Abstract
In this work, nanosized Cu and Ni Schiff-base complexes, namely ahpvCu, ahpnbCu, and ahpvNi, incorporating imine ligands derived from the condensation of 2-amino-3-hydroxypyridine, with either 3-methoxysalicylaldehyde (ahpv) or 4-nitrobenzaldehyde (ahpnb), were synthesized using sonochemical approach. The structure and properties of the new ligands and their complexes with Ni(II) and Cu(II) were determined via infrared (IR), nuclear magnetic resonance (NMR), electronic spectra (UV-Vis), elemental analysis (CHN), thermal gravimetric analysis (TGA), molar conductivity (Λm), and magnetic moment (μeff). The combined results revealed the formation of 1:1 (metal: ligand) complexes for ahpvCu and ahpvNi and 1:2 for ahpnbCu. Additionally, CuO and NiO nanoparticles were prepared by calcination of the respective nanosized Cu/Ni complexes at 500 °C, and characterized by powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). Significantly, the as-prepared nanosized Schiff-base Cu/Ni complexes and their oxides showed remarkable catalytic activity towards the selective oxidation of benzyl alcohol (BzOH) in aqueous H2O2/ dimethylsulfoxide (DMSO) solution. Thus, catalytic oxidation of BzOH to benzaldehyde (BzH) using both ahpvCu complex and CuO nanoparticles in H2O2/DMSO media at 70 °C for 2 h yielded 94% and 98% BzH, respectively, with 100% selectivity.
1. Introduction
Owing to their wide-spread application in biology, biomedicine, and catalysis, complexes bearing Schiff-base ligands are among the most intensively explored coordination compounds [1,2,3,4]. A large number of Schiff-base transition-metal complexes are commonly employed as oxidation catalysts in a variety of important organic transformations, because of their facile synthesis and excellent chemical and thermal stability [2,5,6,7,8]. Given their inherent conducting and magnetic properties, Schiff-base complexes are also employed as precursors in many technologies, including electrochromic display screens, organic batteries, and microelectronic devices [9,10,11,12,13].
Selective oxidation of alcohols to aldehydes is an important and widely used reaction, as aldehydes are crucial intermediates for many organic syntheses and essential precursors for making vitamins, drugs, and fragrances. Benzaldehyde (BzH), in particular, is commonly employed in the manufacture of flavors, odorants, and other pharmaceutical products [14,15,16,17,18]. Previous studies have shown that manipulation of the reaction conditions (e.g., reactant concentration, temperature, and pressure) and the choice of solvent and oxidation catalyst are essential factors for controlling the reaction rate, along with the nature and quantity of the generated side products [17,19]. However, in view of their pivotal role in redox chemistry, tremendous efforts have been devoted in recent years to design and develop novel types of catalysts to improve the selectivity and chemical yield of such reactions [15,16,20,21].
Among the endeavors in the exploration of smart materials in catalysis, inorganic precursors, such as metal oxides (MOs) have received considerable interest over the past few decades, due to their ability to withstand extremely harsh reaction conditions and their promising catalytic activity toward many important transformation processes [21,22,23,24,25,26,27]. The catalytic properties of these metal oxides rely, in part, on their high surface area and the relative acidity and basicity of the atoms present on their surface, which can be tuned via coordination of metal cations and oxygen anions [28,29,30,31]. However, the great challenge in the synthesis of nanotechnology-based materials lies in the production of nanostructures with desired properties that can be tailored and/or tuned to meet specific applications [32,33,34,35,36,37].
In this contribution, we explored the catalytic activity of nanosized Ni(II) and Cu(II) complexes comprising Schiff-base ligands derived from the condensation of 2-amino-3-hydroxypyridine with 3-methoxysalicylaldehyde or 4-nitrobenzaaldehyde along with their generated MO nanoparticles (MO = NiO and CuO) [1] on the oxidation of benzyl alcohol (BzOH) under homogeneous conditions. Other experimental parameters, such as the effect temperature, concentration, and various solvents on the catalytic oxidation of BzOH, were also investigated. Our findings revealed that the parent complexes together with their respective MO showed efficient catalytic activity for oxidation of BzOH into BzH with approximately 100% selectivity under mild conditions.
2. Results and Discussion
2.1. Physicochemical Characteristics
All the prepared complexes are hydrated, air-stable solids at ambient temperature. The physicochemical and analytical results of Schiff-base ligands and their M(II)-complexes (M = Cu and Ni) are described in details in Table S1 in the Supplementary Materials. Complexes ahpvCu and ahpvNi display a 1:1 (metal: ligand) ratio, whereas the ahpnbCu complex presents a 1:2 stoichiometry (vide infra).
2.2. 1H-NMR Spectroscopy
The 1H-NMR spectra of the prepared Schiff-base ahpv and ahpnb ligands in dimethylsulfoxide (DMSO)-d6 show a singlet signal at 9.44 and 9.39 ppm for the azomethine (CH=N) proton, multiple signals at 6.85–8.00 and 7.25–8.46 for six and seven aromatic protons. Moreover, the ahpnb ligand displays only a singlet signal at 10.28 for the OH proton, whereas the ahpv ligand shows similar singlet signal at 10.23 and 6.41 for the OH protons in the pyridine ring and adjacent to OCH3, respectively, along with a singlet signal at 3.34 for the three OCH3 protons [1,38,39].
2.3. Infrared and Electronic Spectra
The characteristic infrared (IR) frequencies of the ligands and their complexes, together with their assignments, are shown in Table S2. The bands assigned to –OH and –CH=N groups are distinguishable and provide insight into the structure of the ligands and their bonding to the metal ion. The bands at 1613 and 1621 cm−1 in the ahpv and ahpnb ligands, respectively, are attributed to the −C=N stretching vibration. Upon coordination, these bands shift to lower wavenumbers by 5–12 cm−1. This negative shift is indicative of the direct coordination of the azomethine nitrogen atoms to the metal ion [1,40,41]. This conclusion is supported by the appearance of strong bands within 527–656 cm−1 range, corresponding to the stretching of the M–N bond.
Additionally, the IR bands at 3447 and 3475 cm−1 observed for both ahpv and ahpnb ligands are consistent with the stretching vibration of free –OH. The IR spectra of all the prepared complexes show broad bands at 3450 and 3490 cm−1, assigned to the υ(OH) stretching vibration of the hydrated water molecules in the complexes, as evidenced by the elemental analysis data listed in Table S1. However, the observation of IR bands at 814–976 cm–1 (OH rocking) implies the existence of coordinated H2O in the prepared complexes. The Schiff-base (ahpv and ahpnb) ligands also display absorption bands at 1307 and 1288 cm−1, respectively, which are assigned to the stretching vibration of the phenolic C–O group. The shift of that band to lower wavenumbers by 9–35 cm−1 for ahpv and 3 cm−1 for ahpnb upon complexation implies that the oxygen atoms of deprotonated phenolic groups are directly coordinated to the metal ion. The involvement of such bands in binding to the metal ion is further supported by the appearance of strong non ligand bands within 723–736 cm−1 range, assigned to the stretching vibration of the M–O bond. As reported for related complexes, the bands observed in the region of 3047–3079 cm−1 are assigned to υ(C–H) aromatic stretching vibrations [1,42].
Figure S1 showed representative electronic absorption spectra of the ahpvCu complex and its components in N,N′-dimethylformamide (DMF) in the wavelength range of 200–800 nm at 25 °C. The absorption bands below 300 nm are assigned to π–π* transitions in the aromatic rings, whereas the absorption bands at λmax = 314–349 nm are attributed to n–π* transitions of the imine group in the Schiff-base ligands [43]. As for the complexes, the absorption bands at λmax = 239–391 nm are consistent with a charge transfer in the Schiff-base ligands [44], whereas the broad bands at λmax = 430–506 nm are indicative of d–d transitions in the complexes [44].
2.4. Magnetic Moment Measurements and Thermal Analysis
Magnetic susceptibility provides valuable insights on the geometric structure of compounds. In this context, the magnetic susceptibility results (Table S1) revealed that all prepared complexes exhibit paramagnetism and octahedral geometry, except for the ahpvCu complex, which presents a tetrahedral geometry [45]. The thermal behavior of the as-prepared complexes revealed the loss of the hydrated water molecules in the first step; then, in subsequent steps, the coordinated water molecules are released and the ligands decomposed, as shown in Table S3 [46]. The final decomposition products were identified as the metallic species [47,48].
2.5. Spectrophotometric Determination of the Stoichiometry of the Prepared Complexes
The stoichiometry of the prepared Schiff-base complexes was determined using two spectrophotometric methods, namely, the continuous variation and molar ratio methods [49]. The curves obtained from the continuous variation method revealed a maximum absorbance at a mole fraction Xligand = 0.43–0.68, thereby suggesting metal-to-ligand complexation in a molar ratio of either 1:1 or 1:2, as illustrated in Figure 1a. Similarly, the results obtained from the molar ratio method supported the above findings for the three prepared complexes, as shown in Figure 1b. Consistent with the aforementioned characterization, the data obtained from the two methods clearly confirm that the stoichiometry (metal: ligand) is 1:1 for both ahpvCu and ahpvNi complexes, and 1:2 for the ahpnbCu congener.
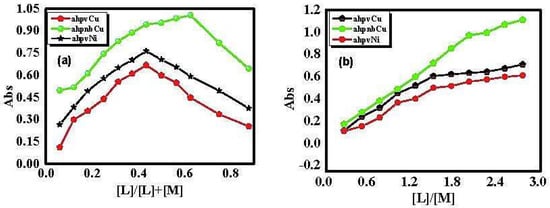
Figure 1.
(a) Continuous variation, and (b) molar ratio plots for the prepared complexes in aqueous ethanolic solution at [M] = [L] = 1 × 10−3 M and 298 K.
2.6. Formation Constants and pH Stability Range of the Complexes
The formation constant (Kf) of the prepared Schiff-base Cu-and Ni-complexes in solution was determined via spectrophotometric measurements using the continuous variation method and Equation (1) [50]:
where Am is the absorption at the maximum formation of the complex, A denotes arbitrary absorbance values on either side of the absorbance curve, and C is the elementary concentration of the metal. As summarized in Table 1, the obtained Kf values reflect the high stability of the prepared complexes. Importantly, the negative values of the Gibbs free energy (ΔG≠) mean that the reactions are spontaneous and favorable.

Table 1.
Formation constant (Kf), stability constant (pK), and Gibbs free energy (ΔG≠) values of the synthesized complexes in aqueous ethanol at 298 K.
Furthermore, the pH profiles (absorbance vs. pH, Figure S2) of the prepared complexes established their marked stability in a wide pH range (4–11). This behavior indicates that the stability of the Schiff-base ligands is strongly enhanced by formation of the corresponding complexes, thereby making them suitable for various applications (vide infra).
2.7. Particle Size of the Prepared Complexes and Their Metal Oxides
Cu(II) and Ni(II) oxide nanoparticles were synthesized at 500 °C using the Schiff-base complexes as precursors and their structure and morphology were examined by transmission electron microscopy (TEM) and powder X-ray diffraction (PXRD). Based on the TEM images and the calculated histograms (Figure 2), it is evident that the prepared complexes have an average particle size of 46 and 65 nm, for ahpvCu and ahpvNi, respectively. The corresponding metal oxides (CuO and NiO) have a particle size of 42 and 16 nm as illustrated in Figure S3. These results clearly confirm that the prepared compounds have high surface area, which is essential for application in catalysis, as discussed in details below [51].
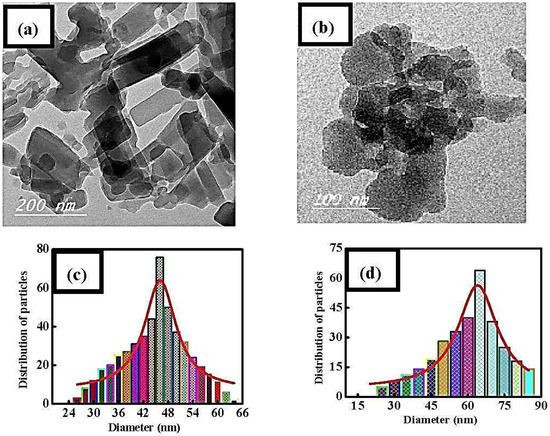
Figure 2.
(a,b) Transmission electron microscopy (TEM) images of the ahpvCu and ahpvNi complexes, and (c,d) their calculated histograms, respectively.
The X-ray diffraction (XRD) patterns of the synthesized CuO and NiO nanoparticles, as shown in Figure 3. The obtained XRD data are consistent with the reported values for CuO and NiO, thereby confirming the formation of pure phases of these materials [12]. The mean grain size (D) of the particles was estimated from the XRD line broadening using the Scherrer equation [52]:
where λ is the wavelength (Cu Kα), β is the full width at the half-maximum (FWHM) of the CuO and NiO lines, and θ is the diffraction angle. Importantly, the annealing temperature was found to greatly affect the particle morphology of the as-prepared CuO and NiO powders.
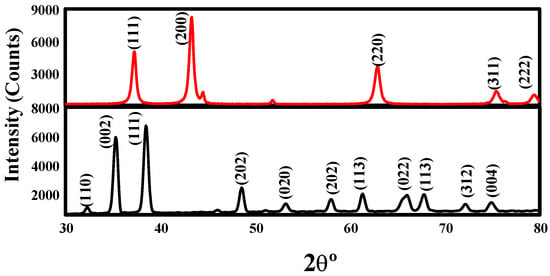
Figure 3.
X-ray diffraction (XRD) patterns of the CuO (black) and NiO (red) nanoparticles.
2.8. Catalytic Oxidation of Benzyl Alcohol Using Schiff-Base M(II) Complexes and Their Oxides
The catalytic oxidation of BzOH in DMSO and other organic solvents was performed using the prepared nanosized Cu and Ni Schiff-base complexes (ahpvCu, ahpnbCu, and ahpvNi) and their metal oxide (CuO and NiO) nanoparticles in the presence of aqueous H2O2 as the oxidant under different experimental conditions, as described in Tables S4–S6. The obtained results clearly demonstrate that the prepared nanosized complexes and their MO exhibit efficient and highly selective catalytic oxidation of benzyl alcohol (BzOH) to the corresponding benzaldehyde (BzH) as the main product, compared to other conceptually and structurally related catalysts as illustrated in Table 2. This finding implied the crucial role of sonochemical approach in providing much higher surface area nanocatalysts compared to other conventional methods of synthesis.

Table 2.
Comparison of the catalytic activity for BzOH oxidation using Schiff-base complexes and metal oxides.
2.8.1. Effect of Temperature
The effect of temperature on the catalytic activity of the prepared Schiff base-M(II) (M = Cu and Ni) complexes and their metal oxides (MO) toward the oxidation of BzOH in DMSO, using aqueous H2O2 as the oxygen source, was evaluated and optimized at different temperatures (60, 70, 80, and 90 °C) and time intervals. Tables S4 and S5 summarize the data obtained from using both ahpvCu and its corresponding CuO as catalysts presented in Figure 4. For all prepared nanocatalysts, gas chromatography (GC) confirmed that BzH was the solely generated product for BzOH oxidation reaction.
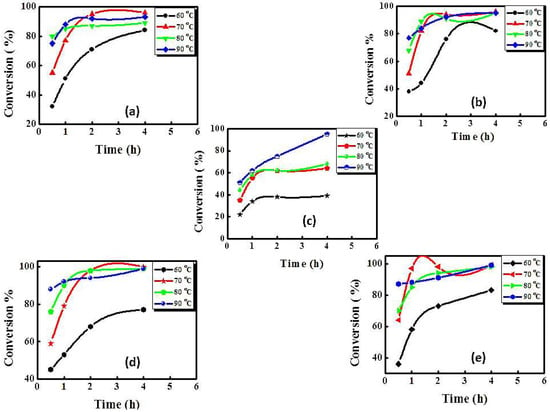
Figure 4.
Oxidation of benzyl alcohol (1.0 mmol) catalyzed by the prepared Schiff base-M(II) complexes and their respective metal oxide (MO) (0.03 mmol) using aqueous H2O2 (3.0 mmol) in dimethylsulfoxide (DMSO) at designated temperatures using: (a) ahpvCu, (b) ahpnbCu, (c) ahpvNi complexes, (d) CuO, and (e) NiO.
Control experiments using only H2O2 (i.e., in the absence of the Schiff base-M(II) complexes or their MO) showed that no other carbonyl products were formed to a measurable extent under similar conditions. Importantly, as can be seen from Figure 4d,e, it is clearly established that the MOs exhibit higher catalytic activity toward BzOH oxidation than their parent complexes at all the investigated temperatures [53]. In the case of CuO (Figure 4d), for instance, the rate of conversion is remarkably increased with the reaction temperature, meanwhile the selectivity toward BzH remained constant. According to the results shown in Table S5, after 30 min, the conversion of BzOH to BzH increased from 45% to 88% upon increasing the temperature from 60 to 90 °C, while the selectivity for generation of BzH was nearly 100% under both conditions.
2.8.2. Effect of the Solvent
Previous studies have shown that the nature of the solvent plays a crucial role in the catalytic oxidation of alcohols and the control of the stereo- and chemo-selectivity, as well as the conversion yield [19,54,55,56]. In the present study, the catalytic behavior of the prepared Schiff-base M(II) complexes and their MOs toward the oxidation of BzOH was investigated in various solvents, including acetonitrile (AN), acetone (AC), N,N′-dimethylformamide (DMF), and dimethylsulfoxide (DMSO). The catalytic reactions were conducted under the optimized conditions for all catalysts, and the obtained results are presented in Table 3. As can be seen from these data, the catalytic activity is prominently influenced by the nature of the solvent, with DMSO being the best solvent for all catalysts affording 94–98% conversion of BzOH into BzH. This is probably, due to the fact that DMSO is the most polar of all the studied solvents and has the greatest coordinating ability, which strongly activated the catalyst toward the oxidation of BzOH. Although NiO yielded 92–97% of BzH, its parent Ni-complex gave the lowest conversion (~55%). Therefore, under these conditions, the Schiff-base Cu-complexes are more efficient catalysts than their Ni-counterparts.

Table 3.
Summary of the optimized parameters for oxidation of BzOH using the five prepared nanocatalysts.
In contrast to DMSO, all catalysts showed drastically poor catalytic activity when acetone was used as the solvent, with the highest selectivity (~69%) and conversion (~36%) for BzOH oxidation being achieved when ahpnbCu complex is used. In view of these results, it can be concluded that the most suitable solvent for the efficient catalytic oxidation of benzyl alcohol by the ahpvCu, ahpnbCu, and ahpvNi complexes, along with CuO and NiO, is DMSO, as previously reported [57].
2.8.3. Effect of the Catalyst Concentration
The amount of catalyst used for the oxidation of primary alcohols, such as benzyl alcohol has a profound impact on the kinetics and yield of the produced products, particularly benzaldehyde [17,19,56]. In this work, the effect of the catalyst concentration was probed by deliberately adding different molar ratios (0.01, 0.02, 0.03, and 0.04 mmol) of the three complexes (ahpvCu, ahpnbCu, and ahpvNi) and their oxides (CuO and NiO) to a BzOH solution in DMSO in the presence of aqueous H2O2, while the other experimental parameters were kept at their optimum values. For all catalysts, the obtained results (Table S6) suggest that the catalytic activity is enhanced by increasing catalyst concentration from 0.01 to 0.03 mmol, while it decays at values greater than 0.04 mmol.
2.8.4. Mechanistic Aspects of the Catalytic Oxidation of Benzyl Alcohol
The data obtained for the five investigated nanocatalysts, under various experimental conditions, revealed several important features on the catalytic oxidation of BzOH. Firstly, the catalytic performance of the ahpvNi complex is markedly lower than that of both copper complexes (ahpvCu and ahpnbCu). This behavior implies that oxygen transfer from H2O2 to the catalyst is much easier in the case of the copper complexes than in the Ni congener, as previously reported for structurally-related systems [19,21,58,59]. Secondly, the catalytic activity of the MOs is greater than that of their parent Schiff-base complexes. This is explained by the fact that the oxides afford comparatively smaller-sized nanoparticles than the parent complexes, with consequently larger surface-to-volume ratios, thereby offering more efficient means for catalytic activity. Thirdly, in most catalytic processes, the major oxidation product was BzH and DMSO was found to be the best solvent for this catalytic oxidation reaction as both benzyl alcohol and the oxidant (H2O2) are soluble in it. Finally, from catalyst recovery viewpoint, the separation and/or recycling of MO nanoparticles from the reaction mixture upon completion of the catalytic reaction was successfully achieved at least 3 times and showed negligible decrease in their catalytic performance. Given their solubility in all employed organic solvents, the recovery of Schiff-based Cu-and Ni-complexes was not possible, as expected for homogeneous catalysis.
The catalytic activity of the prepared complexes correlates with their high stability in different solvents, which are able to accommodate the octahedral structure of ahpnbCu and ahpvNi and tetrahedral geometry of ahpvCu (Scheme S1). In view of these proposed structures, the complexes contain open coordination sites for the oxidant (H2O2), thus replacing the H2O molecules and binding directly to the central cation (M2+). This allowed oxygen to be transferred from H2O2 to M2+ [19,58,59,60] (Scheme 1). This behavior can be detected spectroscopically (UV-Vis) by monitoring the changes in the characteristic peaks of each complex in DMSO at 70 °C, before and after addition of H2O2, as shown in Figures S4 and S5. Putatively, these spectral changes can be attributed to the substitution of H2O by H2O2 molecules, as shown in the proposed mechanism in Scheme 1.
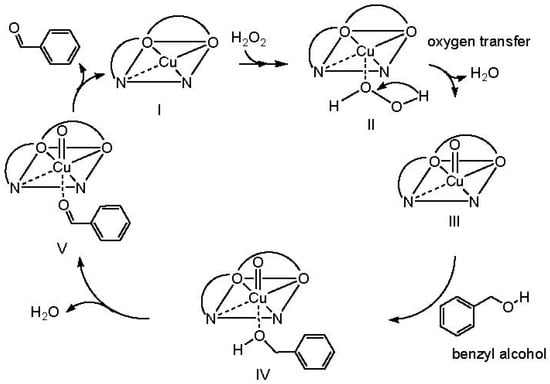
Scheme 1.
Proposed mechanism for the catalytic oxidation of benzyl alcohol by ahpnbCu.
As for the catalytic oxidation of BzOH into BzH using MO nanoparticles (MO = CuO and NiO) catalysts, it is proposed that DMSO abstracts a proton (H+) from H2O2 to generate a perhydroxyl anion (OOH−), which then combines with MO to yield MO–OOH. This species attacks BzOH to form peroxycarboximidic acid intermediate (step IV in Scheme 2), which is then converted into BzH with concomitant regeneration of the catalyst. In view of the proposed mechanistic pathways for the catalytic oxidation of BzOH, it can be concluded that both the phenyl ring and OH group of benzyl alcohol may interact with the metal center of MO (M2+), whereas the inner active sites remain intact. However, the interaction of the phenyl ring and OH group with the outer metal ions of MO is also possible via adsorption of the phenyl ring on MO, as reported in ref. [56,61,62]. Importantly, the active sites of the catalyst can be regenerated by the oxidant. This leads to desorption of the desired product (BzH) molecules, thereby favoring the oxidation of BzOH to BzH. On this basis, it is likely that BzOH, DMSO, and H2O2 interact efficiently with MO, affording the eventual formation of BzH, as described by step V in Scheme 2 [17,19,55].
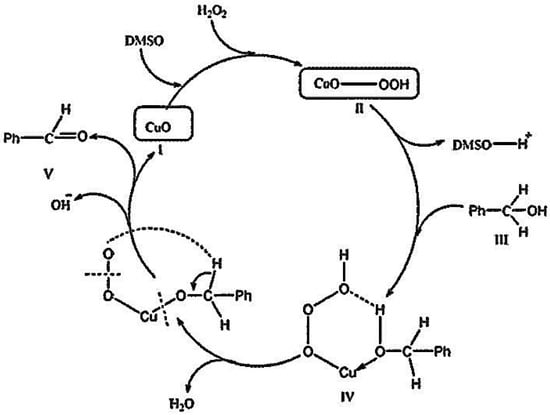
Scheme 2.
Proposed mechanism for the catalytic oxidation of benzyl alcohol by CuO.
3. Materials and Methods
3.1. Chemicals
All solvents, chemicals, and starting materials utilized in this work, such as DMSO, DMF, acetone, acetonitrile, benzyl alcohol, 3-methoxysalicylaldehyde, 2-amino-3-hydroxypyridine, 4-nitrobenzaaldehyde, copper acetate, nickel nitrate hexahydrate, hydrogen peroxide, manganese dioxide, and sodium sulfite were obtained from Sigma-Aldrich Chemie (Darmstadt, Germany), and used as received.
3.2. Synthesis of Schiff-Base Ligands
The Schiff-base ligands were prepared, as shown in Scheme S2, by condensation of 3-methoxysalicylaldehyde (0.152 g, 1.0 mmol) or 4-nitrobenzaaldehyde (0.151 g, 1.0 mmol) with 2-amino-3-hydroxypyridine (0.110 g, 1.0 mmol) in a 1:1 molar ratio in 10 mL of ethanol. The reaction mixtures were then refluxed for 1 h, after which the obtained solid precipitates were filtered off, rinsed with distilled water, dried, and then recrystallized from ethanol to afford an overall yield of 88–90%.
3.3. Preparation of Nanosized Cu- and Ni-Schiff Base Complexes via a Sonochemical Approach
An ethanolic solution (10 mL, 0.1 M) of the metal salts Cu(CH3COO)2⋅H2O, 0.199 g or Ni(NO3)2⋅6H2O, 0.291 g was placed in an ultrasonic probe operated at 24 kHz with a maximum force output of 400 W. A solution (10 mL, 0.1 M) of the Schiff base ahpv ligand (0.260 g) or 10 mL of a 0.2 M solution of the ahpnb ligand was added dropwise to the metal salt solution. The reaction mixture was then exposed to 10–15 min of sonochemical irradiation, allowing for nanoparticles formation of the desired complex. The obtained precipitate was filtered off, rinsed with ethanol, and then dried in air (yield: 72–84%). The proposed structures of the synthesized complexes are illustrated in Scheme S1.
3.4. Preparation of Nanosized NiO and CuO
NiO and CuO nanoparticles were prepared by calcination of 0.05 g of the ahpvCu, ahpvNi, and ahpnbCu complexes in air at 500 °C at a heating rate of 10 °C min−1. The resulting MOs were washed with ethanol and dried in a desiccator. The MO structure was confirmed by TEM and XRD analyses.
3.5. Physical Measurements
The decomposition temperatures, as well as the melting point of the Schiff-base ligands, and corresponding complexes were determined using a Gallenkamp (London, UK) instrument. The IR spectra were recorded as KBr pellets of the compounds in the range of 4000–400 cm−1 on a Shimadzu FTIR model 8101 spectrophotometer. Molar conductivity experiments were performed via a JENWAY conductivity meter model 4320 at 298 K using DMF as the solvent. UV-visible (UV-Vis) spectra of the compounds in DMF were recorded in a 10-mm quartz cell using a PG spectrophotometer model T+80. 1H-NMR spectra, in which tetramethylsilane (TMS) was used as internal standard (δ ppm) and DMSO-d6 as the solvent were obtained through a BRUKER model 400 MHz. The C, H, and N elemental analysis of the Schiff-base ligands and their Ni(II) and Cu(II) complexes was carried out using a Perkin-Elmer (model 240 C) elemental analyzer (Mount Holly, NJ, USA). Magnetic measurements were conducted on a Gouy’s balance and diamagnetic corrections were performed using Pascal’s constants using Hg[Co(SCN)4] as a calibrant. Thermogravimetric testing was undertaken at a heating rate of 10 °C min−1 under N2 atmosphere using a Shimadzu corporation 60H analyzer (Kyoto, Japan). The value of the absorbance of a 5 × 10−3 M solution of each complex was recorded over wide range of pH values. The pH of a series of Britton–Robinson (BR) universal buffers was determined using a HANNA 211 pH meter at 298 K.
Transmission electron microscope (TEM-2100) at the Faculty of Science, Alex University was employed to obtain the TEM images of all prepared nanocatalysts. Ultrasonic irradiation was obtained via an ultrasonic generator (Dr. Hielscher UP400 S ultrasonic processor) prepared with an “H22” sonotrode of diameter 22 mm, working at 24 kHz at a maximum force output of 400 W. XRD measurements were performed using a Philips diffractometer with monochromatized CuKα radiation. Image Launcher of the Broken Symmetry Software, version 1.4.3.6.7 was employed to determine the particle size distribution of the prepared Ni/Cu-complexes and their corresponding MOs.
3.6. Catalytic Oxidation Experiments
The catalytic oxidation of BzOH to the corresponding BzH by the nanosized Ni/Cu-Schiff base complexes and their NiO/CuO nanoparticles was studied in the presence of H2O2. In a typical reaction, BzOH (0.1 mL, 1.0 mmol) was added to a solution of the M(II) complex or metal oxide (0.03 mmol) in 10 mL of DMSO under stirring conditions at different temperatures (60, 70, 80, and 90 °C) in a water bath. The reaction was initiated by adding aqueous H2O2 (30%) at each temperature and monitored by GC. The obtained oxidation products were identified by matching their retention times with those of authentic samples. Control experiments were conducted by treating a withdrawn sample (ca. 2 mL) of the reaction mixture with solid MnO2 (to quench the excess H2O2) and then adding anhydrous Na2SO4 (to absorb the excess water molecules) under the same conditions to those of the catalytic runs. The resulting slurry was filtered off and the obtained filtrate was injected into the GC. This protocol allowed independent measurements for each sample. The amount of generated oxidation product (BzH) obtained upon chemical conversion of BzOH was calculated using computerized standard calibration curves.
4. Conclusions
In this study, two Schiff-base ligands derived from the condensation of 2-amino-3-hydroxypyridine with 3-methoxysalicylaldehyde or 4-nitrobenzaldehyde were synthesized, and their nanosized Cu(II) and Ni(II) complexes, namely ahpvCu, ahpnbCu, and ahpvNi were obtained via a sonochemical approach. The results obtained by a wide range of structural tools revealed the formation of 1:1 (metal: ligand) complexes in the case of ahpvCu and ahpvNi, while a 1:2 stoichiometric ratio was found for the ahpnbCu congener. The prepared nanosized Schiff-base complexes, along with their MOs, exhibit excellent catalytic performance in the oxidation of BzOH to BzH, particularly when carried out in DMSO using H2O2 as the oxidizing agent. The present finding makes the prepared complexes good candidates for the investigation of the catalytic conversions of other organic compounds and alcohols.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/8/10/452/s1, Figures S1–S5: Electronic spectra, pH-profiles, TEM images, and repetitive scans for designated complexes and their MOs, Tables S1–S6: Physical and analytical properties, as well as IR and thermal analysis data for the ligands and their respective complexes along with data for catalytic oxidation of benzyl alcohol under various conditions. Schemes S1 and S2: Proposed structures and procedure for the prepared complexes.
Author Contributions
Conceptualization, L.H.A.R. and A.N.; Data curation, A.M.A.-D. and S.M.A.-F.; Formal analysis, T.M.A.; Investigation, S.M.A.-F. and A.M.A.; Methodology, A.M.A.-D. and A.N.; Project administration, T.M.A.; Resources, A.M.A.; Supervision, S.I.A.-S., L.H.A.R. and A.N.
Acknowledgments
We extend our sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this project through Research Group (RG #236) and RSSU for their technical support. We also thank the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for funding of this work through Research Group (RGP-1438-0005).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. Sonochemical synthesis, DNA binding, antimicrobial evaluation and in vitro anticancer activity of three new nano-sized Cu (II), Co (II) and Ni (II) chelates based on tri-dentate NOO imine ligands as precursors for metal oxides. J. Photochem. Photobiol. B 2016, 162, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.H.; El-Khatib, R.M.; Nassr, L.A.; Abu-Dief, A.M.; Lashin, F.E.-D. Design, characterization, teratogenicity testing, antibacterial, antifungal and DNA interaction of few high spin Fe (II) Schiff base amino acid complexes. Spectrochim. Acta Part A 2013, 111, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Türkkan, B.; Sarıboğa, B.; Sarıboğa, N. Synthesis, characterization and antimicrobial activity of 3,5-di-tert-butylsalicylaldehyde-S-methylthiosemicarbazones and their Ni (II) complexes. Transit. Met. Chem. 2011, 36, 679–684. [Google Scholar] [CrossRef]
- Boghaei, D.M.; Mohebi, S. Synthesis, characterization and study of vanadyl tetradentate Schiff base complexes as catalyst in aerobic selective oxidation of olefins. J. Mol. Catal. A Chem. 2002, 179, 41–51. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Ramesh, R. Synthesis, characterization, catalytic oxidation and biological activity of ruthenium (III) Schiff base complexes derived from 3-acetyl-6-methyl-2H-pyran-2,4-(3H)-dione. Polyhedron 2006, 25, 3095–3103. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Guan, N.; Li, L. Palladium on graphene as efficient catalyst for solvent-free aerobic oxidation of aromatic alcohols: Role of graphene support. Appl. Catal. B 2013, 136, 177–185. [Google Scholar] [CrossRef]
- Chevrot, C.; Henri, T. Electrosynthesis and oxidation of new oligoazomethines containing N-ethylcarbazole groups. Synth. Met. 2001, 118, 157–166. [Google Scholar] [CrossRef]
- Tieke, B. Coordinative supramolecular assembly of electrochromic thin films. Curr. Opin. Colloid Interface Sci. 2011, 16, 499–507. [Google Scholar] [CrossRef]
- Khalaji, A.D. Preparation and characterization of NiO nanoparticles via solid-state thermal decomposition of nickel (II) Schiff base complexes [Ni (salophen)] and [Ni (Me-salophen)]. J. Clust. Sci. 2013, 24, 209–215. [Google Scholar] [CrossRef]
- Xu, H.; Xu, Z.F.; Yue, Z.Y.; Yan, P.F.; Wang, B.; Jia, L.W.; Li, G.M.; Sun, W.B.; Zhang, J.W. A novel deep blue-emitting Zn(II) complex based on carbazole-modified 2-(2-hydroxyphenyl) benzimidazole: Synthesis, bright electroluminescence, and substitution effect on photoluminescent, thermal, and electrochemical properties. J. Phys. Chem. C 2008, 112, 15517–15525. [Google Scholar] [CrossRef]
- Ramesh, R. Spectral and catalytic studies of ruthenium (III) Schiff base complexes. Inorg. Chem. Commun. 2004, 7, 274–276. [Google Scholar] [CrossRef]
- Bordoloi, A.; Sahoo, S.; Lefebvre, F.; Halligudi, S. Heteropoly acid-based supported ionic liquid-phase catalyst for the selective oxidation of alcohols. J. Catal. 2008, 259, 232–239. [Google Scholar] [CrossRef]
- Maity, P.; Gopinath, C.S.; Bhaduri, S.; Lahiri, G.K. Applications of a high performance platinum nanocatalyst for the oxidation of alcohols in water. Green Chem. 2009, 11, 554–561. [Google Scholar] [CrossRef]
- Mahdavi, V.; Mardani, M. Selective oxidation of benzyl alcohol with tert-butylhydroperoxide catalysed via Mn (II) 2,2-bipyridine complexes immobilized over the mesoporous hexagonal molecular sieves (HMS). J. Chem. Sci. 2012, 124, 1107–1115. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Adam, M.S.S.; Hamdan, S.K. Some new nano-sized mononuclear Cu (II) Schiff base complexes: Design, characterization, molecular modeling and catalytic potentials in benzyl alcohol oxidation. Catal. Lett. 2016, 146, 1373–1396. [Google Scholar] [CrossRef]
- Putla, S.; Amin, M.H.; Reddy, B.M.; Nafady, A.; Al Farhan, K.A.; Bhargava, S.K. MnOx Nanoparticle-Dispersed CeO2 Nanocubes: A Remarkable Heteronanostructured System with Unusual Structural Characteristics and Superior Catalytic Performance. ACS Appl. Mater. Interfaces 2015, 7, 16525–16535. [Google Scholar] [CrossRef] [PubMed]
- Poreddy, R.; Engelbrekt, C.; Riisager, A. Copper oxide as efficient catalyst for oxidative dehydrogenation of alcohols with air. Catal. Sci. Technol. 2015, 5, 2467–2477. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Fujita, S.-I.; Ikushima, Y.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides, and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity. Appl. Catal. A 2001, 219, 259–266. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mondal, K.C. CO2 reforming of methane combined with steam reforming or partial oxidation of methane to syngas over NdCoO3 perovskite-type mixed metal-oxide catalyst. Appl. Energy 2006, 83, 1024–1032. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Zhang, M.; De Respinis, M.; Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem. 2014, 6, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Jampaiah, D.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Selvakannan, P.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Ceria-zirconia modified MnOx catalysts for gaseous elemental mercury oxidation and adsorption. Catal. Sci. Technol. 2016, 6, 1792–1803. [Google Scholar] [CrossRef]
- Gervasini, A.; Auroux, A. Acidity and basicity of metal oxide surfaces II. Determination by catalytic decomposition of isopropanol. J. Catal. 1991, 131, 190–198. [Google Scholar] [CrossRef]
- Lavalley, J. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Martin, D.; Duprez, D. Mobility of surface species on oxides. 1. Isotopic exchange of 18O2 with 16O of SiO2, Al2O3, ZrO2, MgO, CeO2, and CeO2-Al2O3. Activation by noble metals. Correlation with oxide basicity. J. Phys. Chem. 1996, 100, 9429–9438. [Google Scholar] [CrossRef]
- Watanabe, M.; Osada, M.; Inomata, H.; Arai, K.; Kruse, A. Acidity and basicity of metal oxide catalysts for formaldehyde reaction in supercritical water at 673 K. Appl. Catal. A 2003, 245, 333–341. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Meyyappan, M. Nanotechnology: Role in emerging nanoelectronics. Solid-State Electron. 2006, 50, 536–544. [Google Scholar] [CrossRef]
- Guo, Y.G.; Hu, J.S.; Wan, L.J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Park, J.Y. Colloid science of metal nanoparticle catalysts in 2D and 3D structures. Challenges of nucleation, growth, composition, particle shape, size control and their influence on activity and selectivity. Top. Catal. 2008, 49, 126–135. [Google Scholar] [CrossRef]
- Kandjani, A.E.; Sabri, Y.M.; Periasamy, S.R.; Zohora, N.; Amin, M.H.; Nafady, A.; Bhargava, S.K. Controlling core/shell formation of nanocubic p-Cu2O/n-ZnO toward enhanced photocatalytic performance. Langmuir 2015, 31, 10922–10930. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Srivastava, A.; Srivastava, S. Synthetic, structural and antifungal studies of coordination compounds of Ru (III), Rh (III) and Ir (III) with tetradentate Schiff bases. J. Serb. Chem. Soc. 2006, 71, 917–928. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman spectra of inorganic and coordination compounds. In Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Agarwal, S.K. Synthesis and characterization of some mixed ligand complexes of Pd (II), Rh (III) and Pt (IV) with carboxylic hydrazones as primary and dithiooxamide as co-ligand. Asian J. Chem. 2007, 19, 2581–2585. [Google Scholar]
- Vatsa, G.; Pandey, O.; Sengupta, S. Synthesis, spectroscopic and toxicity studies of titanocene chelates of isatin-3-thiosemicarbazones. Bioinorg. Chem. Appl. 2005, 3, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kusanur, R.A.; Ghate, M.; Kulkarni, M.V. Synthesis of spiro[indolo-1,5-benzodiazepines] from 3-acetyl coumarins for use as possible antianxiety agents. J. Chem. Sci. 2004, 116, 265–270. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 71–125. ISBN 978-0-470-61637-6. [Google Scholar]
- Labisbal, E.; Sousa, A.; Castiñeiras, A.; García-Vázquez, J.A.; Romero, J.; West, D.X. Spectral and structural studies of metal complexes of isatin 3-hexamethyleneiminylthiosemicarbazone prepared electrochemically. Polyhedron 2000, 19, 1255–1262. [Google Scholar] [CrossRef]
- Al-Maydama, H.; El-Shekeil, A.; Khalid, M.; Al-Karbouly, A. Thermal degradation behaviour of some polydithiooxamide metal complexes. Ecletica Quím. 2006, 31, 45–52. [Google Scholar] [CrossRef]
- Montazerozohori, M.; Jahromi, S.M.; Naghiha, A. Thermal analyses data and antimicrobial screening of some new nano-structure five coordinated cadmium complexes. J. Ind. Eng. Chem. 2015, 22, 248–257. [Google Scholar] [CrossRef]
- Diefallah, E.-H.M. Kinetic analysis of thermal decomposition reactions: Part VI. Thermal decomposition of manganese (II) acetate tetrahydrate. Thermochim. Acta 1992, 202, 1–16. [Google Scholar] [CrossRef]
- Carmody, W.R. Demonstrating Job’s method with colorimeter or spectrophotometer. J. Chem. Educ. 1964, 41, 615–616. [Google Scholar] [CrossRef]
- Türkel, N. Stability constants of mixed ligand complexes of nickel (II) with adenine and some amino acids. Bioinorg. Chem. Appl. 2015. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W. X-Ray powder diffraction analysis film and Geiger counter techniques. Science 1949, 110, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Bestimmung der Grösse und der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen, Nachrichten von der Gesellschaft der Wissenschaften. Göttingen. Mathematisch-Physikalische Klasse 1918, 2, 98–100. [Google Scholar]
- Adam, M.S.S. Catalytic potentials of homodioxo-bimetallic dihydrazone complexes of uranium and molybdenum in a homogeneous oxidation of alkenes. Monatsh. Chem. 2015, 146, 1823–1836. [Google Scholar] [CrossRef]
- Monfared, H.H.; Bikas, R.; Mayer, P. Homogeneous green catalysts for olefin oxidation by mono oxovanadium (V) complexes of hydrazone Schiff base ligands. Inorg. Chim. Acta 2010, 363, 2574–2583. [Google Scholar] [CrossRef]
- Ragupathi, C.; Vijaya, J.J.; Kennedy, L.J. Synthesis, characterization of nickel aluminate nanoparticles by microwave combustion method and their catalytic properties. Mater. Sci. Eng. B 2014, 184, 18–25. [Google Scholar] [CrossRef]
- Ragupathi, C.; Vijaya, J.J.; Narayanan, S.; Jesudoss, S.; Kennedy, L.J. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by cobalt aluminate catalysis: A comparison of conventional and microwave methods. Ceram. Int. 2015, 41, 2069–2080. [Google Scholar] [CrossRef]
- Noshiranzadeh, N.; Bikas, R.; Ślepokura, K.; Mayeli, M.; Lis, T. Synthesis, characterization and catalytic activity of new Cr (III) complex in oxidation of primary alcohols to aldehydes. Inorg. Chim. Acta 2014, 421, 176–182. [Google Scholar] [CrossRef]
- Enamullah, M.; Islam, M.K. Syntheses, spectroscopy, optical properties, and diastereoselectivity of copper(II)-complexes with chiral aminoalcohol based Schiff bases. J. Coord. Chem. 2013, 66, 4107–4118. [Google Scholar] [CrossRef]
- Zueva, E.; Walton, P.H.; McGrady, J.E. Catalytic alcohol oxidation by an unsymmetrical 5-coordinate copper complex: Electronic structure and mechanism. Dalton Trans. 2006, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Banu, K.S.; Chattopadhyay, T.; Banerjee, A.; Bhattacharya, S.; Zangrando, E.; Das, D. Catechol oxidase activity of dinuclear copper (II) complexes of Robson type macrocyclic ligands: Syntheses, X-ray crystal structure, spectroscopic characterization of the adducts and kinetic studies. J. Mol. Catal. A 2009, 310, 34–41. [Google Scholar] [CrossRef]
- Ma, C.Y.; Cheng, J.; Wang, H.L.; Hu, Q.; Tian, H.; He, C.; Hao, Z.P. Characteristics of Au/HMS catalysts for selective oxidation of benzyl alcohol to benzaldehyde. Catal. Today 2010, 158, 246–251. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Cheng, L.; Wu, Z.; Yang, J. DFT studies on the mechanism of veratryl alcohol oxidation catalyzed by Cu-phen complexes. RSC Adv. 2014, 4, 30558–30565. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).