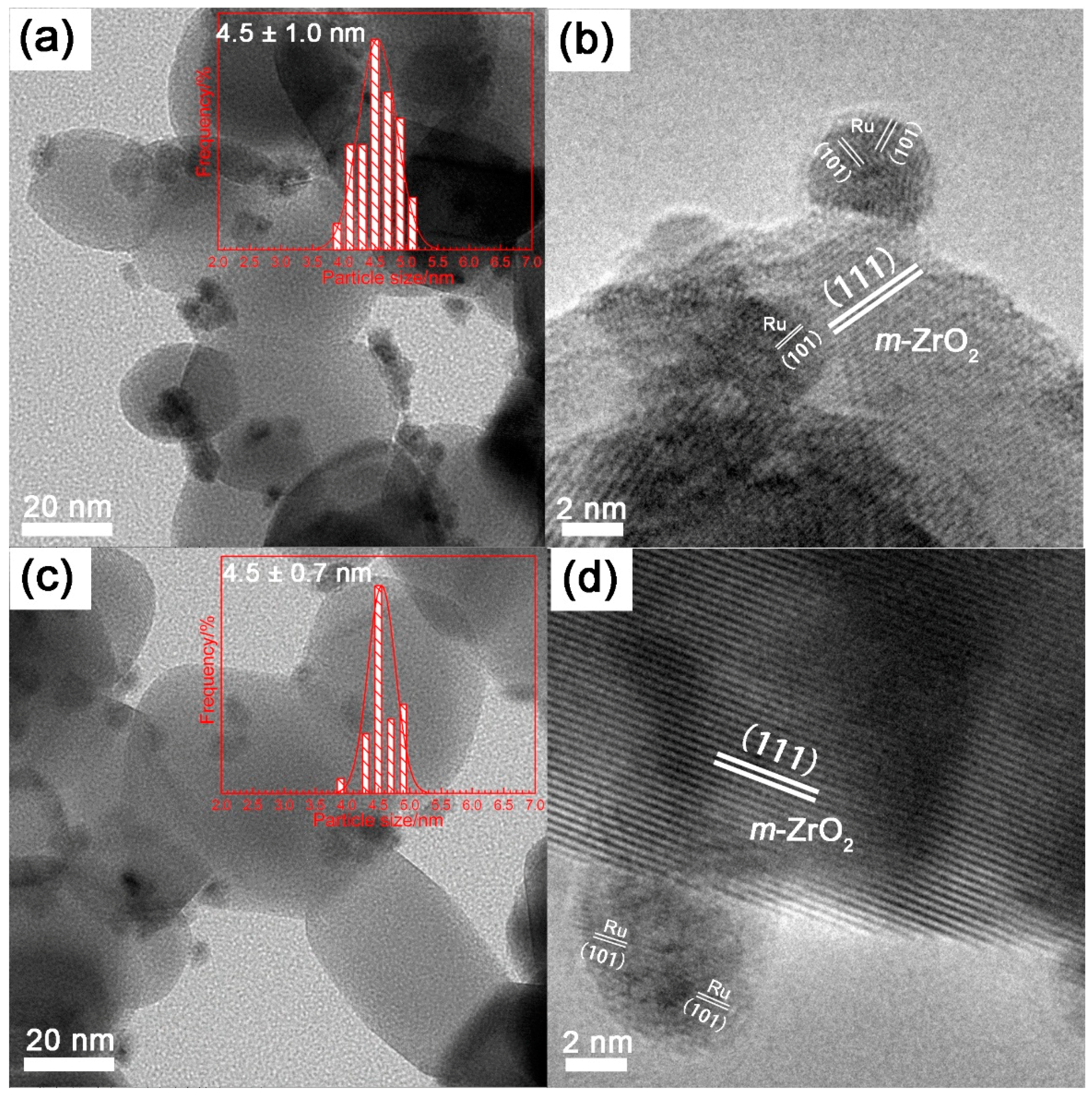
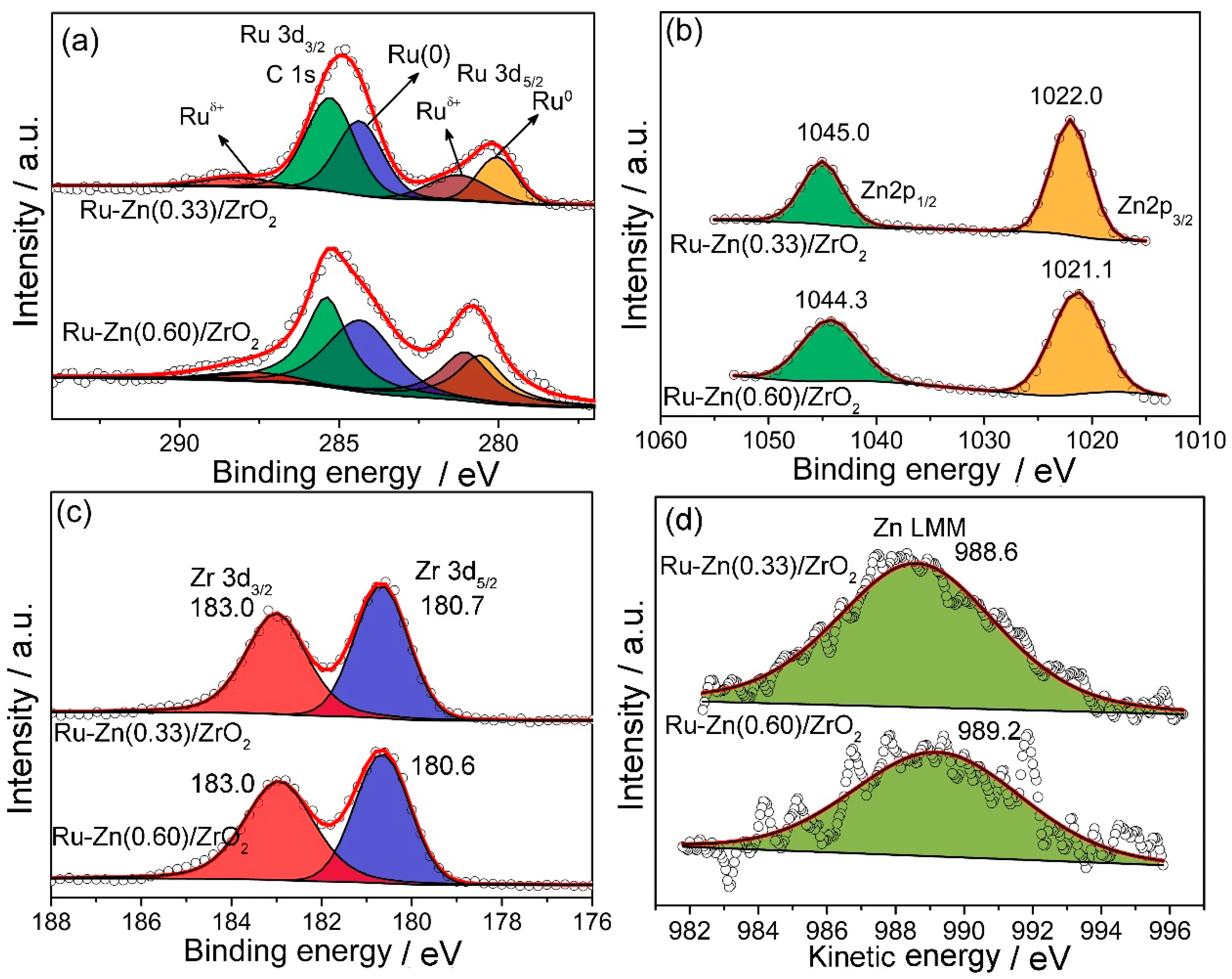
2.1. Effect of Zn Content
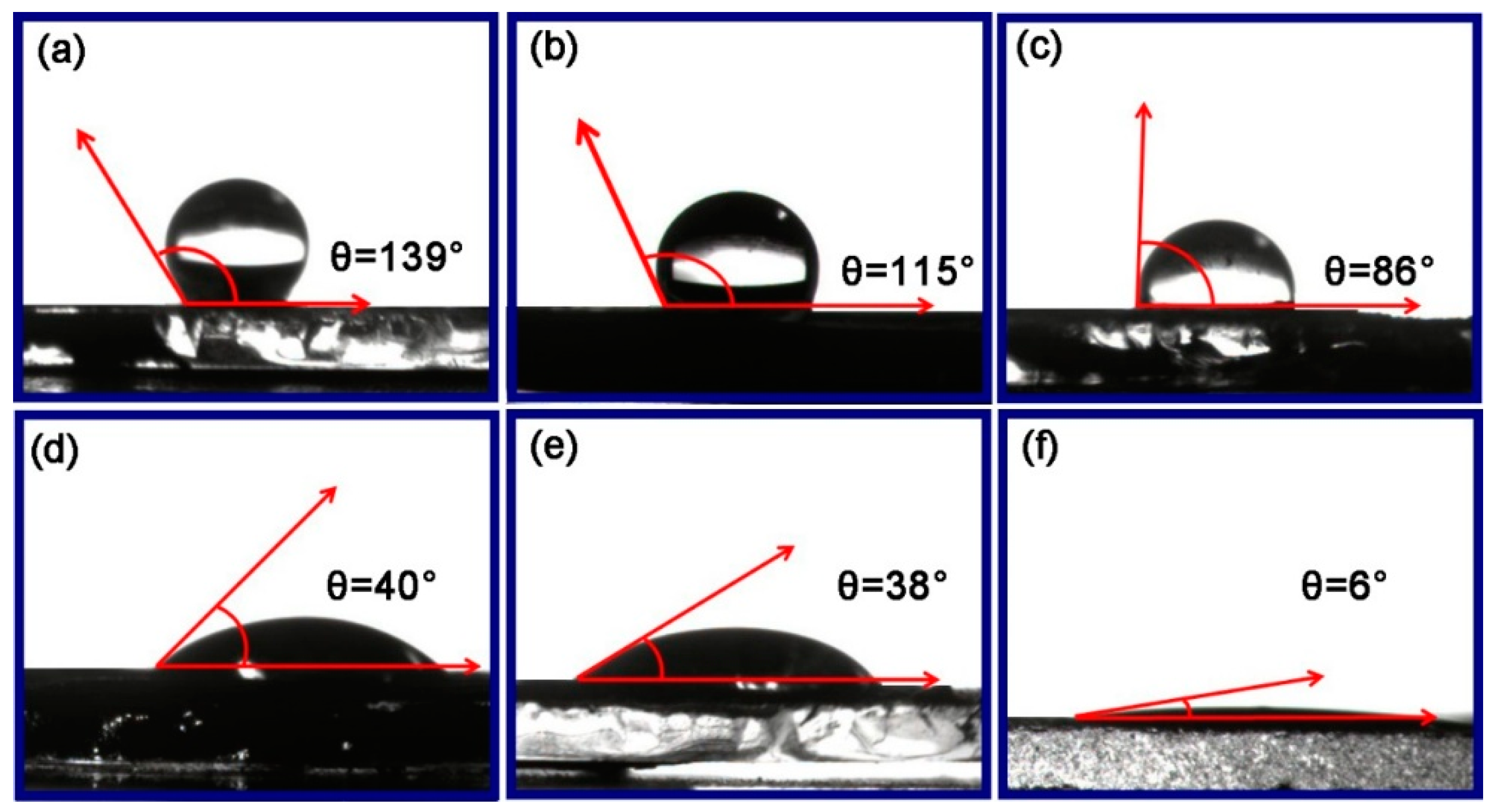
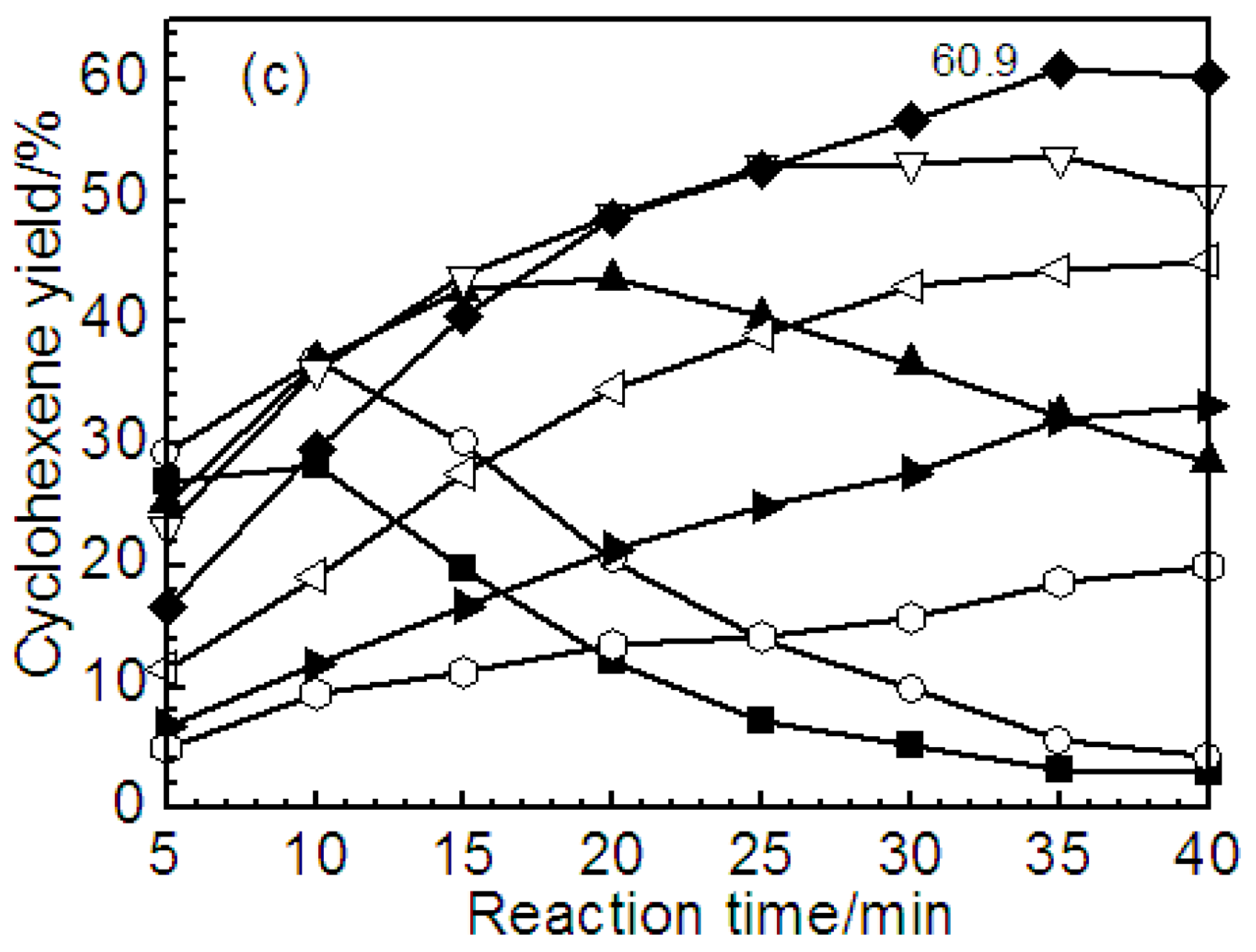
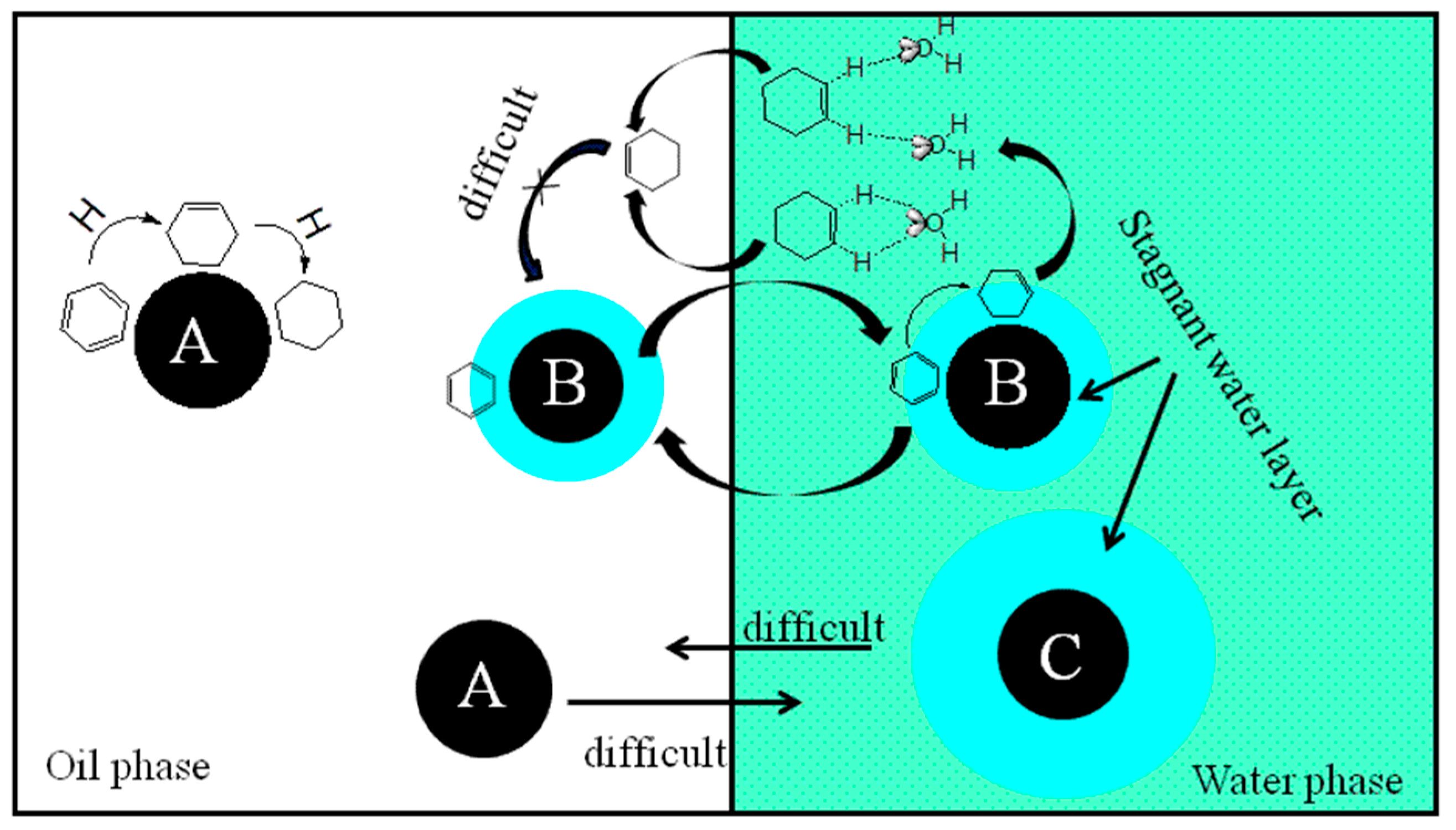
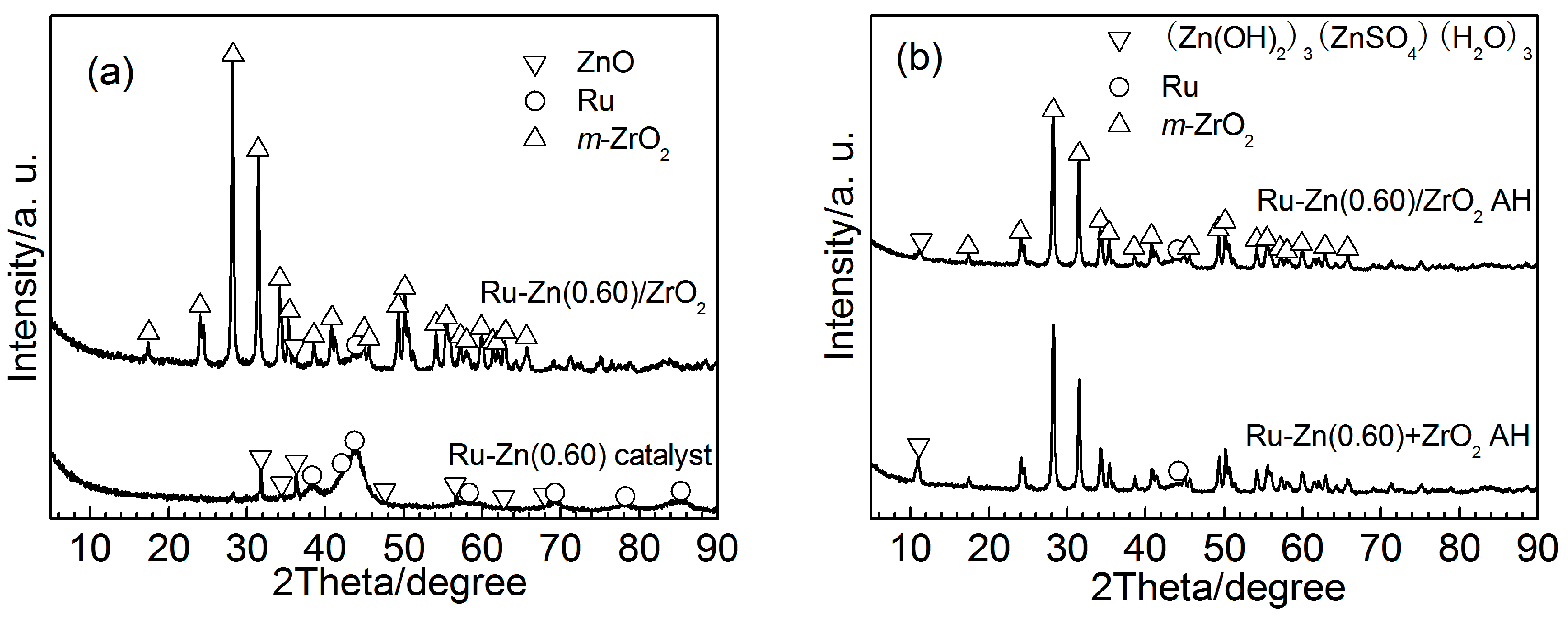
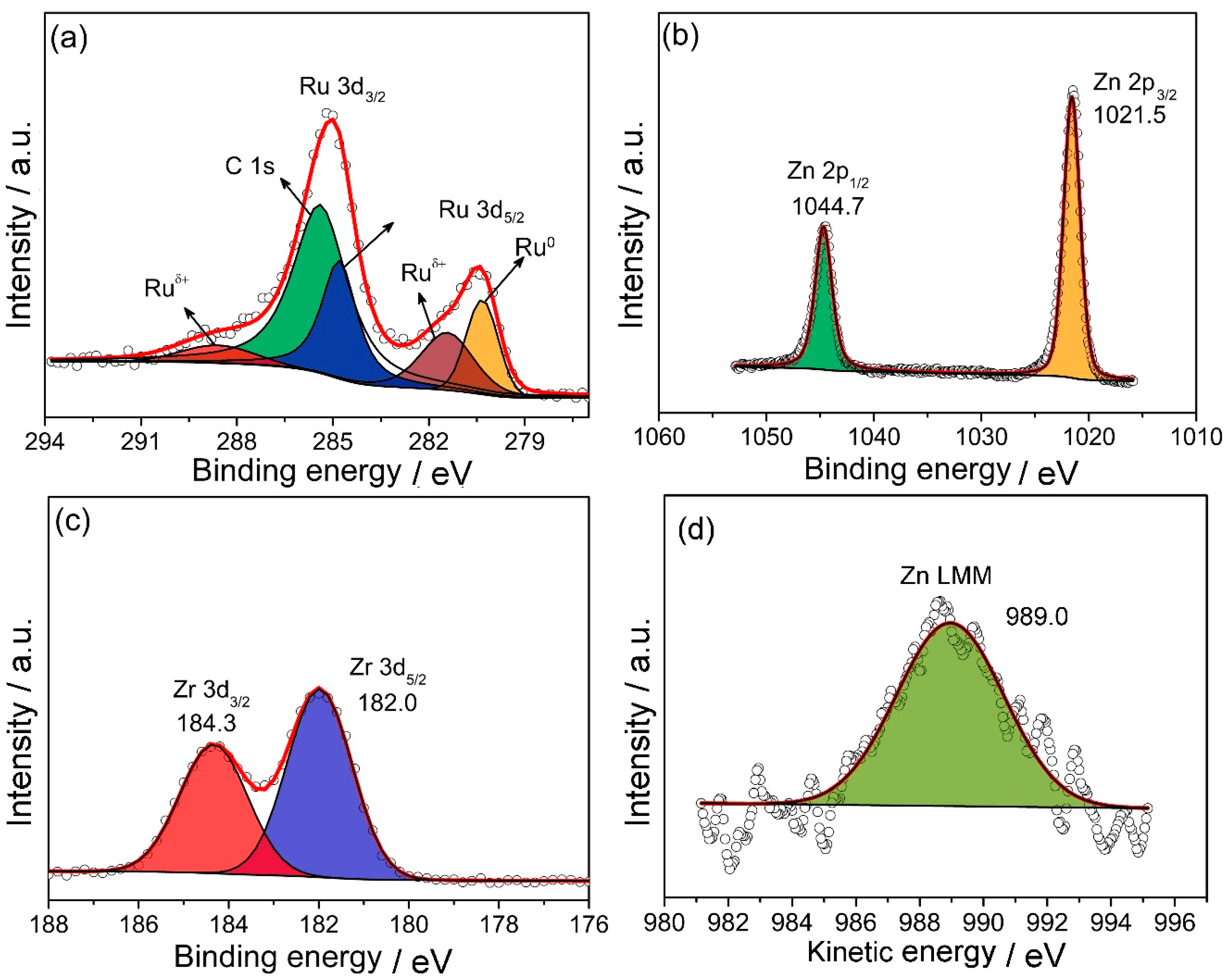
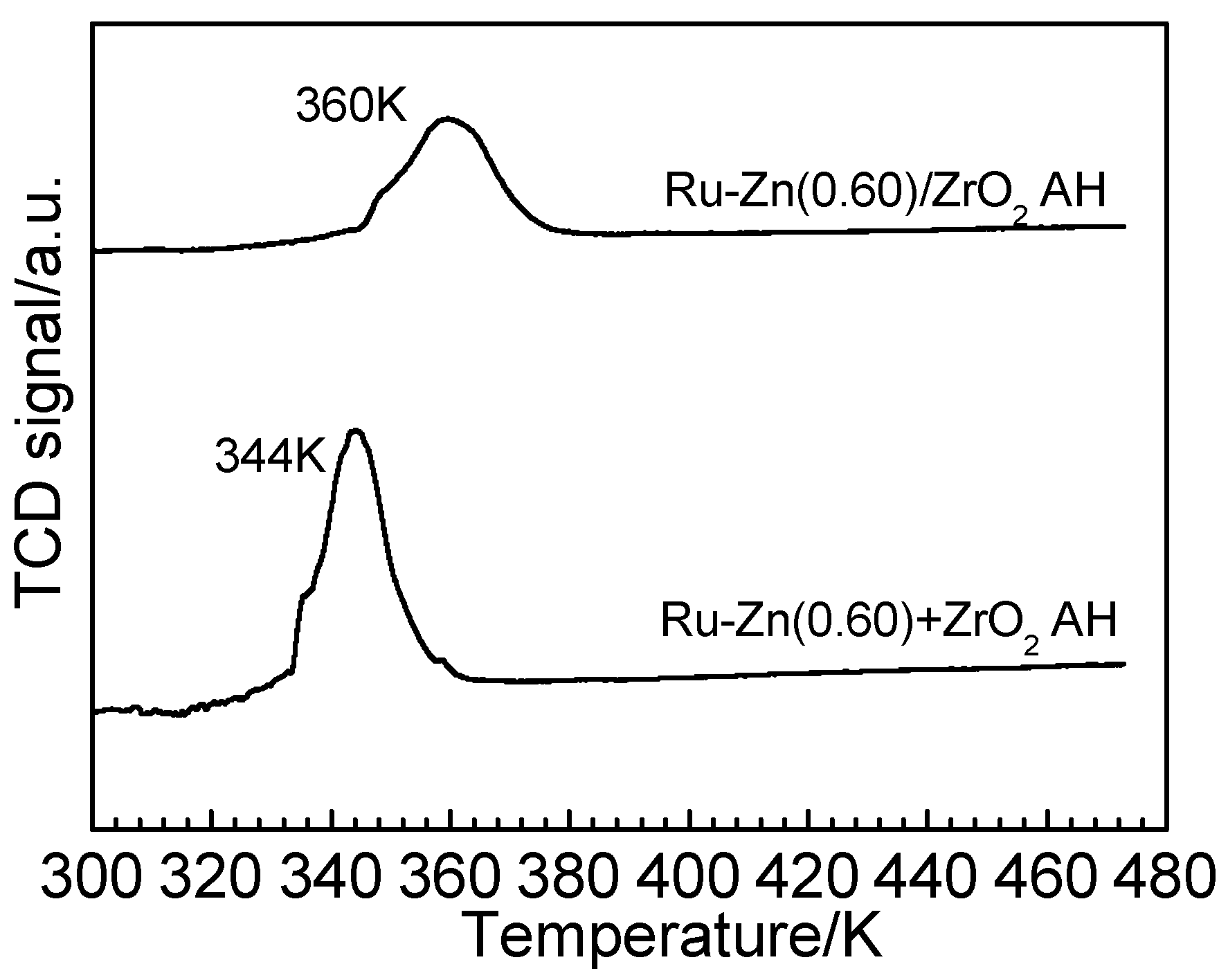
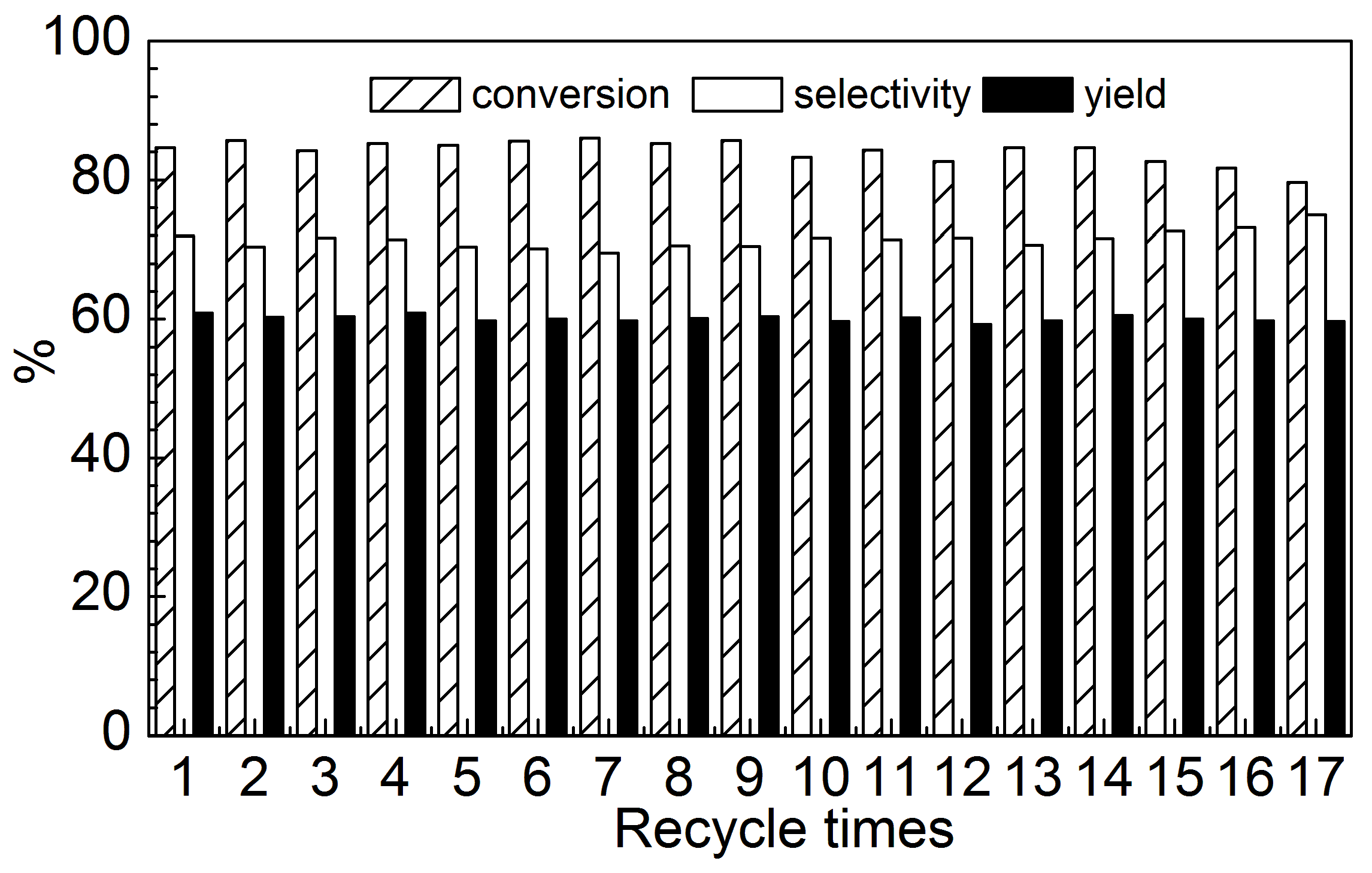
2.2. Effect of ZrO2 as Support and Dispersant
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Catalysts
3.3. Catalytic Experimental Procedure
3.4. Catalysts Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, X.H.; Zhang, Q.; Zhu, M.Q.; Wang, Z.B. Selective hydrogenation of benzene to cyclohexene over Ru-Zn/ZrO2 catalysts prepared by a two step impreganation method. J. Mol. Catal. A Chem. 2016, 413, 85–93. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.D.S.; Carabineiro, S.A.C.; Wang, J.; Rocha, B.G.M.; Maldonado-Hódar, F.J.; Pombeiro, A.J.L.D.O. Supported Gold Nanoparticles as Reusable Catalysts for Oxidation Reactions of Industrial Significance. ChemCatChem 2017, 9, 1211–1221. [Google Scholar] [CrossRef]
- Martins, L.M.; de Almeida, M.P.; Carabineiro, S.A.; Figueiredo, J.L.; Pombeiro, A.J. Heterogenisation of a C-Scorpionate FeII Complex on Carbon Materials for Cyclohexane Oxidation with Hydrogen Peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Martins, L.M.D.R.S.; Avalos-Borja, M.; Buijnsters, J.G.; Pombeiro, A.J.L.; Figueiredo, J.L. Gold nanoparticles supported on carbon materials for cyclohexane oxidation with hydrogen peroxide. Appl. Catal. A Gen. 2013, 467, 279–290. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, Z.H.; Li, C.G.; Chen, L.X.; Peng, Z.K.; Liu, Z.Y.; Liu, S.C. Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Mechanism Investigation on NaOH as a Reaction Additive. Catalysts 2018, 8, 104. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, Z.H.; Li, C.G.; Chen, L.X.; Li, Y.; Peng, Z.K.; Liu, Z.Y.; Liu, S.C. Selective Hydrogenation of Benzene to Cyclohexene over Monometallic Ru Catalysts: Investigation of ZnO and ZnSO4 as Reaction Additives as Well as Particle Size Effect. Catalysts 2018, 8, 172. [Google Scholar] [CrossRef]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Appl. Surf. Sci. 1997, 121–122, 448–451. [Google Scholar] [CrossRef]
- Zhou, G.B.; Wang, H.; Pei, Y.; Qiao, M.H.; Sun, B.; Zong, B.N. Pore size effect of Ru-Zn/ZrO2 catalyst on partial hydrogenation of benzene to cyclohexene. Acta Chim. Sin. 2017, 75, 321–328. [Google Scholar] [CrossRef]
- Peng, Z.K.; Liu, X.; Li, S.H.; Li, Z.J.; Li, B.J.; Liu, Z.Y.; Liu, S.C. Heterophase-structured nanocrystals as superior supports for Ru-based catalysts in selective hydrogenation of benzene. Sci. Rep. 2017, 7, 39847. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Liu, Z.Y.; Wang, Z.; Wu, Y.M.; Yuan, P. Characterization and study on performance of the Ru–La–B/ZrO2 amorphous alloy catalysts for benzene selective hydrogenation to cyclohexene under pilot conditions. Chem. Eng. J. 2008, 139, 157–164. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, Y.; Liu, J.; Pei, Y.; Li, Z.H.; Li, H.; Li, H.X.; Qiao, M.H.; Fan, K.N. Discrimination of the roles of CdSO4 and ZnSO4 in liquid phase hydrogenation of benzene to cyclohexene. J. Catal. 2009, 268, 100–105. [Google Scholar] [CrossRef]
- Fan, G.F.; Li, R.X.; Li, X.J.; Chen, H. Effect of organic on partial hydrogenation of benzene. Catal. Commun. 2008, 9, 1394–1397. [Google Scholar] [CrossRef]
- Sun, H.J.; Wang, H.X.; Jiang, H.B.; Li, S.H.; Liu, S.C.; Liu, Z.Y.; Yuan, X.M.; Yang, K.J. Eeffect of (Zn(OH)2)3(ZnSO4)(H2O)5 on the performance of Ru-Zn catalyst for benzene selective hydrogenation to cyclohexene. Appl. Catal. A Gen. 2013, 450, 160–168. [Google Scholar] [CrossRef]
- Sun, H.J.; Jiang, H.B.; Dong, Y.Y.; Wang, H.X.; Pan, Y.J.; Liu, S.C.; Tang, M.S.; Liu, Z.Y. Effect of alcohols as additives on the performance of a nano-sized Ru-Zn(2.8%) catalyst for selective hydrogenation of benzene to cyclohexene. Chem. Eng. J. 2013, 218, 415–424. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Jiang, H.B.; Li, S.H.; Zhang, Y.X.; Liu, S.C.; Liu, Z.Y. Effect of transition metals (Cr, Mn, Fe, Co, Ni, Cu and Zn) on the hydrogenation properties of benzene over Ru-based catalyst. Appl. Catal. A Gen. 2013, 464–465, 1–9. [Google Scholar] [CrossRef]
- Da-Silva, J.W.; Cobo, A.J.G. The role of the titania and silica supports in Ru-Fe catalysts to partial hydrogenation of benzene. Appl. Catal. A Gen. 2003, 252, 9–16. [Google Scholar] [CrossRef]
- Fan, G.Y.; Jiang, W.D.; Wang, J.B.; Li, R.X.; Chen, H.; Li, X.J. Selective hydrogenation of benzene to cyclohexene over RuCoB/γ-Al2O3 without additive. Catal. Commn. 2008, 10, 98–102. [Google Scholar] [CrossRef]
- Sun, H.J.; Jiang, H.B.; Li, S.H.; Wang, H.X.; Pan, Y.J.; Dong, Y.Y.; Liu, S.C.; Liu, Z.Y. Selective hydrogenation of benzene to cyclohexene over nanocomposite Ru-Mn/ZrO2 catalyst. Chin. J. Catal. 2013, 34, 684–694. [Google Scholar] [CrossRef]
- Sun, H.J.; Dong, Y.Y.; Li, S.H.; Jiang, H.B.; Zhang, Y.X.; Liu, Z.Y.; Liu, S.C. The role of La in improving the selectivity to cyclohexene of Ru catalyst for hydrogenation of benzene. J. Mol. Catal. A Chem. 2013, 368–369, 119–124. [Google Scholar] [CrossRef]
- Liao, H.G.; Ouyang, D.H.; Zhang, J.; Xiao, Y.J.; Liu, P.L.; Hao, F.; You, K.Y.; Luo, H.A. Benzene hydrogenation over oxide-modified MCM-41 supported ruthenium-lanthanum catalyst: The influence of zirconia crystal form and surface hydrophilicity. Chem. Eng. J. 2014, 243, 207–216. [Google Scholar] [CrossRef]
- Sun, H.J.; Pan, Y.J.; Li, S.H.; Zhang, Y.X.; Dong, Y.Y.; Liu, S.C.; Liu, Z.Y. Selective hydrogenation of benzene to cyclohexene over Ce-promoted Ru catalysts. J. Energy Chem. 2013, 22, 710–716. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, L.J.; Pei, Y.; Zhuang, J.H.; Li, H.; Li, H.X.; Qiao, M.H.; Fan, K.N. Ce-promoted Ru/SBA-15 catalysts prepared by a “two solvent” impregnation method for selective hydrogenation of benzene to cyclohexene. Appl. Catal. A Gen. 2009, 353, 282–287. [Google Scholar] [CrossRef]
- Zhou, G.B.; Tan, X.H.; Pei, Y.; Fan, K.N.; Qiao, M.H.; Sun, B.; Zong, B.N. Structural and Catalytic Properties of Alkaline Post-Treated Ru/ZrO2 Catalysts for Partial Hydrogenation of Benzene to Cyclohexene. ChemCatChem 2013, 5, 2425–2435. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, Y.Z.; Xie, S.H.; Qiao, M.H.; Li, H.X.; Fan, K.N. Partial hydrogenation of benzene to cyclohexene on a Ru-Zn/m-ZrO2 nanocomposite catalyst. Appl. Catal. A Gen. 2004, 272, 29–36. [Google Scholar] [CrossRef]
- Mazzieri, V.A.; L’Argentiѐre, P.C.; Coloma-Pascual, F.; Fígoli, N.S. Effect of Chlorine on the Properties of Ru/Al2O3. Ind. Eng. Chem. Res. 2003, 42, 2269–2272. [Google Scholar] [CrossRef]
- Ramos-Fernández, E.V.; Ferreira, A.F.P.; Sepúlveda-Escribano, A.; Kapteijn, F.; Rodríguez-Reinoso, F. Enhancing the catalytic performance of Pt/ZnO in the selective hydrogenation of cinnamaldehyde by Cr addition to the support. J. Catal. 2008, 258, 52–60. [Google Scholar] [CrossRef]
- Evans, S. Energy calibration secondary standards for X-ray photoelectron spectrometers. Surf. Interface Anal. 1985, 7, 299–302. [Google Scholar] [CrossRef]
- Mizuno, N.; Fujii, H.; Igarashi, H.; Misono, M. Formation of lanthanum cobalt oxide (LaCoO3) highly dispersed on zirconium dioxide. J. Am. Chem. Soc. 1992, 114, 7151–7158. [Google Scholar] [CrossRef]
- Struijk, J.; d’Angremond, M.; Lucas-de Regt, W.J.M.; Scholten, J.J.F. Partial liquid phase hydrogenation of benzene to cyclohexene over ruthenium catalysts in the presence of an aqueous salt solution I. Preparation, characterization of the catalyst and study of a number of process variables. Appl. Catal. A Gen. 1992, 83, 263–295. [Google Scholar] [CrossRef]
- Milone, C.; Neri, G.; Donato, A.; Musolino, M.G.; Mercadant, L. Selective Hydrogenation of Benzene to Cyclohexene on Ru/γ-Al2O3. J. Catal. 1996, 159, 253–258. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Sun, H.J.; Wang, D.B.; Guo, W.; Zhou, X.L.; Liu, Z.Y.; Li, Z.J. Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalyst with Nanosized Zirconia as Dispersant. Chin. J. Catal. 2010, 31, 150–152. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, L.X.; Huang, Z.X.; Sun, L.L.; Li, Y.Y.; Liu, S.C.; Liu, Z.Y. Effect of reduction medium and reduction temperature on the performance of Ru-Zn catalysts for selective hydrogenation of benzene to cyclohexene. Chem. Ind. Eng. Prog. 2017, 36, 2962–2970. [Google Scholar]





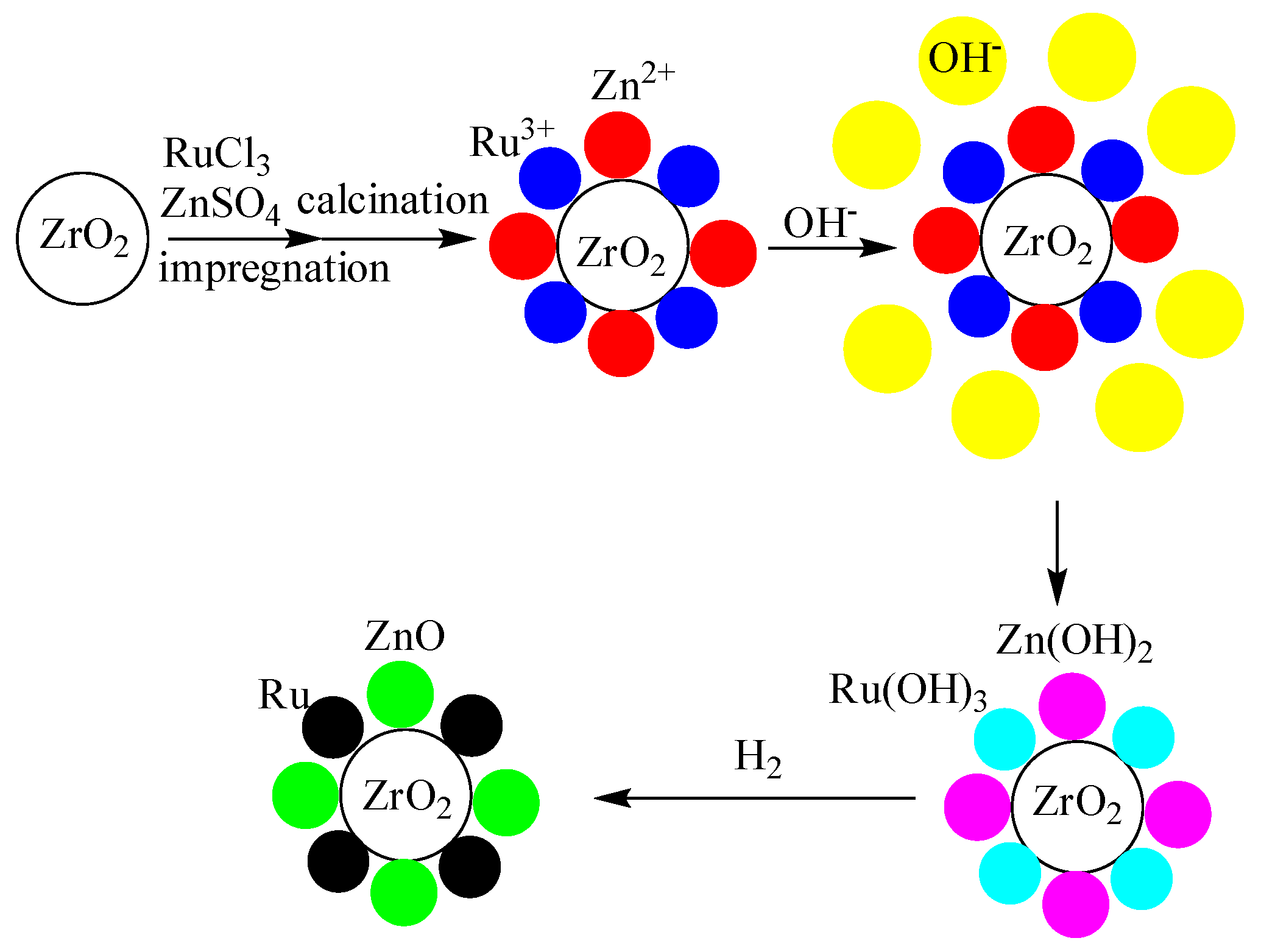
| Catalyst | SBET/(m2·g−1) 1 | Vpore/(cm3·g−1) 1 | dpore/(nm) 1 | nZn/nRu 2 | nZr/nRu 2 | pH 3 |
|---|---|---|---|---|---|---|
| ZrO2 | 34 | 0.13 | 16.0 | - | - | - |
| Ru(0)/ZrO2 | 31 | 0.10 | 10.7 | 0 | 5.19 | - |
| Ru-Zn(0.06)/ZrO2 | 30 | 0.09 | 9.8 | 0.06 | 5.30 | - |
| Ru-Zn(0.33)/ZrO2 | 30 | 0.09 | 9.7 | 0.33 | 5.26 | - |
| Ru-Zn(0.47)/ZrO2 | 30 | 0.10 | 9.6 | 0.47 | 5.24 | - |
| Ru-Zn(0.60)/ZrO2 | 30 | 0.09 | 9.7 | 0.60 | 5.38 | - |
| Ru-Zn(0.69)/ZrO2 | 28 | 0.08 | 9.6 | 0.69 | 5.26 | - |
| Ru-Zn(0.86)/ZrO2 | 28 | 0.08 | 9.1 | 0.86 | 5.25 | - |
| Ru-Zn(1.02)/ZrO2 | 29 | 0.08 | 9.1 | 1.02 | 5.16 | - |
| Ru(0)/ZrO2 AH | 30 | 0.11 | 9.5 | 0.20 | 5.21 | 4.25 |
| Ru-Zn(0.06)/ZrO2 AH | 30 | 0.10 | 9.8 | 0.25 | 5.29 | 4.25 |
| Ru-Zn(0.33)/ZrO2 AH | 30 | 0.09 | 9.9 | 0.37 | 5.38 | 5.38 |
| Ru-Zn(0.47)/ZrO2 AH | 30 | 0.08 | 9.8 | 0.55 | 5.36 | 5.38 |
| Ru-Zn(0.60)/ZrO2 AH | 30 | 0.08 | 10.1 | 0.68 | 5.32 | 5.38 |
| Ru-Zn(0.69)/ZrO2 AH | 28 | 0.07 | 10.3 | 0.86 | 5.30 | 5.44 |
| Ru-Zn(0.86)/ZrO2 AH | 29 | 0.08 | 10.6 | 1.10 | 5.26 | 5.82 |
| Ru-Zn(1.02)/ZrO2 AH | 29 | 0.06 | 11.4 | 1.25 | 5.31 | 5.73 |
| Catalyst | SBET/(m2·g−1) 1 | Vpore/(cm3·g−1) 1 | dpore/(nm) 1 | nZn/nRu 2 | nZr/nRu 2 | pH 3 |
|---|---|---|---|---|---|---|
| Ru-Zn(0.60)/ZrO2 | 30 | 0.09 | 9.7 | 0.60 | 5.38 | - |
| Ru-Zn(0.60)/ZrO2 AH | 30 | 0.08 | 10.1 | 0.68 | 5.33 | 5.38 |
| Ru-Zn(0.60) | 65 | 0.19 | 11.7 | 0.61 | 0 | - |
| Ru-Zn(0.60)+ZrO2 AH | 33 | 0.12 | 14.6 | 0.65 | 5.35 | 5.29 |
| ZrO2 | 34 | 0.13 | 16.0 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Chen, Z.; Chen, L.; Li, H.; Peng, Z.; Liu, Z.; Liu, S. Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Investigations on the Effect of Zn Content and ZrO2 as the Support and Dispersant. Catalysts 2018, 8, 513. https://doi.org/10.3390/catal8110513
Sun H, Chen Z, Chen L, Li H, Peng Z, Liu Z, Liu S. Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Investigations on the Effect of Zn Content and ZrO2 as the Support and Dispersant. Catalysts. 2018; 8(11):513. https://doi.org/10.3390/catal8110513
Chicago/Turabian StyleSun, Haijie, Zhihao Chen, Lingxia Chen, Huiji Li, Zhikun Peng, Zhongyi Liu, and Shouchang Liu. 2018. "Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Investigations on the Effect of Zn Content and ZrO2 as the Support and Dispersant" Catalysts 8, no. 11: 513. https://doi.org/10.3390/catal8110513
APA StyleSun, H., Chen, Z., Chen, L., Li, H., Peng, Z., Liu, Z., & Liu, S. (2018). Selective Hydrogenation of Benzene to Cyclohexene over Ru-Zn Catalysts: Investigations on the Effect of Zn Content and ZrO2 as the Support and Dispersant. Catalysts, 8(11), 513. https://doi.org/10.3390/catal8110513





