Immobilization of Lipases on Magnetic Collagen Fibers and Its Applications for Short-Chain Ester Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. The Effects on Immobilization Process
2.2.1. Initial Concentration of Lipase Solution
2.2.2. Incubation Time
2.2.3. Temperature
2.3. Operational Activity of Immobilized Lipase
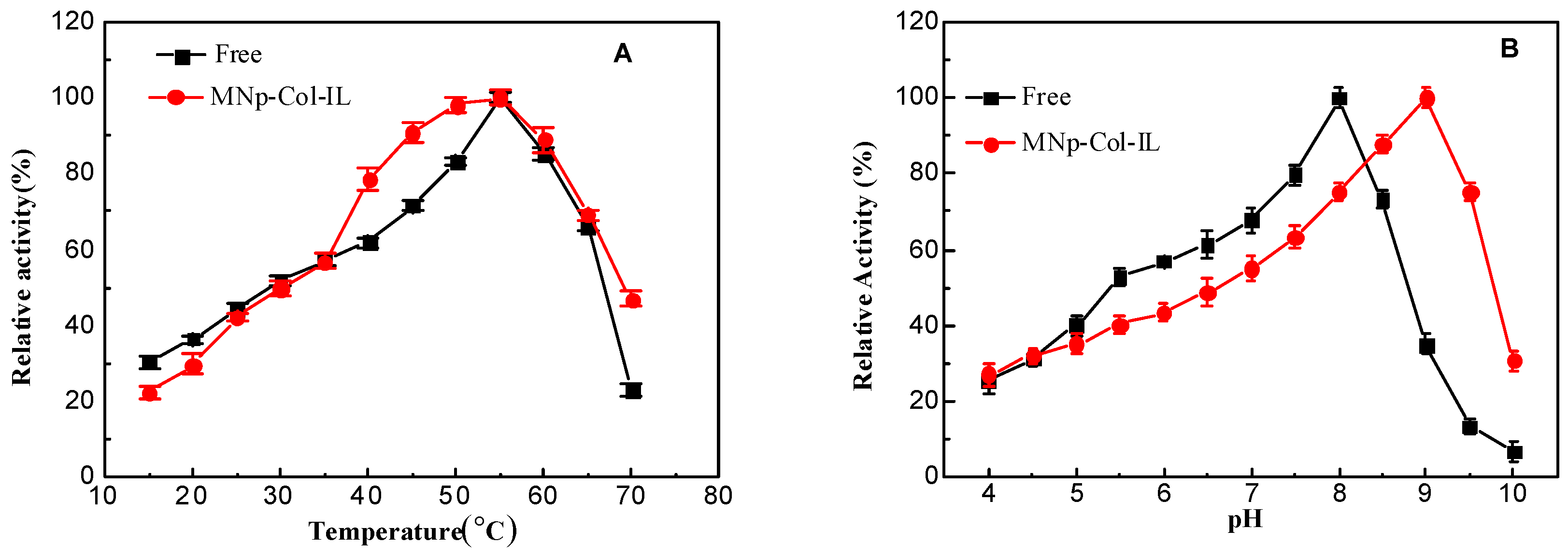
2.3.1. Temperature
2.3.2. Effect of pH
2.3.3. The Kinetic Parameters
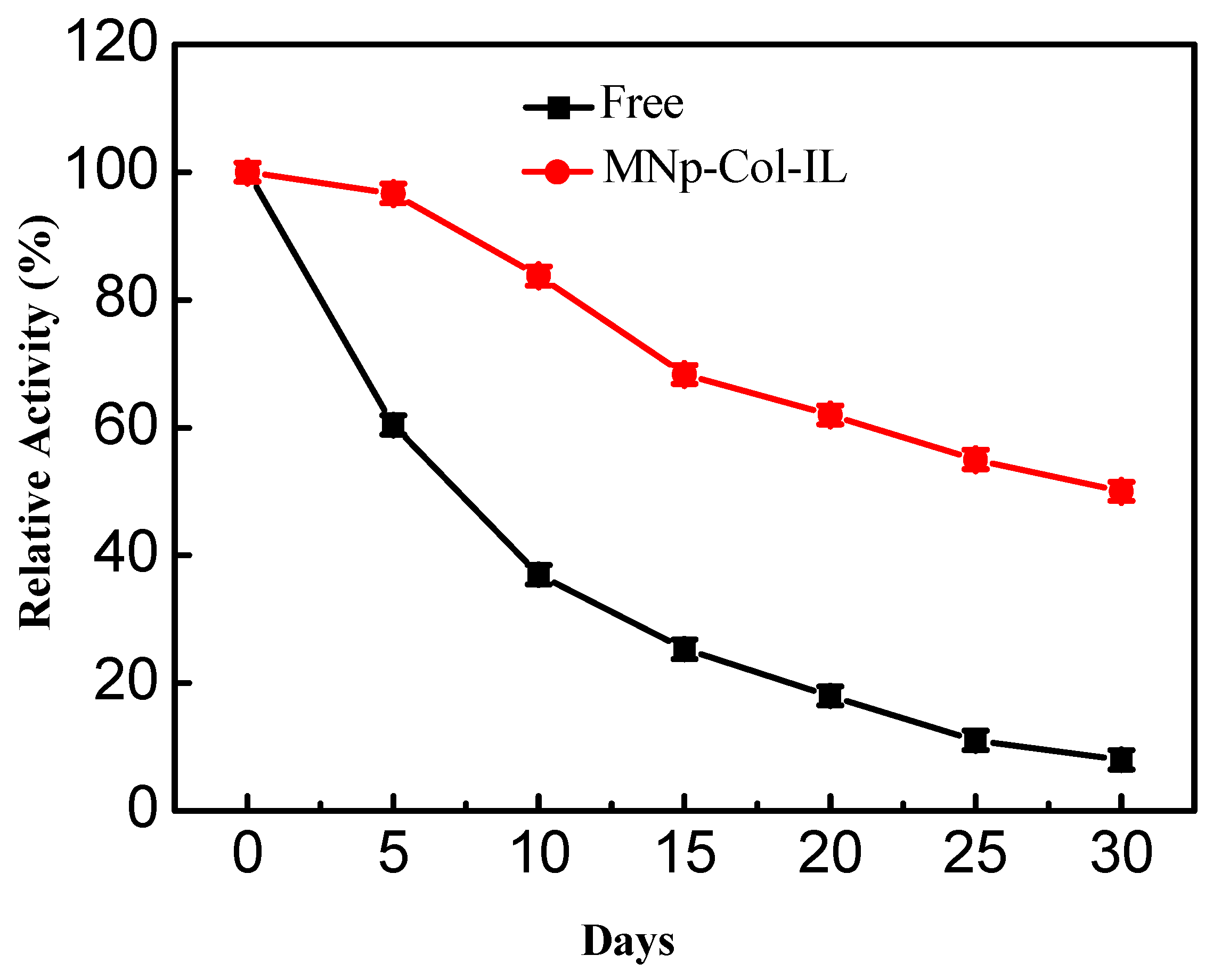
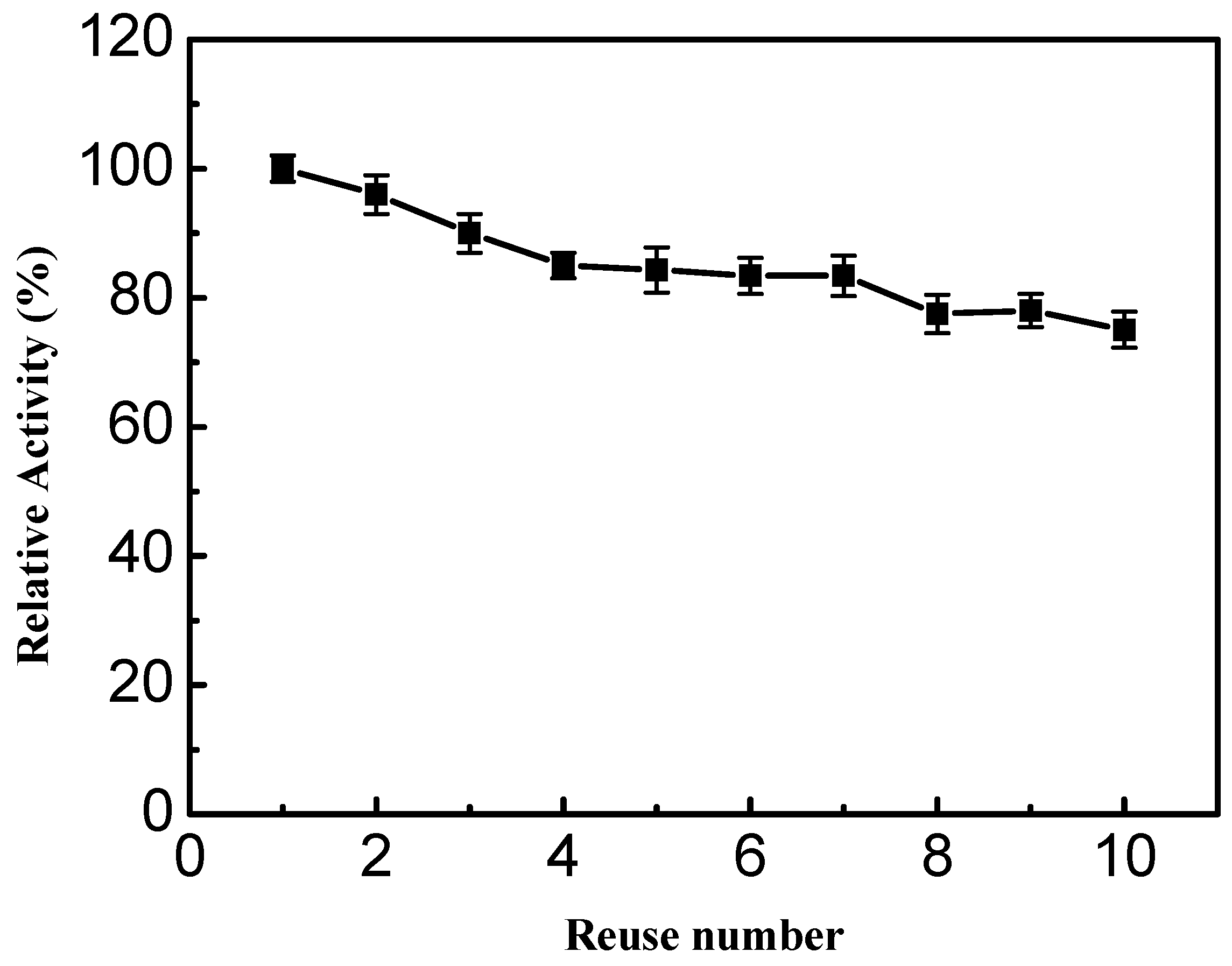
2.3.4. Storage Stability and Reusability Examinations
2.4. Synthesis of Butyrate by MNp-Col-IL
3. Materials and Methods
3.1. Materials
3.2. Preparation of Magnetic Fe3O4 Particles
3.3. Preparation of Magnetic Collagen Support and Enzyme Immobilization
3.4. Hydrolytic Activity Assays
3.5. Characterizations of MNp-Col Supports and Immobilized Lipase
3.6. Determination of Storage Stability, and Reusability
3.7. Kinetic Constants Determination
3.8. Immobilized Lipase-Catalyzed Ester Synthesis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tibrewal, N.; Tang, Y. Biocatalysts for natural product biosynthesis. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 5347–5366. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Sov. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Sander, V.P. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Robert, D.C.; Joseph, M.A.; Poulose, A.J. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar]
- Gupta, R.; Gupta, N.; Rathi, P. Bacterial lipases: An overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 2004, 64, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.B.; Silva, A.M.; Schein, M.F.; Garcia-Galan, C. Comparison of the performance of commercial immobilized lipases in the synthesis of different flavor esters. J. Mol. Catal. B Enzym. 2014, 105, 18–25. [Google Scholar] [CrossRef]
- Yassin, A.; Mohamed, I.; Ibrahim, M.; Yousoff, M. Effect of enzymatic interesterification on melting point of palm olein. Appl. Biochem. Biotechnol. 2003, 110, 45–52. [Google Scholar] [CrossRef]
- Minovska, V.; Winkelhausen, E.; Kuzmanove, S. Lipase immobilized by different techniques on various support materials applied in oil hydrolysis. J. Serb. Chem. Soc. 2005, 70, 609–624. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. : Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Mendes, A.A.; Freitas, L.; Carvalhoak, A.K.; Oliveira, P.C.; Castro, H.F. Immobilization of a commercial lipase from Penicillium camembertii (Lipase G) by different strategies. Enzyme Res. 2011, 40, 611–618. [Google Scholar]
- Santos, J.C.S.D.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, D.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M.P. Chitosan-collagen coated magnetic nanoparticles for lipase immobilization—New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Chen, S.; Song, N.; Liao, X.P.; Shi, B. Immobilization of catalase on Fe(III) modified collagen fiber. Chin. J. Biotechnol. 2011, 27, 1076–1081. [Google Scholar]
- Song, D.W.; Chen, M.; Cheng, H.M. Collagen-immobilized lipases show good activity and reusability for butyl butyrate synthesis. Appl. Biochem. Biotechnol. 2016, 180, 826–840. [Google Scholar]
- Jain, M.; Sebatini, A.M.; Radha, P. Synthesis, characterization and kinetic analysis of chitosan coated magnetic nanobiocatalyst and its application on glucose oleate ester synthesis. J. Mol. Catal. B Enzym. 2016, 128, 1–9. [Google Scholar] [CrossRef]
- Motevalizadeh, S.F.; Khoobi, M.; Sadighi, A. Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. J. Mol. Catal. B Enzym. 2015, 120, 75–83. [Google Scholar] [CrossRef]
- Yue, W.; Wang, Y.; Luo, G. In situ preparation of magnetic Fe3O4 -chitosan nanoparticles for lipase immobilization by cross-linking and oxidation in aqueous solution. Bioresour. Technol. 2009, 100, 3459–3464. [Google Scholar]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica immobilized on a silica-lignin matrix as a stable and reusable biocatalytic system. Catalysts 2017, 7, 14. [Google Scholar] [CrossRef]
- Huang, X.J.; Chen, P.C.; Huang, F.; Ou, Y.; Chen, M.R.; Xu, Z.K. Immobilization of Candida rugosa lipase on electrospun cellulose nanofiber membrane. J. Mol. Catal. B Enzym. 2011, 70, 95–100. [Google Scholar] [CrossRef]
- Yu, W.H.; Tong, D.S.; Fang, M.; Shao, P.; Zhou, C.H. Immobilization of Candida rugosa lipase on MSU-H type mesopours silica for selective esterifiction of conjugated linoleic acid isomers with ethanol. J. Mol. Catal. B Enzym. 2015, 111, 43–50. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Versatility of glutaraldehyde to immobilize lipases: Effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012, 471, 1220–1227. [Google Scholar] [CrossRef]
- Walt, D.R.; Agayn, V.I. The chemistry of enzyme and protein immobilization with glutaraldehyde. Trend Anal. Chem. 1994, 13, 425–430. [Google Scholar] [CrossRef]
- Cunha, A.G.; Besteti, M.D.; Manoel, E.A.; Silva, A.; Freire, D. Preparation of core–shell polymer supports to immobilize lipase B from Candida antarctica: Effect of the support nature on catalytic properties. J. Mol. Catal. B: Enzym. 2014, 100, 59–67. [Google Scholar] [CrossRef]
- Hilal, N.; Kochkodan, V.; Nigmatullin, R. Lipase-immobilized biocatalytic membranes for enzymatic esterification: Comparison of various approaches to membrane preparation. J. Membrane Sci. 2006, 268, 198–207. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Activities, stabilities and reaction, kinetics of three free and chitosan-clay composite immobilized enzyme. Enzyme Microb. Technol. 2005, 36, 75–82. [Google Scholar] [CrossRef]
- Frenkel-Mullerad, H.; Avnir, D. Sol-Gel materials as efficient enzyme protectors: Preserving the activity of phosphatases under extreme pH conditions. J. Am. Chem. Soc. 2005, 127, 8077–8081. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.L.; Wang, J.L. Enzymatic production of biodiesel from soybean oil by using immobilized lipase on Fe3O4/Poly (styrene-methacrylic acid) magnetic microsphere as a biocatalyst. Energy Fuels 2014, 28, 2624–2631. [Google Scholar] [CrossRef]
- Tuzmen, N.; Kalburcu, T.; Denizli, A. Immobilization of catalase via adsorption onto metal–chelated affinity cryogels. Process Biochem. 2012, 47, 26–33. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.; Huang, W.; Zhang, S. Immobilization of laccase in a sponge-like hydrogel for enhanced durability in enzymatic degradation of dye pollutants. J. Colloid Interface Sci. 2015, 450, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhao, H.; Zhang, S.; Tian, C.; Di, Z.; Du, P. High catalytic activity of immobilized laccase on core–shell magnetic nanoparticles by dopamine self-polymerization. J. Mol. Catal. B Enzym. 2015, 112, 15–24. [Google Scholar] [CrossRef]
- Yahya, A.R.M.W.; Anderson, A.; Moo-Young, M. Ester synthesis in lipase-catalyzed reactions. Enzyme Microb. Technol. 1998, 23, 438–450. [Google Scholar] [CrossRef]
- Chulalaksananukul, W.; Condoret, J.S.; Combes, D. Geranyl acetate synthesis by lipase-catalyzed transesterification in supercritical carbon dioxide. Enzyme Microb. Technol. 1993, 15, 691–698. [Google Scholar] [CrossRef]
- Manjon, A.; Iborra, J.L.; Arocas, A. Short-chain flavor ester synthesis by immobilized lipase in organic media. Biotechnol. Lett. 1991, 13, 339–344. [Google Scholar] [CrossRef]
- Bezbradica, D.; Karalazjc, I.; Ognjanovic, N.; Mijin, D.; Siler-Marikovic, S.; Knezevic, Z. Studies on the specificity of Candida rugosa lipase catalyzed esterification reactions in organic media. J. Serb. Chem. Soc. 2006, 71, 31–41. [Google Scholar] [CrossRef]
- De Barros, D.P.C.; Lemos, F.; Fonseca, L.P.; Cabral, J.M.S. Kinetic cutinase-catalyzed esterification of caproic acid in organic solvent system. J. Mol. Catal. B Enzym. 2010, 66, 285–293. [Google Scholar] [CrossRef]
- Kumar, R.; Modak, J.; Madras, G. Effect of chain length of the acid on the enzymatic synthesis of flavors in supercritical carbon dioxide. Biochem. Eng. J. 2005, 23, 199–202. [Google Scholar] [CrossRef]
- Martins, A.; Friedrich, J.; Cavalheiro, J.; Garcia-Galan, C.; Barbosa, O.; Ayub, M.; Fernandez-Lafuente, R.; Rodrigues, R. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads. Bioresour. Technol. 2013, 134, 417–422. [Google Scholar]
- Lai, D.T.; O’Connor, C.J. Studies on synthesis of short chain alkyl esters catalyzed by goat pregastric lipase. J. Mol. Catal. B Enzym. 1999, 6, 411–420. [Google Scholar] [CrossRef]
- Langrand, G.; Rondot, N.; Triantaphylides, C.; Baratti, J. Short-chain flavour esters synthesis by microbial lipases. Biotechnol. Lett. 1990, 12, 581–586. [Google Scholar] [CrossRef]
- Gandhi, N.N.; Sawant, S.B.; Joshi, J.B. Specificity of a lipase in ester synthesis: Effect of alcohol. Biotechnol. Progr. 1995, 11, 282–287. [Google Scholar] [CrossRef]
- Cheng, H.M.; Chen, M.; Liao, L.L.; Li, Z.Q. Chemical and physical behavior of collagen fiber in alkaline solutions. J. Sci. Leather Technol. Chem. 2009, 93, 140–144. [Google Scholar]
- Hou, A.J.; Xu, B.B.; Liang, L.; Li, Y.H.; Peng, B.Y. A modified colorimetric assay of lipase activity using emulsified olive oil as the substrate. Leather Sci. Eng. 2011, 21, 22–27. [Google Scholar]
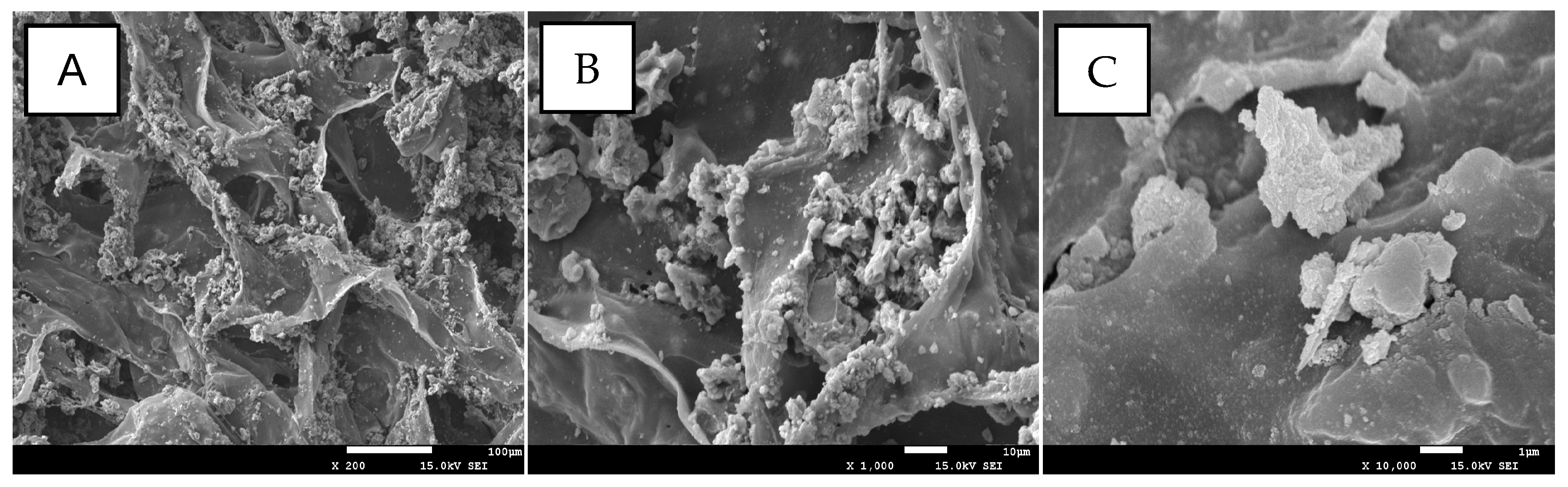
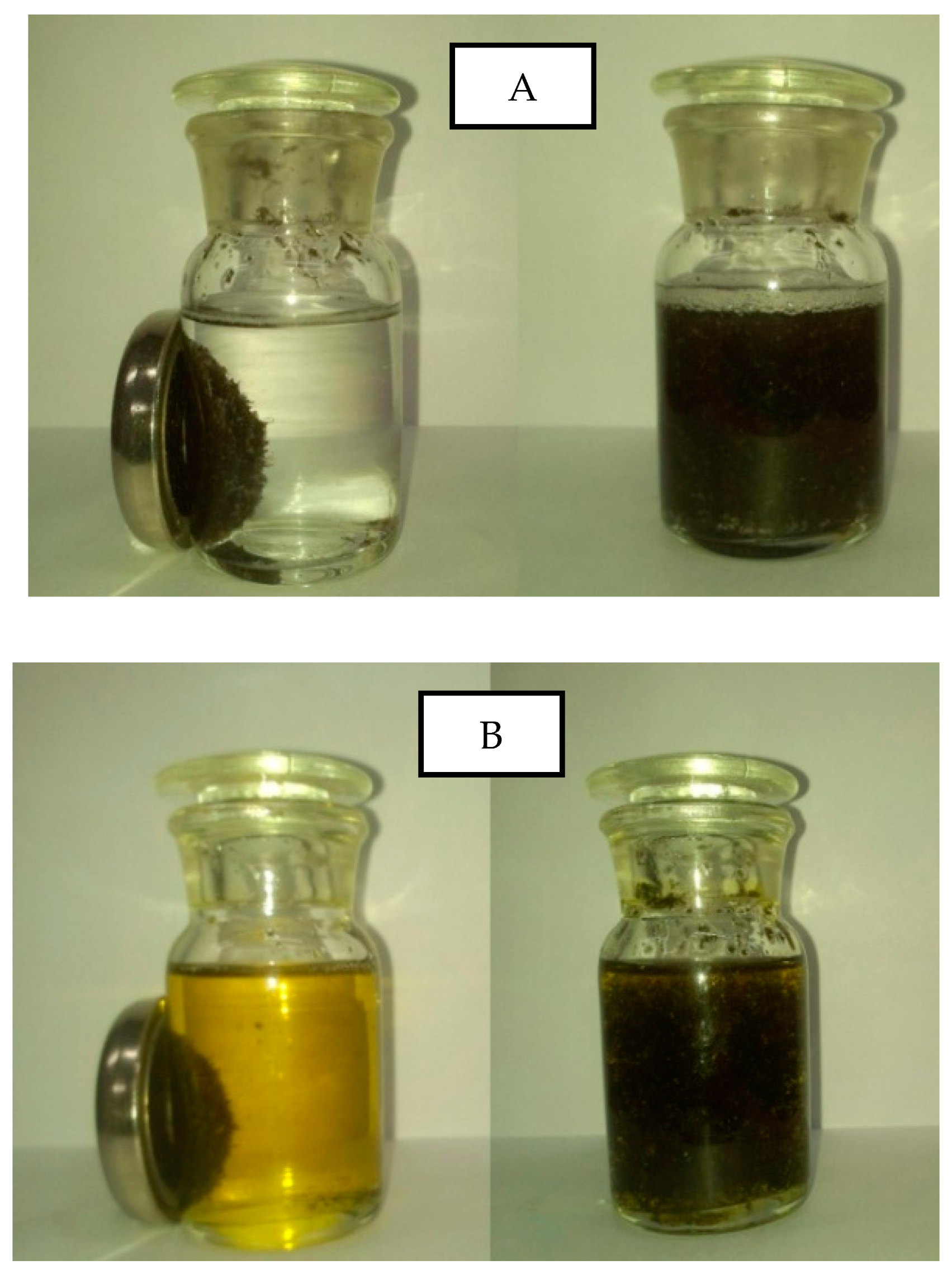
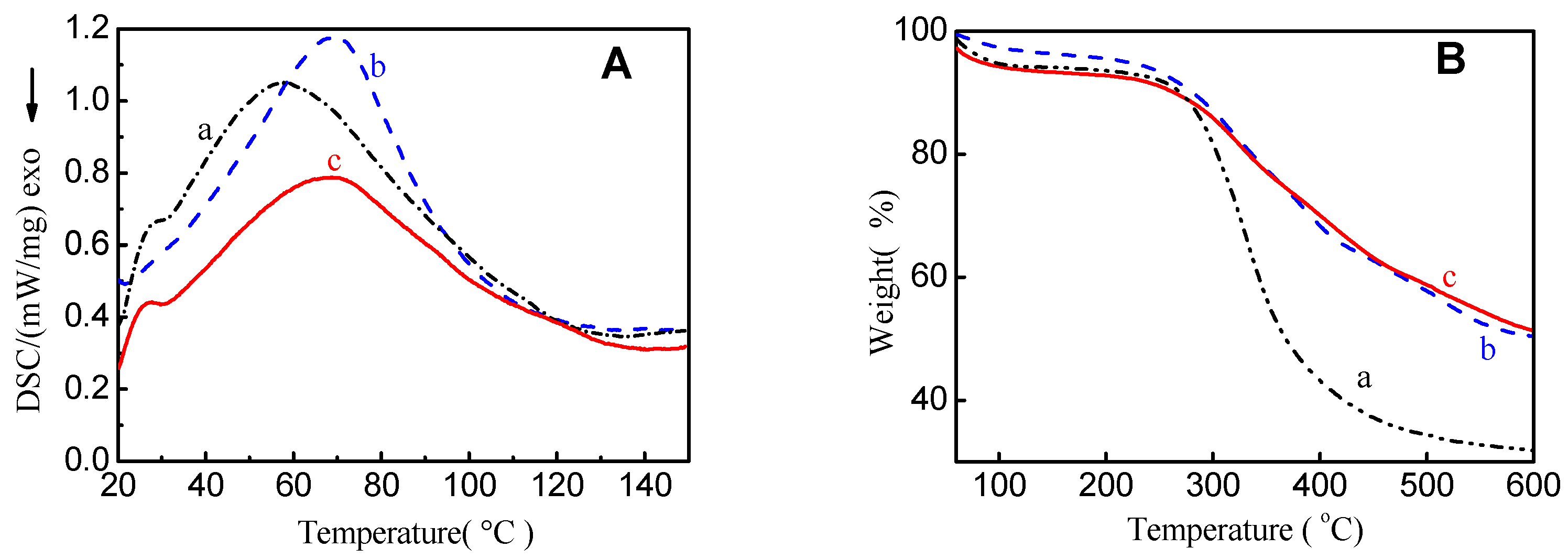
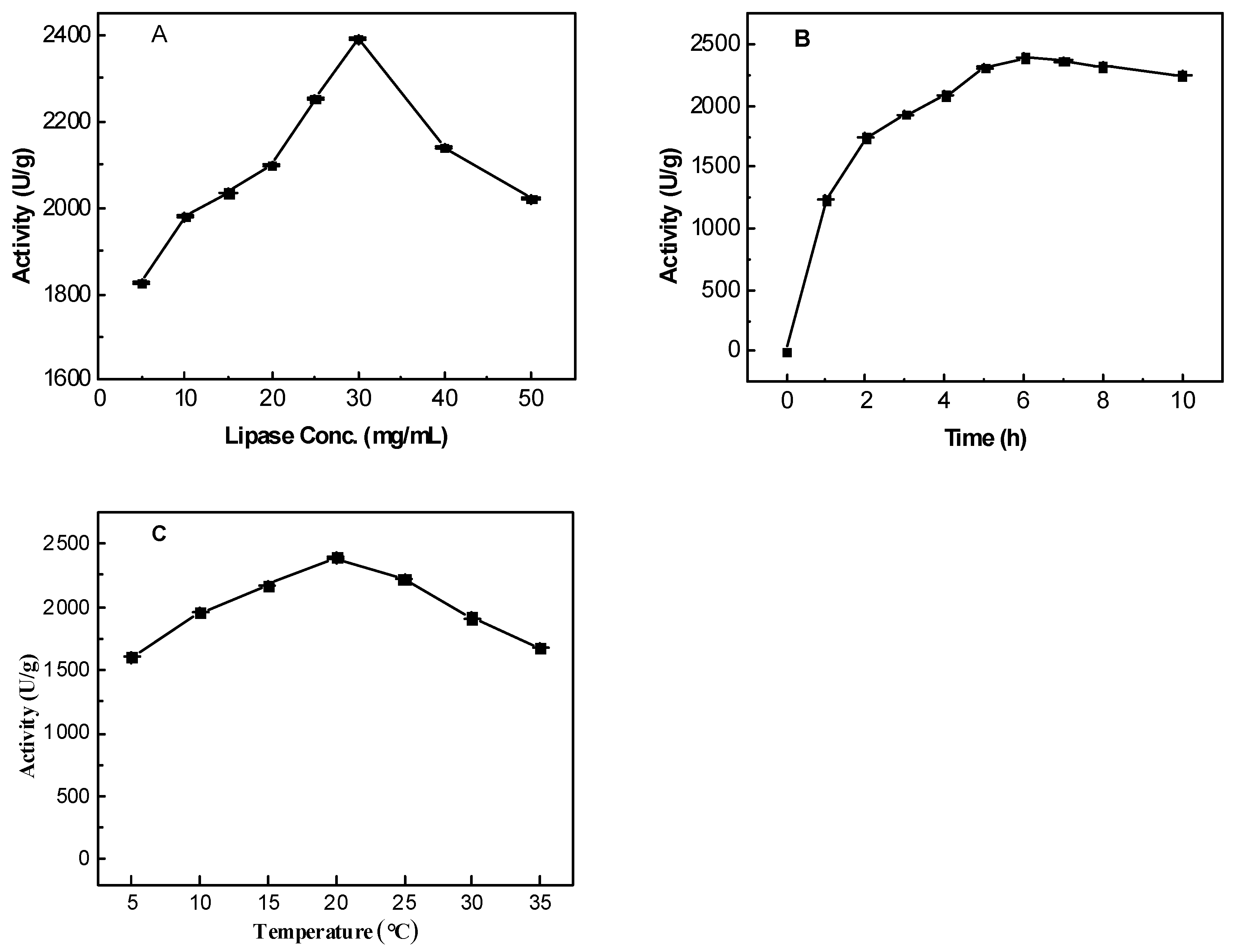

| Samples | BET Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| Native collagen [15] | 2.10 | 13.7 | 0.0049 |
| MNp-Col support | 11.59 | 10.47 | 0.042 |
| MNp-Col-IL | 7.63 | 14.65 | 0.038 |
| Lipase | Km (mM) | Vmax (mM/min) | Vmax/Km | Relative Activity (%) |
|---|---|---|---|---|
| Free | 27.54 | 10.25 | 0.37 | 100 |
| MNp-Col-IL | 36.11 | 3.97 | 0.11 | 30 |
| Alcohol | Yield (%) | Alcohol | Yield (%) |
|---|---|---|---|
| Methanol | 39.8 | Iso-butanol | 61.9 |
| Ethanol | 44.5 | Tert-butanol | 6.2 |
| N-propanol | 56.1 | N-amyl alcohol | 63.4 |
| Iso-propanol | 22.3 | Iso-amyl alcohol | 49.1 |
| N-butanol | 82.7 | N-hexyl alcohol | 36.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Song, D.; Chen, M.; Cheng, H. Immobilization of Lipases on Magnetic Collagen Fibers and Its Applications for Short-Chain Ester Synthesis. Catalysts 2017, 7, 178. https://doi.org/10.3390/catal7060178
He S, Song D, Chen M, Cheng H. Immobilization of Lipases on Magnetic Collagen Fibers and Its Applications for Short-Chain Ester Synthesis. Catalysts. 2017; 7(6):178. https://doi.org/10.3390/catal7060178
Chicago/Turabian StyleHe, Shengsheng, Dewei Song, Min Chen, and Haiming Cheng. 2017. "Immobilization of Lipases on Magnetic Collagen Fibers and Its Applications for Short-Chain Ester Synthesis" Catalysts 7, no. 6: 178. https://doi.org/10.3390/catal7060178
APA StyleHe, S., Song, D., Chen, M., & Cheng, H. (2017). Immobilization of Lipases on Magnetic Collagen Fibers and Its Applications for Short-Chain Ester Synthesis. Catalysts, 7(6), 178. https://doi.org/10.3390/catal7060178





