Rapid Suzuki-Miyaura Couplings with ppm Level of Palladium Catalyst in a High-Pressure and High-Temperature Water System
Abstract
1. Introduction
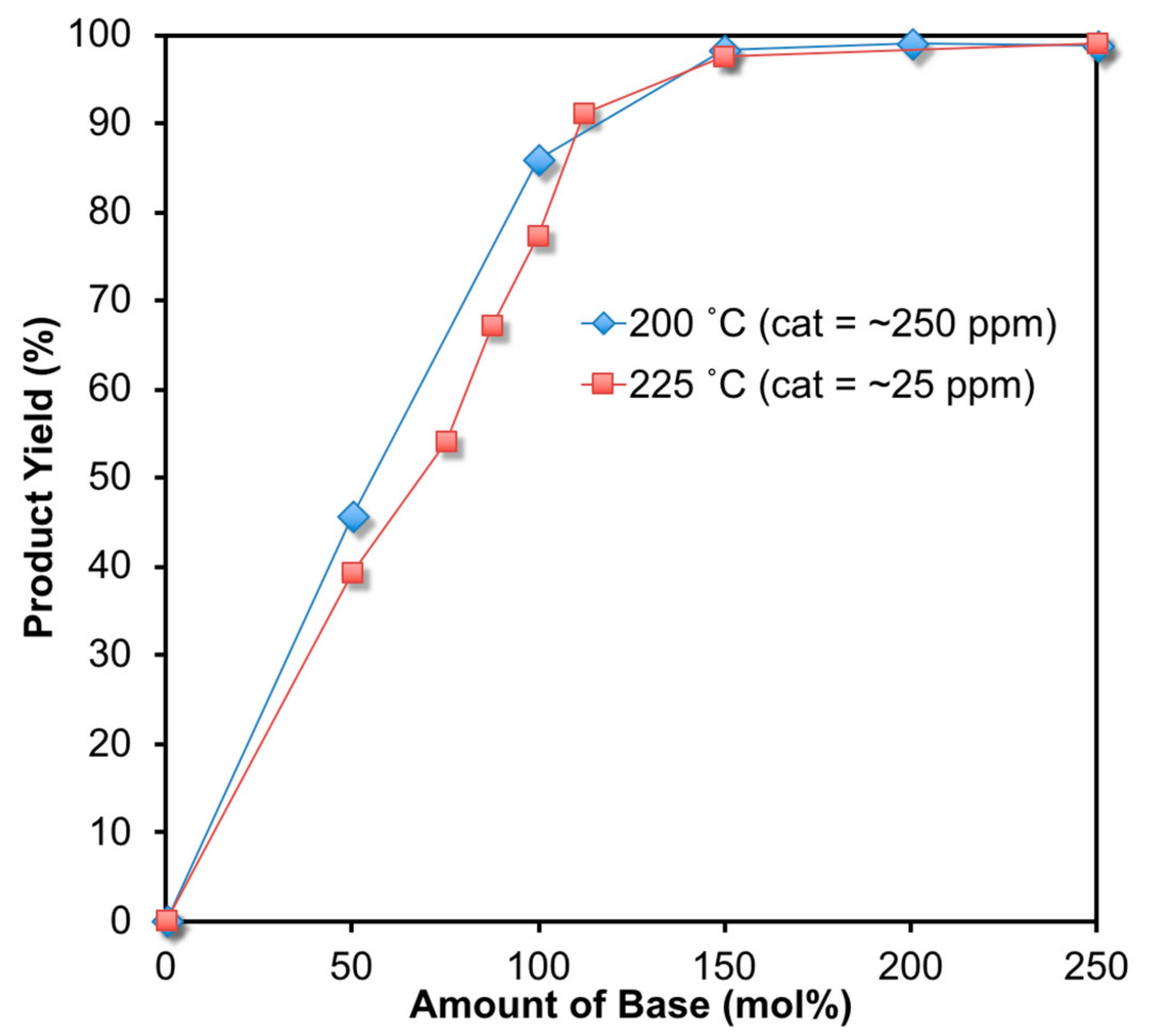
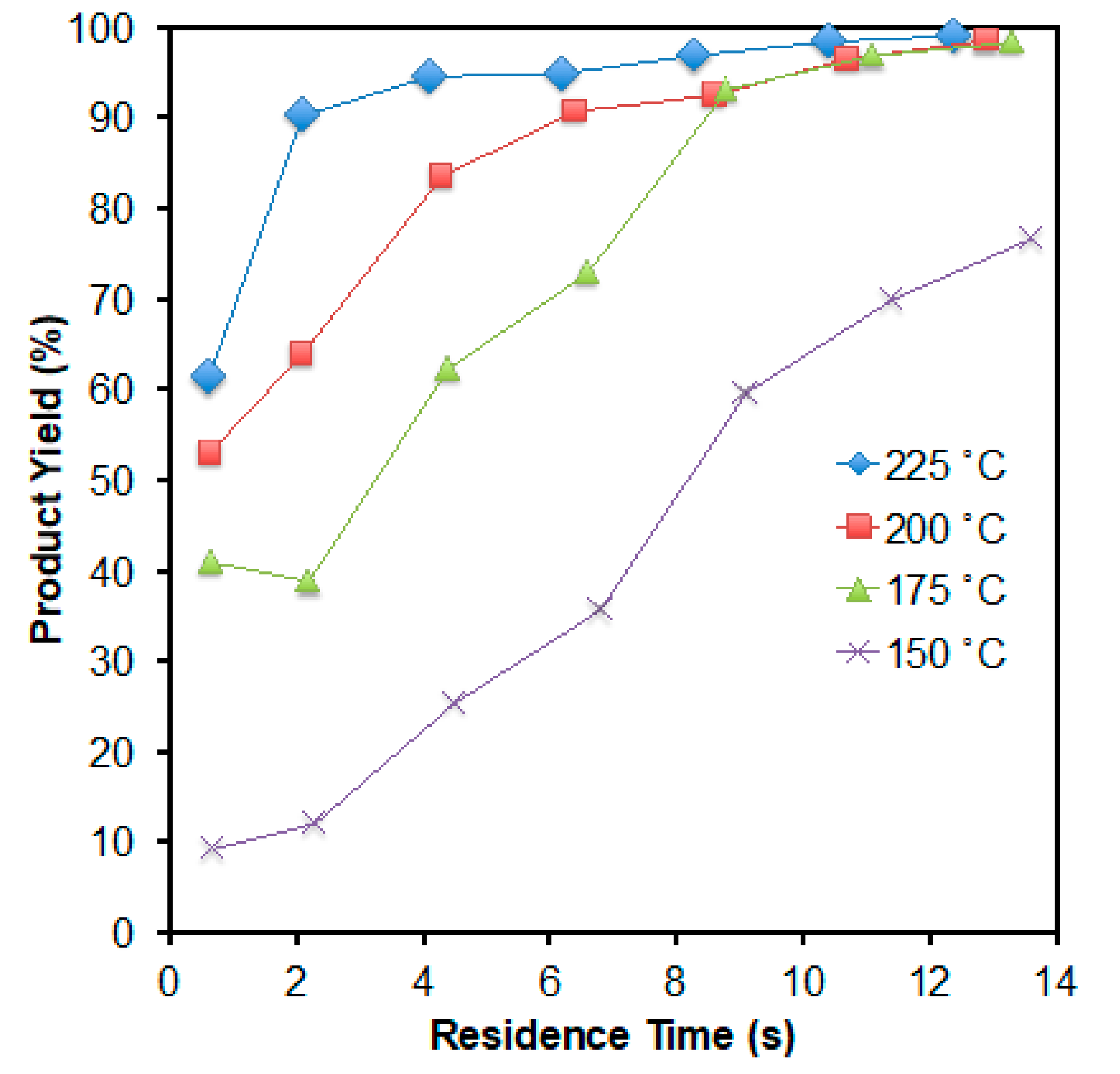
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lipshutz, B.H.; Ghorai, S.; Cortes-Clerget, M. The Hydrophobic Effect Applied to Organic Synthesis: Recent Synthetic Chemistry “in Water”. Chem. Eur. J. 2018, 24, 6672–6695. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Gallou, F.; Handa, S. Evolution of Solvents in Organic Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Saikia, B.; Boruah, P.R.; Ali, A.A.; Sarma, D. ‘On-water’ organic synthesis: A green, highly efficient, low cost and reusable catalyst system for biaryl synthesis under aerobic conditions at room temperature. RSC Adv. 2015, 5, 50655–50659. [Google Scholar] [CrossRef]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki–Miyaura Cross-Coupling Reaction in Water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Hailes, C.H. Reaction Solvent Selection: The Potential of Water as a Solvent for Organic Transformations. Org. Process Res. Dev. 2007, 11, 114–120. [Google Scholar] [CrossRef]
- Rao, K.U.; Appa, R.M.; Lakshmidevi, J.; Vijitha, R.; Rao, K.S.V.K.; Narasimhulu, M.; Venkateswarlu, K. C(sp2)-C(sp2) Coupling in Water: Palladium (II) Complexes of N-Pincer Tetradentate Porphyrins as Effective Catalyst. Asian J. Chem. 2017, 6, 751–757. [Google Scholar] [CrossRef]
- Weingartner, H.; Franck, E.U. Supercritical water as a solvent. Angew. Chem. Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.E. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.B.; Kaul, C.; Krämer, A.; Krammer, P.; Richter, T.; Jung, M.; Vogel, H.; Zehner, P. Chemistry in Supercritical Water. Angew. Chem. Int. Ed. 1999, 38, 2998–3014. [Google Scholar]
- Marcus, Y. Supercritical Water, a Green Solvent: Properties and Uses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 1118310276. [Google Scholar]
- Akizuki, M.; Fujii, T.; Hayashi, R.; Oshima, Y. Effects of water on reactions for waste treatment, organic synthesis, and bio-refinery in sub- and supercritical water. J. Biosci. Bioeng. 2014, 117, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Siskin, M.; Katritzky, A.R. Reactions in High-Temperature Aqueous Media. Chem. Rev. 2001, 101, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Kus, N.S. Organic reactions in subcritical and supercritical water. Tetrahedron 2012, 68, 949–958. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Inomata, H.; Smith, R.L., Jr.; Arai, K.; Kruse, A.; Dinjus, E. Chemical Reactions of C1 Compounds in Near-Critical and Supercritical Water. Chem. Rev. 2004, 104, 5803–5821. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Kristof, A.J.; Vasudevan, V.; Genzer, J.; Srogl, J.; Abolhasani, M. Microfluidic Synthesis of Leastomeric Microparticles: A Case Study in Catalysis of Palladium-Mediated Cross-Coupling. AIChE J. 2018, 64, 3188–3197. [Google Scholar] [CrossRef]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding whether to go with the flow: Evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef] [PubMed]
- Malet-Sanz, L.; Susanne, F. Continuous flow synthesis. A pharma perspective. J. Med. Chem. 2012, 55, 4062–4098. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Hübner, S.; Steinfeldt, N.; Jähnisch, K. Synthesis of Fine Chemicals. In Microreactors in Preparative Chemistry; Reschetilowski, W., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 115–164. [Google Scholar]
- Hessel, V.; Cortese, B.; de Croon, M.H.J.M. Novel process windows—Concept, proposition and evaluation methodology, and intensified superheated processing. Chem. Eng. Sci. 2011, 66, 1426–1448. [Google Scholar] [CrossRef]
- Hessel, V.; Kralisch, D.; Kockmann, N.; Noel, T.; Wang, Q. Novel process windows for enabling, accelerating, and uplifting flow chemistry. ChemSusChem 2013, 6, 746–789. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, H.; Matsushima, K.; Sato, M.; Ikushima, Y. Rapid and highly selective copper-free sonogashira coupling in high-pressure, high-temperature water in a microfluidic system. Angew. Chem. Int. Ed. 2007, 46, 5129–5132. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Matsushima, K.; Kawanami, H.; Ikuhsima, Y. A highly selective, high-speed, and hydrolysis-free O-acylation in subcritical water in the absence of a catalyst. Angew. Chem. Int. Ed. 2007, 46, 6284–6288. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Matsushima, K.; Kawanami, H.; Chatterjee, M.; Yokoyama, T.; Ikuhsima, Y.; Suzuki, T.M. Highly efficient chemoselective N-acylation with water microreaction system in the absence of catalyst. Lab Chip 2009, 9, 2877–2880. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at ppm to ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Rough, A. Fine Chemicals. Chem. Eng. News 2004, 82, 49–67. [Google Scholar]
- Wang, H.-L.; Katon, J.; Balan, C.; Bannon, A.W.; Bernard, C.; Doherty, E.M.; Dominguez, C.; Gavva, N.R.; Gore, V.; Ma, V.; et al. Novel Vanilloid Receptor-1 Antagonists: 3. The Identification of a Second-Generation Clinical Candidate with Improved Physicochemical and Pharmacokinetic Propertiesq. J. Med. Chem. 2007, 50, 3528–3539. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H. Applying the Hydrophobic Effect to Transition Metal-Catalyzed Couplings in Water at Room Temperature. In Transition Metal-Catalyzed Couplings in Process Chemistry; Wiley-VCH: Weinheim, Germany, 2013; pp. 299–312. ISBN 9783527332793. [Google Scholar]
- Winkle, D.D.; Schaab, K.M. Suzuki Reaction of a Diarylborinic Acid: One-Pot Preparation and Cross-Coupling of Bis(3,5-dimethylphenyl)borinic Acid. Org. Process Res. Dev. 2001, 5, 450–451. [Google Scholar] [CrossRef]
- Cole, K.P.; Campbell, M.B.; Forst, M.B.; McClary Groh, J.; Hess, M.; Johnson, M.D.; Miller, R.D.; Mitchell, D.; Polster, C.S.; Reizman, B.J.; et al. An Automated Intermittent Flow Approach to Continuous Suzuki Coupling. Org. Process Res. Dev. 2016, 20, 820–830. [Google Scholar] [CrossRef]
- Bullock, K.M.; Mitchell, M.B.; Toczko, J.F. Optimization and Scale-Up of a Suzuki-Miyaura Coupling Reaction: Development of an Efficient Palladium Removal Technique. Org. Process Res. Dev. 2008, 12, 896–899. [Google Scholar] [CrossRef]
- Nagao, I.; Ishizaka, T.; Kawanami, H. Rapid production of benzazole derivatives by a high-pressure and high-temperature water microflow chemical process. Green Chem. 2016, 18, 3494–3498. [Google Scholar] [CrossRef]
- Handa, S.; Andersson, M.P.; Gallou, F.; Reilly, J.; Lipshutz, B.H. HandaPhos: A General Ligand Enabling Sustainable ppm Levels of Palladium-Catalyzed Cross-Couplings in Water at Room Temperature. Angew. Chem. Int. Ed. 2016, 55, 4914–4918. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Padrell, A.E.; Morthensen, S.T.; Lewandowski, D.J.; Skovby, T.; Kiil, S.; Gernaey, K.V. Continuous Hydrolysis and Liquid–Liquid Phase Separation of an Active Pharmaceutical Ingredient Intermediate Using a Miniscale Hydrophobic Membrane Separator. Org. Process Res. Dev. 2012, 16, 888–900. [Google Scholar] [CrossRef]
- Sahoo, H.R.; Kralj, J.G.; Jensen, K.F. Multistep continuous-flow microchemical synthesis involving multiple reactions and separations. Angew. Chem. Int. Ed. 2007, 46, 5704–5708. [Google Scholar] [CrossRef] [PubMed]
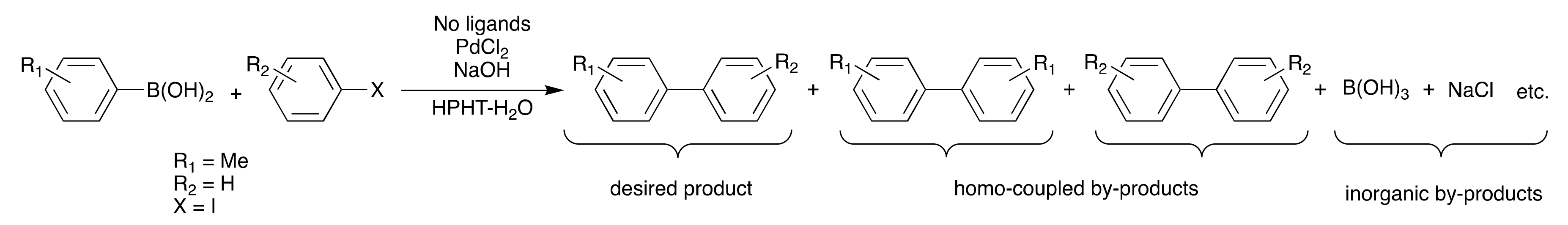
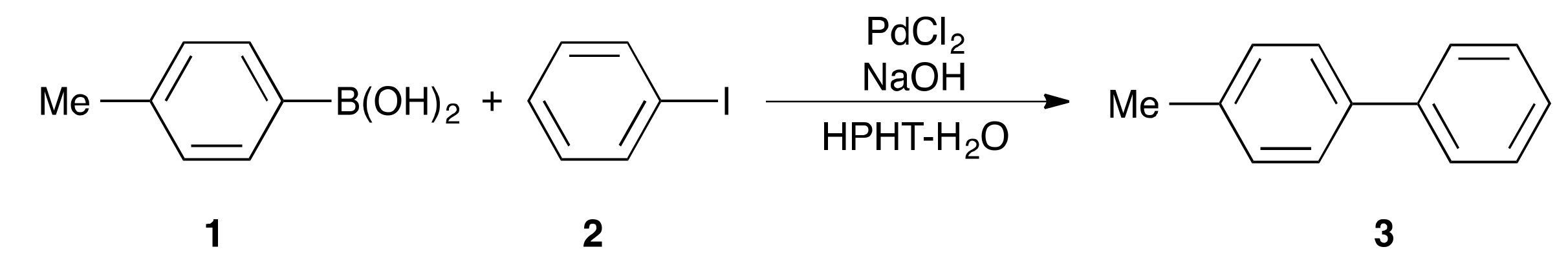
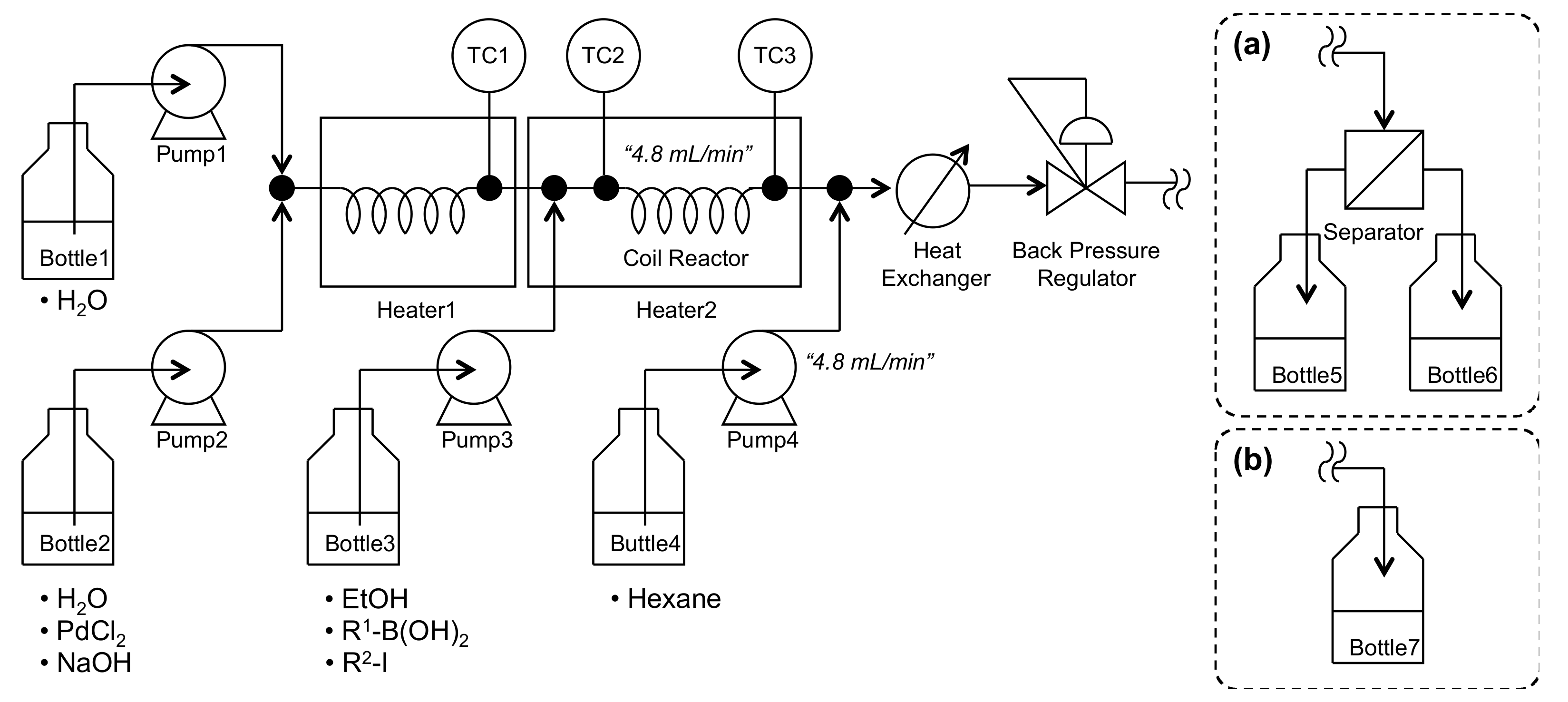
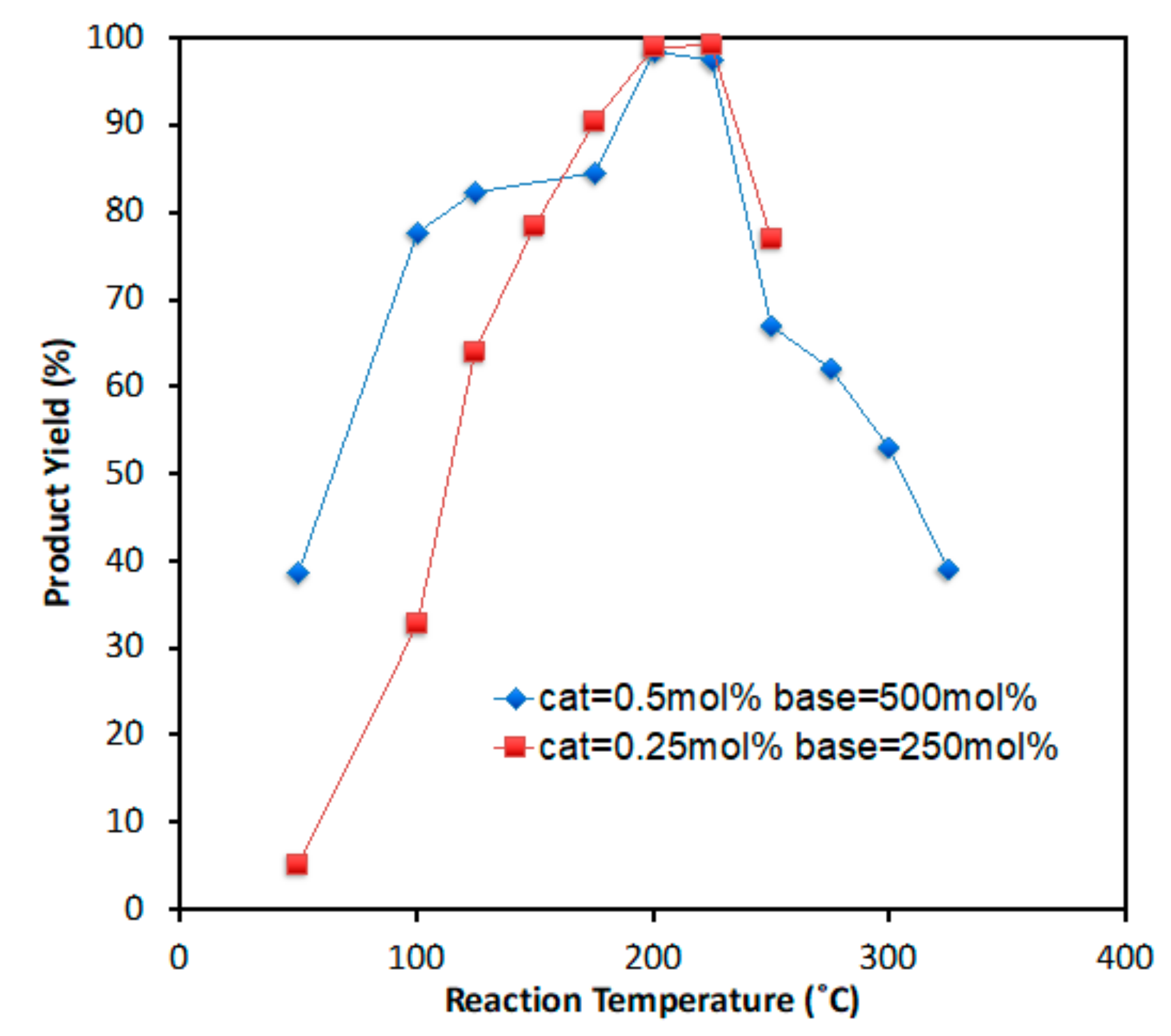
| Entry | Temp. (°C) | PdCl2 (mol %) | NaOH (mol %) | Time (s) | Yield of 3 (%) |
|---|---|---|---|---|---|
| 1 | 50 | 5.0 × 10−1 | 500 | 24.4 | 38 |
| 2 | 100 | 5.0 × 10−1 | 500 | 23.7 | 75 |
| 3 | 125 | 5.0 × 10−1 | 500 | 23.2 | 82 |
| 4 | 175 | 5.0 × 10−1 | 500 | 22.1 | 83 |
| 5 | 200 | 5.0 × 10−1 | 500 | 21.5 | 98 |
| 6 | 200 | 2.5 × 10−1 | 500 | 21.5 | 98 |
| 7 | 200 | 2.5 × 10−1 | 250 | 21.5 | 96 |
| 8 | 200 | 1.25 × 10−1 | 250 | 21.5 | 97 |
| 9 | 200 | 2.5 × 10−2 | 250 | 21.5 | 98 |
| 10 | 200 | 2.5 × 10−3 | 250 | 21.5 | 99 |
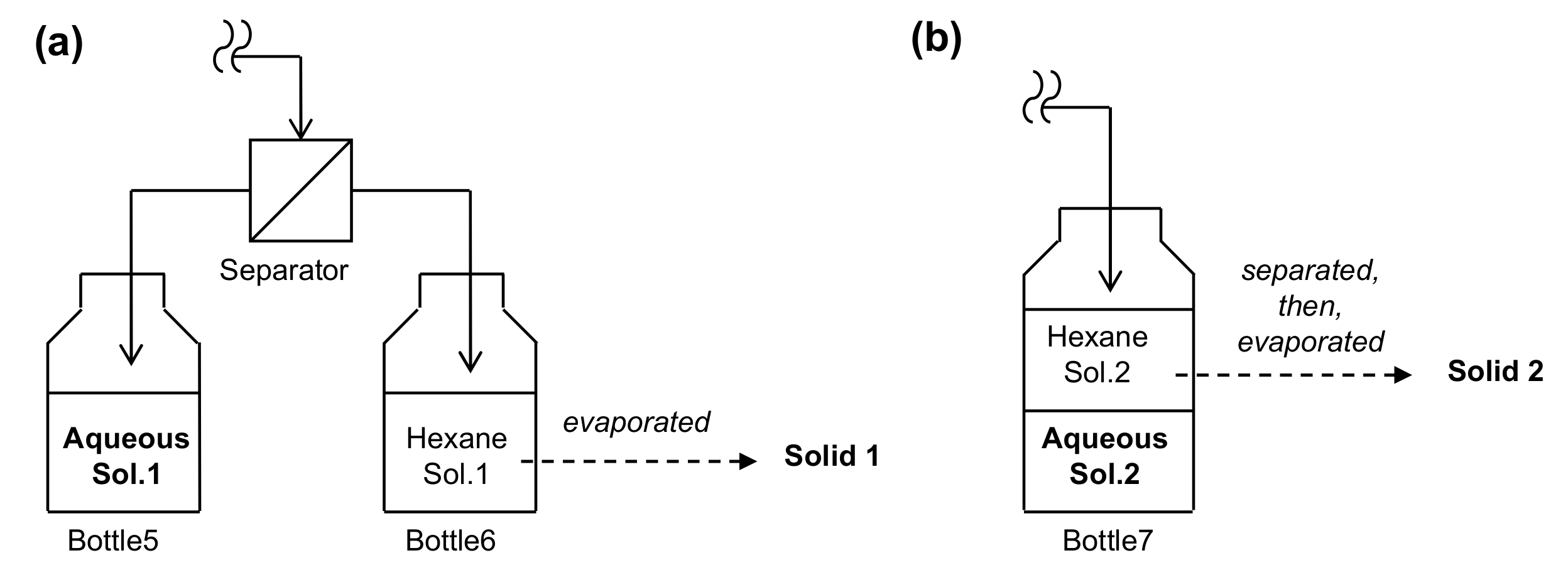
| Entry | Separator | Sample | Pd Content | ||
|---|---|---|---|---|---|
| Conc. | Molar Ratio | Pd Yield | |||
| 1 | Membrane | Solid 1 | 7.4 × 10−6 wt % | 1.2 × 10−5 mol % | 0.47% |
| 2 | Aq. Sol. 1 | >20 mg/L | - | <44% | |
| 3 | Non | 6.1 × 10−5 wt % | 9.6 × 10−5 mol % | 3.9% | |
| 4 | data | >20 mg/L | - | <44% | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagao, I.; Chatterjee, M.; Kawanami, H. Rapid Suzuki-Miyaura Couplings with ppm Level of Palladium Catalyst in a High-Pressure and High-Temperature Water System. Catalysts 2018, 8, 451. https://doi.org/10.3390/catal8100451
Nagao I, Chatterjee M, Kawanami H. Rapid Suzuki-Miyaura Couplings with ppm Level of Palladium Catalyst in a High-Pressure and High-Temperature Water System. Catalysts. 2018; 8(10):451. https://doi.org/10.3390/catal8100451
Chicago/Turabian StyleNagao, Ikuhiro, Maya Chatterjee, and Hajime Kawanami. 2018. "Rapid Suzuki-Miyaura Couplings with ppm Level of Palladium Catalyst in a High-Pressure and High-Temperature Water System" Catalysts 8, no. 10: 451. https://doi.org/10.3390/catal8100451
APA StyleNagao, I., Chatterjee, M., & Kawanami, H. (2018). Rapid Suzuki-Miyaura Couplings with ppm Level of Palladium Catalyst in a High-Pressure and High-Temperature Water System. Catalysts, 8(10), 451. https://doi.org/10.3390/catal8100451






