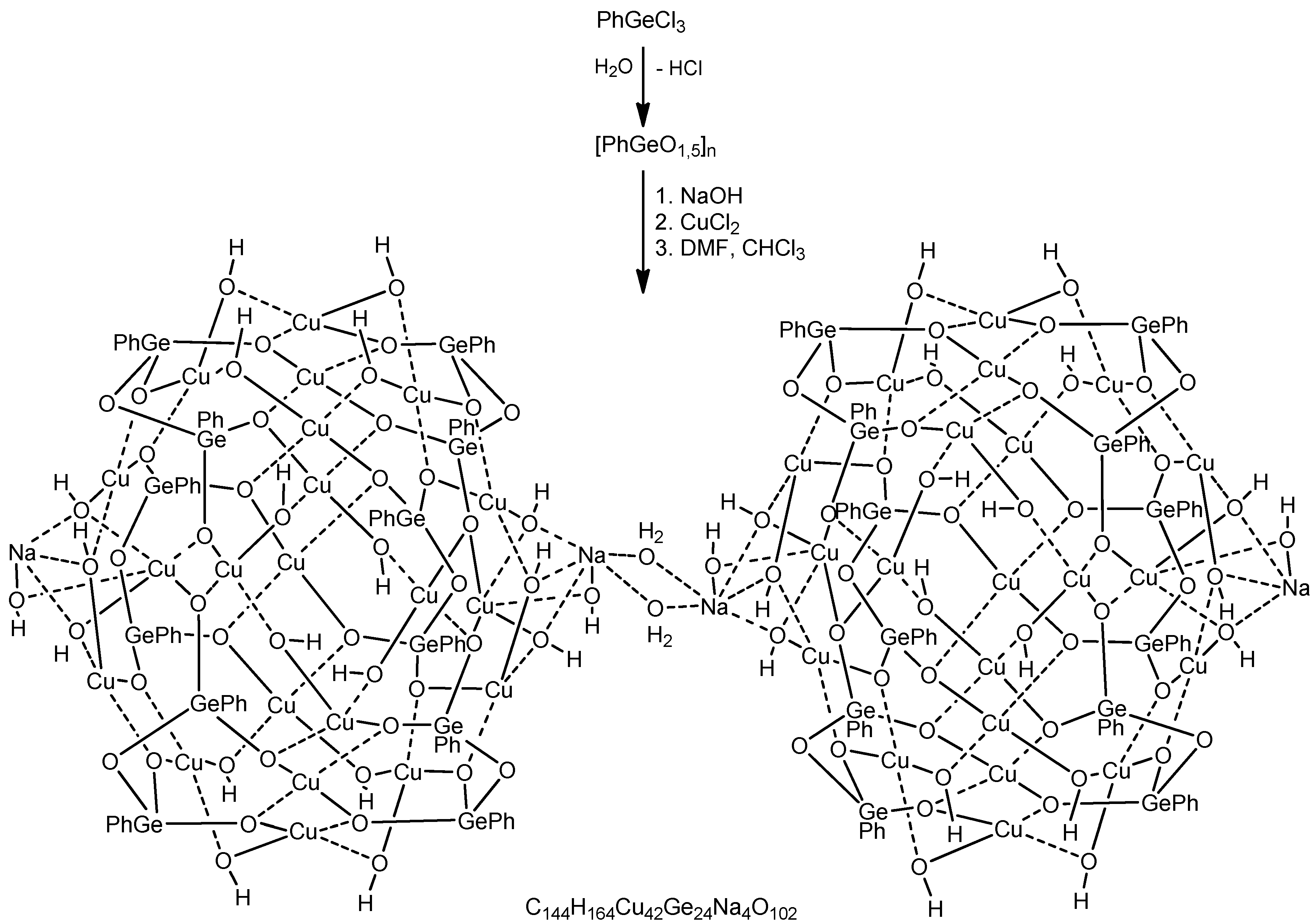
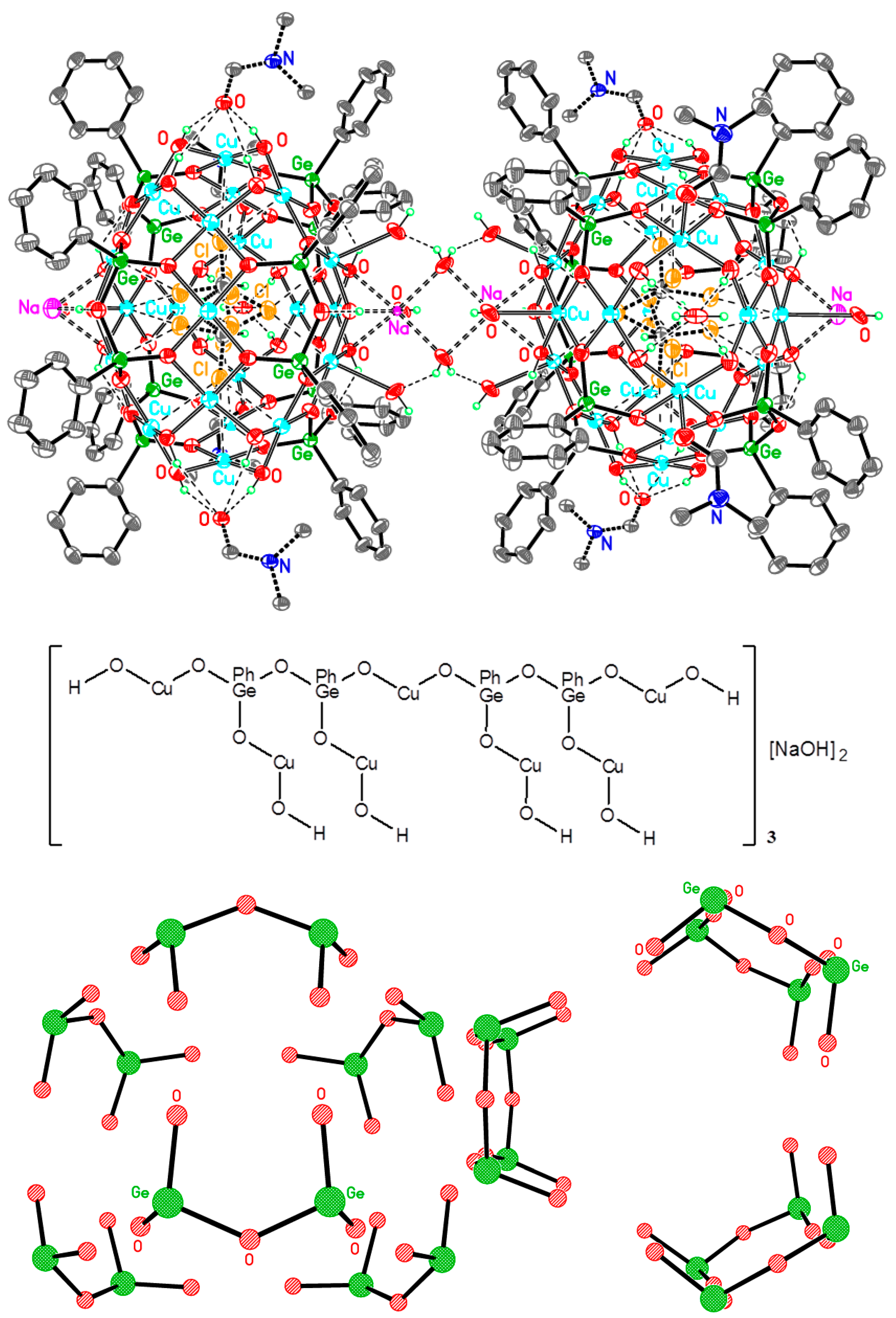
Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity
Abstract
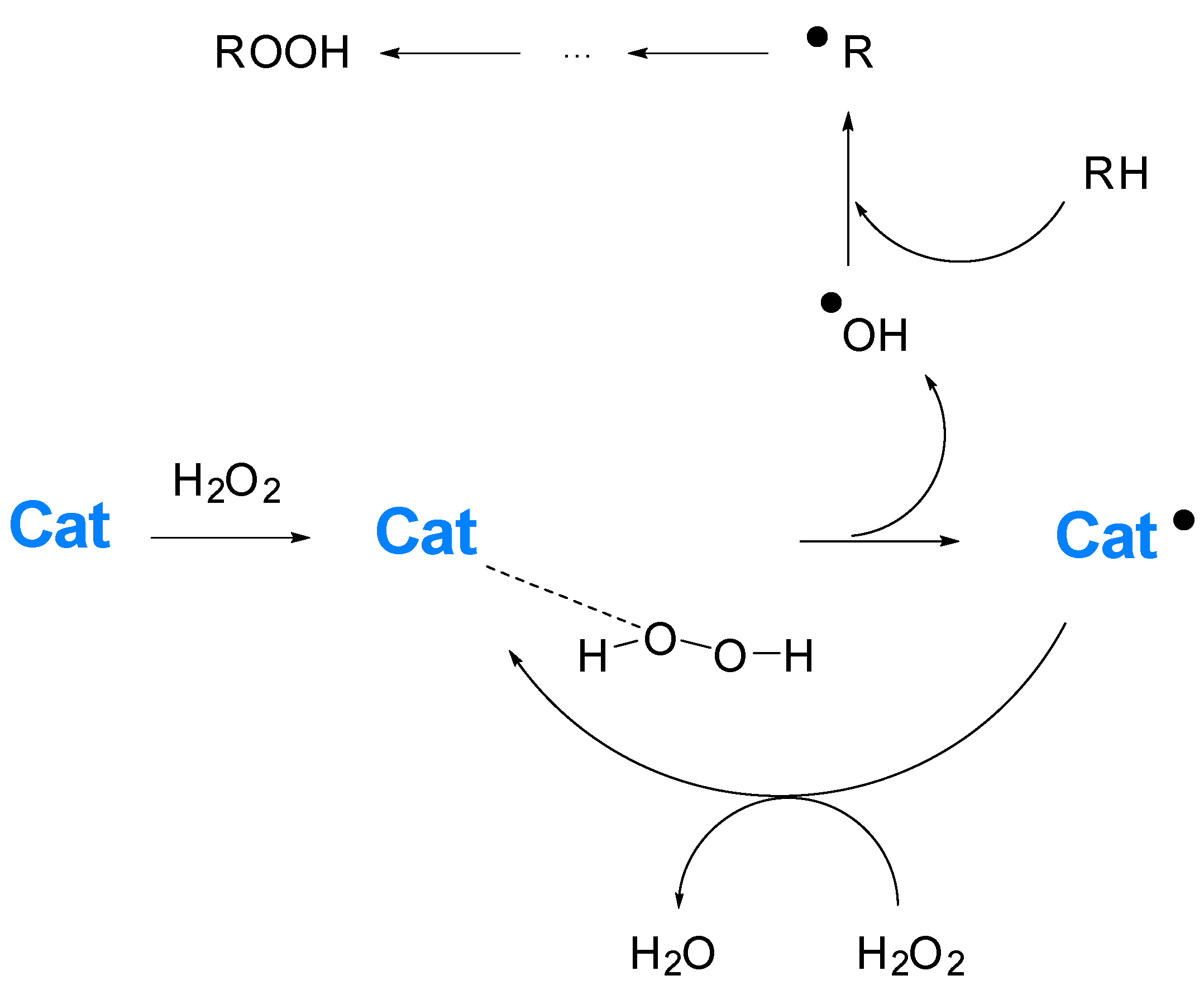
1. Introduction
2. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References and Note
- Fiedler, D.; Leung, D.H.; Bergman, R.G.; Raymond, K.N. Selective molecular recognition, C–H bond activation, and catalysis in nanoscale reaction vessels. Acc. Chem. Res. 2005, 38, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Zarra, S.; Wood, D.M.; Roberts, D.A.; Nitschke, J.R. Molecular containers in complex chemical systems. Chem. Soc. Rev. 2015, 44, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Yusubov, M.; Verpoort, F. Self-assembled metal-organic polyhedra: An overview of various applications. Coord. Chem. Rev. 2016, 306, 171–194. [Google Scholar] [CrossRef]
- Carbocyclic Cage Compounds: Chemistry and Applications; Osawa, E., Yonemitsu, O., Eds.; Wiley-VCH: Hoboken, NJ, USA, 1992; p. 409. [Google Scholar]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Mastalerz, M. Organic cage compounds—From shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Santolini, V.; Miklitz, M.; Berardo, E.; Jelfs, K.E. Topological landscapes of porous organic cages. Nanoscale 2017, 9, 5280–5298. [Google Scholar] [CrossRef] [PubMed]
- Smulders, M.M.J.; Riddell, I.A.; Browne, C.; Nitschke, J.R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.D.; Raithby, P.R. Functional behaviour from controlled self-assembly: Challenges and prospects. Chem. Soc. Rev. 2013, 42, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.M.; Clever, G.H. Integrative self-sorting of coordination cages based on ‘naked’ metal ions. Chem. Commun. 2017, 53, 8506–8516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Gao, W.-X.; Lin, L.; Jin, G.-X. Recent advances in the construction and applications of heterometallic macrocycles and cages. Coord. Chem. Rev. 2017, 344, 323–344. [Google Scholar] [CrossRef]
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and metallasiloxanes derived from silanediols, disilanols, silanetriols, and trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and polyhedral metallasilsesquioxanes of the early transition metals and f-elements. Coord. Chem. Rev. 2000, 206–207, 321–368. [Google Scholar] [CrossRef]
- Pinkert, D.; Limberg, C. Iron silicates, iron-modulated zeolite catalysts, and molecular models thereof. Chem. Eur. J. 2014, 20, 9166–9175. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Roesky, H.W.; Anantharaman, G.; Chandrasekhar, V.; Jancik, V.; Singh, S. Control of molecular topology and metal nuclearity in multimetallic assemblies: Designer metallosiloxanes derived from silanetriols. Chem. Eur. J. 2004, 10, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. Adv. Organomet. Chem. 2005, 53, 101–153. [Google Scholar]
- Edelmann, F.T. Metallasilsesquioxanes. Synthetic and Structural Studies. In Silicon Chemistry: From the Atom to Extended Systems; Jutzi, P., Schubert, U., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 383–394. [Google Scholar]
- He, H.; Cao, G.-J.; Zheng, S.-T.; Yang, G.-Y. Lanthanide germanate cluster organic frameworks constructed from {Ln8Ge12} or {Ln11Ge12} cage cluster building blocks. J. Am. Chem. Soc. 2009, 131, 15588–15589. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-J.; Zheng, S.-T.; Zhao, N.; Sun, J.-K.; Yang, G.-Y. Metal−organogermanate frameworks built by two kinds of infinite Ge−O chains with high thermostability and luminescent properties. Inorg. Chem. 2010, 49, 10211–10213. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Jargstorff, C.; Wriedt, S. Two new crystalline organogermanate-based inorganic-organic hybrid compounds. Z. Anorg. Allg. Chem. 2011, 637, 572–577. [Google Scholar] [CrossRef]
- Schmidt, C.; Lieb, A.; Stock, N. Synthesis and characterization of the organogermanate-based inorganic-organic hybrid compound Ca2[(OOCC2H4Ge)2O3]2·3H2O. Z. Anorg. Allg. Chem. 2011, 637, 2163–2168. [Google Scholar] [CrossRef]
- Schmidt, C.; Stock, N. High-throughput and in situ energy dispersive X-ray diffraction investigation on the formation of the new metal organogermanate Cu(OOCC2H4Ge)2O3. Cryst. Growth Des. 2011, 11, 5682–5687. [Google Scholar] [CrossRef]
- Li, L.L.; Pan, R.; Zhao, J.W.; Yang, B.F.; Yang, G.Y. A series of lanthanide germanate cluster organic frameworks. Dalton Trans. 2016, 45, 11958–11967. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Cao, G.-J.; Zhao, J.-W.; He, H.; Yang, B.-F.; Yang, G.-Y. Lanthanide germanate cluster organic frameworks based on {Ln8Ge12} clusters: From one-dimensional chains to two-dimensional layers and three-dimensional frameworks. Inorg. Chem. 2016, 55, 5671–5683. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, X.-F.; Zhou, J. Hydrothermal syntheses and crystal structure of a new organic hybrid holmium–germanate oxo-cluster. J. Clust. Sci. 2017, 28, 3209–3215. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Shul’pina, L.S.; Ikonnikov, N.S.; Trigub, A.L.; Dorovatovskii, P.V.; et al. Cage-like Fe,Na-germsesquioxanes: Structure, magnetism, and catalytic activity. Angew. Chem. Int. Ed. 2016, 55, 15360–15363. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.N.; Bilyachenko, A.N.; Korlyukov, A.A.; Long, J.; Levitsky, M.M.; Shubina, E.S.; Guari, Y.; Larionova, J. New Ni4Na2-phenylgermsesquioxane architecture: Synthesis, structure and slow dynamic behaviour. Dalton Trans. 2018, 47, 6893–6897. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Kulakova, A.N.; Bantreil, X.; Lamaty, F.; Levitsky, M.M.; Gutsul, E.I.; Shubina, E.S.; et al. Heptanuclear Fe5Cu2-phenylgermsesquioxane containing 2,2-bipyridine: Synthesis, structure, and catalytic activity in oxidation of CH compounds. Inorg. Chem. 2018, 57, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Yang, Y.; Chu, C.; Zhu, H.; Roesky, H.W. Cu24O24Si8R8: Organic soluble 56-membered copper(I) siloxane cage and its use in homogeneous catalysis. J. Am. Chem. Soc. 2010, 132, 12231–12233. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hu, D.; Yang, H.; Li, D.; Wu, T. PCU-type copper-rich open-framework chalcogenides: Pushing up the length limit of the connection mode and the first mixed-metal [Cu7GeSe13] cluster. Inorg. Chem. Front. 2017, 4, 387–392. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhang, B.; Li, W.-A.; Feng, M.-L.; Huang, X.-Y. Two new 3D heterometallic chalcogenides based on copper-rich selenogermanate clusters. Inorg. Chem. Commun. 2017, 85, 41–44. [Google Scholar] [CrossRef]
- Krautscheid, H.; Fenske, D.; Baum, G.; Semmelmann, M. A new copper selenide cluster with PPh3 ligands: [Cu146Se73(PPh3)30]. Angew. Chem. Int. Ed. 1993, 32, 1303–1305. [Google Scholar] [CrossRef]
- Fenske, D. [Cu96P30{P(SiMe3)2}6(PEt3)18], a New Phosphorus-Bridged Copper Cluster. Angew. Chem. Int. Ed. 1994, 33, 1290–1292. [Google Scholar] [CrossRef]
- Zhu, N.; Fenske, D. Novel Cu–Se clusters with Se–layer structures: [Cu32Se7(SenBu)18(PiPr3)6], [Cu50Se20(SetBu)10(PiPr3)10], [Cu73Se35(SePh)3(PiPr3)21], [Cu140Se70(PEt3)34] and [Cu140Se70(PEt3)36]. J. Chem. Soc. Dalton Trans. 1999, 1067–1076. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.; Chen, Z.; Chen, M.; Xu, Y.; Zhang, H.; Zhao, D. A large 24-membered-ring germanate zeolite-type open-framework structure with three-dimensional intersecting channels. Angew. Chem. Int. Ed. 2001, 113, 2224–2226. [Google Scholar] [CrossRef]
- Huang, S.; Christensen, K.; Peskov, M.V.; Yang, S.; Li, K.; Zou, X.; Sun, J. Two open-framework germanates with nickel complexes incorporated into the framework. Inorg. Chem. 2011, 50, 9921–9923. [Google Scholar] [CrossRef] [PubMed]
- Inge, A.K.; Sun, J.; Moraga, F.; Guo, B.; Zou, X. Three low-dimensional open-germanates based on the 44 net. CrystEngComm. 2012, 14, 5465–5471. [Google Scholar] [CrossRef]
- Lin, Y.; Massa, W.; Dehnen, S. “Zeoball” [Sn36Ge24Se132]24–: A molecular anion with zeolite-related composition and spherical shape. J. Am. Chem. Soc. 2012, 134, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Santner, S.; Yogendra, S.; Weigand, J.J.; Dehnen, S. Exploring the chemical reaction space at the formation of chalcogenidometalate superspheres in ionic liquids. Chem. Eur. J. 2017, 23, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, Y.; Belaya, I.; Krämer, R. The Encapsulation Phenomenon. Synthesis, Reactivity and Applications of Caged Ions and Molecules; Springer: Midtown Manhattan, NY, USA, 2016; p. 638. [Google Scholar]
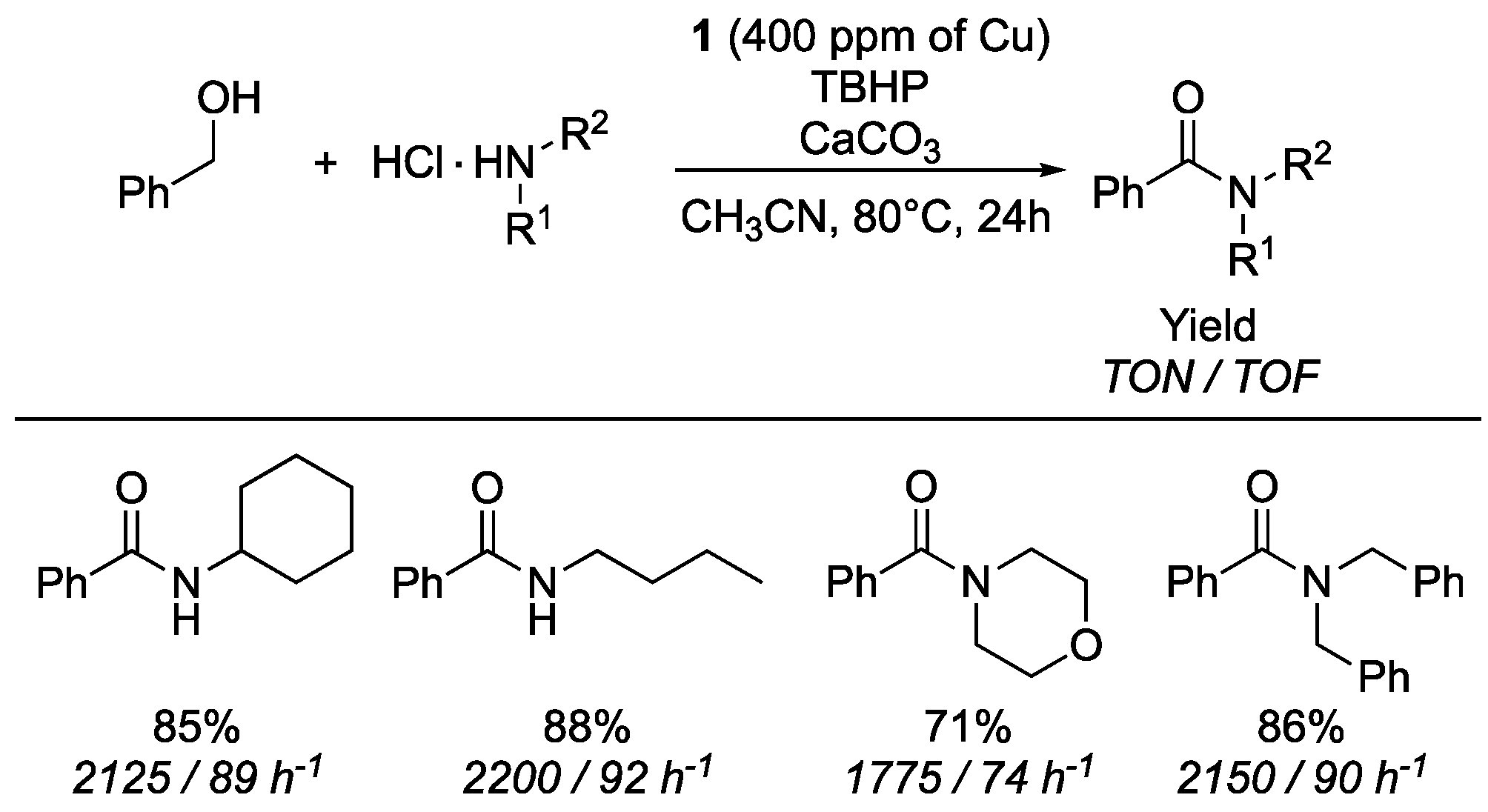
- De Figueiredo, R.M.; Suppo, J.-S.; Campagne, J.-M. Nonclassical routes for amide bond formation. Chem. Rev. 2016, 116, 12029–12122. [Google Scholar] [CrossRef] [PubMed]
- See ESI (Section 2.4. Calculation of TON and TOF) for details on TON and TOF.
- Bantreil, X.; Fleith, C.; Martinez, J.; Lamaty, F. Copper-catalyzed direct synthesis of benzamides from alcohols and amines. ChemCatChem 2012, 4, 1922–1925. [Google Scholar] [CrossRef]
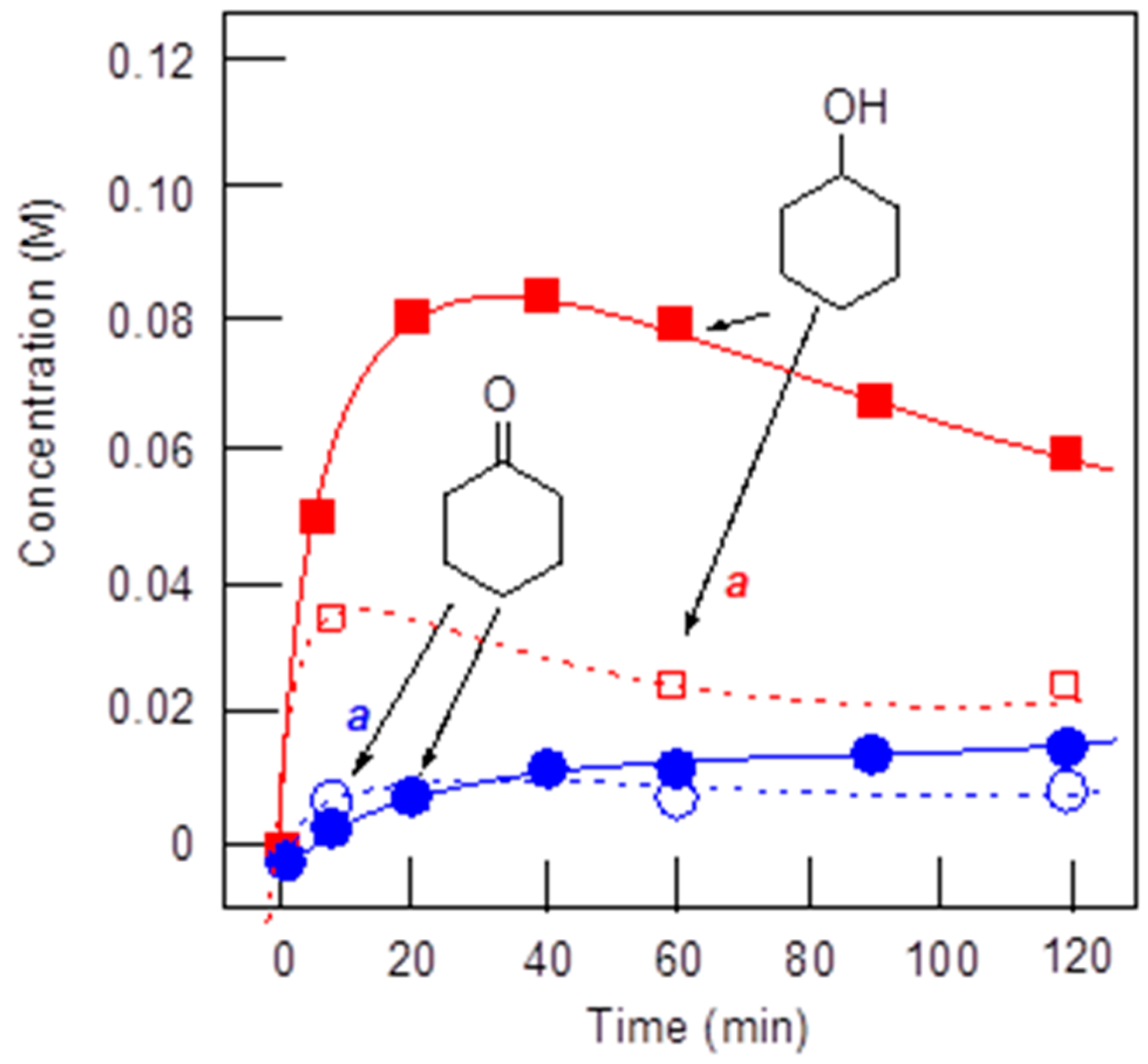
- Shul’pin, G.B. Alkane-Oxidizing Systems Based on Metal Complexes. Radical versus Non-Radical Mechanisms. In Alkane Functionalization; Pombeiro, A.J.L., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2018; Chapter 2; pp. 3–13. [Google Scholar]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. New trends in oxidative functionalization of carbon–hydrogen bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulakova, A.N.; Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Shul’pina, L.S.; Bantreil, X.; Lamaty, F.; Shubina, E.S.; Levitsky, M.M.; et al. Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity. Catalysts 2018, 8, 484. https://doi.org/10.3390/catal8100484
Kulakova AN, Bilyachenko AN, Khrustalev VN, Zubavichus YV, Dorovatovskii PV, Shul’pina LS, Bantreil X, Lamaty F, Shubina ES, Levitsky MM, et al. Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity. Catalysts. 2018; 8(10):484. https://doi.org/10.3390/catal8100484
Chicago/Turabian StyleKulakova, Alena N., Alexey N. Bilyachenko, Victor N. Khrustalev, Yan V. Zubavichus, Pavel V. Dorovatovskii, Lidia S. Shul’pina, Xavier Bantreil, Frédéric Lamaty, Elena S. Shubina, Mikhail M. Levitsky, and et al. 2018. "Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity" Catalysts 8, no. 10: 484. https://doi.org/10.3390/catal8100484
APA StyleKulakova, A. N., Bilyachenko, A. N., Khrustalev, V. N., Zubavichus, Y. V., Dorovatovskii, P. V., Shul’pina, L. S., Bantreil, X., Lamaty, F., Shubina, E. S., Levitsky, M. M., & Shul’pin, G. B. (2018). Cu42Ge24Na4—A Giant Trimetallic Sesquioxane Cage: Synthesis, Structure, and Catalytic Activity. Catalysts, 8(10), 484. https://doi.org/10.3390/catal8100484







