Abstract
This review describes new reactions catalyzed by recently discovered types of metal complexes and catalytic systems (catalyst + co-catalyst). Works of recent years (mainly 2010–2016) devoted to the oxygenations of saturated, aromatic hydrocarbons and other carbon–hydrogen compounds are surveyed. Both soluble metal complexes and solid metal compounds catalyze such transformations. Molecular oxygen, hydrogen peroxide, alkyl peroxides, and peroxy acids were used in these reactions as oxidants.
1. Introduction
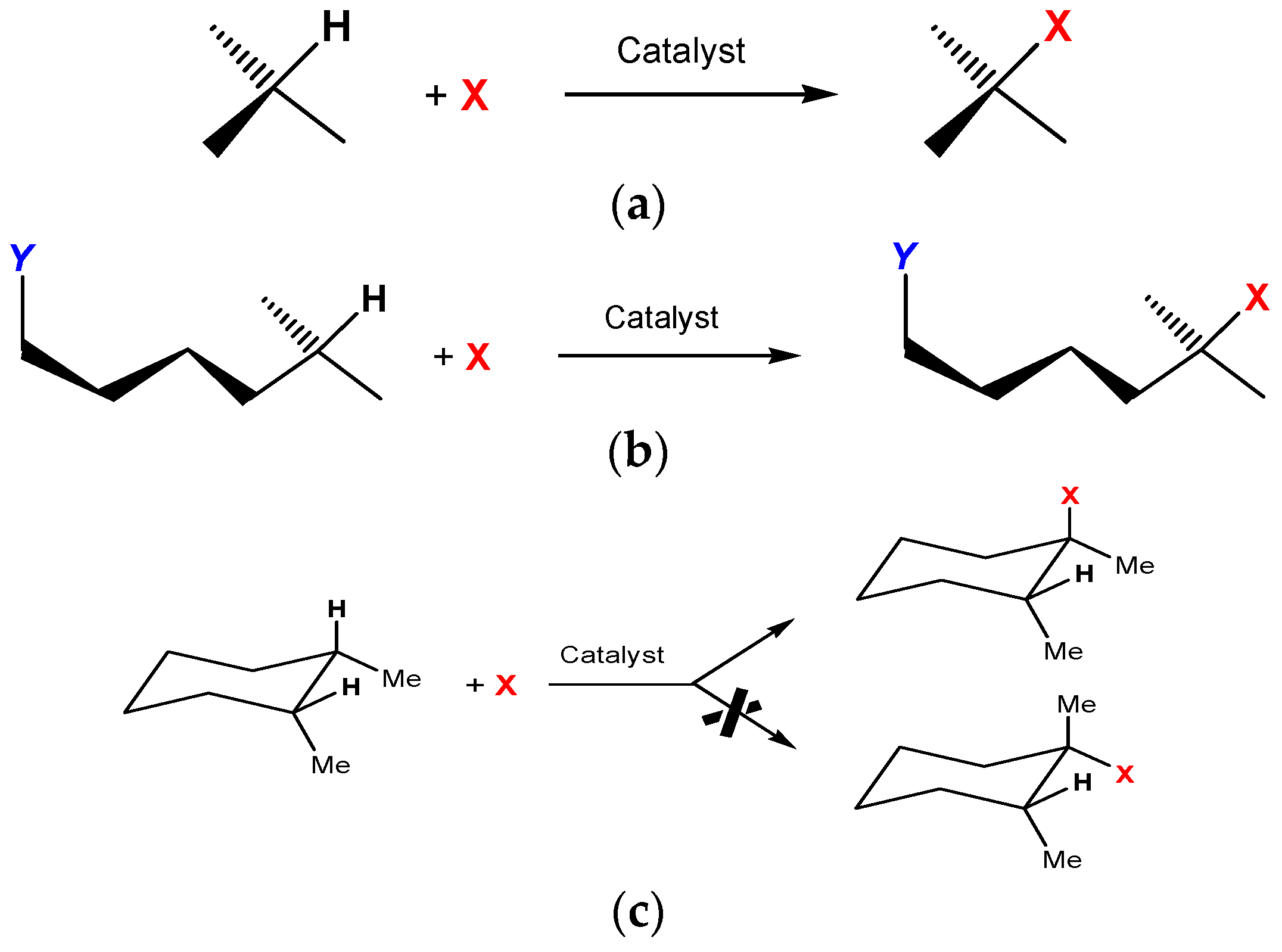
This review is devoted to functionalization of carbon–hydrogen bonds [1,2,3,4,5,6,7,8,9,10,11,12,13] in various organic compounds, which is a very important process both from academic and practical point of view. Replacing hydrogen atoms in carbon–hydrogen compounds by functional groups X leads to valuable functionalized C–X compounds (Scheme 1a).
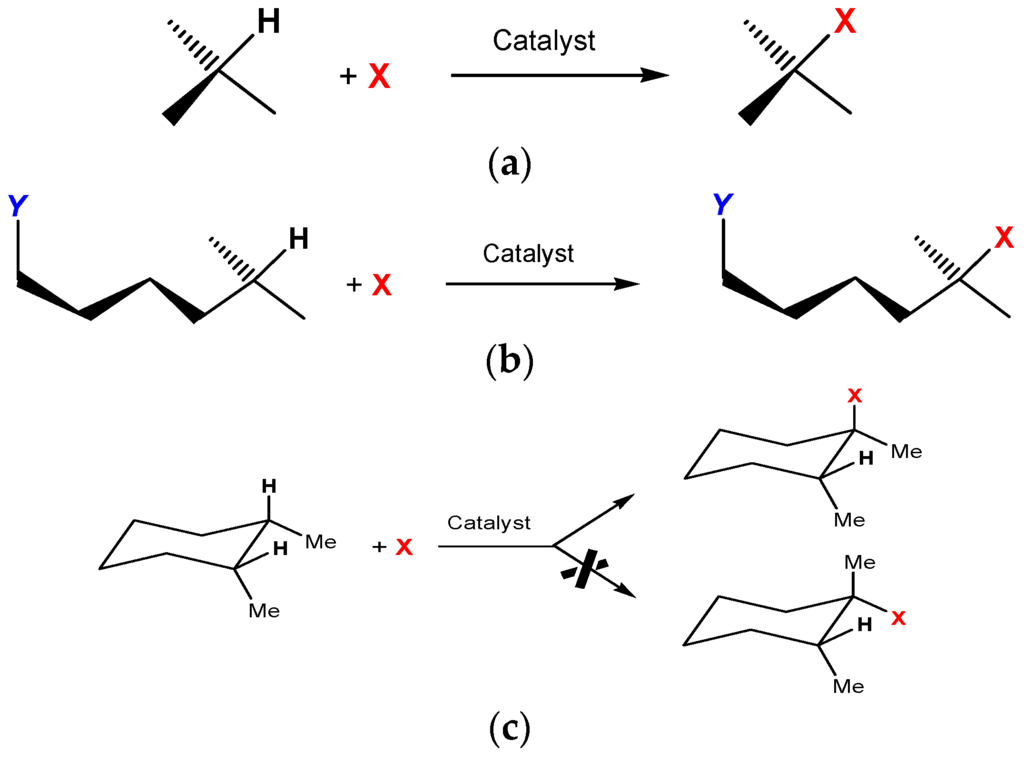
Scheme 1.
Functionalization of various C–H bonds.
Remote functionalization of carbon–hydrogen compounds bearing functional groups Y under the action of metal complex catalysts can give valuable difunctionalized alkanes (Scheme 1b).
Some catalytic systems are capable of functionalizing carbon–hydrogen bonds stereospecifically (Scheme 1c).
Functionalization of the carbon–hydrogen bond primarily proceeds via its activation when the reactivity of this σ-bond increases toward a reagent. As a result, the bond is capable of splitting, and in many cases this rupture of a saturated carbon–hydrogen bond is implied in the literature when the term “activation” is used. However, it is necessary to emphasize that splitting the bond is actually a consequence of its activation [1]. Alternative definitions have been proposed by Periana and colleagues who
and wrote about“define CH activation as a facile CH cleavage reaction with an ‘MX’ species that proceeds by coordination of an alkane to the inner-sphere of ‘M’ (either via an intermediate ‘alkane complex’ or a transition state) leading to a M–C intermediate”[14]
Labinger and Bercaw gave the definition of“the CH activation reaction as a coordination reaction with a reactive species ‘M’, that proceeds without the involvement of free radicals, carbocations, or carbanions to generate discrete M–R intermediates”.[14,15,16,17]
and Crabtree wrote:“reactions that have clearly been shown to involve bond formation between a metal and an alkane carbon”[18]
“Activation is considered to be the binding of a substrate to a metal center. This can be followed by a functionalization step in which the substrate is transformed...The term ‘CH activation’ emphasizes the distinct reactivity pattern of low valent metal complexes from that of classical organic reagents which break CH bonds by electrophilic or radical routes. In a classical route, radicals such as •OH readily abstract an H• atom from alkanes, RH, to give the alkyl radical R•. Included in this group are some metal catalyzed oxidations, such as the Gif reaction and Fenton chemistry”.[2]
Finally, the term “C−H activation” refers to a metal-mediated pathway involving the formation of metal-carbon bonds [19].
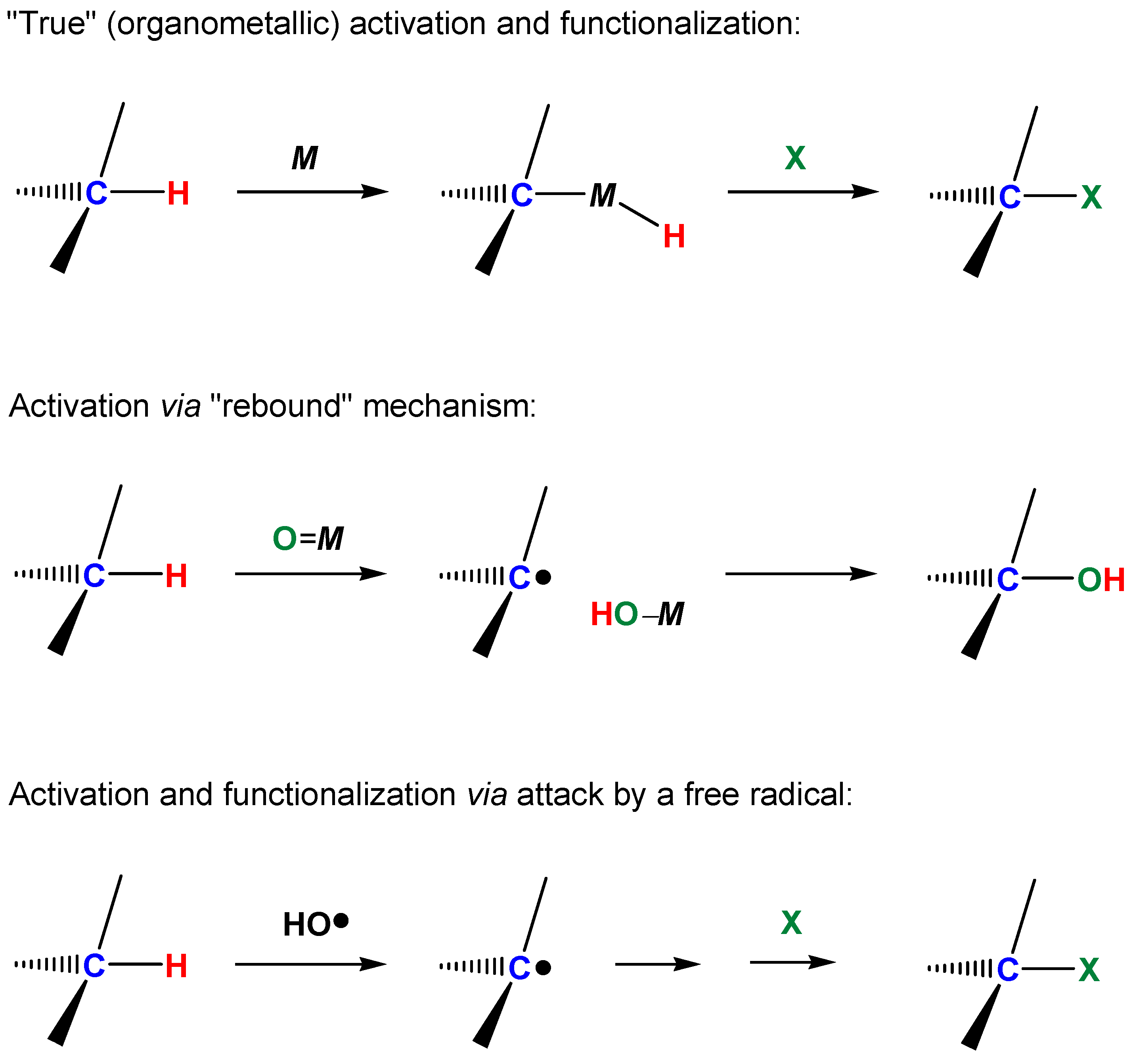
In recent years and decades, many reactions of inert saturated hydrocarbons with various metal compounds under mild conditions have been described [1]. Such processes with the participation of metal complexes occur at relatively low temperatures and can be selective. The oxidations of hydrocarbons with molecular oxygen and donors of an oxygen atom (hydrogen peroxide, alkyl hydroperoxides, and peroxy acids) constitute an important field of contemporary catalytic chemistry. We divide all carbon–hydrogen functionalization processes into three types taking into account the mechanistic point of view [1]. The first group includes reactions involving “true” or “organometallic” functionalization of the carbon–hydrogen bond. In these reactions compounds containing metal-carbon σ-bonds (organometallic derivatives) are formed as intermediates or as final products. We include in the second group reactions in which the contact between the complex and the carbon–hydrogen bond is only via a ligand of the complex during the whole process of the carbon–hydrogen bond cleavage. The σ-C–M bond is not generated directly at any stage. In these reactions the function of the metal complex usually consists in abstracting an electron or a hydrogen atom from the carbon–hydrogen compound. In the processes that belong to the third type, a complex activates initially not the hydrocarbon but it reacts with another reactant (for example, with hydrogen peroxide or molecular oxygen). The formed reactive species (for example, hydroxyl radical) then attacks the hydrocarbon molecule without any participation in the latter process of the metal complex. The “direct activation” in this case does not involve the participation of the metal catalyst [1]. The three categories of metal-mediated C–H functionalization processes are shown below Scheme 2.
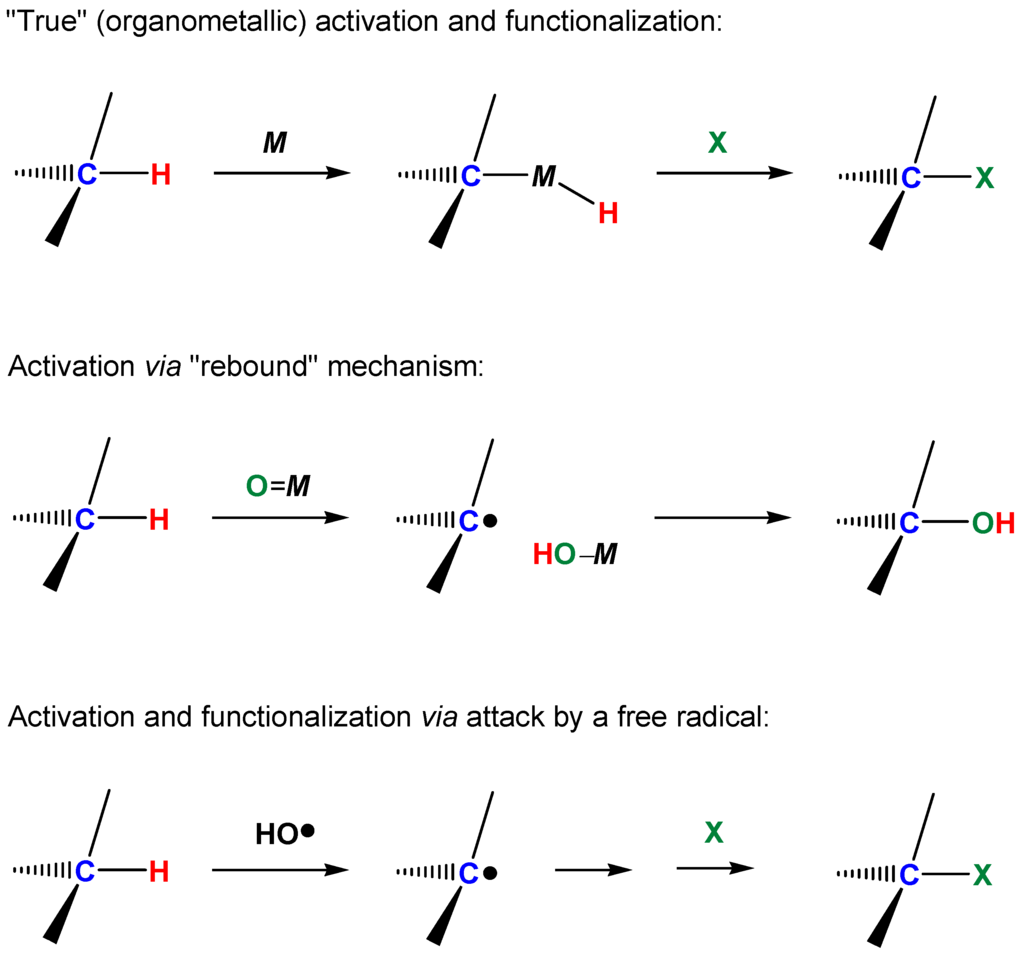
Scheme 2.
Three types of metal-mediated C–H functionalization.
In living organisms under the action of certain metal-containing enzymes, the carbon–hydrogen oxidations proceed as reactions of the second or third type [1]. Although these oxidations occur via the formation of reactive radicals, they are rather selective and produce what is necessary for microorganisms compounds and energy. Biodegradation of hydrocarbons requires also metal-containing enzymes.
The Shilov reaction [1] was the first example of the organometallic (first type) activation which allows us to oxidize alkanes in aqueous solutions under catalysis by platinum(II) complexes. Curiously, the mechanism of this first reaction is very complex and is not completely clear. It will be discussed in more detail later. Numerous publications have appeared since Shilov’s discovery which described unambiguous formation of σ-alkyl derivatives in alkane activation by metal complexes. In this review, however, we will discuss mainly the processes of oxygenation of carbon–hydrogen bonds to afford compounds with C‒O bonds. All hydrocarbon oxidations occurring in living organisms [1,20,21,22,23,24,25,26,27,28,29] from the mechanistic point of view are also of the second or third types. Some non-organometallic complexes, for example, metal derivatives of porphyrins can play the role of models of certain metal-containing enzyme centers. In this review, we will consider recent publications devoted to metal-catalyzed liquid-phase reactions of carbon–hydrogen compounds. Many reviews on some aspects of carbon–hydrogen functionalization have been published in recent years [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
2. Main Characteristics of Catalysts and Catalyzed Reactions
2.1. Definitions
A reaction can be characterized [53] by the yield (%) (Equation (1)):
(amount of a product/amount of either starting substrate or reagent) × 100
Important parameters [54,55] characterizing the catalyst are turnover number (TON) (Equation (2))
and turnover frequency (TOF) (Equation (3))
TON = moles of product/moles of catalyst
TOF = TON/time.
In functionalization of inert hydrocarbons, these substrate compete with a solvent. In these cases, usually acetic and trifluoroacetic acid [56], acetonitrile [57], and water [58,59] are employed. Alcohols are more easily oxidizable compounds in comparison with alkanes. It is noteworthy, however, that, in the aerobic oxidation of alkanes catalyzed by FeCl3 under visible light irradiation, alcohols can be used as solvents [60]. In the initial stage, photohomolysis of the FeIII–Cl bonds leads to the generation of reactive Cl• radicals [61] which attack both the alkane and the alcohols used as a solvent with comparable rates. This leads to the formation of oxygenation products from the alkanes in quite high yield.
Ionic liquids can be presented as examples [63,64,65,66].“To strengthen the relationship between catalysis and green chemistry, methods making use of neoteric solvents or alternative media rather than hazardous solvents, are more and more considered and represent unique challenges and opportunities for the future of catalysis”.[62]
In recent decades, traditional methods of stimulating catalyzed reactions (heating) new methods were added: photochemical initiation [67,68,69,70,71,72], microwave heating [73], electrocatalytic [74], and sonochemical [75] transformations.
2.2. Selectivity
Typically alkanes are transformed into derivatives which are more reactive in comparison with parent hydrocarbons, and this makes the selectivity one of the most important problems in functionalizations of saturated hydrocarbons as well as aromatic and other carbon–hydrogen compounds.
We will begin our discussion here from the term “substrate selectivity.” It is especially important to discuss the substrate selectivity in the oxidations in living systems because the cell contains myriad of various compounds that might be potential substrates.
The term “product selectivity” is a concept more important for the reactions made in vitro. Indeed, the same interaction of a few reactants can lead depending on conditions to different distribution of products. By optimizing the conditions, a chemist can obtain predominantly one desirable product (isomer).
Usually, reactions give rise to the formation of a few possible positional isomers, such as monosubstituted benzenes. Electrophilic substitution in monosubstituted benzenes C6H5X gives comparable amounts of the three isomers. The distribution of isomers is ortho ≈ para > meta if X is an electron-releasing group and meta > ortho ≈ para if X is an electron-withdrawing group. In reactions of homolytic arylation, both withdrawing and releasing groups X direct the substituent mainly into ortho- and para-positions. Normal alkanes contain carbon–hydrogen bonds of two types: primary carbon–hydrogen bonds of two terminal methyl groups and secondary carbon–hydrogen bonds of internal methylene groups. Reactivities of methylene carbon–hydrogen bonds relative reactivity of the methyl group often depend on the nature of an oxidizing species and thus can give valuable information on the mechanism of the oxidation. Parameter C(1):C(2):C(3):C(4) is relative normalized (calculated taking into account the number of hydrogen atoms at each carbon) reactivities of hydrogen atoms at carbons 1, 2, 3, and 4 of the chain of normal alkane. This parameter is often discussed in mechanistic studies. Substitution of hydrogen atoms in normal alkanes with the participation of very reactive radicals like Cl• or HO• proceeds non-selectively, which is reflected by low selectivity parameter: C(1):C(2):C(3):C(4) ≈ 1:5:5:5. The stochiometric uncatalyzed oxygenations of normal alkanes with peracids occur without the participation of free radicals and exhibit high selectivity; more inert methyl groups are not oxidized at all. Some catalytic systems direct the substituent predominantly to only one position (region). We say that such reactions are “regioselective” or regiospecific. Regioselectivity is the preferential formation of one constitutional isomer over another (constitutional isomers of the same molecular formula differ in how their atoms are connected).
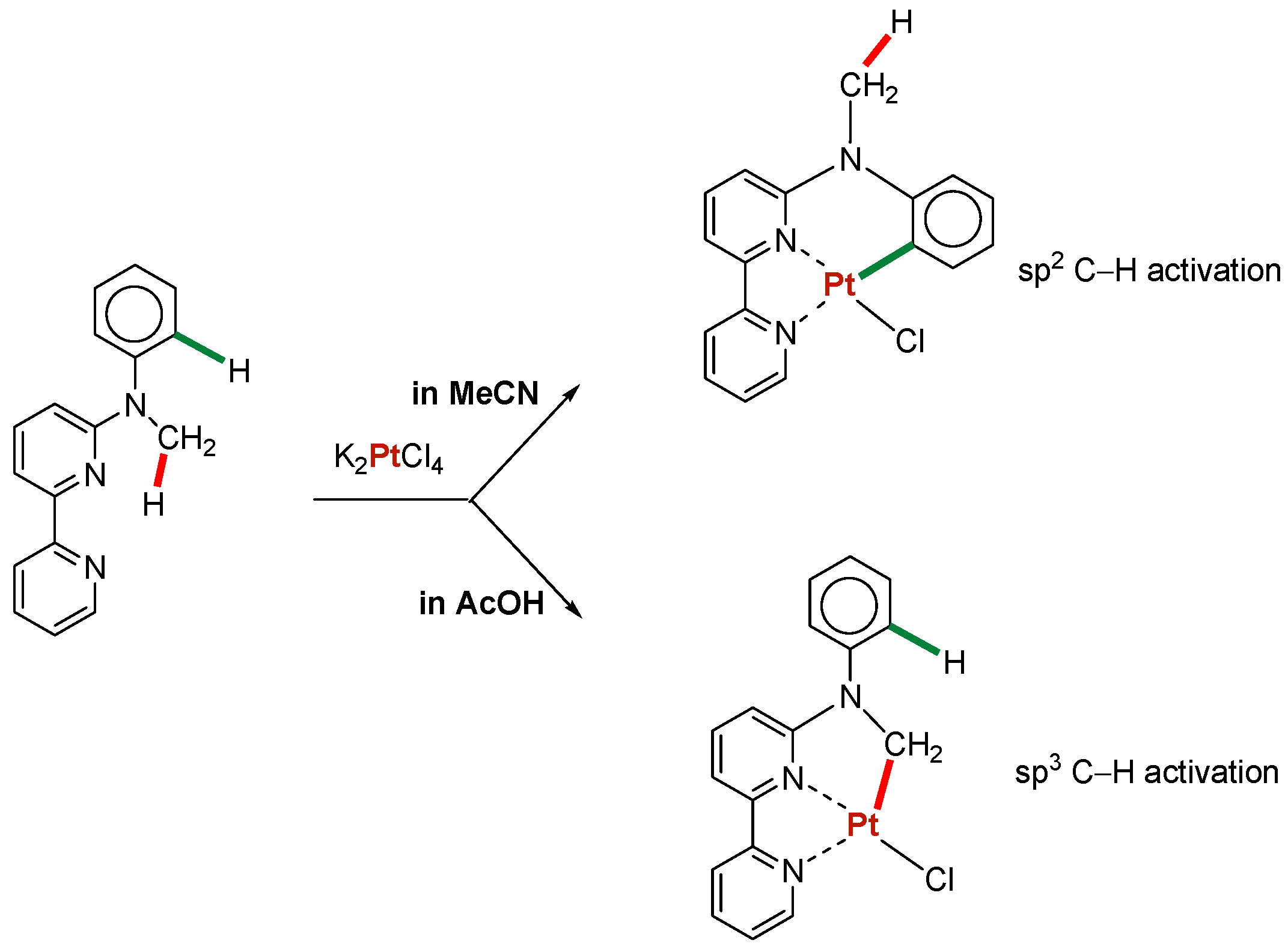
In reactions of branched alkanes, the bond selectivity parameter 1°:2°:3° is widely used. This value is relative normalized reactivities of hydrogen atoms at primary, secondary, and tertiary carbons of a branched alkane. Reactions that involve free radicals are usually less selective compared to reactions that proceed via concerted non-radical mechanism. In some reactions, the nature of a solvent can influence dramatically changing preferable carbon–hydrogen bonds in the same molecule [76] Scheme 3:
Scheme 3.
Activation of aryl or methyl C–H groups by the Pt(II) complex.
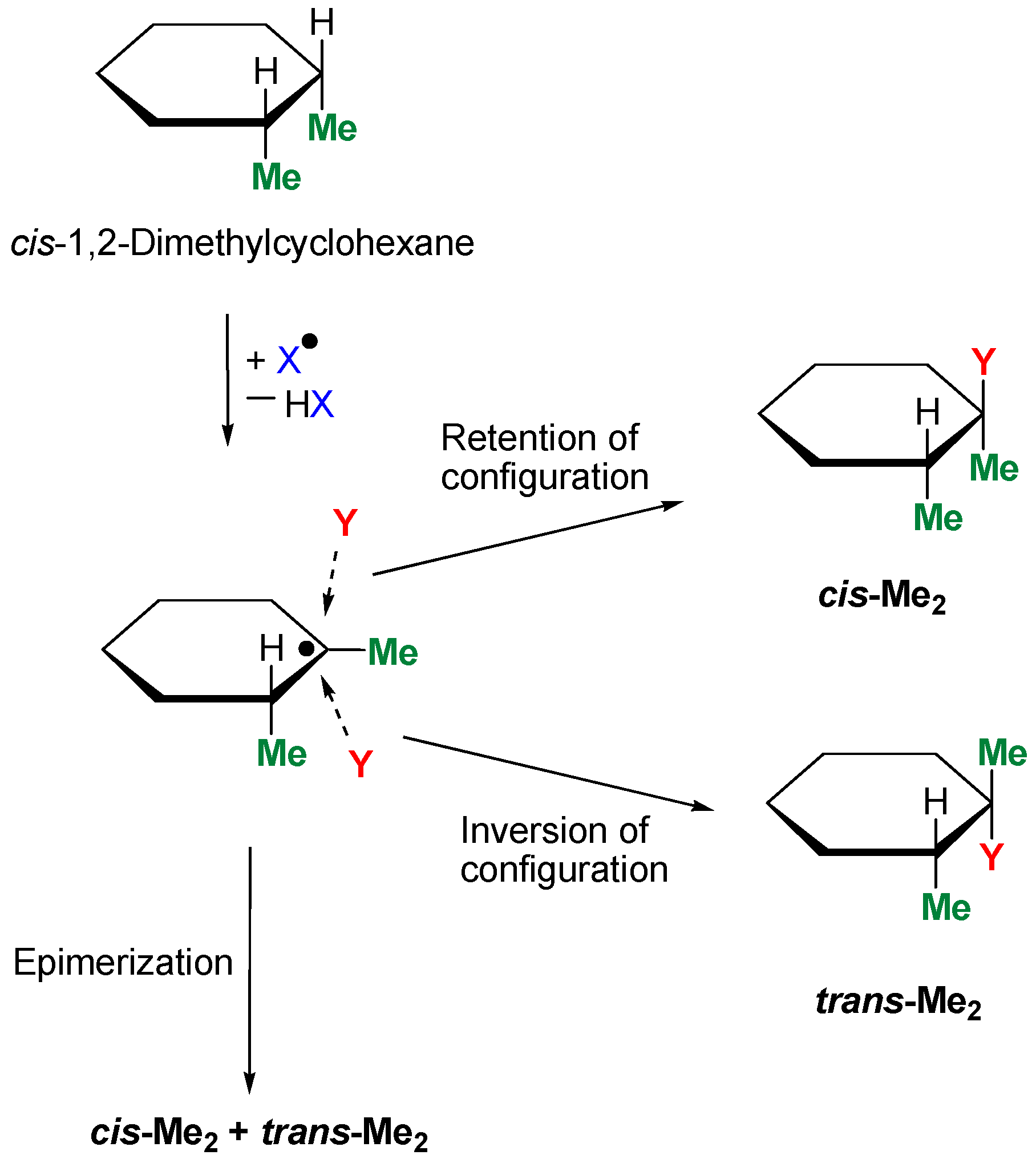
An investigation of “stereoselectivity” helps to understand the mechanism of the interaction between the active species and carbon–hydrogen bonds. The transformation of a test compound cis-1,2-dimethylcyclohexane with or without retention of configuration is shown in Scheme 4.
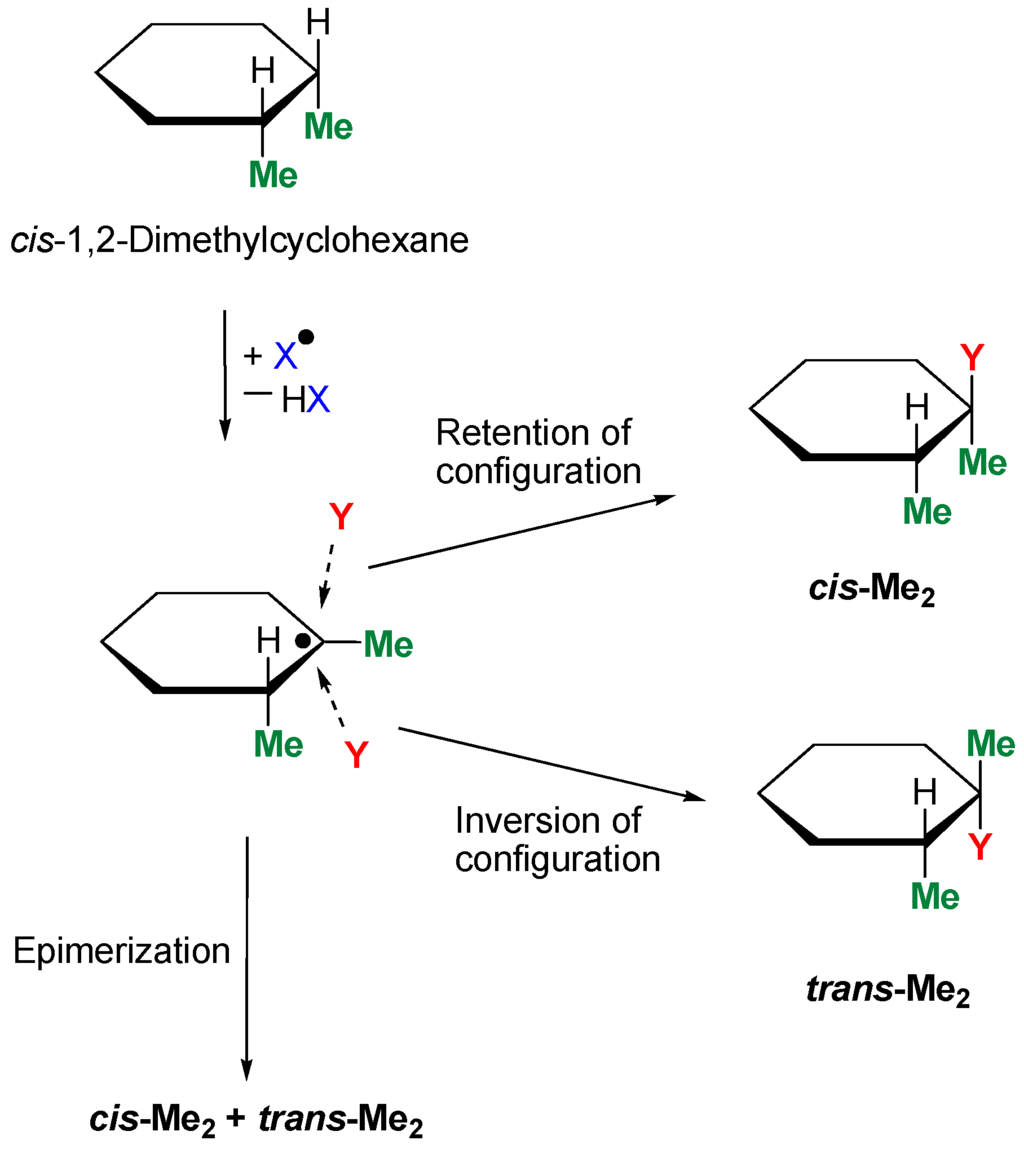
Scheme 4.
Activation of cis-1,2-dimethylcyclohexane.
If two methyl groups in the product (in the case Y = OH) are in cis-orientation, we say that the reaction proceeds with retention of configuration. In the second case, if methyls are in mutual trans-orientation, we say about inversion of configuration. Usually, both reactions proceed with comparable rates and give cis-Me2 and trans-Me2 isomers in approximately equal amounts (epimerization). If the reaction proceeds without the participation of reactive radicals but via a concerted mechanism (as in the cases of peracids), the products are generated with partial or complete retention of configuration.
2.3. Effect of Additives: Pyrazine-2-Carboxylic Acid (PCA)
It is known that the addition of some compounds can dramatically change the reaction rate (increasing or decreasing it) and also affect the selectivity of the reaction. Thus, pyridine present in the solution of Gif systems induces the transformation of initially formed alkyl hydroperoxide into the corresponding ketone (“ketonization” of the alkane occurs). Light irradiation of the emulsion of cyclohexane with an aqueous solution of Fe(ClO4)3 in air exclusively gives cyclohexanone [77]. One can propose that water, like pyridine, dehydrates the cyclohexyl hydroperoxide. This is supported by finding that irradiation of the solution of cyclohexyl hydroperoxide and Fe(ClO4)3 in water selectively gave cyclohexanone.
Some time ago, we found [78,79,80] that pyrazine-2-carboxylic acid (PCA) and analogous compounds (2,3-pyrazinedicarboxylic, picolinic, and dipicolinic acids) can be used as highly active co-catalysts in the vanadate-catalyzed oxidations of alkanes, arenes, alcohols, and other substrates, under mild conditions by peroxides in air. The [VO3]‒/PCA/H2O2 system has been studied in detail [81,82,83,84,85,86,87,88,89,90,91,92]. We then continued studies of alkane oxidations in the presence of PCA under catalysis by complexes of some other metals: iron [93,94,95,96], manganese [97,98], and rhenium [99].
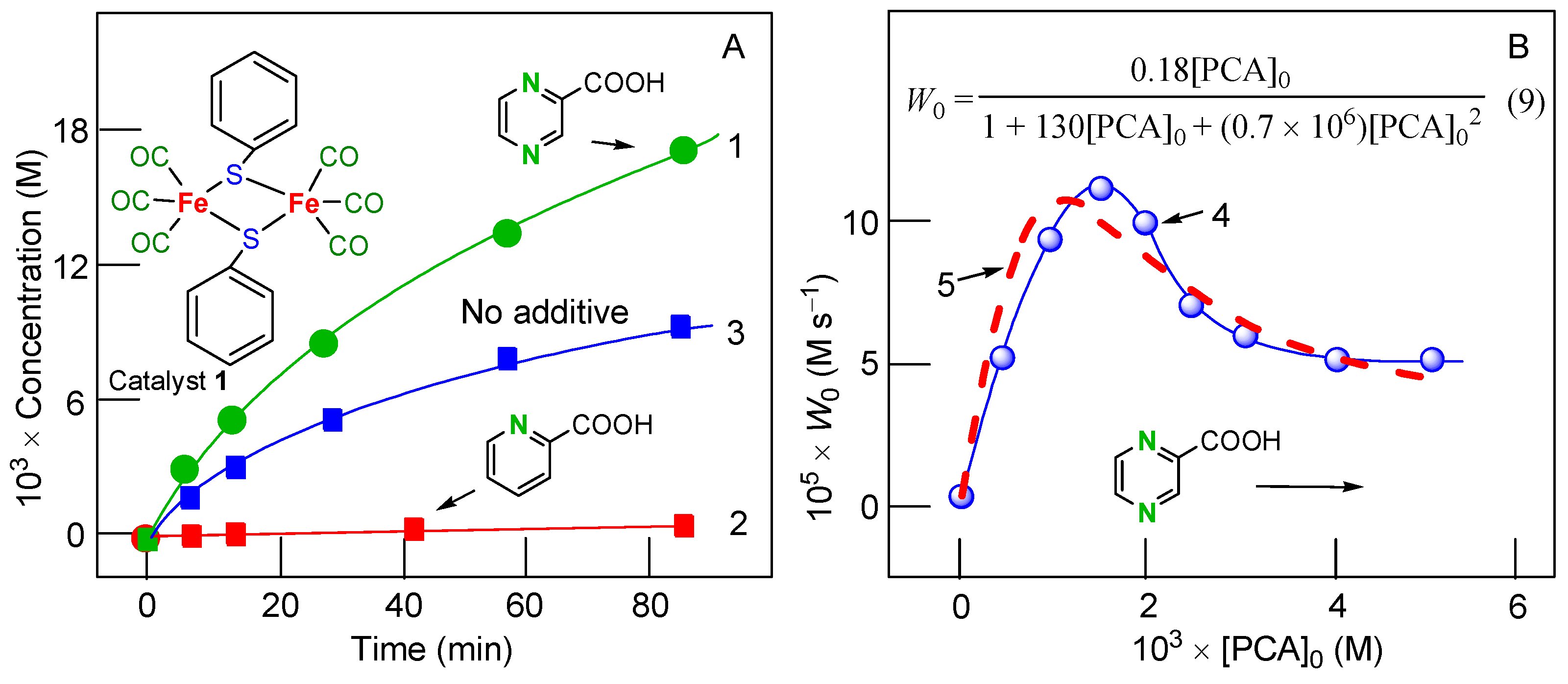
The complex (OC)3Fe(μ-PhS)2Fe(CO)3 (compound 1; see Figure 1) catalyzes the oxygenation of alkanes and benzene with hydrogen peroxide in air in an acetonitrile solution [100]. Alkyl hydroperoxides are formed as the main reaction products in the alkane oxidation (turnover numbers of 2300 were attained); benzene is transformed into phenol. The addition of PCA improves the oxidation. The duration of the lag period in the oxygenate accumulation can be substantially reduced if pyridine is added. Some dependencies are shown in Figure 1. Interestingly, the addition of picolinic acid (PIC) instead of PCA leads to the complete oxidation inhibition (Figure 1A).
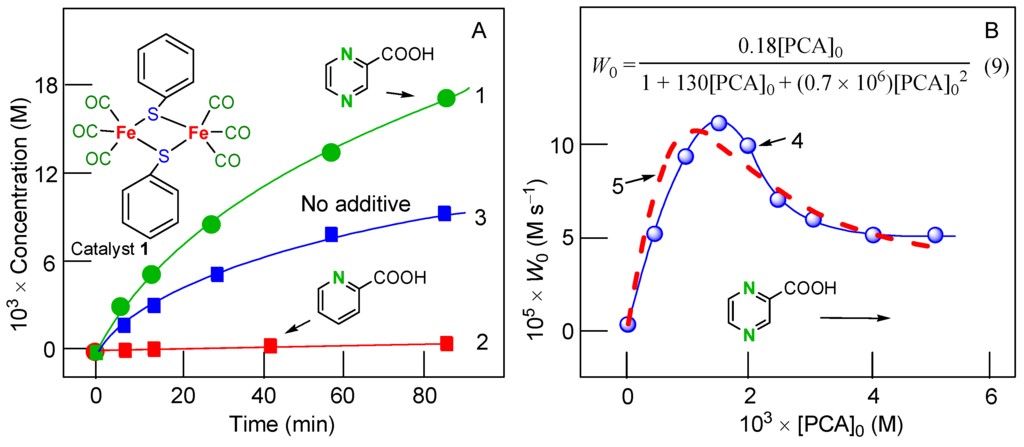
Figure 1.
Graph (A). Accumulation of oxygenates (cyclohexyl hydroperoxide + cyclohexanol + cyclohexanone) with time in the cyclohexane oxidation with H2O2 catalyzed by compound 1 in acetonitrile in the presence of two different co-catalysts: PCA (3.0 × 10−3 M; curve 1) and PIC (3.0 × 10−3 M; curve 2) or in the absence of additives (curve 3). Conditions: [1]0 = 2.0 × 10−4 M, [pyridine]0 = 0.2 M, [H2O2]0 = 0.64 M, [cyclohexane]0 = 0.37 M, 50 °C. Graph (B). Curve 4: dependence of the initial rate W0 of oxygenate accumulation in the cyclohexane oxidation with H2O2 catalyzed by 1 in acetonitrile on the initial concentrations of PCA. Curve 5: simulated dependence of the initial rate W0 on [PCA]0 corresponding to Equation (6). Conditions: [1]0 = 2.0 × 10−4 M, [pyridine]0 = 0.1 M, [H2O2]0 = 0.64 M, [cyclohexane]0 = 0.37 M, [H2O]total = 1.21 M, 50 °C. Adapted from Ref. [100].
The dependence of the initial oxidation rate W0 on the initial concentration of added co-catalyst PCA in the presence of pyridine is presented by curve 1 in Figure 1B. For the kinetic Equations (4)–(7).
where X is a species active in the cyclohexane oxidation, we have Equation (8)
It is easy to obtain Equation (9) shown in Figure 1B for conditions of the discussed experiments.
More recently, further research of other chemists has resulted in new interesting catalytic systems based on the combination of PCA. For example, Pombeiro and colleagues described catalytic oxidations with complexes of iron, copper, vanadium as well as gold nanoparticles [101,102,103,104] (see also reviews [105,106]).
3. New Catalysts and Ligands
New types of catalysts for efficient oxidation of carbon–hydrogen compounds have been reported in recent years: heteropolycompounds [107,108,109,110], supported complex catalysts [111,112], and porous materials [113,114,115,116,117,118,119,120,121,122].
3.1. Metal Ions Most Active in Oxidation Catalysis
In the early stages of development of homogeneous carbon–hydrogen oxidations only a few transition metals were more or less carefully studied as catalysts [1]. These ions were iron, copper, manganese, etc. During the past several decades, many metals have been studied in catalytic activation and functionalization of carbon–hydrogen bonds. The reader is directed to selected recent examples with gold [123], cobalt [124,125,126] chromium [127], copper [128,129,130,131] iron [132,133,134,135], iridium [136], manganese [137,138,139], molybdenum [140], nickel [141], osmium [142,143,144,145,146,147,148], palladium [149,150,151], rhenium [152], rhodium [153,154], ruthenium [155,156], and vanadium [157,158].
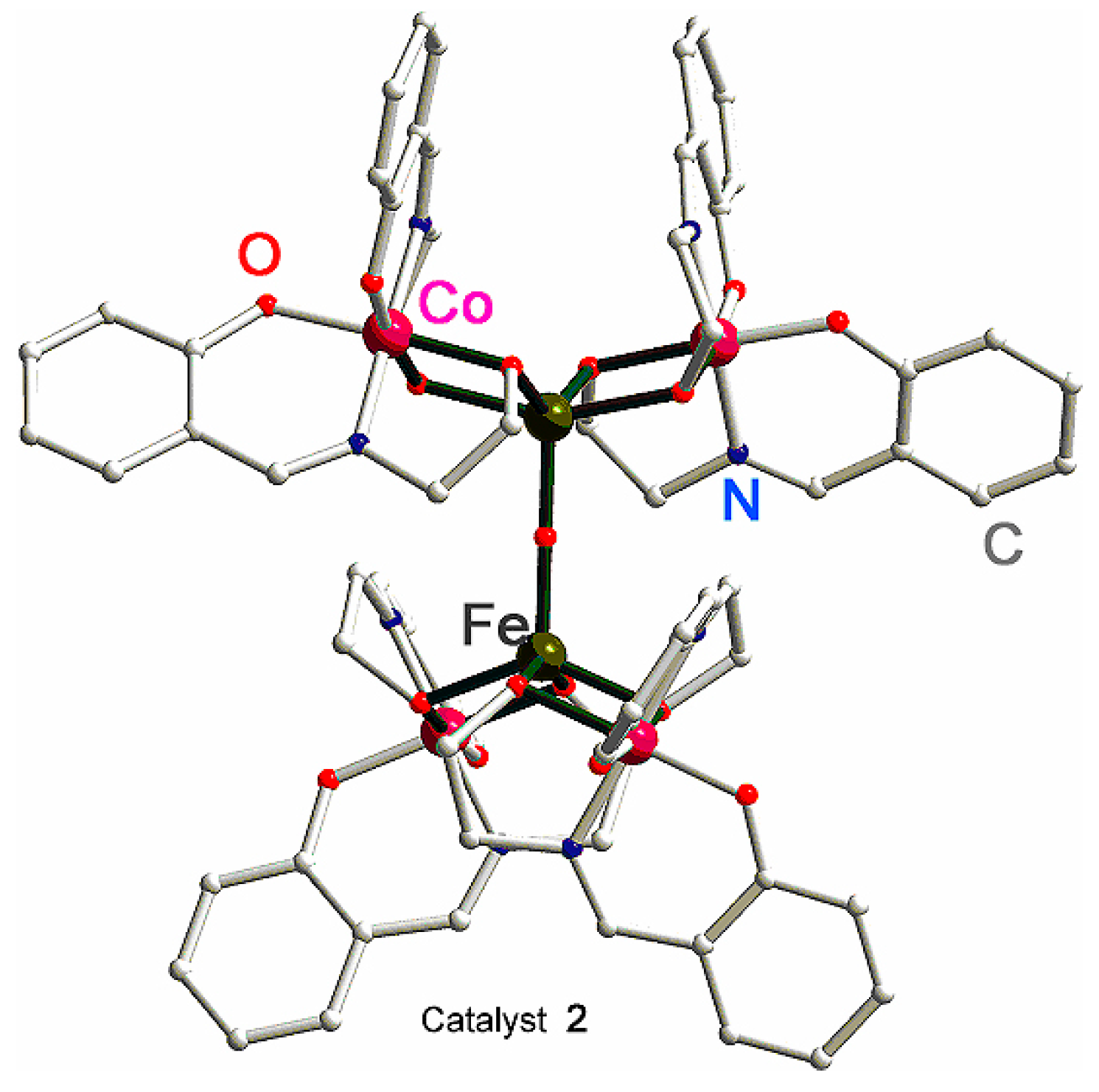
A new method of synthesis of heterometallic coordination compounds by spontaneous self-assembly (SSA) [159] led recently to complexes which turned out to be very efficient catalysts in oxidations with peroxides. For example, Nesterov et al. prepared the heterobimetallic complex with a Schiff-base ligand [Co4Fe2OSae8] (compound 2; where Sae = L4 and H2Sae is salicylidene-2-ethanolamine (Figure 2)) [160]. In oxidation of cyclohexane into cyclohexyl hydroperoxide under the action of H2O2, this compounds exhibits a very high TON of 3570, which is the highest value ever observed for an iron-containing catalyst. It has been indicated [160] via mass spectral and comprehensive kinetic studies that hexanuclear 2 dissociates when dissolved in CH3CN to form a trinuclear particle [Co2Fe(L4)4]+. This particle, observed in concentrated (1 × 10−4 M) solutions of 2, is the catalytically active species responsible for the fast reaction rates greater than 3 × 104 M·s−1 (maximum turnover frequency TOF = 11,200 h−1 = 3.1 s−1). The catalytic system is believed to proceed by free-radical pathways with the hydroxyl radical as the main attacking species.
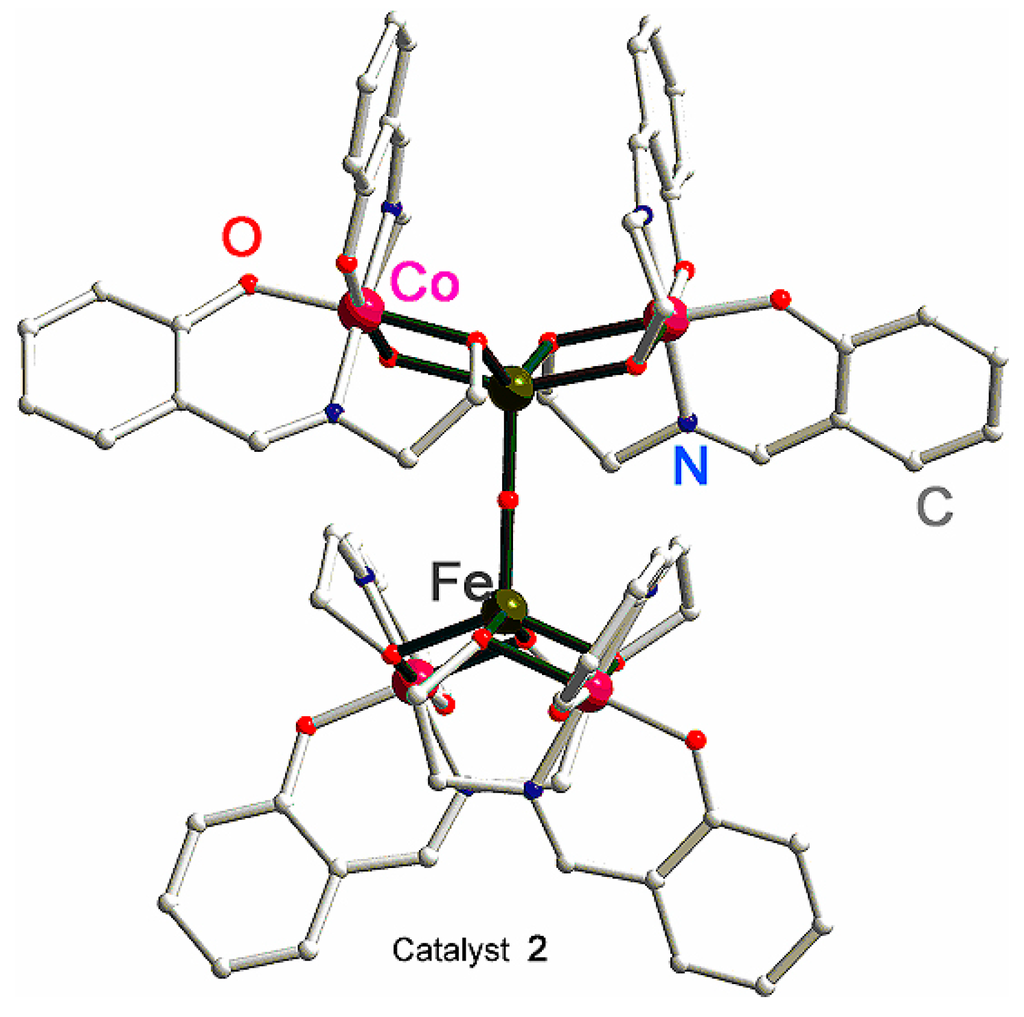
Figure 2.
Molecular structure of [CoIII4FeIII2O(L4)8]·4DMF·H2O (complex 2). Solvated DMF and water molecules have been omitted for clarity. Adapted from Ref. [160].
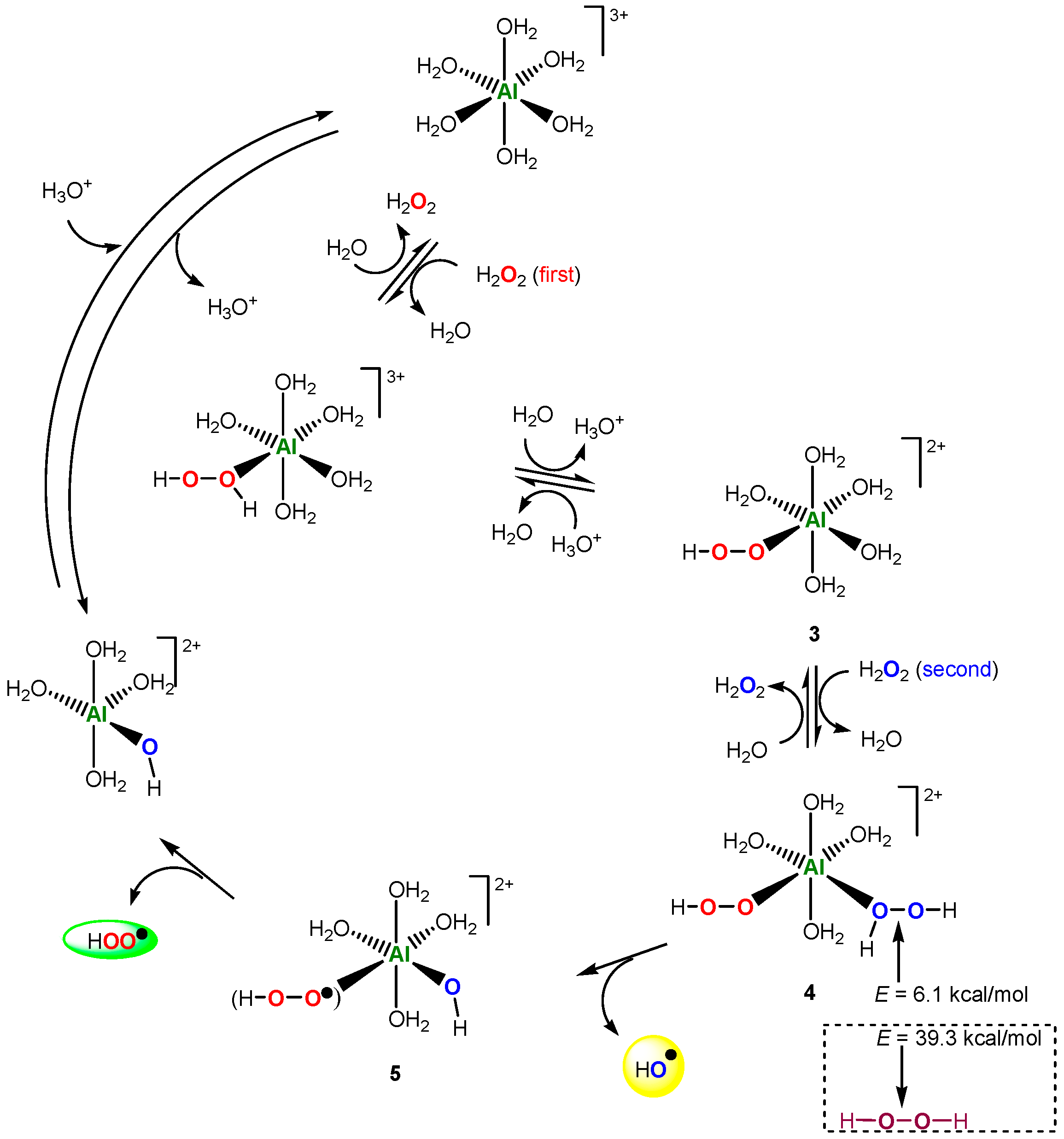
A few years ago, we discovered that the salt of non-transition metal aluminum catalyzes the alkane hydroperoxidation with hydrogen peroxide [161]. It has been proposed that interacting with an aluminum derivative hydrogen peroxide is decomposed to afford hydroxyl radicals that attack the alkane molecule. This reaction is by no means a Fenton-like process because, in a Fenton reaction with Fe(II) or a Fenton-like reaction (for example with FeIII), a transition metal can change its oxidation state (FeII ⇌ FeIII) in the course of the hydrogen peroxide decomposition. Aluminum cation is unable to change its valency. To explain the catalytic action of aluminum cation, our co-authors Kozlov and Kuznetsov [162] assumed that, in the case of AlIII, not one but simultaneously two H2O2 molecules interact (entering into cation’s coordination sphere). The first H2O2 molecule—as a model of the electron-rich FeII ion—delivers one electron (like in the Fenton mechanism) to the system, and this leads to the splitting the O–O bond in the second H2O2 molecule. Figure 3 presents a catalytic cycle generating two free radicals, HO• and HOO•. According to DFT calculations, H2O2 molecule in complex 4 is highly activated toward the homolysis in the free hydrogen peroxide (6.1 vs. 39.4 kcal/mol). This approach has been used for some other non-transition metals [163,164,165,166,167]. The overall activation barriers (kcal/mol) of the generation of hydroxyl radicals were calculated: Ga (19.5) < Sc (22.2) < In (23.5) ≈ Y (23.7) < La (25.2) ≈ Al (25.6).
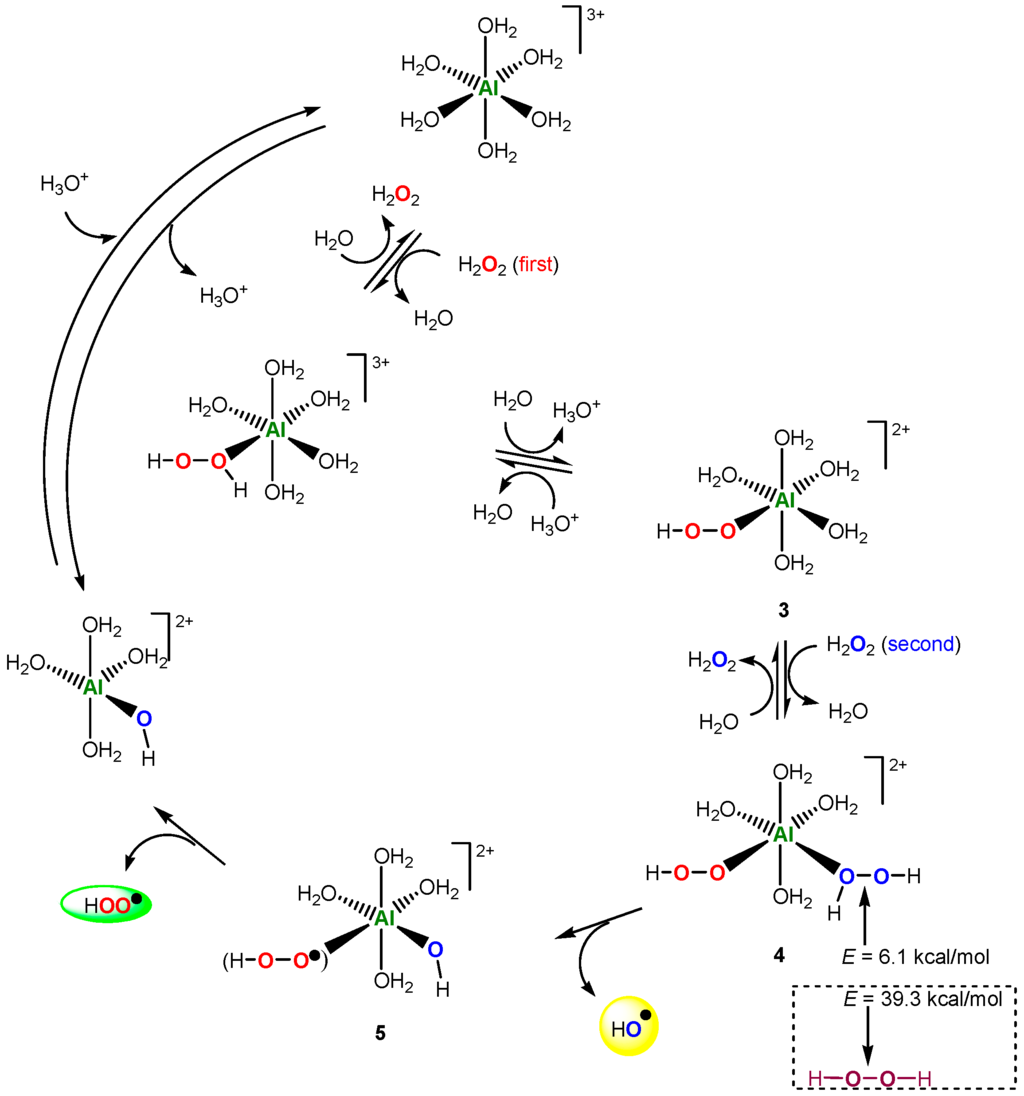
Figure 3.
The Al-catalyzed decomposition of hydrogen peroxide. Adapted from Ref. [162].
Very recently, Meyerstein and colleagues used our approach involving two H2O2 molecules in DFT calculations of Fenton-like reactions with the participation of transition metals [168]. It was also shown that the Fenton-like reaction of Co(H2O)62+ proceeds via the binding of three H2O2 molecules to the cobalt(II) ion forming the transient (H2O)3CoII(OOH)2(H2O2) [169]. Harvey studied the mechanism of the Fenton reaction via computational methods [170].
3.2. New Types of Ligands and Catalysts
In this section, we will briefly consider work that has been published in recent years describing new types of ligands and catalysts. Some examples have been discussed in previous sections (for example, see Figure 3).
Heme and nonheme oxidation catalysts have been intensively used in oxidations of alkanes aromatics, as well as in epoxidations [171,172,173,174]. Metalloporphyrins [175,176,177,178] play a very important role in such studies; they are models of enzyme reaction centers.
Sorokin and colleagues used phthalocyanines as catalysts in oxidations with peroxides [179,180]. Thus, mild oxidation of ethane to acetic acid by H2O2 was catalyzed by supported mu-nitrido diiron phthalocyanes [181], methane was oxidized to methanol, formaldehyde, and formic acid [182]. Maksimov, Karakhanov, and colleagues used analogous catalysts containing ions of iron and copper [183].
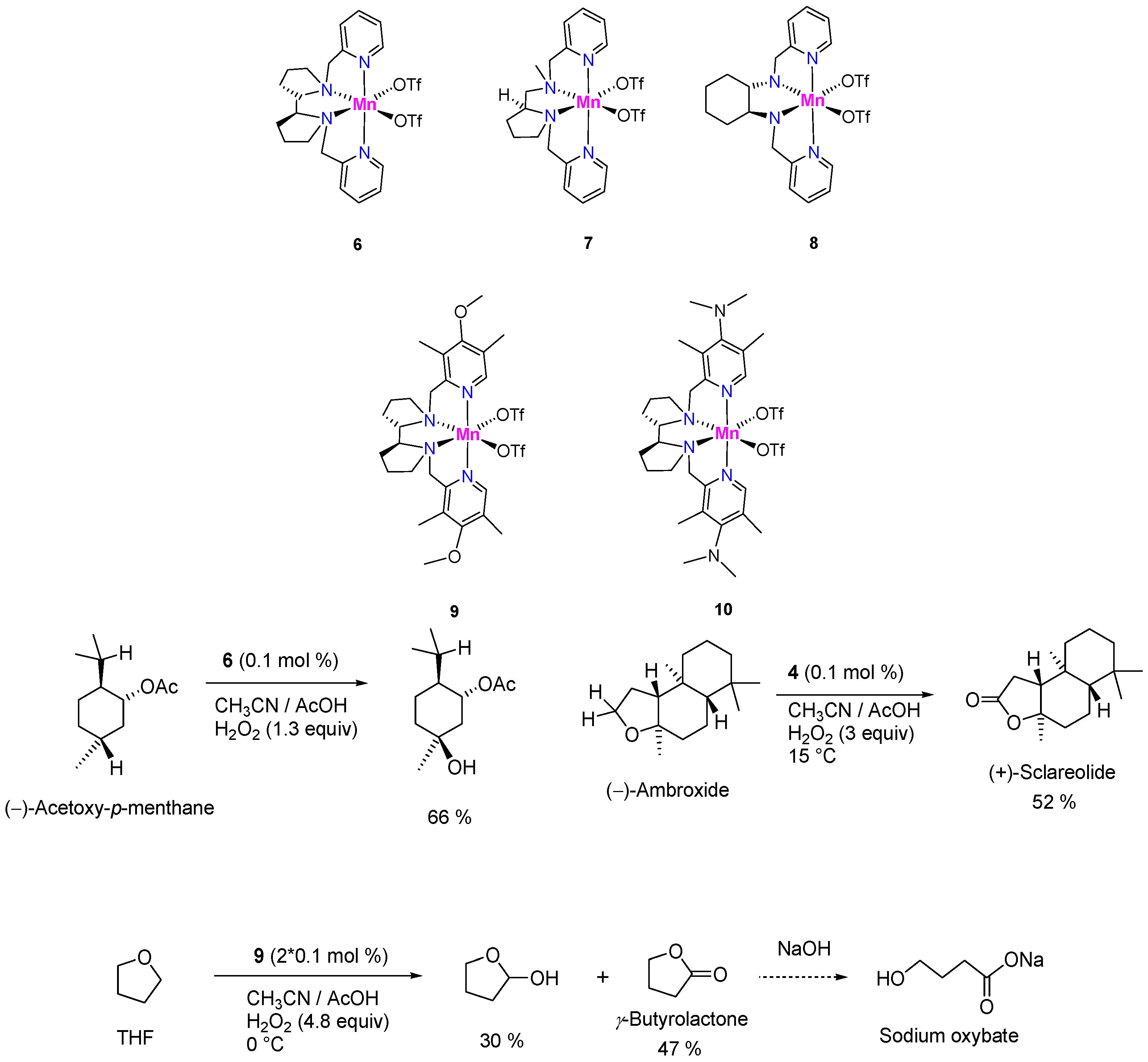
Talsi, Bryliakov, and colleagues published a series of papers on the C–H oxidations with H2O2 in the presence of aminopyridine complexes of manganese(II) of the types 6–10. It was shown that complexes 6–8 efficiently (with TON up to 970) induce the oxidation of substrates containing aliphatic carbon–hydrogen groups, such as cyclohexane, adamantane, and (‒)-acetoxy-p-menthane (Figure 4), with high regioselectivity (preferential oxidation of tertiary C–H bonds) [184]. Along with a few more catalysts, Mn-aminopyridines have been regarded as demonstrating good promise for practical use [185]. The introduction of electron-donating groups (complexes 9 and 10) improved the oxidation selectivity; furthermore, catalysts 9 and 10 surprisingly demonstrated a much better efficiency in the benzylic oxidation of cumenes and ethylbenzene compared to the parent catalyst 6 [186]. All Mn-aminopyridine catalysts were active in acetonitrile solutions with the addition of co-catalytic additive (acetic acid), and typically required only 0.1 mol. % catalyst loadings and 1.3 equiv. of H2O2 (vs. substrate). Some examples of preparative oxidations on catalysts of this type are presented in Figure 4.
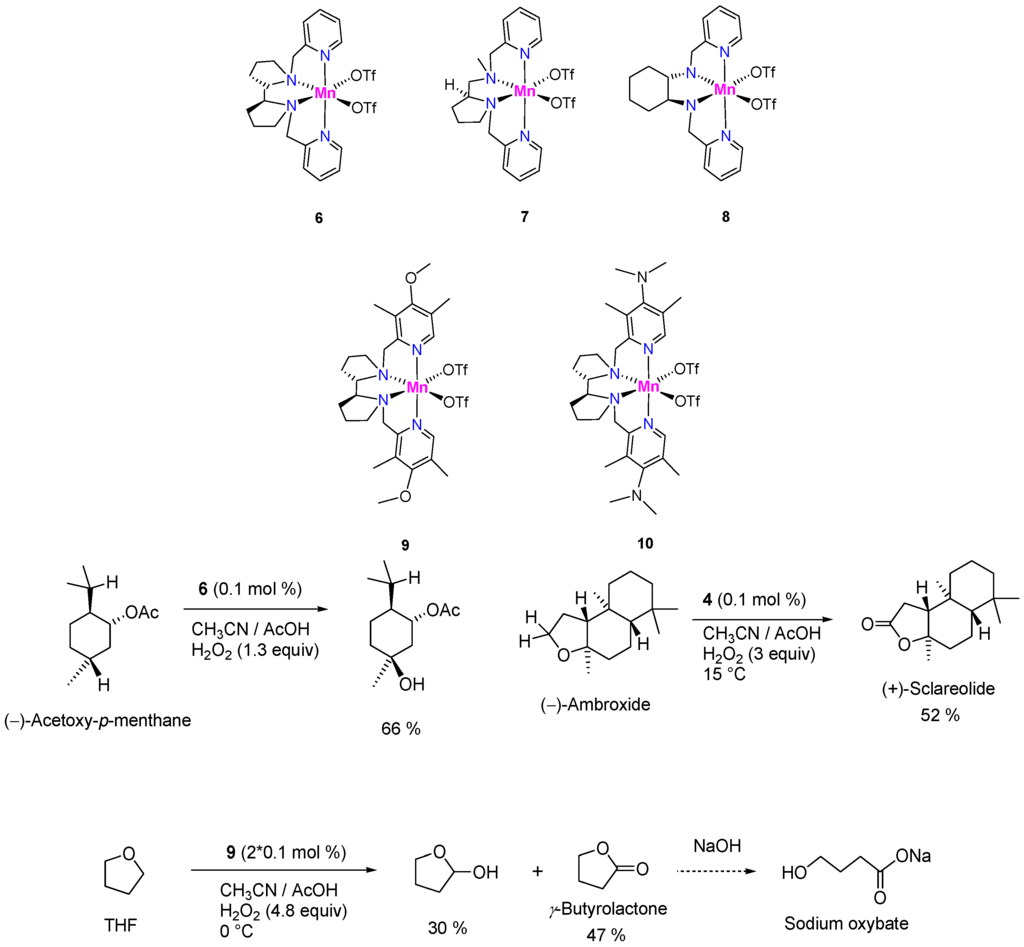
Figure 4.
Examples of preparative-scale selective C–H oxidations catalyzed by Mn-aminopyridine complexes.
The mechanism of carbon–hydrogen bond oxidation in the presence of Mn-aminopyridine complexes was considered. The authors confirmed the electrophilic nature of the active oxidizing species. The latter demonstrated high 3°/2° ratios (40–49), alcohol/ketone ratios > 5, and high stereospecificity in the oxidation of cis-1,2-dimethylcyclohexane [187]; kinetic studies revealed a sizable kinetic isotopic effect (kH/kD 4.5–4.7), indicating that that the H atom lies on the reaction coordinate and participates in the C–H bond breaking step. Altogether, the experimental data provided indirect evidence in favor of the participation of a high-valent (presumably oxometal(V) [188]) species, ruling out the contribution of a free-radical-driven oxidation pathway. The key findings pointing to the oxomanganese(V) active species were (i) the incorporation of 18O from added H218O into the oxidizing product, corroborating the “water-assisted” mechanism and (ii) partial inversion of cis configuration during the oxidation of cis-1,2-dimethylcyclohexane, apparently proceeding via formation of relatively short-lived carbon-centered tertiary radicals typical of the classic “rebound mechanism,” postulated for the oxoferryl-driven cytochrome P450 reaction cycle. So far, no direct spectroscopic evidence for the oxomanganese(V) reactive species in Mn-aminopyridine-based systems has been reported. However, in catalyst systems relying on isostructural Fe catalysts, such evidence was gained by EPR spectroscopy taking advantage of the low-spin (S = 1/2) structure of known oxoiron(V) intermediates [189].
Costas in recent years has carried out many valuable works on functionalization of carbon–hydrogen bond catalyzed by nonheme complexes with polyamino ligands [190,191,192,193]. Additionally, very important works on complexes with polydentate ligands as catalysts have been reported by Nam and colleagues [194].
Complexes with ligands of new types have been recently synthesized, and in some cases these complexes turned out to be efficient catalysts for C–H functionalization. We have to mention here TpXM complexes which were used by Pérez and other authors for alkane functionalization by carbene insertion [195,196,197,198,199,200].
The nature of ligand and ligand robustness play a very important role in metal-complex catalysis [201]. New types of ligands have been proposed in recent decades which form efficient catalysts with transition metal ions. These ligands are: N-heterocyclic carbene (NHC) [202,203], pincer-like [204,205,206,207,208], redox-active ligands [209,210,211,212], and scorpionates [213,214,215,216,217,218].
3.3. Polynuclear Metal Complexes As Catalysts
Multi-metallic metal complexes often have higher catalytic activity in comparison with mononuclear compounds [219,220].
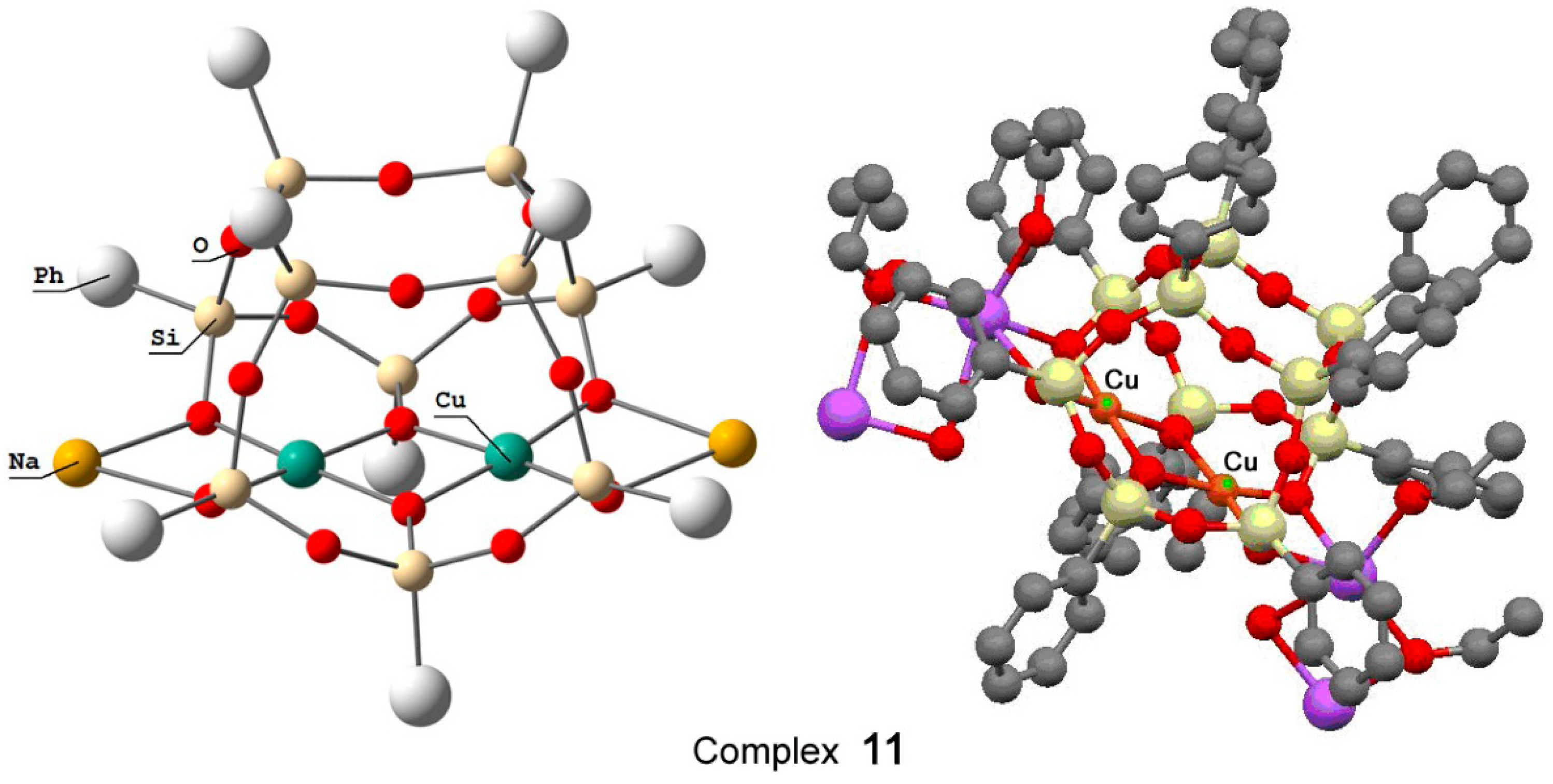
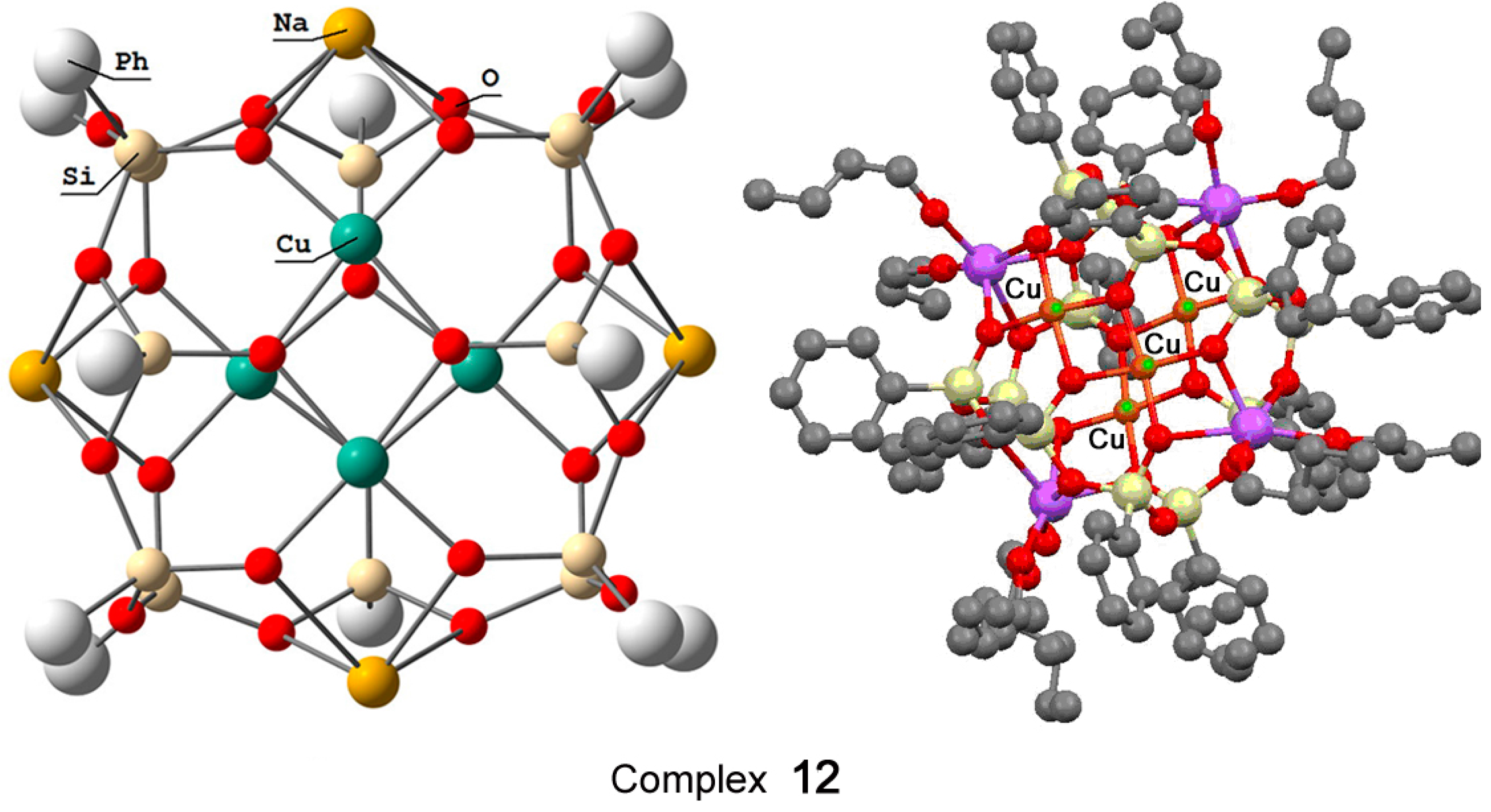
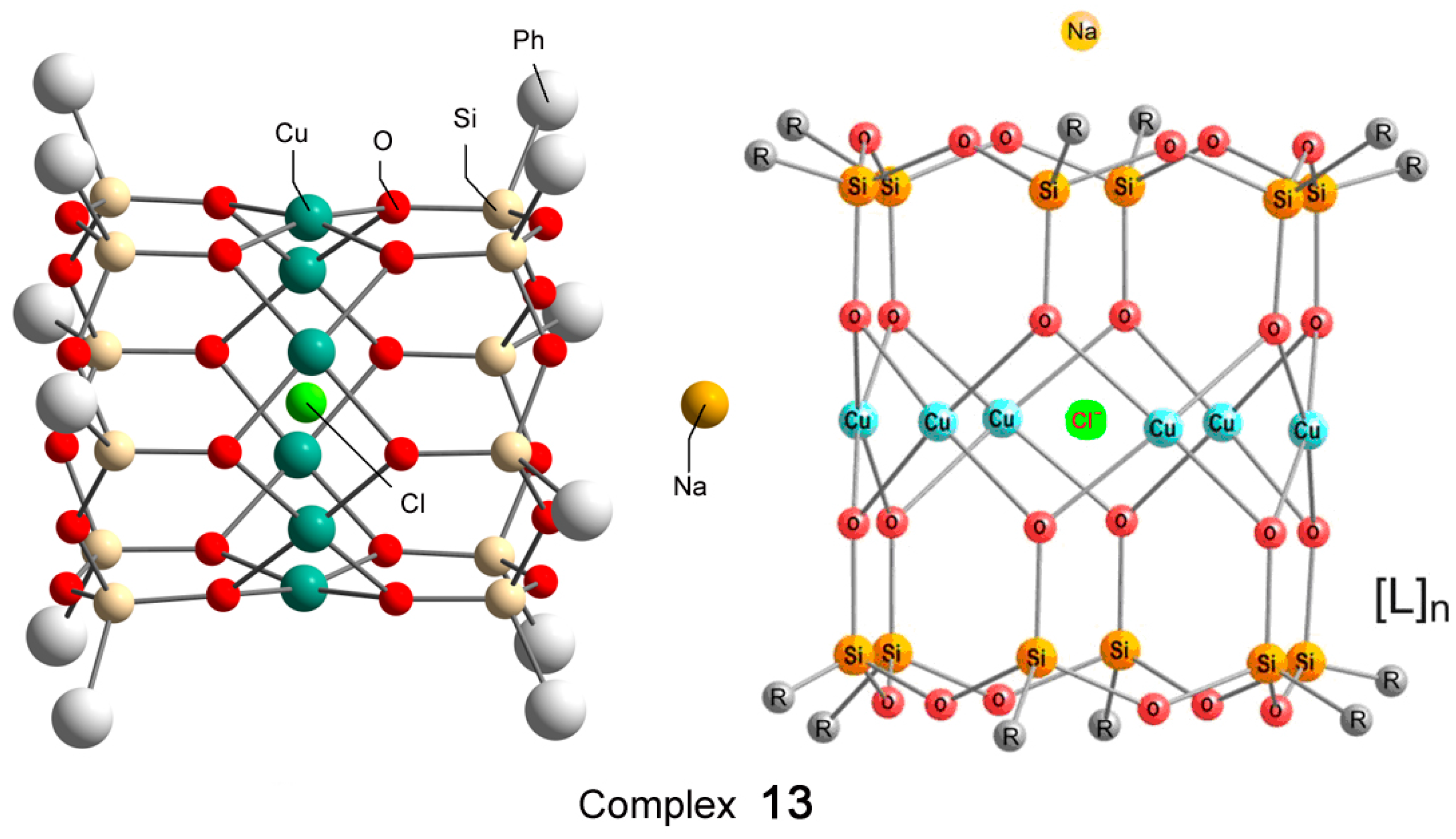
Recently, Bilyachenko et al. have proposed a new convenient route to metallasilsesquioxanes and synthesized a series of complexes containing various transition metal ions. The most simple compound is the binuclear cage-like copper silsesquioxane [(PhSiO1.5)10(CuO)2(NaO0.5)2 4EtOH] (complex 11) with a “Cooling Tower” structure (Figure 5) [221]. In addition, isomers containing four [222] and six [223] copper ions have been isolated. Their structures are presented in Figure 6 and Figure 7, respectively. Al these compounds exhibited high catalytic activity in oxidation of benzene, alkanes, and alcohols with peroxides [224].
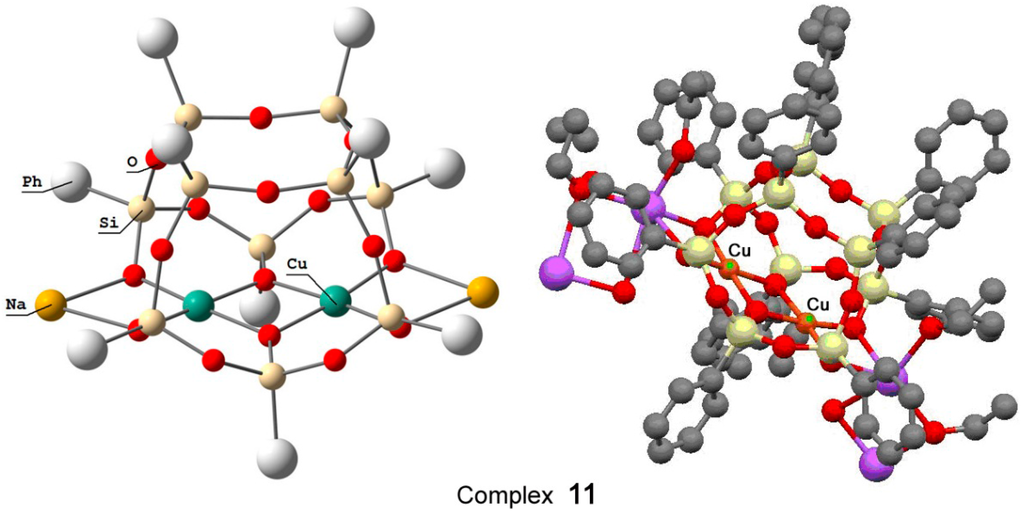
Figure 5.
The structure of compound [(PhSiO1.5)10(CuO)2(NaO0.5)2 4EtOH] (11) (left) and a fragment of polymeric structure (right). Adapted from Ref. [221].
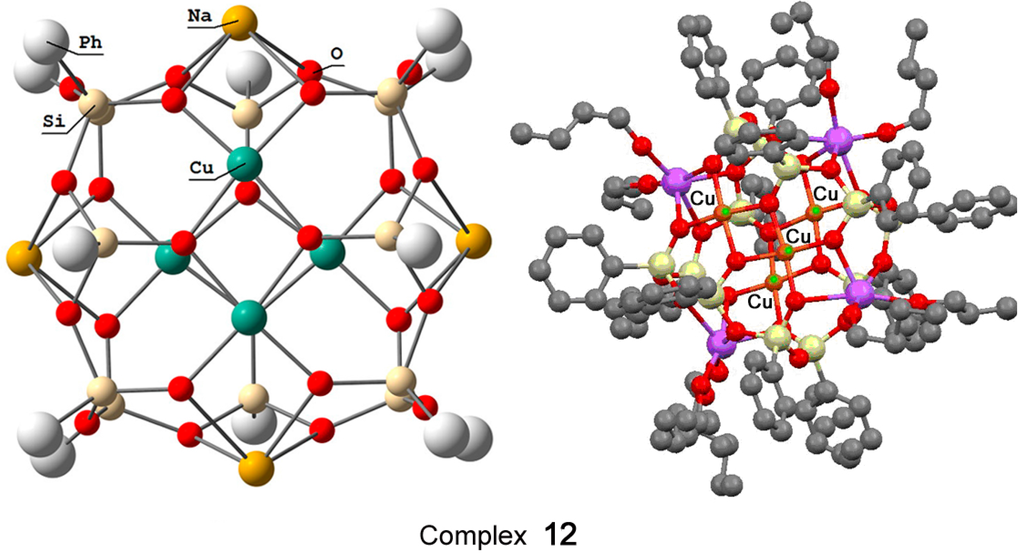
Figure 6.
The structure of “Globule”-like compound [(PhSiO1.5)12(CuO)4(NaO0.5)4] (12). Adapted from Ref. [222].
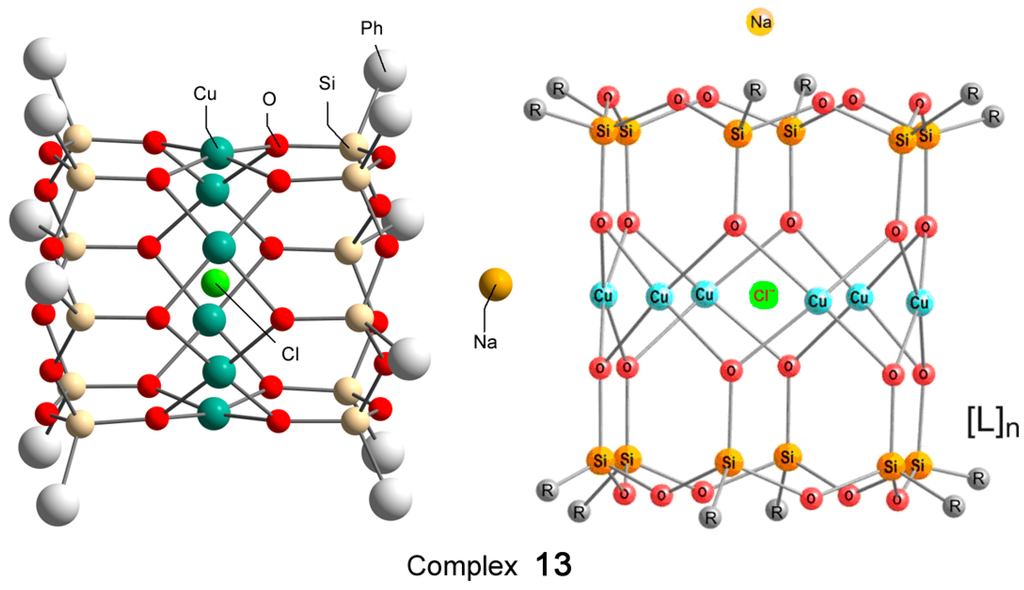
Figure 7.
The structure of hexanuclear cylinder-like copper silsesquioxane [(PhSiO1.5)12(CuO)6(NaCl)(C4H8O2)12(H2O)2] (13), L = 1,4-dioxane/H2O. Adapted from Ref. [223].
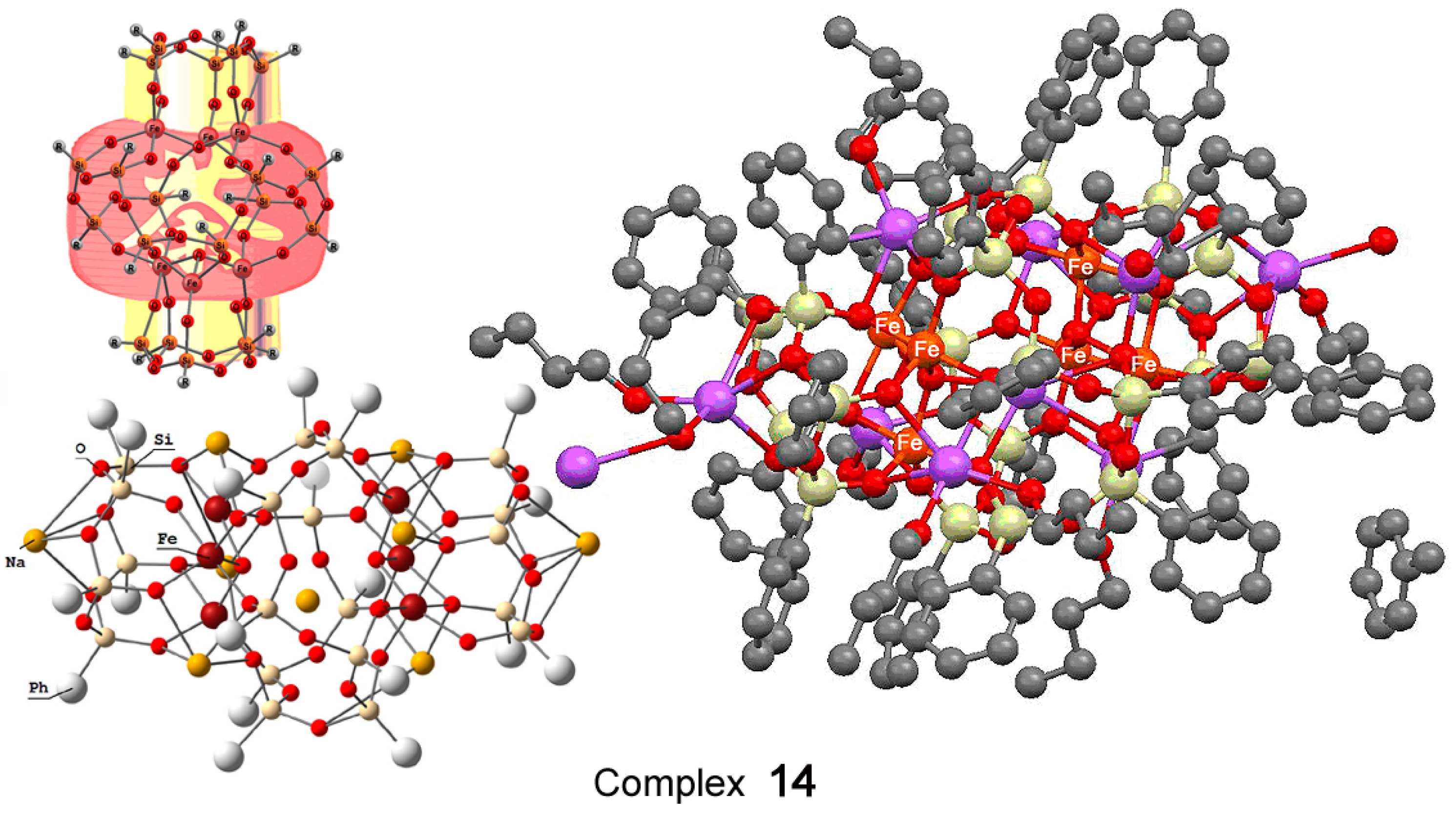
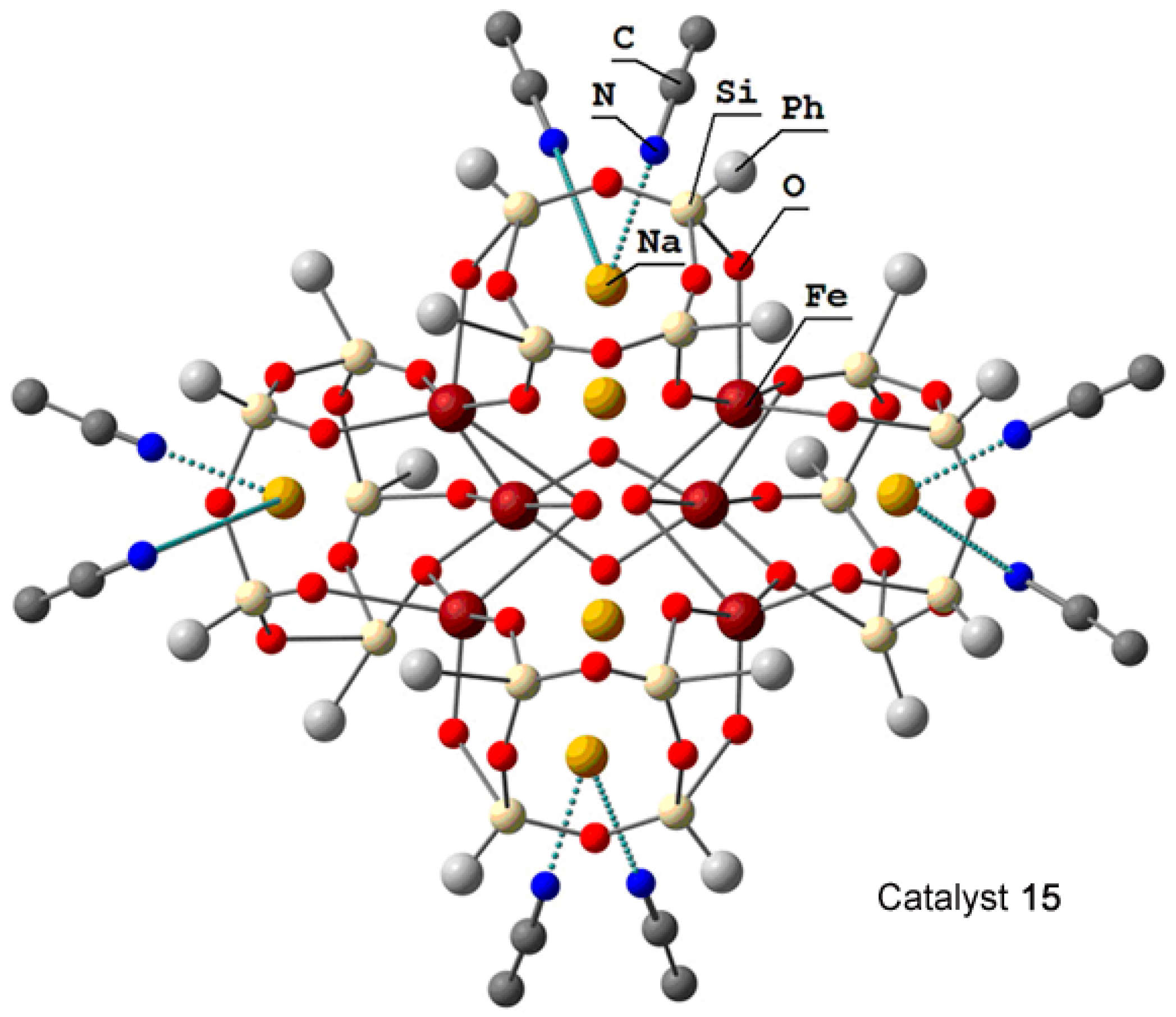
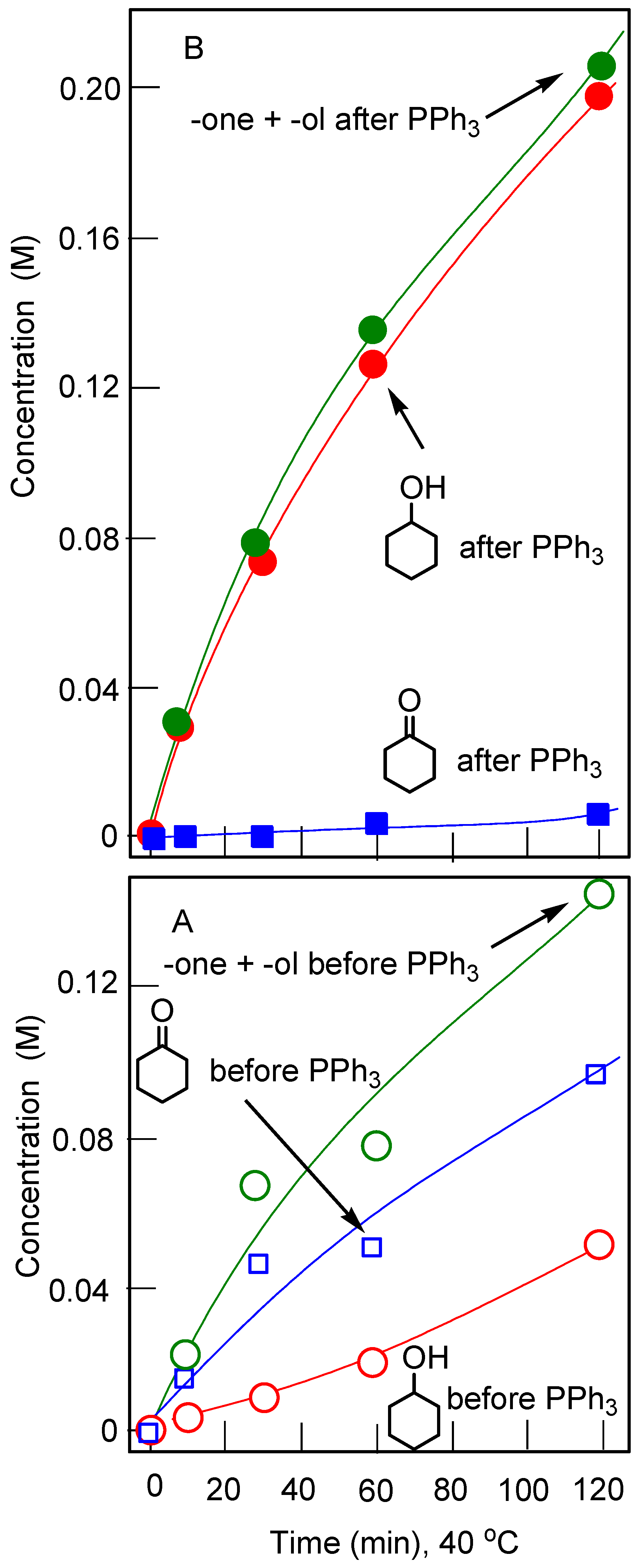
The hexanuclear oxo cluster [(PhSiO1.5)20(FeO1.5)6(NaO0.5)8(C7H8)(C4H9OH)9] 14 (for the strucure, see Figure 8) containing iron and sodium cations efficiently catalyzed oxidation of cyclohexane and benzene in acetonitrile solution to the corresponding alkyl hydroperoxides and phenol, respectively, by hydrogen peroxide in air in the presence of nitric acid [225]. Another hexairon complex 15 [226] (for the structure, see Figure 9) catalyzed the oxidation of cyclohexane affording the products in 46% yield (Figure 10), which is very high for oxidation of alkanes.
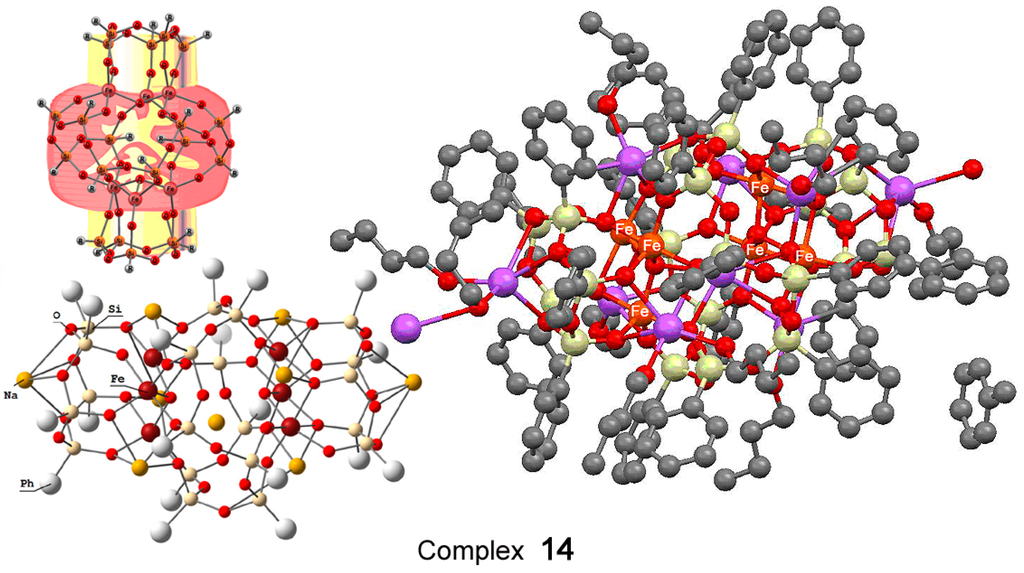
Figure 8.
The structure of heteronuclear Na8Fe6-oxo cluster bearing silsesquioxane ligands [(PhSiO1.5)20(FeO1.5)6(NaO0.5)8(C7H8)(C4H9OH)9] (14). Adapted from Ref. [225].
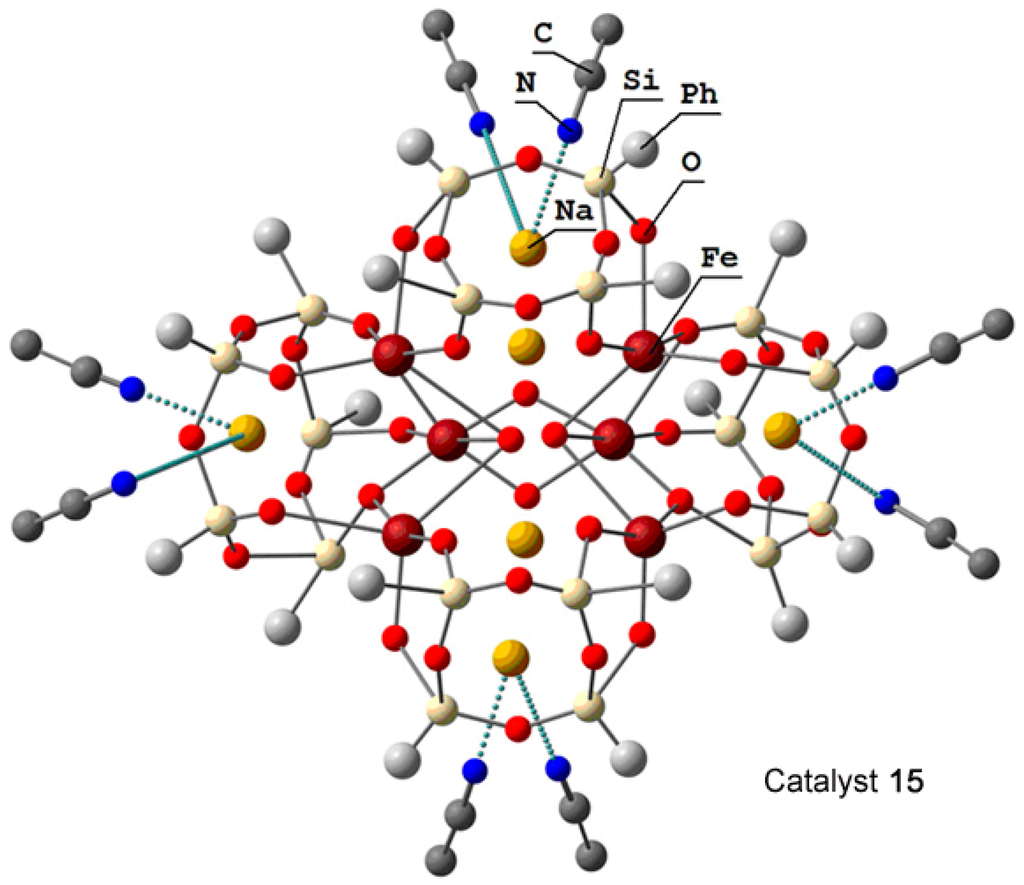
Figure 9.
Molecular structure of hexairon compound 15. Adapted from Ref. [226].
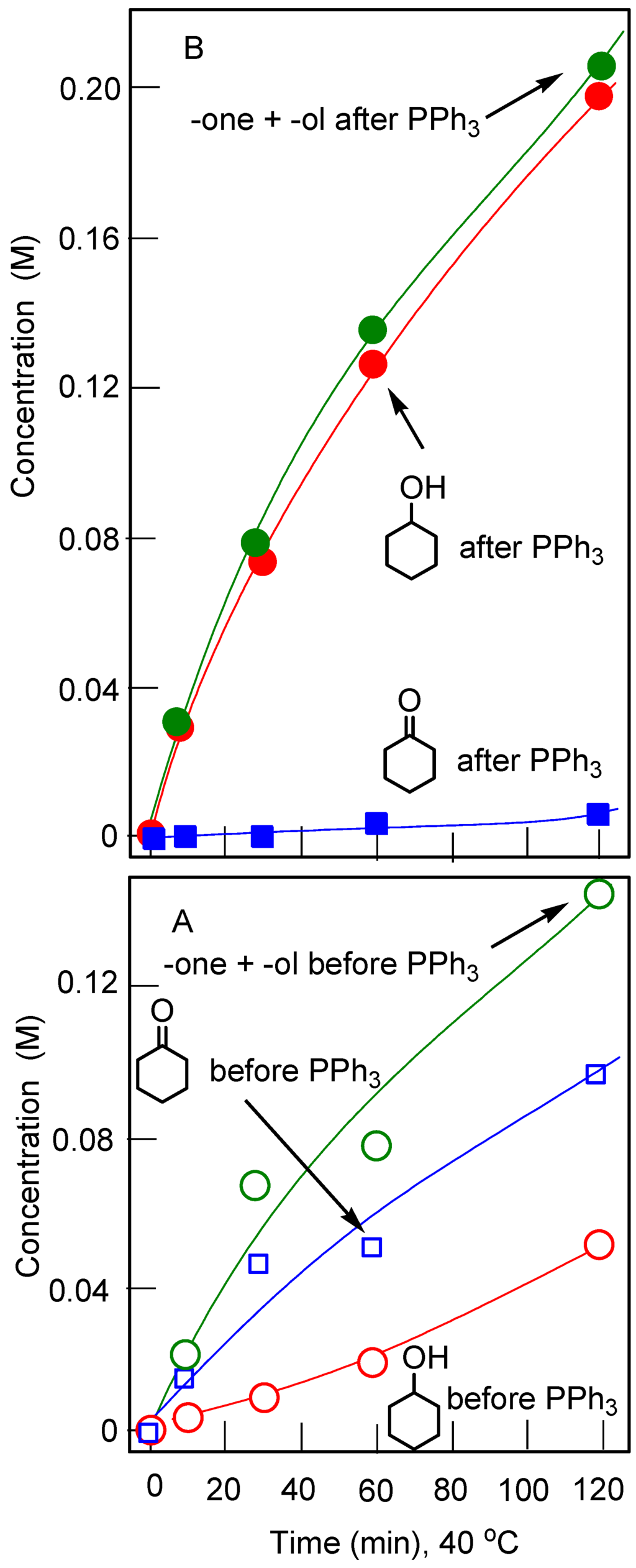
Figure 10.
Accumulation of cyclohexanol and cyclohexanone with time in the cyclohexane (0.45 M) oxidation with H2O2 (50%, aqueous, 1.5 M) catalyzed by compound 15 (5 × 10‒4 M) in the presence of CF3COOH (0.05 M). Solvent was acetonitrile (total volume of the reaction solution was 5 mL); temperature was 40 °C. Concentrations measured before (Graph A) and after (Graph B) reduction of the samples with PPh3 are shown.
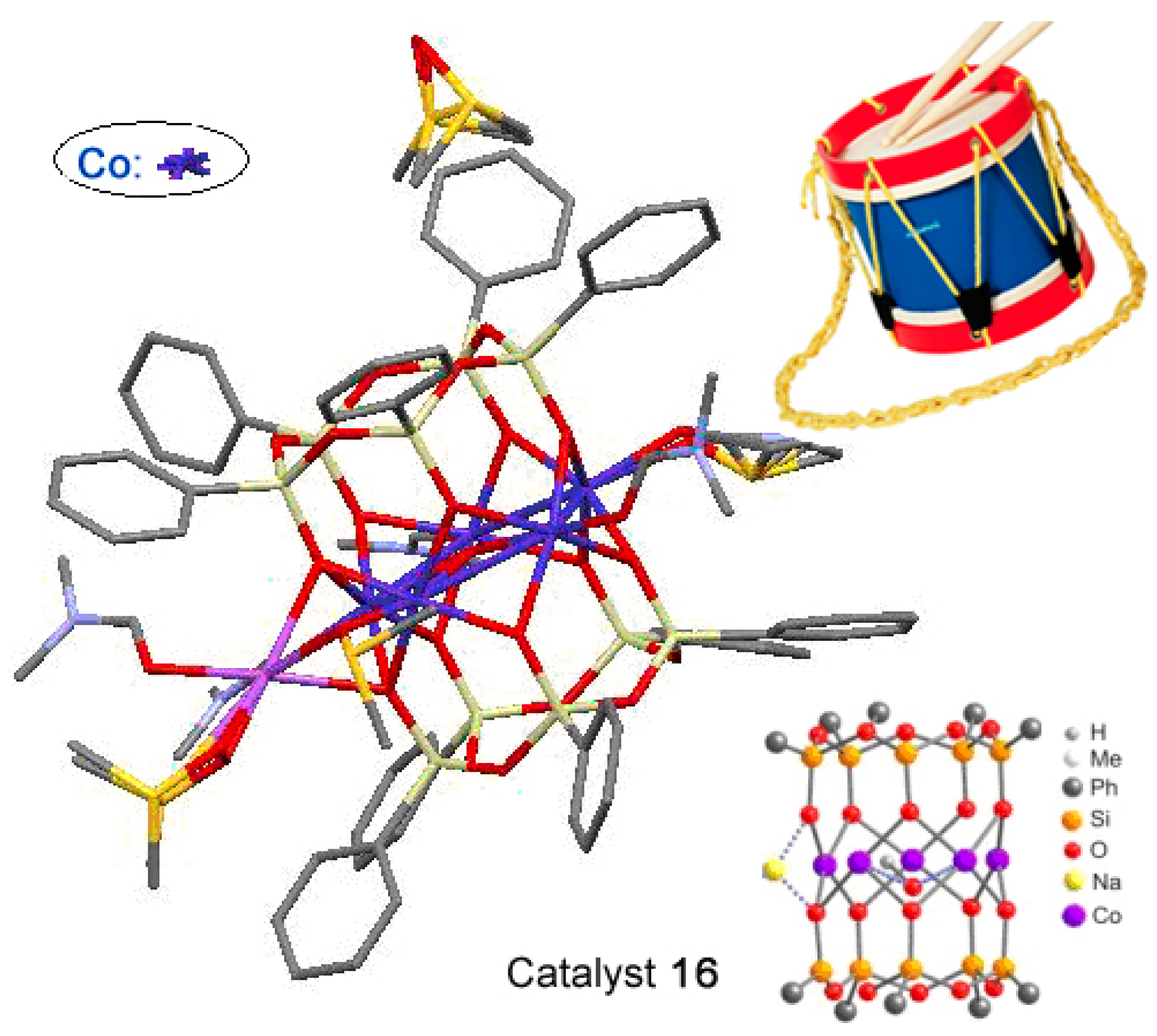
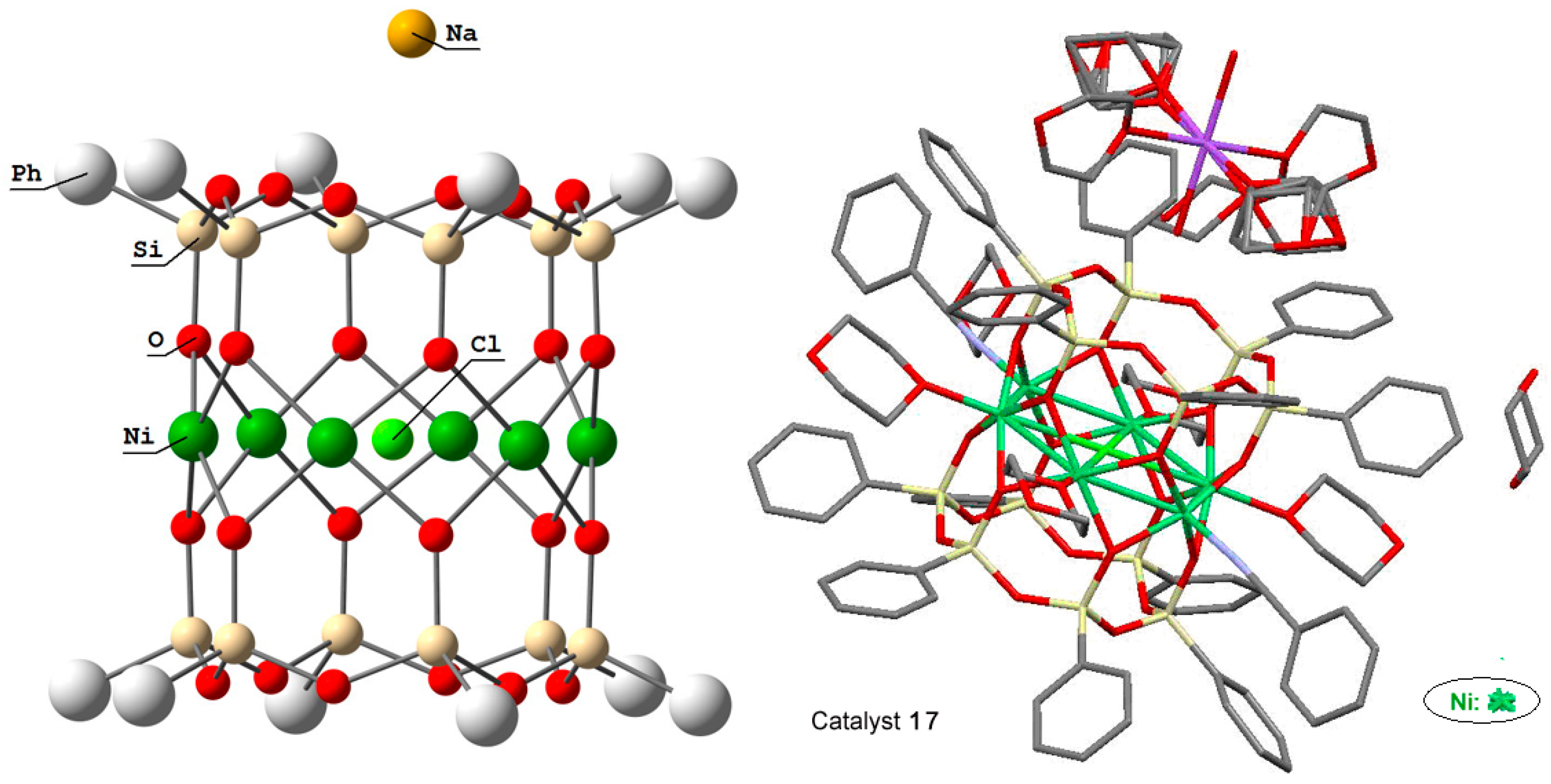
Cations of cobalt and nickel gave cylinder-type complexes phenylsilsesquioxane [(PhSiO1,5)10(CoO)5(NaOH)] (compound 16) [227] and (PhSiO1.5)12(NiO)6(NaCl) (compound 17) [228]. Their “drum-like” structures are presented in Figure 11 and Figure 12, respectively. Compound 16 exhibited activity in catalytic highly stereoselective oxidation of 1,2-cyclohexane with m-CPBA (Table 1).
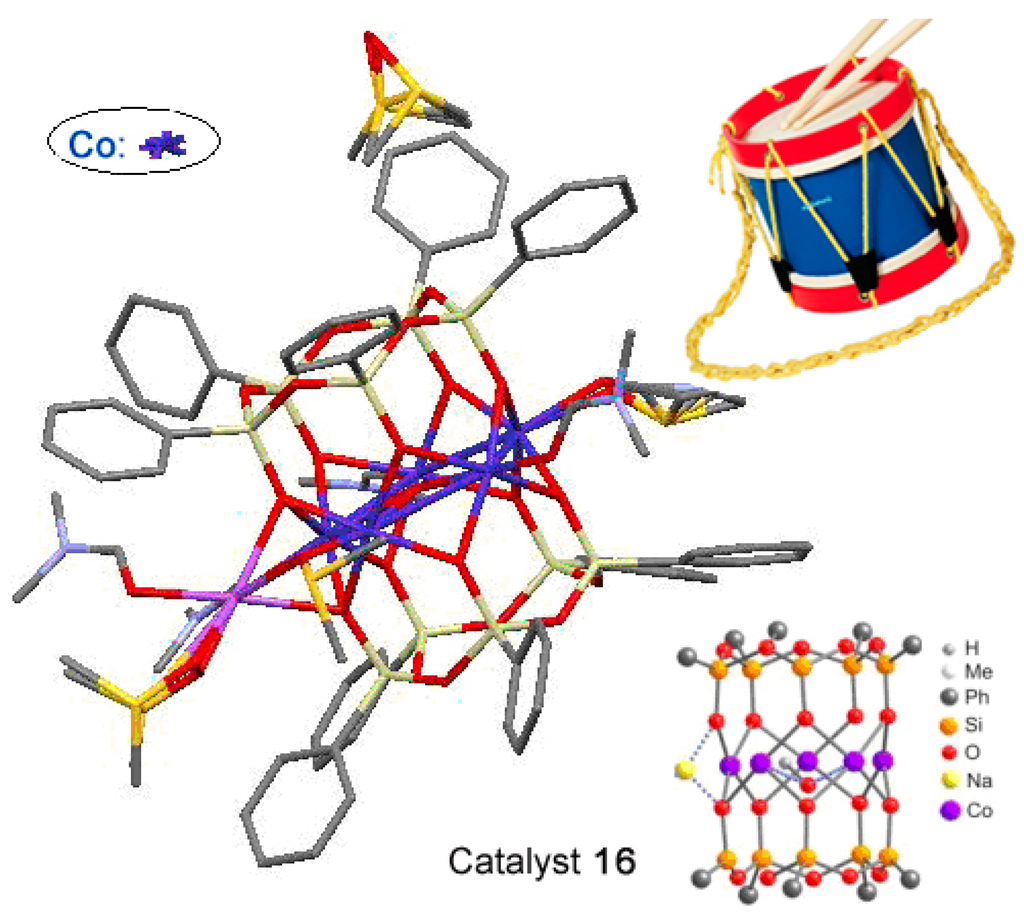
Figure 11.
Molecular structure of compound [(PhSiO1,5)10(CoO)5(NaOH)], 16. Adapted from Ref. [227].
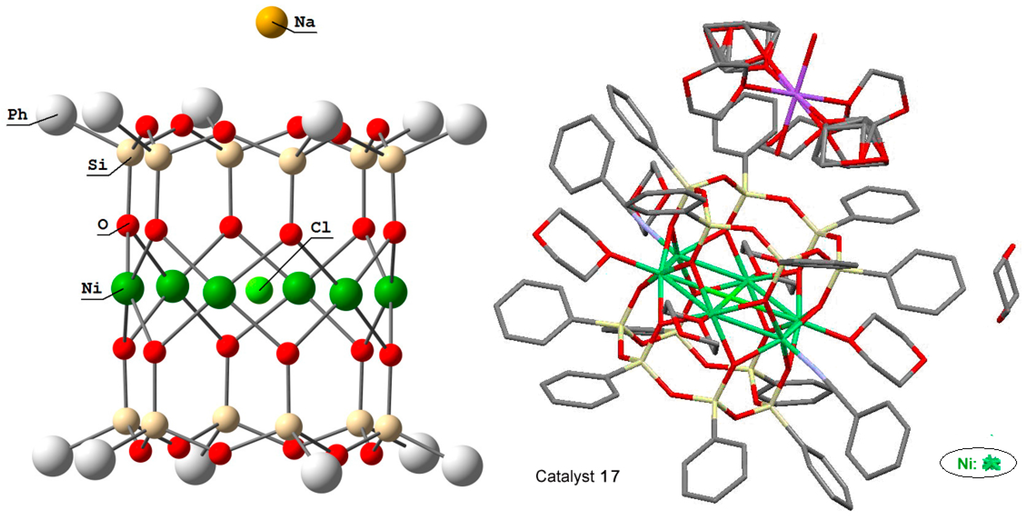
Figure 12.
Molecular structure of compound (PhSiO1.5)12(NiO)6(NaCl), 17. Adapted from Ref. [228].

Table 1.
Oxygenation of isomeric dimethylcyclohexanes with meta-chloroperoxybenzoic acid (m-CPBA) catalyzed by hexanuclear cobalt complex 16 [227] a.
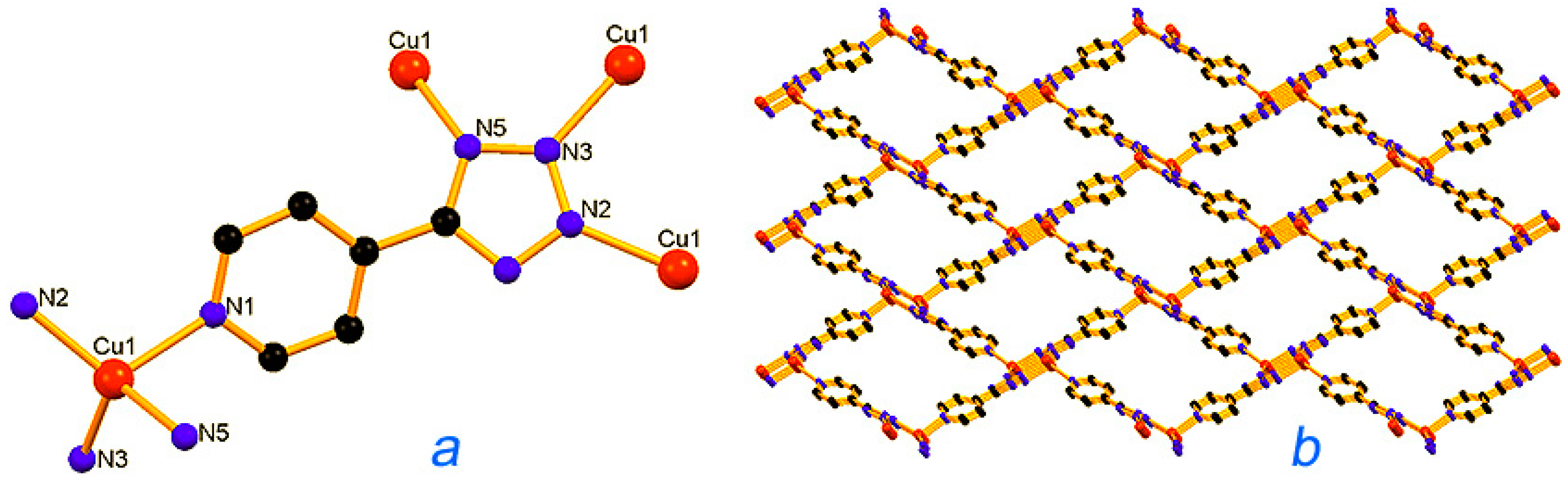
Metal-organic frameworks (MOFs), or porous coordination polymers, have attracted the attention of chemists [229,230,231,232,233]. Pombeiro, Martins, Guedes da Silva [234,235,236,237,238], Kirillov [234,235,239,240,241], and other scientists [242] synthesized various polynuclear complexes, coordination polymers, and derived metal integrated mesoporous matrices and demonstrated high catalytic activity of these materials in oxidations with peroxides. Figure 13, Figure 14 and Figure 15 show the examples of such catalysts.
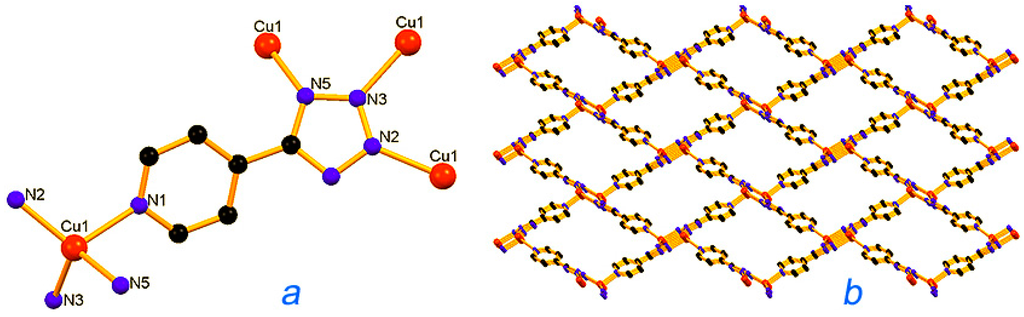
Figure 13.
Structural fragments of the Cu(I) catalyst [Cu(mu4-4-ptz)]n [4-ptz = 5-(4-pyridyl)tetrazolate], 18, representing (a) the basic unit; (b) a view of rhomboid voids along the a-axis. Adapted from Ref. [238].
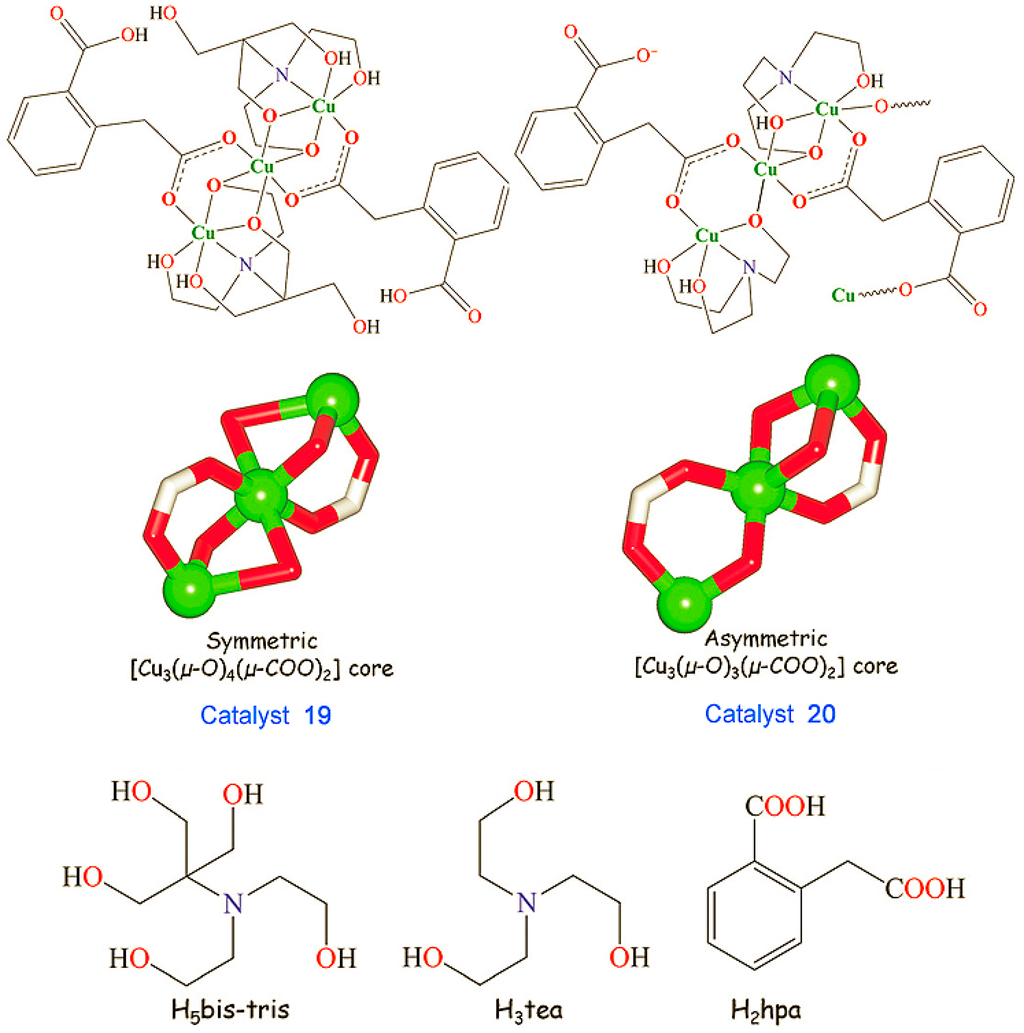
Figure 14.
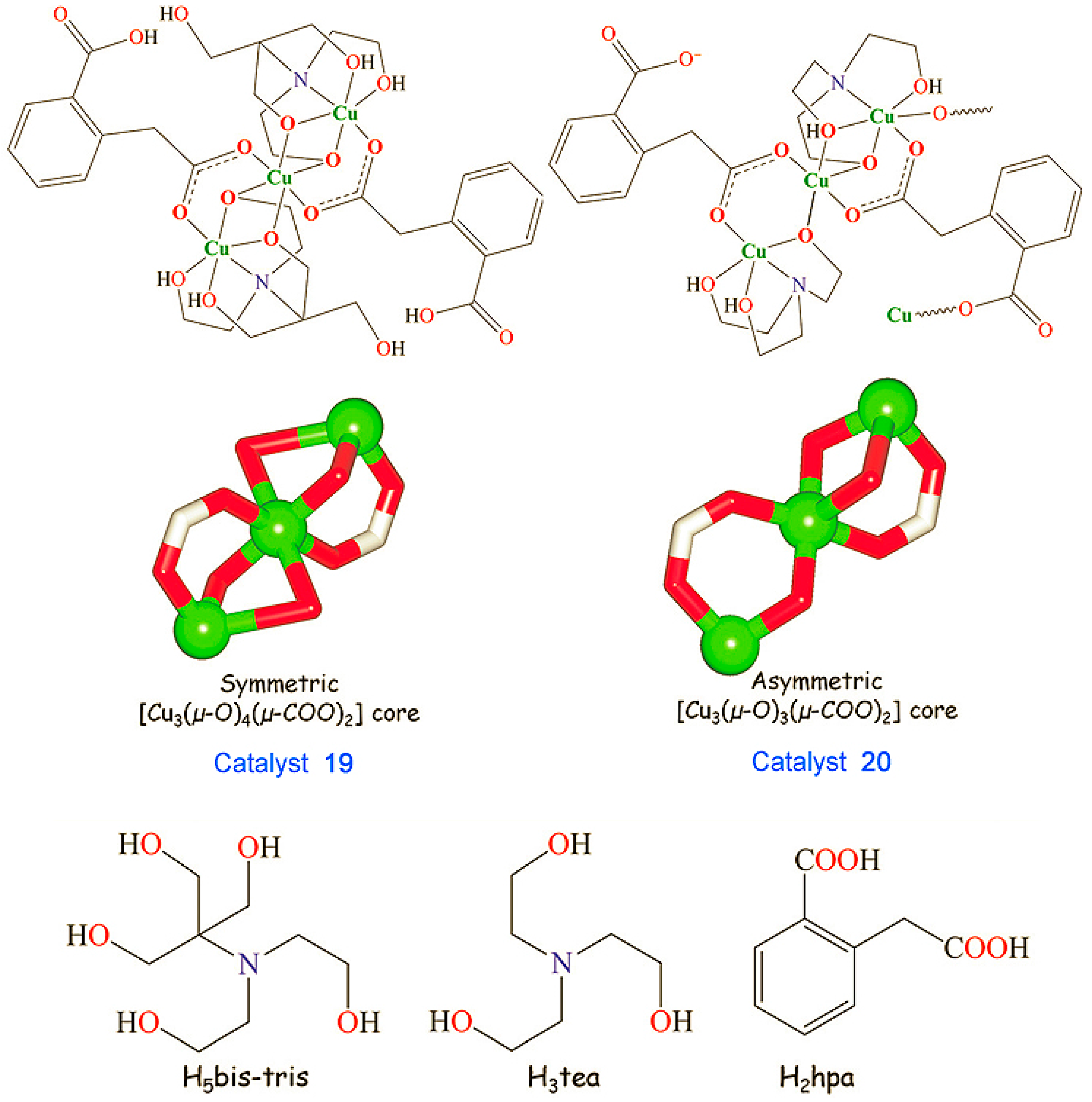
Catalysts [Cu3(μ2-H3bis-tris)2(μ2-Hhpa)2]·H2O (19) and [Cu3(μ2-H2tea)2(μ2-hpa)(μ3-hpa)]n (20) bearing distinct tricopper(II) cores where H3tea is triethanolamine, H2hpa is homophthalic acid, H5bistris is bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane. Adapted from Ref. [241].
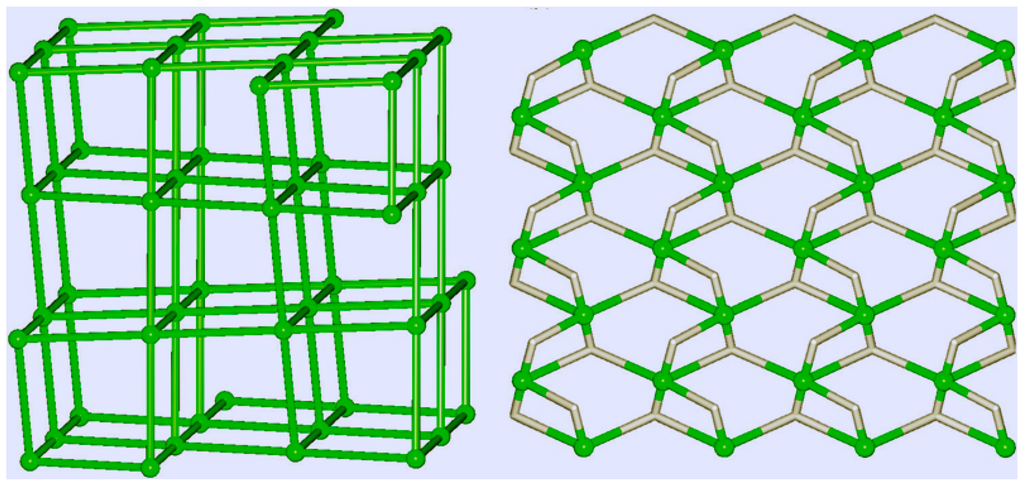
Figure 15.
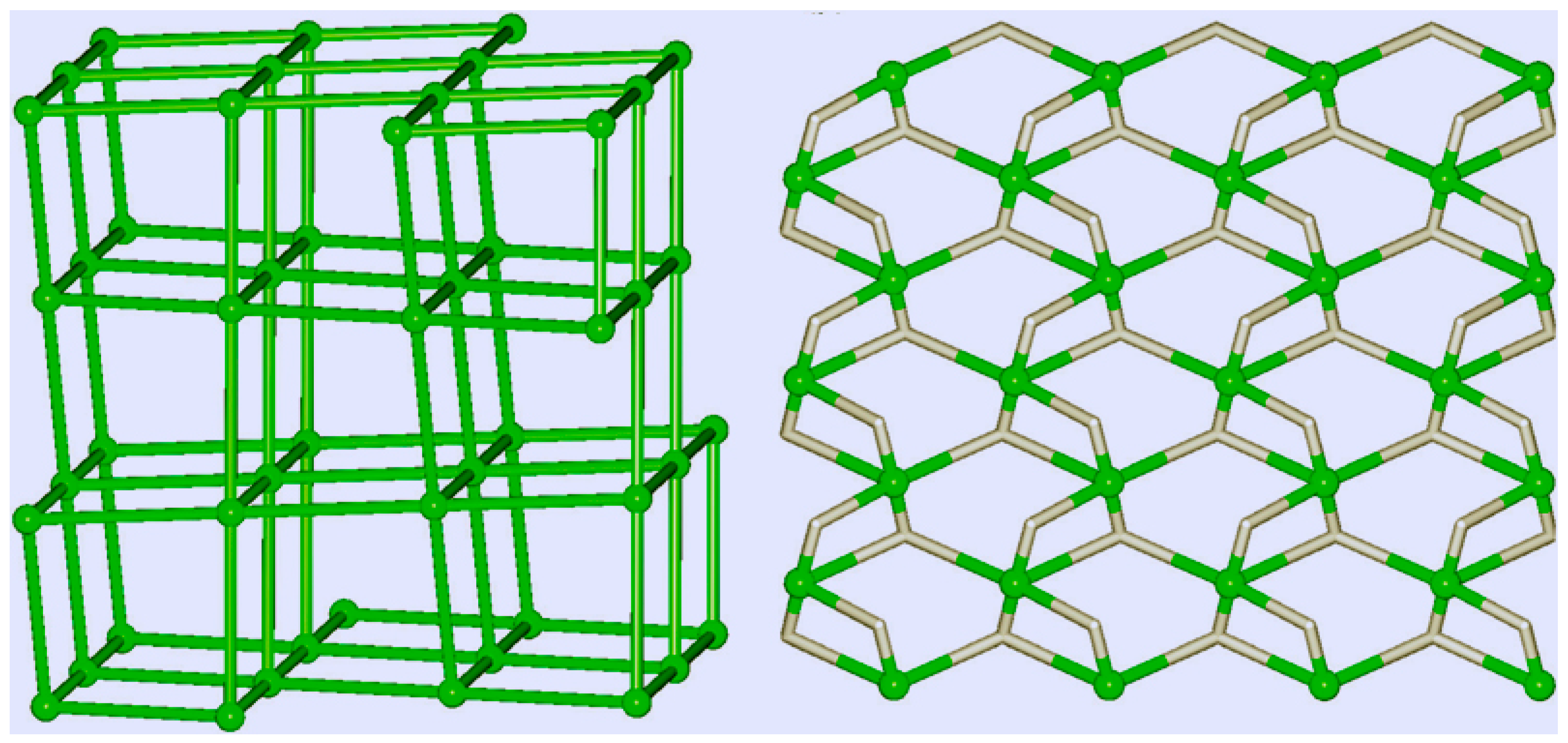
Topological representations of the H-bonded networks in catalysts 19 (left) and 20 (right). Green balls correspond to centroids of 6-connected or 5-connected molecular nodes. Adapted from Ref. [241].
4. Mechanism of Oxidation by Peroxides: Radical, or not Radical, That is the Question
In many cases, organic reactions occur with the formation of free carbon- and oxygen-centered radicals [243,244,245,246], and alkyl hydroperoxides are formed. A few tests can be used to detect the formation of ROOH from RH.
4.1. Detection and Determination of Alkyl Hydroperoxides Comparing GC before and after Treating Samples with PPh3
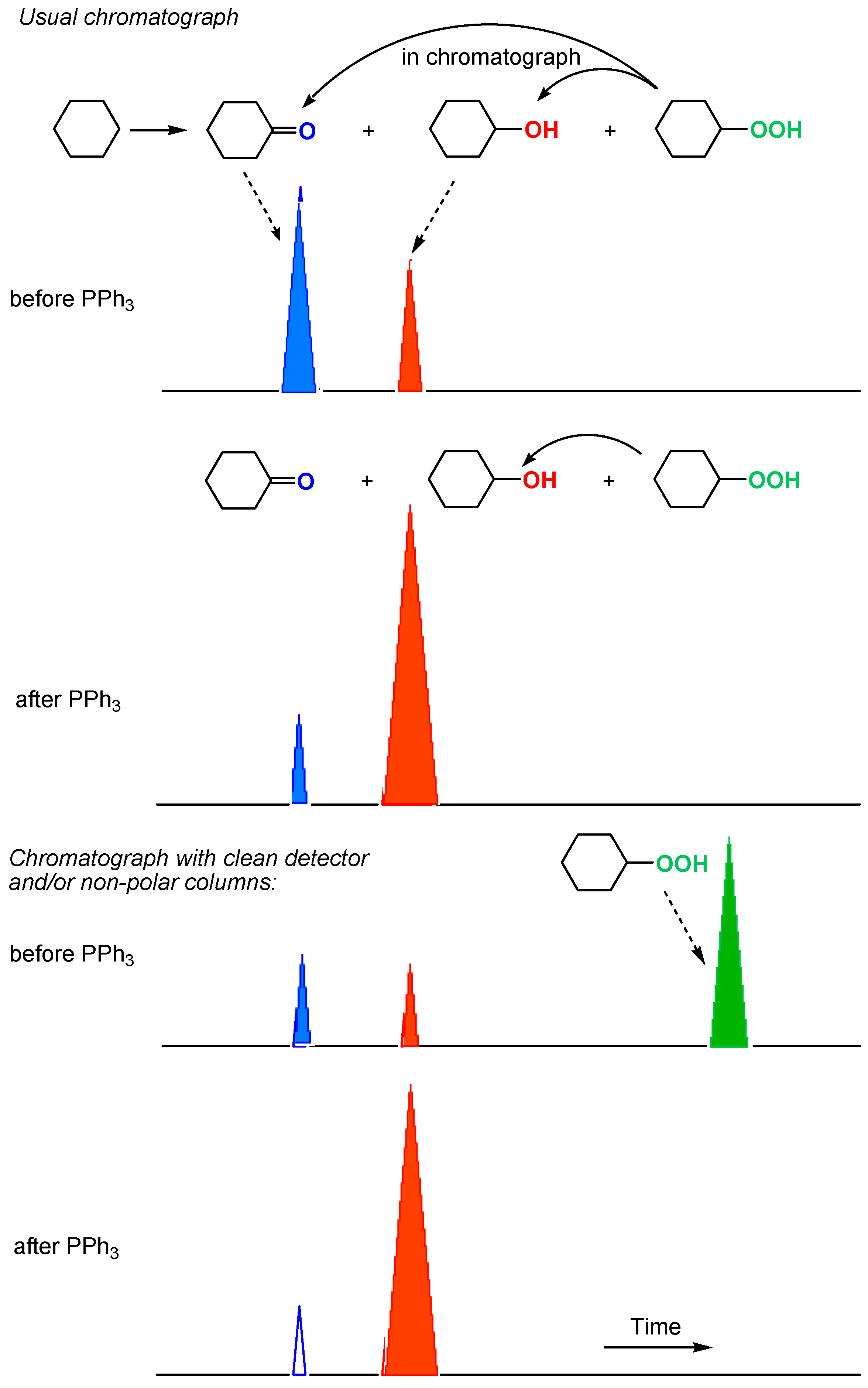
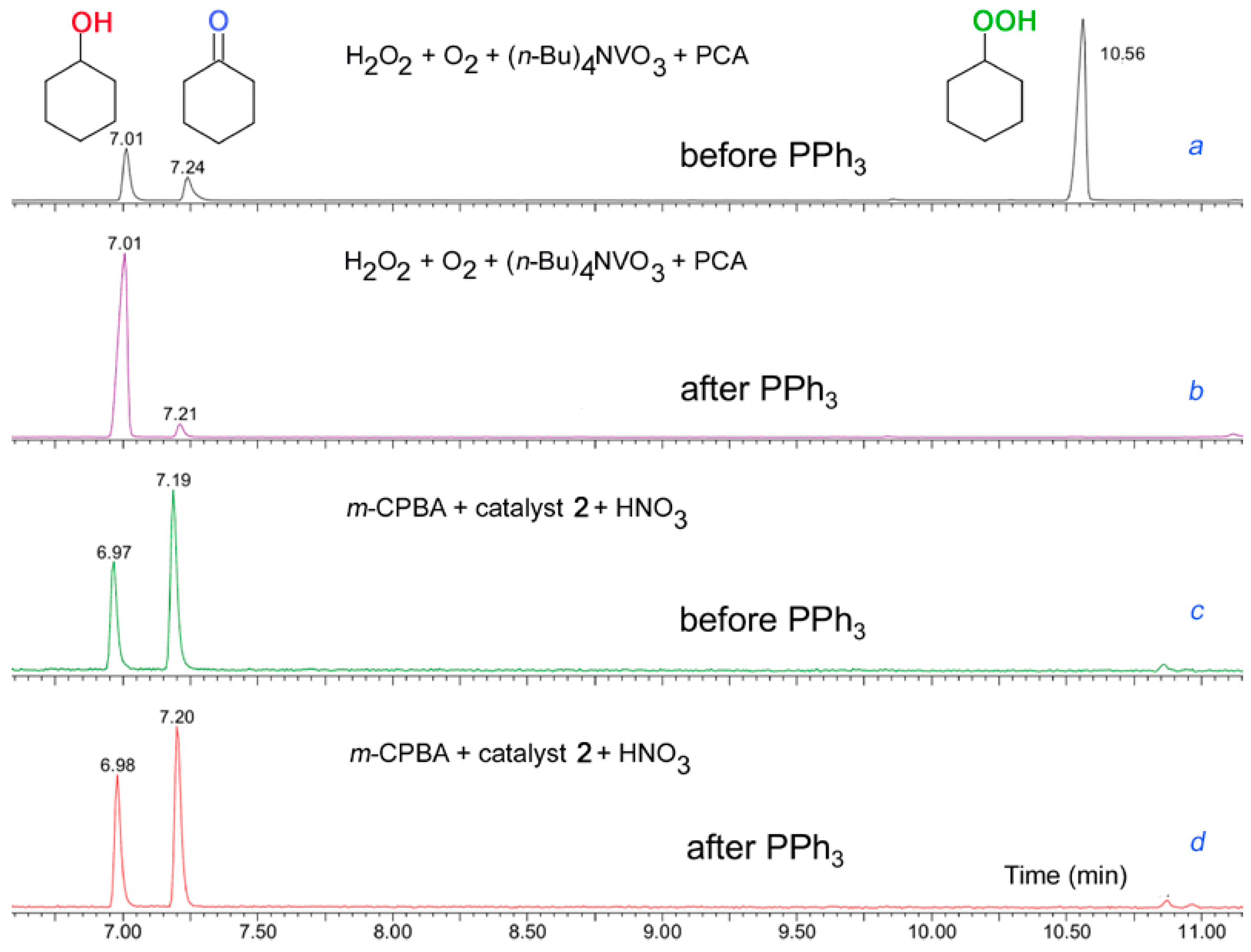
We proposed and developed a simple method for analysis of alkyl hydroperoxides generated in various alkane oxidations in solutions [11,12,13,247,248,249,250,251,252]). A chemist can find that, if the direct injection of a reaction sample into the chromatograph gave comparable amounts of cyclohexanol and cyclohexanone (Figure 16, Figure 17a), the reduction of the sample with PPh3 prior to GC analysis led to the noticeable predominance of the alcohol (Figure 17b). The comparison of the results obtained before and after the reduction clearly indicates that cyclohexyl hydroperoxide was formed as the main primary product. We can estimate the real concentrations of the alkyl hydroperoxide, alcohol, and ketone present in the reaction solution comparing the intensities of peaks attributed to the alcohol and ketone before and after the reduction. This method gives the very valuable information on the existence or non-existence of alkyl peroxides in the reaction mixture and allows us to estimate concentrations of the three products (ketone, alcohol, and alkyl hydroperoxide) formed in the reaction. In some cases, using non-polar columns and/or very clean detector, we can see in chromatogram a peak of alkyl hydroperoxide (Figure 16, Figure 17a), which disappears under the action of PPh3 (Figure 17b).
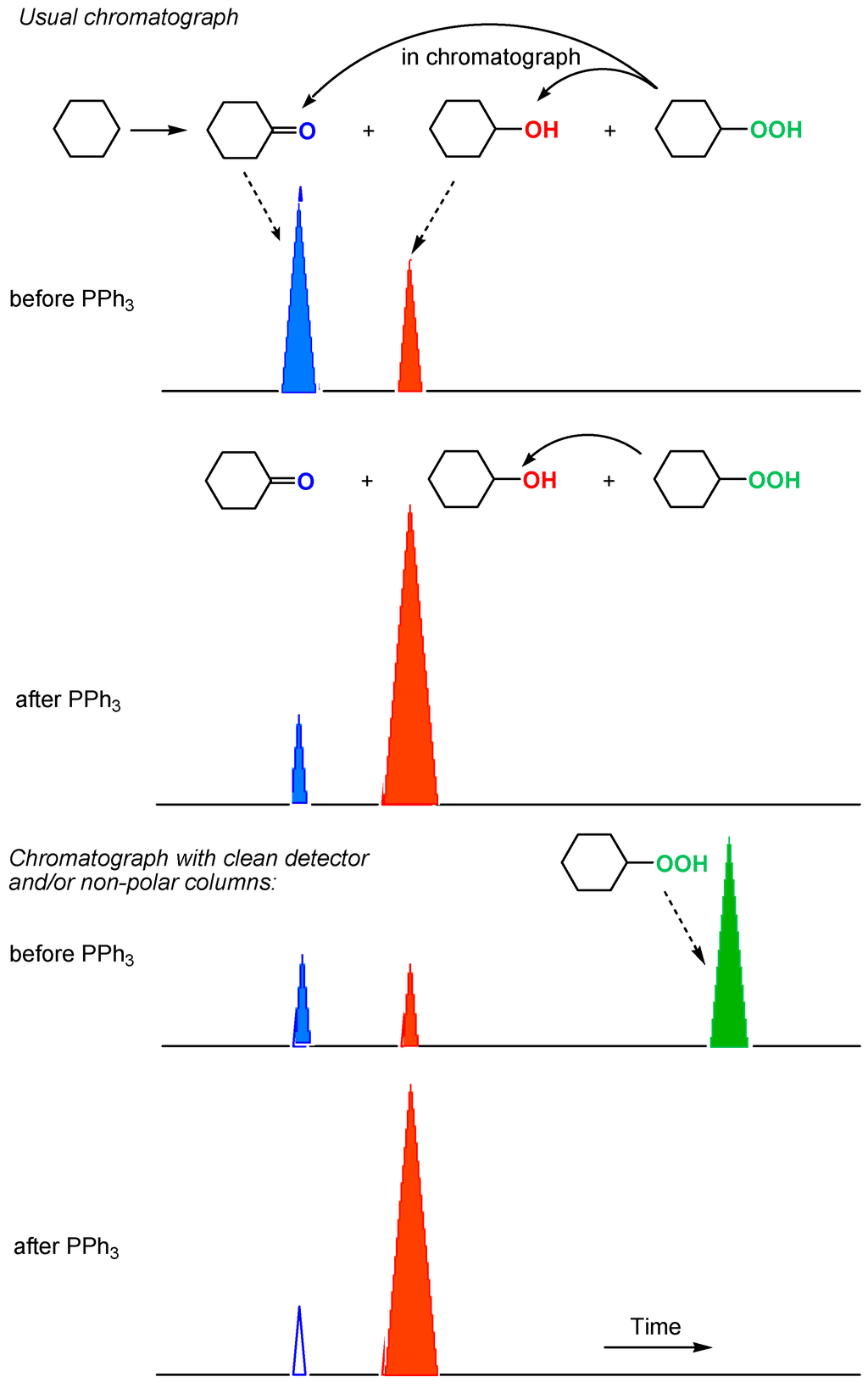
Figure 16.
Detection of alkyl hydroperoxide ROOH by GC.
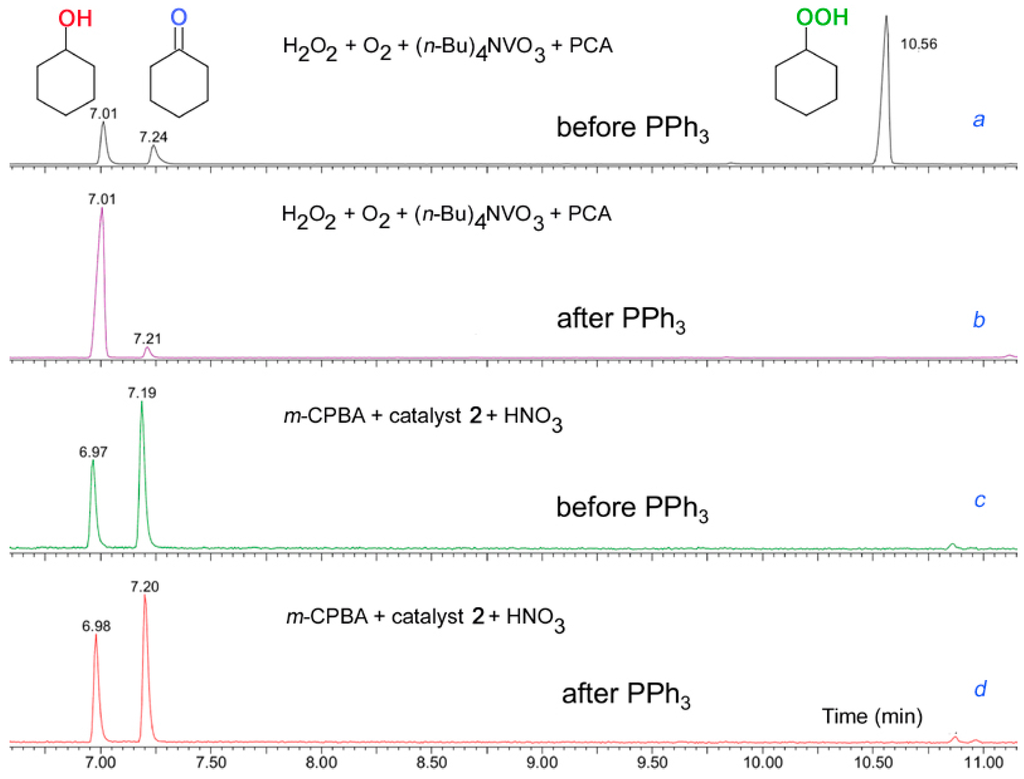
Figure 17.
Chromatograms of reaction products obtained in the cyclohexane oxidation in acetonitrile by the H2O2/O2/vanadate anion/PCA reagent [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92] and the m-CPBA/catalyst 2/HNO3 system (see below [160]). For both systems the chromatograms were recorded before and after the addition of PPh3. Conditions: [cyclohexane]0 = 0.46 M; (a and b): [H2O2]0 = 0.75 M, [(n-Bu)4NVO3]0 = 1 × 10−4 M, [PCA]0 = 4 × 10−4 M, 40 °C; (c and d): [m-CPBA]0 = 0.028 M, [complex 2]0 = 2.7 × 10−6 M, [HNO3]0 = 5.5 × 10−3 M, 50 °C. A Perkin-Elmer Clarus 600 gas chromatograph, equipped with non-polar capillary columns (SGE BPX5; 30 m × 0.32 mm × 25 μm) was used for analyses of the reaction mixtures. Adapted from Ref. [160].
Generally speaking, the [alcohol]/[ketone] ratio (presented in many publication as the A/K ratio) has no meaning and gives valuable information only if [alkyl hydroperoxide] = 0 [253]. Thus, it is more correct to write “the A/K ratio determined after reduction of the sample with PPh3” [254,255]. Evidently, in this case, [A] = [alcohol] + [alkyl hydroperoxide].
When we have only the chromatogram obtained after the addition of PPh3, we cannot definitively say that ROOH is present in the reaction solution (for example, [256]). In cases when the difference between chromatograms obtained before and after reduction of samples with PPh3 indicates the formation of cyclohexyl hydroperoxide (that is when the investigator knows definitely that ROOH is present in the reaction mixture), it is convenient to characterize the reaction by the following ratio: ([alkyl hydroperoxide] + [alcohol])/[ketone]. This ratio is measured by GC for the samples after treating with PPh3, and ([alkyl hydroperoxide] + [alcohol]) = [alcohol]after PPh3. Although this rough parameter (see, for example, a recent paper by Kühn [257]) does not give the information on the amount of alkyl hydroperoxide that is present in the reaction mixture, it approximately describes the selectivity relative to the three products (alkyl hydroperoxide, alcohol and ketone).
The so-called Shul’pin method [257,258,259] of analysis of oxygenates discussed above in this section and based on GC and PPh3 has been used by other authors (selected examples are given in Refs. [260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278]).
Finally, if the GC analysis shows that there is no difference between chromatograms obtained before and after reduction with PPh3, it indicates that the samples do not contain alkyl hydroperoxides. Consequently, the reaction proceeds without the participation of free radicals, unless it was proposed, for example, for an oxidation with hydrogen peroxide via hydroxyl radicals (Fenton-like scheme): H2O2 → HO•; HO• + RH → R•; R• + O2 → ROO•; ROO• → → ROOH. Possibly, we have a concerted mechanism in this case. Examples of such reactions are given below in Table 1 and Figure 17c,d for the catalyzed oxidation of 1,2-dimethylcyclohexane with m-CPBA.
4.2. Competitive Oxidation of Alkane and Solvent
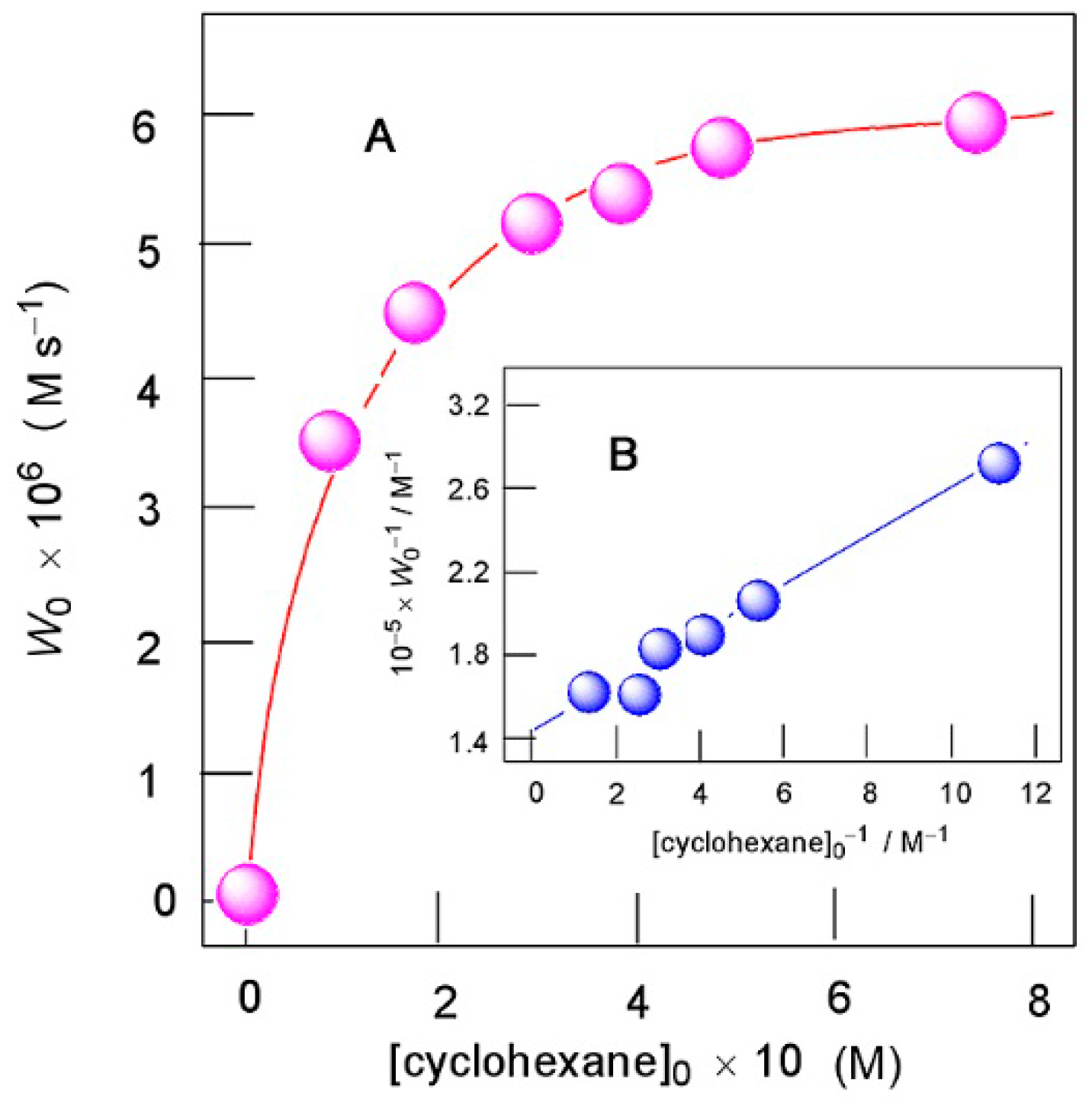
Kozlov demonstrated that the mode of dependence of the initial rate of oxygenate formation W0 on initial concentration of cyclohexane (W0 is approaching to a plateau at relatively high concentrations of cyclohexane; an example is shown in Figure 18A) can lead to the conclusion on the participation of hydroxyl radicals in the reaction (see, for, example, Ref. [160]) by the competitive interaction of hydroxyl radicals both with cyclohexane and acetonitrile. The following kinetic scheme describes the dependence of W0 on [cyclohexane]0 with the plateau:
H2O2 + catalyst → HO• (rate W5)
HO• + CyH → Cy• → → CyOOH (constant k6)
HO• + CH3CN → →products (constant k7)
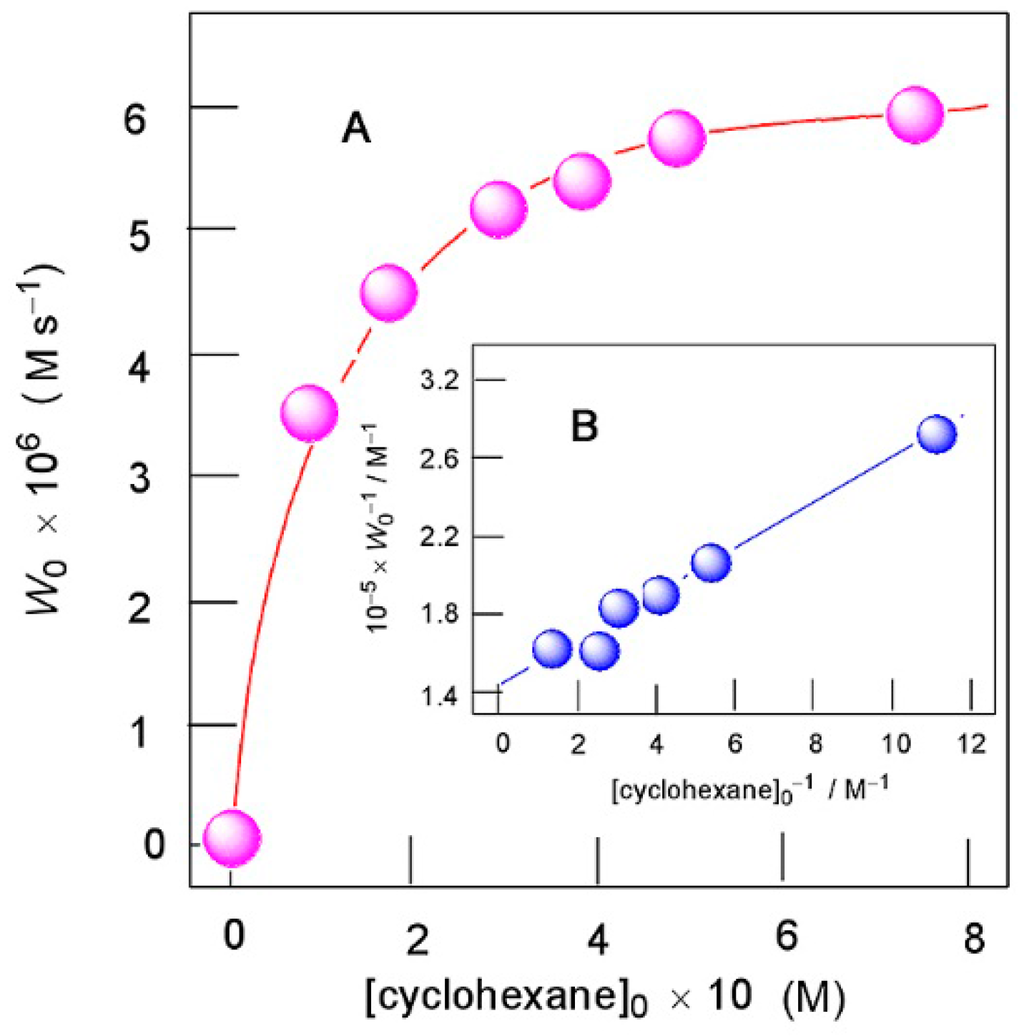
Figure 18.
Oxidation of cyclohexane with H2O2 (1.0 M) catalyzed by compound 2 (3.2 × 10−5 M) in the presence of HNO3 (0.04 M) in MeCN at 50 °C. Graph A: dependence of the initial rate of oxygenate formation W0 on initial concentration of cyclohexane. Graph B: anamorphosis of curve shown in Graph A in coordinates [cyclohexane]0−1 – W0−1. Adapted from Ref. [160].
Here, the initiation stage (10) corresponds to the catalytic generation of hydroxyl radicals with rate W5, stage (11) corresponds to the sequence of transformations of CyH into CyOOH (with kinetically rate-limiting interaction between HO• and CyH), and stage (12) is the reaction of HO• with acetonitrile. The analysis of this kinetic scheme in the quasi-stationary approximation relative concentration of hydroxyl radicals leads to the following equation:
The term
should be a linear function of 1/[CyH]0 (see Figure 18B). The following ratio can be obtained:
If we assume [MeCN] = 18 M in the reaction solution, we obtain k7/k6 = 4.4 × 10−3. Values k7[MeCN]/k6 (0.08 M) and k7/k6 (0.0044) are close to the corresponding parameters obtained for oxygenation of cyclohexane in acetonitrile with H2O2. The experimental data are in agreement with this dependence, which is shown in Figure 18A.
4.3. On the Rebound and Concerted Mechanisms
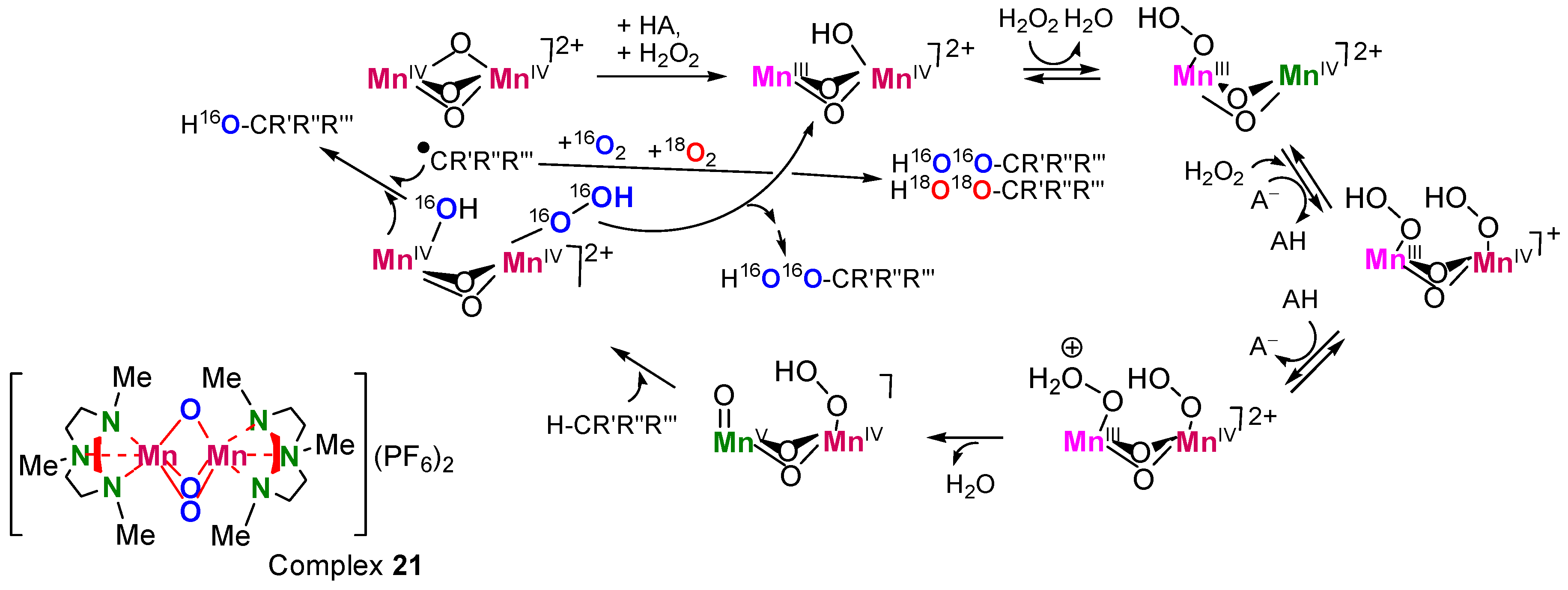
Radicals can be generated in the solvent cage, as in the so-called “oxygen rebound mechanism” [279,280,281,282]: M=O + RH → M–OH + R• → M + R–OH. The regioselectivity of such reactions is relatively high, e.g., C(1):C(2):C(3):C(4) ≈ 1:40:40:40 (compare with the parameter typical for free-radical oxidation: 1:5:5:5). Reactions of such type are in many cases stereoselective and even stereospecific, i.e., for the oxidation of cis-1,2-dimethylcyclohexane parameter trans/cis <<1. However, if radicals R• partly leave the solvent cage and react with O2, the formation of ROOH is possible. The H2O2/[Mn2(TMTACN)2O3]2+ (21)/RCOOH system has been assumed to oxidize via a “crypto-radical” (“hydroperoxo-rebound”) mechanism (Figure 19) [283].
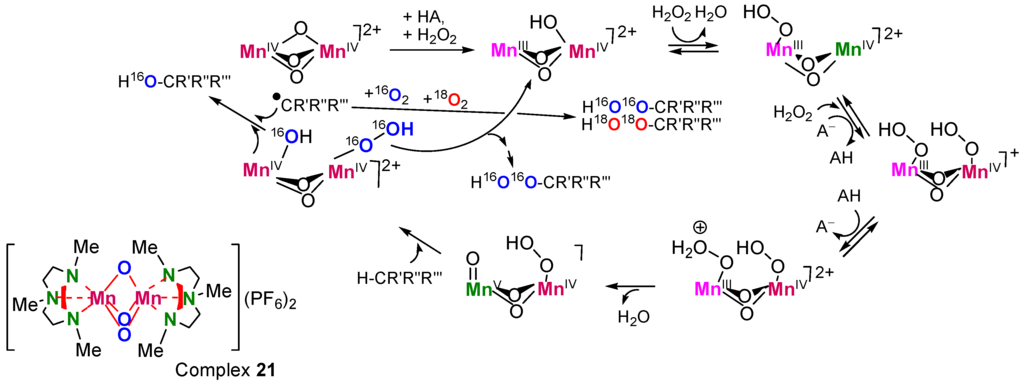
Figure 19.
A catalytic cycle proposed for the alkane hydroperoxidation catalyzed by complex 21 in the presence of 18O2 (in an atmosphere 16O2+18O2). AH is a carboxylic acid where A− is its anion. Adapted from Ref. [283].
m-Chloroperbenzoic acid in the presence of certain metal compounds efficiently oxidizes alkanes at low temperature with a very high degree of stereoselectivity (trans/cis << 1) [160,227,284,285]. Apparently, radicals are not produced intermediately in the course of such oxidation, which occurs via a “concerted” mechanism.
5. Some New Reactions
5.1. Shilov Chemistry
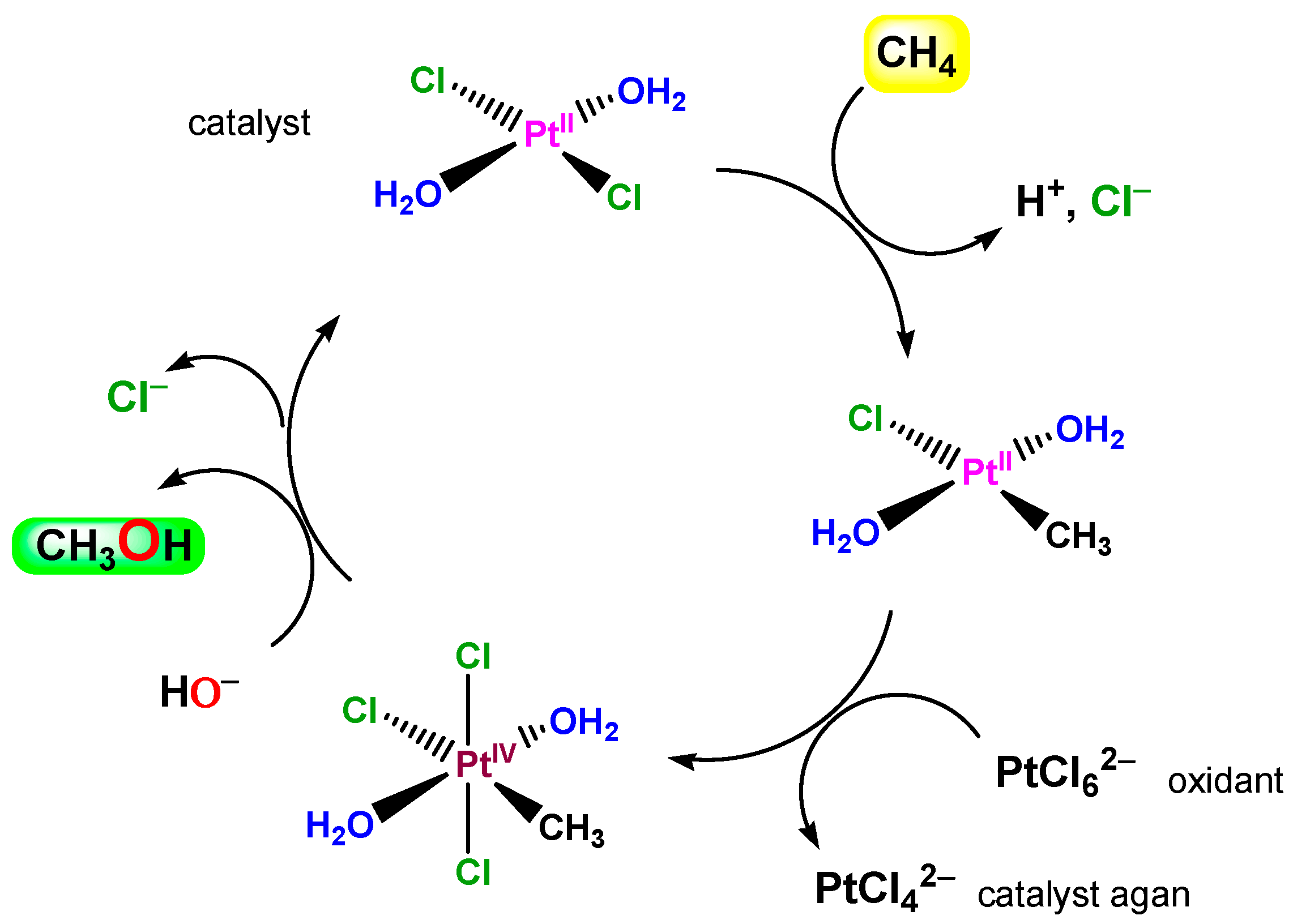
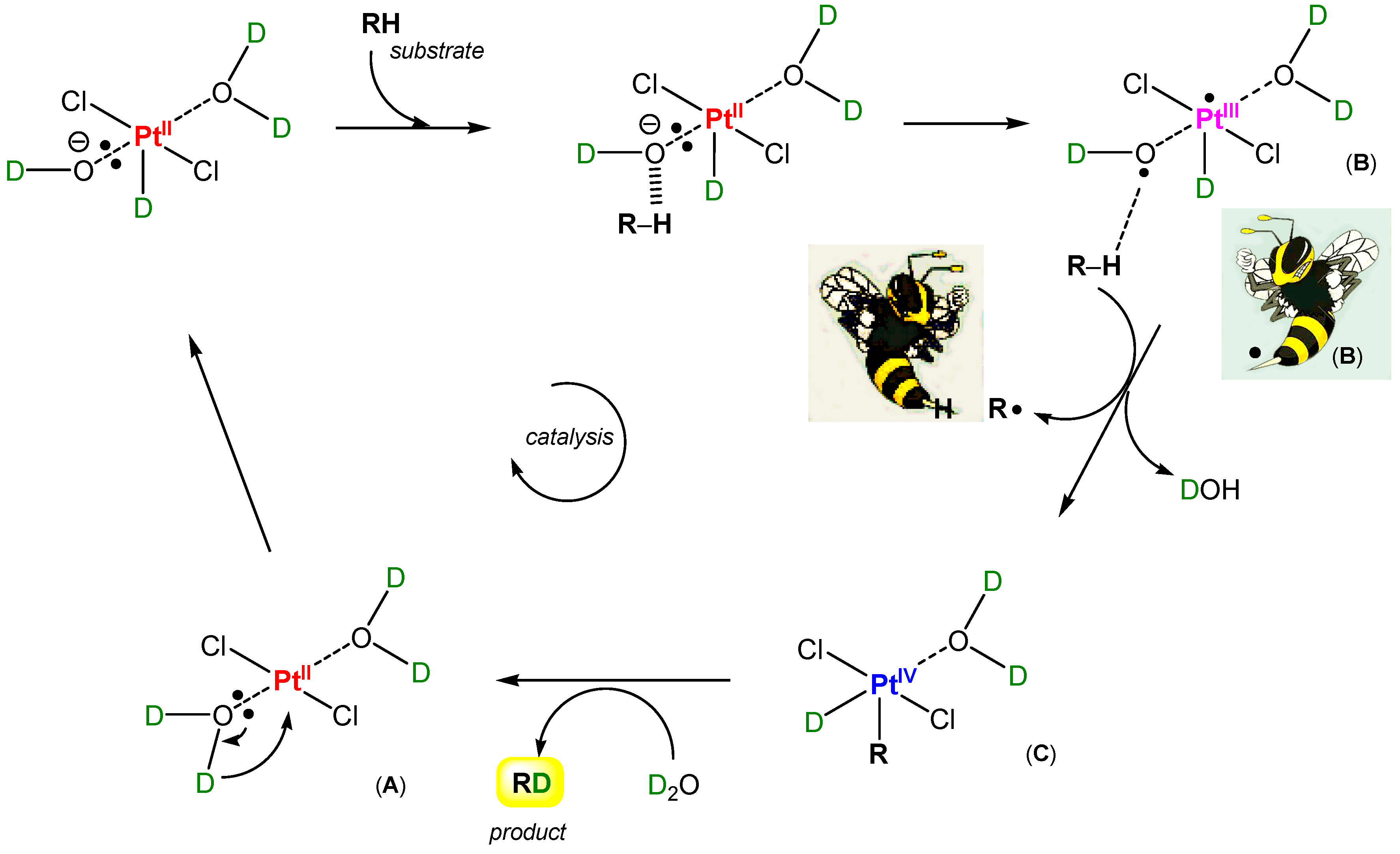
So-called Shilov chemistry [1,286,287,288,289,290,291] includes two reactions (discovered in 1969 and 1972 [1]). It was demonstrated that PtIICl42− ion catalyzes H/D exchange in methane in a D2O/CD3COOD solution and, if PtIVCl62− is added, the latter oxidizes methane to methanol. The catalytic cycle in which σ-methyl complexes of platinum(II) and platinum(IV) are involved is shown in Figure 20.
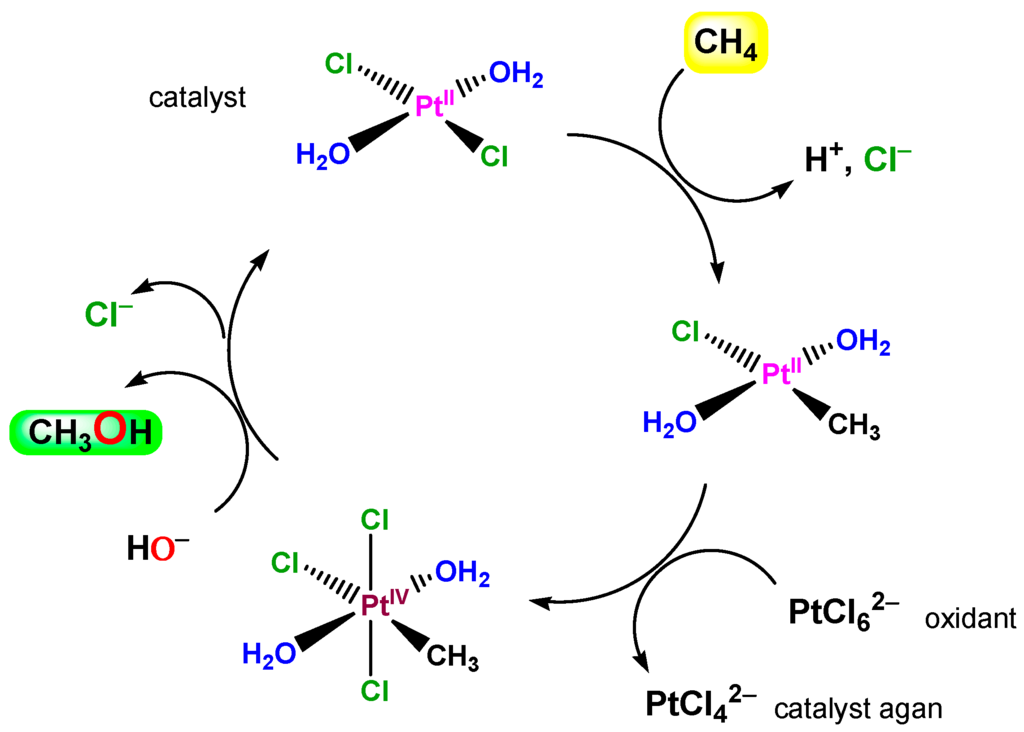
Figure 20.
A schematically depicted catalytic cycle proposed for Shilov chemistry (the methane oxidation to methanol by PtIVCl62− catalyzed by PtIICl42−).
The initial step of the reaction between alkane and ion PtIICl42− is the formation of a σ-alkyl platinum(II) derivative, which in the second step is oxidized by platinum(IV) present in the solution to give alkanol (and also alkyl chloride):
Alk–H + Cl–PtII–Cl → Alk–PtII–Cl + HCl
Alk–PtII–Cl + Pt(IV) + H2O → Alk–OH + PtII + Pt(II) + HCl
Recently, Rudakov [287] proposed an intimate mechanism for the first step of organometallic alkane activation by the PtII species (Figure 21). An active form of the aquachloride species Cl2(H2O)2PtII (A) is an adduct of the hydride •PtIII derivative with hydroxyl radical in the form of unseparated radical pair Cl2(H2O)H(•PtIII)…O•H (B). This coordinatively unsaturated complex has a vacancy to add an R fragment from the reacting alkane, RH. Indeed, coordinated hydroxyl radical can abstract a hydrogen atom from RH to produce radical R•. A collapse of radical pair (•PtIII) and R• affords an alkylhydride derivative of platinum(IV) (C) postulated for one stage of the catalytic cycle [1,287]. Thus, considering such a hypothesis, we can say that one of the main peculiarities of Shilov chemistry is a crypto-radical character of its intimate mechanism (“rebound mechanism”; see Section 4.3). It is possible to compare the platinum complex Cl2PtII(OH2)2 (A) with a wasp, which “pricks” (activates) alkanes using its sting (coordinated hydroxyl radical). It is useful to remember that Bach and Dmitrenko [292] proposed a mechanism of alkane oxidation catalyzed by cytochrome P450. It involves the rearrangement (somersault motion) of the metal hydroperoxide to its inverted isomeric form with a hydroxyl radical that is hydrogen bonded to the metal oxide: MO‒OH → M=O…HO•. The coordinated hydroxyl radical abstracts a hydrogen atom from the alkane carbon–hydrogen bond. This mechanism has similarities with the hypothetical platinum “wasp’s sting mechanism” described in this paragraph (Figure 21).
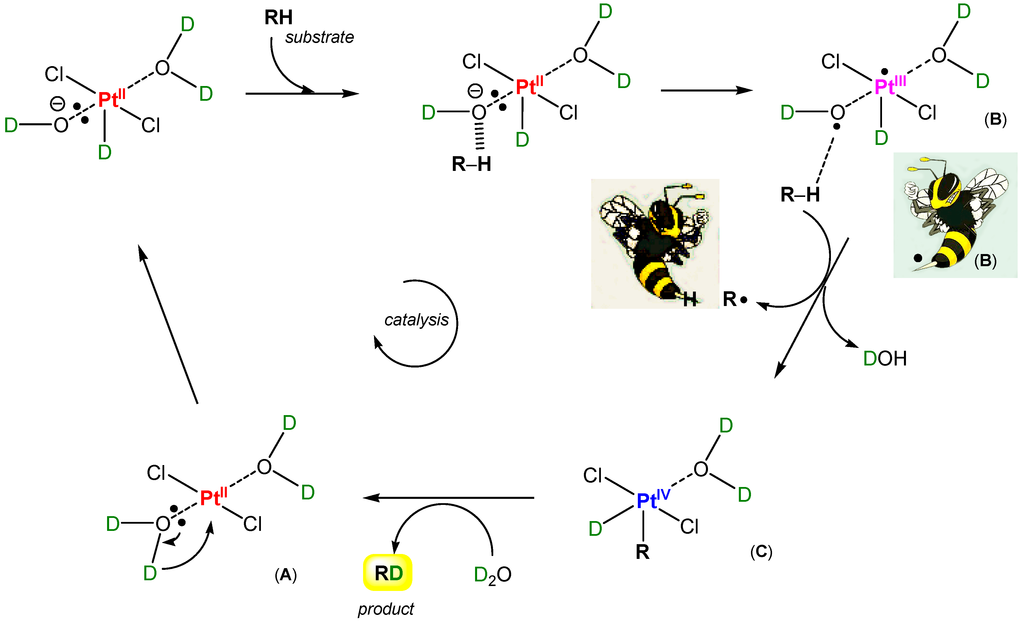
Figure 21.
A hypothetical “wasp’s sting mechanism” of the first step of Shilov chemistry (interaction between methane and PtIICl42−).
Hexachloroplatinate used originally as the stoichiometric oxidant in alkane reactions is a very expensive reagent and is not convenient to use in the synthesis. The Pt(II)–Pt(IV)–H2O2 system used by Thorn and colleagues to hydroxylate n-propanol selectively to 1,3-propanediol [293] exhibited very low efficiency: The amount of 1,3-propandiol corresponded to 1.3 turnovers of the entire platinum content (about 0.09 h−1).
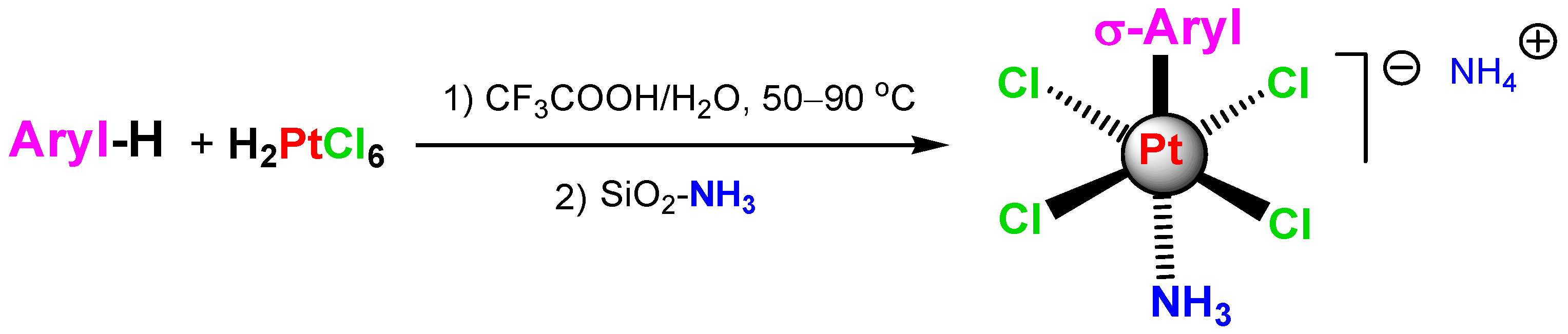
Sigma-aryl complexes of platinum(IV) obtained via the electrophilic substitution reaction between H2PtCl6 and arenes in CF3COOH or CH3COOH (so-called Shul’pin reaction as noted by Matsumoto [294], Sanford [295], and Jones [296]) (Scheme 5) turned out to be more stable than sigma-alkyl complexes and have been isolated in the form stabilized by NH3 ligands. These complexes were demonstrated to play the role of models of sigma-alkyl platinum complexes generated as intermediates in the reactions with alkanes [1,287,297,298,299]. For selected recent original publications on organoplatinum chemistry including carbon–hydrogen bond functionalizations, see [300,301,302,303,304].
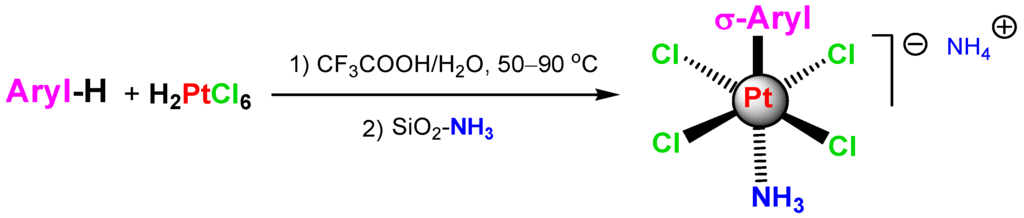
Scheme 5.
The reaction of H2PtCl6 with arenes to afford σ-aryl derivatives of Pt(IV).
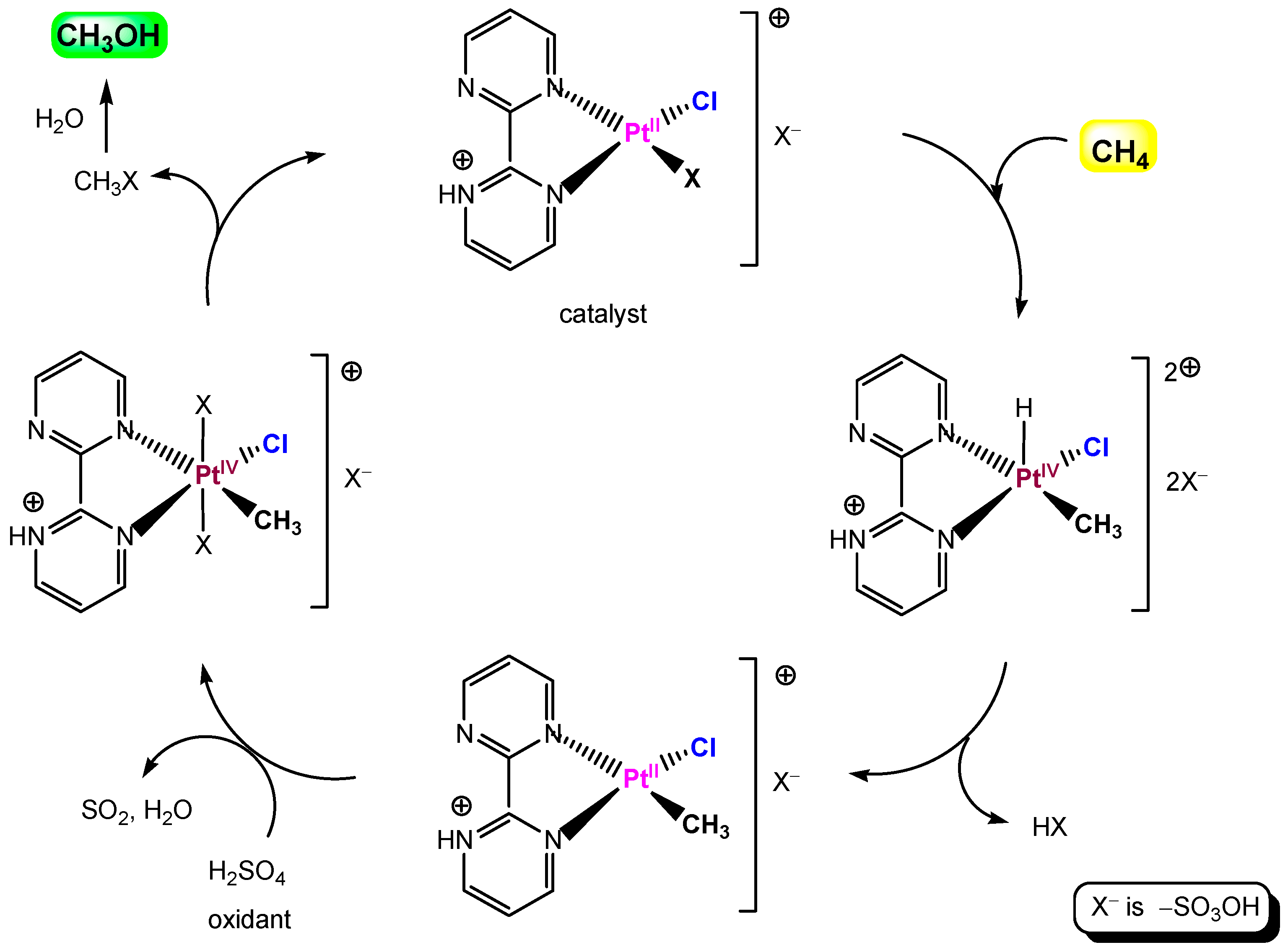
5.2. Periana System
Platinum complexes derived from the bidiazine ligand family, discovered by Periana and colleagues [305,306,307], catalyze oxidation of methane by sulfuric acid to a methanol derivative in >70% one-pass yield based on methane. These complexes are very stable in concentrated sulfuric acid at relatively high temperature and are among the most effective catalysts for methane conversion. A simplified scheme of the catalytic cycle is shown in Figure 22.
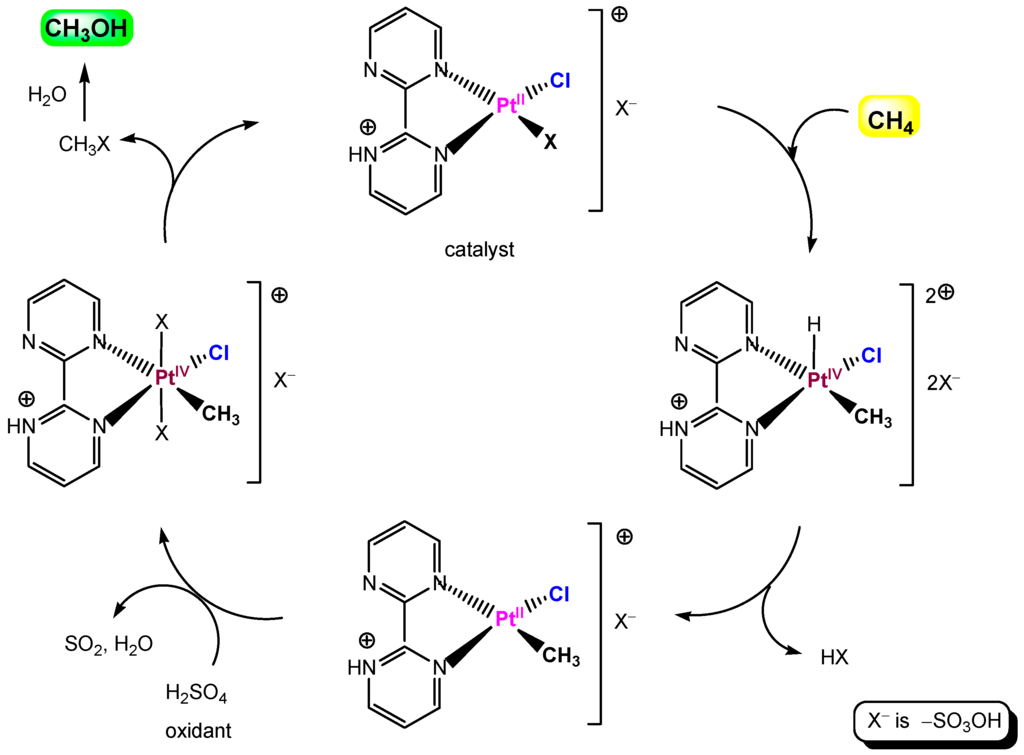
Figure 22.
A catalytic cycle for the methane oxidation by Periana system.
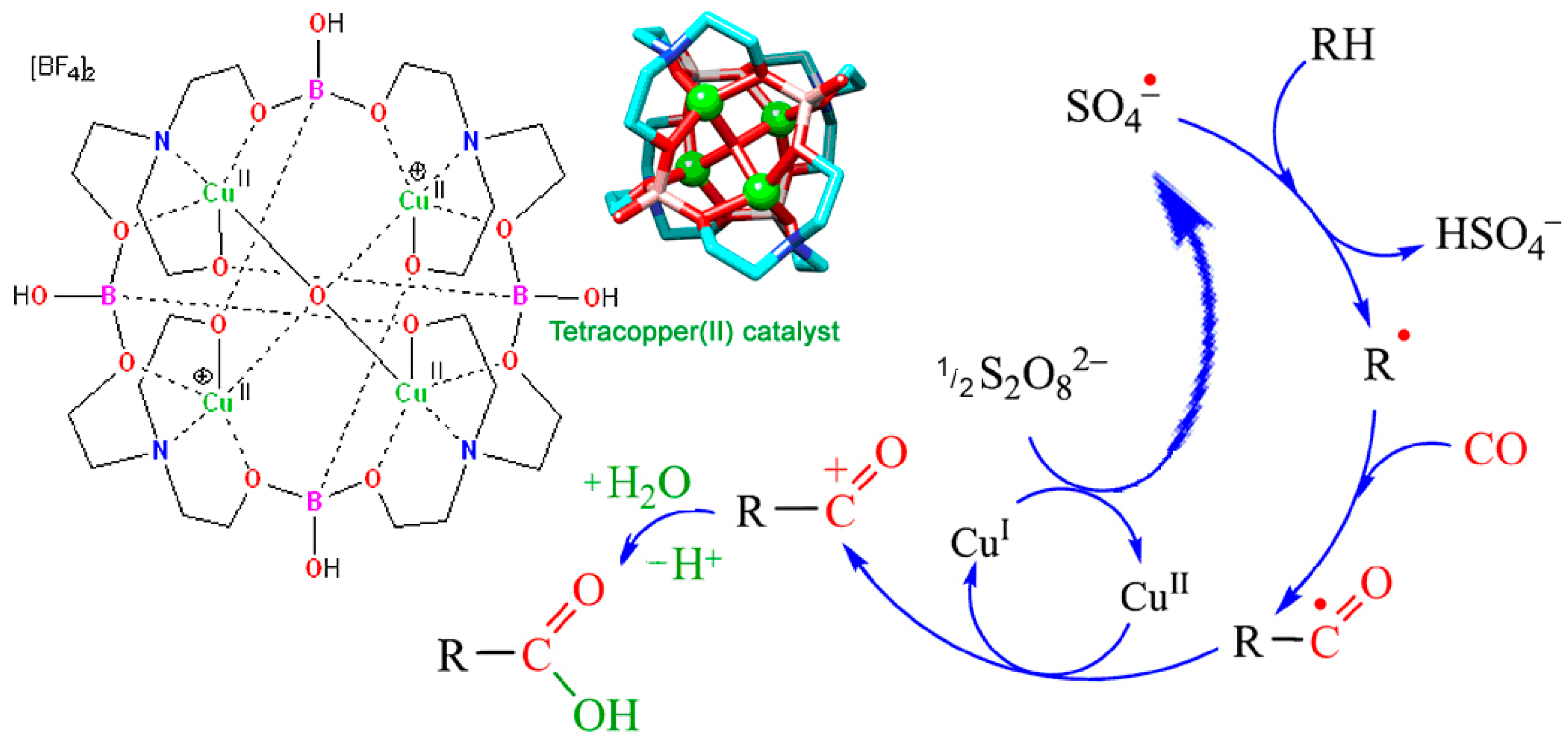
5.3. Carboxylation of C–H Compounds (Sen-Fujiwara-Pombeiro Reaction)
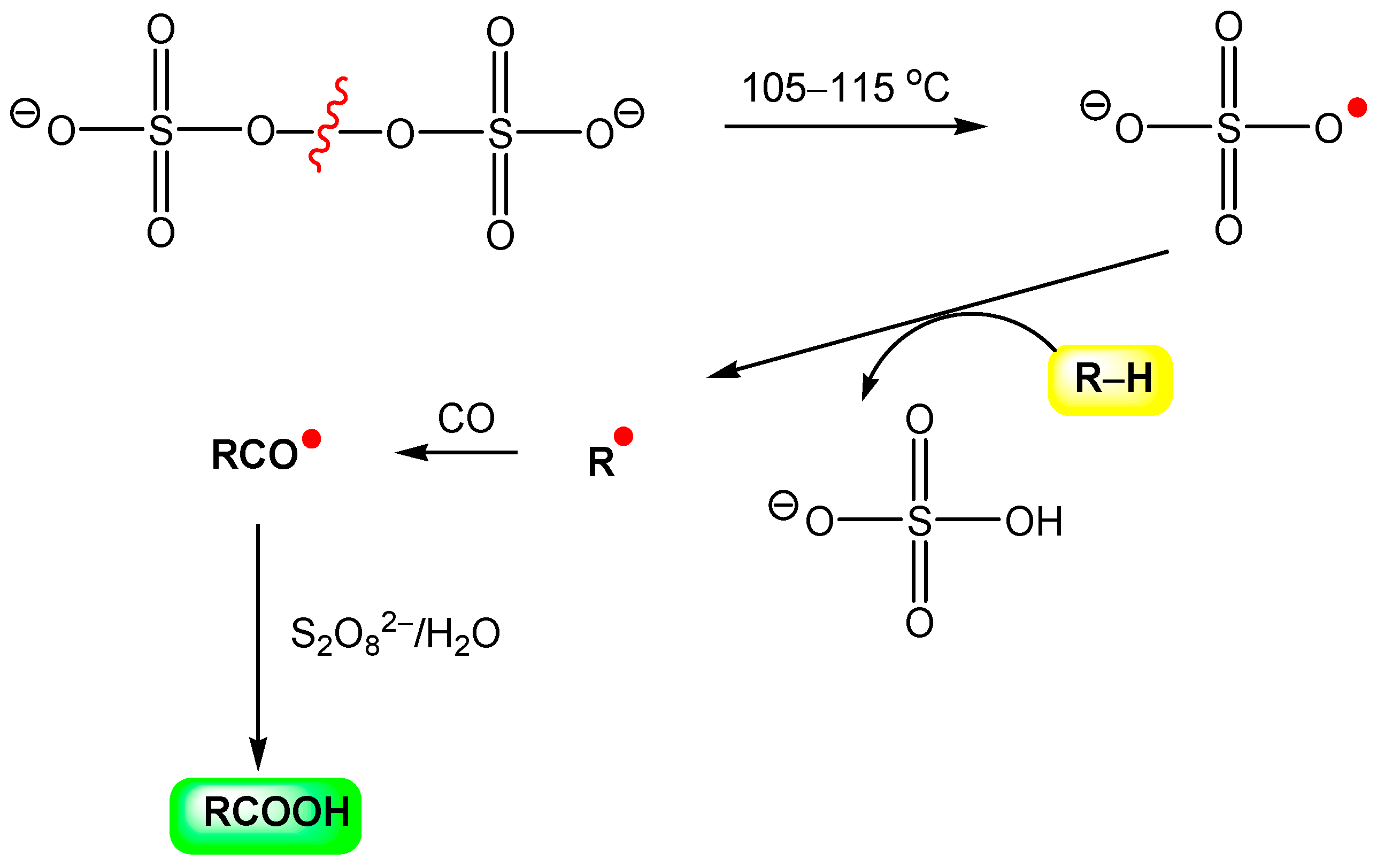
In 1992, Sen reported that, in aqueous medium anion-radical SO4‒• (generated from S2O82‒) abstracts,
“a hydrogen atom from methane and ethane to form the corresponding alkyl radicals which could be trapped efficiently by carbon monoxide, the resultant acyl radicals being ultimately converted into the homologous carboxylic acids”.[308]
The reaction can be presented by Figure 23.
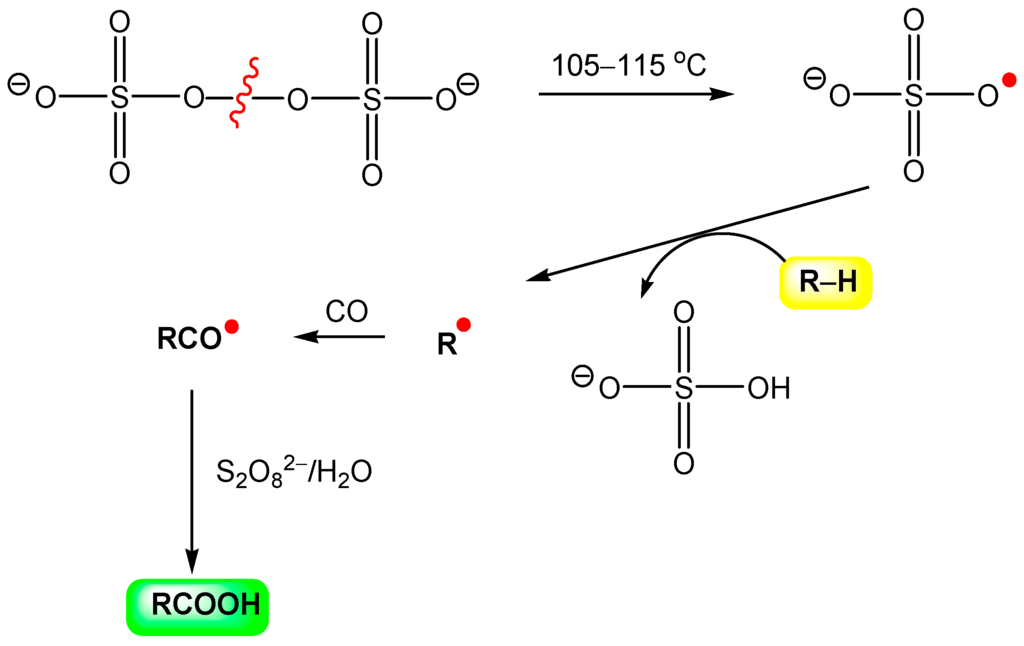
Figure 23.
Hydrocarbonylation of alkane, RH, with hexaoxo-μ-peroxodisulfat, S2O82‒, and CO. Adapted from Ref. [308].
Later, Fujiwara [309,310,311] and Pombeiro [312,313,314,315] demonstrated that this reaction can be sufficiently improved if various catalysts are used. Derivatives of copper, vanadium, and other metals served as catalysts. A simplified scheme of this reaction is shown in Figure 24. A number of works have been published by other authors in recent years [316].
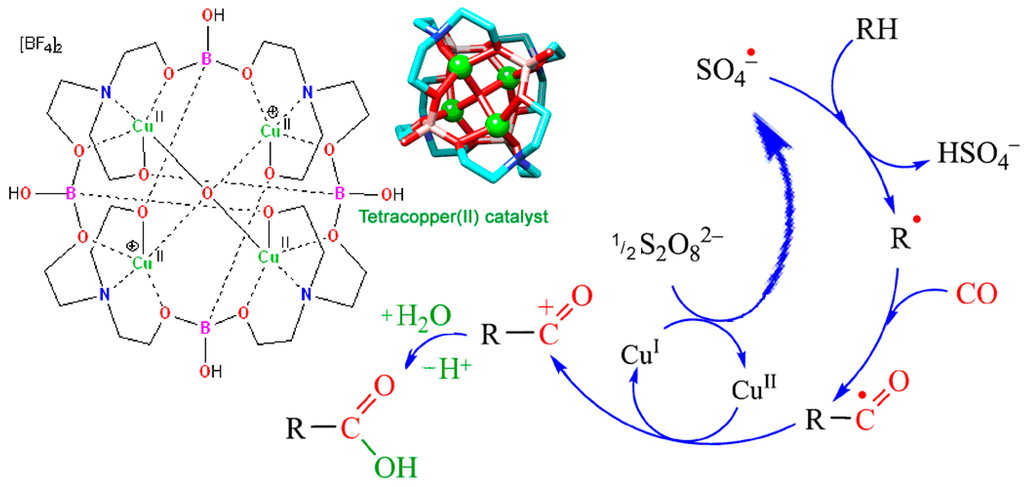
Figure 24.
Alkane (RH) carboxylation with S2O82‒/CO system catalyzed by a copper salt. Adapted from Refer. [313].
6. Outlook
We can see a few routes to further develop the field of metal-catalyzed functionalization of C–H compounds. One of these ways is the search of complexes of as yet unexplored metals. It has been found recently that osmium complexes catalyze the alkane oxidation with huge TONs [144,145,146,148]. For example, the oxidation of cyclohexane with hydrogen peroxide at 60 °C and low concentration of Os-catalyst (10−7 M) gave a turnover number (TON) of 200,000 after 24 h. [146].
Polynuclear metal complexes containing voluminous organic [63,66,118,119,129,130,131,186,213,214,215,216,217,218,234,235,236,237,238,239,240,241] or siloxane [221,222,223,224,225,226,227,228] ligands are also very efficient in oxidations of organic compounds including saturated hydrocarbons with peroxides. For example, dinuclear copper complex 11 [221,224] and hexairon complex 15 (for the structure, see Figure 9) affording the products of cyclohexane oxidation in a very high yield of 46% (see Figure 10 [226]) can be considered as “inorganic models of methane monooxygenases.” These enzymes contain dinuclear copper [317,318] and iron [319] reaction centers, respectively. A new method of synthesis of heterometallic coordination compounds by spontaneous self-assembly (SSA) gave complexes which are very efficient catalysts in oxidations with peroxides (turnover frequency TOF was up to 11200 h−1 = 3.1 s−1) [160]. This approach modeling natural enzymes can be called biomimetic route. Various polynuclear complexes [320,321,322] including siloxane derivatives [221,222,223,224,225,226,227,228] containing bulky ligands are especially attractive as catalysts in oxidations with peroxides. Bulky ligands around the reaction centers give rise in these cases to the selectivity enhancement [12,323].
Another route that uses as oxidants dioxygen or even concentrated sulfuric acid [305,306,307,308] that require high temperatures seems to be more attractive from the point of view of industrial applications. It is noteworthy that the Periana reaction occurring under severe conditions employs a metal complex catalyst (but not a simple salt). We may hope that new practically useful systems will be discovered in the near future.
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (grant No. 16-03-00254) and the “Science without Borders Program, Brazil-Russia,” CAPES (grant A017-2013).
Conflicts of Interest
The author declares no conflict of interest.
References
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, Holland; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Crabtree, R.H. Organomeallic alkane CH activation. J. Organomet. Chem. 2004, 689, 4083–4091. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biochemistry; Taylor & Francis: Boca Raton, FL, USA; London, UK; New York, NY, USA; Singapore, 2005. [Google Scholar]
- Hartwig, J.F. Evolution of C–H bond functionalization from methane to methodokogy (Review). J. Am. Chem. Soc. 2016, 138, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M.L.; Morton, D. Recent advances in C–H functionalization. J. Org. Chem. 2016, 81, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, T.; Baran, P.S. If C–H bonds could talk: Selective C–H bond oxidation. Angew. Chem. Int. Ed. 2011, 50, 3362–3374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Crabtree, R.H. C–H Oxidation by platinum group metal oxo or peroxo species. Chem. Soc. Rev. 2011, 40, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Bolaño, T.; Gunnoe, B. Catalytic oxy-functionalization of methane and other hydrocarbons: Fundamental advancement and new strategies. ChemSusChem 2011, 4, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Chepaikin, E.G. Homogeneous catalysis in the oxidative functionalization of alkanes in protic media. Russ. Chem. Rev. 2011, 80, 363–396. [Google Scholar] [CrossRef]
- Labinger, J.A.; Bercaw, J.E. The role of higher oxidation state species in platinum-mediated C–H bond activation and functionalization. Top. Organomet. Chem. 2011, 35, 29–60. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon oxygenations with peroxides catalyzed by metal compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. C–H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Periana, R.A.; Bhalla, G.; Tenn, W.J., III; Young, K.J.H.; Liu, X.Y.; Mironov, O.; Jones, C.J.; Ziatdinov, V.R. Perspectives on some challenges and approaches for developing the next generation of selective, low temperature, oxidation catalysts for alkane hydroxylation based on the CH activation reaction. J. Mol. Catal. A 2004, 220, 7–25. [Google Scholar] [CrossRef]
- Conley, B.L.; Ganesh, S.K.; Gonzales, J.M.; Ess, D.H.; Nielsen, R.J.; Ziatdinov, V.R.; Oxgaard, J.; Goddard, W.A., III; Periana, R.A. Facile oxy-functionalization of a nucleophilic metal alkyl with a cis- dioxo metal species via a (2+3) transition state. Angew. Chem. Int. Ed. 2008, 47, 7849–7852. [Google Scholar] [CrossRef] [PubMed]
- Bischof, S.M.; Ess, D.H.; Meier, S.K.; Oxgaard, J.; Nielsen, R.J.; Bhalla, G.; Goddard, W.A., III; Periana, R.A. Benzene C–H bond activation in carboxylic acids catalyzed by O-donor iridium(III) complexes: An experimental and density functional study. Organometallics 2010, 29, 742–756. [Google Scholar] [CrossRef]
- Hashiguchi, B.G.; Young, K.J.H.; Yousufuddin, M.; Goddard, W.A., III; Periana, R.A. Acceleration of nucleophilic CH activation by strongly basic solvents. J. Am. Chem. Soc. 2010, 132, 12542–12545. [Google Scholar] [CrossRef] [PubMed]
- Labinger, J.A.; Bercaw, J.E. Understanding and exploiting C–H bond activation. Nature 2002, 417, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Munz, D.; Webster-Gardiner, M.; Fu, R.; Strassner, T.; Goddard, W.A., III; Gunnoe, T.B. Proton or metal? The H/D exchange of arenes in acidic solvents. ACS Catal. 2015, 5, 769–775. [Google Scholar] [CrossRef]
- Burton, S.G. Oxidizing enzymes as biocatalysts. Trends Biotechnol. 2003, 21, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Coon, M.J. Omega oxygenases: Nonheme-iron enzymes and P450 cytochromes. Biochem. Biophys. Res. Commun. 2005, 338, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Borovik, A.S. Lessons from nature: Unraveling biological C–H bond activation. Curr. Opin. Chem. Biol. 2009, 13, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Austin, R.N.; Groves, J.T. Alkane-oxidizing metalloenzymes in the carbon cycle. Metallomics 2011, 3, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ding, Y. Recent advances in biocatalyst discovery, development and applications. Bioorg. Med. Chem. 2014, 22, 5604–5612. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Hirota, S. Artificial enzymes with protein scaffolds: Structural design and modification. Bioorg. Med. Chem. 2014, 22, 5638–5656. [Google Scholar] [CrossRef] [PubMed]
- Doble, M.V.; Ward, A.C.C.; Deuss, P.J.; Jarvis, A.G.; Kamer, P.C.J. Catalyst design in oxidation chemistry; from KMnO4 to artificial metalloenzymes. Bioorg. Med. Chem. 2014, 22, 5657–5677. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, M.R.A.; Borowsky, T.; Himo, F.; Liao, R.-Z.; Siegbahn, P.E.M. Quantum chemical studies of mechanisms for metalloenzymes. Chem. Rev. 2014, 114, 3601–3658. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Heme enzyme structure and function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [PubMed]
- Zhuk, T.S.; Goldman, M.; Hofmann, J.; Pohl, J.C.S.; Zorn, H. Preparative aerobic oxidations with basidiomycetous enzymes: CH-functionalization of adamantane. J. Mol. Catal. B 2015, 122, 87–92. [Google Scholar] [CrossRef]
- Ishii, Y.; Sakaguchi, S. Recent progress in aerobic oxidation of hydrocarbons by N-hydroxyimides. Catal. Today 2006, 117, 105–113. [Google Scholar] [CrossRef]
- Vastin, B.A.; Hall, M.B. The molecular and electronic structure of carbon–hydrogen bond activation and transition metal assisited hydrogen transfer. Coord. Chem. Rev. 2009, 253, 1202–1218. [Google Scholar] [CrossRef]
- Gunay, A.; Theopold, H.H. C–H bond activations by metal oxo compounds. Chem. Rev. 2010, 110, 1060–1081. [Google Scholar] [CrossRef] [PubMed]
- Chepaikin, E.G. Oxidative functionalization of alkanes under dioxygen in the presenceof homogeneous noble metal catalysts. J. Mol. Catal. A 2014, 385, 160–174. [Google Scholar] [CrossRef]
- Alkane C‒H Activation by Single-Site Metal Catalysis; Pérez, P.J. (Ed.) Springer: Heidelberg, Germany, 2012.
- Cavaliere, V.N.; Mindiola, D.J. Methane: A new frontier in organometallic chemistry. Chem. Sci. 2012, 3, 3356–3365. [Google Scholar] [CrossRef]
- Campbell, A.N.; Stahl, S.S. Overcoming the “Oxidant Problem”: Strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalysed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Borylation and silylation of C–H bonds: A platform for diverse C–H bond functionalizations. Acc. Chem. Res. 2012, 45, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, B.G.; Bischof, S.M.; Konnick, M.M.; Periana, R.A. Designing catalysts for functionalization of unactivated C–H bonds based on the CH activation reaction. Acc. Chem. Res. 2012, 45, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.L.; Harvey, M.E.; Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 2012, 45, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishna, A.; Suman, P.; Goud, E.V.; Janardan, S.; Sravani, C.; Sandep, T.; Vijayakrishna, K.; Clayton, H.S. Review: Active homogeneous reagents and catalysts in n-alkane activation reactions. J. Coord. Chem. 2013, 66, 2091–2109. [Google Scholar] [CrossRef]
- Caballero, A.; Pérez, P.J. Methane as raw material in synthetic chemistry: The final frontier. Chem. Soc. Rev. 2013, 42, 8809–8820. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, J.; Putaj, P.; Caps, V.; Lefebre, F.; Pelletier, J.; Basset, J.-M. Catalytic oxidation of light alkanes (C1-C4) by heteropoly compounds. Chem. Rev. 2014, 114, 981–1019. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Singh, T. Synthesis of biaryls through aromatic C–H bond activation: A review of recent developments. Adv. Synth. Catal. 2014, 356, 1661–1696. [Google Scholar] [CrossRef]
- Guan, W.; Sayyed, F.B.; Zeng, G.; Sakaki, S. σ-Bond activation of small molecules and reactions catalyzed by transition-metal complexes: Theoretical understanding of electronic processes. Inorg. Chem. 2014, 53, 6444–6457. [Google Scholar] [CrossRef] [PubMed]
- Landaeta, V.R.; Rodríguez-Lugo, R.E. Catalytic oxygenation of organic substrates: Toward greener ways for incorporating oxygen. Inorg. Chim. Acta 2015, 431, 21–47. [Google Scholar] [CrossRef]
- Horn, R.; Schlogl, R. Methane activation by heterogeneous catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Souillart, L.; Cramer, N. Catalytic C–C bond activation via oxidative addition to transition metals. Chem. Rev. 2015, 115, 9410–9464. [Google Scholar] [CrossRef] [PubMed]
- Kuninobu, Y.; Sueki, S. C–H Bond transformations leading to the synthesis of organic functional materials. Synthesis 2015, 47, 3823–3845. [Google Scholar] [CrossRef]
- Reay, A.J.; Fairlamb, J.S. Catalytic C–H bond functionalisation chemistry: The case for quasi-heterogeneous catalysis. Chem. Commun. 2015, 51, 16289–16307. [Google Scholar] [CrossRef] [PubMed]
- Chepaikin, E.G.; Borsch, V.N. Rhodium complexes in homogeneous catalytic systems for oxidative functionalization of alkanes: Experiment and quantum-chemical calculations. J. Organomet. Chem. 2015, 793, 78–82. [Google Scholar] [CrossRef]
- Munz, D.; Strassner, T. Alkane C–H functionalization and oxidation with molecular oxygen. Inorg. Chem. 2015, 54, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Haque, A.; Al-Suti, M.K.; Raithby, P.R. Recent advances in the application of group-10 transition metal based catalysts in C–H activation and functionalization. J. Organomet. Chem. 2015, 793, 114–133. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M.; Chadwick, J.C. Homogeneous Catalysis: Activity, Stability and Deactivation; Wiley: Weinheim, Germany, 2011. [Google Scholar]
- Kozuch, S.; Shaik, S. How to conceptualize catalytic cycles? The energetic span model. Acc. Chem. Res. 2011, 44, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kozuch, S.; Martin, J.M.L. “Turning over” definitions in catalytic cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Direct and efficient transformation of gaseous alkanes into carboxylic acids catalyzed by vanadium containing heteropolyacids. Appl. Catal. A 2007, 332, 159–165. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kirillova, M.V.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Pombeiro, A.J.L. Decamethylosmocene-catalyzed efficient oxidation of saturated and aromatic hydrocarbons and alcohols with hydrogen peroxide in the presence of pyridine. Part 3 from the series “Oxidations Catalyzed by Osmium Compounds”. J. Catal. 2011, 277, 164–172. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Arutyunov, V.S.; dos Santos, A.C.M.; Ferreira, A.C.T.; Mandelli, D. Oxidations by the system “hydrogen peroxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–oxalic acid.” Part 6. Oxidation of methane and other alkanes and olefins in water. J. Organomet. Chem. 2005, 690, 4498–4504. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Kudrik, E.V.; Alvarez, L.X.; Afanasiev, P.; Millet, J.M.M.; Bouchu, D. Oxidation of methane and ethylene in water at ambient conditions. Catal. Today 2010, 157, 149–154. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Druzhinina, A.N. Iron(III) chloride catalysed photooxygenation of alcohol solutions of alkanes by atmospheric oxygen. Mendeleev Commun. 1992, 2, 36–37. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N. Photochemical aerobic oxidation of alkanes promoted by iron complexes. Part 32 of the series “Photoinduced Reactions of Organic Compounds with Metal Complexes”. New J. Chem. 1996, 20, 1243–1256. [Google Scholar]
- Lamaty, F.; Dupont, J. Preface to the special issue on “Catalysis in neoteric solvent or alternative media”. Catal. Commun. 2015, 63, 1. [Google Scholar] [CrossRef]
- Jlassi, R.; Ribeiro, A.P.C.; Guedes da Silva, M.F.C.; Mahmudov, K.T.; Kopylovich, M.N.; Anisimova, T.B.; Pombeiro, A.J.L. Polynuclear copper(II) complexes as catalysts for the peroxidative oxidation of cyclohexane in a room-temperature ionic liquid. Eur. J. Inorg. Chem. 2014, 4541–4550. [Google Scholar] [CrossRef]
- Chepaikin, E.G.; Bezruchenko, A.P.; Menchikova, G.N.; Moiseeva, N.I.; Gekhman, A.E. Direct catalytic oxidation of lower alkanes in ionic liquid media. Petrol. Chem. 2014, 54, 374–381. [Google Scholar] [CrossRef]
- Lentini, S.; Galloni, P.; Garcia-Bosch, I.; Costas, M.; Conte, V. Ionic liquids as reaction media in catalytic oxidations with manganese and iron pyridyl triazacyclononane complexes. Inorg. Chim. Acta 2014, 410, 60–64. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. C. R. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Transforming CdS into an efficient visible light photocatalyst for selective oxidation of saturated primary C–H bonds under ambient conditions. Chem. Sci. 2012, 3, 2812–2822. [Google Scholar] [CrossRef]
- Ohtani, B. Titania photocatalysis beyond recombination: A critical review. Catalysts 2013, 3, 942–953. [Google Scholar] [CrossRef]
- Vaiano, V.; Sannino, D.; Almeida, A.R.; Mul, G.; Ciambelli, P. Investigation of the deactivation phenomena occurring in the cyclohexane photocatalytic oxidative dehydrogenation on MoOx/TiO2 through gas phase and in situ DRIFTS analyses. Catalysts 2013, 3, 978–997. [Google Scholar] [CrossRef]
- Hoffmann, N. Combining photoredox and metal catalysis. ChemCatChem 2015, 7, 393–394. [Google Scholar] [CrossRef]
- Protti, S.; Fagnoni, M.; Ravelli, D. Photocatalytic C–H activation by hydrogen-atom transfer in synthesis. ChemCatChem 2015, 7, 1516–1523. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yuan, Q.; He, J.; Au, C.-T.; Yin, S.-F. A green and efficient photocatalytic route for the highly-selective oxidation of saturated alpha-carbon C–H bonds in aromatic alkanes over flower-like Bi2WO6. Chem. Commun. 2016, 52, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, J.M.; Menendez, J.A.; Arenillas, A.; Martínez-Palou, R.; Antonio, A.; Romero, A.A.; Luque, R. Graphene oxide-catalysed oxidation reaction of unsaturated compounds under microwave irradiation. Catal. Commun. 2015, 72, 133–137. [Google Scholar] [CrossRef]
- Joglekar, M.; Nguyen, V.; Pylypenko, S.; Ngo, C.; Li, Q.; O’Reilly, M.E.; Gray, T.S.; Hubbard, W.A.; Gunnoe, T.B.; Herring, A.M.; Trewyn, B.G. Organometallic complexes anchored to conductive carbon for electrocatalytic oxidation of methane at low temperature. J. Am. Chem. Soc. 2016, 216, 116–125. [Google Scholar]
- Cravotto, G.; Borretto, E.; Oliverio, M.; Procopio, A.; Penoni, A. Organic reactions in water or biphasic aqueous systems under sonochemical conditions. A review on catalytic effects. Catal. Commun. 2015, 63, 2–9. [Google Scholar] [CrossRef]
- Garner, A.W.; Harris, C.F.; Vezzu, D.A.K.; Pike, R.D.; Huo, S. Solvent-controlled switch of selectivity between sp2 and sp3 C–H bond activation by platinum(II). Chem. Commun. 2011, 47, 1902–1904. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Nizova, G.V. Selective photochemical ketonization of cyclohexane by air in aqueous emulsion in the presence of iron ions. Mendeleev Commun. 1995, 143–145. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Efficient H2O2 oxidation of alkanes and arenes to alkyl peroxides and phenols catalyzed by the system vanadate-pyrazine-2-carboxylic acid. J. Catal. 1993, 142, 147–152. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Oxidations by a H2O2-VO3−-pyrazine-2-carboxylic acid reagent. 1. Oxidations of alkanes in CH3CN to produce alkyl peroxides. Russ. Chem. Bull. 1993, 42, 55–59. [Google Scholar] [CrossRef]
- Nizova, G.V.; Shul’pin, G.B. Oxidation by a H2O2-vanadium complex-2-pyrazinecarboxylic acid reagent. 3. Evidence for hydroxyl radical formation. Russ. Chem. Bull. 1994, 43, 1146–1148. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Drago, R.S.; Gonzalez, M. Oxidations by a “H2O2-vanadium complex–pyrazine-2-carboxylic acid” reagent. 5. Oxidation of lower alkanes with the formation of carbonyl compounds. Russ. Chem. Bull. 1996, 45, 2386–2388. [Google Scholar]
- Shul’pin, G.B.; Guerreiro, M.C.; Schuchardt, U. Oxidations by the reagent O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid. Part 7. Hydroperoxidation of higher alkanes. Tetrahedron 1996, 52, 13051–13062. [Google Scholar] [CrossRef]
- Nizova, G.V.; Süss-Fink, G.; Shul’pin, G.B. Oxidations by the reagent «O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid»-8. Efficient oxygenation of methane and other lower alkanes in acetonitrile. Tetrahedron 1997, 53, 3603–3614. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Ishii, Y.; Sakaguchi, S.; Iwahama, T. Oxidations with the “O2-H2O2-vanadium complex-pyrazine-2-carboxylic acid” reagent. 11. Oxidation of styrene, phenylacetylene, and their derivatives with the formation of benzaldehyde and benzoic acid. Russ. Chem. Bull. 1999, 48, 887–890. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiy, A.; Kulikova, V.S. Oxidations by the reagent "O2-H2O2-vanadium derivative–pyrazine-2-carboxylic acid" Part 12. Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar]
- De la Cruz, M.H.C.; Kozlov, Y.N.; Lachter, E.R.; Shul’pin, G.B. Oxidations by the reagent “O2-H2O2-vanadium derivative-pyrazine-2-carboxylic acid.” Part 13. Kinetics and mechanism of the benzene hydroxylation. New J. Chem. 2003, 27, 634–638. [Google Scholar] [CrossRef]
- Khaliullin, R.Z.; Bell, A.T.; Head-Gordon, M. A density functional theory study of the mechanism of free radical generation in the system vanadate/PCA/H2O2. J. Phys. Chem. B 2005, 109, 17984–17992. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, Y.N.; Romakh, V.B.; Kitaygorodskiy, A.; Buglyó, P.; Süss-Fink, G.; Shul’pin, G.B. Oxidation of 2-propanol and cyclohexane by the reagent “Hydrogen peroxide-vanadate anion-pyrazine-2-carboxylic acid”: Kinetics and mechanism. J. Phys. Chem. A 2007, 111, 7736–7752. [Google Scholar] [CrossRef] [PubMed]
- Romakh, V.B.; Süss-Fink, G.; Shul’pin, G.B. Vanadate ion-catalyzed oxidation of methane with hydrogen peroxide in an aqueous solution. Petrol. Chem. 2008, 48, 440–443. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of oxidations with H2O2 catalyzed by vanadate anion or oxovanadium(V) triethanolaminate (vanadatrane) in combination with pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Menini, L.; Parreira, L.A.; Mesquita, R.A.; Kozlov, Y.N.; Shul’pin, G.B. Oxidation of isoeugenol to vanillin by the “H2O2-vanadate-pyrazine-2-carboxylic acid” reagent. J. Mol. Catal. A 2012, 363–364, 140–147. [Google Scholar] [CrossRef]
- Sutradhar, M.; Shvydkiy, N.V.; da Silva, M.F.C.G.; Kirillova, M.V.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. New binuclear oxovanadium(V) complex as a catalyst in combination with pyrazinecarboxylic acid (PCA) for efficient alkane oxygenation by H2O2. Dalton Trans. 2013, 42, 11791–11803. [Google Scholar] [CrossRef] [PubMed]
- Nizova, G.V.; Krebs, B.; Süss-Fink, G.; Schindler, S.; Westerheide, L.; Gonzalez Cuervo, L.; Shul’pin, G.B. Hydroperoxidation of methane and other alkanes with H2O2 catalysed by a dinuclear iron complex and an amino acid. Tetrahedron 2002, 58, 9231–9237. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Gonzalez Cuervo, L.; Süss-Fink, G. Hydrogen peroxide oxygenation of alkanes including methane and ethane catalyzed by iron complexes in acetonitrile. Adv. Synth. Catal. 2004, 346, 317–332. [Google Scholar] [CrossRef]
- Romakh, V.B.; Therrien, B.; Süss-Fink, G.; Shul’pin, G.B. Synthesis, molecular structure and catalytic potential of the tetrairon complex [Fe4(N3O2-L)4(μ-O)2]4+ (L = 1-carboxymethyl-4,7-dimethyl-1,4,7-triazacyclononane). Inorg. Chem. 2007, 46, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D.; Carvalho, W.A.; Kozlov, Y.N.; Vinogradov, M.M.; Ikonnikov, N.S.; Shul’pin, G.B. Oxidation of alkanes and benzene with hydrogen peroxide catalyzed by ferrocene in the presence of acids. J. Organomet. Chem. 2015, 793, 217–231. [Google Scholar] [CrossRef]
- Nizova, G.V.; Shul’pin, G.B. A unique rate-accelerating effect of certain amino acids in the H2O2 oxidation of alkanes catalyzed by a dinuclear manganese complex containing 1,4,7-trimethyl-1,4,7-triazacyclononane. Part 9 from the series “Oxidations by the system ‘hydrogen peroxide–[Mn2L2O3][PF6]2 (L =1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid”. Tetrahedron 2007, 63, 7997–8001. [Google Scholar]
- Shul’pin, G.B.; Matthes, M.G.; Romakh, V.B.; Barbosa, M.I.F.; Aoyagi, J.L.T.; Mandelli, D. Oxidations by the system “hydrogen peroxide–[Mn2L2O3][PF6]2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)–carboxylic acid.” Part 10: Co-catalytic effect of different carboxylic acids in the oxidation of cyclohexane, cyclohexanol, and acetone. Tetrahedron 2008, 64, 2143–2152. [Google Scholar] [CrossRef]
- Schuchardt, U.; Mandelli, D.; Shul’pin, G.B. Methyltrioxorhenium catalyzed oxidation of saturated and aromatic hydrocarbons by H2O2 in air. Tetrahedron Lett. 1996, 37, 6487–6490. [Google Scholar] [CrossRef]
- Karslyan, E.E.; Shul’pina, L.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxygenation of saturated and aromatic hydrocarbons with H2O2 catalyzed by the carbonyl thiophenolate iron complex (OC)3Fe(PhS)2Fe(CO)3. Catal. Today 2013, 218–219, 93–98. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Kirillova, M.V.; da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Oxidations of cycloalkanes and benzene by hydrogen peroxide catalyzed by an {FeIIIN2S2} centre. Appl. Catal. A 2009, 353, 107–112. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; Guedes da Silva, M.F.C.; da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Mild alkane C–H and O-H oxidations catalysed by mixed-N,S copper, iron and vanadium systems. Appl. Catal. A 2011, 402, 110–120. [Google Scholar] [CrossRef]
- Milunovic, M.N.M.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; Krachler, R.; Trettenhahn, G.; Turta, C.; Shova, S.; Arion, V.B. Hexanuclear and undecanuclear iron(III) carboxylates as catalyst precursors for cyclohexane oxidation. Dalton Trans. 2013, 40, 14388–14401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carabineiro, S.A.C.; Martins, L.M.D.R.S.; Avalos-Borja, M.; Buijnsterse, J.G.; Pombeiro, A.J.L.; Figueiredo, J.L. Gold nanoparticles supported on carbon materials for cyclohexane oxidation with hydrogen peroxide. Appl. Catal. A 2013, 467, 279–290. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M. 2-Pyrazinecarboxylic Acid. In Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; Wiley: Chichester, UK.
- Piquemal, J.-Y.; Briot, E.; Brégeault, J.-M. Preparation of materials in the presence of hydrogen peroxide: From discrete or “zero-dimensional” objects to bulk materials. Dalton Trans. 2013, 42, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Khenkin, A.M.; Neumann, R. Carbon–hydrogen bond activation of arenes and alkylarenes by electron transfer followed by oxygen transfer catalyzed by vanadium substituted polyoxometalates—A comparative study of the reactivity of different polyoxometalate compounds. J. Organomet. Chem. 2015, 793, 134–138. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Evtushok, V.Y.; Maksimov, G.M.; Kholdeeva, O.A. Selective oxidation of pseudocumene and 2-methylnaphthalene with aqueous hydrogen peroxide catalyzed by Keggin divanadium-substituted polyoxotungstate. J. Organomet. Chem. 2015, 793, 210–216. [Google Scholar] [CrossRef]
- Jing, F.; Katryniok, B.; Bordes-Richard, E.; Dumeignil, F.; Paul, S. Structural evolution under reaction conditions of supported (NH4)3HPMo11VO40 catalysts for the selective oxidation of isobutane. Catalysts 2015, 5, 460–477. [Google Scholar] [CrossRef]
- Alberto, J.; Garcia, H. Doped graphenes in catalysis. J. Mol. Catal. A 2015, 408, 296–309. [Google Scholar] [CrossRef]
- Gruselle, M. Apatites: A new family of catalysts in organic synthesis. J. Organomet. Chem. 2015, 793, 93–101. [Google Scholar] [CrossRef]
- Rahman, A.K.M.L.; Kumashiro, M.; Ishihara, T. Direct synthesis of formic acid by partial oxidation of methane on H-ZSM-5 solid acid catalyst. Catal. Commun. 2011, 12, 1198–1200. [Google Scholar] [CrossRef]
- Wannakao, S.; Warakulwit, C.; Kongpatpanich, K.; Probst, M.; Limtrakul, J. Methane activation in gold cation-exchanged zeolites: A DFT study. ACS Catal. 2012, 2, 986–992. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; Hammond, C.; He, Q.; Jenkins, R.L.; Kondrat, S.A.; Dimitratos, N.; Lopez-Sanchez, J.A.; Tylor, S.H.; Willock, D.; et al. Partial oxidation of ethane to oxygenates using Fe- and Cu-containing ZSM-5. J. Am. Chem. Soc. 2013, 135, 11087–11099. [Google Scholar] [CrossRef] [PubMed]
- Liquid Phase Oxidation via Heterogeneous Catalysis; Clerici, M.G.; Kholdeeva, O.A. (Eds.) Wiley: Hoboken, NJ, USA, 2013.
- Kholdeeva, O.A.; Skobelev, I.Y.; Ivanchikova, I.D.; Kovalenko, K.A.; Fedin., V.P.; Sorokin, A.B. Hydrocarbon oxidation over Fe- and Cr-containing metal-organic frameworks MIL-100 and MIL-101—A comparative study. Catal. Today 2014, 238, 54–61. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Recent developments in liquid-phase selective oxidation using environmentally benign oxidants and mesoporous metal silicates. Catal. Sci. Technol. 2014, 4, 1869–1889. [Google Scholar] [CrossRef]
- Alayon, E.M.C.; Nachtegaal, M.; Bodi, A.; Rabocchiari, M.; van Bokhoven, J.A. Bis(mu-oxo) versus mono(mu-oxo)dicopper cores in a zeolite for converting methane to methanol: An in situ XAS and DFT investigation. Phys. Chem. Chem. Phys. 2015, 17, 7681–7693. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Sun, Q.; Fan, D.; Fu, W.; Liu, L.; Wang, R. Facile synthesis of Yolk/Core-Shell structured TS-1@Mesosilica composites for enhanced hydroxylation of phenol. Catalysts 2015, 5, 2134–2146. [Google Scholar] [CrossRef]
- Dragomirova, R.; Wohlrab, S. Zeolite membranes in catalysis—From separate units to particle coatings. Catalysts 2015, 5, 2161–2222. [Google Scholar] [CrossRef]
- Hammond, C.; Hermans, I.; Dimitratos, N. Biomimetic oxidation with Fe-ZSM-5 and H2O2? Identification of an active, extra-framework binuclear core and an FeIII-OOH intermediate with resonance-enhanced Raman spectroscopy. ChemCatChem 2015, 7, 434–440. [Google Scholar] [CrossRef]
- Xie, J.; Pan, C.; Abdukader, A.; Zhu, C. Gold-catalyzed C(sp3)–H bond functionalization. Chem. Soc. Rev. 2014, 43, 5245–5256. [Google Scholar] [CrossRef] [PubMed]
- Strassner, T.; Ahrens, S.; Muehlhofer, M.; Munz, D.; Zeller, A. Cobalt-catalyzed oxidation of methane to methyl trifluoroacetate by dioxygen. Eur. J. Inorg. Chem. 2013, 3659–3663. [Google Scholar] [CrossRef]
- Bellows, S.M.; Thomas, R.; Cundari, T.R.; Jones, W.D. Methane is the best substrate for C(sp3)−H activation with Cp*(PMe3)Co(Me)(OTf): A density functional theory study. Organometallics 2015, 34, 4032–4038. [Google Scholar] [CrossRef]
- Moselage, M.; Li, J.; Ackermann, L. Cobalt-catalyzed C–H activation. ACS Catal. 2016, 6, 498–525. [Google Scholar] [CrossRef]
- Goo, Y.R.; Maity, A.C.; Cho, K.-B.; Lee, Y.-M.; Seo, M.S.; Park, Y.J.; Cho, J.; Nam, W. Tuning the reactivity of chromium(III)-superoxo species by coordinating axial ligands. Inorg. Chem. 2015, 54, 10513–10520. [Google Scholar] [CrossRef] [PubMed]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Nunes, A.C.C.; Mahmudov, K.T.; Haukka, M.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Complexes of copper(II) with 3-(ortho-substituted phenylhydrazo)pentane-2,4-diones: Syntheses, properties and catalytic activity for cyclohexane oxidation. Dalton Trans. 2011, 40, 2822–2836. [Google Scholar] [CrossRef] [PubMed]
- Perraud, O.; Sorokin, A.B.; Dutasta, J.P.; Martinez, A. Oxidation of cycloalkanes by H2O2 using a copper-hemicryptophane complex as a catalyst. Chem. Commun. 2013, 49, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Nguyen, K.D.; Doan, S.H.; Phan, N.T.S. Efficient and recyclable Cu2(BPDC)2(DABCO)-catalyzed directamination of activated sp3 C–H bonds by N–H heterocycles. Appl. Catal. A 2016, 510, 27–33. [Google Scholar] [CrossRef]
- Bauer, E.B. Iron catalysis: Historic overview and current tends. Top. Organomet. Chem. 2015, 50, 1–18. [Google Scholar]
- Martínez-Ferraté, O.; Britovsek, G.J.P.; Carmen Claver, C.; van Leeuwen, PW.N.M. C–H benzylic oxidation promoted by dinuclear iron DBDOC iminopyridine complexes. Inorg. Chim. Acta 2015, 431, 156–160. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Catalytic behaviour of a novel Fe(III) Schiff base complex in the mild oxidation of cyclohexane. Catal. Sci. Technol. 2015, 5, 1801–1812. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Scott, B.L.; Viswanathan, H.S.; Sutton, A.D. Synthesis and reactivity of cis-FeH2(dcpe)2 (dcpe = 1,2-bis(dicyclohexylphosphino)ethane). Inorg. Chem. Commun. 2016, 63, 57–60. [Google Scholar] [CrossRef]
- D’Amato, E.M.; Neumann, C.N.; Ritter, T. Selective aromatic C–H hydroxylation enabled by η6-coordination to iridium(III). Organometallics 2015, 34, 4626–4631. [Google Scholar] [CrossRef] [PubMed]
- Wegermann, C.A.; Ribeiro, R.R.; Ucoski, G.M.; Nakagaki, S.; Nunes, F.S.; Drechsel, S.M. Study of the catalytic activity of non-heme manganese complexes toward oxidation of cyclooctene and cyclohexene. Appl. Catal. A 2014, 471, 56–62. [Google Scholar] [CrossRef]
- De Paula, R.; Santos, I.C.M.S.; Simões, M.M.Q.; Graça, M.; Neves, P.M.S.; Cavaleiro, J.A.S. An immobilized imidazolyl manganese porphyrin for the oxidation of olefins. J. Mol. Catal. A 2015, 404–405, 156–166. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Jeon, S.H.; Lee, Y.-M.; Seo, M.S.; Ohkubo, K.; Fukuzumi, S.; Nam, W. An amphoteric reactivity of a mixed-valent bis(μ-oxo)dimanganese(III,IV) complex acting as an electrophile and a nucleophile. Dalton Trans. 2016, 45, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.C.; Richard, R.; Schrock, R.R.; Kundu, S.; Goldman, A.S.; Huang, Z.; Brookhart, M. Evaluation of molybdenum and tungsten metathesis catalysts for homogeneous tandem alkane metathesis. Organometallics 2009, 28, 355–360. [Google Scholar] [CrossRef]
- Nagataki, T.; Ishii, K.; Tachi, Y.; Itoh, S. Ligand effects on NiII-catalysed alkane-hydroxylation with m-CPBA. Dalton Trans. 2007, 11, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kudinov, A.R.; Shul’pina, L.S.; Petrovskaya, E.A. Oxidations catalyzed by osmium compounds. Part 1: Efficient alkane oxidation with peroxides catalyzed by an olefin carbonyl osmium(0) complex. J. Organomet. Chem. 2006, 691, 837–845. [Google Scholar] [CrossRef]
- Yuan, Q.; Deng, W.; Zhang, Q.; Wang, Y. Osmium-catalyzed selective oxidations of methane and ethane with hydrogen peroxide in aqueous medium. Adv. Synth. Catal. 2007, 349, 1199–1209. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Süss-Fink, G.; Shul’pina, L.S. Oxygenation of alkanes with hydrogen peroxide catalysed by osmium complexes. Chem. Commun. 2000, 1131–1132. [Google Scholar]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Carvalho, W.; Mandelli, D. Oxidation reactions catalyzed by osmium compounds. Part 4. Highly efficient oxidation of hydrocarbons and alcohols including glycerol by the H2O2/Os3(CO)12/pyridine reagent. RSC Adv. 2013, 3, 15065–15074. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Kozlov, Y.N.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxidation of hydrocarbons with H2O2/O2 catalyzed by osmium complexes containing π-cymene ligands in acetonitrile. Catal. Sci. Technol. 2014, 4, 3214–3226. [Google Scholar] [CrossRef]
- Chen, M.; Yi, P.; Kwong, H.-K.; Zeng, R.J.; Lau, K.-C.; Lau, T.-C. Catalytic oxidation of alkanes by a (salen)osmium(VI) nitrido complex using H2O2 as the terminal oxidant. Chem. Commun. 2015, 51, 13686–13689. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, M.M.; Shul’pina, L.S.; Kozlov, Y.N.; Kudinov, A.R.; Ikonnikov, N.S.; Shul’pin, G.B. Oxidation of hydrocarbons and alcohols with peroxides catalyzed by new π-cymene osmium complexes. J. Organometal. Chem. 2015, 784, 52–61. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Musaev, D.G.; Figg, T.M.; Kaledin, A.L. Versatile reactivity of Pd-catalysts: Mechanistic features of the mono-N-protected amino acid ligand and cesium-halide base in Pd-catalyzed C–H bond functionalization. Chem. Soc. Rev. 2014, 43, 5009–5031. [Google Scholar] [CrossRef] [PubMed]
- Zultanski, S.L.; Stahl, S.S. Palladium-catalyzed aerobic acetoxylation of benzene using NOx-based redox mediators. J. Organomet. Chem. 2015, 793, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Gryca, I.; Machura, B.; Małecki, J.G.; Kusz, J.; Shul’pina, L.S.; Ikonnikov, N.S.; Shul’pin, G.B. p-Tolylimido rhenium(V) complexes with phenolate-based ligands: Synthesis, X-ray studies and catalytic activity in oxidation with tert-butylhydroperoxide. Dalton Trans. 2016, 45, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Muratov, D.V.; Shul’pina, L.S.; Kudinov, A.R.; Strelkova, T.V.; Petrovskiy, P.V. Oxygenation of aromatic hydrocarbons with hydrogen peroxide catalyzed by rhodium carbonyl complexes. Appl. Organomet. Chem. 2008, 22, 684–688. [Google Scholar]
- Lin, Y.; Zhu, L.; Lan, Y.; Rao, Y. Development of a rhodium(II)-catalyzed chemoselective C(sp3)-H oxygenation. Chem. Eur. J. 2015, 21, 14937–14942. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Darcel, C.; Roisnel, T.; Dixneuf, P.H. Cycloruthenation of aryl imines and N-heteroaryl benzenes via C–H bond activation with Ru(II) and acetate partners. J. Organomet. Chem. 2015, 793, 200–209. [Google Scholar] [CrossRef]
- Ackermann, L. Robust ruthenium(II)-catalyzed C–H arylations: Carboxylate assistance for the efficient synthesis of angiotensin-II-Receptor blockers. Org. Process Res. Dev. 2015, 19, 260–269. [Google Scholar] [CrossRef]
- da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Oxovanadium complexes in catalytic oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Ma, X.T.; Xing, N.; Yan, Z.; Zhang, X.; Wu, Q.; Xing, Y.H. Peroxo- and oxovanadium(IV) complexes with tridentate N-heterocycle ligands: Synthesis, structure, and catalytic performance. New J. Chem. 2015, 39, 1067–1074. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Kokozay, V.N.; Pombeiro, A.J.L. Polynuclear Heterometallic Complexes from Metal Powders: The “Direct Synthesis” Approach. Eur. J. Inorg. Chem. 2014, 4496–4517. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Chygorin, E.N.; Kokozay, V.N.; Bon, V.V.; Boča, R.; Kozlov, Y.N.; Shul’pina, L.S.; Jezierska, J.; Ozarowski, A.; Pombeiro, A.J.L.; et al. Heterometallic CoIII4FeIII2 schiff base complex: Structure, electron paramagnetic resonance, and alkane oxidation catalytic activity. Inorg. Chem. 2012, 51, 9110–9122. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, D.; Chiacchio, K.C.; Kozlov, Y.N.; Shul’pin, G.B. Hydroperoxidation of alkanes with hydrogen peroxide catalyzed by aluminium nitrate in acetonitrile. Tetrahedron Lett. 2008, 49, 6693–6697. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Kozlov, Y.N.; Mandelli, D.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of Al3+-catalyzed oxidations of hydrocarbons: Dramatic activation of H2O2 toward O–O homolysis in complex [Al(H2O)4(OOH)(H2O2)]2+ explains the formation of HO• radicals. Inorg. Chem. 2011, 50, 3996–4005. [Google Scholar] [CrossRef] [PubMed]
- Novikov, A.S.; Kuznetsov, M.L.; Pombeiro, A.J.L.; Bokach, N.A.; Shul’pin, G.B. Generation of HO radical from hydrogen peroxide catalyzed by aqua complexes of the group III metals [M(H2O)n]3+ (M = Ga, In, Sc, Y, or La): A theoretical study. ACS Catal. 2013, 3, 1195–1208. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Teixeira, F.A.; Bokach, N.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Radical decomposition of hydrogen peroxide catalyzed by aqua complexes [M(H2O)n]2+ (M = Be, Zn, Cd). J. Catal. 2014, 313, 135–148. [Google Scholar] [CrossRef]
- Rocha, B.G.M.; Kuznetsov, M.L.; Kozlov, Y.N.; Pombeiro, A.J.L.; Shul’pin, G.B. Simple soluble Bi(III) salts as efficient catalysts for the oxidation of alkanes with H2O2. Catal. Sci. Technol. 2015, 5, 2174–2187. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Rocha, B.G.M.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxidation of olefins with hydrogen peroxide catalyzed by bismuth salts: A mechanistic study. ACS Catal. 2015, 5, 3823–3835. [Google Scholar] [CrossRef]
- Novikov, A.S.; Kuznetsov, M.L.; Rocha, B.G.M.; Pombeiro, A.J.L.; Shul’pin, G.B. Oxidation of olefins with H2O2 catalysed by salts of group III metals (Ga, In, Sc, Y and La): Epoxidation versus hydroperoxidation. Catal. Sci. Technol. 2016, 6, 1343–1356. [Google Scholar] [CrossRef]
- Popivker, I.; Zilbermann, I.; Maimon, E.; Cohen, H.; Meyerstein, D. The “Fenton like” reaction of MoO43− involves two H2O2 molecules. Dalton Trans. 2013, 42, 16666–16668. [Google Scholar] [CrossRef] [PubMed]
- Burg, A.; Shusterman, I.; Kornweitz, H.; Meyerstein, D. Three H2O2 molecules are involved in the “Fenton-like” reaction between Co(H2O)62+ and H2O2. Dalton Trans. 2014, 43, 9111–9115. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.S.; Pennifold, R.C.R.; Harvey, J.N. Electronic structure and formation of simple ferryloxo complexes: Mechanism of the Fenton reaction. Inorg. Chem. 2014, 53, 6473–6481. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Britovsek, G.J.P. High-valent iron in biomimetic alkane oxidation catalysis. Top. Organomet. Chem. 2015, 50, 145–172. [Google Scholar]
- Liu, W.; Groves, J.T. Manganese catalyzed C–H halogenation. Acc. Chem. Res. 2015, 48, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Oloo, W.N.; Que, L., Jr. Bioinspired nonheme iron catalysts for C–H and C=C bond oxidation: Insights into the nature of the metal-based oxidants. Acc. Chem. Res. 2015, 48, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Lindhorst, A.C.; Haslinger, S.; Kühn, F.E. Molecular iron complexes as catalysts for selective C–H bond oxygenation reactions. Chem. Commun. 2015, 51, 17193–17212. [Google Scholar] [CrossRef] [PubMed]
- Costas, M. Selective C–H oxidation catalyzed by metalloporphyrins. Coord. Chem. Rev. 2011, 255, 2912–2932. [Google Scholar] [CrossRef]
- Che, C.-M.; Lo, V.K.-Y.; Ahou, C.-Y.; Huang, J.-S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 2011, 40, 1950–1975. [Google Scholar] [CrossRef] [PubMed]
- Kudrik, E.V.; Afanasiev, P.; Alvarez, L.X.; Dubourdeaux, P.; Clémancey, M.; Latour, J.-M.; Blondin, G.; Bouchu, D.; Albrieux, F.; Nefedov, S.E.; et al. An N-bridged high-valent diiron–oxo species on a porphyrin platform that can oxidize methane. Nat. Chem. 2012, 4, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.S.; Meireles, A.M.; da Silva Martins, D.C.; Rebouças, J.S.; DeFreitas-Silva, G.; Idemori, Y.M. Effect of imidazole on biomimetic cyclohexane oxidation by first-, second-, and third-generation manganese porphyrins using PhIO andPhI(OAc)2 as oxidants. Appl. Catal. A 2015, 491, 17–27. [Google Scholar] [CrossRef]
- Kudrik, E.V.; Sorokin, A.B. μ-Nitrido bridged diiron phthalocyanines: Old complexes for new catalytic applications. Macroheterocycles 2011, 4, 154–160. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.X.; Sorokin, A.B. Mild oxidation of ethane to acetic acid by H2O2 catalyzed by supported μ-nitrido diiron phthalocyanines. J. Organomet. Chem. 2015, 793, 139–144. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Kudrik, E.V.; Bouchu, D. Bio-inspired oxidation of methane in water catalyzed by N-bridged diiron phthalocyanine complex. Chem. Commun. 2008, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, A.L.; Kardasheva, Y.S.; Predeina, V.V.; Kluev, M.V.; Ramazanov, D.N.; Talanova, M.Y.; Karakhanov, E.A. Iron and copper complexes with nitrogen_containing ligands as catalysts for cyclohexane oxidation with hydrogen peroxide under mild reaction conditions. Petrol. Chem. 2012, 52, 318–326. [Google Scholar] [CrossRef]
- Ottenbacher, R.V.; Samsonenko, D.G.; Talsi, E.P.; Bryliakov, K.P. Highly efficient, regioselective, and stereospecific oxidation of aliphatic C–H groups with H2O2, catalyzed by aminopyridine manganese complexes. Org. Lett. 2012, 14, 4310–4313. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.M.; Du Bois, J.; Malik, H.A. Comparative study of the limitations and challenges in atom-transfer C−H oxidations. Org. Lett. 2015, 17, 6066–6069. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, R.V.; Talsi, E.P.; Bryliakov, K.P. Mechanism of Selective C–H hydroxylation mediated by manganese-aminopyridine enzyme models. ACS Catal. 2015, 5, 39–44. [Google Scholar] [CrossRef]
- Talsi, E.P.; Ottenbacher, R.V.; Bryliakov, K.P. Bioinspired oxidations of aliphatic C–H groups with H2O2 in the presence of manganese complexes. J. Organomet. Chem. 2015, 793, 102–107. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P. Active sites and mechanisms of bioinspired oxidation with H2O2, catalyzed by non-heme Fe and related Mn complexes. Coord. Chem. Rev. 2014, 276, 73–96. [Google Scholar] [CrossRef]
- Lyakin, O.Y.; Zima, A.M.; Samsonenko, D.G.; Bryliakov, K.P.; Talsi, E.P. EPR Spectroscopic detection of the elusive FeV=O intermediates in selective catalytic oxofunctionalizations of hydrocarbons mediated by biomimetic ferric complexes. ACS Catal. 2015, 5, 2702–2707. [Google Scholar] [CrossRef]
- Gómez, L.; Canta, M.; Font, D.; Prat, I.; Ribas, X.; Costas, M. Regioselective oxidation of nonactivated alkyl C−H groups using highly structured non-heme iron catalysts. J. Org. Chem. 2013, 78, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Prat, I.; Company, A.; Corona, T.; Parella, T.; Ribas, X.; Costas, M. Assessing the impact of electronic and steric tuning of the ligand in the spin state and catalytic oxidation ability of the FeII(Pytacn) family of complexes. Inorg. Chem. 2013, 52, 9229–9244. [Google Scholar] [CrossRef] [PubMed]
- Canta, M.; Font, D.; Gómez, L.; Ribas, X.; Costas, M. The iron(II) complex [Fe(CF3SO3)2(mcp)] as a convenient, readily available catalyst for the selective oxidation of methylenic sites in alkanes. Adv. Synth. Catal. 2014, 356, 818–830. [Google Scholar] [CrossRef]
- Codola, Z.; Lloret-Fillol, J.; Costas, M. Aminopyridine iron and manganese complexes as molecular catalysts for challenging oxidative transformations. Prog. Inorg. Chem. 2014, 59, 447–531. [Google Scholar]
- Nam, W. Synthetic mononuclear nonheme iron−oxygen intermediates. Acc. Chem. Res. 2015, 48, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.A.; Munoz, B.K.; Jacob, K.; Vendier, L.; Caballero, A.; Etienne, M.; Pérez, P.J. Functionalization of non-activated C–H bonds of alkanes: An effective and recyclable catalytic system based on fluorinated silver catalysts and solvents. Chem. Eur. J. 2013, 19, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Pérez, P.J. Catalyst design in the alkane C–H bond functionalization of alkanes by carbene insertion with TpxM complexes (Tpx = hydrotrispyrazolylborate ligand; M = Cu, Ag). J. Organomet. Chem. 2015, 793, 108–113. [Google Scholar] [CrossRef]
- Gava, R.; Olmos, A.; Noverges, B.; Varea, T.; Alvarez, E.; Belderrain, T.R.; Caballero, A.; Asensio, G.; Pérez, P.J. Discovering copper for methane C–H bond functionalization. ACS Catal. 2015, 5, 3726–3730. [Google Scholar] [CrossRef]
- Caballero, A.; Díaz-Requejo, M.M.; Fructos, M.R.; Olmos, A.; Urbano, J.; Pérez, P.J. Catalytic functionalization of low reactive C(sp3)-H and C(sp2)-H bonds of alkanes and arenes by carbene transfer from diazo compounds. Dalton Trans. 2015, 44, 20295–20307. [Google Scholar] [CrossRef] [PubMed]
- Gava, R.; Olmos, A.; Noverges, B.; Varea, T.; Funes-Ardoiz, I.; Belderrain, T.R.; Caballero, A.; Maseras, F.; Asensio, G.; Pérez, P.J. Functionalization of CnH2n+2 alkanes: Supercritical carbon dioxide enhances the reactivity towards primary carbon–hydrogen bonds. ChemCatChem 2015, 7, 3254–3260. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Dash, C.; Jayaratna, N.B.; Ridlen, S.G.; Khani, S.K.; Das, A.; Kou, X.; Yousufuddin, M.; Cundari, T.R.; Dias, H.V.R. Zinc(II)-Mediated carbene insertion into C–H bonds in alkanes. Inorg. Chem. 2015, 54, 11043–11045. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. The stability of organometallic ligands in oxidation catalysis. J. Organomet. Chem. 2014, 751, 174–180. [Google Scholar] [CrossRef]
- Davies, H.M.L.; Manning, J.R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 2008, 451, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Cazin, C.S.J.; Nolan, S.P. N-Heterocyclic carbene gold(I) and copper(I) complexes in C–H bond activation. Acc. Chem Res. 2012, 45, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Haibach, M.C.; Kundu, S.; Brookhart, M.; Goldman, A.S. Alkane metathesis by tandem alkane dehydrogenation-olefin metathesis catalysis and related chemistry. Acc. Chem. Res. 2012, 45, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Organometallic Pincer Chemistry; van Koten, G.; Milstein, D. (Eds.) Springer: Heidelberg, Germany, 2013.
- Pincer and Pincer-Type Complexes: Application in Organic Synthesis and Catalysis; Szabo, K.J.; Wendt, O.F. (Eds.) Wiley-VCH: Weinheim, Germany, 2014.
- Schaefer, B.A.; Margulieux, G.W.; Small, B.L.; Chirik, P.J. Evaluation of cobalt complexes bearing tridentate pincer ligands for catalytic C–H borylation. Organometallics 2015, 34, 1307–1320. [Google Scholar] [CrossRef]
- Andrew, R.E.; González-Sebastián, L.; Chaplin, A.B. NHC-Based pincer ligands: Carbenes with a bite. Dalton Trans. 2016, 45, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W. Manifestations of noninnocent ligand behavior. Inorg. Chem. 2011, 50, 9752–9765. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, S.; Derat, E.; Desage-El Murr, M.; Fensterbank, L.; Malacria, M.; Desage-El Murr, M.; Mouriès-Mansuy, V. Non-innocent ligands: New opportunities in iron catalysis. Eur. J. Inorg. Chem. 2012, 2012, 376–389. [Google Scholar] [CrossRef]
- Gambarotta, S.; Budzelaar, P.H.M. Redox-active ligands and organic radical chemistry. Inorg. Chem. 2011, 50, 9879–9887. [Google Scholar]
- Olatunji-Ojo, O.A.; Cundari, T.R. C–H Activation by multiply bonded complexes with potentially noninnocent ligands: A computational study. Inorg. Chem. 2013, 52, 8106–8113. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.; De Almeida, M.P.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-scorpionate FeII complex on carbon materials for cyclohexane oxidation with hydrogen peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Schiavon, M.A.; Pombeiro, A.J.L. Pyrazole or tris(pyrazolyl)ethanol oxo-vanadium(IV) complexes as homogeneous or supported catalysts for oxidation of cyclohexane under mild conditions. J. Mol. Catal. A 2013, 367, 52–60. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1-yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Kuznetov, M.L.; Fernandes, A.R.; Silva, A.; Pan, C.-J.; Lee, J.-F.; Hwang, B.-J.; Pombeiro, A.J.L. Cobalt complexes with pyrazole ligands as catalyst precursors for the peroxidative oxidation of cyclohexane: X-ray absorption spectroscopy studies and biological applications. Chem. Asian J. 2014, 9, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Ritter, T. Bimetallic redox synergy in oxidative palladium catalysis. Acc. Chem. Res. 2012, 45, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D.; Rassolov, V.; Wong, Y.O. Binuclear aromatic C–H bond activation at a Dirhenium Site. Angew. Chem. Int. Ed. 2016, 55, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Korlyukov, A.A.; Shul’pina, L.S.; Arkhipov, D.E.; Shubina, E.S.; Levitsky, M.M.; Kirilin, A.D.; Shul’pin, G.B. New binuclear cage-like copper(II) silsesquioxane (“Cooling Tower”); Its high catalytic activity in oxidation of benzene and alcohols. Eur. J. Inorg. Chem. 2013, 2013, 5240–5246. [Google Scholar] [CrossRef]
- Dronova, M.S.; Bilyachenko, A.N.; Yalymov, A.I.; Kozlov, Y.N.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; Levitsky, M.M.; Shubina, E.S.; Shul’pin, G.B. Solvent-controlled synthesis of tetranuclear cage-like copper(II) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides. Dalton Trans. 2014, 43, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Dronova, M.S.; Yalymov, A.I.; Lamaty, F.; Bantreil, X.; Martinez, J.; Bizet, C.; Shul’pina, L.S.; Korlyukov, A.A.; Arkhipov, D.E.; et al. Cage-like Copper(II) silsesquioxanes: Transmetalation reactions, structural, quantum chemical and catalytic studies. Chem. Eur. J. 2015, 21, 8758–8770. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, M.M.; Kozlov, Y.N.; Bilyachenko, A.N.; Nesterov, D.S.; Shul’pina, L.S.; Zubavichus, Y.V.; Pombeiro, A.J.L.; Levitsky, M.M.; Yalymov, A.I.; Shul’pin, G.B. Alkane oxidation with peroxides catalyzed by cage-like copper(II) silsesquioxanes. New J. Chem. 2015, 39, 187–199. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Vologzhanina, A.V.; Kozlov, Y.N.; Shul’pina, L.S.; Nesterov, D.N.; Pombeiro, A.J.L.; Lamaty, F.; et al. A heteronuclear Na8Fe6-oxo cluster of unusual “Asian Lantern”-like type: Its synthesis, structure, spin glass behavior and high catalytic activity. 2016. in preparation. [Google Scholar]
- Bilyachenko, A.N.; Yalymov, A.I.; Levitsky, M.M.; Korlyukov, A.A.; Shul’pina, L.S.; Es’kova, M.A.; Khrustalev, V.N.; Dorovatovsky, P.V.; Lamaty, F.; Bantreil, X.; et al. Unusual heterometallic (Fe6Na7 and Fe6Na6) cage silsesquioxanes: Synthesis, structure and high catalytic activity. 2016. in preparation. [Google Scholar]
- Bilyachenko, A.N.; Yalymov, A.I.; Levitsky, M.M.; Korlyukov, A.A.; Es’kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Shul’pina, L.S.; Ikonnikov, N.I.; et al. First pentanuclear Co(II) silsesquioxane: Synthesis, structure, slow dynamic behaviour of the magnetization and high activity in stereoselective alkane oxidations with peroxides. 2016. in preparation. [Google Scholar]
- Bilyachenko, A.N.; Yalymov, A.I.; Shul’pina, L.S.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’kova, M.A.; Shubina, E.S.; Levitsky, M.M.; Shul’pin, G.B. Novel cage-like hexanuclear nickel(II) silsesquioxane. Synthesis, structure and catalytic activity. 2016. in preparation. [Google Scholar]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X. Metal organic framework catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Van Der Voort, P.; Leus, K.; Liu, Y.-Y.; Vandichel, M.; Van Spebroeck, V.; Waroquier, M.; Biswas, S. Vanadium metal–organic frameworks: Structures and applications. New J. Chem. 2014, 38, 1853–1867. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Skobelev, I.Y.; Kholdeeva, O.A. Kinetics and mechanism of anthracene oxidation with tert-butyl hydroperoxide over metal-organic frameworks Cr-MIL-101 and Cr-MIL-100. J. Organomet. Chem. 2015, 793, 175–181. [Google Scholar] [CrossRef]
- Ruano, D.; Díaz-García, M.; Alfayate, A.; Sánchez-Sánchez, M. Nanocrystalline M–MOF-74 as heterogeneous catalysts in the oxidation of cyclohexene: Correlation of the activity and redox potential. ChemCatChem 2015, 7, 674–681. [Google Scholar] [CrossRef]
- Yao, R.-X.; Quiao, X.-H.; Cui, X.; Jia, X.-X.; Zhang, X.-M. Hexagonal Co6 and zigzag Co4 cluster based magnetic MOFs with a pcu net for selective catalysis. Inorg. Chem. Front. 2016, 3, 78–85. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Homogeneous multicopper catalysts for oxidation and hydrocarboxylation of alkanes. Adv. Inorg. Chem. 2013, 65, 1–31. [Google Scholar]
- Gupta, S.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Kirillov, A.M. Alkali metal directed assembly of heterometallic Vv/M (M = Na, K, Cs) coordination polymers: Structures, topological analysis, and oxidation catalytic properties. Inorg. Chem. 2013, 52, 8601–8611. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mukherjee, S.; Lopes, L.M.; Ilharco, L.M.; Ferraria, A.M.; do Rego, A.M.B.; Pombeiro, A.J.L. Synthesis, characterization and heterogeneous catalytic application of copper integrated mesoporous matrices. Dalton Trans. 2014, 43, 3215–3226. [Google Scholar] [CrossRef] [PubMed]
- Timokhin, I.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel coordination polymers with (Pyrazolato)-based tectons: Catalytic activity in the peroxidative oxidation of alcohols and cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Martins, L.; Nasani, R.; Saha, M.; Mobin, S.; Mukhopadhyay, S.; Pombeiro, A. Greener selective cycloalkane oxidations with hydrogen peroxide catalyzed by copper-5-(4-pyridyl)tetrazolate metal-organic frameworks. Molecules 2015, 20, 19203–19220. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Kłak, J.; Kirillova, M.V.; Kirillov, A.M. Copper(II) coordination polymers self-assembled from aminoalcohols and pyromellitic acid: Highly active precatalysts for the mild water-promoted oxidation of alkanes. Inorg. Chem. 2016, 55, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tetracopper(II) cubane cores driven by a diamino alcohol: Self-assembly synthesis, structural and topological features, and magnetic and catalytic oxidation properties. Inorg. Chem. 2015, 54, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tricopper(II) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: Synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5-C8 alkanes. Inorg. Chem. Front. 2015, 2, 525–537. [Google Scholar] [CrossRef]
- Palomas, D.; Kalamaras, C.; Haycock, P.; White, A.J.P.; Hellgardt, K.; Horton, A.; Crimmin, M.R. Re-evaluating selectivity as a determining factor in peroxidative methane oxidation by multimetallic copper complexes. Catal. Sci. Technol. 2015, 5, 4108–4115. [Google Scholar] [CrossRef]
- Schreiner, P.R.; Fokin, A.A. Selective alkane C–H-bond functionalizations utilizing oxidative single-electron transfer and organocatalysis. Chem. Rec. 2004, 3, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Limberg, C. The role of radicals in metal-assisted oxygenationreactions. Angew. Chem. Int. Ed. 2003, 42, 5932–5954. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-L.; Wu, X.-N.; Zhao, Y.-X.; He, S.-G. C–H bond activation by oxygen-centered radicals over atomic clusters. Acc. Chem. Res. 2012, 45, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.; Curran, D.P. Catalysis of radical reactions: A radical chemistry perspective. Angew. Chem. Int. Ed. 2016, 55, 58–102. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Druzhinina, A.N. Hydroperoxidation of alkanes by atmospheric oxygen in the presence of hydroquinone or quinone catalyzed by copper(II) acetate under visible light irradiation. React. Kinet. Catal. Lett. 1992, 47, 207–211. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes. React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal A 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely efficient alkane oxidation by a new catalytic reagent H2O2/Os3(CO)12/pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(μ-H)2. Appl. Organometal. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Das, B.; Al-Hunaiti, A.; Haukka, M.; Demeshko, S.; Meyer, S.; Shteinman, A.A.; Meyer, F.; Repo, T.; Norlander, E. Catalytic oxidation of alkanes and alkenes by H2O2 with a μ-oxido diiron(III) complex as catalyst/catalyst precursor. Eur. J. Inorg. Chem. 2015, 3590–3601. [Google Scholar] [CrossRef]
- Contaldi, S.; Di Nicola, C.; Garau, F.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. New coordination polymers based on the triangular [Cu3(μ3-OH)(μ-pz)3]2+ unit and unsaturated carboxylates. Dalton Trans. 2009, 4928–4941. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kirillov, A.M.; Mandelli, D.; Carvalho, W.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Mild homogeneous oxidation of alkanes and alcohols including glycerol with tert-butyl hydroperoxide catalyzed by a tetracopper(II) complex. J. Catal. 2010, 272, 9–17. [Google Scholar] [CrossRef]
- Xue, S.; Chen, G.; Long, Z.; Zhou, Y.; Wang, J. Efficient and recyclable multi-cationic polyoxometalate-based hybrid catalyst for heterogeneous cyclohexane oxidation with H2O2. RSC Adv. 2015, 5, 19306–19314. [Google Scholar] [CrossRef]
- Haslinger, S.; Raba, A.; Cokoja, M.; Pöthig, A.; Kühn, F.E. Iron-catalyzed oxidation of unreactive C–H bonds: Utilizing bio-inspired axial ligand modification to increase catalyst stability. J. Catal. 2015, 331, 147–153. [Google Scholar] [CrossRef]
- Olivo, G.; Nardi, M.; Vidal, D.; Barbieri, A.; Lapi, A.; Gomez, L.; Lanzalunga, O.; Costas, M.; Di Stefano, S. C–H bond oxidation catalyzed by an imine-based iron complex: A mechanistic insight. Inorg. Chem. 2015, 54, 10141–10152. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Mahmudov, K.T.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear CuII structural isomers: Coordination, magnetism, electrochemistry and catalytic activity towards the oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 3959–3969. [Google Scholar] [CrossRef]
- Vanoppen, D.L.; de Vos, D.E.; Genet, M.J.; Rouxhet, P.G.; Jacobs, P.A. Cobalt-containing molecular sieves as catalysts for the low conversion autoxidation of pure cyclohexane. Angew. Chem. Int. Ed. Engl. 1995, 34, 560–563. [Google Scholar] [CrossRef]
- Knops-Gerrits, P.P.; Trujillo, C.A.; Zhan, B.Z.; Li, X.Y.; Rouxhet, P.; Jacobs, P.A. Oxidation catalysis with well-characterised vanadyl bis-bipyridine complexes encapsulated in NaY zeolite. Top. Catal. 1996, 3, 437–449. [Google Scholar] [CrossRef]
- Bonchio, M.; Carraro, M.; Scorrano, G.; Kortz, U. Microwave-assisted fast cyclohexane oxygenation catalyzed by iron-substituted polyoxotungstates. Adv. Synth. Catal. 2005, 347, 1909–1912. [Google Scholar] [CrossRef]
- De Castries, A.; Magnier, E.; Momotton, S.; Fensterbank, H.; Larpent, C. An expedient synthesis of perfluorinated tetraazamacrocycles: New ligands for copper-catalyzed oxidation under fluorous biphasic conditions. Eur. J. Org. Chem. 2006, 4685–4692. [Google Scholar] [CrossRef]
- Fornal, E.; Giannotti, C. Photocatalyzed oxidation of cyclohexane with heterogenized decatungstate. Photochem. Photobiol. A 2007, 188, 279–286. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Y.; Yang, X.; Su, Y.; Zhang, W.; Xu, J. Electronic effect of substituent of quinones on their catalytic performance in hydrocarbons oxidation. Catal. Lett. 2008, 125, 154–159. [Google Scholar] [CrossRef]
- Aprile, C.; Corma, A.; Domine, M.E.; Garcia, H.; Mitchell, C. A cascade aerobic epoxidation of alkenes over Au/CeO2 and Ti-mesoporous material by “in situ” formed peroxides. J. Catal. 2009, 264, 44–53. [Google Scholar] [CrossRef]
- Jiang, D.; Mallat, T.; Meier, D.M.; Urakawa, A.; Baiker, A. Copper metal–organic framework: Structure and activity in the allylic oxidation of cyclohexene with molecular oxygen. J. Catal. 2010, 270, 26–33. [Google Scholar] [CrossRef]
- Lloyd, R.; Jenkins, R.L.; Piccinini, M.; He, Q.; Kiely, C.J.; Carley, A.F.; Golunski, S.E.; Bethell, D.; Bartley, J.K.; Hutchings, G.J. Low-temperature aerobic oxidation of decane using an oxygen-free radical initiator. J. Catal. 2011, 283, 161–167. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Cyclohexane selective oxidation over metal-organic frameworks of MIL-101 family: Superior catalytic activity and selectivity. Chem. Commun. 2012, 48, 6812–6814. [Google Scholar] [CrossRef] [PubMed]
- Skobelev, I.Y.; Kovalenko, K.A.; Fedin, V.P.; Sorokin, A.B.; Kholdeeva, O.A. Allylic oxidation of alkenes with molecular oxygen catalyzed by porous coordination polymers Fe-MIL-101 and Cr-MIL-1011. Kinet. Catal. 2013, 54, 607–614. [Google Scholar] [CrossRef]
- Skobelev, I.V.; Sorokin, A.B.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Solvent-free allylic oxidation of alkenes with O2 mediated by Fe- and Cr-MIL-101. J. Catal. 2013, 298, 61–69. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, H.; Peng, F.; Wang, H. Selective allylic oxidation of cyclohexene catalyzed by nitrogen-doped carbon nanotubes. ACS Catal. 2014, 4, 1617–1625. [Google Scholar] [CrossRef]
- Mavrogiorgou, A.; Papastergiou, M.; Deligiannakis, Y.; Louloudi, M.J. Activated carbon functionalized with Mn(II) schiff base complexes asefficient alkene oxidation catalysts: Solid support matters. J. Mol. Catal. A 2014, 393, 8–17. [Google Scholar] [CrossRef]
- Makgwane, P.R.; Ray, S.S. Efficient room temperature oxidation of cyclohexane over highly active hetero-mixed WO3/V2O5 oxide catalyst. Catal. Commun. 2014, 54, 118–123. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Yu, H.; Peng, F.; Wang, H. Aerobic oxidation of α-pinene catalyzed by carbon nanotubes. Catal. Sci. Technol. 2015, 5, 3935–3944. [Google Scholar] [CrossRef]
- Liu, X.; Xing, N.; Song, J.; Wu, Q.; Yan, Z.; Zhang, Y.; Xing, Y. Two novel oxomolybdenum(V)-trispyrazolylborate complexes: Synthesis, structure, and catalytic performance in the cyclohexane oxidation. Polyhedron 2015, 102, 386–393. [Google Scholar] [CrossRef]
- Yan, Z.D.; Xing, N.; Zhang, Y.; Ma, X.T.; Song, J.; Liu, X.; Xing, Y.H. Oxidovanadium(IV) complexes with tridentate N-heterocycle ligands: Synthesis, structure, and efficient catalyst for cyclohexane oxidation to cyclohexanone. Polyhedron 2015, 102, 600–608. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, K.; Zhang, W.; Yao, J.; Li, H. Efficient metal-free oxidation of ethylbenzene with molecular oxygen utilizing the synergistic combination of NHPI analogues. J. Mol. Catal. A 2015, 402, 79–82. [Google Scholar] [CrossRef]
- Kojima, T.; Nakayama, K.; Ikemura, K.; Ogura, T.; Fukuzumi, S. Formation of a ruthenium(iv)-oxo complex by electron-transfer oxidation of a coordinatively saturated ruthenium(II) complex and detection of oxygen-rebound intermediates in C–H bond oxygenation. J. Am. Chem. Soc. 2011, 133, 11692–11700. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-B.; Wu, X.; Lee, Y.-M.; Kwon, Y.H.; Shaik, S.; Nam, W. Evidence for an alternative to the oxygen rebound mechanism in C–H bond activation by non-heme FeIVO complexes. J. Am. Chem. Soc. 2012, 134, 20222–20225. [Google Scholar] [CrossRef] [PubMed]
- Postils, V.; Company, A.; Solà, M.; Costas, M.; Luis, J.M. Computational insight into the mechanism of alkane hydroxylation by non-heme Fe(PyTACN) iron complexes. Effects of the substrate and solvent. Inorg. Chem. 2015, 54, 8223–8236. [Google Scholar] [PubMed]
- Cho, K.-B.; Nam, W. A theoretical study into a trans-dioxo MnV porphyrin complex that does not follow the oxygen rebound mechanism in C–H bond activation reactions. Chem. Commun. 2016, 52, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation by the system “H2O2‒O2‒[Mn2L2O3][PF6]2 (L=1,4,7-trimethyl-1,4,7-triazacyclononane)–acid” with involvement of atmospheric dioxygen. 2016. in preparation. [Google Scholar]
- Shul’pin, G.B.; Stoeckli-Evans, H.; Mandelli, D.; Kozlov, Y.N.; Tesouro Vallina, A.; Woitiski, C.B.; Jimenez, R.S.; Carvalho, W.A. Oxidation of alkanes with m-chloroperbenzoic acid catalyzed by iron(III) chloride and a polydentate amine. J. Mol. Catal. A 2004, 219, 255–264. [Google Scholar]
- Nesterova, O.V.; Nesterov, D.S.; Kokozay, V.N.; Shul’pin, G.B.; Pombeiro, A.J.L. Highly efficient stereospecific alkane hydroxylation with m-chloroperbenzoic acid catalyzed by a heterometallic Co/Fe complex. 2016. in preparation. [Google Scholar]
- Shul’pin, G.B.; Adams, R.D. Biosketch—Shilov special issue. J. Organomet. Chem. 2015, 793, 2–3. [Google Scholar] [CrossRef]
- Rudakov, E.S.; Shul’pin, G.B. Stable organoplatinum complexes as intermediates and models in hydrocarbon functionalization (review). J. Organomet. Chem. 2015, 793, 4–16. [Google Scholar] [CrossRef]
- Shestakov, A.F.; Goldshleger, N.F. Catalysts for C–H functionalization: Platinum, gold and even nanodiamonds. J. Organomet. Chem. 2015, 793, 17–33. [Google Scholar] [CrossRef]
- Shteinman, A.A. Shilov alkane platinum chemistry: 45 years. J. Organomet. Chem. 2015, 793, 34–40. [Google Scholar] [CrossRef]
- Crabtree, R.H. A.E. Shilov’s influence on early work in organometallic CH activation and functionalization. J. Organomet. Chem. 2015, 793, 41–46. [Google Scholar] [CrossRef]
- Labinger, J.A.; Bercaw, J.E. Mechanistic studies on the Shilov system: A retrospective. J. Organomet. Chem. 2015, 793, 47–53. [Google Scholar] [CrossRef]
- Bach, R.D.; Dmitrenko, O. The “Somersault” mechanism for the P-450 hydroxylation of hydrocarbons. The intervention of transient inverted metastable hydroperoxides. J. Am. Chem. Soc. 2006, 128, 1474–1488. [Google Scholar] [CrossRef] [PubMed]
- DeVries, N.; Roe, D.C.; Thorn, D.L. Catalytic hydroxylation using chloroplatinum compounds. J. Mol. Catal. A 2002, 189, 17–22. [Google Scholar] [CrossRef]
- Ochiai, M.; Fukui, K.; Iwatsuki, S.; Ishihara, K.; Matsumoto, K. Synthesis of aryl-platinum dinuclear complexes via ortho C–H bond activation of phenol and transmetalation of arylboronic acid. Organometallics 2005, 24, 5528–5536. [Google Scholar] [CrossRef]
- Wagner, A.M.; Hickman, A.J.; Sanford, M.S. Platinum-catalyzed C–H arylation of simple arenes. J. Am. Chem. Soc. 2013, 135, 15710–15713. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.; Brennessel, W.W.; Jones, W.D. Electrophilic C–H activation of benzene with a Shilov-inspired rhodium(III) diimine complex. J. Organomet. Chem. 2015, 793, 192–199. [Google Scholar] [CrossRef]
- Shul’pin, G.B. The reaction of H2PtCl6 with aromatic compounds affording the sigma-aryl complexes of Pt(IV). III. The synthesis of Pt(IV) complexes of benzenes containing electron-withdrawing substituents. J. Organometal. Chem. 1981, 212, 267–274. [Google Scholar]
- Shul’pin, G.B. Reaction of aromatic compounds with H2PtCl6 in CF3COOH-H2O system leading to the preparation of anionic sigma-aryl complexes of Pt(IV). J. Gen. Chem. USSR 1981, 51, 1808–1818. [Google Scholar]
- Shul’pin, G.B.; Nizova, G.V.; Nikitaev, A.T. The reaction of PtCl62− with aromatic compounds to afford anionic sigma-aryl complexes of Pt(IV). VIII. Kinetics and mechanisms of thermal, photochemical and gamma-induced reactions with arenes and arylmercury compounds (electrophilic substitution involving electron transfer). J. Organomet. Chem. 1984, 276, 115–153. [Google Scholar]
- Albert, J.; Bosque, R.; Crespo, M.; Granell, J.; Rodriguez, J.; Zafrilla, J. Platinum-mediated C−H bond activation of arene solvents and subsequent C−C bond formation. Organometallics 2010, 29, 4619–4627. [Google Scholar] [CrossRef]
- Prince, B.M.; Cundari, T.R. C−H Bond Activation of Methane by PtII−N-Heterocyclic Carbene Complexes. The importance of having the ligands in the right place at the right time. Organometallics 2012, 31, 1042–1048. [Google Scholar] [CrossRef]
- Pal, S.; Zavalij, P.Y.; Vedernikov, A.N. Concurrent B to-Pt methyl migration and B-center retention in aerobic oxidation of methylborato platinum(II) complexes. Organometallics 2015, 34, 5183–5190. [Google Scholar] [CrossRef]
- Furukawa, T.; Tobisu, M.; Chatani, N. C–H Functionalization at sterically congested positions by the platinum-catalyzed borylation of arenes. J. Am. Chem. Soc. 2015, 137, 12211–12214. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sanford, M.S. Platinum-catalyzed, terminal-selective C(sp3)-H oxidation of aliphatic amines. J. Am. Chem. Soc. 2015, 137, 12796–12799. [Google Scholar] [CrossRef] [PubMed]
- Gunsalus, N.J.; Konnick, M.M.; Hashiguchi, B.G.; Periana, R.A. Discrete molecular catalysis for methane functionalization. Isr. J. Chem. 2014, 54, 1467–1480. [Google Scholar] [CrossRef]
- Mironov, O.A.; Bischof, S.M.; Konnick, M.M.; Hashiguchi, B.G.; Ziatdinov, V.R.; Goddard, W.A., III; Ahlquist, M.; Periana, R.A. Using reduced catalysts for oxidation reactions: Mechanistic studies of the “Periana-Catalytica” system for CH4 oxidation. J. Am. Chem. Soc. 2013, 135, 14644–14658. [Google Scholar] [CrossRef] [PubMed]
- Konnick, M.M.; Bischhof, S.M.; Yousufuddin, M.; Hashiguchi, B.G.; Ess, D.H.; Periana, R.A. A mechanistic change results in 100 times faster CH functionalization for ethane versus methane by a homogeneous Pt catalyst. J. Am. Chem. Soc. 2014, 136, 10085–10094. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Sen, A. Oxidation and oxidative carbonylation of methane and ethane by hexaoxo-μ-peroxodisulfate(2‒) ion in aqueous medium. A model for alkane oxidation through the hydrogen-atom abstraction pathway. J. Chem. Soc. Chem. Commun. 1992, 892–893. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Nakata, K.; Takaki, K.; Fujiwara, Y. Transition metal catalyzed acetic acid synthesis from methane and CO. Chem. Lett. 1992, 21, 1141–1142. [Google Scholar] [CrossRef]
- Nakata, K.; Yamaoka, Y.; Miyata, T.; Taniguchi, Y.; Takaki, K.; Fujiwara, Y. Palladium and/or copper-catalyzed carboxylation of small alkanes such as methane and ethane with carbon monoxide. J. Organomet. Chem. 1994, 473, 329–334. [Google Scholar] [CrossRef]
- Jia, C.; Kitamura, T.; Fujiwara, Y. Catalytic functionalization of arenes and alkanes via C–H bond activation. Acc. Chem. Res. 2001, 34, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.M.; Silva, J.A.L.; Palavra, A.F.; Frausto da Silva, J.J.R.; Pombeiro, A.J.L. Vanadium-catalyzed carboxylation of linear and cyclic C5 and C6 alkanes. J. Catal. 2005, 235, 333–340. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Pombeiro, A.J.L. Mild, single-pot hydrocarboxylation of linear C5–C9 alkanes into branched monocarboxylic C6–C10 acids in copper-catalyzed aqueous systems. Appl. Catal. A 2011, 401, 106–113. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Tautomeric effect of hydrazone Schiff bases in tetranuclear Cu(II) complexes: Magnetism and catalytic activity towards mild hydrocarboxylation of alkanes. Dalton Trans. 2013, 42, 16578–16587. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, O.V.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Boča, R.; Pombeiro, A.J.L. How to force a classical chelating ligand to a metal non-chelating bridge: The observation of a rare coordination mode of diethanolamine in the 1D complex {[Cu2(Piv)4(H3tBuDea)](Piv)}n. CrystEngComm 2014, 16, 775–783. [Google Scholar] [CrossRef]
- Phan, A.; Czaja, A.U.; Gándara, F.; Knobler, C.B.; Yaghi, O.M. Metal-organic frameworks of vanadium as catalysts for conversion of methane to acetic acid. Inorg. Chem. 2011, 50, 7388–7390. [Google Scholar] [CrossRef] [PubMed]
- Itoyama, S.; Doitomi, K.; Kamachi, T.; Shiota, Y.; Yoshizawa, K. Possible peroxo state of the dicopper site of particulate methane monooxygenase from combined quantum mechanics and molecular mechanics calculations. Inorg. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Culpepper, M.A.; Cutsail III, G.E.; Gunderson, W.A.; Hoffman, B.M.; Rosenzweig, A.C. Identification of the valence and coordination environment of the particulate methane monooxygenase copper centers by advanced EPR characterization. J. Am. Chem. Soc. 2014, 136, 11767–11775. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lippard, S.J. Diiron oxidation state control of substrate access to the active site of soluble methane monooxygenase mediated by the regulatory component. J. Am. Chem. Soc. 2014, 136, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kirillov, A.M.; Martins, A.N.C.; Graiff, C.; Tiripicchio, A.; Pombeiro, A.J.L. Topologically unique heterometallic CuII/Li coordination polymers self-assembled from N,N-bis(2-Hydroxyethyl)-2-aminoethanesulfonic acid biobuffer: Versatile catalyst precursors for mild hydrocarboxylation of alkanes to carboxylic acids. Inorg. Chem. 2012, 51, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Karabach, Y.Y.; Kirillova, M.V.; Haukka, M.; Pombeiro, A.J.L. Topologically unique 2D heterometallic CuII/Mg coordination polymer: Synthesis, structural features, and catalytic use in alkane hydrocarboxylation. Cryst. Growth Des. 2012, 12, 1069–1074. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Dias, S.S.P.; Kirillova, M.V.; Kirillov, A.M. New aqua-soluble dicopper(II) aminoalcoholate cores for mild and water-assisted catalytic oxidation of alkanes. Catal. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Chapter 6.04 Selectivity in C–H functionalizations. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, K.J., Poeppelmeier, L.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 6, pp. 79–104. [Google Scholar]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).