Selective Dehydration of Glucose into 5-Hydroxymethylfurfural by Ionic Liquid-ZrOCl2 in Isopropanol
Abstract
:1. Introduction
2. Results & Discussion
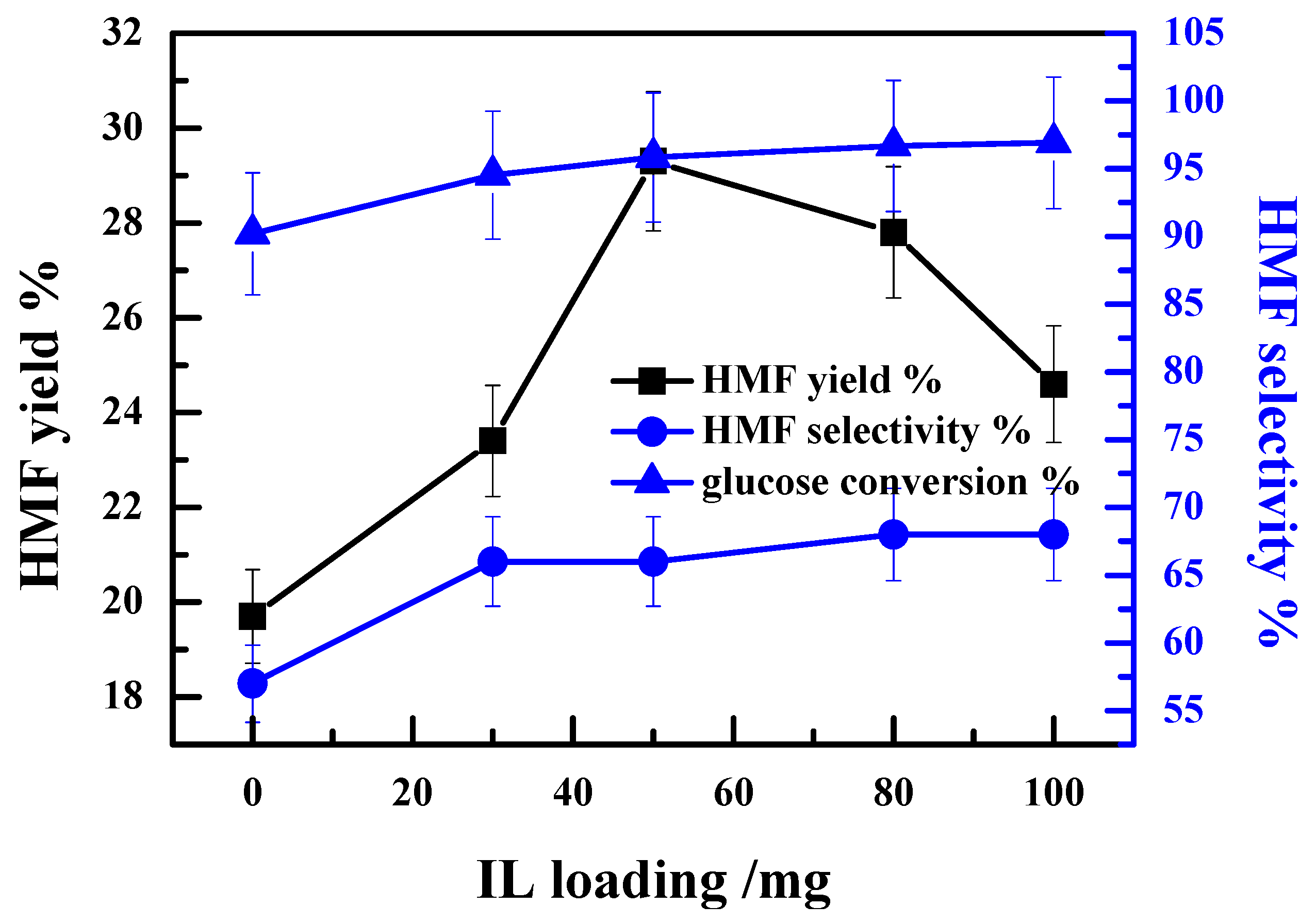
2.1. Effect of IL [HO2CMMIm]Cl Loading on the Catalytic Performance
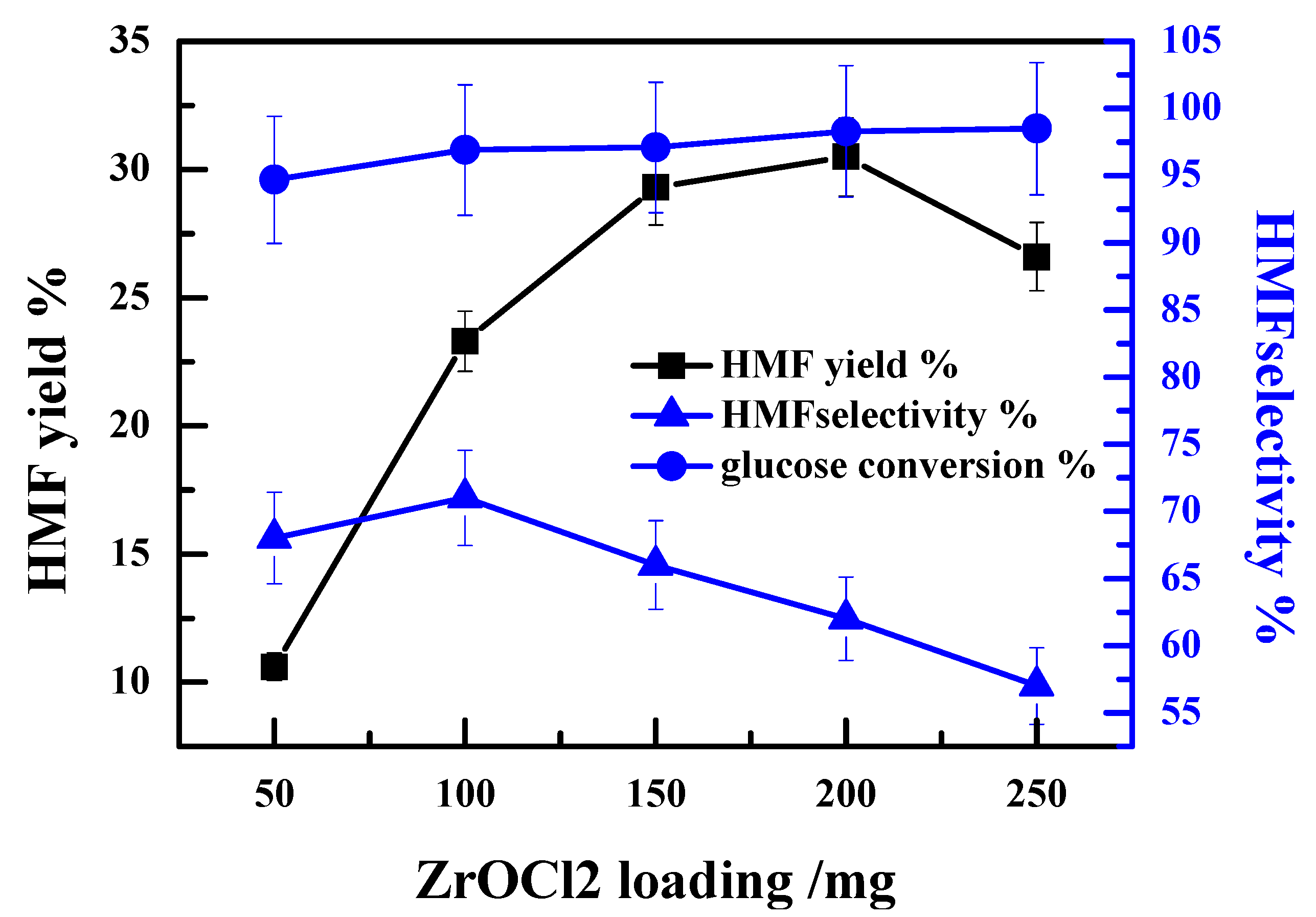
2.2. Effect of ZrOCl2 Loading on the Catalytic Performance
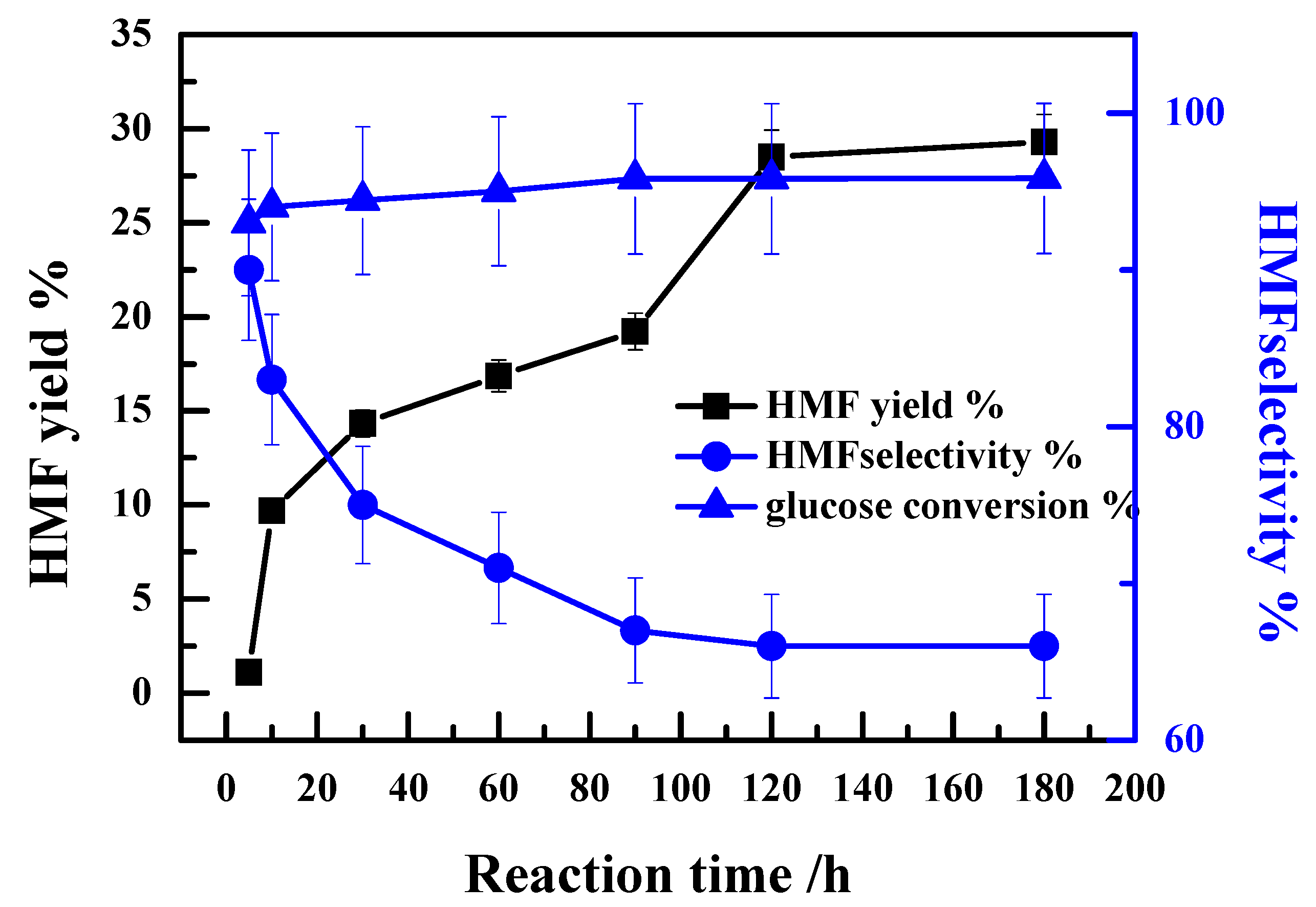
2.3. Influence of the Reaction Time on the Catalytic Performance
2.4. Effect of the Reaction Temperature on the Catalytic Performance
2.5. Effect of Substrate Loading on HMF synthesis
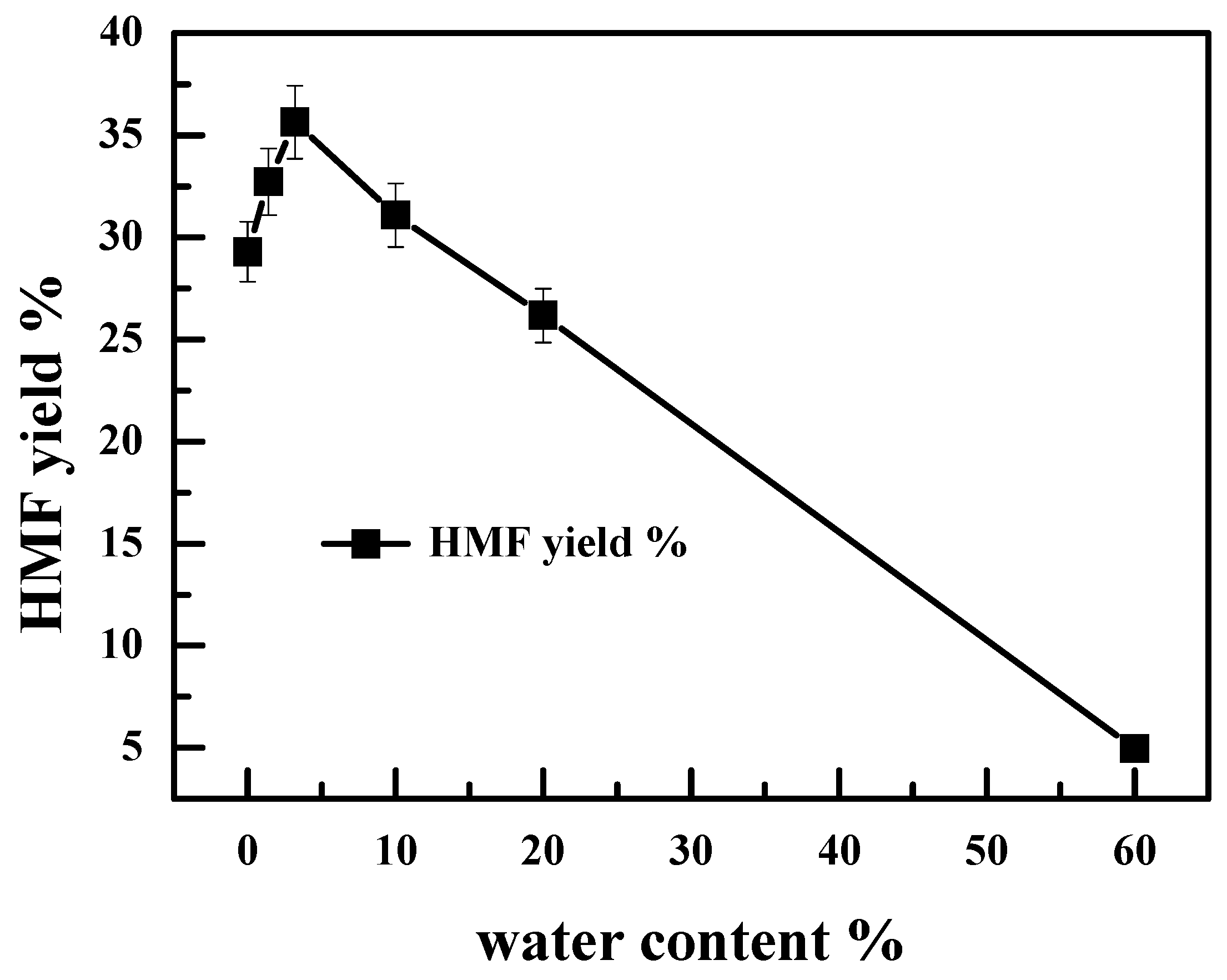
2.6. Effect of Water Content on the Catalytic Performance
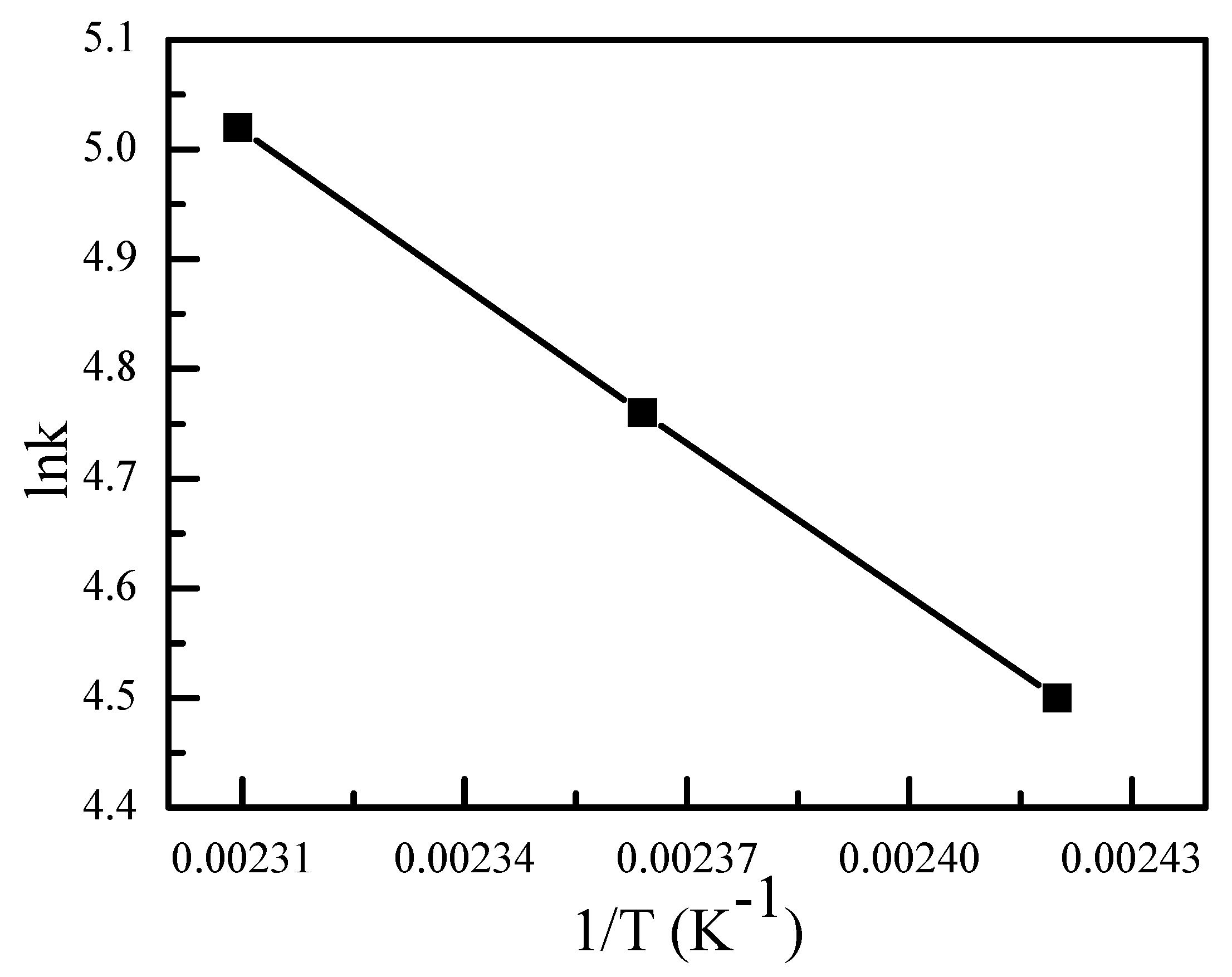
2.7. Kinetics Studies
2.8. Recycling of the Catalysts
3. Experimental Section
3.1. Materials
3.2. Reaction Testing
3.3. Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Antal, M.J., Jr.; Leesomboon, T.; Mok, W.S.; Richards, G.N. Mechanism of formation of 2-furaldehyde from D-xylose. Carbohydr. Res. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Ren, J.; Liu, X.; Lu, G.; Wang, Y. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678–2681. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P.; Rivalier, P.; Ros, P.; Avignon, G. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl. Catal. A Gener. 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ren, J.; Liu, X.; Li, X.; Xia, Y.; Lu, G.; Wang, Y. Mesoporous niobium phosphate: An excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2012, 2, 2485–2491. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Williams, L.D.; Ebede, C.C. Mechanism of the dehydration of d-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 C: An NMR study. Carbohydrate research. Carbohydr. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Moreau, C.; Finiels, A.; Vanoye, L. Dehydration of fructose and sucrose into 5-hydroxymethylfurfural in the presence of 1-H-3-methyl imidazolium chloride acting both as solvent and catalyst. J. Mol. Catal. A Chem. 2006, 253, 165–169. [Google Scholar] [CrossRef]
- Hansen, T.S.; Mielby, J.; Riisager, A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Crisci, A.J.; Tucker, M.H.; Lee, M.-Y.; Jang, S.G.; Dumesic, J.A.; Scott, S.L. Acid-Functionalized SBA-15-Type Silica Catalysts for Carbohydrate Dehydration. ACS Catal. 2011, 1, 719–728. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.K.; Wang, Q. Catalytic Conversion of Inulin and Fructose into 5-Hydroxymethylfurfural by Lignosulfonic Acid in Ionic Liquids. ChemSusChem 2012, 5, 901–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Zhang, B.; Shi, J.; Ma, J.; Yang, G.; Han, B. Dehydration of Carbohydrates to 5-Hydroxymethylfurfural in Ionic Liquids Catalyzed by Hexachlorotriphosphazene. Chin. J. Chem. 2012, 30, 2079–2084. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Huang, X.; Chen, L.; Ma, L.; Li, X. Conversion of fructose into 5-hydroxymethylfurfural and alkyl levulinates catalyzed by sulfonic acid-functionalized carbon materials. Green Chem. 2013, 15, 2895–2903. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y. Efficient and Selective Dehydration of Fructose to 5-Hydroxymethylfurfural Catalyzed by Brønsted-Acidic Ionic Liquids. ChemSusChem 2010, 3, 350–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Ma, Y.; Li, Y. An efficient catalytic dehydration of fructose and sucrose to 5-hydroxymethylfurfural with protic ionic liquids. Carbohydr. Res. 2010, 345, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, B.; Zhang, Z.; Chen, L. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by acidic ionic liquids in dimethyl sulfoxide. Ind. Crops Prod. 2013, 50, 264–269. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; He, Z.C.; Wang, H.W.; You, F.; Li, K.N. Study on self-tuning tyre friction control for developing main-servo loop integrated chassis control system. IEEE Access 2017, 5, 6649–6660. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Meng, W.; Zhang, R.; Li, K.; Peng, T. Primary resonance analysis and vibration suppression for the harmonically excited nonlinear suspension system using a pair of symmetric viscoelastic buffers. Nonlinear Dyn. 2018, 94, 1243–1265. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; You, F.; Peng, T.; He, Z.; Li, K. Exploring to direct the reaction pathway for hydrogenation of levulinic acid into γ-valerolactone for future Clean-Energy Vehicles over a magnetic Cu-Ni catalyst. Int. J. Hydrog. Energy 2017, 42, 25185–25194. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, H.; Ma, Y.; Zhang, R. High selective production of 5-hydroxymethylfurfural from fructose by sulfonic acid functionalized SBA-15 catalyst. Compos. Part B Eng. 2019, 156, 88–94. [Google Scholar] [CrossRef]
- Deng, T.; Cui, X.; Qi, Y.; Wang, Y.; Hou, X.; Zhu, Y. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by ZnCl2 in water. Chem. Commun. 2012, 48, 5494–5496. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Dutta, S.; Saha, B. Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride catalyst in water. Green Chem. 2011, 13, 2859–2868. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, B.; Zhang, L.-J.; Da, Y.-X.; Quan, Z.-J.; Yang, L.-J.; Wang, X.-C. Conversion of carbohydrates into 5-hydroxymethylfurfural using polymer bound sulfonic acids as efficient and recyclable catalysts. RSC Adv. 2013, 3, 9201–9205. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Wang, T.; Glasper, J.A.; Shanks, B.H. Kinetics of glucose dehydration catalyzed by homogeneous Lewis acidic metal salts in water. Appl. Catal. A Gener. 2015, 498, 214–221. [Google Scholar] [CrossRef]
- Xiong, H.; Zhu, X.; Zhang, R. Energy Recovery Strategy Numerical Simulation for Dual Axle Drive Pure Electric Vehicle Based on Motor Loss Model and Big Data Calculation. Complexity 2018. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Xia, Y.; Li, W.; Wang, H.; Zheng, C. Efficient process for the direct transformation of cellulose and carbohydrates to 5-(hydroxymenthyl)furfural with dual-core sulfonic acid ionic liquids and co-catalysts. RSC Adv. 2013, 3, 7782–7790. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Wu, L.; Song, J.; Zhang, B.; Zhou, B.; Zhou, H.; Fan, H.; Yang, Y.; Han, B. Very efficient conversion of glucose to 5-hydroxymethylfurfural in DBU-based ionic liquids with benzenesulfonate anion. Green Chem. 2014, 16, 3935–3941. [Google Scholar] [CrossRef]
- Liu, W.; Holladay, J. Catalytic conversion of sugar into hydroxymethylfurfural in ionic liquids. Catal. Today 2013, 200, 106–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Xie, H.; Liu, W.; Zhao, Z. Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Germanium(IV) Chloride in Ionic Liquids. ChemSusChem 2011, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Q.; Yue, M.; Bai, X.; Du, Y. Tantalum compounds as heterogeneous catalysts for saccharide dehydration to 5-hydroxymethylfurfural. Chem. Commun. 2011, 47, 4469–4471. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, T.; Rodriguez-Rodriguez, S.; Fristrup, P.; Riisager, A. Metal-Free Dehydration of Glucose to 5-(Hydroxymethyl)furfural in Ionic Liquids with Boric Acid as a Promoter. Chem. Eur. J. 2011, 17, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Huang, C.; Song, Y.; Zhang, J.; Chen, B. Efficient dehydration of glucose to 5-hydroxymethylfurfural catalyzed by the ionic liquid,1-hydroxyethyl-3-methylimidazolium tetrafluoroborate. Bioresour. Technol. 2012, 121, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Heguaburu, V.; Franco, J.; Reina, L.; Tabarez, C.; Moyna, G.; Moyna, P. Dehydration of carbohydrates to 2-furaldehydes in ionic liquids by catalysis with ion exchange resins. Catal. Commun. 2012, 27, 88–91. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glucose dehydration to 5-hydroxymethylfurfural on zirconium containing mesoporous MCM-41 silica catalysts. Fuel 2014, 118, 265–271. [Google Scholar] [CrossRef]
- Kruger, J.S.; Nikolakis, V.; Vlachos, D.G. Aqueous-phase fructose dehydration using Brønsted acid zeolites: Catalytic activity of dissolved aluminosilicate species. Appl. Catal. A Gener. 2014, 469, 116–123. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Chen, E.Y.X. Chromium(0) Nanoparticles as Effective Catalyst for the Conversion of Glucose into 5-Hydroxymethylfurfural. ChemSusChem 2013, 6, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Osatiashtiani, A.; Lee, A.F.; Brown, D.R.; Melero, J.A.; Morales, G.; Wilson, K. Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose. Catal. Sci. Technol. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Teckchandani-Ortiz, A.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Selective dehydration of glucose to 5-hydroxymethylfurfural on acidic mesoporous tantalum phosphate. Appl. Catal. B Environ. 2014, 144, 22–28. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Thermo-kinetic assessment of glucose decomposition to 5-hydroxymethyl furfural and levulinic acid over acidic functionalized ionic liquid. Chem. Eng. J. 2018, 335, 221–230. [Google Scholar] [CrossRef]
- Alam, M.I.; De, S.; Khan, T.S.; Haider, M.A.; Saha, B. Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crops Prod. 2018, 123, 629–637. [Google Scholar] [CrossRef]
- Chinnappan, A.; Baskar, C.; Kim, H. Biomass into chemicals: Green chemical conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids. RSC Adv. 2016, 6, 63991–64002. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.; Li, W.; Ju, M. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B Environ. 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Alam, M.I.; Abu-Omar, M.M.; Saha, B. Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J. Catal. 2012, 288, 8–15. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic Liquid-Mediated Formation of 5-Hydroxymethylfurfural—A Promising Biomass-Derived Building Block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qing, S.; Wang, L.; Islam, N.; Guan, S.; Gao, Z.; Mamat, X.; Li, H.; Eli, W.; Wang, T. Production of 5-hydroxymethylfurfural from fructose by a thermo-regulated and recyclable Bronsted acidic ionic liquid catalyst. RSC Adv. 2015, 5, 47377–47383. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Dumesic, J.A. Analyses of Reaction Schemes Using De Donder Relations. J. Catal. 1999, 185, 496–505. [Google Scholar] [CrossRef]
- Dumesic, J.A. Reply to Finding the Rate-Determining Step in a Mechanism: Comparing DeDonder Relations with the “Degree of Rate Control”. J. Catal. 2001, 204, 525–529. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.R.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent Effects in Acid-Catalyzed Biomass Conversion Reactions. Angew. Chem. Int. Ed. 2014, 53, 11872–11875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.-P. Kinetic and Mechanistic Study of Glucose Isomerization Using Homogeneous Organic Brønsted Base Catalysts in Water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef]
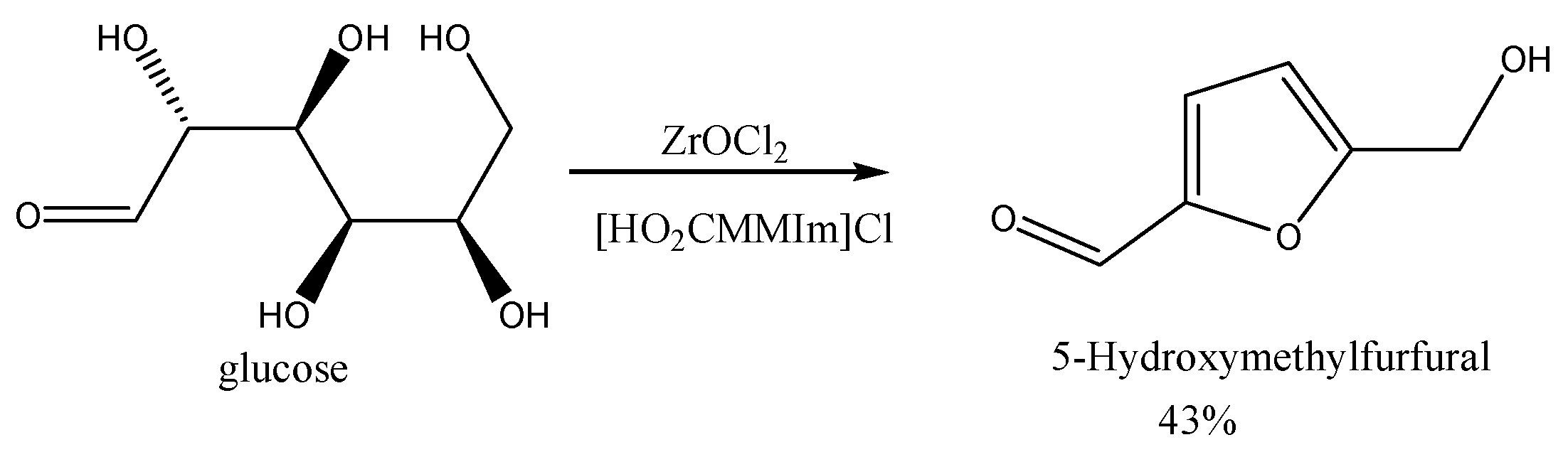


| Entry | Temperature °C | k × 103 (min−1) | Correlation Coefficient |
|---|---|---|---|
| 1 | 140 | 89.1 | 0.986 |
| 2 | 150 | 118.7 | 0.989 |
| 3 | 160 | 152.4 | 0.977 |
| Entry | Reaction Temperature | ΔH/kJ mol−1 |
|---|---|---|
| 1 | 140 | 47.976 |
| 2 | 150 | 47.975 |
| 3 | 160 | 47.974 |
| Run | Glucose Conversion % | HMF Yield % |
|---|---|---|
| 1 | 99 | 43 |
| 2 | 99 | 41 |
| 3 | 99 | 40 |
| 4 | 99 | 39 |
| 5 | 99 | 38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, L.; Li, H.; Wang, T.; Zhang, R. Selective Dehydration of Glucose into 5-Hydroxymethylfurfural by Ionic Liquid-ZrOCl2 in Isopropanol. Catalysts 2018, 8, 467. https://doi.org/10.3390/catal8100467
Ma Y, Wang L, Li H, Wang T, Zhang R. Selective Dehydration of Glucose into 5-Hydroxymethylfurfural by Ionic Liquid-ZrOCl2 in Isopropanol. Catalysts. 2018; 8(10):467. https://doi.org/10.3390/catal8100467
Chicago/Turabian StyleMa, Yubo, Lei Wang, Hongyi Li, Tianfu Wang, and Ronghui Zhang. 2018. "Selective Dehydration of Glucose into 5-Hydroxymethylfurfural by Ionic Liquid-ZrOCl2 in Isopropanol" Catalysts 8, no. 10: 467. https://doi.org/10.3390/catal8100467
APA StyleMa, Y., Wang, L., Li, H., Wang, T., & Zhang, R. (2018). Selective Dehydration of Glucose into 5-Hydroxymethylfurfural by Ionic Liquid-ZrOCl2 in Isopropanol. Catalysts, 8(10), 467. https://doi.org/10.3390/catal8100467




