Abstract
As a favorably clean fuel, syngas (synthesis gas) production has been the focus of concern in past decades. Substantial literatures reported the syngas production by various catalytic reforming reactions particularly in methane or ethanol reforming. Among the developed catalysts in these reforming processes, Ni-based catalysts from hydrotalcite-like compounds (HTLcs) precursors have drawn considerable attention for their preferable structural traits. This review covers the recent literature reporting syngas production with Ni-based catalysts from HTLc precursors via methane or ethanol reforming. The discussion was initiated with catalyst preparation (including conventional and novel means), followed by subsequent thermal treatment processes, then composition design and the addition of promoters in these catalysts. As Ni-based catalysts have thermodynamic potential to deactivate because of carbon deposition or metal sintering, measures for dealing with these problems were finally summarized. To obtain optimal catalytic performances and resultantly better syngas production, based on analyzing the achievements of the references, some perspectives were finally proposed.
1. Introduction
As a consequence of global environmental pollution and fuel crisis issues, many efforts have been made to produce energy in a clean manner. Synthesis gas (simplified as syngas), a mixture of CO and H2, has attracted broad attention as clean energy and important raw material of F-T synthesis, ammonia, methanol, and dimethyl ether (DME) synthesis [1]. In recent decades, various processes have been developed for syngas production such as the catalytic reforming of hydrocarbons including steam or carbon dioxide, varying in the final ratio of H2/CO. Among all the attempts, methane-reforming processes may be the most attractive. Methane-reforming processes are a batch of reactions, such as partial oxidation of methane (simplified as POM, Equation (1)), steam reforming of methane (simplified as SRM, Equation (2)), autothermal reforming that is a combination of partial oxidation and steam reforming, and carbon dioxide reforming of methane (simplified as CRM, Equation (3)).
CH4 + 1/2O2 → CO + 2H2, ΔH298 K = −35.6 kJ/mol
CH4 + H2O → CO + 3H2, ΔH298 K = 206 kJ/mol
CH4 + CO2 → 2CO + 2H2, ΔH298 K = 246 kJ/mol
Besides, the combination of these reforming processes can also be performed to manipulate the syngas composition purposefully, e.g., the tri-reforming means the co-occurrence of three reforming of POM, SRM, and CRM. Other reforming such as steam or carbon dioxide reforming of ethanol or glycerol have also been extensively investigated. The steam reforming of hydrocarbon is the most extensive industrial route for the production of syngas. This route occupies more than 60% of raw material sources in any petrochemical production plants like ammonia, methanol, and gas-to-liquid fuel plants [2]. Catalytic ethanol steam reforming is also regarded as the basis of new developments in H2 fuel cell technology [3]. However, hydrocarbon reforming with CO2—a greenhouse gas—as a feed stock has been widely considered for the advantages of chemical value of CO2 and natural gas.
To effectively fulfill these catalytic reforming processes, the choice of appropriate catalyst is crucially important. Although catalysts based on Pt, Pd, Ru, Rh, Ir [4,5,6], and many other noble metals for syngas production via various reforming processes have been studied, the relatively high cost of noble metals would still confine their applications in commercial fields. Despite their excellent catalytic behavior, noble metals cannot be served as the wise choice for economic concerns. In recent years, Ni-based catalysts with fine catalytic performances have been widely investigated and employed as satisfactory alternatives for noble metals in these reforming processes [7,8]. When preparing Ni-based catalysts, the use of precursors is also extensively explored, by which highly dispersed and stable metal particles can be more easily obtained on the catalyst’s surface via calcination and reduction [9].
Among the Ni-based catalyst precursors, promising results were found for hydrotalcite-like compounds (HTLcs) [10,11,12]. HTLcs are a batch of compounds composed of positively charged brucite like layers with an interlayer region containing charge compensating anions and solvation molecules. The general formula for HTLcs is always presented as: [M(II)1−xM(III)x(OH)2]x+ (An− x/n)·mH2O, where M(II) is commonly Mg2+, Zn2+, or Ni2+, and M(III) is commonly Al3+, Fe3+, Ti4+, or Mn3+. An− is an inorganic or organic anion existing for charge encountering—e.g., CO32−, Cl−, NO3−—and x is usually set in the range of 0.2 to 0.4. The structure of HTLcs is originated from the brucite structure of Mg(OH)2, where octahedral of Mg2+ share edges to form sheets. Mg2+ is coordinated with OH− ligands. The sheets stack on each other and are held by hydrogen bonding to form a well-organized layer structure. When a Mg2+ ion is replaced by a trivalent cation, such as Al3+, a positive charge will be generated, and this extra positive charge can be counter-balanced by interlayer anions as CO32−. Being used as the catalysts, HTLc precursors will be treated under high temperature to transform the uniform layer structure into complex metallic oxides, which possess some significant characteristics, such as high surface area-to-volume ratio, high homogeneity, and good synergetic effects between the elements. Moreover, the high dispersion and small-size of the particles of catalysts derived from HTLc precursors, can confine the sintering of Ni particles [13,14]. Based on their favorable properties, the as-acquired metal oxides with HTLcs as precursors are often employed as catalysts in many catalytic reactions. Ni-based catalysts with HTLcs as precursors were extensively used in catalytic reforming reactions for syngas production. For optimal catalytic performance, it is however necessary to tailor-design the catalysts during the preparation as well as the subsequent treatment processes.
Herein, we will cover literature on the catalytic performances of Ni-based catalysts using hydrotalcites as precursors (denoted as Ni-based HTLcs) in syngas production via reforming reactions, particularly in methane or ethanol reforming, including the influences of preparation methods, thermal treatment, reduction process, composition content, and promoters.
2. Results and Discussion
2.1. Preparation Method Effect of the Ni-Based HTLc Precursors
The preparation method of Ni-HTLcs significantly governs the catalytic behaviors of catalysts using them as precursors. Different preparation processes have been established for synthesizing HTLcs, among which the most frequently employed are co-precipitaion [15,16,17,18] (the precipitaion agent varied among sodium hydroxide, sodium carbonate, urea, etc.), impregnation, or sol-gel methods.
2.1.1. Co-Precipitation
Co-precipitation is the most commonly used method for preparing HTLcs, which is usually performed at slightly raised temperature and at a fixed pH with the co-precipitation of metallic nitrate solution and precipitation agent. The precipitation process is performed at low supersaturation by slowly adding the mixed solutions of M(II) and M(III) metal salts into a reactor containing an aqueous solution of the desired interlayer anion. An alkali solution is then added into the reactor simultaneously and the pH of the aqueous solution is kept constant.
Serrano-Lotina et al. [19] synthesized hydrotalcite-like precursors and evaluated the catalytic performances of their subsequently derived catalyst for methane reforming of carbon dioxide. They demonstrated the dependence of the structure and properties of the precursors upon the addition of lanthanum, as well as on the activity and stability of catalysts. As for the catalytic performance, at 700 °C, with a feed ratio of CH4 to CO2 being equal, fine catalytic activity was obtained. The catalysts did not deactivate and no coke deposition appeared during 50-h trial. Similar results were also described by Yu et al. [20] who also investigated the catalytic performances of La-doped Ni–Mg–Al complex oxides catalysts. They also prepared the catalysts from the HTLc precursors via coprecipitation, by which the size distribution of Ni metal particle in the catalysts was confined in a narrow scope of 5–11 nm. The presence of lanthanum effectively improved reforming activity as evaluated from the catalytic performance in the carbon dioxide reforming of methane. De Souza et al. [21] prepared M–Ni–Al HTLc catalysts (M = Mg, Zn, Mo, Co) by the continuous co-precipitation method with two aqueous solutions, the catalytic performances of the samples were investigated in steam reforming of ethanol. Abdelsadek et al. [22] reported a novel approach for preparing catalysts derived from NiAl hydrotalcite, in which the precursor was regenerated in situ from a former precursor by co-precipitation method. By that attempt, the conversion of CH4 and CO2 in methane reforming of carbon dioxide achieved an increase of 15.7% and 17.3%, respectively. Ashok et al. [15] investigated the bi-functional properties of NiO-CaO-Al2O3 catalysts derived from HTLc precursors by the coprecipitation method for steam reforming of biomass reactions and toluene. A catalyst of Ni–Ca–Al with a composition ratio 8:62:30 afforded excellent catalytic behavior in terms of stability and activity in both the reforming reactions. Li et al. [23] introduced a MgAl2O4-supported Ni catalyst with the MgAl2O4 support synthesized by coprecipitation method. When performing the precipitation process, for comparison, three routes were adopted by constant pH, decreasing pH precipitation, and increasing pH to the appropriate pH, respectively. They found that the precipitation procedure greatly influenced the physical property of spinel. The MgAl2O4 synthesized by constant pH precipitation appeared to be a more preferable support than those fabricated by changing pH precipitation (no matter increasing or decreasing), because the former exhibited much higher Brunauer–Emmett–Teller(BET) specific surface area than the latter ones. Besides, the Ni catalysts supported by MgAl2O4 afforded a fine dispersion in Ni nanoparticles and presented stable activity for the methane reforming of carbon dioxide. Zhang et al. [24] investigated Ni/Co catalysts originated from HTLcs which were prepared in situ on the surface of γ-Al2O3 by urea precipitation method for dry reforming of methane. By this in-situ co-precipitation method, highly homogeneous dispersed and small particles of the active component and of large specific surface area provided by γ-Al2O3 were achieved for these Ni/Co catalysts. Xu et al. [25] reported an in situ controllable assembly procedure of Ni-based nanocatalysts derived from HTLcs. In this procedure, the mesoporous γ-Al2O3 was activated in a solution of metal nitrates by urea hydrolysis under hydrothermal conditions. Subsequently, undergoing a homogeneous co-precipitation process, NiAl-HTLcs and Ni–Mg–Al-HTLcs were produced in situ on the surface of the γ-Al2O3, as shown in Figure 1. The developed nanohybrid presented fine activity and stability in methane reforming of carbon dioxide.
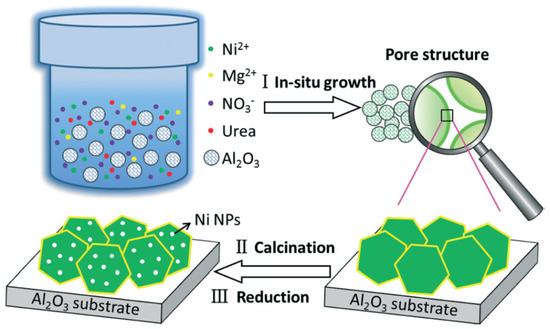
Figure 1.
Schematic synthesis route of the catalysts from HTLc precursors grown in situ on an alumina substrate. Reproduced from [25] with permission of The Royal Society of Chemistry. Copyright 2014.
Our group previously prepared Ni–Mg–Al catalysts [26] from HTLc precursor via a surfactant-assisted co-precipitation method and evaluated their catalytic performances in methane reforming of carbon dioxide at 800 °C. In that work, three surfactants were adopted to modify the properties and catalytic performances of the catalyst. As results showed, the obtained hydroxide with the addition of tetrapropylammonium during the co-precipitation process promoted the growth of the Ni(200), the crystallinity degree of which was responsible for the activation of methane. However, the addition of (EO)20(PO)70(EO)20 triblock copolymer (P123), polyvinylpyrrolidone (PVP), and cetyltrimethylammonium bromide restrained the growth of Ni(200) plane.
Resini et al. [27] prepared Ni–Zn–Al and Ni–Mg–Al catalysts from HTLc precursors by urea hydrolysis method for the steam reforming of ethanol. Both processes achieved fine crystallized HTLc precursors. After undergoing thermal treatment process, the two as-acquired mixed oxides presented the lamellar morphology of the precursors. IR spectroscopy suggested that the composition of the two obtained mixed oxides was different. The calcination products were a mixture of NiO, ZnO, and ZnAl2O4 for Ni–Zn–Al HTLcs and NiO–MgO solid solution for Ni–Mg–Al HTLcs. Both Ni–Zn–Al and Ni–Mg–Al catalysts afforded fine activities for steam reforming of ethanol with flow reactor. The authors found that the yield composition was influenced by temperature. When temperature was above 750–800 K, the products were H2, CO2, CO, and CH4, where the production of methane and carbon monoxide confined the yield of hydrogen to no more than 90% even if in the optimal condition. To get additional information on improving hydrogen yield as well as reaction mechanism, the author group further investigated steam reforming of ethanol reaction over the Ni–Zn–Al catalyst with atomic ratio of 49.6:21.7:28.7 [28]. The reaction experiments were carried out from 575 K to almost 1173 K with flow reactor. As the results addressed, the production distribution had close connection with thermodynamic equilibrium between reverse water gas shift and methane steam reforming above 800 K and hydrogen yield as high as 95% was obtained at 853 K. Moreover, in another work [29], catalytic performances evaluation for ethanol steam reforming of Ni(Co)–Zn–Al and Ni–Mg–Al catalysts from HTLc precursors was carried out and compared. Except for the above mentioned influential factors, they also pointed out that the formation of methane would affect the hydrogen production. The formation of methane could be confined by activation of C2 carbon species of ethanol or of its partial decomposition products and resultantly improved hydrogen yield. A similar mechanism and conclusion for affecting hydrogen production can also be achieved in another paper [30] on investigating the catalytic performance of Ni–Co–Zn–Al catalyst derived from HTLc precursor for ethanol steam reforming. After calcining at 973 K, the as-acquired mixed oxides was composed of NiO, ZnO, and a spinel. At 770 K, the catalyst with a composition around Zn0.58Ni0.42[Al0.44Co0.56]2O4 and minor amount of NiO and ZnO presented the optimal catalytic performance. At 823 K, with this catalyst and a feed ratio of water to ethanol being 6, a hydrogen yield beyond 90% could be obtained.
2.1.2. Impregnation
Wet impregnation synthesis [31,32] is the most commonly adopted preparation method, especially when addition of promoters is involved. However, for Ni-based catalysts, this process is prone to cause heterogeneous distribution of metal surface and a poor dispersion of metallic species, which promotes coke formation. Shiratori et al. [33] prepared an inorganic fiber network in which the MgAl hydrotalcite is dispersed by a paper-making process, and Ni was then loaded in this paper matrix via an impregnation process. Because Ni is more apt to agglomerating under the reducing conditions, to restrict Ni loading on the inorganic fibers, Ni would be purposely loaded on the hydrotalcite in the impregnation process. Thus, the tolerance of this paper-structured catalyst to sulfur impurities in biogas can be enhanced. By incipient wetness impregnation method, Carrero et al. [34] prepared Cu−Ni supported catalysts and confirmed their excellent catalytic performance at 600 °C for steam reforming of ethanol. The result suggested that the product distribution is susceptible to metal content, metal particles sizes and the composition ratio of Ni/Cu. Ren et al. [35] synthesized mixed oxide (NMA) of NiO, MgO, and Al2O3 from the HTLc precursors, and ceria was respectively added into NMA by the incipient impregnation (Ce/NMA) method and co-precipitation (NMACe). The catalytic performance of these two as-prepared catalysts in pressurized methane reforming of carbon dioxide showed difference that Ce/NMA afforded superior activity and stability.
2.1.3. Sol-Gel
Sol-gel method is another widely-adopted method for catalyst preparation. González et al. [36] synthesized nano-structured complex oxides with different nickel loads from nanocapsular HTLc precursors via sol-gel process. Besides, they systematically analyzed the optimal conditions for HTLcs preparation and the properties of their resulting complex oxides. Furthermore, the catalytic performances of the as-obtained catalysts were evaluated in the methane reforming of carbon dioxide. As demonstrated, the catalysts with 19% nickel load and thermally treated at 650 °C provided high activity as well as stability. Notably, minimal carbon deposition could be found on the catalysts. Based on the X-ray adsorption near edge structure (XANES) analysis, they also found that the formation of NiAl2O4 was inhibited by this synthetic method.
2.1.4. Others
Despite of the above-mentioned methods for the synthesis of Ni-based HTLc precursors, some other synthesis methods have also been reported for Ni-based catalysts.
As well acquainted, “memory effect” is a peculiar and important feature of HTLc precursors. That is, when the resultant complex oxides derived from HTLc precursors are treated with steam, aqueous water, or other aqueous solutions at certain conditions, the HTLc structure can be totally or partially regenerated. This aspect plays a fundamental role in the structure of the catalysts during the thermal treatments. Daza et al. [37] considered and applicated the trait of ‘memory effect’. They prepared Ce-promoted Ni–Mg–Al catalysts via a method that involved the doping of Ni–Mg–Al complex oxides derived from hydrotalcites with [Ce(EDTA)]− (EDTA is Ethylene Diamine Tetraacetic Acid). They firstly prepared Ni–Mg–Al complex oxides from HTLc precursor and then reconstructured the hydrotalcites with anion of [Ce(EDTA)]−.Finally, the newly synthesized HTLc precursor underwent the calcination and thermal treatment process and produced the target catalyst. The characteriztion results suggested that the periclase phase was partially reconstructed during the doping process with [Ce(EDTA)]− and a mixture of fluorite phases and crystalline periclase was produced after calcination. The catalyst showed stable catalytic performance in dry reforming of methane during 100, 20, and 15 h with Gas Hour Space Velocity (GHSV) of 24, 48, and 96 L·g−1·h−1, respectively.
Takehira et al. [38] successfully prepared an egg-shell type Ni–Mg–Al catalyst by adopting “memory effect” of Mg–Al hydrotalcite(HT). The authors first synthesized Mg–Al hydrotalcite by co-precipitation method with ratio of Mg2+ to Al3+ being 3. After calcining Mg–Al HT at 850 °C for 1 h, Mg–Al complex oxides were obtained. Subsequently, the Mg–Al HT was reconstructed with a part of the Mg(II) sites substituted by Ni(II) by immersing the Mg–Al complex oxide particles in Ni(II) solution. Finally, the object Ni–Mg–Al catalyst was achieved by calcining the regenerated Mg–Al HT with Ni loading and reducing the as-acquired mixed oxides. The catalytic performance of the catalyst was evaluated in autothermal reforming of CH4 at 800 °C with the space velocity from 6 to 30 L·g−1·h−1. The catalyst afforded excellent activity because of high dispersion of Ni metal particle in the catalyst surface.
Similarly, a shell-core Ni/Mg–Al catalyst was also prepared via the reconstruction of a HT-derived Mg–Al mixed oxide in a Ni2+ nitrate solution in the work of Zeng [39]. Characterization showed that Ni2+ was partially incorporated into the layered structure. The catalyst was applied in the ethanol steam reforming for syngas production. Catalytic behavior investigation at 700 °C suggested that shell-core catalyst with minor Ni content afforded more preferable performances than that of the bulk of 15 wt % Ni content.
Zhang et al. [40] introduced a new Ni–Mg–Al catalyst acquired from HTLcs which was prepared on γ-Al2O3 by in-situ preparation method as shown in Figure 2. For the construction of HTLc precursor, Ni(NO3)2 and Mg(NO3)2 offered the divalent cation and γ-Al2O3 afforded the trivalent cation, besides, γ-Al2O3 also acted as a substrate for HTLcs to grow on its inner or outer surface. Subsequently, the as-acquired Ni–Mg–Al-HTLcs/γ-Al2O3 was then calcined and reduced by atmospheric H2/Ar cold plasma to get the final catalyst, which afforded better catalytic performance in CO2 reforming of CH4 compared to a reference catalyst of Ni/MgO/γ-Al2O3 prepared by impregnation. They suggested that the excellent performance of the catalyst could be attributed to its relatively larger specific surface area as well as smaller active-crystal particles because of the molecular-order dispersion of active components over the HTLc precursor.
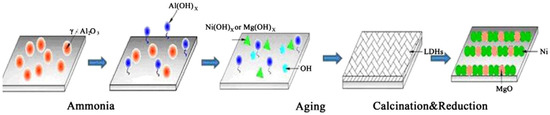
Figure 2.
Schematic synthesis process of NiMgAl-HTLcs/γ-Al2O3 in-situ. Reproduced from [40] with permission of Elsevier. Copyright 2014.
In short, co-precipitation method should be the better option for obtaining the most crystalline phase [41,42] and for efficiently incorporating the transition metal [43], which is also the most extensively employed method so far.
2.2. Effect of Thermal and Reduction Treatment
Besides the preparation method, the subsequent thermal and reduction treatment processes for the Ni-based HTLc precursors both have vital effect on their catalytic performances and so it is essential to optimize the reforming performance. Researchers reported their products such as mixed metallic oxides or spinel which were obtained under different temperatures in the calcination process. Takehira et al. [44] and Gazzano [45] suggested that the optimal thermal condition for HTLc decomposition would be 650 °C. They proposed that the thermal decomposition of NiMgAl-HTLc in air at 650 °C led to a homogeneous mixture of Mg(Al)O oxides. In Mg(Al)O oxides, not only Ni2+ but also Al3+ replaced the octahedral sites of the MgO structure, thus causing the high dispersion of Ni2+. They also addressed that idea that the calcination at 750 °C would lead to the formation of the spinel MgAl2O4 and NiAl2O4. However, when studying catalytic performances of lanthanum promoted catalysts and the structure properties of their precursors, Serrano-Lotina et al. [19] set the calcination temperature as 750 °C to ensure the integrity on methane reforming of carbon dioxide reaction which was performed at 700 °C, when they studied catalytic performances of lanthanum promoted catalysts the structure properties of their precursors. When investigating the effects of iron content (ratio of Ni/Fe ranging from 3 to 1) and the calcination temperature of the catalyst precursor (500 and 800 °C) on the catalytic performance in ethanol steam reforming, Abelló et al. [46] found that the calcination temperature caused changes in phase component composed of NiFe2O4 and Ni(Fe)Ox solid solution, which influenced the dispersion and final size of nickel species generated during the reforming. When calcined at 773 °C and Ni/Fe of 1, the catalyst, a mixture of NiFe2O4 + Ni(Fe)Ox possessing small NiO crystallites and high surface area presented optimal activity. When treated at high calcination temperature (1073 °C), a higher percentage of crystalline NiFe2O4 was attained, which presented lower activity and deactivated quickly. Thus, result might be associated with the lower carbon deposition resistance or easy sintering of NiO of NiFe2O4. Serrano-Lotina et al. [13] reported the influence of calcination temperature on biogas reforming over La-NiMgAl catalysts derived from HTLc structure. The precursor was calcined under six different temperatures 250, 350, 450, 550, 650, and 750 °C and found that the hydrotalcite structure could not be detected for calcination temperature above 250 °C. Increasing the calcination temperature would cause the enhancement of cell parameter value and the grain size. Catalytic evaluation proved that calcination temperature evidently influenced catalytic activity and stability, that is, the stability and catalytic activity decreased with calcination temperatures decreasing below 750 °C, except for the case of 550 °C, where a higher activity was achieved but with a comparatively low stability. Apart from the above-mentioned calcination temperatures, many other temperatures were also adopted, such as 450 °C [22], 500 °C [20,37], 600 °C [21], 650 °C [35], and 750 °C [19,47]. The calcination temperature was varied with the reforming reaction type. In ethanol reforming, the calcination temperature was set lower; while for carbon dioxide reforming of methane, the calcining temperature of the catalysts was set higher to decrease the carbon deposition rate.
Reduction pretreatment temperature is another important parameter for affecting the catalytic performance of the catalyst. When varying the reduction temperatures, the metal dispersion as well as the particle size of the metal could be changed and resultantly affect the activity of the catalyst [48,49]. As the reduction pretreatment is performed to reduce the mixed metallic oxides, thus the phase composition of the metallic oxides which connect closely with the calcination procedure should be emphatically considered. According to the literature, for reduction of Ni-based mixed metallic oxides, the reduction peaks from 370 to 450 °C should be ascribed to the reduction of free NiO [50,51] and reduction peaks appearing in realm of 500–800 °C may be associated with the slight interaction between Ni2+ and the other metallic oxides [52,53]. The peak located at temperatures higher than 800 °C can be attributed to the reduction of Ni2+ in the Ni-based spinel [54,55]. When studying the ethanol steam reforming for hydrogen production over Ni-based catalysts originated from Ni–Mg–Al HTLc precursors, Li et al. [56] suggested that high reduction temperature (>700 °C) was beneficial for the reduction of Ni0 metal, and prominently increased the catalytic stability and activity. They proposed that the optimal reduction temperature should be 800 °C, catalysts owning a preferable amount of Ni0 species and features of effective resistance to carbon deposition could be obtained under those optimal conditions. Pakhare et al. [57] preferred lower reduction temperatures based on some referencing reviews that Pt/ZrO2 catalysts reduced at 200 °C showed higher conversion of CH4 and CO2 than that reduced at 500 °C [58]. They also pointed out that Ru/La2O3-SiO2 reduced at 400 °C could behave better than the same catalyst reduced at 550 °C [48]. Upon the mentioned results above, still no consensus can be reached for selecting optimal calcination conditions (temperature, time, and rates) and reduction temperature to obtain optimal catalytic performance, this concern is still under study.
Besides the temperature setting, some novel means as well as techniques were applied in the thermal or reduction processes, such as combustion [59,60], microwave radiation [61,62], supercritical [63] and plasma treatment [64,65,66]. Compared with the conventional thermal treatment processes, applying microwave radiation affords some benefits of smaller Ni particle sizes, shorter operating times, and decreased effect of the surface properties on the Ni particle size [62]. In recent years, various plasma treatments have been introduced into the preparation of Ni-based catalyst and preferable effect has been achieved. Particularly, plasma has been evidenced as an effective technique in solving the carbon deposition problem of Ni-based catalysts. Long et al. [67] fabricated Ni–Co bimetallic catalysts from HTLc precursors containing Ni, Co, Mg, and Al. For the decomposition of the precursors and the reduction of the as-acquired complex oxides, cold plasma jet was employed. Experimental results showed that the HTLc precursors could be entirely decomposed but partly reduced via cold plasma jet. Moreover, the catalytic performance of the obtained catalysts was investigated for dry reforming of methane and a decent result was achieved. The preferable catalytic activity was ascribed to the smaller metal particle size as well as higher metal dispersion of the catalysts, and the interaction effect between Co and Ni, which were all caused by cold plasma jet treatment. Similar results were also achieved for Zhang et al. [24], who prepared Ni/Co catalyst derived from HTLc precursors with decomposition and reduction process performed by H2/Ar atmospheric plasma jet. Xu et al. [68] prepared Ni(NO3)2/MgO/γ-Al2O3 precursor by impregnation method and the precursor was then treated via two ways for the subsequent calcination and reduction processes: one was decomposition and reduction by direct atmospheric cold plasma jet (as shown in Figure 3) and the other was calcination at 550 °C for 4 h and reduction at 750 °C for 2 h as the conventional method. With the aid of the mixed beneficial effects of plasma reduction and MgO addition, the catalyst treated by cold plasma jet presented better catalytic performance in methane reforming of carbon dioxide. As the results presented that the plasma reduction could decrease the initial Ni crystals of the catalyst via its atomic hydrogen effect and resultantly prevent the increase of Ni crystals in the reduction process. Moreover, by plasma treatment, catalysts with relatively larger surface areas, better metalic dispersion, as well as smaller particles can be obtained.
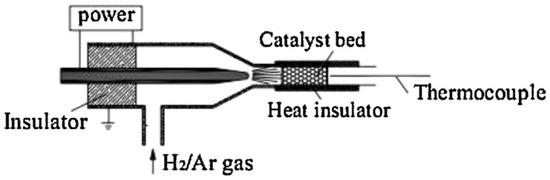
Figure 3.
The schematic of catalyst reduction process by atmospheric cold plasma jet. Reproduced from [68] with permission of Elsevier. Copyright 2013.
2.3. Composition Content Design
Despite of the favorable behaviors of Ni-based catalysts for the reforming reactions, Ni-based catalysts tend to deactivate easily because of coke deposition and metal sintering [69,70]. To optimally fulfill the catalytic activity and stability of Ni-based catalysts, the contents of Ni and other metals as well as the content ratio among these metallic elements in the Ni-based HTLc precursors’ structure should also be considered. Perez-Lopez et al. [71] observed that the catalytic behaviors of Ni–Mg–Al catalysts were more susceptible to the ratio of M(II) to M(III) rather than the ratio of Ni to Mg in carbon dioxide reforming of methane. Many literatures covered the influence of Ni content as well as other composition on the catalytic performances of Ni-based catalysts. Tan et al. [72] studied the effect of nickel content on the surface properties and structure of the catalyst. They found that the Ni particle sizes reduced with enhancing the Ni content and a minimum particle size was got at 18 wt % of Ni content. Abelló et al. [46] investigated the effect of Ni:Fe ratio on catalytic properties of Ni–Fe catalysts from HTLc precursors for ethanol steam reforming and sieved the optimal catalyst with ratio of Ni to Fe being unity. To successfully prepare Ni-based catalysts from HTLc precursors and to achieve the optimal catalytic performances, as the active metallic component, the content of Ni in the catalysts is always stressed. However, except for the content of Ni, many other factors should also be considered. The Mg/Al molar ratios in a pure hydrotalcite structure should be between 2 and 4 [69,73], beyond which range, metal hydroxides would be formed. The partial substitution of Mg by Ni causes the formation of Ni–Mg–Al HTLcs, which is the most widely employed catalysts with HTLc type precursors in the reforming reactions. As reported [69,74], Ni–Mg–Al HTLcs were always fabricated from the nitrates of Mg2+, Al3+ and Ni2+ with the ratios of Ni/Mg being 2 and (Ni + Mg)/Al being 3. Lin et al. [75] prepared Ni–Mg–Al catalysts with Ni contents being 3–18 wt % and evaluated their catalytic performances for the dry reforming. They discovered that the catalyst activity was enhanced when the Ni content increased. However, the catalyst stability, the sintering of Ni metal and coke deposition were influenced by both the Ni content and temperature. The Ni–Mg–Al catalyst with Ni loading of 12 wt % afforded the most preferable performances with high activity, fine stability, and less carbon deposition at high temperature. For the preparation of NiMgAl catalysts from HTLc precursors, the Mg/Al ratio is always fixed [35]. While it is really worth addressing that many researchers have been aware of that the Mg/Al ratio also can greatly influence the catalytic properties. Zhu et al. [76] investigated the effect of the Mg/Al ratio in NiMgAl complex oxide catalyst originated from HTLc precursors for dry reforming. They found that the NiMgAl catalyst with a relatively higher Mg/Al ratio exhibited preferable catalytic activity and carbon deposition resistance. When Mg:Al ratio was equal, the best catalytic results were achieved. As addressed by Shen et al. [77], the ratio of M2+ to M3+ could affect the basicity of catalysts. They proposed that the catalyst with ratio of Mg to Al being 0.5 behaved the best in combined reaction of H2O and CO2 reforming of CH4 [78]. Serrano-Lotina et al. [47] addressed that hydromagnesite phase would form when Mg/Al molar ratio was 4. With the increasing of Mg/Al molar ratio, the thermal stability and free or segregated NiO reducibility increased while the BET surface area, and reducibility of Mg(Ni,Al)O decreased. High Mg/Al molar ratio did not favor catalytic activities, but it raised the stability during reaction tests. During a 120 h test, catalysts with a Mg/Al of 3 being afforded the best stability. Takehira et al. [79] reported that the molar ratio (Mg + Ni)/Al of 3 provided the highest activity and deactivation rate in methane reforming of steam. Koo et al. [80] reported that 20 wt % MgO was the optimum content in MgO-promoted Ni/Al2O3 for inhibiting coke formation in combined carbon dioxide and steam reforming of methane.
2.4. Promoter Effect
Although the Ni-based catalysts from HTLc precursors have attractive properties of high surface area and thermal stability [81,82], Ni0 has the high thermodynamic potential to sinter and to produce carbon deposition when contacting with hydrocarbons, which will result in fast deactivation [83]. To increase the activity and longevity of Ni-based catalysts for the reforming reactions and minimize the formation of coke deposits, the addition of promoters with other proper metals [27] is deemed as an effective means. Many elements such as basic earth metals (Mg, Ca, Sr), noble metals (Rh, Pd, Pt, Ag), rare earth elements (La, Ce, Pr, Y), and other metals (Zr, Zn, Mo or Co) were employed as promoters for catalysts in the reforming reactions. Generally, the addition of these promoters can affect the acid–base properties of the catalyst and the structure and morphology of the Ni particles. The addition of appropriate metals to Ni-based catalysts can often bring about high reforming activity and selectivity to hydrogen. As is found that the addition of Cu to Ni-based catalysts is beneficial to water-gas-shift reaction and dehydrogenation, thus enhances the hydrogen production [84,85]. Lanthanide metals can promote the ease of catalyst reduction and decrease the reduction temperature [86,87]. Among these lanthanide metals, Foo et al. [88] found that Ce had the superiority over Pr and Sm in confining carbon deposition, which is probably because of the electronic traits of Ce with higher energy 5d electrons to eliminate carbon more easily. Moreover, it has also been proposed that the addition of CeO2 can increase dispersion degree of Ni and thus reduce the interactions between Ni and the support [89]. Here, we will mainly focus on the promoters of Ni-based catalysts from HTLc precursors. Yu et al. [90] prepared Ni–Mg–Al and NiMMgAl (M = Zr, Mn, Cu, or Co) catalysts originated from HTLcs and investigated their catalytic performances for the methane reforming of carbon dioxide. They found that the initial activity of the catalysts decreased in the sequence: NiCoMgAl > NiMgAl > NiCuMgAl > NiMnMgAl > NiZrMgAl. The NiCoMgAl catalyst presented the best catalytic activity. It was found that Mn2O3 and ZrO2 in the NiMnMgAl and NiZrMgAl catalysts hindered the Ni-active centers and thus reduced their original catalytic activity. Because of the formation of NiCuMgAl alloy and the depressed rate of carbon deposition, the NiCuMgAl catalyst significantly improved the reaction stability. The NiCoMgAl catalyst afforded excellent stability because it could confine the formation of carbon deposition and the sintering of metal, which could be ascribed to its high metal dispersion and small metal size. However, the stability of NiMnMgAl and NiZrMgAl catalysts were relatively poor due to the enhanced rate of coke formation especially for filaments carbon. Zhang et al. [91] employed Ni–Mg–Mn–Fe–O catalyst derived from HTLc precursor in auto-thermal reforming of ethanol (ATR) for hydrogen production and obtained fine catalytic result with a high H2 yield at 4.0 mol-H2/mol-EtOH. They found that the surface area and reduction behavior of the catalysts could be improved by the synergistic effect from Fe and Mn on the structural and electronic properties and thereby acquired more Ni0 species in ATR test. Daza et al. [37] found that Ce presented a promote effect not only in the reduction degree of Ni but also in the strength and amount of the basic sites. Besides, as no obvious variation could be found in catalytic activity and selectivity or in the size when increasing the Ce load from 1% to 10%, Ce-promoted NiMgAl catalysts behaved better in inhibiting coke formation rather than improving catalytic performances or decreasing the catalyst size. Serrano-Lotina et al. [19] and Yu et al. [20] both investigated the La-promoted NiMgAl catalysts in dry reforming and confirmed the positive effect of the addition of lanthanum. The presence of lanthanum evidently augmented the surface Ni content and the total amount of basic sites, thus inhibiting carbon depositing. De Souza et al. [21] discussed the promotion effect of Co, Mo, and Zn in nickel-based HTLcs in the ethanol reforming of steam for syngas production. It was got that the addition of Co, Mo, and Zn led to changes of acidic sites, crystallinity, surface area, and reduction behavior. For the ethanol reforming of steam, the dehydration and dehydrogenation of ethanol preferred to take place at lower temperatures, while the production of syngas mainly appeared at higher temperatures. The Mo, Co, and Zn-promoted catalysts presented better activity at mediate temperatures, however, Mo-, Co-, and catalysts without promoters exhibited higher selectivity to syngas at high temperatures.
3. Conclusions
The present review mainly covers the application of Ni-based catalysts from hydrotalcite-like compounds precursors in reforming reactions for syngas production. From points of preparation, thermal treatment, reduction process, and composition design, the superiority for Ni-based catalysts from HTLc precursors were discussed. As Ni-based catalysts have the potential to deactivate arisen from carbon deposition and metallic sintering in reforming reactions, it is really necessary to design coke resistance and anti-sintering catalysts to improve the catalyst stability and syngas production. For improving catalytic performances and the coke resistance of the catalysts, the usage of proper promoters, additive sand supports are effective means. The use domain of various promoters and additives is closely correlated with electronic traits of these elements, which have been well acquainted as the former mentioned. For the present topic of Ni-based catalysts derived from HTLc precursors, the support characteristics of HTLcs might be a promising research direction. As most HTLc precursors present as basic supports, this basic property is preferable for the adsorption and activation for reforming reaction of carbon dioxide. However, for reforming reaction of methane, the acidic supports will be more feasible as methane tends to adsorb and activate at acidic sites [88]. Thus, in most cases, catalysts with both acidic and basic bi-functional supports are needed where CH4 and CO2 can be activated on various sites and the resulted intermediates will react at the metal-support interfacial sites. HTLcs are usually applied as basic catalysts or supports with basic sites. To achieve Ni-based acidic and basic bi-functional catalysts, two approaches can be considered, one is by inviting another acidic support together with HTLcs co-employed as the support [92], the other is exploiting acidic and basic bi-functional HTLc-structured catalysts [93]. To inhibit the sintering or aggregation of Ni particles, preparation of Ni-based catalysts smaller size, higher Ni dispersion, and preferable strong metal-support interaction (SMSI) effect between the metal and support are still deemed as feasible means. Some other ways that can control the particle size of Ni particle are still pursued. Kim et al. [94] reported a novel way to confine Ni particles with a uniform size of 10 nm within the pores of mesoporous silica. Thus, both carbon deposition and metal sintering were confined. However, the design of coke resistance and anti-sintering catalysts for reforming processes remain vital challenges world-wide. The conventional catalyst preparation methods, such as coprecipitation, impregnation, and sol-gel method will continue to be developed. Any progress in this field will be definitely important for the syngas production from reforming reactions. This review may serve as a fundamental guideline for the acquaintance of the application of Ni-based HTLc structured catalysts in reforming reactions for syngas production.
Acknowledgments
The project was funded by the National High Technology Research and Development Program of China (863 Program, 2013AA051201); National Key Technology R&D Program(2013BAC14B04); the Natural Science Foundation of Shanxi Province, China (201601D102007); State Key Laboratory of Chemical Resource Engineering (CRE-2015-C-106).
Author Contributions
Ya-Li Du carried out the literature collection and the manuscript writing; Xu Wu took on the preparation processes analysis of hydrotalcite-like compounds; Qiang Chen analyzed the catalytic performance data of the catalysts; Yan-Li Huang completed the typesetting and revision; Wei Huang supervised the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Hardiman, K.M.; Ying, T.T.; Adesina, A.A.; Kennedy, E.M.; Dlugogorski, B.Z. Performance of a Co-Ni catalyst for propane reforming under low steam-to-carbon ratios. Chem. Eng. J. 2004, 102, 119–130. [Google Scholar] [CrossRef]
- Chen, Z.; Elnashaie, S.S.E.H. Steady-state modeling and bifurcation behavior of circulating fluidized bed membrane reformer–regenerator for the production of hydrogen for fuel cells from heptane. Chem. Eng. Sci. 2004, 59, 3965–3979. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, W.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production by steam reforming of ethanol over an Ir/CeO2 catalyst: Reaction mechanism and stability of the catalyst. Int. J. Hydrogen Energy 2008, 33, 4377–4386. [Google Scholar] [CrossRef]
- Deluga, G.A.; Salge, J.R.; Schmidt, L.D.; Verykios, X.E. Renewable hydrogen from ethanol by autothermal reforming. Science 2004, 303, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Galvita, V.V.; Semin, G.L.; Belyaev, V.D.; Semikolenov, V.A.; Tsiakaras, P.; Sobyanin, V.A. Synthesis gas production by steam reforming of ethanol. Appl. Catal. A 2001, 220, 123–127. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Cai, W.; Zhan, E.; Mu, X.; Shen, W. Ethanol steam reforming over Ni and Ni–Cu catalysts. Catal. Today 2009, 146, 31–36. [Google Scholar] [CrossRef]
- Liguras, D.K.; Goundani, K.; Verykios, X.E. Production of hydrogen for fuel cells by catalytic partial oxidation of ethanol over structured Ni catalysts. J. Power Sources 2004, 130, 30–37. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Jerkiewickz, G.; Assaf, E.M. Nickel catalysts promoted with cerium and lanthanum to reduce carbon formation in partial oxidation of methane reactions. Appl. Catal. A 2007, 333, 90–95. [Google Scholar] [CrossRef]
- Gennequin, C.; Safariamin, M.; Siffert, S.; Aboukaïs, A.; Abi-Aad, E. CO2 reforming of CH4 over Co–Mg–Al mixed oxides prepared via hydrotalcite like precursors. Catal. Today 2011, 176, 139–143. [Google Scholar] [CrossRef]
- Muroyama, H.; Nakase, R.; Matsui, T.; Eguchi, K. Ethanol steam reforming over Ni-based spinel oxide. Int. J. Hydrogen Energy 2010, 35, 1575–1581. [Google Scholar] [CrossRef]
- Mas, V.; Dieuzeide, M.L.; Jobbágy, M.; Baronetti, G.; Amadeo, N.; Laborde, M. Ni(II)-Al(III) layered double hydroxide as catalyst precursor for ethanol steam reforming: Activation treatments and kinetic studies. Catal. Today 2008, 133–135, 319–323. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Rodríguez, L.; Muñoz, G.; Martin, A.J.; Folgado, M.A.; Daza, L. Biogas reforming over La-NiMgAl catalysts derived from hydrotalcite-like structure: Influence of calcination temperature. Catal. Commun. 2011, 12, 961–967. [Google Scholar] [CrossRef]
- Daza, C.E.; Moreno, S.; Molina, R. Co-precipitated Ni–Mg–Al catalysts containing Ce for CO2 reforming of methane. Int. J. Hydrogen Energy 2011, 36, 3886–3894. [Google Scholar] [CrossRef]
- Ashok, J.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B 2015, 172–173, 116–128. [Google Scholar] [CrossRef]
- Olafsen, A.; Slagtern, A.; Dahl, I.; Olsbye, U.; Schuurman, Y.; Mirodatos, C. Mechanistic features for propane reforming by carbon dioxide over a Ni/Mg(Al)O hydrotalcite-derived catalyst. J. Catal. 2005, 229, 163–175. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Daza, L. Influence of the operating parameters over dry reforming of methane to syngas. Int. J. Hydrogen Energy 2014, 39, 4089–4094. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Koike, M.; Nakagawa, Y.; Tomishige, K. Steam reforming of tar from pyrolysis of biomass over Ni/Mg/Al catalysts prepared from hydrotalcite-like precursors. Appl. Catal. B 2011, 102, 528–538. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Rodríguez, L.; Muñoz, G.; Daza, L. Biogas reforming on La-promoted NiMgAl catalysts derived from hydrotalcite-like precursors. J. Power Sources 2011, 196, 4404–4410. [Google Scholar] [CrossRef]
- Yu, X.; Wang, N.; Chu, W.; Liu, M. Carbon dioxide reforming of methane for syngas production over La-promoted NiMgAl catalysts derived from hydrotalcites. Chem. Eng. J. 2012, 209, 623–632. [Google Scholar] [CrossRef]
- de Souza, G.; Ávila, V.C.; Marcílio, N.R.; Perez-Lopez, O.W. Synthesis gas production by steam reforming of ethanol over M-Ni-Al hydrotalcite-type catalysts; M = Mg, Zn, Mo, Co. Procedia Eng. 2012, 42, 1805–1815. [Google Scholar] [CrossRef]
- Abdelsadek, Z.; Sehailia, M.; Halliche, D.; Gonzalez-Delacruz, V.M.; Holgado, J.P.; Bachari, K.; Caballero, A.; Cherifi, O. In-situ hydrogasification/regeneration of NiAl-hydrotalcite derived catalyst in the reaction of CO2 reforming of methane: A versatile approach to catalyst recycling. J. CO2 Util. 2016, 14, 98–105. [Google Scholar] [CrossRef]
- Li, D.; Lu, M.; Cai, Y.; Cao, Y.; Zhan, Y.; Jiang, L. Synthesis of high surface area MgAl2O4 spinel as catalyst support via layered double hydroxides-containing precursor. Appl. Clay Sci. 2016, 132–133, 243–250. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhang, Y.; Xu, Y.; Shang, S.; Yin, Y. Ni–Co catalyst derived from layered double hydroxides for dry reforming of methane. Int. J. Hydrogen Energy 2015, 40, 16115–16126. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, N.; Chu, W.; Deng, J.; Luo, S. In situ controllable assembly of layered-double-hydroxide-based nickel nanocatalysts for carbon dioxide reforming of methane. Catal. Sci. Technol. 2015, 5, 1588–1597. [Google Scholar] [CrossRef]
- Tan, P.; Gao, Z.; Shen, C.; Du, Y.; Li, X.; Huang, W. Ni–Mg–Al solid basic layered double oxide catalysts prepared using surfactant-assisted coprecipitation method for CO2 reforming of CH4. Chin. J. Catal. 2014, 35, 1955–1971. [Google Scholar] [CrossRef]
- Resini, C.; Montanari, T.; Barattini, L.; Ramis, G.; Busca, G.; Presto, S.; Riani, P.; Marazza, R.; Sisani, M.; Marmottini, F.; et al. Hydrogen production by ethanol steam reforming over Ni catalysts derived from hydrotalcite-like precursors: Catalyst characterization, catalytic activity and reaction path. Appl. Catal. A 2009, 355, 83–93. [Google Scholar] [CrossRef]
- Barattini, L.; Ramis, G.; Resini, C.; Busca, G.; Sisani, M.; Costantino, U. Reaction path of ethanol and acetic acid steam reforming over Ni–Zn–Al catalysts. Flow reactor studies. Chem. Eng. J. 2009, 153, 43–49. [Google Scholar] [CrossRef]
- Busca, G.; Montanari, T.; Resini, C.; Ramis, G.; Costantino, U. Hydrogen from alcohols: Ir and flow reactor studies. Catal. Today 2009, 143, 2–8. [Google Scholar] [CrossRef]
- Busca, G.; Costantino, U.; Montanari, T.; Ramis, G.; Resini, C.; Sisani, M. Nickel versus cobalt catalysts for hydrogen production by ethanol steam reforming: Ni–Co–Zn–Al catalysts from hydrotalcite-like precursors. Int. J. Hydrogen Energy 2010, 35, 5356–5366. [Google Scholar] [CrossRef]
- Chen, J.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Promoting effect of trace Pd on hydrotalcite-derived Ni/Mg/Al catalyst in oxidative steam reforming of biomass tar. Appl. Catal. B 2015, 179, 412–421. [Google Scholar] [CrossRef]
- Nawfal, M.; Gennequin, C.; Labaki, M.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Hydrogen production by methane steam reforming over Ru supported on Ni–Mg–Al mixed oxides prepared via hydrotalcite route. Int. J. Hydrogen Energy 2015, 40, 1269–1277. [Google Scholar] [CrossRef]
- Shiratori, Y.; Sakamoto, M.; Uchida, T.; Le, H.; Quang-Tuyen, T.; Sasaki, K. Hydrotalcite-dispersed paper-structured catalyst for the dry reforming of methane. Int. J. Hydrogen Energy 2015, 40, 10807–10815. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen production by ethanol steam reforming over Cu-Ni/SBA-15 supported catalysts prepared by direct synthesis and impregnation. Appl. Catal. A 2007, 327, 82–94. [Google Scholar] [CrossRef]
- Ren, H.; Song, Y.; Wang, W.; Chen, J.; Cheng, J.; Jiang, J.; Liu, Z.; Liu, Z.; Hao, Z.; Lu, J. Insights into CeO2-modified Ni–Mg–Al oxides for pressurized carbon dioxide reforming of methane. Chem. Eng. J. 2015, 259, 581–593. [Google Scholar] [CrossRef]
- González, A.R.; Asencios, Y.J.O.; Assaf, E.M.; Assaf, J.M. Dry reforming of methane on Ni–Mg–Al nano-spheroid oxide catalysts prepared by the sol–gel method from hydrotalcite-like precursors. Appl. Surf. Sci. 2013, 280, 876–887. [Google Scholar] [CrossRef]
- Daza, C.E.; Gallego, J.; Mondragón, F.; Moreno, S.; Molina, R. High stability of Ce-promoted Ni/Mg–Al catalysts derived from hydrotalcites in dry reforming of methane. Fuel 2010, 89, 592–603. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Shoro, D.; Murakami, K.; Honda, M.; Kawabata, T.; Takaki, K. Preparation of egg-shell type Ni-loaded catalyst by adopting “Memory Effect” of Mg–Al hydrotalcite and its application for CH4 reforming. Catal. Commun. 2004, 5, 209–213. [Google Scholar] [CrossRef]
- Zeng, G.M. The Investigation of Ethanol Steam Reforming over Hydrotalcite-Derived Ni-Based Catalysts. Ph.D. Thesis, Tianjin University, Tianjin, China, 2012. [Google Scholar]
- Zhang, X.; Wang, N.; Xu, Y.; Yin, Y.; Shang, S. A novel Ni–Mg–Al-LDHs/γ-Al2O3 catalyst prepared by in-situ synthesis method for CO2 reforming of CH4. Catal. Commun. 2014, 45, 11–15. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Graffin, P.; Tichit, D. Synthesis and characterization of sol–gel Mg/Al and Ni/Al layered double hydroxides and comparison with Co-precipitated samples. Micropor. Mesopor. Mater. 2000, 39, 229–247. [Google Scholar] [CrossRef]
- Tsyganok, A.; Sayari, A. Incorporation of transition metals into Mg–Al layered double hydroxides: Coprecipitation of cations vs. their pre-complexation with an anionic chelator. J. Solid State Chem. 2006, 179, 1830–1841. [Google Scholar] [CrossRef]
- Takehira, K.; Kawabata, T.; Shishido, T.; Murakami, K.; Ohi, T.; Shoro, D.; Honda, M.; Takaki, K. Mechanism of reconstitution of hydrotalcite leading to eggshell-type Ni loading on MgAl mixed oxide. J. Catal. 2005, 231, 92–104. [Google Scholar] [CrossRef]
- Gazzano, M.; Kagunya, W.; Matteuzzi, D.; Vaccari, A. Neutron diffraction studies of polycrystalline Ni/Mg/Al mixed oxides obtained from hydrotalcite-like precursors. J. Phys. Chem. B 1997, 101, 4514–4519. [Google Scholar] [CrossRef]
- Abelló, S.; Bolshak, E.; Montané, D. Ni–Fe catalysts derived from hydrotalcite-like precursors for hydrogen production by ethanol steam reforming. Appl. Catal. A 2013, 450, 261–274. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Martin, A.J.; Folgado, M.A.; Daza, L. Dry reforming of methane to syngas over La-promoted hydrotalcite clay-derived catalysts. Int. J. Hydrogen Energy 2012, 37, 12342–12350. [Google Scholar] [CrossRef]
- Faroldi, B.M.; Lombardo, E.A.; Cornaglia, L.M. Surface properties and catalytic behavior of Ru supported on composite La2O3–SiO2 oxides. Appl. Catal. A 2009, 369, 15–26. [Google Scholar] [CrossRef]
- Ghelamallah, M.; Granger, P. Impact of barium and lanthanum incorporation to supported Pt and Rh on α-Al2O3 in the dry reforming of methane. Fuel 2012, 97, 269–276. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, W.; Yuan, X.; Mu, R.; Song, Z.; Wang, X. Ni catalysts derived from Mg–Al layered double hydroxides for hydrogen production from landfill gas conversion. Int. J. Hydrogen Energy 2012, 37, 11488–11494. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Comparative study of Ni-based mixed oxide catalyst for carbon dioxide reforming of methane. Energy Fuels 2008, 22, 3575–3582. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Z.; Zhou, X.; Zhu, K. A unique method to fabricate NixMg1−xO (111) nano-platelet solid solution catalyst for CH4-CO2 dry reforming. Catal. Commun. 2013, 34, 11–15. [Google Scholar]
- Bang, Y.; Han, S.J.; Yoo, J.; Choi, J.H.; Kang, K.H.; Song, J.H.; Seo, J.G.; Jung, J.C.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over trimethylbenzene-assisted ordered mesoporous nickel–alumina catalyst. Int. J. Hydrogen Energy 2013, 38, 8751–8758. [Google Scholar] [CrossRef]
- Dieuzeide, M.L.; Iannibelli, V.; Jobbagy, M.; Amadeo, N. Steam reforming of glycerol over Ni/Mg/γ-Al2O3 catalysts. Effect of calcination temperatures. Int. J. Hydrogen Energy 2012, 37, 14926–14930. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Coke- and sintering-resistant monolithic catalysts derived from in situ supported hydrotalcite-like films on Al wires for dry reforming of methane. Nanoscale 2013, 5, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Li, S.; Wang, S.; Ma, X. Hydrogen production from ethanol steam reforming over nickel based catalyst derived from Ni/Mg/Al hydrotalcite-like compounds. Int. J. Hydrogen Energy 2010, 35, 6699–6708. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Stagg-Williams, S.M.; Soares, R.; Romero, E.; Alvarez, W.E.; Resasco, D.E. Metal-support interaction on Pt/ZrO2 catalysts for the CO2 reforming of CH4. Stud. Surf. Sci. Catal. 2000, 130, 3663–3668. [Google Scholar]
- Gonzalezdelacruz, V.; Holgado, J.; Pereniguez, R.; Caballero, A. Morphology changes induced by strong metal–support interaction on a Ni–ceria catalytic system. J. Catal. 2008, 257, 307–314. [Google Scholar] [CrossRef]
- Gonzalez-Delacruz, V.M.; Ternero, F.; Pereñíguez, R.; Caballero, A.; Holgado, J.P. Study of nanostructured Ni/CeO2 catalysts prepared by combustion synthesis in dry reforming of methane. Appl. Catal. A 2010, 384, 1–9. [Google Scholar] [CrossRef]
- Daza, C.E.; Kiennemann, A.; Moreno, S.; Molina, R. Dry reforming of methane using Ni–Ce catalysts supported on a modified mineral clay. Appl. Catal. A 2009, 364, 65–74. [Google Scholar] [CrossRef]
- Fidalgo, B.; Zubizarreta, L.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Synthesis of carbon-supported nickel catalysts for the dry reforming of CH4. Fuel Process. Technol. 2010, 91, 765–769. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Q.; Hao, Z.; Zhang, T.; Xie, Z. Development of a Co–Ni bimetallic aerogel catalyst for hydrogen production via methane oxidative CO2 reforming in a magnetic assisted fluidized bed. Int. J. Hydrogen Energy 2010, 35, 8494–8502. [Google Scholar] [CrossRef]
- Chai, X.; Shang, S.; Liu, G.; Tao, X.; Li, X.; Bai, M.; Dai, X.; Yin, Y. Characterization of Ni/γ-Al2O3 catalyst prepared by atmospheric high frequency cold plasma jet for CO2 reforming of CH4. Chin. J. Catal. 2010, 31, 353–359. [Google Scholar]
- Shang, S.; Liu, G.; Chai, X.; Tao, X.; Li, X.; Bai, M.; Chu, W.; Dai, X.; Zhao, Y.; Yin, Y. Research on Ni/γ-Al2O3 catalyst for CO2 reforming of CH4 prepared by atmospheric pressure glow discharge plasma jet. Catal. Today 2009, 148, 268–274. [Google Scholar] [CrossRef]
- Oukacine, L.; Gitzhofer, F.; Abatzoglou, N.; Gravelle, D. Application of the induction plasma to the synthesis of two dimensional steam methane reforming Ni/Al2O3 catalyst. Surf. Coat. Technol. 2006, 201, 2046–2053. [Google Scholar] [CrossRef]
- Long, H.; Xu, Y.; Zhang, X.; Hu, S.; Shang, S.; Yin, Y.; Dai, X. Ni-Co/Mg–Al catalyst derived from hydrotalcite-like compound prepared by plasma for dry reforming of methane. J. Energy Chem. 2013, 22, 733–739. [Google Scholar] [CrossRef]
- Xu, Y.; Long, H.; Wei, Q.; Zhang, X.; Shang, S.; Dai, X.; Yin, Y. Study of stability of Ni/MgO/γ-Al2O3 catalyst prepared by plasma for CO2 reforming of CH4. Catal. Today 2013, 211, 114–119. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chang, V.W.; Schumacher, D.J. CO2 reforming of methane to syngas I: Evaluation of hydrotalcite clay-derived catalysts. Appl. Clay. Sci. 1998, 13, 317–328. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of composition and thermal pretreatment on properties of Ni–Mg–Al catalysts for CO2 reforming of methane. Appl. Catal. A 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Hu, Y.; Shang, X.; Zhang, L.; Zou, X.; Ding, W.; Lu, X. Influence of nickel content on structural and surface properties, reducibility and catalytic behavior of mesoporous γ-alumina-supported Ni–Mg oxides for pre-reforming of liquefied petroleum gas. Catal. Sci. Technol. 2016, 6, 3049–3063. [Google Scholar] [CrossRef]
- Reichle, W.T.; Kang, S.Y.; Everhardt, D.S. The nature of the thermal decomposition of a catalytically active anionic clay mineral. J. Catal. 1986, 101, 352–359. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Tsunoda, T.; Hamakawa, S.; Suzuki, K.; Takehira, K.; Hayakawa, T. Dry reforming of methane over catalysts derived from nickel-containing Mg–Al layered double hydroxides. J. Catal. 2003, 213, 191–203. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Lu, M.; Chen, C.; Li, D.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ni catalysts prepared from Ni–Mg–Al layered double hydroxides: Influence of Ni loadings. Fuel 2015, 162, 271–280. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Chen, B.; Zhang, Z.; Shi, C. Effect of Mg/Al ratio of NiMgAl mixed oxide catalyst derived from hydrotalcite for carbon dioxide reforming of methane. Catal. Today 2016, 264, 163–170. [Google Scholar] [CrossRef]
- Shen, C.; Huang, W.; Qin, L.; Hu, X.; Lu, C.; Li, X. Effect of the ratio of M2+/M3+ on CH4 reforming of CO2 over Ni–Mg–Al catalyst. Chem. Ind. Eng. Prog. 2015, 34, 138–142. [Google Scholar]
- Koo, K.Y.; Roh, H.-S.; Jung, U.H.; Seo, D.J.; Seo, Y.-S.; Yoon, W.L. Combined H2O and CO2 reforming of CH4 over nano-sized Ni/MgO-Al2O3 catalysts for synthesis gas production for gas to liquid (GTL): Effect of Mg/Al mixed ratio on coke formation. Catal. Today 2009, 146, 166–171. [Google Scholar] [CrossRef]
- Takehira, K.; Ohi, T.; Miyata, T.; Shiraga, M.; Sano, T. Steam reforming of CH4 over Ni-Ru catalysts supported on Mg–Al mixed oxide. Top. Catal. 2007, 42–43, 471–474. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.-S.; Seo, Y.T.; Seo, D.J.; Yoon, W.L.; Park, S.B. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Appl. Catal. A 2008, 340, 183–190. [Google Scholar] [CrossRef]
- Holgado, M.J.; Rives, V.; San Román, M.S. Characterization of Ni–Mg–Al mixed oxides and their catalytic activity in oxidative dehydrogenation of n-butane and propene. Appl. Catal. A 2001, 214, 219–228. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, B.; Jin, Y.; Cheng, Y. Dry reforming of methane in a dielectric barrier discharge reactor with Ni/Al2O3 catalyst: Interaction of catalyst and plasma. Energy Fuels 2009, 23, 4196–4201. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Yu, X.; Chu, W.; Wang, N.; Ma, F. Hydrogen production by ethanol steam reforming on NiCuMgAl catalysts derived from hydrotalcite-like precursors. Catal. Lett. 2011, 141, 1228–1236. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Oktar, O.; Ozkan, U.S. Effect of lanthanide promotion on catalytic performance of sol–gel Ni/Al2O3 catalysts in steam reforming of propane. J. Mol. Catal. A 2005, 241, 133–146. [Google Scholar] [CrossRef]
- Gallego, G.S.; Marín, J.G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Influence of Pr and Ce in dry methane reforming catalysts produced from La1−xAxNiO3−δ perovskites. Appl. Catal. A 2009, 369, 97–103. [Google Scholar] [CrossRef]
- Foo, S.Y.; Cheng, C.K.; Nguyen, T.-H.; Adesina, A.A. Evaluation of lanthanide-group promoters on Co–Ni/Al2O3 catalysts for CH4 dry reforming. J. Mol. Catal. A 2011, 344, 28–36. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Role of CeO2 in Ni/CeO2–Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. B 1998, 19, 267–277. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Chu, W. Effect of a second metal (Co, Cu, Mn or Zr) on nickel catalysts derived from hydrotalcites for the carbon dioxide reforming of methane. RSC Adv. 2016, 6, 70537–70546. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M.; Yang, L.; Ye, S.; Huang, L. Ni–Mg–Mn–Fe–O catalyst derived from layered double hydroxide for hydrogen production by auto-thermal reforming of ethanol. Catal. Commun. 2014, 43, 6–10. [Google Scholar] [CrossRef]
- Zuo, Z.; Shen, C.; Tan, P.; Huang, W. Ni based on dual-support Mg–Al mixed oxides and SBA-15 catalysts for dry reforming of methane. Catal. Commun. 2013, 41, 132–135. [Google Scholar] [CrossRef]
- Wu, X.; Du, Y.; An, X.; Xie, X. Fabrication of NiFe layered double hydroxides using urea hydrolysis—Control of interlayer anion and investigation on their catalytic performance. Catal. Commun. 2014, 50, 44–48. [Google Scholar] [CrossRef]
- Kim, D.H.; Sim, J.K.; Lee, J.; Seo, H.O.; Jeong, M.-G.; Kim, Y.D.; Kim, S.H. Carbon dioxide reforming of methane over mesoporous Ni/SiO2. Fuel 2013, 112, 111–116. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).