The Preparation of a Highly Efficient Ag3PO4/Ag/Bi2O2CO3 Photo-Catalyst and the Study of Its Photo-Catalytic Organic Synthesis Reaction Driven by Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phase Stucture (XRD)
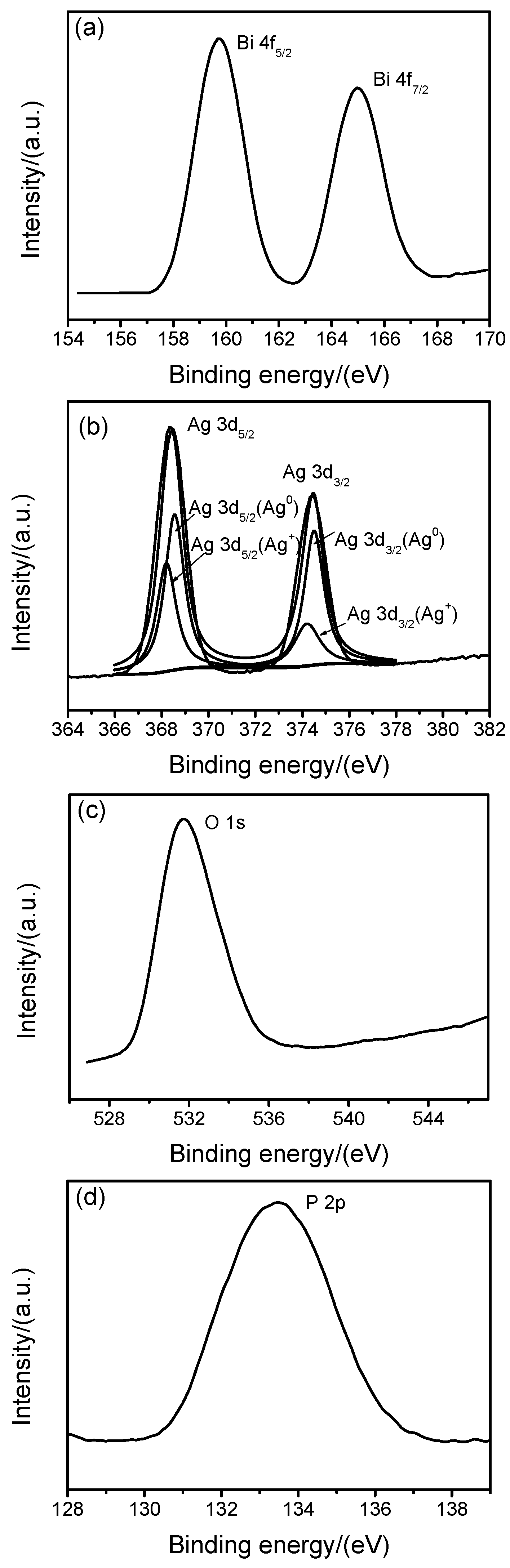
2.2. Chemical Composition (XPS)
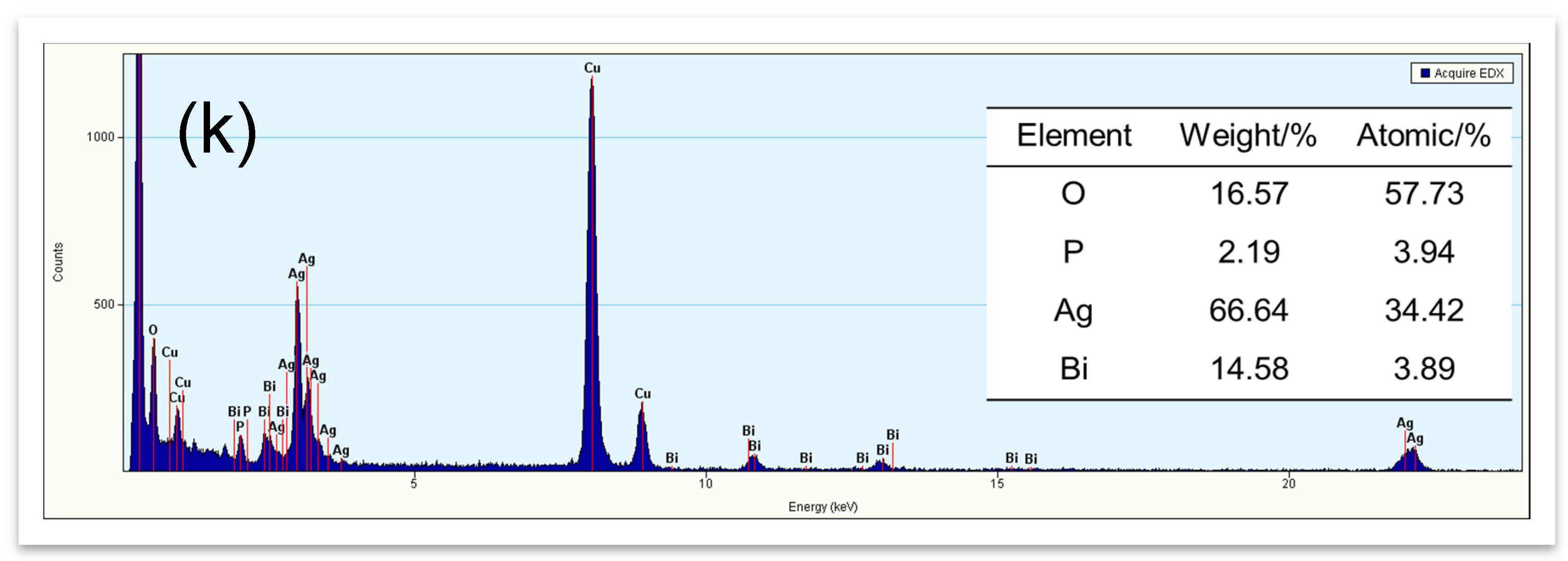
2.3. Morphological Structure (TEM, Energy Dispersive X-ray Spectrum (EDX), and SEM)
2.4. Specific BET Surface Areas
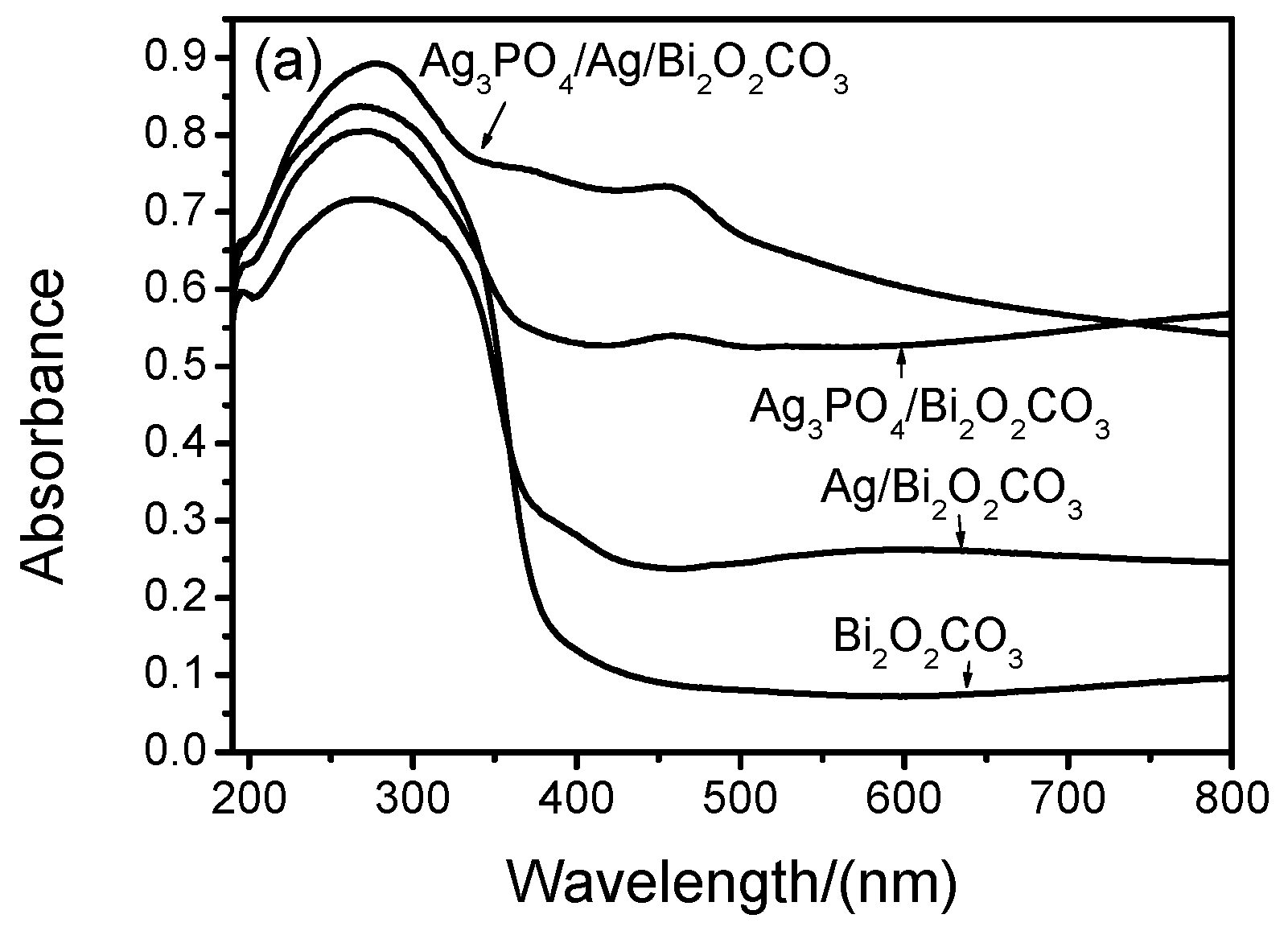
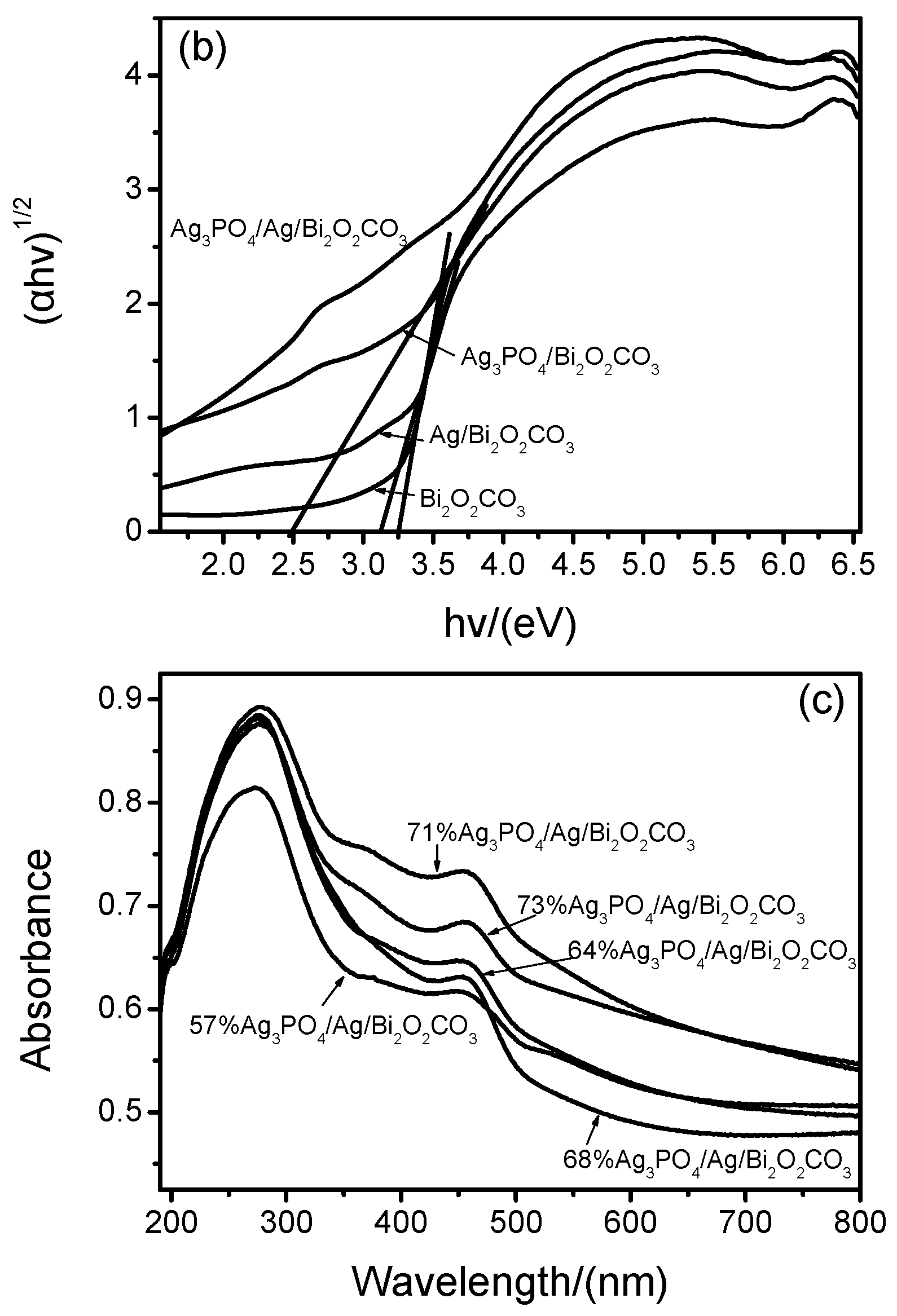
2.5. UV-Vis DRS and Band Gap
2.6. Photo-Catalytic Activities
2.6.1. Esterification of Aldehydes and Alcohols
2.6.2. Esterification of Alcohols and Alcohols
2.6.3. Amination of Alcohols and Amines
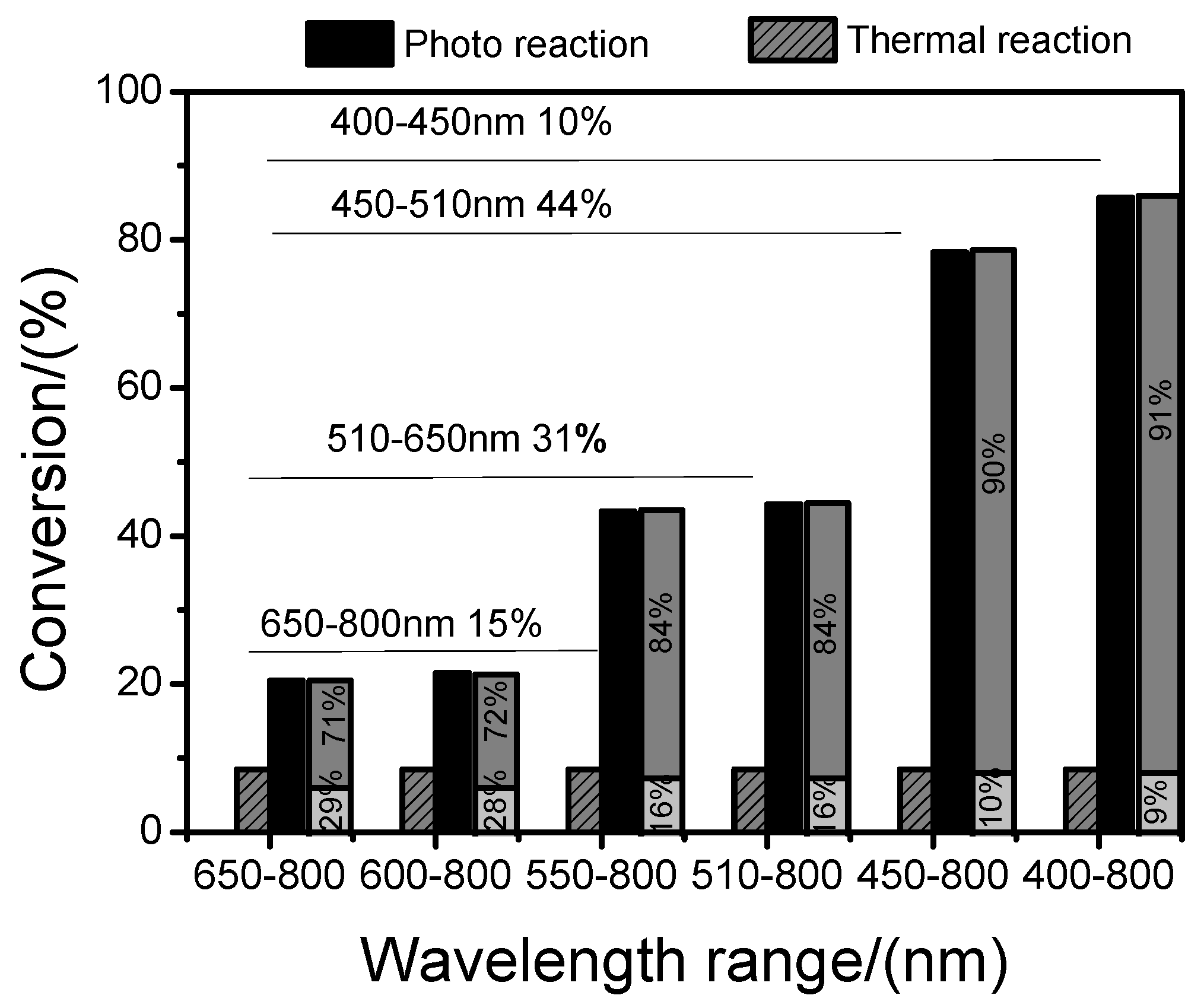
2.7. Effect of Light Intensity and Wavelength
2.8. The Recyclability of the Prepared Catalysts
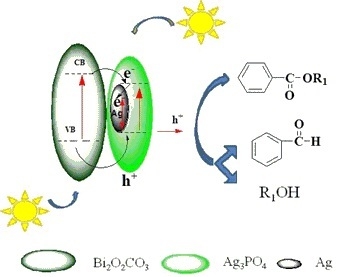
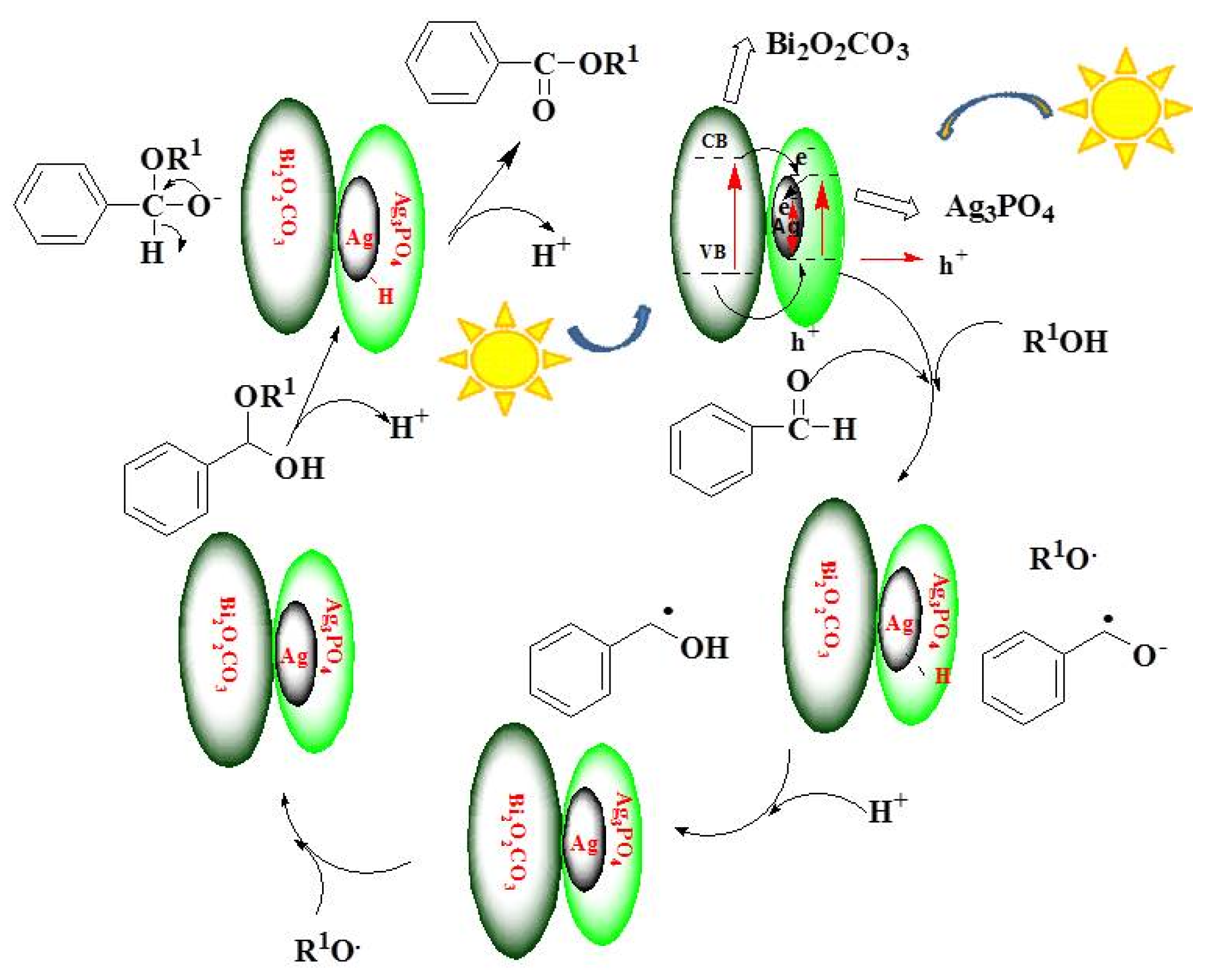
2.9. Reaction Mechanism
3. Materials and Methods
3.1. Preparation of Composites
3.1.1. Preparation of Bi2O2CO3 Porous Microspheres
3.1.2. Preparation of the Ag3PO4/Ag/Bi2O2CO3 Composite
3.1.3. Preparation of the Ag3PO4/Bi2O2CO3 Composite
3.1.4. Preparation of the Ag/Bi2O2CO3 Composite
3.2. Characterization
3.3. Photo-Catalytic Activity Test
3.3.1. The Esterification Reaction of Aldehydes (or Alcohols) and Alcohols
3.3.2. The Amination Reaction of Alcohols and Amines
3.4. Reuse of the Catalyst
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jagadeesh, R.V.; Junge, H.; Pohl, M.M.; Radnik, J.; Bruckner, A.; Beller, M. Selective oxidation of alcohols to ester using heterogeneous Co3O4-N@C catalysts under mild conditions. J. Am. Chem. Soc. 2013, 135, 10776–10782. [Google Scholar] [CrossRef] [PubMed]
- Larock, R.C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations; Wiley-VCH Press: New York, NY, USA, 1989. [Google Scholar]
- Sarkar, S.D.; Grimme, S.; Studer, A. NHC catalyzed oxidations of aldehydes to esters: Chemoselective acylation of alcohols in presence of amines. J. Am. Chem. Soc. 2010, 132, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Xiao, Z.F.; Yao, C.Z.; Zheng, H.X.; Kang, Y.B. Direct alkylation of amines with alcohols catalyzed by base. Org. Lett. 2015, 17, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.S.; Rosa, J.N.; Veiros, L.F.; Caddick, S.; Gois, P.M.P. NHC/iron cooperative catalysis: Aerobic oxidative esterification of aldehydes with phenols. Org. Biomol. Chem. 2011, 9, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Fujita, K.I.; Yamaguchi, R. N-alkylation of amines with alcohols catalyzed by a water soluble Cp*iridium complex: An efficient method for the synthesis of amines in aqueous media. Adv. Synth. Catal. 2011, 353, 1161–1168. [Google Scholar] [CrossRef]
- Kiyooka, S.I.; Wada, Y.; Ueno, M.; Yokoyama, T.; Yokoyama, R. [IrCl(cod)]2-catalyzed direct oxidative esterification of aldehydes with alcohols. Tetrahedron 2007, 63, 12695–12701. [Google Scholar] [CrossRef]
- Bolm, C.; Legros, J.; Paih, J.L.; Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.K.; Guin, S.; Ghara, K.K.; Banerjee, A.; Patel, B.K. Copper catalyzed oxidative esterification of aldehydes with alkylbenzenes via cross dehydrogenative coupling. Org. Lett. 2012, 14, 3982–3985. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Li, C.J. Copper-catalyzed oxidative esterification of alcohols with aldehydes activated by lewis acids. Tetrahedron Lett. 2007, 48, 1033–1035. [Google Scholar] [CrossRef]
- Liu, C.; Tang, S.; Zheng, L.W.; Liu, D.; Zhang, H.; Lei, A.W. Covalently bound benzyl ligand promotes selective palladium catalyzed oxidative esterification of aldehydes with alcohols. Angew. Chem. Int. Ed. 2012, 51, 5662–5666. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, C.; Hensen, E.J.M. Efficient tandem synthesis of methyl esters and imines by using versatile hydrotalcite-supported gold nanoparticles. Chem. Eur. J. 2012, 18, 12122–12129. [Google Scholar] [CrossRef] [PubMed]
- Abiko, A.; Roberts, J.C.; Takemasa, T.; Masamune, S. KMnO4 revisited: Oxidation of aldehydes to carboxylic acids in the tert-butyl alcohol-aqueous NaH2PO4 system. Tetrahedron Lett. 1986, 27, 4537–4540. [Google Scholar] [CrossRef]
- Garegg, P.J.; Olsson, L.; Oscarson, S. Synthesis of methyl (ethyl 2-O-acyl-3,4-di-O-benzyl-1-thio-β-d-glucopyranosid) uronates and evaluation of their use as reactive β-selective glucuronic acid donors. J. Org. Chem. 1995, 60, 2200–2204. [Google Scholar] [CrossRef]
- Qian, G.; Zhao, R.; Ji, D.; Lu, G.M.; Qi, Y.X.; Suo, J.S. Facile oxidation of aldehydes to esters using S-SnO2/SBA-1-H2O2. Chem. Lett. 2004, 33, 834–835. [Google Scholar] [CrossRef]
- Travis, B.R.; Sivakumar, M.; Hollist, G.O.; Borhan, B. Facile oxidation of aldehydes to acids and esters with oxone. Org. Lett. 2003, 5, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Holcomb, H.; Kennedy, K.; Kirkpatrick, E.; Leathers, T.; Vanemon, P. N-iodosuccinimide-mediated conversion of aldehydes to methyl esters. J. Org. Chem. 1989, 54, 1213–1215. [Google Scholar] [CrossRef]
- Gopinah, R.; Barkakaty, B.; Talukdar, B.; Patel, B.K. Peroxovanadium-catalyzed oxidative esterification of aldehydes. J. Org. Chem. 2003, 68, 2944–2947. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Junge, K.; Beller, M. Sustainable metal catalysis with iron: From rust to a rising star. Angew. Chem. Int. Ed. 2008, 47, 3317–3321. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Chong, Y.Z.; Li, S.R.; Yang, H.Q. Controlled synthesis of Au nanoparticles in the nanocages of SBA-16: Improved activity and enhanced recyclability for the oxidative esterification of alcohols. J. Phys. Chem. C 2012, 116, 6512–6519. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xiao, Q.; Bao, Y.S.; Zhang, Y.J.; Bottle, S.; Sarina, S.; Bao, Z.R.G.T.; Zhu, H.Y. Direct photocatalytic conversion of aldehydes to esters using supported gold nanoparticles under visible light irradiation at room temperature. J. Phys. Chem. C 2014, 118, 19062–19069. [Google Scholar] [CrossRef]
- Bu, Y.Y.; Chen, Z.Y.; Sun, C.J. Highly efficient Z-scheme Ag3PO4/Ag/WO3−x photocatalyst for its enhanced photocatalytic performance. Appl. Catal. B 2015, 179, 363–371. [Google Scholar] [CrossRef]
- Zhang, L.W.; Man, Y.; Zhu, Y.F. Effects of Mo replacement on the structure and visible-light-induced photocatalytic performances of Bi2WO6 photocatalyst. ACS Catal. 2011, 1, 841–848. [Google Scholar] [CrossRef]
- Ruan, Q.J.; Zhang, W.D. Tunable morphology of Bi2Fe4O9 crystals for photocatalytic oxidation. J. Phys. Chem. C 2009, 113, 4168–4173. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.D. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity. J. Mater. Chem. 2010, 20, 5866–5870. [Google Scholar] [CrossRef]
- Dong, F.; Ho, W.K.; Lee, S.C.; Wu, Z.B.; Fu, M.; Zou, S.C.; Huang, Y. Template-free fabrication and growth mechanism of uniform (BiO)2CO3 hierarchical hollow microspheres with outstanding photocatalytic activities under both UV and visible light irradiation. J. Mater. Chem. 2011, 21, 12428–12436. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Z.Y.; Huang, B.B.; Yang, K.S.; Zhang, X.Y.; Qin, X.Y.; Dai, Y. Preparation, electronic structure, and photocatalytic properties of Bi2O2CO3 nanosheet. Appl. Surf. Sci. 2010, 257, 172–175. [Google Scholar] [CrossRef]
- Chen, R.; So, M.H.; Yang, J.; Deng, F.; Che, C.M.; Sun, H.Z. Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate. Chem. Commun. 2006, 21, 2265–2267. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cheng, G.; So, M.H.; Wu, J.L.; Lu, Z.; Che, C.M.; Sun, H.Z. Bismuth subcarbonate nanoparticles fabricated by water-in-oil microemulsion-assisted hydrothermal process exhibit anti-helicobacter pylori properties. Mater. Res. Bull. 2010, 45, 654–658. [Google Scholar] [CrossRef]
- Cheng, H.F.; Huang, B.B.; Yang, K.S.; Wang, Z.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Facile template-free synthesis of Bi2O2CO3 hierarchical microflowers and their associated photocatalytic activity. Chem. Phys. Chem. 2010, 11, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.F.; Zhang, L.; Chen, X.T.; Xue, Z.L. Persimmon-like (BiO)2CO3 microstructures: Hydrothermal preparation, photocatalytic properties and their conversion into Bi2S3. Cryst. Eng. Commun. 2011, 13, 1939–1945. [Google Scholar] [CrossRef]
- Dong, F.; Lee, S.C.; Wu, Z.B.; Huang, Y.; Fu, M.; Ho, W.K.; Zou, S.C.; Wang, B. Rose-like monodisperse bismuth subcarbonate hierarchical hollow microspheres: One-pot template-free fabrication and excellent visible light photocatalytic activity and photochemical stability for NO removal in indoor air. J. Hazard. Mater. 2011, 195, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.Y.; Zai, J.T.; Xu, M.; Zou, Q.; Su, Y.Z.; Wang, K.X.; Qian, X.F. Hierarchical Bi2O2CO3 microspheres with improved visible-light-driven photocatalytic activity. Cryst. Eng. Commun. 2011, 13, 4010–4017. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.R.; Zhang, J.; Yu, J.G.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nano composites with enhanced visible-light photocatalytic activity. Appl. Catal. B 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Madhusudan, P.; Yu, J.G.; Wang, W.G.; Cheng, B.; Liu, G. Facile synthesis of novel hierarchical graphene-Bi2O2CO3 composites with enhanced photocatalytic performance under visible light. Dalton Trans. 2012, 41, 14345–14353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.Q.; Hojamberdievb, M.; Katsumata, K.I.; Cai, X.; Matsushita, N.; Okada, K.; Liu, P.; Zhou, J.P. Heterostructured Fe3O4/Bi2O2CO3 photocatalyst: Synthesis, characterization and application in recyclable photodegradation of organic dyes under visible light irradiation. Mater. Chem. Phys. 2013, 142, 95–105. [Google Scholar] [CrossRef]
- Zhang, X.C.; Guo, T.Y.; Wang, X.W.; Wang, Y.W.; Fan, C.M.; Zhang, H. Facile composition-controlled preparation and photocatalytic application of BiOCl/Bi2O2CO3 nanosheets. Appl. Catal. B 2014, 150, 486–495. [Google Scholar] [CrossRef]
- Bi, Y.P.; Ouyang, S.X.; Umezawa, N.; Cao, J.Y.; Ye, J.H. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J. Am. Chem. Soc. 2011, 133, 6490–6492. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, X.Y.; Zhao, Q.D.; Chen, G.H.; Raston, C.L. Role of hydroxyl radicals and mechanism of escherichia coli inactivation on Ag/AgBr/TiO2 nanotube array electrode under visible light irradiation. Environ. Sci. Technol. 2012, 46, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Hu, C.; Peng, T.W.; Zhou, X.F.; Qu, J.H. Plasmon-induced inactivation of enteric pathogenic microorganisms with Ag-AgI/Al2O3 under visible-light irradiation. Environ. Sci. Technol. 2010, 44, 7058–7062. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Saraswathi, A.; Indi, S.S.; Hoti, S.L.; Vasan, H.N. Ag@AgI, core@shell structure in agarose matrix as hybrid: Synthesis, characterization, and antimicrobial activity. Langmuir 2012, 28, 8550–8561. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.X.; Ye, J.H. β-AgAl1-xGaxO2 solid-solution photocatalysts: Continuous modulation of electronic structure toward high-performance visible-light photoactivity. J. Am. Chem. Soc. 2011, 133, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, N.; Ouyang, S.X.; Ye, J.H. Theoretical study of high photocatalytic performance of Ag3PO4. Phys. Rev. B 2011, 83, 035202(1–8). [Google Scholar] [CrossRef]
- Boltersdorf, J.; Wong, T.; Maggard, P.A. Synthesis and optical properties of Ag(I), Pb(II), and Bi(III) tantalate-based photocatalysts. ACS Catal. 2013, 3, 2943–2953. [Google Scholar] [CrossRef]
- Yi, Z.G.; Ye, J.H.; Kikugawa, N.; Kako, T.; Ouyang, S.X.; Williams, H.S.; Yang, H.; Cao, J.Y.; Luo, W.J.; Li, Z.S.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhu, G.Q.; Hojamberdiev, M.; Luo, X.C.; Tan, C.W.; Peng, J.H.; Wei, X.M.; Li, J.P.; Liu, P. A plasmonic Ag-AgBr/Bi2O2CO3 composite photocatalyst with enhanced visible-light photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 13718–13727. [Google Scholar]
- Chen, X.; Zheng, Z.F.; Ke, X.B.; Jaatinen, E.; Xie, T.F.; Wang, D.J.; Guo, C.; Zhao, J.C.; Zhu, H.Y. Supported silver nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Green Chem. 2010, 12, 414–419. [Google Scholar] [CrossRef]
- Yu, C.L.; Zhou, W.Q.; Zhu, L.H.; Li, G.; Yang, K.; Jin, R.C. Integrating plasmonic Au nanorods with dendritic like α-Bi2O3/Bi2O2CO3 heterostructures for superior visible-light-driven photocatalysis. Appl. Catal. B 2016, 184, 1–11. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, W.L.; Zhang, Z.G.; Fang, X.M. High-efficiency visible-light-driven Ag3PO4/AgI photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic activity. J. Phys. Chem. C 2013, 117, 19346–19352. [Google Scholar] [CrossRef]
- Zhu, M.S.; Chen, P.L.; Liu, M.H. Visible-light-driven Ag/Ag3PO4-based plasmonic photocatalysts: Enhanced photocatalytic performance by hybridization with graphene oxide. Chin. Sci. Bull. 2013, 58, 84–91. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.J.; Fu, M.; Ho, W.K.; Lee, S.C.; Wu, Z.B. Novel in situ N-doped (BiO)2CO3 hierarchical microspheres self-assembled by nanosheets as efficient and durable visible light driven photocatalyst. Langmuir 2012, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, H.; Sakai, T.; Suzuki, T.; Kaneco, S. Highly efficient photocatalytic activity of g-C3N4/Ag3PO4 hybrid photocatalysts through Z-scheme photocatalytic mechanism under visible light. Ind. Eng. Chem. Res. 2014, 53, 8018–8025. [Google Scholar] [CrossRef]
- Li, W.J.; Li, D.Z.; Chen, Z.X.; Huang, H.J.; Sun, M.; He, Y.H.; Fu, X.Z. High-efficient degradation of dyes by ZnxCd1−xS solid solutions under visible light irradiation. J. Phys. Chem. C 2008, 112, 14943–14947. [Google Scholar] [CrossRef]
- Sarina, S.; Zhu, H.Y.; Xiao, Q.; Jaatinen, E.; Jia, J.F.; Huang, Y.M.; Zheng, Z.F.; Wu, H.S. Viable photocatalysts under solar-spectrum irradiation: Nonplasm onic metal nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, J.Y.; Wei, H.L.; Zheng, J.W.; Su, H.Q.; Wang, X.J. Hydrotalcite-supported gold catalysts for a selective aerobic oxidation of benzyl alcohol driven by visible light. J. Mol. Catal. A 2014, 395, 128–136. [Google Scholar] [CrossRef]
- Wei, H.L.; Li, J.Y.; Yu, J.; Zheng, J.W.; Su, H.Q.; Wang, X.J. Gold nanoparticles supported on metal oxides as catalysts for the direct oxidative esterification of alcohols under mild conditions. Inorg. Chim. Acta 2015, 427, 33–40. [Google Scholar] [CrossRef]
- Shimura, K.; Shimizu, K.I. Transfer hydrogenation of ketones by ceria-supported Ni catalysts. Green Chem. 2012, 14, 2983–2985. [Google Scholar] [CrossRef]
- Cano, R.; Yus, M.; Ramón, D.J. First practical cross-alkylation of primary alcohols with a new and recyclable impregnated iridium on magnetite catalyst. Chem. Commun. 2012, 48, 7628–7630. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamaguchi, T.; Matsushita, K.; Iitsuka, C.; Miura, J.; Akaogi, T.; Ishida, H. Aerobic oxidative esterification of aldehydes with alcohols by gold-nickel oxide nanoparticle catalysts with a core-shell structure. ACS Catal. 2013, 3, 1845–1849. [Google Scholar] [CrossRef]
- Bowling, N.P.; Mcmahon, R.J. Enediyne isomers of tetraethynylethene. J. Org. Chem. 2006, 71, 5841–5847. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, N.Y.; Cheon, C.H. Beyond benzoin condensation: Trimerization of aldehydes via metal-free aerobic oxidative esterification of aldehydes with benzoin products in the presence of cyanide. Org. Lett. 2014, 16, 2514–2517. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.W.; Li, J.Y.; Wei, H.L.; Yu, J.; Su, H.Q.; Wang, X.J. The investigation of gold/zirconia as a photocatalyst for the direct synthesis of imine from alcohols and aniline. Mater. Sci. Semicond. Process. 2015, 32, 131–136. [Google Scholar] [CrossRef]






| Entry | Aldehyde | Alcohol | In the Visible Light | In the Dark | ||
|---|---|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | Conversion (%) | Selectivity (%) | |||
| 1 | 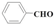 | Methanol | 88.3 | >99 | 8.2 | >99 |
| 2 | 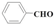 | Ethanol | 86.9 | >99 | 3.5 | >99 |
| 3 | 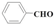 | Isopropanol | 61.7 | >99 | 1.6 | >99 |
| 4 | 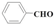 | Butanol | 86.3 | >99 | 0.1 | >99 |
| 5 | 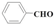 | Octanol | 37.0 | 84.3 | 3.5 | 40.2 |
| 6 | 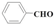 | 2-Octanol | 15.9 | 50.3 | 5.2 | 30.1 |
| 7 |  | Methanol | 95.5 | >99 | 0.6 | >99 |
| 8 |  | Methanol | 99.4 | >99 | 0.7 | >99 |
| 9 |  | Methanol | 66.2 | >99 | 0.7 | >99 |
| 10 | 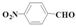 | Methanol | 48.6 | >99 | 1.2 | >99 |
| 11 |  | Methanol | 52.6 | >99 | 0.4 | >99 |
| 12 | 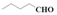 | Methanol | 40.1 | >99 | 1.3 | >99 |
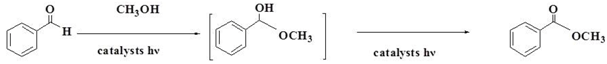
| Entry | Catalysts | In the Visible Light | In the Dark | ||
|---|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | Conversion (%) | Selectivity (%) | ||
| 1 | Bi2O2CO3 | 33.3 | >99 | 13.1 | >99 |
| 2 | 10%Ag/Bi2O2CO3 | 60.8 | >99 | 12.2 | >99 |
| 3 | 64%Ag3PO4/Bi2O2CO3 | 67.8 | >99 | 4.4 | >99 |
| 4 | 57%Ag3PO4/Ag/Bi2O2CO3 | 25.7 | >99 | 4.7 | >99 |
| 5 | 64%Ag3PO4/Ag/Bi2O2CO3 | 73.0 | >99 | 6.4 | >99 |
| 6 | 68%Ag3PO4/Ag/Bi2O2CO3 | 76.0 | >99 | 13.1 | >99 |
| 7 | 71%Ag3PO4/Ag/Bi2O2CO3 | 88.3 | >99 | 8.2 | >99 |
| 8 | 73%Ag3PO4/Ag/Bi2O2CO3 | 74.8 | >99 | 4.4 | >99 |
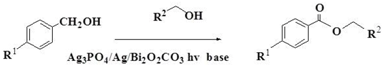
| Entry | Alcohol | Alcohol | In the Visible Light | In the Dark | ||
|---|---|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | Conversion (%) | Selectivity (%) | |||
| 1 | 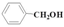 | Methanol | 93.5 | 75.6 | 11.1 | 51.3 |
| 2 | 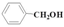 | Ethanol | 83.4 | 73.2 | 6.8 | 48.0 |
| 3 | 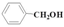 | Isopropanol | 8.1 | 21.5 | 3.4 | 0 |
| 4 | 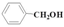 | Butanol | 71.2 | 54.0 | 20.4 | 21.2 |
| 5 | 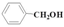 | Octanol | 40.0 | 61.8 | 0.1 | 0 |
| 6 | 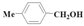 | Methanol | 72.6 | 81.8 | 23.3 | >99 |
| 7 | 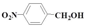 | Methanol | 37.2 | 78.6 | 12.5 | >99 |
| 8 | 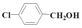 | Methanol | 55.0 | 62.7 | 16.0 | >99 |
| 9 | 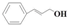 | Methanol | 52.5 | >99 | 20.1 | >99 |
| 10 | 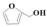 | Methanol | 40.1 | >99 | 11.4 | >99 |
| 11 | 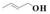 | Methanol | 13.6 | >99 | 1.4 | >99 |


| Entry | Alcohol | Amine | In the Visible Light | In the Dark | ||
|---|---|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | Conversion (%) | Selectivity (%) | |||
| 1 | 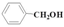 |  | 80.5 | >99 | 10.1 | >99 |
| 2 | 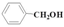 |  | 92.0 | >99 | 6.8 | >99 |
| 3 | 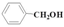 |  | 94.0 | >99 | 3.4 | >99 |
| 4 | 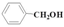 |  | 81.2 | >99 | 10.4 | >99 |
| 5 | 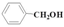 | 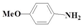 | 55.9 | >99 | 0.1 | >99 |
| 6 |  |  | 71.8 | 80.7 | 13.3 | >99 |
| 7 |  |  | 94.0 | 83.7 | 12.5 | >99 |
| 8 |  |  | 79.1 | 77.2 | 11.0 | >99 |
| 9 | 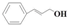 |  | 62.5 | >99 | 10.1 | >99 |
| 10 | 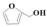 |  | 40.1 | >99 | 11.4 | >99 |
| 11 | 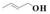 |  | 65.6 | >99 | 1.4 | >99 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Xin, H.; Ma, J.; Bai, M.; Wang, Y.; Li, J. The Preparation of a Highly Efficient Ag3PO4/Ag/Bi2O2CO3 Photo-Catalyst and the Study of Its Photo-Catalytic Organic Synthesis Reaction Driven by Visible Light. Catalysts 2017, 7, 276. https://doi.org/10.3390/catal7090276
Guo Z, Xin H, Ma J, Bai M, Wang Y, Li J. The Preparation of a Highly Efficient Ag3PO4/Ag/Bi2O2CO3 Photo-Catalyst and the Study of Its Photo-Catalytic Organic Synthesis Reaction Driven by Visible Light. Catalysts. 2017; 7(9):276. https://doi.org/10.3390/catal7090276
Chicago/Turabian StyleGuo, Zhi, Hui Xin, Jingjing Ma, Meifen Bai, Yan Wang, and Jingyi Li. 2017. "The Preparation of a Highly Efficient Ag3PO4/Ag/Bi2O2CO3 Photo-Catalyst and the Study of Its Photo-Catalytic Organic Synthesis Reaction Driven by Visible Light" Catalysts 7, no. 9: 276. https://doi.org/10.3390/catal7090276
APA StyleGuo, Z., Xin, H., Ma, J., Bai, M., Wang, Y., & Li, J. (2017). The Preparation of a Highly Efficient Ag3PO4/Ag/Bi2O2CO3 Photo-Catalyst and the Study of Its Photo-Catalytic Organic Synthesis Reaction Driven by Visible Light. Catalysts, 7(9), 276. https://doi.org/10.3390/catal7090276