Surface Modification of Hematite Photoanodes for Improvement of Photoelectrochemical Performance
Abstract
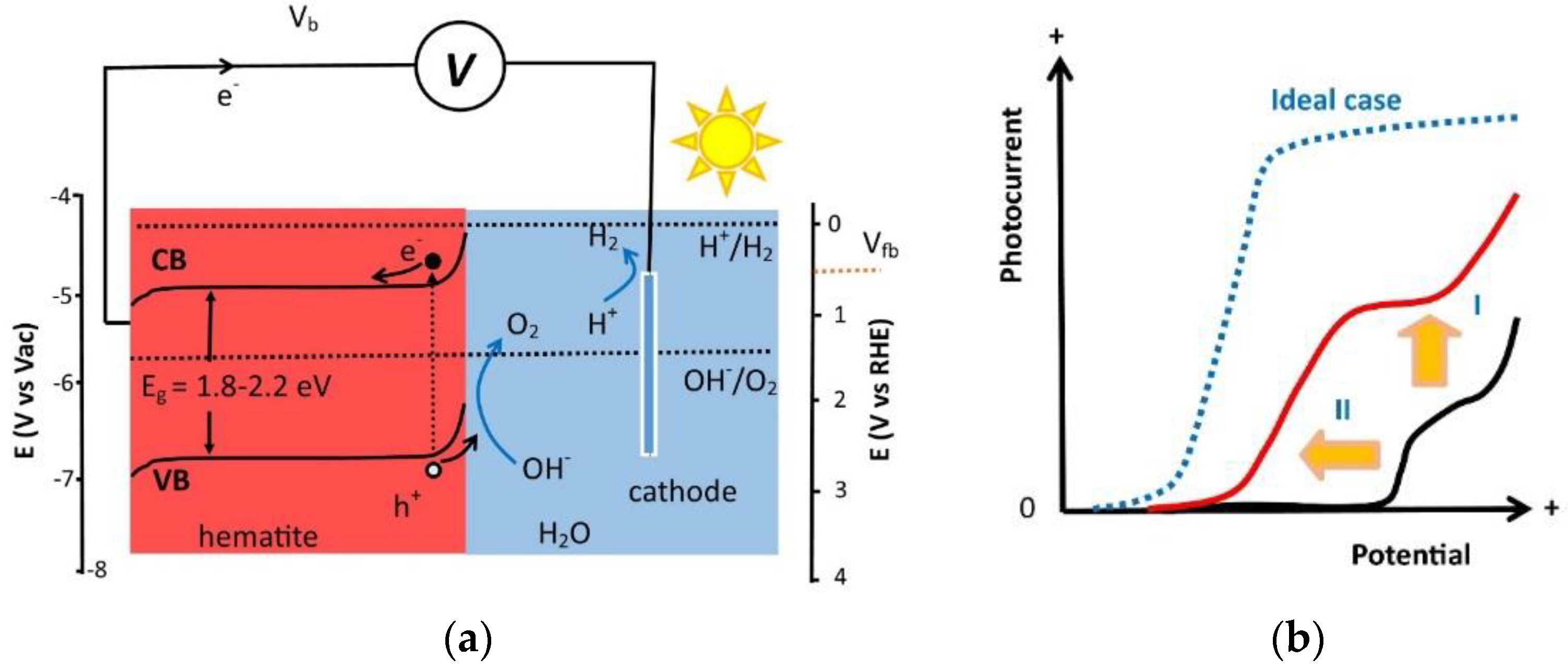
1. Introduction
2. Surface Modification Strategies
2.1. Co-Catalyst
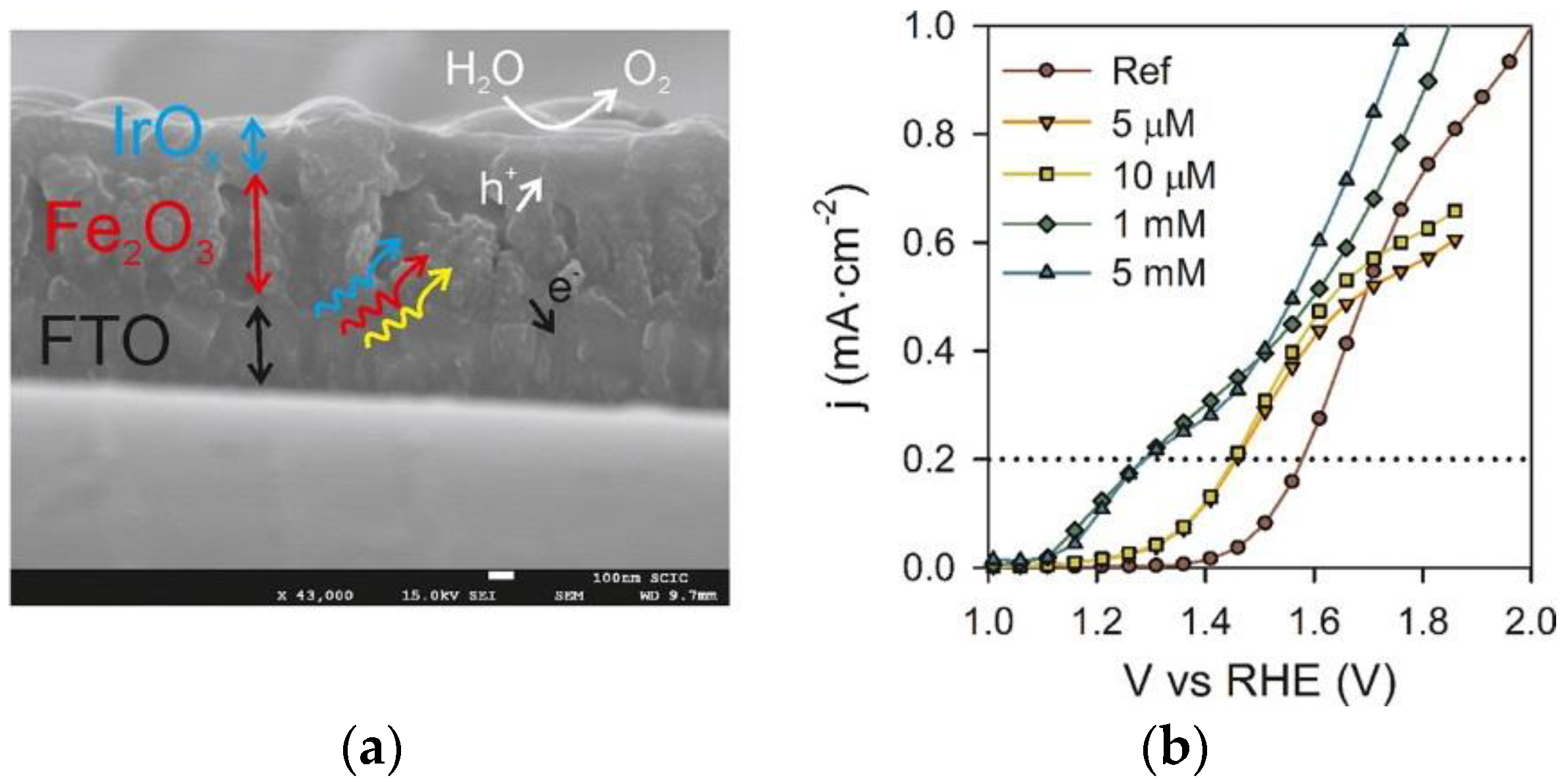
2.1.1. Noble Metal Based Co-Catalysts
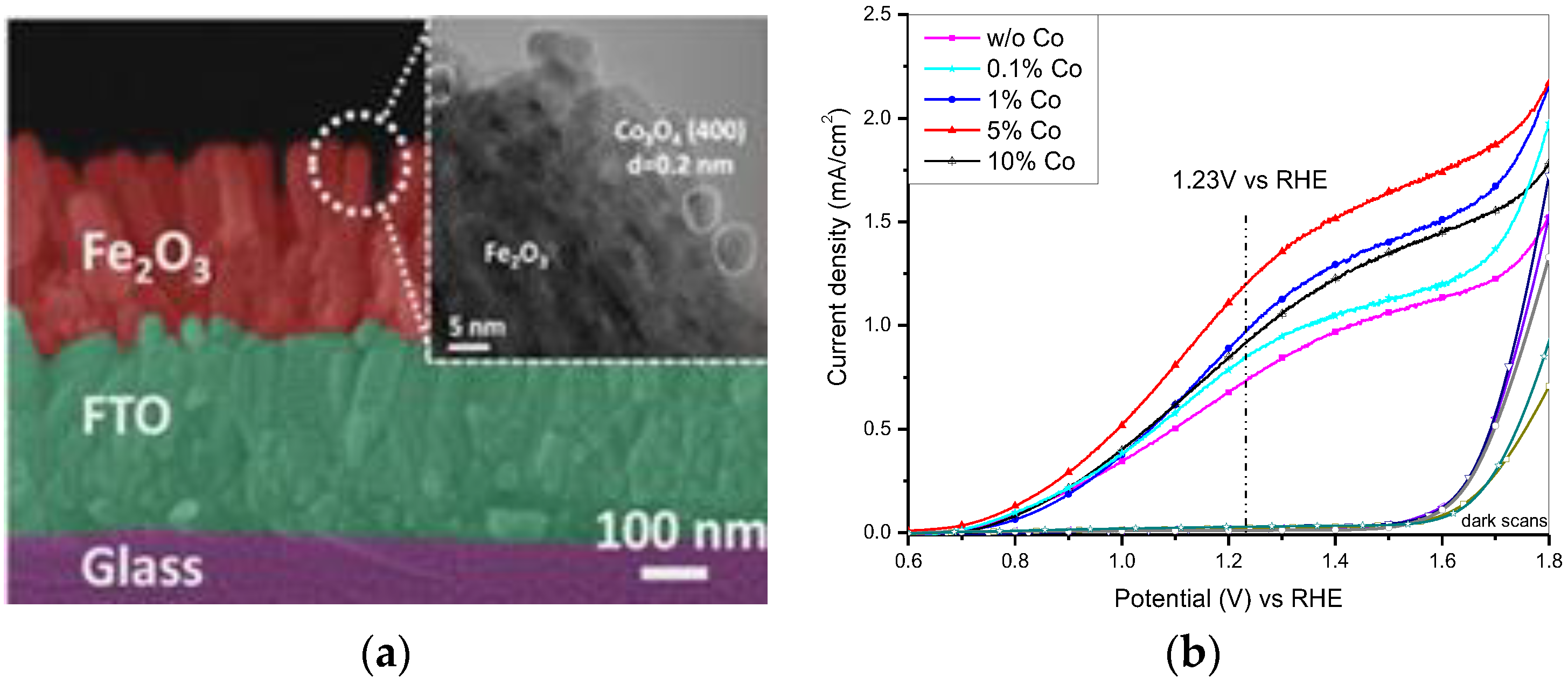
2.1.2. Earth Abundant Metal Based Co-Catalysts
2.2. Enhancing Photoabsorption
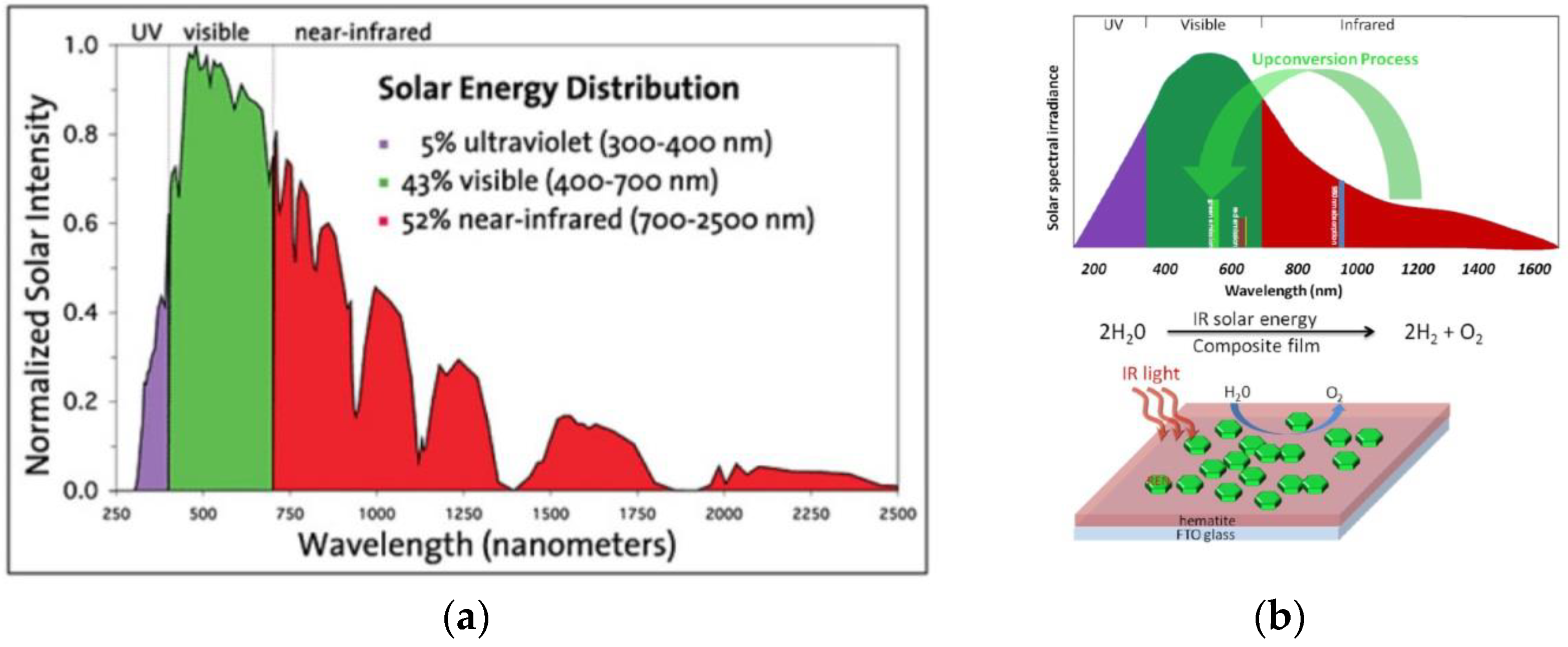
2.2.1. Rare Earth Element Decoration
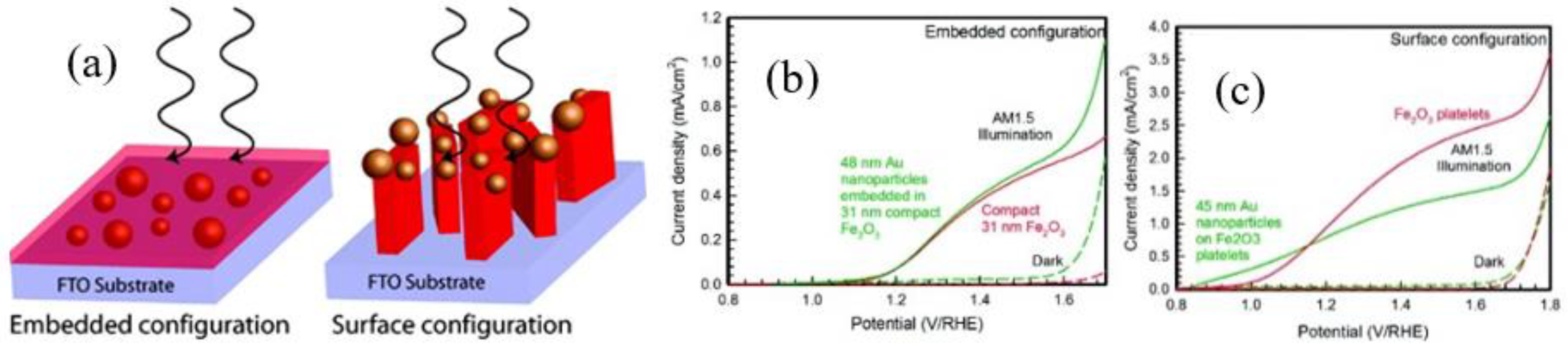
2.2.2. Surface Plasmonic Metal Decorated Hematite
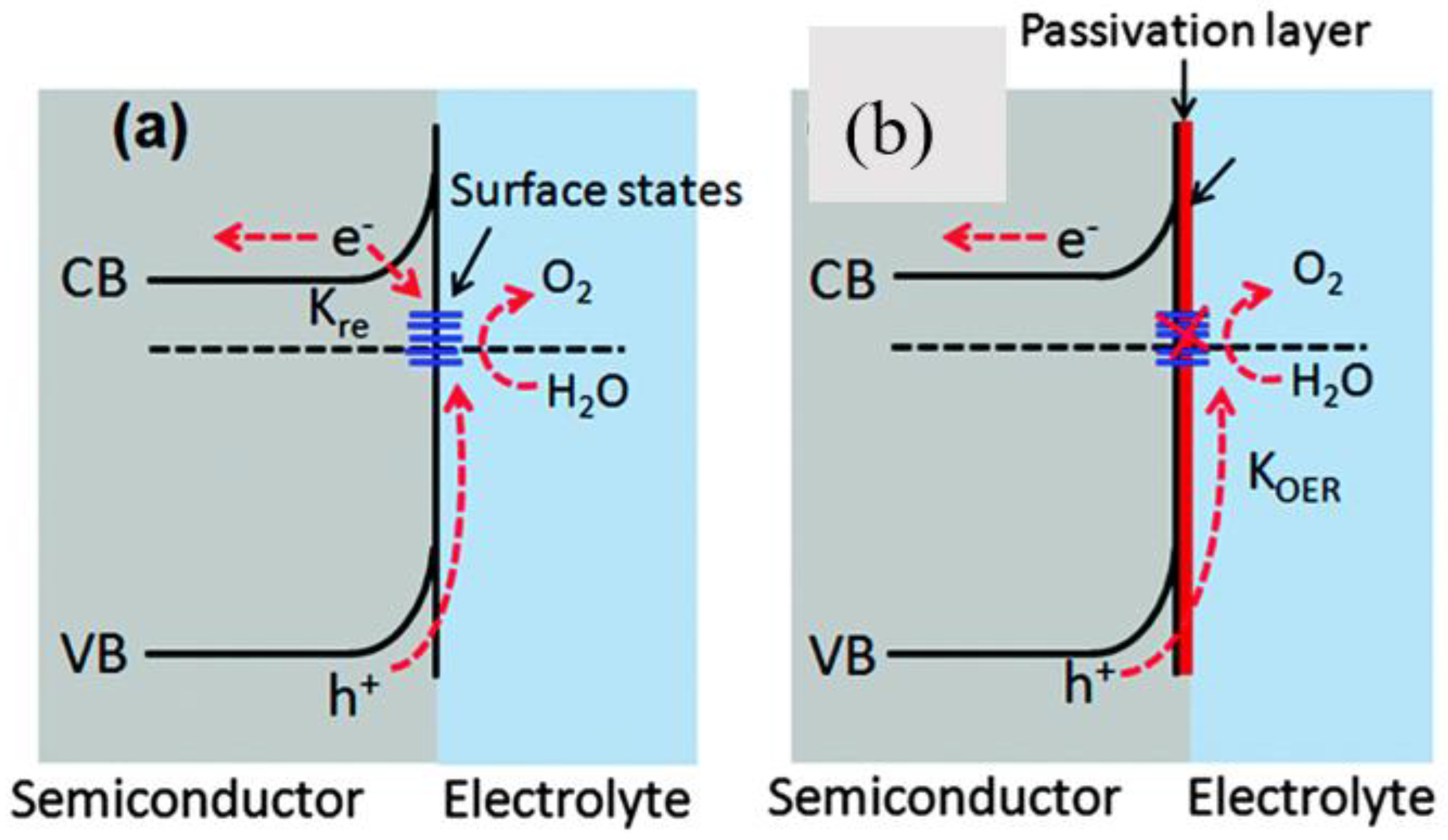
2.3. Surface Passivation
2.4. Surface Chemical Etching
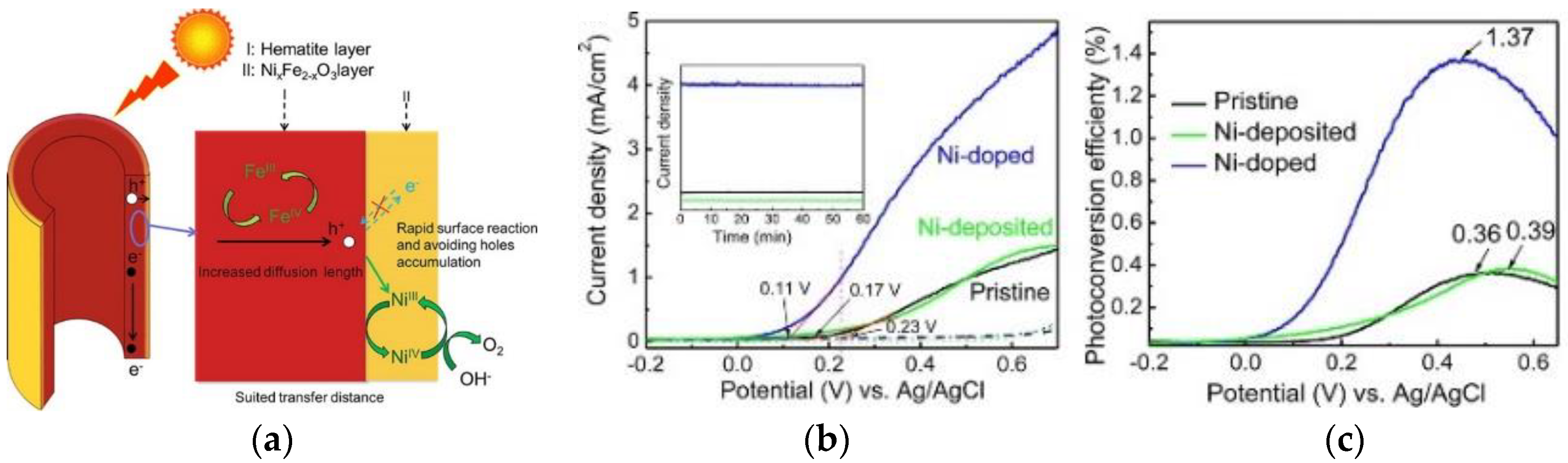
2.5. Surface Doping
3. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Química Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Yang, J.H.; Wang, D.G.; Han, H.X.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Schwanke, C.; Zhou, D.; Drevon, D.; van de Krol, R.; Lange, K.M. In situ XAS study of CoBi modified hematite photoanodes. Dalton Trans. 2017, 46, 15719–15726. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.B.; Barnes, P.R.F.; Randeniya, L.K.; Plumb, I.C.; Grey, I.E.; Horne, M.D.; Glasscock, J.A. Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen Energy 2006, 31, 1999–2017. [Google Scholar] [CrossRef]
- Le Formal, F.; Tetreault, N.; Cornuz, M.; Modehl, T.; Grätzel, M.; Sivula, K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737–743. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Schwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Tilley, S.D.; Cornuz, M.; Sivula, K.; Grätzel, M. Light-induced water splitting with hematite: Improved nanostructure and iridium oxide catalysis. Angew. Chem. Int. Ed. 2010, 49, 6405–6408. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Chiam, S.Y.; Mak, W.F.; Tran, P.D.; Barber, J.; Loo, S.C.J.; Wong, L.H. A novel strategy for surface treatment on hematite photoanode for efficient water oxidation. Chem. Sci. 2013, 4, 164–169. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. WO3−Fe2O3 photoanodes for water splitting: A host scaffold, guest absorber approach. Chem. Mater. 2009, 21, 2862–2867. [Google Scholar] [CrossRef]
- Wang, K.X.; Yu, Z.; Liu, V.; Brongersma, M.L.; Jaramillo, T.F.; Fan, S. Nearly total solar absorption in ultrathin nanostructured iron oxide for efficient photoelectrochemical water splitting. ACS Photonics 2014, 1, 235–240. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.N.; Hwang, B.J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Wu, H.Y.; Ren, F.; Xing, Z.; Zheng, X.D.; Wu, L.; Jiang, C.Z. Cathodic shift of onset potential for water oxidation of WO3 photoanode by Zr+ ions implantation. J. Appl. Phys. 2017, 121, 085305. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Y. Review of Sn-doped hematite nanostructures for photoelectrochemical water splitting. Part. Part. Syst. Charact. 2014, 31, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Ma, W.; Chen, C.; Song, W.; Zhao, J. Doping-promoted solar water oxidation on hematite photoanodes. Molecules 2016, 21, 868. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zheng, Z.; Spurgeon, J.; Yang, X. Enhanced photoelectrochemical water-splitting performance of semiconductors by surface passivation layers. Energy Environ. Sci. 2014, 7, 2504–2517. [Google Scholar] [CrossRef]
- Badia-Bou, L.; Mas-Marza, E.; Rodenas, P.; Barea, E.M.; Fabregat-Santiago, F.; Gimenez, S.; Peris, E.; Bisquert, J. Water oxidation at hematite photoelectrodes with an iridium-based catalyst. J. Phys. Chem. C 2013, 117, 3826–3833. [Google Scholar] [CrossRef]
- Li, W.; Sheehan, S.W.; He, D.; He, Y.; Yao, X.; Grimm, R.L.; Brudvig, G.W.; Wang, D. Hematite-based solar water splitting in acidic solutions: Functionalization by mono- and multilayers of iridium oxygen-evolution catalysts. Angew. Chem. Int. Ed. 2015, 54, 11428–11432. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.A.; Khan, S.U.M. Photoelectrolysis of water at bare and electrocatalyst covered thin film iron oxide electrode. Int. J. Hydrogen Energy 1994, 19, 881–887. [Google Scholar] [CrossRef]
- Fan, K.; Li, F.; Wang, L.; Daniel, Q.; Chen, H.; Gabrielsson, E.; Sun, J.; Sun, L. Immobilization of a molecular ruthenium catalyst on hematite nanorod arrays for water oxidation with stable photocurrent. ChemSusChem 2015, 8, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, X.; Liu, Z.; Zhuang, L.; Lu, J. Promoting the photoanode efficiency for water splitting by combining hematite and molecular Ru catalysts. Electrochem. Commun. 2013, 27, 148–151. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Li, Z.; Yu, T.; Zou, Z. Surface modification of hematite photoanode films with rhodium. Rare Met. 2011, 30, 38–41. [Google Scholar] [CrossRef]
- Warwick, M.E.A.; Barreca, D.; Bontempi, E.; Carraro, G.; Gasparotto, A.; Maccato, C.; Kaunisto, K.; Ruokozmo, T.-P.; Lemmetyinen, H.; Sada, C.; et al. Pt-functionalized Fe2O3 photoanodes for solar water splitting: The role of hematite nano-organization and the platinum redox state. Phys. Chem. Chem. Phys. 2015, 17, 12899–12907. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Seabold, J.A.; Choi, K.S. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem. Mater. 2011, 23, 1105–1112. [Google Scholar] [CrossRef]
- Pijpers, J.J.; Winkler, M.T.; Surendranath, Y.; Buonassisi, T.; Nocera, D.G. Light-induced water oxidation at silicon electrodes functionalized with a cobalt oxygen-evolving catalyst. Proc. Natl. Acad. Sci. USA 2011, 108, 10056–10061. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Sun, J.; Inumaru, H.; Gamelin, D.R. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes. J. Am. Chem. Soc. 2009, 131, 6086–6087. [Google Scholar] [CrossRef] [PubMed]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Hamann, T.W. Photoelectrochemical and impedance spectroscopic investigation of water oxidation with “Co-Pi”-coated hematite electrodes. J. Am. Chem. Soc. 2012, 134, 16693–16700. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Bassi, P.S.; Chiam, S.Y.; Mak, W.F.; Tran, P.D.; Barber, J.; Chye Loo, J.S.; Wong, L.H. Co3O4-decorated hematite nanorods as an effective photoanode for solar water oxidation. J. Phys. Chem. C 2012, 116, 13884–13889. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Zhai, T.; Qian, F.; Tong, Y.; Li, Y. A mechanistic study into the catalytic effect of Ni(OH)2 on hematite for photoelectrochemical water oxidation. Nanoscale 2013, 5, 4129–4133. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.R.; Liu, Z.; Al-Bukhari, S.F.; Lee, C.J.; Yung, D.L.; Chi, D.; Hor, T.S. Effect of oxygen evolution catalysts on hematite nanorods for solar water oxidation. Chem. Commun. 2011, 47, 10653–10655. [Google Scholar] [CrossRef] [PubMed]
- Halaoui, L. Photoelectrochemical investigation of the mechanism of enhancement of water oxidation at the hematite nanorod array modified with “NiBi”. J. Phys. Chem. C 2016, 120, 22766–22776. [Google Scholar] [CrossRef]
- Young, K.M.; Hamann, T.W. Enhanced photocatalytic water oxidation efficiency with Ni(OH)2 catalysts deposited on α-Fe2O3 via ALD. Chem. Commun. 2014, 50, 8727–8730. [Google Scholar] [CrossRef] [PubMed]
- Malara, F.; Minguzzi, A.; Marelli, M.; Morandi, S.; Psaro, R.; Santo, V.D.; Naldoni, A. α-Fe2O3/NiOOH: An effective heterostructure for photoelectrochemical water oxidation. ACS Catal. 2015, 5, 5292–5300. [Google Scholar] [CrossRef]
- Yang, X.; Du, C.; Liu, R.; Xie, J.; Wang, D. Balancing photovoltage generation and charge-transfer enhancement for catalyst-decorated photoelectrochemical water splitting: A case study of the hematite/MnOx combination. J. Catal. 2013, 304, 86–91. [Google Scholar] [CrossRef]
- Deng, J.; Lv, X.; Zhang, H.; Zhao, B.; Sun, X.; Zhong, J. Loading the FeNiOOH cocatalyst on Pt-modified hematite nanostructures for efficient solar water oxidation. Phys. Chem. Chem. Phys. 2016, 18, 10453–10458. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Rui, Y.; Li, Y.; Zhang, Q.; Wang, H. Zn-Co layered double hydroxide modified hematite photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2015, 358, 436–442. [Google Scholar] [CrossRef]
- Selvaraj, S.; Moon, H.; Kim, D.H. Combined effect of nano-structured NiCo2S4 coated hematite photoanodes for efficient photoelectrochemical water oxidation. Catal. Today 2018, in press. [Google Scholar] [CrossRef]
- Kuang, Y.; Yamada, T.; Domen, K. Surface and interface engineering for photoelectrochemical water oxidation. Joule 2017, 1, 290–305. [Google Scholar] [CrossRef]
- Leduc, J.; Gönüllü, Y.; Raauf, A.; Fischer, T.; Mathur, S. Rare-earth-containing materials for photoelectrochemical water splitting applications. In Semiconductors for Photocatalysis; Mi, Z., Wang, L., Jagadish, C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 97, pp. 185–215. [Google Scholar] [CrossRef]
- Enhance Solar Water Splitting Performance by Utilizing Near Infrared Radiation with Composite Films of Hematite and Rare Earth Doped Upconversion Materials. 2012. Available online: http://icam-i2cam.org/images/uploads/Zhang_Presentation.pdf (accessed on 22 July 2018).
- Zhang, M.; Lin, Y.; Mullen, T.J.; Lin, W.-F.; Sun, L.-D.; Yan, C.-H.; Patten, T.E.; Wang, D.; Liu, G.-Y. Improving hematite’s solar water splitting efficiency by incorporating rare-earth upconversion nanomaterials. J. Phys. Chem. Lett. 2012, 3, 3188–3192. [Google Scholar] [CrossRef] [PubMed]
- Cots, A.; Gómez, R. Ytterbium modification of pristine and molybdenum-modified hematite electrodes as a strategy for efficient water splitting photoanodes. Appl. Catal. B 2017, 219, 492–500. [Google Scholar] [CrossRef]
- Liu, R.; Yang, X.; Anfuso, C.L.; Huang, Z.; Han, L. Plasmonic α-Fe2O3 photoanodes for solar water splitting. Rev. Adv. Sci. Eng. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Hermann, L.O.; Baumberg, J.J. Size dependent plasmonic effect on BiVO4 photoanodes for solar water splitting. Sci. Rep. 2015, 5, 16660. [Google Scholar] [CrossRef] [PubMed]
- Thimsen, E.; Le Formal, F.; Grätzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe2O3 electrodes for water splitting. Nano Lett. 2011, 11, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Archana, S.A.; Pachauri, N.; Shan, Z.C.; Pan, S.L.; Gupta, A. Plasmonic enhancement of photoactivity by gold nanoparticles embedded in hematite films. J. Phys. Chem. C 2015, 119, 15506–15516. [Google Scholar] [CrossRef]
- Shinde, P.S.; Choi, S.H.; Kim, Y.; Ryu, J.; Jang, J.S. Onset potential behavior in α-Fe2O3 photoanodes: The influence of surface and diffusion Sn doping on the surface states. Phys. Chem. Chem. Phys. 2016, 18, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Le Formal, F.; Cornuz, M.; Brillet, J.; Tétreault, N.; Sivula, K.; Grätzel, M. Cathodic shift in onset potential of solar oxygen evolution on hematite by 13-group oxide overlayers. Energy Environ. Sci. 2011, 4, 2512–2515. [Google Scholar] [CrossRef]
- Le Formal, F.; Sivula, K.; Grätzel, M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. J. Phys. Chem. C 2012, 116, 26707–26720. [Google Scholar] [CrossRef]
- Xi, L.F.; Bassi, P.S.; Chiam, S.Y.; Mak, W.F.; Tran, P.D.; Barber, J.; Chye Loo, J.S.; Wong, L.H. Surface treatment of hematite photoanodes with zinc acetate for water oxidation. Nanoscale 2012, 4, 4430–4433. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, R.; Du, C.; Dai, P.; Zheng, Z.; Wang, D. Improving hematite-based photoelectrochemical water splitting with ultrathin TiO2 by atomic layer deposition. ACS Appl. Mater. Interfaces 2014, 6, 12005–12011. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Carraro, G.; Gasparotto, A.; Maccato, C.; Warwick, M.E.A.; Kaunisto, K.; Sada, C.; Turner, S.; Gönüllü, Y.; Ruoko, T.-P. Fe2O3-TiO2 nano-heterostructure photoanodes for highly efficient solar water oxidation. Adv. Mater. Interfaces 2015, 2, 1500313. [Google Scholar] [CrossRef]
- Xiong, D.; Li, W.; Wang, X.; Liu, L. Passivation of hematite nanorod photoanodes with a phosphorus overlayer for enhanced photoelectrochemical water oxidation. Nanotechnology 2016, 27, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Antony, R.P.; Wong, L.H.; Ng, D.H.L. Promotional effects of cetyltrimethylammonium bromide surface modification on a hematite photoanode for photoelectrochemical water splitting. RSC Adv. 2015, 5, 100142–100146. [Google Scholar] [CrossRef]
- Chernomordik, B.D.; Russell, H.B.; Cvelbar, U.; Jasinski, J.B.; Kumar, V.; Deutsch, T.; Sunkara, M.K. Photoelectrochemical activity of as-grown, α-Fe2O3 nanowire array electrodes for water splitting. Nanotechnology 2012, 23, 194009. [Google Scholar] [CrossRef] [PubMed]
- Rioult, M.; Belkhou, R.; Magnan, H.; Stanescu, D.; Stanescu, S.; Maccherozzi, F.; Rountree, C.; Barbier, A. Local electronic structure and photoelectrochemical activity of partial chemically etched Ti-doped hematite. Surf. Sci. 2015, 641, 310–313. [Google Scholar] [CrossRef]
- Cao, D.; Luo, W.; Feng, J.; Zhao, X.; Li, Z.; Zou, Z. Cathodic shift of onset potential for water oxidation on a Ti4+ doped Fe2O3 photoanode by suppressing the back reaction. Energy Environ. Sci. 2014, 7, 752–759. [Google Scholar] [CrossRef]
- Li, M.; Deng, J.; Pu, A.; Zhang, P.; Zhang, H.; Gao, J.; Hao, Y.; Zhong, J.; Sun, X. Hydrogen-treated hematite nanostructures with low onset potential for highly efficient solar water oxidation. J. Mater. Chem. A 2014, 2, 6727–6733. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Kleiman-Shwarsctein, A.; Stucky, G.D.; McFarland, E.W. Improved photoelectrochemical performance of Ti-doped α-Fe2O3 thin films by surface modification with fluoride. Chem. Commun. 2009, 2652–2654. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zong, X.; Wang, Z.; Li, C. A hematite photoanode with gradient structure hows an unprecedentedly low onset potential for photoelectrochemical water oxidation. Phys. Chem. Chem. Phys. 2014, 16, 23544–23548. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Han, H.S.; Logar, M.; Park, J.; Zheng, X.L. Enhancing low-bias performance of hematite photoanodes for solar water splitting by simultaneous reduction of bulk, interface, and surface recombination pathways. Adv. Energy Mater. 2016, 6, 1501840. [Google Scholar] [CrossRef]
- Cheng, W.; He, J.; Sun, Z.; Peng, Y.; Yao, T.; Liu, Q.; Jiang, Y.; Hu, F.; Xie, Z.; He, B.; et al. Ni-doped overlayer hematite nanotube: A highly photoactive architecture for utilization of visible light. J. Phys. Chem. C 2012, 116, 24060–24067. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, J.; Dong, C.L.; Hu, Y.; Tseng, E.N.; Guo, P.; Guo, L.; Mao, S.S. Surface engineered doping of hematite nanorod arrays for improved photoelectrochemical water splitting. Sci. Rep. 2014, 4, 6627. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Wang, F.; Schwanke, C.; Abdi, F.F.; Golnak, R.; Fiechter, S.; Ellmer, K.; van de Krol, R.; Lange, K.M. In situ structural study of MnPi modified BiVO4 photoanodes by soft X-ray absorption spectroscopy. J. Phys. Chem. C 2017, 121, 19668–19676. [Google Scholar] [CrossRef]
| Noble Metal | Preparation | Electrolyte | Onset Potential (VRHE) | Onset Potential shift (mV) | Photocurrent at 1.23 VRHE (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|
| IrO2 | Electrophoresis | 1 M NaOH | 0.8 | 200 | ~3.2 | [9] |
| IrOx | Electrodeposition | 0.1 M KPi (pH 7) | ~1.1 | 300 | 0.2 | [20] |
| IrOx | Photoelectrodeposition | 0.1 M KNO3 (pH = 1.01) | 0.6 | 250 | 0.66 | [21] |
| RuO2 | Spray pyrolysis | 0.2 M NaOH containing 0.5 M Na2SO4 (pH 13) | 0.22 a | 120 | ~0.68 b | [22] |
| Ru complex | Dip coating | 1 M NaOH | 0.72 | ~100 | ~2.2 | [23] |
| Ru complex | Self-assembly | 0.1 M KHC8H4O4-HCl buffer (pH 3) | 0.7 | ~200 | ~0.12 | [24] |
| Rh(OH)x | Precipitation | 1 M NaOH | ~0.2 | ~300 | 1.5 c | [25] |
| Pt | Sputtering | 0.1 M NaOH | 0.8 | No data | 0.638 | [26] |
| Transition Metal | Catalyst Preparation | Electrolyte | Onset Potential (VRHE) | Onset Potential shift (mV) | Photocurrent at 1.23 VRHE (mA/cm2) | Ref. | |
|---|---|---|---|---|---|---|---|
| Co | Co3O4 | in situ hydrothermal growth | 1 M NaOH | 0.66 | 40 | 1.20 | [32] |
| Co-Bi | photoelectrochemical deposition | 0.1 M NaBi (pH 9.2) | 0.8 | 50 | 1.12 | [4] | |
| Co-Pi | electrochemical deposition | 1 M NaOH | ~0.8 | 350 | 0.6 | [30] | |
| Co-Pi | photoelectrochemical deposition | 0.1M KPi (pH 6.9) with 0.2 M KCl | ~0.9 | ~240 | 0.4 | [31] | |
| Ni | Ni(OH)2 | ALD | 1 M KOH | 0.9 | ~300 | 0.4 | [36] |
| Ni(OH)2 | Dip coating | 1 M KOH and 0.1 M glucose | 0.7 | ~200 | 3.0 | [33] | |
| NiOOH | Photoelectrodeposition | 1 M NaOH | 0.62 | 150 | 0.625 | [37] | |
| Ni-Bi | Photodeposition | 1 M NaOH | 0.7 | ~200 | 0.55 | [34] | |
| Ni-Bi | Photodeposition | 1 M KBi (pH~9.2) | 0.6 | 140 | 0.65 | [35] | |
| Others | MnOx | ALD | 1 M NaOH | 0.1.6 | ~100 | <0.01 | [38] |
| FeNiOOH | Electrodeposition | 1 M NaOH | 0.57 | 190 | 2.21 | [39] | |
| ZnCo LDHs | Electrodeposition | 1 M KOH | ~1.0 | −100 | 1.73 | [40] | |
| NiCo2S4 | hydrothermal method | 1 M KOH | 0.51 | ~330 | 1.51 | [41] | |
| Passivation Materials | Preparation | Electrolyte | Onset Potential (VRHE) | Onset Potential shift (mV) | Photocurrent at 1.23 VRHE (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|
| Al2O3 | CBD | 1 M NaOH | 0.91 | 200 | 0.4 | [52] |
| Al2O3 | ALD | 1 M NaOH | 0.8 | 100 | 2.8 | [7] |
| Al2O3 | ALD | 1 M NaOH | 0.97 | ~90 | ~2.2 | [53] |
| Ga2O3 | CBD | 1 M NaOH | 0.8 | ~210 | ~0.45 | [52] |
| In2O3 | CBD | 1 M NaOH | 0.89 | ~100 | ~0.47 | [52] |
| ZnAc | Spin coating | 1 M NaOH | 0.76 | 170 | 1.08 | [54] |
| TiO2 | ALD | 1 M NaOH | ~0.9 | 100 | 1.014 | [55] |
| TiO2 | ALD | 1 M NaOH | 0.8 | ~250 | 2 | [56] |
| NaPO2H2 | Decompose | 1 M NaOH | 0.7 | 100 | 1.1 | [57] |
| CTAB | Hydrothermal | 1 M KOH | 0.6 | 0 | 0.45 | [58] |
| Modification Material | Preparation | Electrolyte | Onset Potential (VRHE) | Onset Potential Shift (mV) | Photocurrent at 1.23 VRHE (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|
| HF | Dip in HF solution | 1 M KOH | ~0.8 | No data | ~0.18 | [59] |
| HCl | Dip in HCl solution | 0.1 M NaOH | ~1.0 | 100 | ~1.0 | [60] |
| HCl | Dip in HCl solution | 1 M NaOH | 0.87 | 100 | 1.5 | [61] |
| NaBH4 | Pyrolysis by NaBH4 solution | 1 M NaOH | 0.87 | 120 | 2.28 | [62] |
| CoF3 | Dip in CoF3 solution | 1 M NaOH | 0.6 | 200 | ~1.5 | [63] |
| H2-O2 a | React in a H2-O2 flame | 1 M NaOH | 0.50 | No data | ~0.45 | [64] |
| Oxalic acid | Dip in oxalic acid solution | 1 M NaOH with and without H2O2 added | ~0.7 | <20 | ~1.4 | [65] |
| Modification Material | Preparation | Electrolyte | Onset Potential (VRHE) | Onset Potential Shift (mV) | Photocurrent at 1.23 VRHE (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|
| Sn4+ aqueous solution | Drop the solution on top and then anneal at high temperature | 1 M NaOH | 0.62 | 100 | 2.25 | [11] |
| Ni2+ aqueous solution | Electrodeposit and then anneal at high temperature | 1 M KOH | ~1.0 | 100 | ~1.0 | [66] |
| Ag+ aqueous solution | Dip in the Ag+ solution, dry and then anneal at high temperature | 0.5 M NaCl (pH 6.7) | 0.40 | 100 | ~0.1 | [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, L.; Lange, K.M. Surface Modification of Hematite Photoanodes for Improvement of Photoelectrochemical Performance. Catalysts 2018, 8, 497. https://doi.org/10.3390/catal8110497
Xi L, Lange KM. Surface Modification of Hematite Photoanodes for Improvement of Photoelectrochemical Performance. Catalysts. 2018; 8(11):497. https://doi.org/10.3390/catal8110497
Chicago/Turabian StyleXi, Lifei, and Kathrin M. Lange. 2018. "Surface Modification of Hematite Photoanodes for Improvement of Photoelectrochemical Performance" Catalysts 8, no. 11: 497. https://doi.org/10.3390/catal8110497
APA StyleXi, L., & Lange, K. M. (2018). Surface Modification of Hematite Photoanodes for Improvement of Photoelectrochemical Performance. Catalysts, 8(11), 497. https://doi.org/10.3390/catal8110497





