Copolymers Based on Indole-6-Carboxylic Acid and 3,4-Ethylenedioxythiophene as Platinum Catalyst Support for Methanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
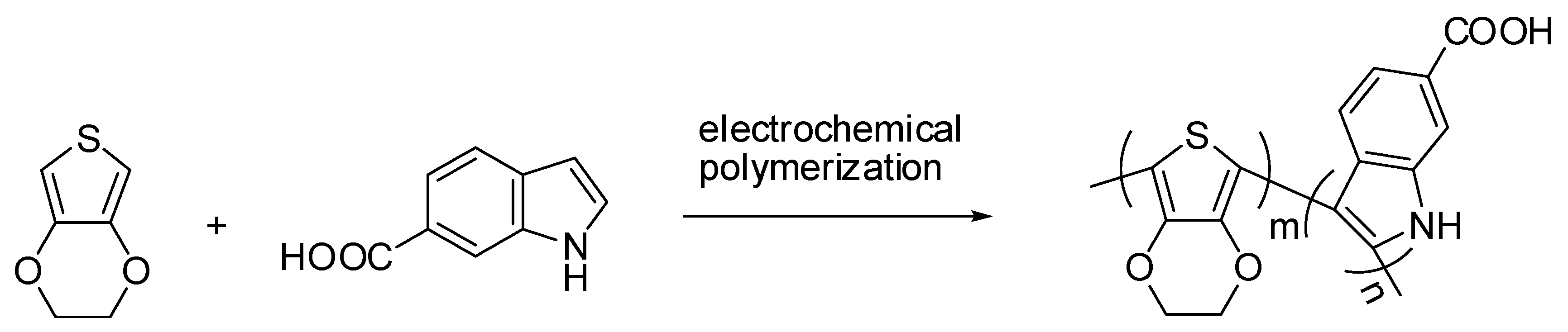
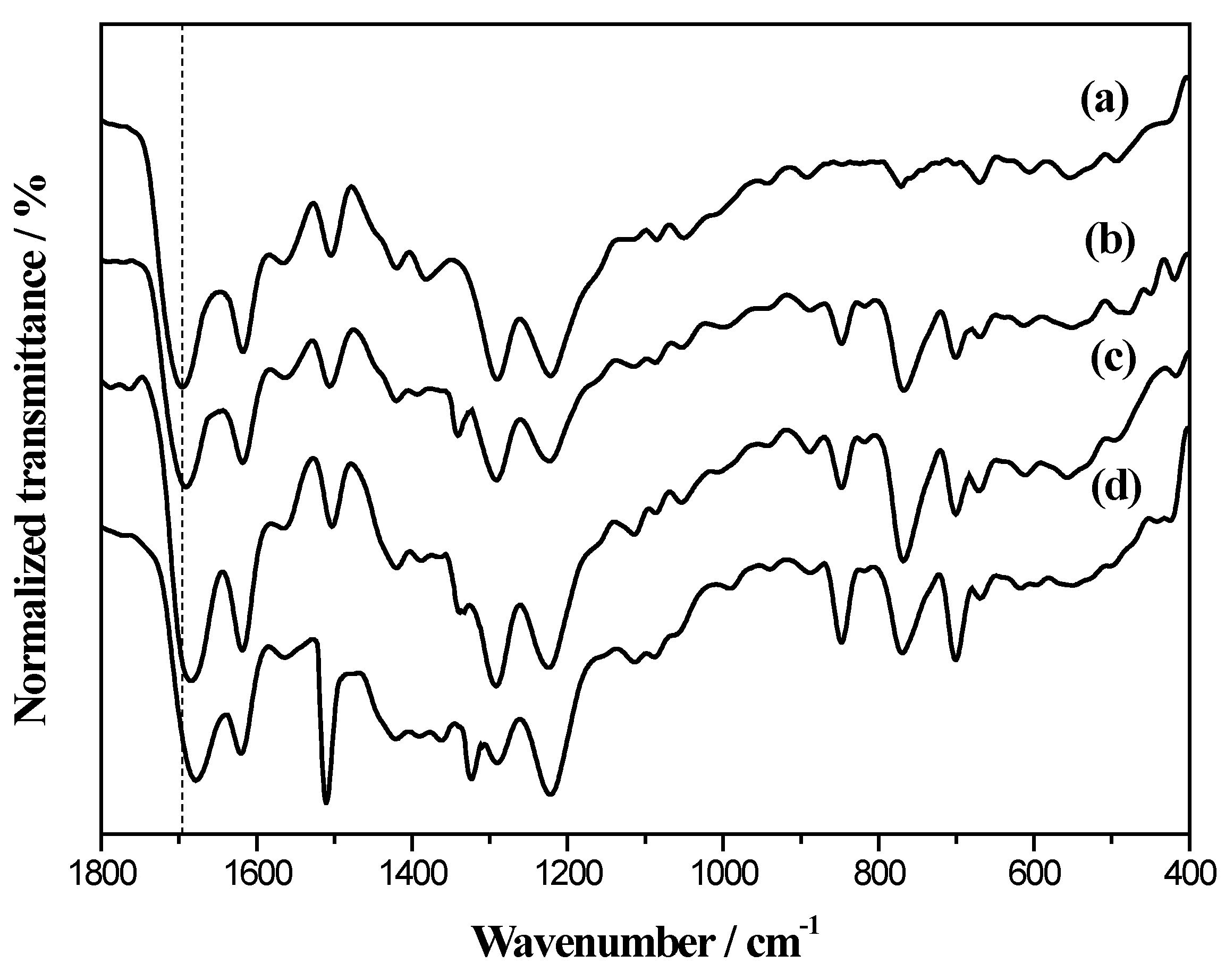
2.1. Electrochemical Polymerization and Characterizations

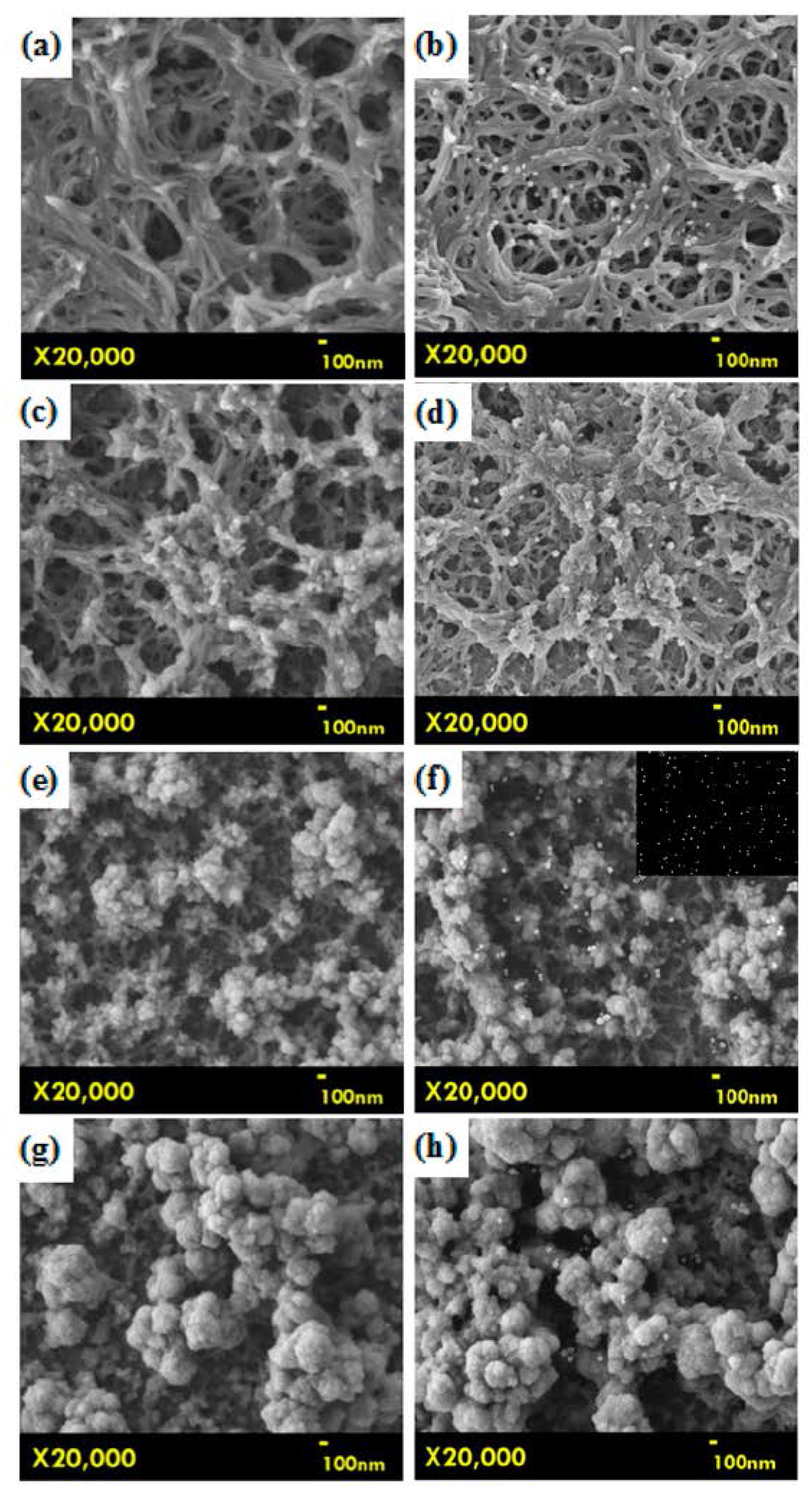
2.2. Surface Morphology
2.3. XRD Patterns

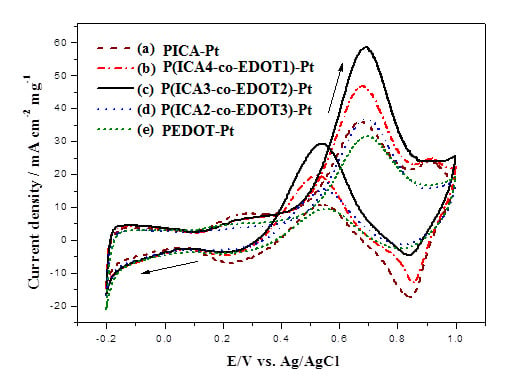
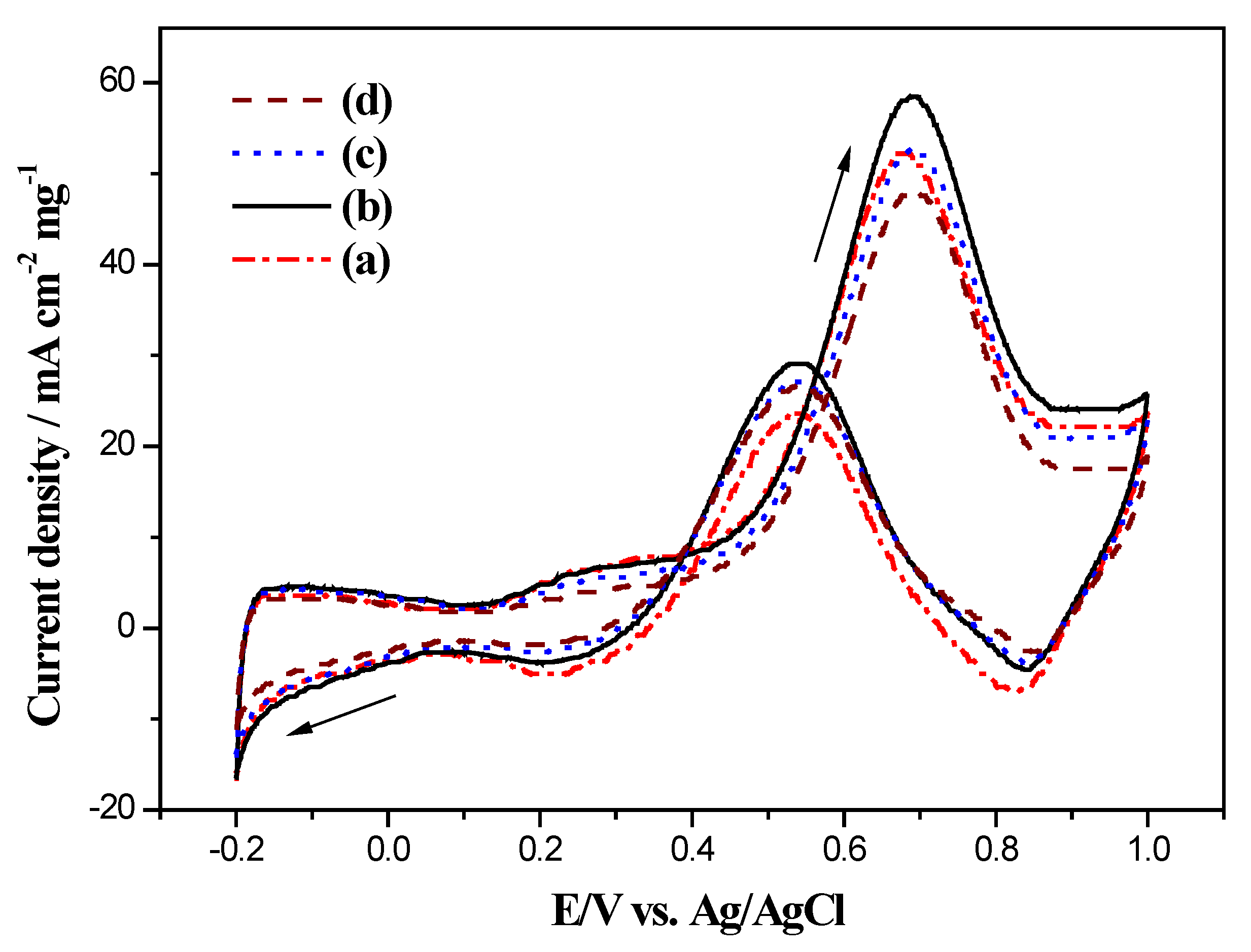
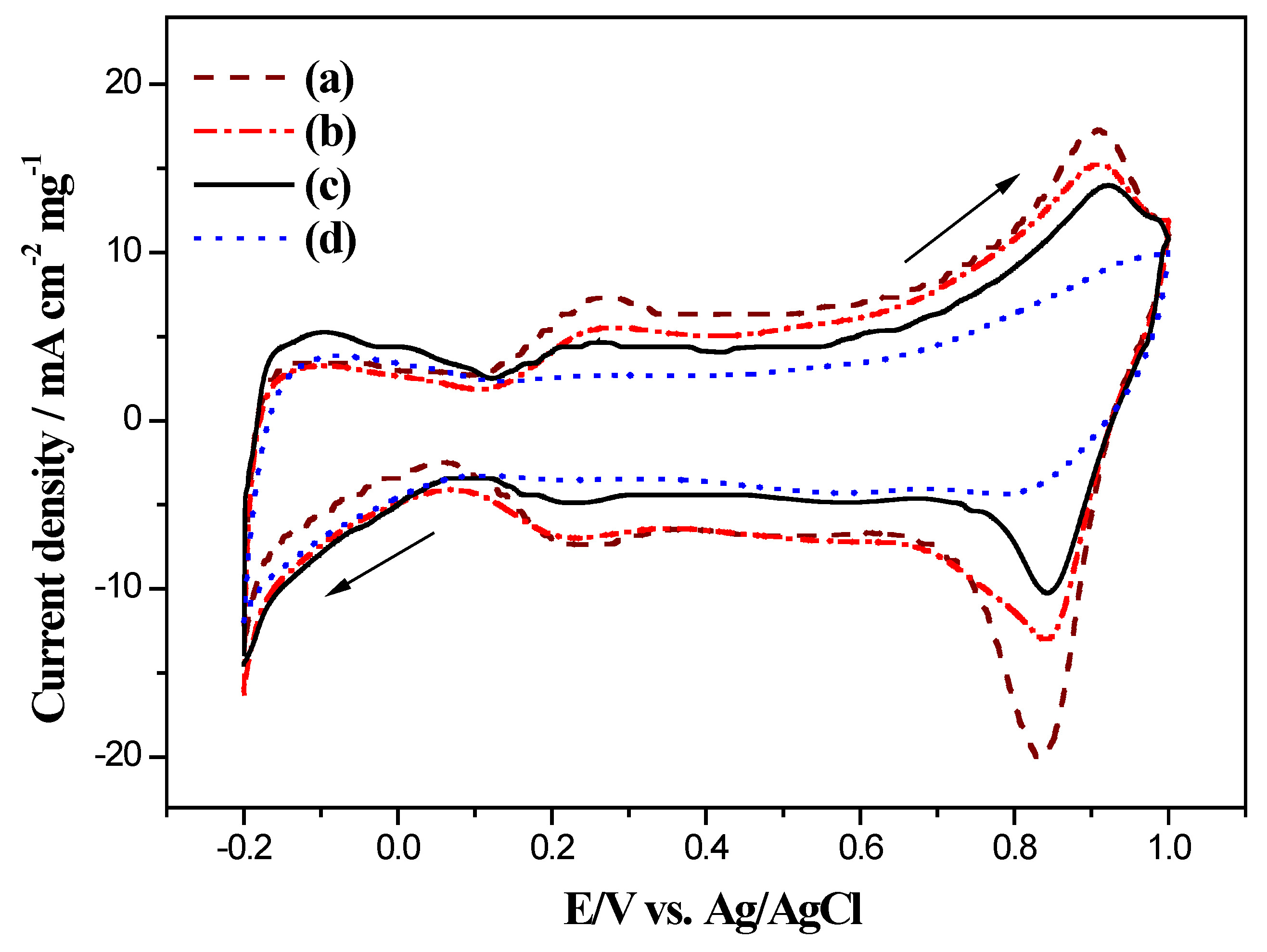
2.4. Electrocatalytic Activity of Electrodes for Methanol Oxidation
| Electrodes | QH (mC cm-2·mg-1) | ESA (cm2·mg-1) |
|---|---|---|
| PICA-Pt | 48.9 | 230 |
| P(ICA4-co-EDOT1)-Pt | 60.1 | 286 |
| P(ICA3-co-EDOT2)-Pt | 71.4 | 340 |
| P(ICA2-co-EDOT3)-Pt | 60.0 | 283 |
| Electrodes | CH2SO4/CMethanol (M/M) | ν (mV·s−1) | Eonset (V) (c) | Ipa (mA·mg−1) | Ref. |
|---|---|---|---|---|---|
| PANI-300PSS-Pt (b) | 0.5/0.1 | 10 | 0.4 | 19 | [32] |
| Pt/PANI/MGCE (a) | 0.5/0.5 | 5 | 0.3 | 32 | [33] |
| Pt/Nano-PDAN/MGCE (a) | 0.5/2.4 | 50 | 0.2 | 28 | [34] |
| PANI-PSS-Pt (b) | 0.5/1.0 | 10 | 0.4 | 31 | [11] |
| nanotube-Pt (b) | 0.5/1.0 | 50 | - | 141 | [35] |
| P(ICA3-co-EDOT2)-Pt (b) | 0.5/0.5 | 50 | 0.4 | 58 | This work |
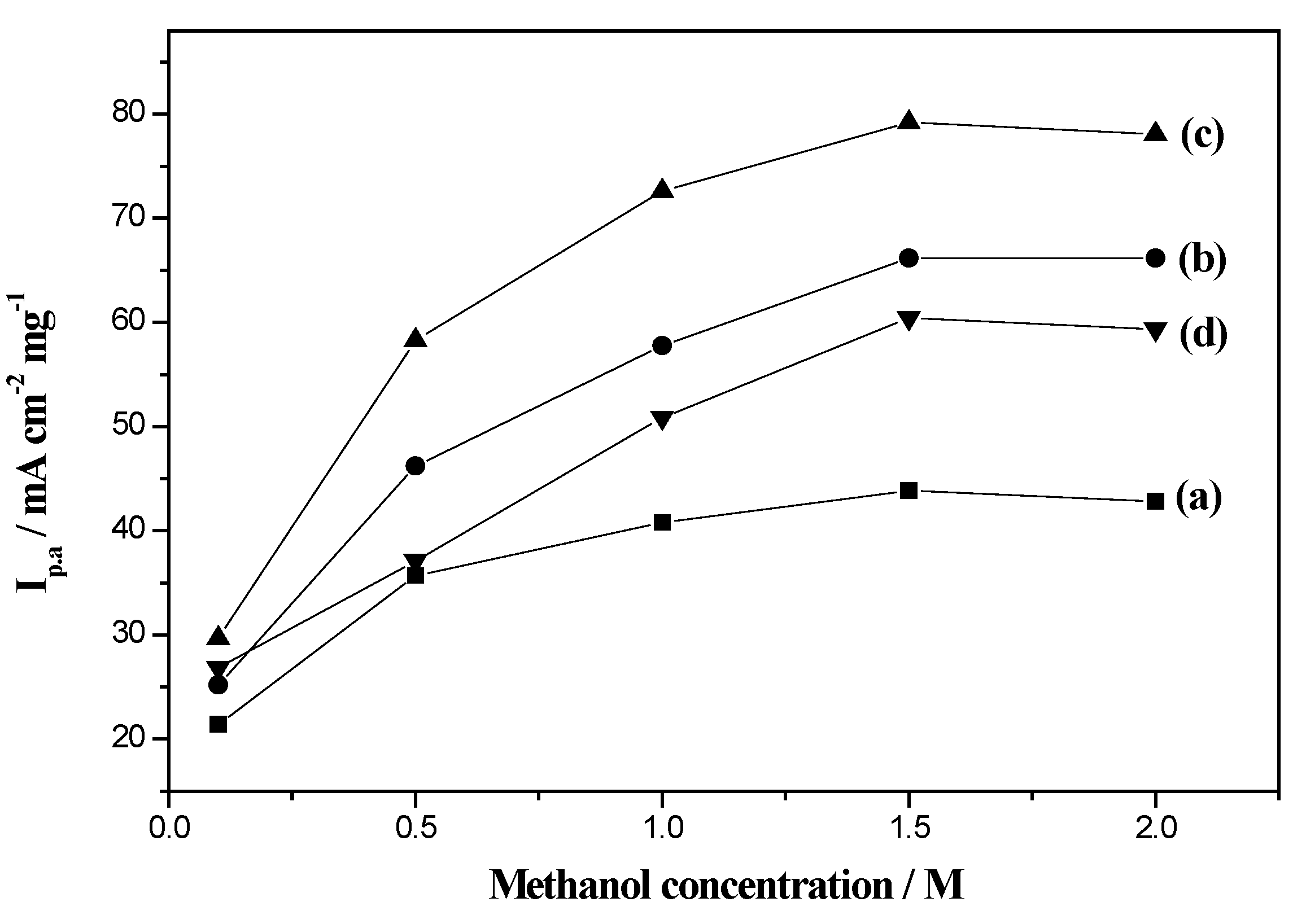
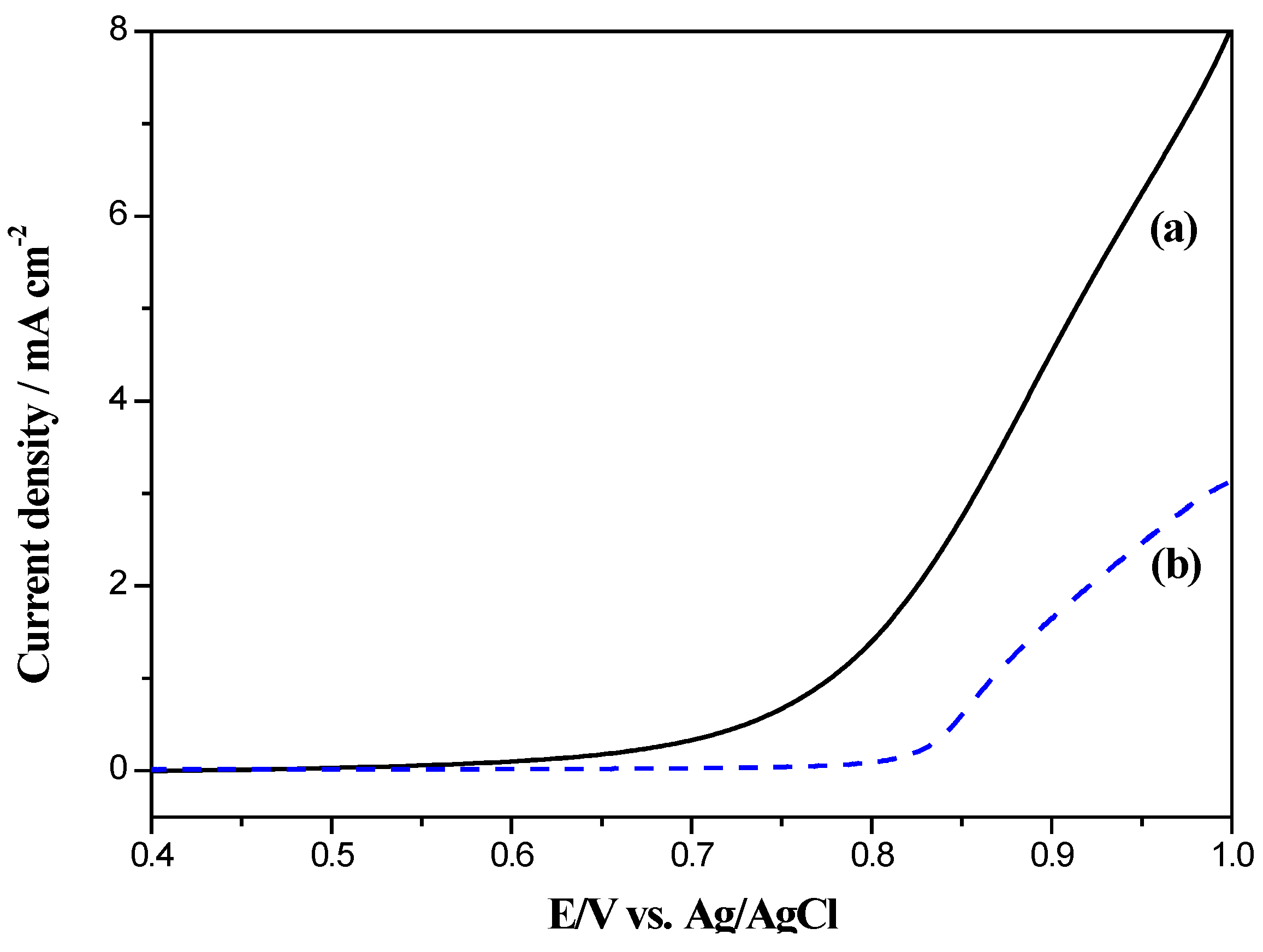
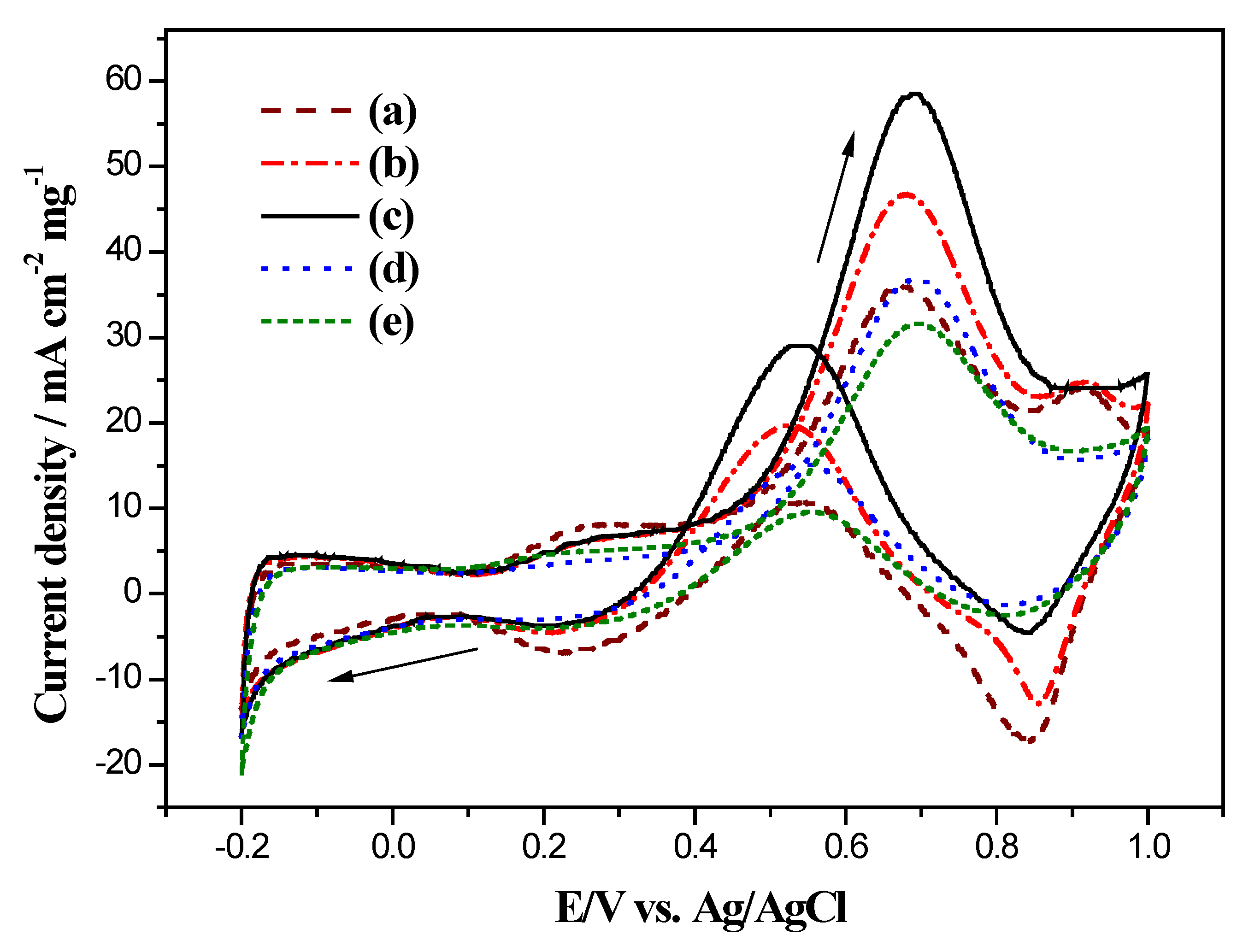
2.5. Electrocatalytic Long-Term Stability of Electrodes for Methanol Oxidation
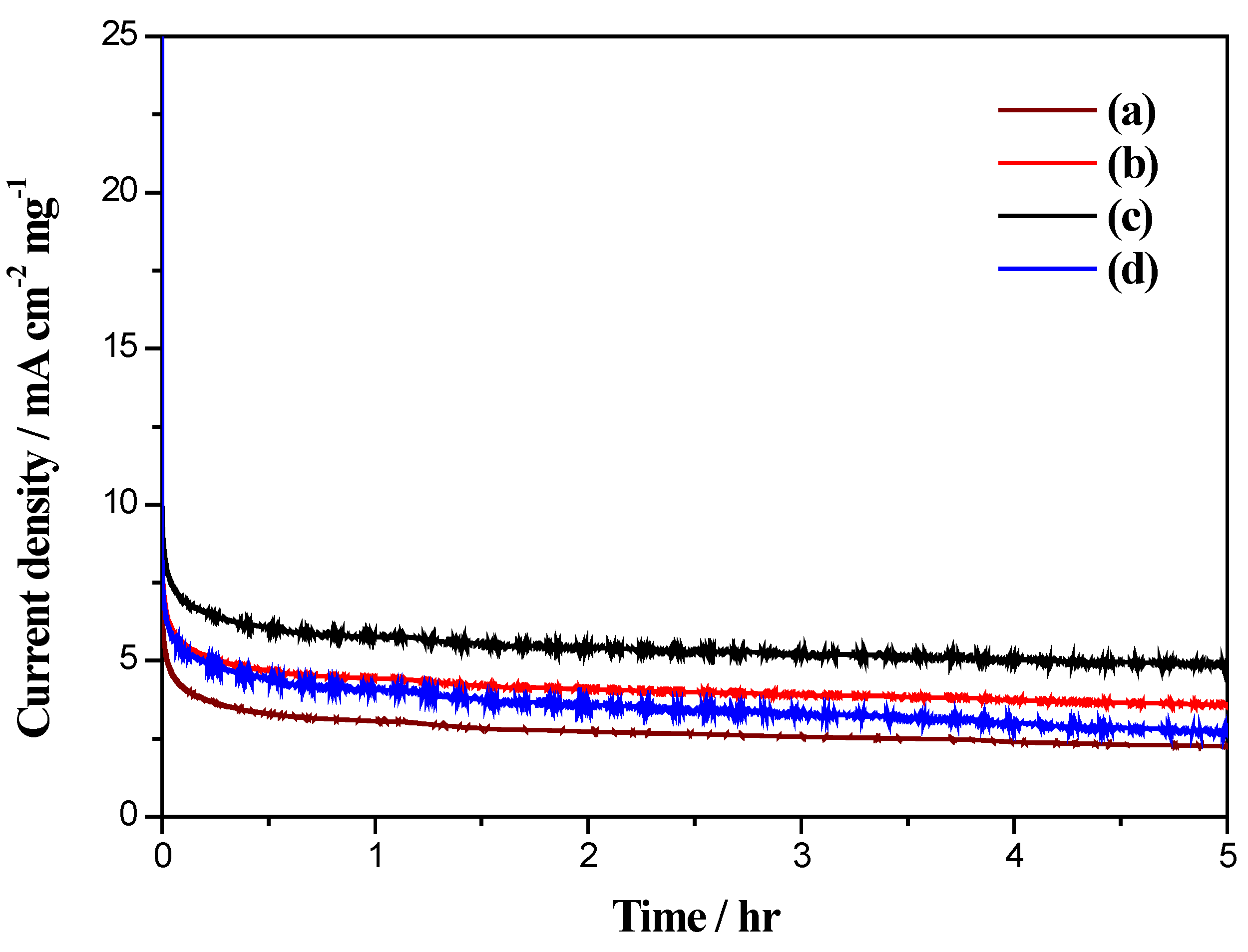
3. Experimental Section
3.1. Preparation of PICA, P(ICA4-co-EDOT1), P(ICA3-co-EDOT2), and P(ICA2-co-EDOT3) Films
3.2. Deposition of Pt into PICA, P(ICA4-co-EDOT1), P(ICA3-co-EDOT2), and P(ICA2-co-EDOT3) Matrices
3.3. Physical and Electrochemical Characterizations
3.4. Methanol Electro-Oxidation and Stability of Composite Electrodes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alegre, C.; Gálvez, M.E.; Moliner, R.; Lázaro, M.J. Influence of the synthesis method for Pt catalysts supported on highly mesoporous carbon xerogel and vulcan carbon black on the electro-oxidation of methanol. Catalysts 2015, 5, 392–405. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Wang, Y.; Bai, Q.; Gao, Y.; Zhang, Z. Synthesis and electrocatalytic performance of multi-component nanoporous PtRuCuW alloy for direct methanol fuel cells. Catalysts 2015, 5, 1003–1015. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Chang, J.K.; Chen, P.R.; Kuo, C.W. Nanostructured poly(aniline-co-metanilic acid) as platinum catalyst support for electro-oxidation of methanol. Int. J. Hydrogen Energy 2015, 40, 2631–2640. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, Y.H.; Zang, J.B.; Lu, J.; Xu, X.P. A novel support of nano titania modified graphitized nanodiamond for Pt electrocatalyst in direct methanol fuel cell. Int. J. Hydrogen Energy 2015, 40, 4540–4547. [Google Scholar] [CrossRef]
- Kamarudina, S.K.; Achmada, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2010, 34, 6902–6916. [Google Scholar] [CrossRef]
- Chou, H.Y.; Yeh, T.K.; Tsai, C.H. Electrodeposited Pt and PtRu Nanoparticles without hydrogen evolution reaction on mesoporous carbon for methanol oxidation. Int. J. Electrochem. Sci. 2014, 9, 5763–5775. [Google Scholar]
- Shen, P.K.; Tseung, A.C.C. Anodic oxidation of methanol on Pt/WO3 in acidic media. J. Electrochem. Soc. 1994, 141, 3082–3090. [Google Scholar] [CrossRef]
- Wu, T.Y.; Kuo, Z.Y.; Jow, J.J.; Kuo, C.W.; Tsai, C.J.; Chen, P.R.; Chen, H.R. Co-electrodeposition of platinum and rhodium in poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid) as electrocatalyst for methanol oxidation. Int. J. Electrochem. Sci. 2012, 7, 8076–8090. [Google Scholar]
- Caballero-Manrique, G.; Brillas, E.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.-L. Electrochemical oxidation of the carbon support to synthesize Pt(Cu) and Pt-Ru(Cu) core-shell electrocatalysts for low-temperature fuel cells. Catalysts 2015, 5, 815–837. [Google Scholar] [CrossRef]
- Qin, H.; Qian, X.; Meng, T.; Lin, Y.; Ma, Z. Pt/MOx/SiO2, Pt/MOx/TiO2, and Pt/MOx/Al2O3 catalysts for CO oxidation. Catalysts 2015, 5, 606–633. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, B.K.; Tseng, Y.H.; Hsieh, T.H.; Ho, K.S.; Wu, T.Y.; Chen, H.R. A comparative study of poly(acrylic acid) and poly(styrenesulfonic acid) doped into polyaniline as platinum catalyst support for methanol electro-oxidation. J. Taiwan Inst. Chem. Eng. 2012, 43, 798–805. [Google Scholar] [CrossRef]
- Habibi, B.; Pournaghi-Azar, M.H.; Abdolmohammad-Zadeh, H.; Razmi, H. Electrocatalytic oxidation of methanol on mono and bimetallic composite films: Pt and Pt-M (M = Ru, Ir and Sn) nano-particles in poly(o-aminophenol). Int. J. Hydrogen Energy 2009, 34, 2880–2892. [Google Scholar] [CrossRef]
- Kuo, C.W.; Tsai, C.J.; Chen, W.P.; Chen, P.R.; Wu, T.Y.; Tseng, C.G. Nano-composite based on platinum particles and modified polyaniline for methanol, formic acid, and ethanol oxidation. J. Chin. Chem. Soc. 2014, 61, 819–826. [Google Scholar] [CrossRef]
- Sun, C.L.; Su, J.S.; Tang, J.H.; Lin, M.C.; Wu, J.J.; Pu, N.W.; Shi, G.N.; Ger, M.D. Investigation of the adsorption of size-selected Pt colloidal nanoparticles on high-surface-area graphene powders for methanol oxidation reaction. J. Taiwan Inst. Chem. Eng. 2014, 45, 1025–1030. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Mahendiran, C.; Chaitanya, K.; Tyagi, R.; Nawaz Khan, F. Electro-catalytic performance of Pt-supported poly (o-phenylenediamine) microrods for methanol oxidation reaction. Res. Chem. Intermed. 2012, 38, 383–391. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Dong, X.; Chen, P.; Wang, X. Electrodeposited Pt on three-dimensional interconnected graphene as a free-standing electrode for fuel cell application. J. Mater. Chem. 2012, 22, 5286–5290. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Viswanathan, B. Synthesis, characterization and electrocatalytic activity of Pt supported on poly(3,4-ethylenedioxythiophene)-V2O5 nanocomposites electrodes for methanol oxidation. Mater. Chem. Phys. 2010, 121, 165–171. [Google Scholar] [CrossRef]
- Yang, C.C.; Wu, T.Y.; Chen, H.R.; Hsieh, T.H.; Ho, K.S.; Kuo, C.W. Platinum particles embedded into nanowires of polyaniline doped with poly(acrylic acid-co-maleic acid) as electrocatalyst for methanol oxidation. Int. J. Electrochem. Sci. 2011, 6, 1642–1654. [Google Scholar]
- Selvaraj, V.; Alagar, M.; Hamerton, I. Electrocatalytic properties of monometallic and bimetallic nanoparticles-incorporated polypyrrole films for electro-oxidation of methanol. J. Power Sources 2006, 160, 940–948. [Google Scholar] [CrossRef]
- Fernandez-Blanco, C.; Ibanez, D.; Colina, A.; Ruiz, V.; Heras, A. Spectroelectrochemical study of the electrosynthesis of Pt nanoparticles/poly(3,4-(ethylenedioxythiophene) composite. Electrochim. Acta 2014, 145, 139–147. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsai, C.J.; Tseng, L.Y.; Chen, S.J.; Hsieh, T.H.; Kuo, C.W. Nanocomposite of platinum particles embedded into nanosheets of polycarbazole for methanol oxidation. J. Chin. Chem. Soc. 2014, 61, 860–866. [Google Scholar] [CrossRef]
- Nagashree, K.L.; Raviraj, N.H.; Ahmed, M.F. Carbon paste electrodes modified by Pt and Pt-Ni microparticles dispersed in polyindole film for electrocatalytic oxidation of methanol. Electrochim. Acta 2010, 55, 2629–2635. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, Y. Synthesis and optical and electrochemical properties of novel copolymers containing alternate 2,3-quinoxaline and hole-transporting units. J. Polym. Sci. A 2002, 40, 4570–4580. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Cassidy, B.; Hofstetter, N.; Bergquist, L.; Taylor, A.; Rider, D.A. Block copolymer template synthesis of core-shell PtAu bimetallic nanocatalysts for the methanol oxidation reaction. Chem. Mater. 2014, 26, 6928–6940. [Google Scholar] [CrossRef]
- Kham, K.; Sadki, S.; Chevrot, C. Oxidative electropolymerizations of carbazole derivatives in the presence of bithiophene. Synth. Met. 2004, 145, 135–140. [Google Scholar] [CrossRef]
- Gaupp, C.L.; Reynolds, J.R. Multichromic copolymers based on 3,6-bis(2-(3,4-ethylenedioxythiophene))-N-alkylcarbazole derivatives. Macromolecules 2003, 36, 6305–6315. [Google Scholar] [CrossRef]
- Nie, T.; Leng, J.; Bai, L.; Lu, L.; Xu, J.; Zhang, K. Synthesis and characterization of benzene sulfonate derivatives doped poly(3,4-ethylenedioxythiophene) films and their application in electrocatalysis. Synth. Met. 2014, 189, 161–172. [Google Scholar] [CrossRef]
- Kuo, C.W.; Sivakumar, C.; Wen, T.C. Nanoparticles of Pt/HxMoO3 electrodeposited in poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid) as the electrocatalyst for methanol oxidation. J. Power Sources 2008, 185, 807–814. [Google Scholar] [CrossRef]
- Radmilovic, V.; Gasteiger, H.A.; Ross, P.N. Structure and chemical composition of a supported Pt-Ru electrocatalyst for methanol oxidation. J. Catal. 1995, 154, 98–106. [Google Scholar] [CrossRef]
- Qiu, L.H.; Liu, B.Q.; Peng, Y.J.; Yan, F. Fabrication of ionic liquid-functionalized polypyrrole nanotubes decorated with platinum nanoparticles and their electrocatalytic oxidation of methanol. Chem. Commun. 2011, 47, 2934–2936. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.W.; Chen, S.J.; Chen, P.R.; Wu, T.Y.; Tsai, W.T.; Tseng, C.G. Doping process effect of polyaniline doped with poly(styrenesulfonic acid) supported platinum for methanol oxidation. J. Taiwan Inst. Chem. Eng. 2013, 44, 497–504. [Google Scholar] [CrossRef]
- Niu, L.; Li, Q.; Wei, F.; Wu, S.; Liu, P.; Cao, X. Electrocatalytic behavior of Pt-modified polyaniline electrode for methanol oxidation: Effect of Pt deposition modes. J. Electroanal. Chem. 2005, 578, 331–337. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Hosseini, S.R. Electrocatalytic oxidation of methanol onto platinum particles decorated nanostructured poly(1,5-diaminonaphthalene) film. J. Solid State Electrochem. 2012, 16, 2699–2708. [Google Scholar] [CrossRef]
- Maiyalagan, T. Electrochemical synthesis, characterization and electro-oxidation of methanol on platinum nanoparticles supported poly(o-phenylenediamine) nanotubes. J. Power Sources 2008, 179, 443–450. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-Y.; Kuo, C.-W.; Chen, Y.-L.; Chang, J.-K. Copolymers Based on Indole-6-Carboxylic Acid and 3,4-Ethylenedioxythiophene as Platinum Catalyst Support for Methanol Oxidation. Catalysts 2015, 5, 1657-1672. https://doi.org/10.3390/catal5041657
Wu T-Y, Kuo C-W, Chen Y-L, Chang J-K. Copolymers Based on Indole-6-Carboxylic Acid and 3,4-Ethylenedioxythiophene as Platinum Catalyst Support for Methanol Oxidation. Catalysts. 2015; 5(4):1657-1672. https://doi.org/10.3390/catal5041657
Chicago/Turabian StyleWu, Tzi-Yi, Chung-Wen Kuo, Yu-Lun Chen, and Jeng-Kuei Chang. 2015. "Copolymers Based on Indole-6-Carboxylic Acid and 3,4-Ethylenedioxythiophene as Platinum Catalyst Support for Methanol Oxidation" Catalysts 5, no. 4: 1657-1672. https://doi.org/10.3390/catal5041657
APA StyleWu, T.-Y., Kuo, C.-W., Chen, Y.-L., & Chang, J.-K. (2015). Copolymers Based on Indole-6-Carboxylic Acid and 3,4-Ethylenedioxythiophene as Platinum Catalyst Support for Methanol Oxidation. Catalysts, 5(4), 1657-1672. https://doi.org/10.3390/catal5041657