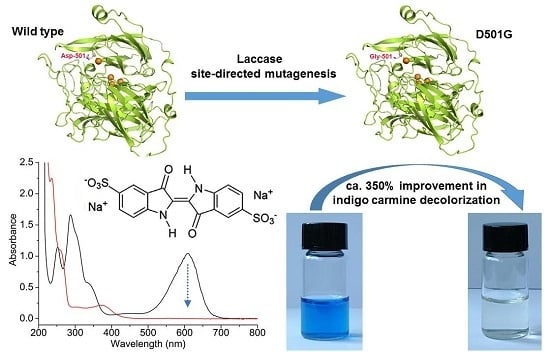
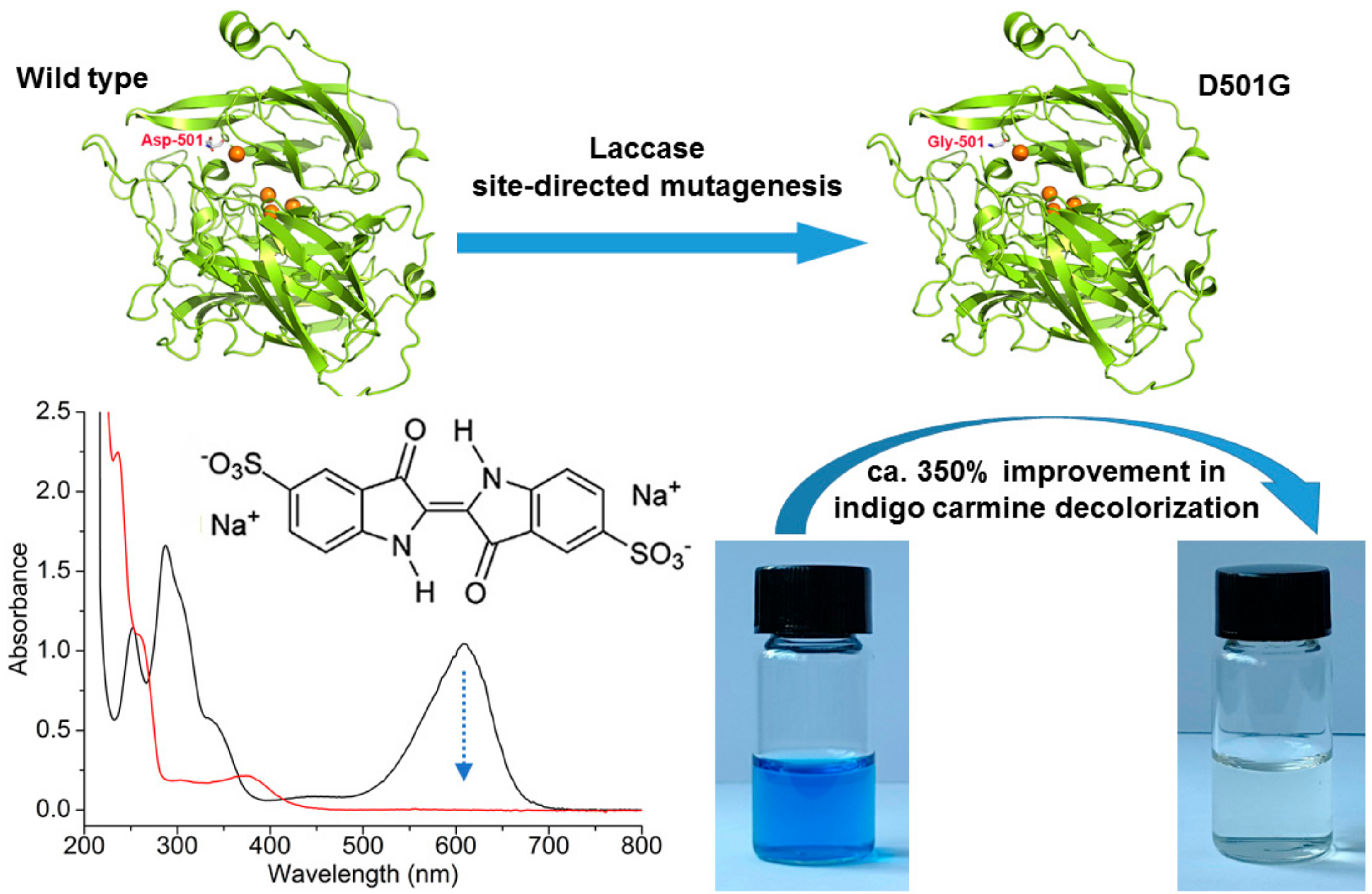
Improving the Indigo Carmine Decolorization Ability of a Bacillus amyloliquefaciens Laccase by Site-Directed Mutagenesis
Abstract
:1. Introduction
2. Results and Discussion
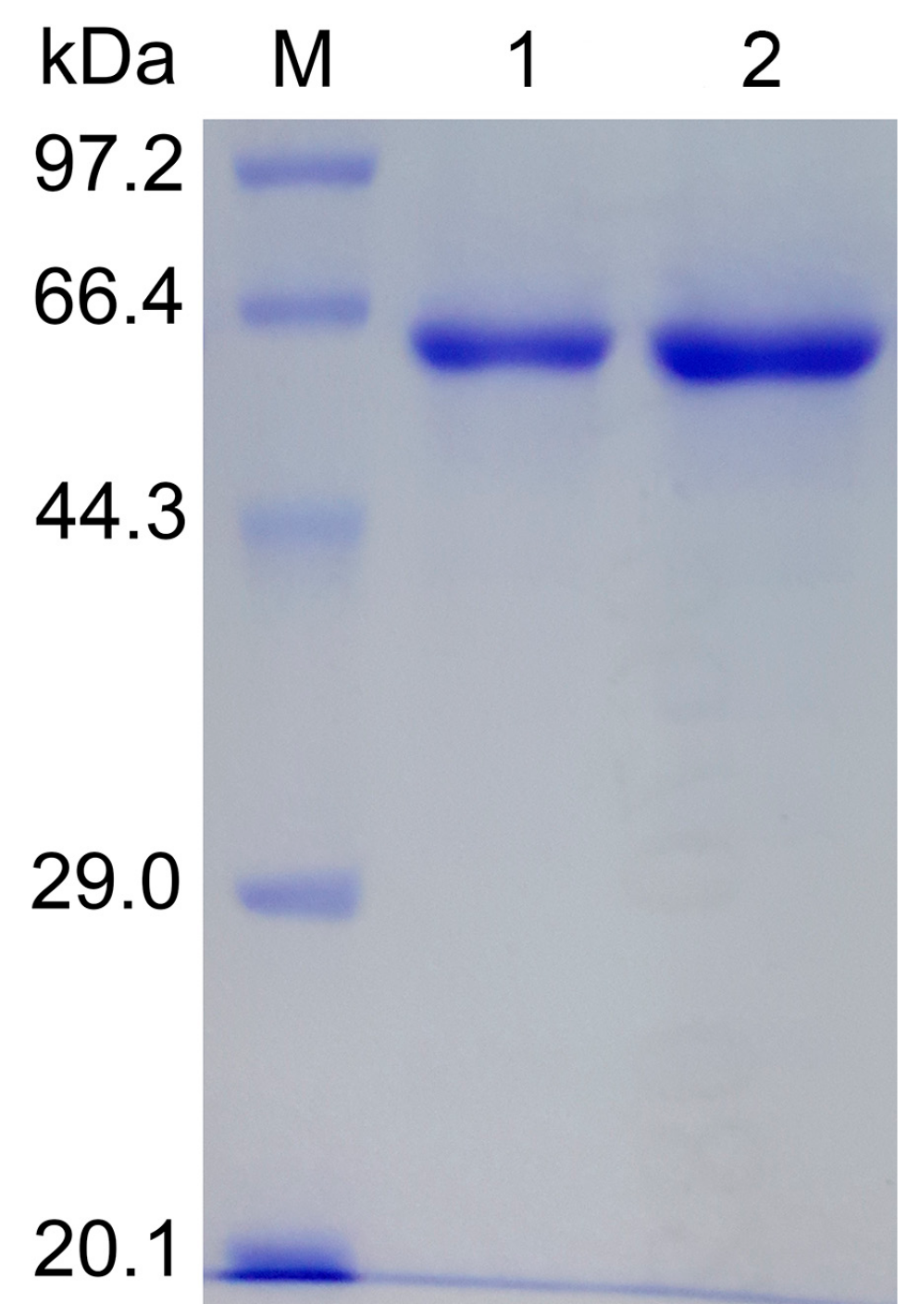
2.1. Site-Directed Mutagenesis and Expression in E. coli
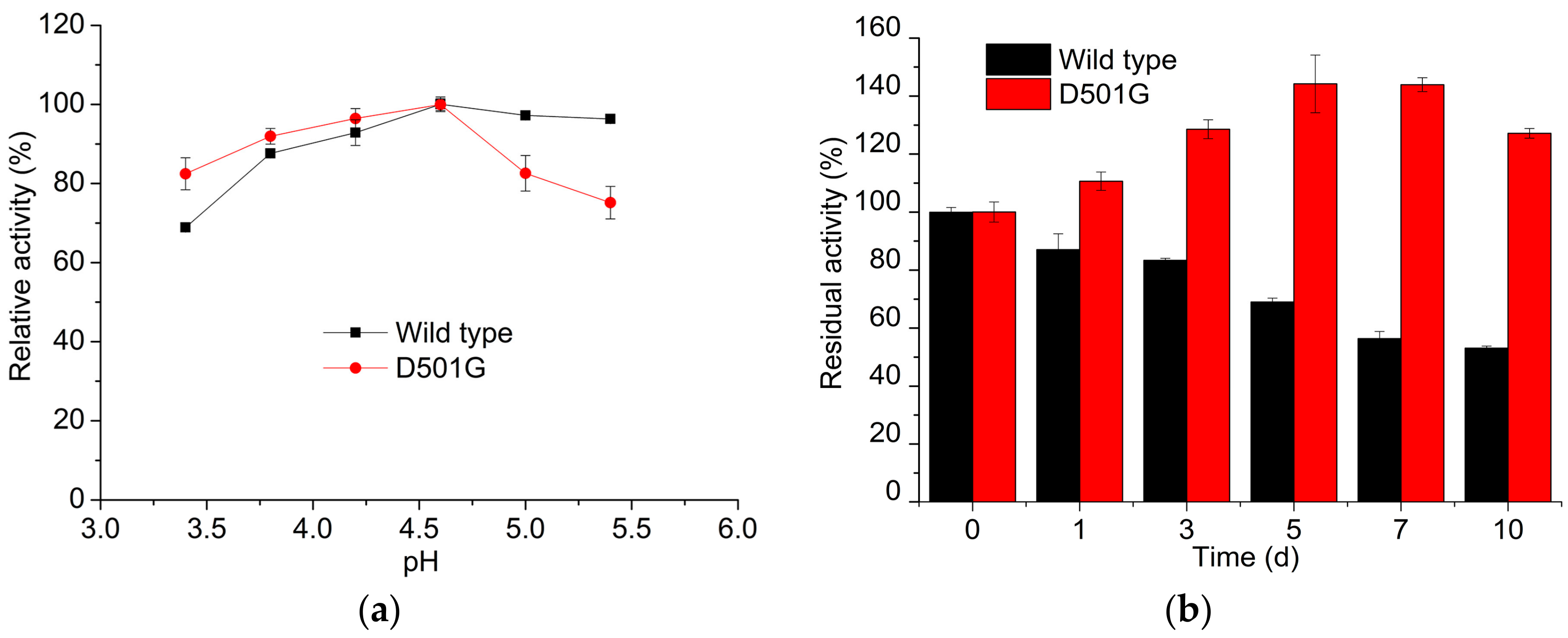
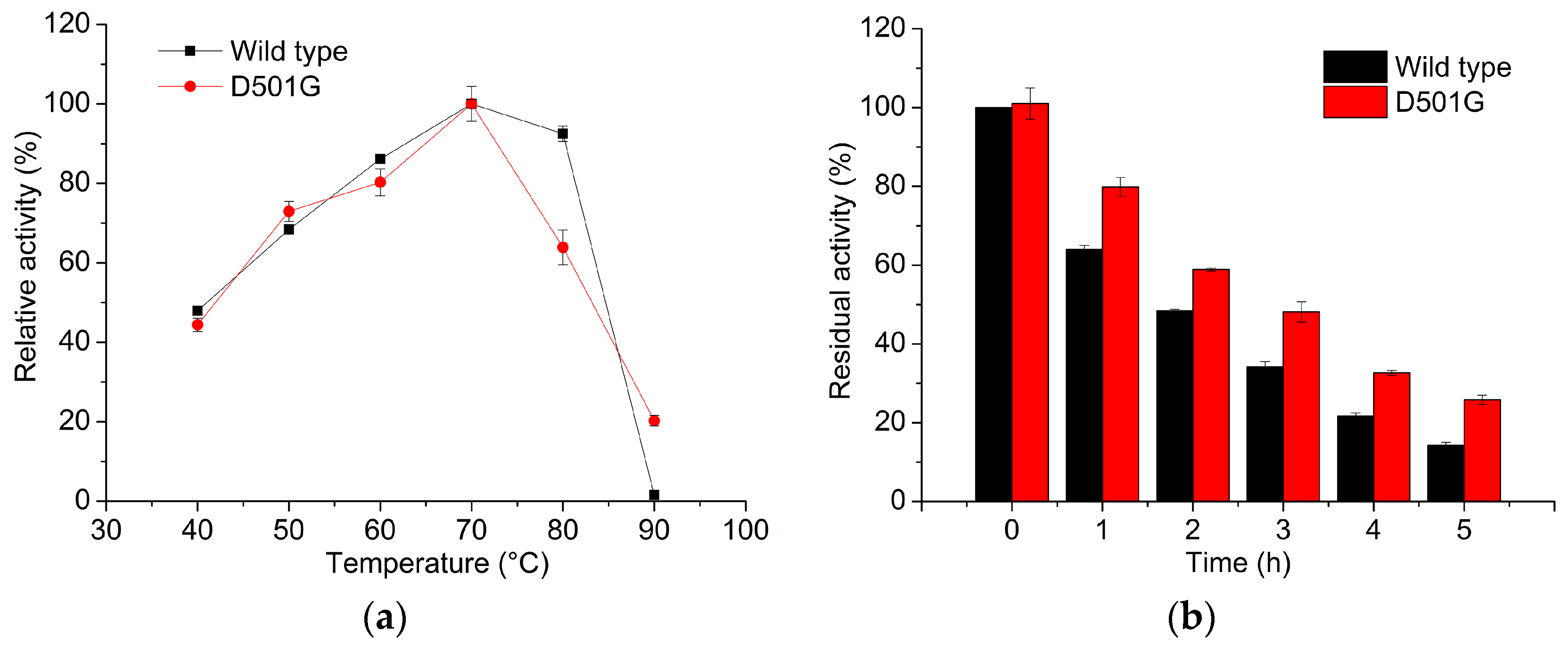
2.2. Enzyme Characterization
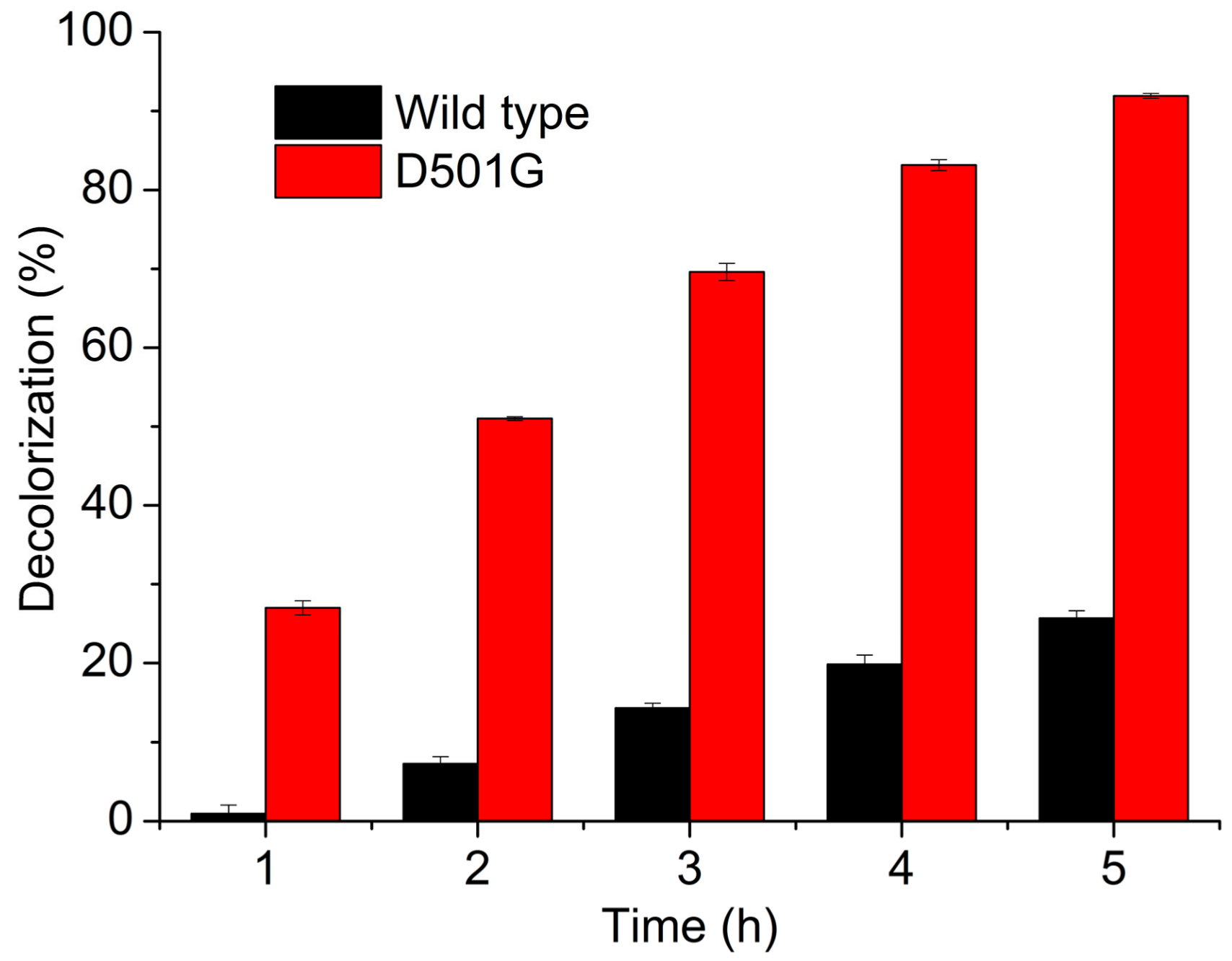
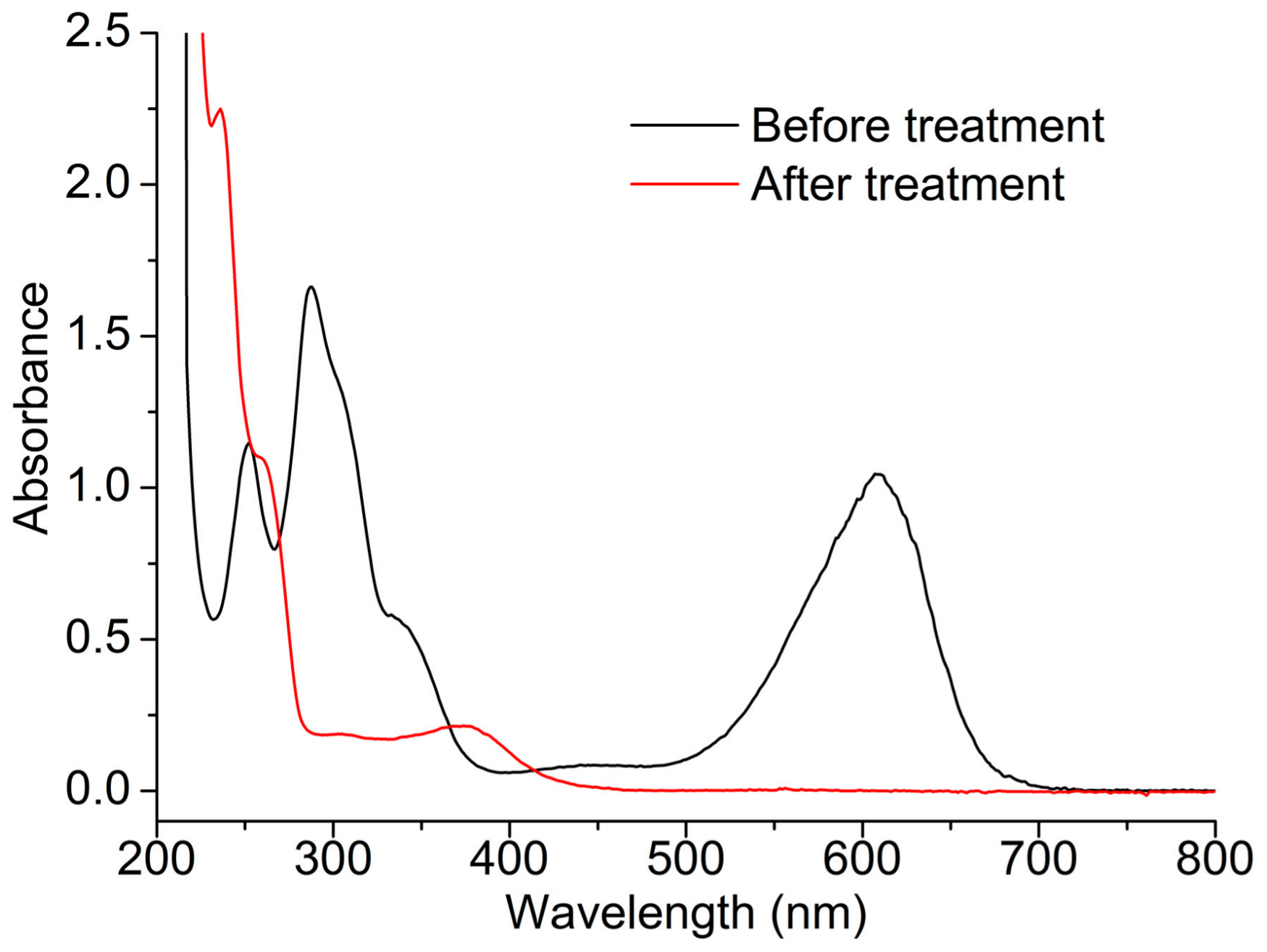
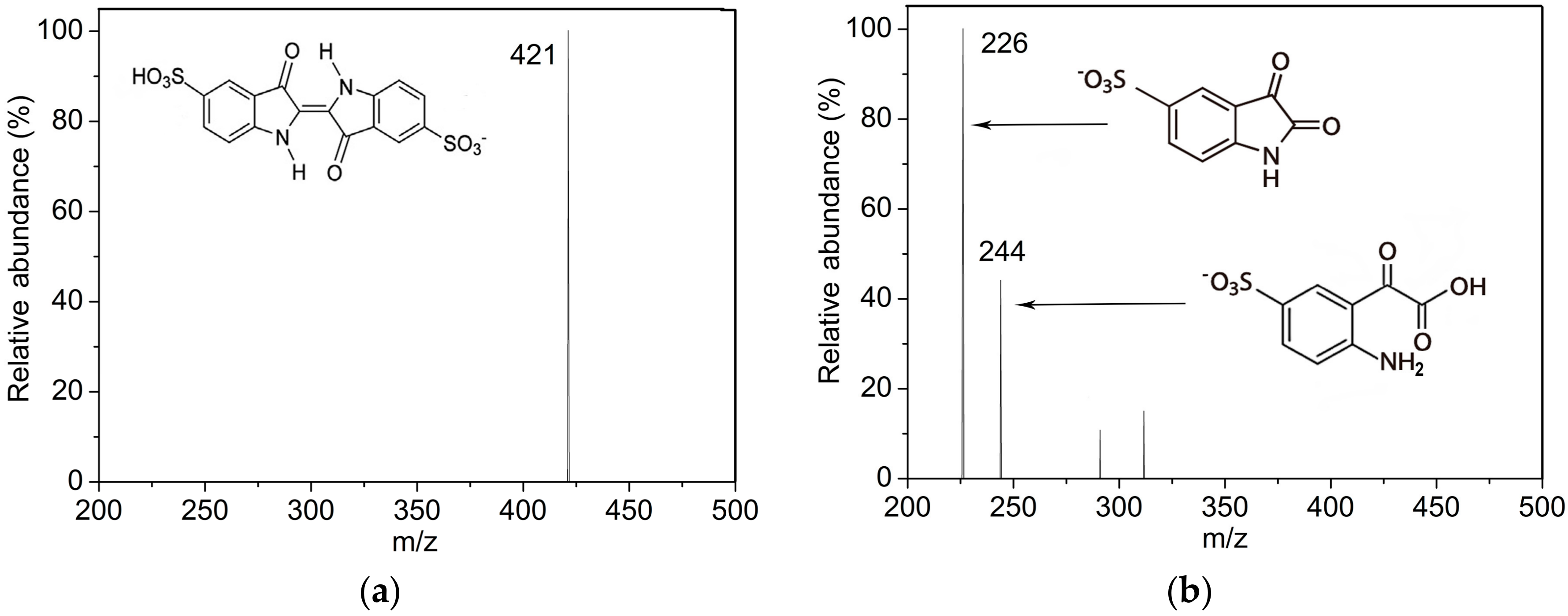
2.3. Indigo Carmine Decolorization
3. Materials and Methods
3.1. Materials
3.2. Site-Directed Mutagenesis
3.3. Protein Expression and Purification
3.4. Enzyme Assay and Characterization
3.5. Indigo Carmine Decolorization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Imran, M.; Crowley, D.E.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Arshad, M. Microbial biotechnology for decolorization of textile wastewaters. Rev. Environ. Sci. Biotechnol. 2015, 14, 73–92. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M.N. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants-A review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef] [PubMed]
- China Dyestuff Industry Association Secretariat. The development situation and trends of China’s dyestuff industry in “Twelfth Five-Year”. Fine Spec. Chem. 2016, 24, 5–12. [Google Scholar]
- Hernández-Gordillo, A.; Rodríguez-González, V.; Oros-Ruiz, S.; Gómez, R. Photodegradation of indigo carmine dye by CdS nanostructures under blue-light irradiation emitted by LEDs. Catal. Today 2016, 266, 27–35. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Silva-Agredo, J.; González, I.; Torres-Palma, R.A. Comparative degradation of indigo carmine by electrochemical oxidation and advanced oxidation processes. Electrochim. Acta 2014, 140, 427–433. [Google Scholar] [CrossRef]
- Morali, E.K.; Uzal, N.; Yetis, U. Ozonation pre and post-treatment of denim textile mill effluents: Effect of cleaner production measures. J. Clean. Prod. 2016, 137, 1–9. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.O.; Durão, P.; Brissos, V.; Lindley, P.F. Laccases of prokaryotic origin: Enzymes at the interface of protein science and protein technology. Cell. Mol. Life Sci. 2015, 72, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Ticha, M.B.; Meksi, N.; Drira, N.; Kechida, M.; Mhenni, M.F. A promising route to dye cotton by indigo with an ecological exhaustion process: A dyeing process optimization based on a response surface methodology. Ind. Crops Prod. 2013, 46, 350–358. [Google Scholar] [CrossRef]
- Chen, B.; Xu, W.Q.; Pan, X.R.; Lu, L. A novel non-blue laccase from Bacillus amyloliquefaciens: Secretory expression and characterization. Int. J. Biol. Macromol. 2015, 76, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, K.; Puri, S.; Sharma, P. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl. Microbiol. Biotechnol. 2015, 99, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Enguita, F.J.; Marçal, D.; Martins, L.O.; Grenha, R.; Henriques, A.O.; Lindley, P.F.; Carrondo, M.A. Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J. Biol. Chem. 2004, 279, 23472–23476. [Google Scholar] [CrossRef] [PubMed]
- Nasoohi, N.; Khajeh, K.; Mohammadian, M.; Ranjbar, B. Enhancement of catalysis and functional expression of a bacterial laccase by single amino acid replacement. Int. J. Biol. Macromol. 2013, 60, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mollania, N.; Khajeh, K.; Ranjbar, B.; Rashno, F.; Akbari, N.; Fathi-Roudsari, M. An efficient in vitro refolding of recombinant bacterial laccase in Escherichia coli. Enzyme Microb. Technol. 2013, 52, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, S.Y.; Park, H.; Jeon, S.J. Expression, refolding, and characterization of a small laccase from Thermus thermophilus HJ6. Protein Expr. Purif. 2015, 114, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jain, K.K.; Rani, S.; Bhardwaj, K.N.; Goel, M.; Kuhad, R.C. In Vitro refolding and characterization of recombinant laccase (CotA) from Bacillus pumilus MK001 and its potential for phenolics degradation. Mol. Biotechnol. 2016, 58, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Haitjema, C.H.; Lee, R.; Boock, J.T.; DeLisa, M.P. An engineered survival-selection assay for extracellular protein expression uncovers hypersecretory phenotypes in Escherichia coli. ACS Synth. Biol. 2017, 6, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, T.N.; Xu, T.F.; Wang, J.Y.; Wang, C.L.; Zhao, M. Cloning and expression of thermo-alkali-stable laccase of Bacillus licheniformis in Pichia pastoris and its characterization. Bioresour. Technol. 2013, 134, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.N.; Lu, L.; Wang, J.Y.; Xu, T.F.; Li, J.; Zhao, M. Enhanced expression of an industry applicable CotA laccase from Bacillus subtilis in Pichia pastoris by non-repressing carbon sources together with pH adjustment: Recombinant enzyme characterization and dye decolorization. Process Biochem. 2015, 50, 97–103. [Google Scholar] [CrossRef]
- Wang, T.N.; Zhao, M. A simple strategy for extracellular production of CotA laccase in Escherichia coli and decolorization of simulated textile effluent by recombinant laccase. Appl. Microbiol. Biotechnol. 2017, 101, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Koschorreck, K.; Schmid, R.D.; Urlacher, V.B. Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis. BMC Biotechnol. 2009, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Namanja, A.T.; Chen, Y. Conformational flexibility and changes underlying activation of the SUMO-specific protease SENP1 by remote substrate binding. Nat. Commun. 2014, 5, 4968. [Google Scholar] [CrossRef] [PubMed]
- Kazuyo, F.; Hong, S.Y.; Yeon, Y.J.; Joo, J.C.; Yoo, Y.J. Enhancing the activity of Bacillus circulans xylanase by modulating the flexibility of the hinge region. J. Ind. Microbiol. Biotechnol. 2014, 41, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Stoisser, T.; Brunsteiner, M.; Wilson, D.K.; Nidetzky, B. Conformational flexibility related to enzyme activity: Evidence for a dynamic active-site gatekeeper function of Tyr215 in Aerococcus viridans lactate oxidase. Sci. Rep. 2016, 6, 27892. [Google Scholar] [CrossRef] [PubMed]
- Durão, P.; Bento, I.; Fernandes, A.T.; Melo, E.P.; Lindley, P.F.; Martins, L.O. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: Structural, biochemical, enzymatic and stability studies. J. Biol. Inorg. Chem. 2006, 11, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Castillo, P.; Villalonga-Santana, L.; Islas-Flores, I.; Rivera-Muñoz, G.; Ancona-Escalante, W.; Solís-Pereira, S. Synergistic action of laccases from Trametes hirsuta Bm2 improves decolourization of indigo carmine. Lett. Appl. Microbiol. 2015, 61, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Shareck, F.; Hurtubise, Y.; Daneault, C.; Beauregard, M. Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl. Microbiol. Biotechnol. 2008, 79, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Vautier, M.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of dyes in water: Case study of indigo and of indigo carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Kessler, W.; Kessler, R.W. Online UV-visible spectroscopy and multivariate curve resolution as powerful tool for model-free investigation of laccase-catalysed oxidation. Anal. Bioanal. Chem. 2008, 390, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, F.V.; de Lima, G.M.; Augusti, R.; Coelho, M.G.; Ardisson, J.D.; Romero, O.B. A versatile approach to treat aqueous residues of textile industry: The photocatalytic degradation of indigo carmine dye employing the autoclaved cellular concrete/Fe2O3 system. Chem. Eng. J. 2012, 180, 25–31. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Blanco-Tirado, C.; Hinestroza, J.P.; Combariza, M.Y. Biocomposite of nanostructured MnO2 and Fique fibers for efficient dye degradation. Green Chem. 2013, 15, 2920–2928. [Google Scholar] [CrossRef]
- Dalmázio, I.; de Urzedo, A.P.F.M.; Alves, T.M.A.; Catharino, R.R.; Eberlin, M.N.; Nascentes, C.C.; Augusti, R. Electrospray ionization mass spectrometry monitoring of indigo carmine degradation by advanced oxidative processes. J. Mass Spectrom. 2007, 42, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
| Enzyme | Km (μM) | kcat (s−1) | kcat/Km (s−1·μM−1) | Specific Activity (U·mg−1) |
|---|---|---|---|---|
| Wild-type | 436.8 ± 48.2 | 50.1 ± 4.4 | 0.11 | 60.5± 0.7 |
| D501G | 282.6 ± 20.9 | 117.2 ± 4.6 | 0.41 | 81.4± 1.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lu, L.; Feng, F. Improving the Indigo Carmine Decolorization Ability of a Bacillus amyloliquefaciens Laccase by Site-Directed Mutagenesis. Catalysts 2017, 7, 275. https://doi.org/10.3390/catal7090275
Wang J, Lu L, Feng F. Improving the Indigo Carmine Decolorization Ability of a Bacillus amyloliquefaciens Laccase by Site-Directed Mutagenesis. Catalysts. 2017; 7(9):275. https://doi.org/10.3390/catal7090275
Chicago/Turabian StyleWang, Jiayi, Lei Lu, and Fujuan Feng. 2017. "Improving the Indigo Carmine Decolorization Ability of a Bacillus amyloliquefaciens Laccase by Site-Directed Mutagenesis" Catalysts 7, no. 9: 275. https://doi.org/10.3390/catal7090275
APA StyleWang, J., Lu, L., & Feng, F. (2017). Improving the Indigo Carmine Decolorization Ability of a Bacillus amyloliquefaciens Laccase by Site-Directed Mutagenesis. Catalysts, 7(9), 275. https://doi.org/10.3390/catal7090275