Water: Friend or Foe in Catalytic Hydrogenation? A Case Study Using Copper Catalysts
Abstract
1. Introduction
2. Results and Discussion
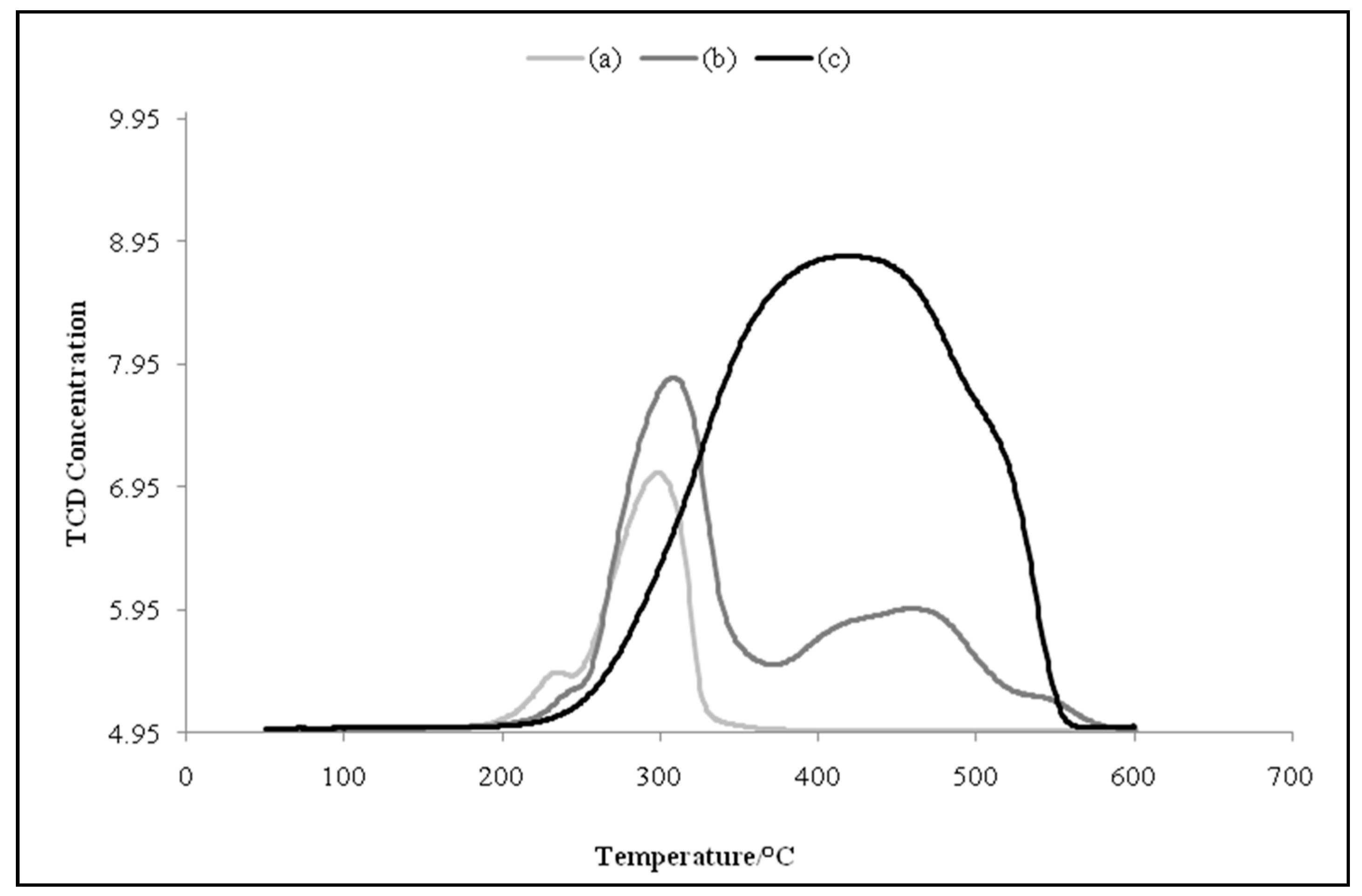
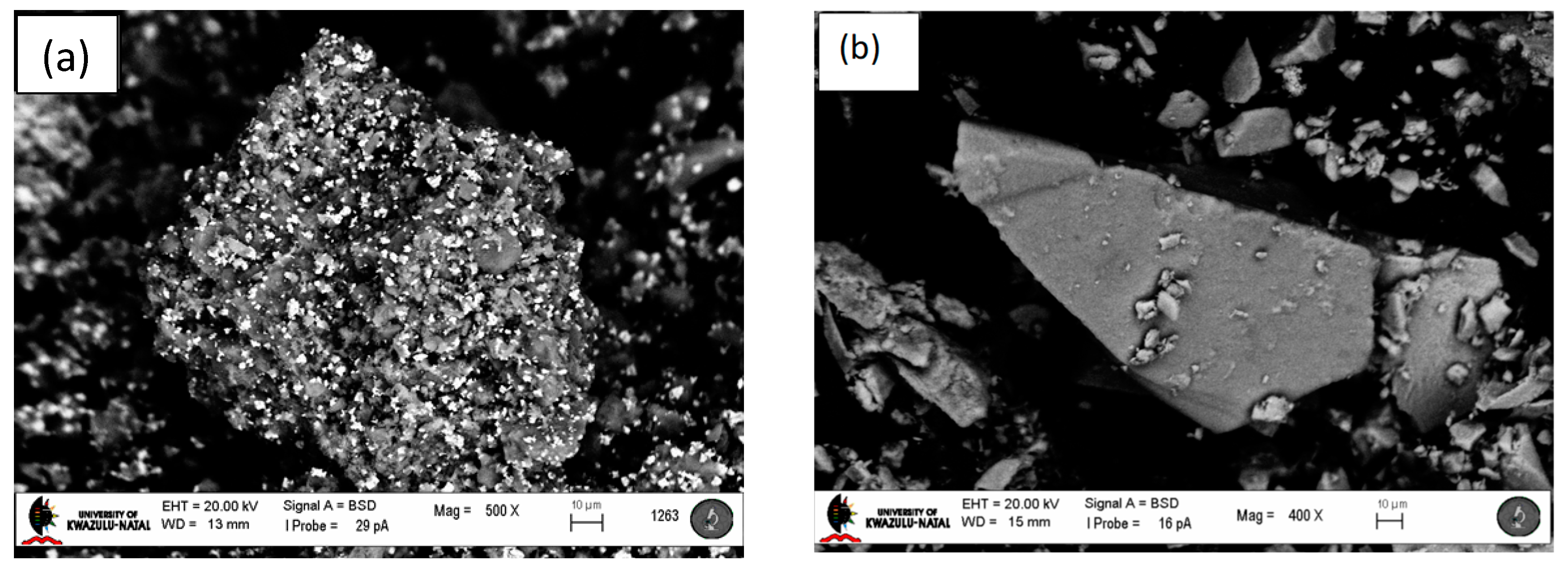
2.1. Catalyst Characterization
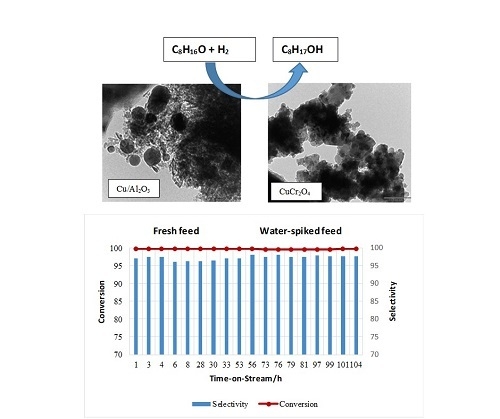
2.2. Catalytic Testing—Water Impact Studies
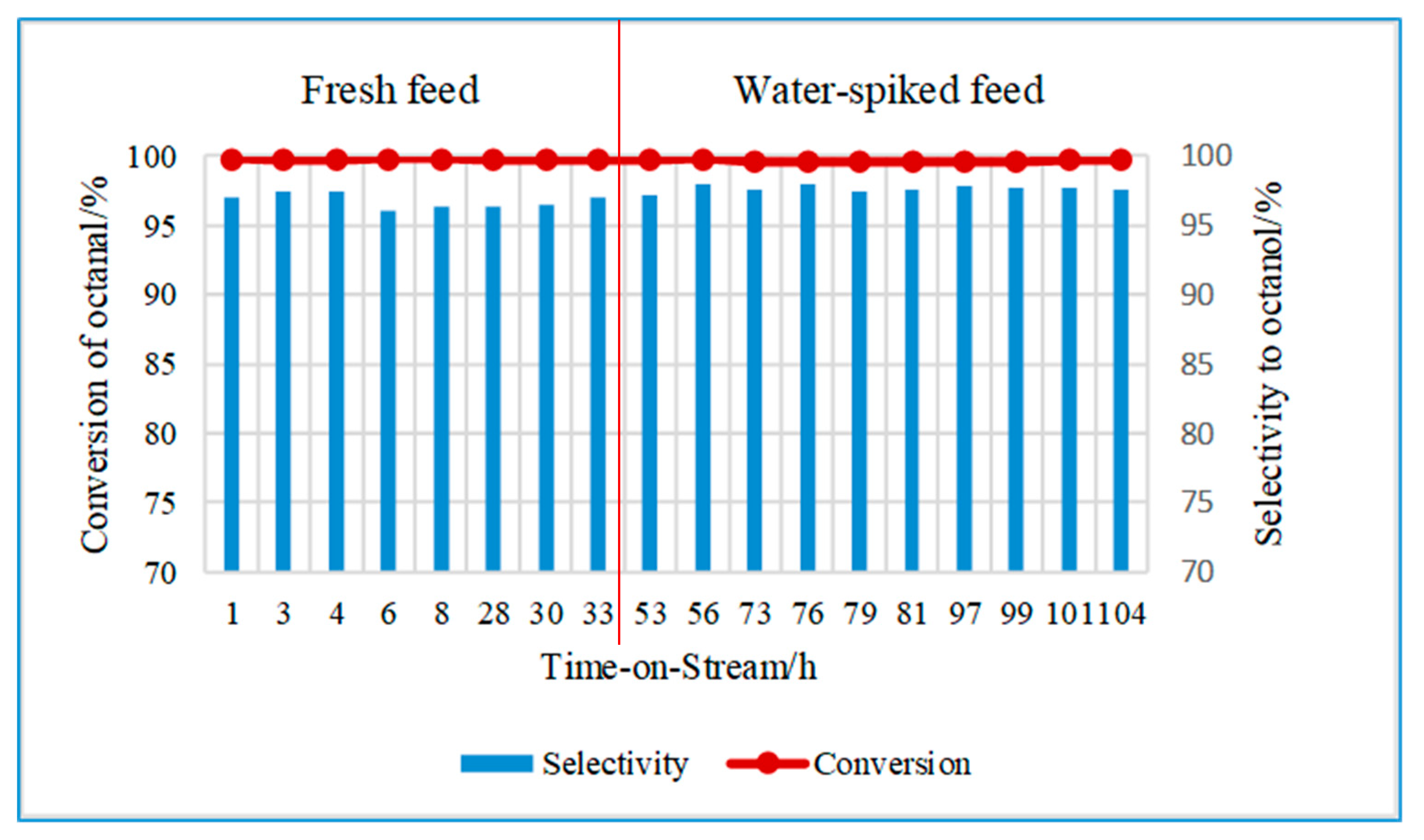
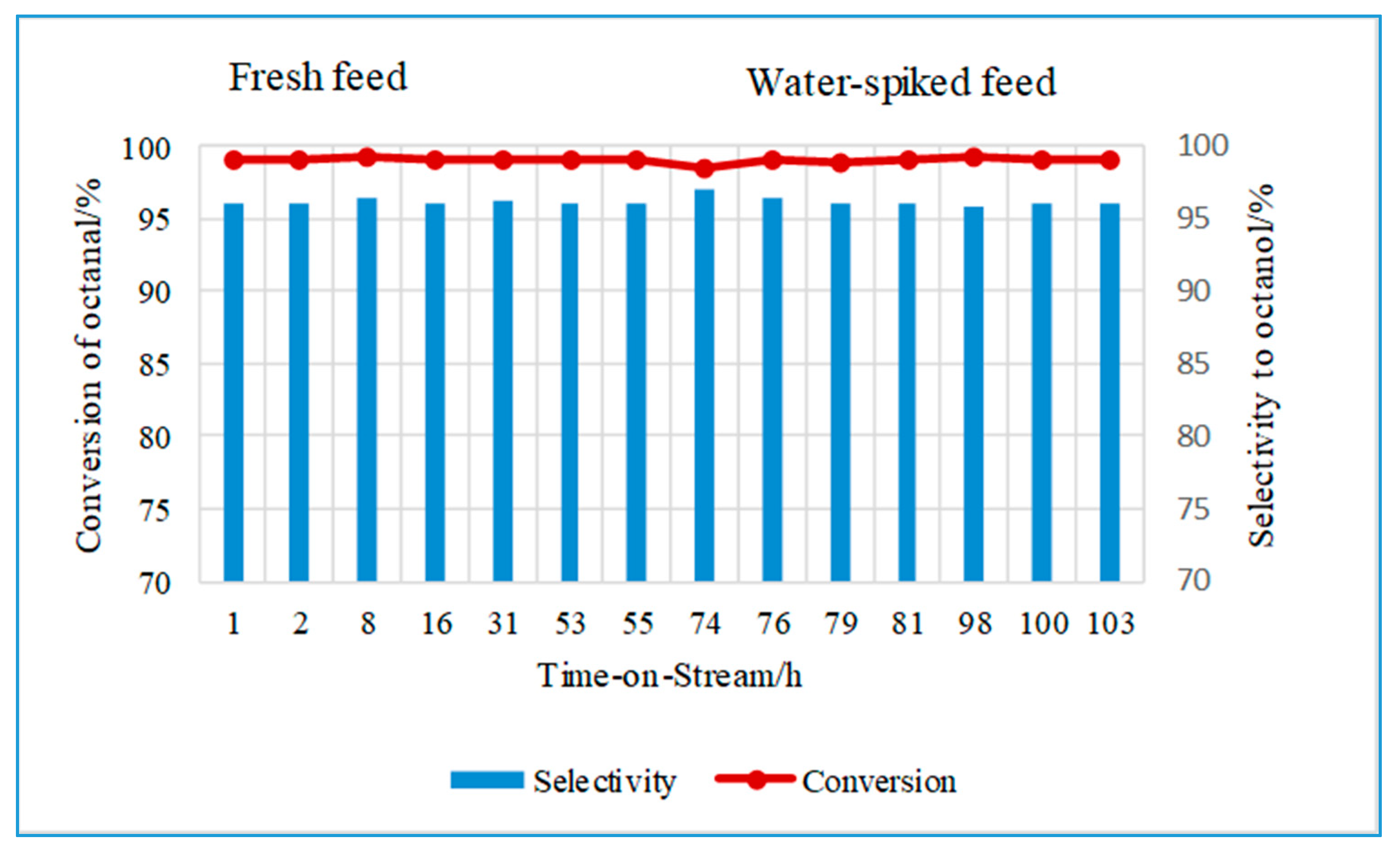
2.2.1. Effect of Water on the Hydrogenation of Octanal Using CuO/Al2O3
2.2.2. Effect of Water on the Hydrogenation of Octanal Using CuCr2O4
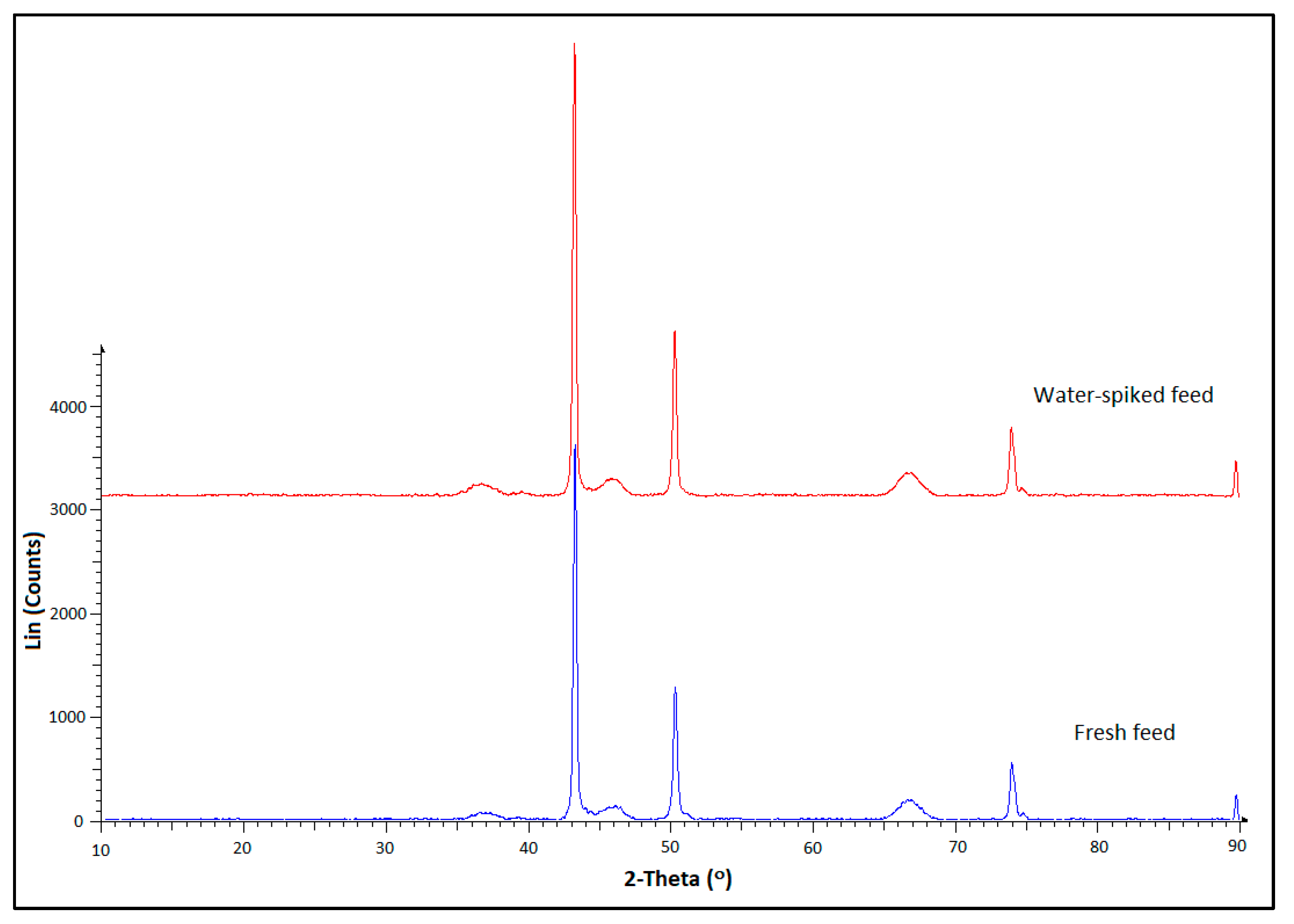
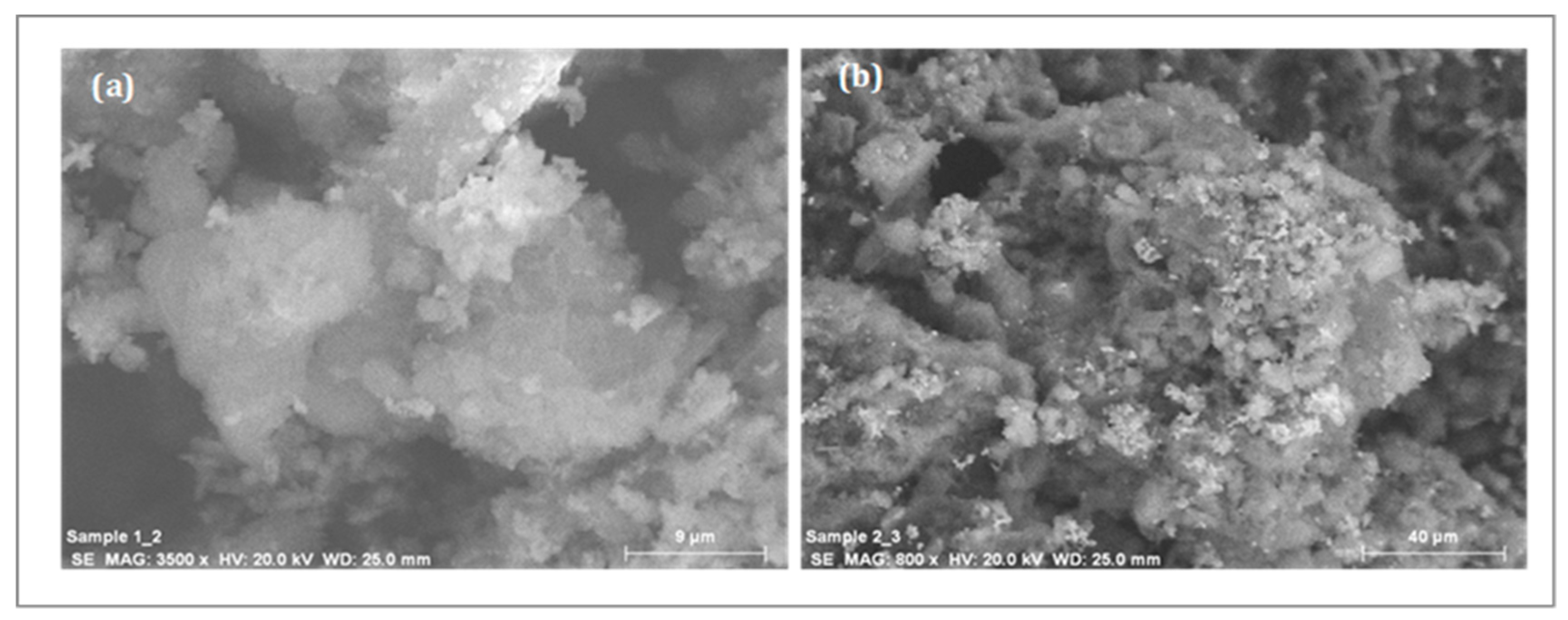
2.3. Used Catalyst Characterization
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rioux, R.M.; Vannice, M.A. Hydrogenation/dehydrogenation reactions: Isopropanol dehydrogenation over copper catalysts. J. Catal. 2003, 216, 362–376. [Google Scholar] [CrossRef]
- Satterfield, C.N. Heterogeneous Catalysis in Industrial Practice; McGraw Hill: New York, NY, USA, 1991. [Google Scholar]
- Lok, M. Structure and Performance of Selective Hydrogenation Catalysts. Available online: https://www.topsoe.com/sites/default/files/martin_lok_structure_and_performance_of_selective_hydrogenation_catalysts.pdf (accessed on 5 October 2018).
- Prasad, R.; Singh, P. Applications and Preparation Methods of Copper Chromite Catalysts: A Review. Bull. Chem. React. Eng. Catal. 2011, 6, 63–113. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, G. Heat Exchanger Network Integration of a Hydrogenation Process of Benzene to Cyclohexene Considering the Reactor Conversion. Chem. Eng. Trans. 2017, 61, 283–288. [Google Scholar]
- Woods, D.R. Successful Trouble Shooting for Process Engineers: A Complete Course in Case Studies; Wiley: Darmstadt, Germany, 2006. [Google Scholar]
- Kister, H.Z. What Caused Tower Malfunctions in the Last 50 Years? Chem. Eng. Res. Des. 2003, 81, 5–26. [Google Scholar] [CrossRef]
- Chang, J.-R.; Lin, T.-B.; Cheng, C.-H. Pd/Δ-Al2O3 Catalysts for Isoprene Selective Hydrogenation: Regeneration of Water-Poisoned Catalysts. Ind. Eng. Chem. Res. 1997, 36, 5096–5102. [Google Scholar] [CrossRef]
- Wang, W.-J.; Qiao, M.-H.; Li, H.-X.; Dai, W.-L.; Deng, J.-F. Study on the deactivation of amorphous NiB/SiO2 catalyst during the selective hydrogenation of cyclopentadiene to cyclopentene. Appl. Catal. A: Gen. 1998, 168, 151–157. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, R.; Liu, C.; Mi, Z. Production of hydrogen peroxide from carbon monoxide, water, and oxygen over alumina supported amorphous ni catalysts. Chem. Lett. 2002, 31, 884–885. [Google Scholar] [CrossRef]
- Twigg, M.V.; Spencer, M.S. Deactivation of supported copper metal catalysts for hydrogenation reactions. Appl. Catal. A Gen. 2001, 212, 161–174. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Rao, R.S.; Walters, A.B.; Vannice, M.A. Influence of crystallite size on acetone hydrogenation over copper catalysts. J. Phys. Chem. B 2005, 109, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, E.S.; Lemonidou, A.A. Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis. Appl. Catal. A Gen. 2011, 396, 177–185. [Google Scholar] [CrossRef]
- Masson, J.; Cividino, P.; Court, J. Selective hydrogenation of acetophenone on chromium promoted raney nickel catalysts. III. The influence of the nature of the solvent. Appl. Catal. A Gen. 1997, 161, 191–197. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Rajkhowa, T.; Marin, G.B.; Thybaut, J.W. A comprehensive kinetic model for Cu catalyzed liquid phase glycerol hydrogenolysis. Appl. Catal. B Environ. 2017, 205, 469–480. [Google Scholar] [CrossRef]
- Mahlaba, S.V.L.; Valand, J.; Mahomed, A.S.; Friedrich, H.B. A study on the deactivation and reactivation of a Ni/Al2O3 aldehyde hydrogenation catalyst: Effects of regeneration on the activity and properties of the catalyst. Appl. Catal. B Environ. 2018, 224, 295–304. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mater. Devices 2016, 1, 343–350. [Google Scholar] [CrossRef]
- Ma, Z.; Xiao, Z.; van Bokhoven, J.A.; Liang, C. A non-alkoxide sol-gel route to highly active and selective Cu-Cr catalysts for glycerol conversion. J. Mater. Chem. 2010, 20, 755–760. [Google Scholar] [CrossRef]
- Chen, L.; Horiuchi, T.; Osaki, T.; Mori, T. Catalytic selective reduction of no with propylene over Cu-Al2O3 catalysts: Influence of catalyst preparation method. Appl. Catal. B: Environ. 1999, 23, 259–269. [Google Scholar] [CrossRef]
- Luo, M.-F.; Fang, P.; He, M.; Xie, Y.-L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, T.; Jiang, X.; Chen, F.; Zheng, X. Temperature-programmed reduction and temperature-programmed desorption studies of CuO/ZrO2 catalysts. Appl. Surf. Sci. 1999, 148, 263–270. [Google Scholar] [CrossRef]
- Chetty, T.; Dasireddy, V.D.B.C.; Callanan, L.H.; Friedrich, H.B. Continuous Flow Preferential Hydrogenation of an Octanal/Octene Mixture Using Cu/Al2O3 Catalysts. ACS Omega 2018, 3, 7911–7924. [Google Scholar] [CrossRef]
- Wang, X.; Saleh, R.Y.; Ozkan, U.S. Reaction network of aldehyde hydrogenation over sulfided Ni–Mo/Al2O3 catalysts. J. Catal. 2005, 231, 20–32. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ko, A.-N. Vapor phase reactions of acetaldehyde over type X zeolites. Appl. Catal. A Gen. 2000, 190, 149–155. [Google Scholar] [CrossRef]
- Luo, S.; Falconer, J.L. Acetone and Acetaldehyde Oligomerization on TiO2 Surfaces. J. Catal. 1999, 185, 393–407. [Google Scholar] [CrossRef]
- Nascimento, M.A. Theoretical Aspects of Heterogeneous Catalysis; Kluwer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Gredig, S.V.; Maurer, R.; Koeppel, R.; Baiker, A. Copper-catalyzed synthesis of methylamines from CO2. H2 and NH3. Influence of support. J. Mol. Catal. A Chem. 1997, 127, 133–142. [Google Scholar] [CrossRef]
- Bando, K.K.; Sayama, K.; Kusama, H.; Okabe, K.; Arakawa, H. In-situ FT-IR study on CO2 hydrogenation over Cu catalysts supported on SiO2, Al2O3, and TiO2. Appl. Catal. A Gen. 1997, 165, 391–409. [Google Scholar] [CrossRef]
- Lang, B.E. Solubility of Water in Octan-1-ol from (275 to 369) K. J. Chem. Eng. Data 2012, 57, 2221–2226. [Google Scholar] [CrossRef]
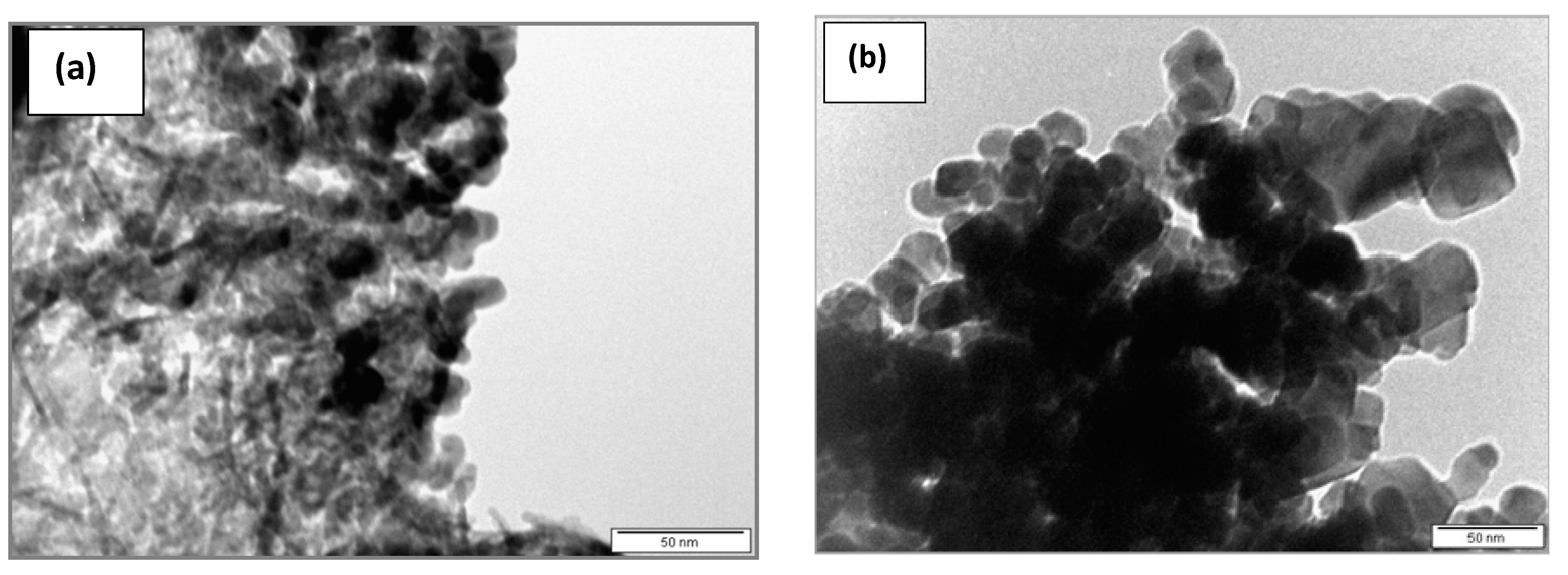


| Catalyst | Cu Loading/wt. % | BET Surface Area/m2 g−1 | Total Pore Volume/cm3 g−1 | Average TEM Particle Size/nm |
|---|---|---|---|---|
| CuO/Al2O3 | 23.5 | 128.8 | 0.4285 (0.6530) a | 16 |
| CuCr2O4 | 26.3 | 25.3 | 0.3697 | 30 |
| Catalyst | Temperature at Maximum (Tm)/°C | Degree of Reduction a/% |
|---|---|---|
| Unsupported CuO (Bulk) | 416 | - |
| CuO/Al2O3 | 231, 298 | 84.4 |
| CuCr2O4 | 308, 468, shoulder peaks at 240 and 541 | 85.8 |
| Acid Strength Temperature (°C) | Acid Sites Concentration (μmol g−1 of Cat) | ||||||
|---|---|---|---|---|---|---|---|
| Catalyst | Tm at A | Tm at B | Tm at C | Acidity at A | Acidity at B | Acidity at C | Total Acidity |
| CuO/Al2O3 | 244 | - | 636 | 1288 | - | 504 | 1791 |
| CuCr2O4 | 261 | 464 | 987 | 847 | 1014 | 78 | 1938 |
| Catalyst | FWHM/° | Average Particle Size/nm | BET Surface Area/m2 g−1 |
|---|---|---|---|
| Cu/Al2O3 fresh feed | 0.264 | 23 | 141.7 |
| Cu/Al2O3 water-spiked feed | 0.258 | 15 | 143.9 |
| CuCr2O4 fresh feed | 0.799 | 33 | 33.5 |
| CuCr2O4 water-spiked feed | 1.335 | 24 | 36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govender, A.; Mahomed, A.S.; Friedrich, H.B. Water: Friend or Foe in Catalytic Hydrogenation? A Case Study Using Copper Catalysts. Catalysts 2018, 8, 474. https://doi.org/10.3390/catal8100474
Govender A, Mahomed AS, Friedrich HB. Water: Friend or Foe in Catalytic Hydrogenation? A Case Study Using Copper Catalysts. Catalysts. 2018; 8(10):474. https://doi.org/10.3390/catal8100474
Chicago/Turabian StyleGovender, Alisa, Abdul S. Mahomed, and Holger B. Friedrich. 2018. "Water: Friend or Foe in Catalytic Hydrogenation? A Case Study Using Copper Catalysts" Catalysts 8, no. 10: 474. https://doi.org/10.3390/catal8100474
APA StyleGovender, A., Mahomed, A. S., & Friedrich, H. B. (2018). Water: Friend or Foe in Catalytic Hydrogenation? A Case Study Using Copper Catalysts. Catalysts, 8(10), 474. https://doi.org/10.3390/catal8100474