Abstract
Composites consisting of carbon nanotubes (CNTs) grown directly on oxygen-deficient anatase TiO2 (TiO2−δ) were synthesized by a two-step chemical vapor deposition (CVD) method and applied in photocatalytic hydrogen production from aqueous methanol solutions using photodeposited Pt as the co-catalyst. Thermogravimetry coupled with mass spectroscopy, X-ray diffraction, scanning electron microscopy, photocurrent analysis, X-ray photoelectron spectroscopy, and (scanning) transmission electron microscopy were performed to investigate the physical and (photo)chemical properties of the synthesized CNT-TiO2−δ composites before and after photocatalytic methanol reforming. The initial photocatalytic activity of TiO2 was found to be significantly improved in the presence of oxygen vacancies. An optimized amount (~7.2 wt%) of CNTs grown on the TiO2−δ surface led to a highly effective stabilization of the photocatalytic performance of TiO2−δ, which is attributed to the improved dispersion and stability of the photodeposited Pt co-catalyst nanoparticles and enhanced separation efficiency of photogenerated electron-hole pairs, rendering the photocatalysts less prone to deactivation.
1. Introduction
Photocatalytic production of hydrogen by water splitting is considered to be a promising route toward efficient conversion of solar energy [1,2]. One of the key issues in solar water-splitting is the development of efficient and stable systems for light-driven reduction of protons to hydrogen. Among other materials, titanium dioxide (TiO2) is comprehensively studied as a photocatalyst for hydrogen production due to its relatively negative position in the conduction band edge allowing for proton reduction, low cost, high stability and the possibility of further functionalization [3,4,5,6,7,8].
One of the commonly observed and applied features for TiO2 is that its activity can be significantly enhanced by surface and/or bulk defects resulting from, for example, the introduction of heteroatoms or the removal of lattice oxygen [3,4,6]. Doping with metallic (Nb, Ag, Pt, W, Mn, etc.) and/or non-metallic (C, N, P, F, etc.) heteroatoms has been proven to be one effective route and is thus widely used in practice [9,10,11,12]. Recently, hydrogenation, i.e., reduction in hydrogen, was found to be another efficient route to create defects and to obtain oxygen-deficient TiO2 (TiO2−δ) [6,13]. As compared to the doping method, hydrogenation of TiO2 seems to be more promising due to its high efficiency and easy operation. For example, in photodegradation reactions, significantly higher activity can be achieved over TiO2−δ than pristine TiO2 [5,6,8]. In photocatalytic water splitting, the efficiency of TiO2−δ is rather moderate [14], and further improvement of the activity and stability is required.
Compositing TiO2 with carbon nanomaterials, such as carbon nanotubes (CNTs) and graphene [15,16], was reported to be an effective tool for enhancing the efficiency of the separation of photogenerated electron-hole (e−-h+) pairs because of the excellent electric conductivity of carbon nanomaterials, which resulted in more active and more stable photocatalyst for dye degradation and water splitting [15,16,17,18,19]. Nevertheless, most of the preparation of carbon-TiO2 composites was in the context of physical mixing, either in solution [18,19] or via mechanical routes [20], and thus, the photocatalytic performance of TiO2 was only limitedly improved due to the insufficient electronic contact between the TiO2 and the carbon nanoparticles. Therefore, in order to further increase the photocatalytic performance, it is necessary to improve the junction quality between carbon nanomaterials and TiO2, which requires more delicately designed preparation strategies.
In this work, a two-step chemical vapor deposition (CVD) approach was applied in the synthesis of CNT-TiO2−δ composites [21]. As compared to those prepared by physical mixing [18,19,20], composites in this work consist of CNTs grown directly on anatase TiO2 particles and are able to significantly improve the electronic contact between the CNTs and TiO2 particles [21]. Furthermore, the oxygen deficiency of TiO2, which is created prior to and during the CNT synthesis under reducing atmosphere, can contribute to the separation of photogenerated e−-h+ pairs. As a result, the CNT-TiO2−δ composite synthesized under optimal conditions displayed significantly improved performance stability in the photocatalytic production of hydrogen from aqueous methanol solutions.
2. Results and Discussion
2.1. Physicochemical Properties
The time for the CVD growth of CNTs was varied from 5 min to 60 min in order to obtain different carbon contents on TiO2−δ. The synthesized CNT-TiO2−δ composites with a growth time of 5, 15 and 60 min were denoted as CNT-TiO2−δ-5, CNT-TiO2−δ-15 and CNT-TiO2−δ-60, respectively. In the case of CNT growth with a shorter time than 60 min, additional treatment in He was performed, aiming at an identical duration of 60 min at 550 °C for all of the samples. The applied reducing atmosphere prior to and during the CNT growth is known to form oxygen-deficient TiO2−δ [21]. For comparison, pristine TiO2 was subjected to the same preparation procedure, except that C2H4 in the CNT growth step was replaced by He. The resulting sample was designated as TiO2−δ.
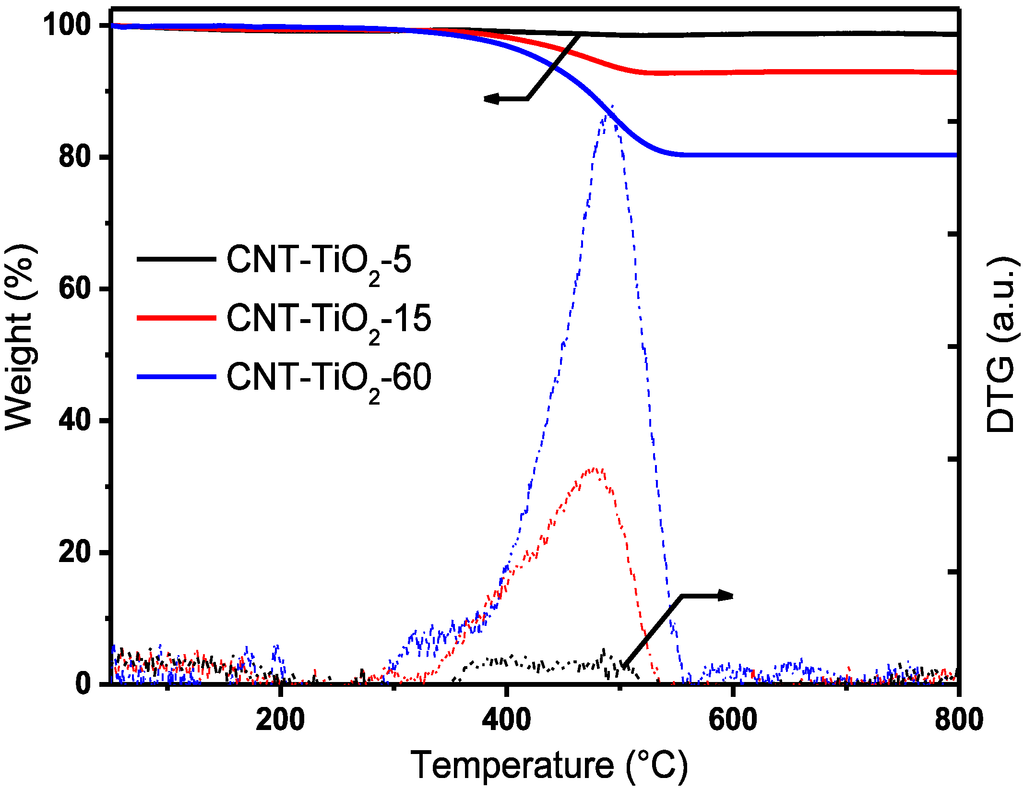
Figure 1.
TG (solid lines) and DTG (dashed lines) curves for the CNT-TiO2−δ composites. Black: CNT-TiO2−δ-5 (5, five minutes); red: CNT-TiO2−δ-15; blue: CNT-TiO2−δ-60.
To determine the amount and the thermal stability of CNTs synthesized by the method used, TG analysis in synthetic air was performed with the obtained CNT-TiO2−δ composites. The TG and differential TG (DTG) curves for the CNT-TiO2−δ composites are shown in Figure 1. It can be seen that the weight loss of the CNT-TiO2−δ-15 and CNT-TiO2−δ-60 composites mainly occurred in the temperature range between 400 and 550 °C (Figure 1), which is characteristic for the combustion of CNTs in air [22]. Correspondingly, DTG curves that are typical for CNT combustion were observed for these CNT-TiO2−δ composites, as well. No significant weight loss was detected at temperatures above 550 °C, indicating the complete total oxidation of CNTs at lower temperatures. The main DTG peak was found to be at much lower temperatures for CNT-TiO2−δ-5 than the other CNT-TiO2−δ composites, which is attributed to the presence of poorly crystallized carbon, e.g., amorphous carbon and carbon deposition due to the incomplete decomposition of C2H4 on the TiO2−δ surface [23].
CNT contents of the CNT-TiO2−δ composites were determined based on the calibrated weight loss, and the results are summarized in Table 1. It can be seen that the CNT content in the CNT-TiO2−δ composites increased with growth time, which is also reflected in the DTG curves (Figure 1) and the mass spectrometry (MS) profiles of m/z 44 corresponding to CO2 resulting from the CNT combustion (Figure S1).
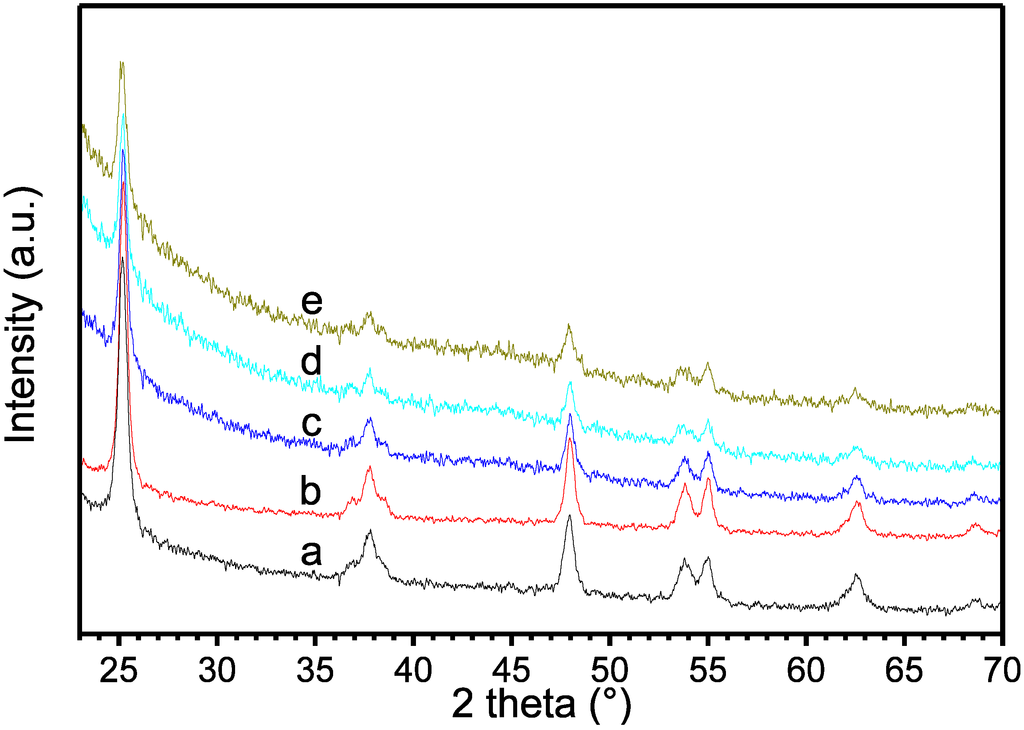
Figure 2.
XRD patterns of pristine TiO2 (a), TiO2−δ (b), CNT-TiO2−δ-5 (c), CNT-TiO2−δ-15 (d) and CNT-TiO2−δ-60 (e).

Table 1.
CNT contents (derived from the weight loss in TG analysis), BET surface areas and the average H2 evolution rates (with Pt co-catalyst) before and after resuming the reaction (r1 and r2).
| Catalyst | C (wt%) | BET (m2 g−1) | r1 (mmol h−1) | r2 (mmol h−1) |
|---|---|---|---|---|
| Pristine TiO2 | - | 104 | 13.4 | 4.2 |
| TiO2−δ | - | 92 | 18.2 | 6.2 |
| CNT-TiO2−δ-5 | 1.4 | 73 | 8.2 | 2.6 |
| CNT-TiO2−δ-15 | 7.2 | 79 | 9.7 | 8.1 |
| CNT-TiO2−δ-60 | 19.3 | 71 | 6.8 | 6.5 |
The BET surface areas of pristine TiO2, TiO2−δ and the CNT-TiO2−δ composites are summarized in Table 1. TiO2−δ and the CNT-TiO2−δ composites show clearly lower BET surface areas than the pristine TiO2, due to the agglomeration of TiO2 particles at high temperatures [21]. However, no significant difference of particle size was observed between TiO2 and TiO2−δ by SEM (Figure S2), indicating that the agglomeration was limited to a small extent. In the CNT-TiO2−δ composites, the covering of TiO2 by CNTs leads to an additional loss of accessible surface area. XRD patterns shown in Figure 2 revealed that the pure anatase phase is present in oxygen-deficient TiO2−δ and all of the CNT-TiO2−δ composites [24]. Clear CNT diffraction peaks were not observed in any of the CNT-TiO2−δ composites, probably due to the insufficiently crystalline structure of the CNTs synthesized at relatively low temperature.
SEM images in Figure 3 show the morphologies of the CNT-TiO2−δ composites with varied growth time. It can be seen that CNTs were hardly detected in CNT-TiO2−δ-5 (Figure 3A). Well-defined CNTs can be clearly observed on TiO2−δ by extending the growth time to 15 min (CNT-TiO2−δ-15, Figure 3B), whereas a growth time of 60 min (CNT-TiO2−δ-60) led to TiO2−δ fully covered by CNTs, as shown in Figure 3C. Although the length of CNTs in CNT-TiO2−δ-60 is significantly greater than that in CNT-TiO2−δ-15, due to a longer growth time, all of the observed CNTs have a diameter of around 40–50 nm and were homogeneously grown on the surface of the TiO2−δ particles. It is supposed that the TiO2 particles are also bridged by CNTs in the micro-scale range [25], because uniformly distributed Fe nanoparticles (indicated by the absence of clear FeOx reflections in the XRD pattern of CVD-synthesized FeOx/TiO2 catalyst in Figure S3) serving as the catalyst for CNT growth form on the surface of TiO2 [21,26]. As a consequence, a different performance from that of TiO2 and TiO2−δ can be expected for the CNT-TiO2−δ composites in photocatalysis.
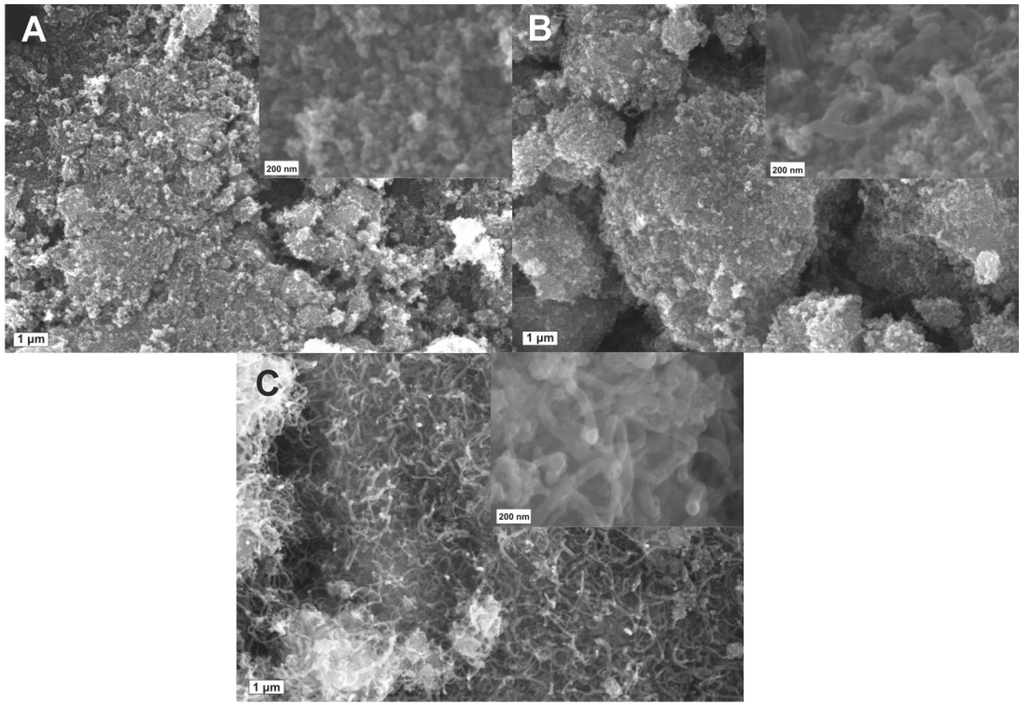
Figure 3.
SEM images for CNT-TiO2−δ composites with a growth time of 5 min (A), 15 min (B) and 60 min (C). The inserted images were taken at a higher magnification.
2.2. Photocatalytic Performance
The activities of CNT-TiO2−δ composites were tested in the photocatalytic production of hydrogen from aqueous methanol solutions. Pristine TiO2 and TiO2−δ were examined for comparison. In the presence of photocatalysts with Pt co-catalyst, the following reactions can occur in the photocatalytic system [27,28]:
2H+ + 2e−→H2
CH3OH + H2O + 6h+→CO2 + 6H+
CH3OH + 4h+→CO + 4H+
CH3OH + 2h+→CH2O + 2H+
CH3OH + H2O + 4h+→HCOOH + 4H+
CH3OH + h+→HCHO + 2H++ e− (TiO2)
Notably, Reaction 6 (injection of the second electron, from the methoxy radical formed upon one-hole oxidation of methanol, into the conduction band of TiO2) is likely to occur in our system, as corroborated by the photocurrent multiplication effect observed upon the addition of methanol (see Figure S2).
In the control experiments (Figure S4), only a negligible amount of H2 (with a H2 evolution rate smaller than 0.002 mmol h−1) was evolved under UV illumination in pure water, and the addition of methanol into the reaction system did not essentially increase the H2 evolution rates (remaining smaller than 0.5 mmol h−1). Subsequently, Pt was deposited under UV irradiation following the protocol by Ohtani and coworkers using H2PtCl6 as the precursor [29,30]. It was confirmed that the complete photoreduction of the precursor to the metallic state and thorough deposition onto the catalyst surface occur in a short time [30], which is corroboratedin this work by the short induction period (less than 5 min) for all of the tested catalysts (Figure 4). Afterwards, drastically enhanced H2 evolution was observed for the photocatalysts used, demonstrating the importance of co-catalyst in this reaction. The amounts of evolved H2 for pristine TiO2, TiO2−δ and all of the CNT-TiO2−δ composites with Pt co-catalyst are displayed as a function of time (the Pt precursor was added into the reaction system at Time 0) in Figure 4.
In the first hour, the pristine TiO2 showed high activity in terms of evolved H2, and the introduction of oxygen deficiency further improved the hydrogen productivity of TiO2 (Figure 4 and Figure S4) [6,13]. However, both the pristine TiO2 and the TiO2−δ photocatalysts were deactivated rapidly (Figure S4), probably due to the build-up of surface contaminates from the reaction, blocking the photoabsorption. In contrast, CNT-TiO2−δ-15 and CNT-TiO2−δ-60 as photocatalysts have clearly lower initial activities, but much higher stabilities in photocatalytic H2 production (Figure 4 and Figure S4). On the one hand, the presence of CNTs leads to poorer photoabsorption of TiO2−δ due to partial blocking of UV light by CNTs, as indicated by the lowered incident photon-to-current efficiency (IPCE) in Figure S5, and consequently lower overall photocatalytic activity of CNT-TiO2−δ composites, especially for CNT-TiO2−δ-60 [19]. On the other hand, the presence of CNTs facilitates the separation of photogenerated e−-h+ pairs in TiO2−δ [17], which prohibits the oxidation of active oxygen-deficient Ti species and consequently leads to higher stability [15,31]. The CNT-TiO2−δ-5 composite, however, showed no substantial improvement in terms of hydrogen productivity, probably due to the presence of a relatively large amount of poorly crystallized carbon (Figure 1) on the surface of TiO2−δ, deteriorating the IPCE (Figure S5) [20].
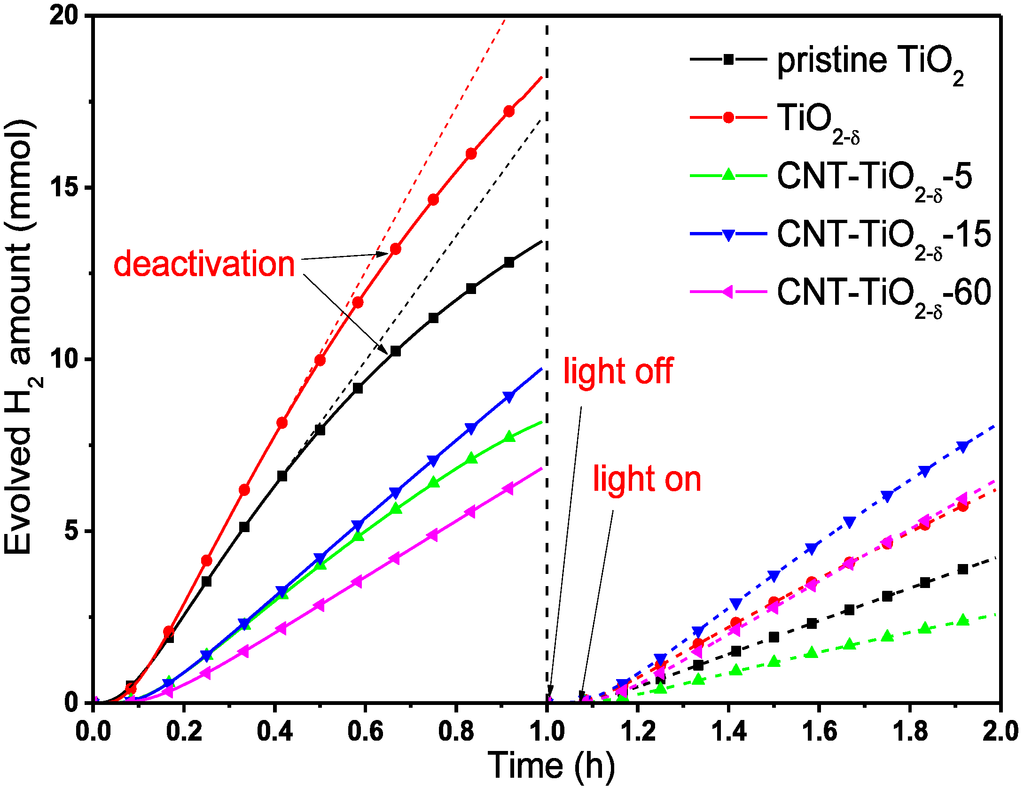
Figure 4.
Evolved H2 amount as a function of reaction time for different photocatalysts in photocatalytic hydrogen production. The reactions were carried out in aqueous methanol solutions (50 mL methanol in 500 mL pure water) using 0.2 g catalyst (pristine TiO2, TiO2−δ or CNT-TiO2−δ composites). Before the reaction, 0.1 wt% Pt was photodeposited on the photocatalyst as the co-catalyst. The irradiation lamp was switched off after 1 h, and the reactor was purged with Ar for ca. 5 min to remove all of the evolved gaseous species. Then, the reaction was resumed, and the evolved H2 amount was re-measured.
After resuming the reactions, lower activities in terms of H2 productivity were observed for all the photocatalysts, which can be ascribed to the adsorbed intermediates of methanol reforming, blocking the surface active sites at TiO2 or TiO2−δ (see the discussion below) [28,32]. Nevertheless, a nearly linear accumulation of H2 was achieved over all of the photocatalysts, indicating more stable photocatalytic performance. As compared to the pristine TiO2, TiO2−δ still demonstrated a higher activity, implying the importance of oxygen deficiency in this reaction [4,5]. Most importantly, CNT-TiO2−δ-15 and CNT-TiO2−δ-60 showed even higher H2 productivity than TiO2−δ. By comparing the average H2 evolution rates before and after resuming the reaction (r1 and r2 values in Table 1, respectively), we notice a decrease of activity by ca. 65% for TiO2−δ, whereas there is only a decrease by ca. 15% for CNT-TiO2−δ-15 and less than 5% for CNT-TiO2−δ-60. This observation clearly proves the beneficial effect of CNTs on the stability of photocatalytic performance and suggests that the presence of CNTs allows for efficient hydrogen evolution, even in the presence of surface contamination by carbon-containing reaction intermediates and products (see Reactions 1–6 above). Similar to the reaction before resuming, the highest photocatalytic activity was observed over CNT-TiO2−δ-15 among the three composites, whereas a detrimental influence resulting from poorly crystallized carbon or excess CNTs on TiO2−δ was observed, as shown by the slower H2 accumulation trends (Figure 4) and average H2 evolution rates (r2 values in Table 1) for CNT-TiO2−δ-5 and CNT-TiO2−δ-60. As a preliminary conclusion, the results imply that high-performance CNT-TiO2−δ composites for photocatalytic hydrogen production can be obtained by delicately controlling the content of CNTs on the TiO2−δ surface, in which a balance is achieved between two factors related to the presence of CNTs: the improved charge separation and stability and the diminished light absorption.
It is worth mentioning that the doping effect of Fe, as reported in the literature [33,34], is not possible in the CNT-TiO2−δ composites, because the temperature used for the preparation and treatment of Fe/TiO2 is not high enough to achieve the diffusion of Fe into the TiO2 bulk [35]. In addition, the growth catalyst particles remaining in CNTs after purification are typically covered by several carbon layers and, therefore, separated from the TiO2 particles [22,36,37].
2.3. XPS and (S)TEM of the Photocatalysts after Reactions
In order to gain further insight into the factors governing the photoactivity of our samples, after photocatalytic reactions, the surface chemistry of three representative photocatalysts, i.e., TiO2−δ, CNT-TiO2−δ-15 and CNT-TiO2−δ-60, was examined by XPS. In all three samples, Ti, O, C and Pt were detected. Both the Ti 2p and O 1s spectra were found to be in typical binding energy positions for TiO2 and without a clear shift between samples (Figure 5A,B), indicating that CNTs on the surface did not change the chemical environment of Ti and O. Nevertheless, their intensities varied significantly between different samples, that is, the Ti 2p and O 1s for CNT-TiO2−δ-60 photocatalyst have clearly lower intensities than the corresponding ones for the TiO2−δ and CNT-TiO2−δ-15 photocatalyst, which is due to the presence of CNTs covering the TiO2−δ particles. Oxygen-deficient Ti species, e.g., Ti3+, were not detected in any of the samples, presumably due to the very low amount and the complexity of the photocatalysts (e.g., the presence of adsorbed intermediate species on the surface).
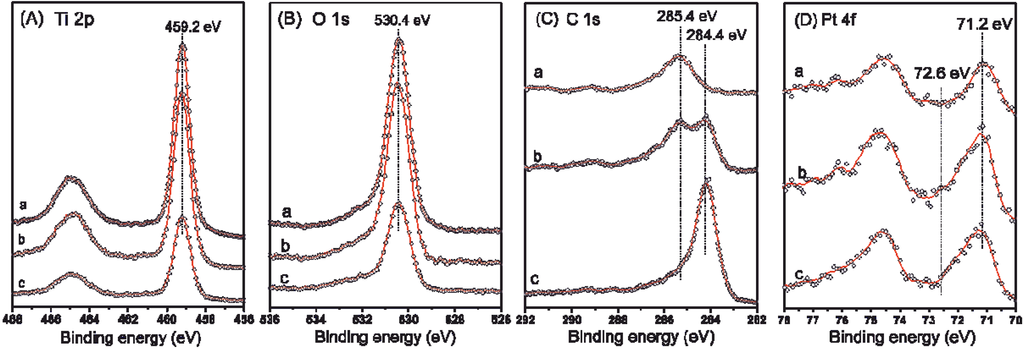
Figure 5.
XP Ti 2p (A), O 1s (B), C 1s (C) and Pt 4f (D) spectra for TiO2−δ (a), CNT-TiO2−δ-15 (b) and CNT-TiO2−δ-60 (c) with photodeposited Pt (0.1 wt%) after photocatalytic reactions.

Table 2.
Surface compositions (in atomic percent, at%) of the photocatalysts after reactions derived from the quantitative XPS studies. Platinum (0.1 wt%) was photodeposited as the co-catalyst.
| Catalyst | C | O | Ti | Pt |
|---|---|---|---|---|
| TiO2−δ | 20.34 | 55.03 | 24.47 | 0.16 |
| CNT-TiO2−δ-15 | 31.94 | 47.22 | 20.52 | 0.32 |
| CNT-TiO2−δ-60 | 54.81 | 32.08 | 12.76 | 0.35 |
Both the intensity and binding energy positions of C 1s spectra were found to vary significantly between different samples (Figure 5C). A major contribution at 285.4 eV was observed in the C 1s spectrum of the TiO2−δ photocatalyst, mainly due to the carbon contamination during the storage and transfer of the samples upon contact with air. However, a minor contribution of the adsorbed carbon species from the photocatalytic reaction in aqueous methanol solution cannot be fully excluded [38]. Similar surface carbon content was observed for pristine TiO2 photocatalyst by XPS (Figure S6 and Table S1). In the case of the CNT-TiO2−δ-15 photocatalyst, in addition to the contribution at 285.4 eV, a second peak positioning at 284.4 eV was observed, as well, and is characteristic for carbon in CNTs [22], which led to an overall double-peak C 1s spectrum. A more intense C 1s spectrum with a shifted peak center at ca. 284.4 eV was recorded for CNT-TiO2−δ-60, indicating that CNTs are the predominant carbon species. The major 4f7/2 peaks for Pt in all three samples were found to be at 71.2 eV and attributed to Pt in the metallic state (Figure 5D), indicating a complete photoreduction of the Pt precursor [22]. A much lower Pt intensity was observed on TiO2−δ than on the two CNT-TiO2−δ-based photocatalysts, which can be ascribed to the covering of Pt nanoparticles by very fine titania particles, being less visible in the surface-sensitive XPS measurements. The relatively more severe agglomeration of Pt nanoparticles on TiO2−δ than on CNT-TiO2−δ composites is believed to be responsible for the lower Pt intensity.
Quantitative analysis of the XPS spectra yielded surface compositions for the three photocatalysts as summarized in Table 2. As compared to TiO2−δ, a significantly higher amount of carbon was found on the surface of CNT-TiO2−δ photocatalysts, obviously due to the grown CNTs. Increasing the CNT growth time led to the increase of surface carbon content and, meanwhile, the decrease of surface O and Ti contents, implying that the TiO2−δ surface was more completely covered, which is in accordance with the SEM results (Figure 3). Notably, significantly higher Pt contents were observed on CNT-TiO2−δ composites than on TiO2−δ, demonstrating the advantage of CNT-TiO2−δ composites as a support for photodeposited Pt nanoparticles [22]. Obviously, the amount and morphology of Pt nanoparticles and their coupling with the light-absorbing TiO2−δ plays a crucial role in photocatalytic hydrogen evolution, particularly in the presence of surface contamination by reaction intermediates/products. Therefore, a closer analysis of the size and distribution of the Pt nanoparticles was carried out.
STEM and TEM analysis revealed that the photodeposited Pt nanoparticles were dispersed uniformly on the surface of TiO2−δ and CNT-TiO2−δ-15 (Figure 6A,B and Figure S7), whereas Pt nanoparticles on CNT-TiO2−δ-60 were observed to agglomerate more severely (Figure 6C and Figure S8). A statistical analysis of particle size was conducted over the TiO2−δ and CNT-TiO2−δ-15 photocatalysts by measuring the size of ca. 100 Pt nanoparticles in representative TEM images, and the histograms yielded are shown in Figure 6D. It can be seen that a relatively smaller mean particle size with a narrower size distribution was obtained for Pt nanoparticles on CNT-TiO2−δ-15 than on TiO2−δ, showing the relevance of the presence of CNTs, which is in agreement with the higher surface concentration of Pt on CNT-TiO2−δ-15 than on TiO2−δ, as demonstrated by the quantitative XPS analysis (Table 2).
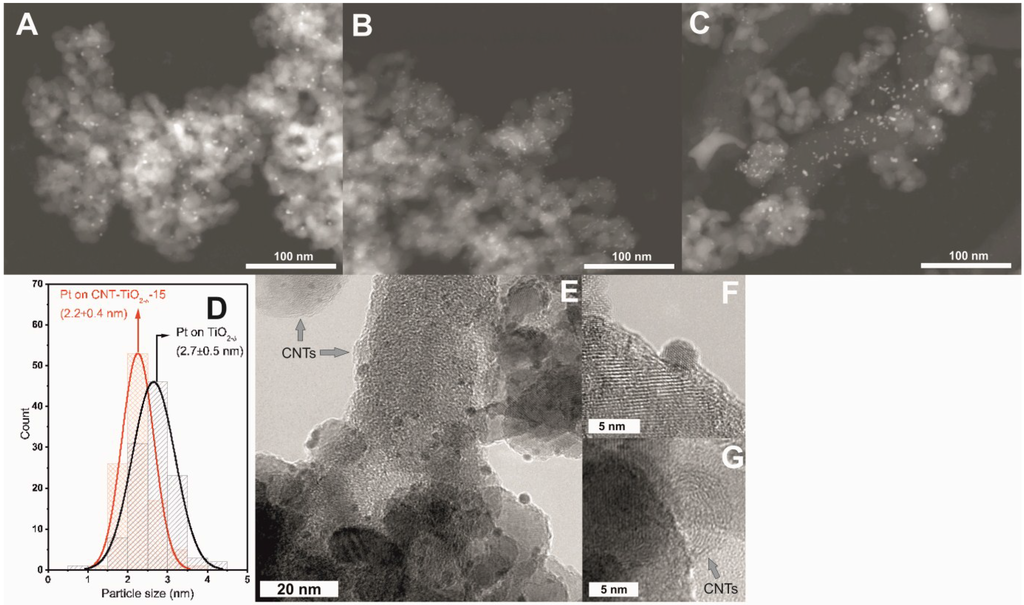
Figure 6.
Representative STEM images for TiO2−δ (A), CNT-TiO2−δ-15 (B) and CNT-TiO2−δ-60 (C) with photodeposited Pt nanoparticles and after photocatalytic reactions; particle size distributions of the Pt nanoparticles on CNT-TiO2−δ-15 and on TiO2−δ (D); TEM image of the CNT-TiO2−δ-15 with photodeposited Pt nanoparticles (E); HRTEM image of Pt nanoparticle on CNT-TiO2−δ-15 (F); HRTEM image of well-structured CNTs bridging two TiO2−δ particles in CNT-TiO2−δ-15 (G).
The TEM image in Figure 6E shows that Pt nanoparticles in the CNT-TiO2−δ-15 photocatalyst are dispersed on both TiO2−δ particles and CNTs without significant preference. The high-resolution TEM (HRTEM) image in Figure 6F shows the typical morphology of a Pt nanoparticle. Well-formed CNTs connecting the TiO2−δ particles were also detected in the TEM analysis (Figure 6E,G). A poorly crystalline structure was observed in CNTs with a larger diameter and greater length (Figure 6E), whereas a highly crystalline structure was formed for CNTs with a shorter length and smaller diameters (Figure 6E,G). The latter is supposed to play a vital role in the transfer and separation of photogenerated e−-h+ pairs [15,17,31].
According to the STEM and TEM results for CNT-TiO2−δ-60, it is clear that increasing the growth time led to the formation of longer and large-diameter CNTs, which had a negative influence on the dispersion of the photodeposited Pt nanoparticles (Figure 6C and Figure S8). Specifically, Pt nanoparticles form larger agglomerates on the long and large-diameter CNTs far from TiO2−δ particles. A large amount of surface defects on these less crystalline CNTs [39], which act as strong anchoring sites for Pt nanoparticles [22], was suggested to be the reason. This is in agreement with the fact that a higher surface Pt concentration was detected on CNT-TiO2−δ-60 by XPS (Figure 5B and Table 2) in spite of a more severe particle agglomeration (Figure 6C). Nevertheless, these Pt nanoparticles on the long and large-diameter CNTs are supposed to contribute negligibly in photocatalytic reactions. On the one hand, the size of the CNTs prohibits the intimate contact between Pt nanoparticles and the neighboring TiO2−δ particles serving as photocatalytically active sites. On the other hand, the long and large-diameter CNTs with poor crystallinity (Figure S8) are not expected to efficiently collect and transfer the photogenerated electrons [15,17,31], which impairs the subsequent functioning of Pt nanoparticles as co-catalyst to a large extent [40]. As a consequence, significantly lower activity in photocatalytic hydrogen production was observed for CNT-TiO2−δ-60 than for CNT-TiO2−δ-15 (Figure 4), in which the good crystallinity of CNTs and the optimized distribution of Pt nanoparticles yielded composite photocatalysts with optimized electron transfer to hydrogen-evolving surface catalytic sites on Pt, enhancing thusly the charge separation and rendering the photocatalyst less prone to deactivation due to the presence of methanol decomposition products.
3. Experimental Section
3.1. Materials and Sample Preparation
Commercial anatase TiO2 (Sachtleben Chemie) with a specific surface area of ca. 100 m2 g−1 was used as the starting material. The CNT-TiO2−δ composites were obtained via a two-step CVD method, which was described elsewhere [21]. Iron oxide (with a nominal Fe loading of 2 wt%) supported on TiO2 was synthesized in a vertically positioned CVD reactor using ferrocene (Merck, 98%) as the Fe precursor [41]. Reduction of the obtained FeOx/TiO2 composites was performed at 550 °C in flowing H2 (50 vol% H2 in He, 100 mL min−1) to obtain metallic Fe and TiO2−δ simultaneously. At the same temperature, CVD growth of CNTs was subsequently carried out using the supported Fe as the catalyst and a mixture of C2H4 and H2 (57 vol% C2H4, 43 vol% H2, 100 mL min−1) as the gas feed [23]. The growth time was varied from 5 to 60 min in order to obtain different carbon contents on TiO2−δ. Prior to any further use, the synthesized CNT-TiO2−δ composites were purified by washing in diluted HNO3 solution [22].
3.2. Physicochemical Characterization
Static N2 physisorption experiments were performed in a Quantachrome Autosorb-1-MP setup. Samples were degassed at 200 °C for 2 h before the measurements. The adsorption-desorption isotherm data were recorded in the relative pressure range of 0.05–1. The specific surface area was calculated according to the BET equation, assuming that the area covered by one N2 molecule is 0.162 nm2. Thermogravimetric (TG) analysis was performed in synthetic air (20.5 vol% O2 in N2) using a Cahn TG 2131 thermobalance in order to examine both the thermal stability and the achieved amount of the synthesized carbon on TiO2−δ. The samples were heated up to 800 °C at a ramp of 2 °C min−1. The evolved gas species resulting from the carbon combustion were monitored by a coupled mass spectrometer in the exhaust stream. The calibrated weight loss was used to determine the carbon content of each CNT-TiO2−δ composite.
X-ray diffraction was measured using a PANalytical MPD diffractometer equipped with Cu Kα radiation, a secondary graphite monochromator and 0.04 rad incident/secondary beam Soller slits. Measurement conditions were 45 kV, 40 mA, 0.5° divergence slit and 0.2-mm receiving slit width. A step width of 0.015° and 20-s scanning time were applied in all of the measurements. A high-resolution thermal field emission scanning electron microscope (SEM, Zeiss, LEO 1530 Gemini) was used to investigate the morphology of the CNT-TiO2−δ samples. The SEM images were acquired using an acceleration voltage of 20 kV. Transmission electron microscopy (TEM) and scanning TEM (STEM) measurements were carried out with a Jeol JEM-2200FS instrument operated at 200 kV. The specimens for TEM and STEM were prepared by ultrasonically dispersing the powder samples in ethanol and dropping the suspension on a carbon-coated Au grid.
X-ray photoelectron spectroscopy (XPS) measurements were performed in an ultrahigh vacuum setup equipped with a high resolution Gammadata Scienta SES 2002 analyzer and a monochromatic Al Kα X-ray source (1486.6 eV, operated at 14.1 kV and 30 mA). The spectra were acquired at 200 eV pass energy (high pass energy mode) at a pressure of 3.5 to 6
10−10 mbar. Charging effects during the measurements were mediated by applying a flood gun (SPECS). The CASA XPS program was employed in the analysis of XP spectra. Calibration of the measured data was performed by positioning the main Ti 2p peak at 459.2 eV for TiO2. Quantitative analysis was performed based on the C 1s, O 1s, Ti 2p and Pt 4f spectra after Shirley background subtraction and by taking the atomic sensitivity factor (ASF) of each element into account, which is consistent with our previous work [22].
3.3. Photocatalytic and Photoelectrochemical Measurements
Photocatalytic hydrogen production was performed in a double-walled inner irradiation-type quartz reactor connected to a closed gas circulation system. The reaction temperature was maintained at 10 °C with a double-walled quartz jacket filled with a flow of cooling water from a thermostat (Lauda) to prevent any thermal effect. A 350-W Hg mid-pressure immersion lamp (Peschl UV-Consulting, 700 W, operated at 50% power) was used as the light source for irradiation. Argon was used as the carrier gas, and a continuous flow of 50 mL min−1 was applied. The evolved gas mixture was analyzed using an online multi-channel analyzer (Emerson) equipped with a thermal conductivity detector for hydrogen. In a typical run, 0.2 g of photocatalyst (TiO2, TiO2−δ or CNT-TiO2−δ composites) powders were added and suspended in aqueous methanol solution (50 mL of methanol in 500 mL of pure water), where methanol was acting as a sacrificial reagent for H2 production. Before irradiation, the whole system, including the photocatalyst, was rinsed with Ar to completely remove dissolved air. Afterwards, Pt (a nominal loading of 0.1 wt%) as the co-catalyst was loaded onto the photocatalysts by in situ photoreduction of the H2PtCl6 precursor (0.42 mg), and the photocatalytic methanol reforming was initiated by switching on the UV lamp. After 1 h of reaction, the irradiation lamp was switched off, and the reactor was purged with Ar. The complete removal of all of the evolved gaseous species was indicated by the detector signal down to the baseline level. Then, the reaction was resumed by switching on the UV lamp, and the evolved H2 amount was re-measured. Control experiments were performed in either pure water or a water-methanol mixture under UV irradiation and in the presence of photocatalysts (i.e., pristine TiO2, TiO2−δ or CNT-TiO2−δ-60) without Pt co-catalyst.
The protocol for the preparation of electrodes for photocurrent measurements was described elsewhere [42]. Briefly, a suspension containing 100 mg of powder sample in 1 mL of ethanol was sonicated for 15 min and then evenly deposited onto ITO glass by doctor blading using scotch tape as the frame and spacer. The electrode was then dried at 80 °C, pressed for 3 min at a pressure of 200 kg cm−2 and heated in flowing He (100 mL min−1) at 400 °C for 60 min in order to achieve a good contact. The photoelectrochemical setup for photocurrent measurements consisted of a SP-300 BioLogic potentiostat and a three-electrode cell using a Pt counter electrode and an Ag/AgCl reference electrode. The photoelectrodes were pressed against an O-ring of the cell, leaving an irradiated area of 0.5 cm2. The electrodes were irradiated from the backside (through the ITO glass) using a tunable monochromatic light source (Instytut Fotonowy) equipped with a 150-W Xenon lamp and a grating monochromator with a bandwidth of ca. 10 nm. Appropriate cut-off filters were used in order to eliminate second-order diffraction radiation. The photocurrent was firstly recorded in a phosphate buffer (pH 7.0) at 0.5 V vs. Ag/AgCl. The measurements were repeated after the addition of 2 mL methanol, and the corresponding IPCE (incident photon-to-current efficiency) values were calculated for each sample.
4. Conclusions
CNT-TiO2−δ composites consisting of CNTs directly grown on TiO2−δ, i.e., oxygen-deficient TiO2, were successfully synthesized via a two-step CVD method. The amount of CNTs can be precisely controlled by varying the growth time. CNTs were found to be uniformly grown on the surface of TiO2−δ particles without altering the crystalline structure.
In the photocatalytic production of hydrogen using Pt as the co-catalyst, TiO2−δ showed a significantly higher activity than pristine TiO2, due to the presence of oxygen vacancies, but strongly deactivated in the absence of CNTs. In contrast, a certain amount of CNTs can effectively stabilize the photocatalytic activity of TiO2−δ due to the beneficial effects of CNTs on the separation of photogenerated e−-h+ pairs and on the dispersion and anchoring of photodeposited Pt nanoparticles acting as co-catalyst. An excess amount of CNTs was found to deteriorate the activity of TiO2−δ, which is attributed to the blocking of light absorption by CNTs fully covering the surface of TiO2−δ and to the agglomeration of the photodeposited Pt nanoparticles at long and large CNTs. While attempts to enhance the initial photocatalytic activity of hydrogen evolving systems are indispensable, the present study highlights the crucial importance of functional stability as a key factor in the development of viable solar hydrogen production schemes and demonstrates that CNT-TiO2−δ composite photocatalysts with high performance stability can be obtained by growing short and small-diameter CNTs on the surface of TiO2−δ.
Acknowledgments
Financial support by the MIWFT-NRW within the project “Anorganische Nanomaterialien für Anwendungen in der Photokatalyse: Wasseraufbereitung und Wasserstoffgewinnung” and by the EU-FP7 Grant “4G-PHOTOCAT” (Grant No. 309636) is gratefully acknowledged. The authors thank Wei Xia for fruitful discussions. P.W. acknowledges the support from the China Scholarship Council for a PhD scholarship.
Author Contributions
P.C., M.M., M.W., and RB developed the project and designed the experiments. P.C. synthesized the photocatalysts. P.C., L.W., P.W., and A.K. performed the measurements. All authors contributed to the manuscript preparation, and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Martin, D.J.; Reardon, P.J.; Moniz, S.J.; Tang, J. Visible light-driven pure water splitting by a nature-inspired organic semiconductor-based system. J. Am. Chem. Soc. 2014, 136, 12568–12571. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.; Bard, A.J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano. Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-H.; Gopal, N.O.; Ke, S.-C. Origin of photoactivity of oxygen-deficient TiO2 under visible light. Appl. Phys. Lett. 2009, 95, 83126–83128. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Lin, B.; Gao, B. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies. Appl. Surf. Sci. 2013, 264, 845–852. [Google Scholar] [CrossRef]
- Leshuk, T.; Parviz, R.; Everett, P.; Krishnakumar, H.; Varin, R.A.; Gu, F. Photocatalytic activity of hydrogenated TiO2. ACS Appl. Mater. Interfaces 2013, 5, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Dozzi, M.V.; Selli, E. Specific facets-dominated anatase TiO2: Fluorine-mediated synthesis and photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Wei, W.; Yaru, N.; Chunhua, L.; Zhongzi, X. Hydrogenation of TiO2 nanosheets with exposed {001} facets for enhanced photocatalytc activity. RSC Adv. 2012, 2, 8286. [Google Scholar] [CrossRef]
- Mei, B.; Sánchez, M.D.; Reinecke, T.; Kaluza, S.; Xia, W.; Muhler, M. The synthesis of Nb-doped TiO2 nanoparticles by spray drying: An efficient and scalable method. J. Mater. Chem. 2011, 21, 11781–11790. [Google Scholar] [CrossRef]
- Jin, Q.L.; Arimoto, H.; Fujishima, M.; Tada, H. Manganese oxide-surface modified titanium(IV) dioxide as environmental catalyst. Catalysts 2013, 3, 444–454. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q. Titania-based photocatalysts—Crystal growth, doping and heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Amer. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Wong, T.J.; Lim, F.J.; Gao, M.; Lee, G.H.; Ho, G.W. Photocatalytic H2 production of composite one-dimensional TiO2 nanostructures of different morphological structures and crystal phases with graphene. Catal. Sci. Technol. 2013, 3, 1086–1093. [Google Scholar] [CrossRef]
- Tsubota, T.; Ono, A.; Murakami, N.; Ohno, T. Characterization and photocatalytic performance of carbon nanotube material-modified TiO2 synthesized by using the hot CVD process. Appl. Catal. B 2009, 91, 533–538. [Google Scholar] [CrossRef]
- Huang, Q.; Tian, S.; Zeng, D.; Wang, X.; Song, W.; Li, Y.; Xiao, W.; Xie, C. Enhanced photocatalytic activity of chemically bonded TiO2/graphene composites based on the effective interfacial charge transfer through the C–Ti bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Li, C.; Chen, W.; Zeng, M. Carbon nanotube/titania composites prepared by a micro-emulsion method exhibiting improved photocatalytic activity. Appl. Catal. A 2012, 427–428, 1–7. [Google Scholar] [CrossRef]
- Ahmmad, B.; Kusumoto, Y.; Somekawa, S.; Ikeda, M. Carbon nanotubes synergistically enhance photocatalytic activity of TiO2. Catal. Commun. 2008, 9, 1410–1413. [Google Scholar] [CrossRef]
- Ventosa, E.; Chen, P.; Schuhmann, W.; Xia, W. CNTs grown on oxygen-deficient anatase TiO2−δ as high-rate composite electrode material for lithium ion batteries. Electrochem. Commun. 2012, 25, 132–135. [Google Scholar] [CrossRef]
- Chen, P.R.; Chew, L.M.; Xia, W. The influence of the residual growth catalyst in functionalized carbon nanotubes on supported Pt nanoparticles applied in selective olefin hydrogenation. J. Catal. 2013, 307, 84–93. [Google Scholar] [CrossRef]
- Becker, M.J.; Xia, W.; Tessonnier, J.-P.; Blume, R.; Yao, L.; Schlögl, R.; Muhler, M. Optimizing the synthesis of cobalt-based catalysts for the selective growth of multiwalled carbon nanotubes under industrially relevant conditions. Carbon 2011, 49, 5253–5264. [Google Scholar] [CrossRef]
- Ventosa, E.; Xia, W.; Klink, S.; La Mantia, F.; Mei, B.; Muhler, M.; Schuhmann, W. Ammonia-annealed TiO2 as a negative electrode material in Li-ion batteries: N doping or oxygen deficiency? Chem. Eur. J. 2013, 19, 14194–14199. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Su, D.; Birkner, A.; Ruppel, L.; Wang, Y.; Wöll, C.; Qian, J.; Liang, C.; Marginean, G.; Brandl, W.; Muhler, M. Chemical vapor deposition and synthesis on carbon nanofibers: sintering of ferrocene-derived supported iron nanoparticles and the catalytic growth of secondary carbon nanofibers. Chem. Mater. 2005, 17, 5737–5742. [Google Scholar] [CrossRef]
- Kundu, S.; Nagaiah, T.C.; Chen, X.X.; Xia, W.; Bron, M.; Schuhmann, W.; Muhler, M. Synthesis of an improved hierarchical carbon-fiber composite as a catalyst support for platinum and its application in electrocatalysis. Carbon 2012, 50, 4534–4542. [Google Scholar] [CrossRef]
- Sánchez, M.D.; Chen, P.; Reinecke, T.; Muhler, M.; Xia, W. The role of oxygen- and nitrogen-containing surface groups on the sintering of iron nanoparticles on carbon nanotubes in different atmospheres. ChemCatChem 2012, 4, 1997–2004. [Google Scholar] [CrossRef]
- Santato, C.; Ulmann, M.; Augustynski, J. Photoelectrochemical properties of nanostructured tungsten trioxide films. J. Phys. Chem. B 2001, 105, 936–940. [Google Scholar] [CrossRef]
- Busser, G.W.; Mei, B.; Muhler, M. Optimizing the deposition of hydrogen evolution sites on suspended semiconductor particles using on-line photocatalytic reforming of aqueous methanol solutions. ChemSusChem 2012, 5, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Iwai, K.; Nishimoto, S.; Sato, S. Role of Platinum Deposits on Titanium(IV) Oxide Particles: Structural and Kinetic Analyses of Photocatalytic Reaction in Aqueous Alcohol and Amino Acid Solutions. J. Phys. Chem. B 1997, 101, 3349–3359. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube-TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Chen, T.; Feng, Z.; Wu, G.; Shi, J.; Ma, G.; Ying, P.; Li, C. Mechanistic studies of photocatalytic reaction of methanol for hydrogen production on Pt/TiO2 by in situ Fourier transform IR and time-Resolved IR spectroscopy. J. Phys. Chem. C 2007, 111, 8005–8014. [Google Scholar] [CrossRef]
- Yu, J.G.; Xiang, Q.J.; Zhou, M.H. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Tatarchuk, B. Physical characterization of Fe/TiO2 model supported catalysts I. Electron microscopic studies of reduction behavior. J. Catal. 1981, 70, 308–322. [Google Scholar] [CrossRef]
- Pumera, M. Carbon nanotubes contain residual metal catalyst nanoparticles even after washing with nitric acid at elevated temperature because these metal nanoparticles are sheathed by several graphene sheets. Langmuir 2007, 23, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfander, N.; Timpe, O.; Su, D.S.; Schlögl, R. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef]
- Lascovich, J.C.; Giorgi, R.; Scaglione, S. Evaluation of the sp2/sp3 ratio in amorphous carbon structure by XPS and XAES. Appl. Surf. Sci. 1991, 47, 17–21. [Google Scholar] [CrossRef]
- Veziri, C.M.; Pilatos, G.; Karanikolos, G.N.; Labropoulos, A.; Kordatos, K.; Kasselouri-Rigopoulou, V.; Kanellopouios, N.K. Growth and optimization of carbon nanotubes in activated carbon by catalytic chemical vapor deposition. Micro. Mesopor. Mater. 2008, 110, 41–50. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Bledowski, M.; Wang, L.; Ramakrishnan, A.; Betard, A.; Khavryuchenko, O.V.; Beranek, R. Visible-light photooxidation of water to oxygen at hybrid TiO2-polyheptazine photoanodes with photodeposited Co-Pi (CoOx) cocatalyst. Chemphyschem 2012, 13, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).