Abstract
Deactivation of heterogeneous catalysts is a ubiquitous problem that causes loss of catalytic rate with time. This review on deactivation and regeneration of heterogeneous catalysts classifies deactivation by type (chemical, thermal, and mechanical) and by mechanism (poisoning, fouling, thermal degradation, vapor formation, vapor-solid and solid-solid reactions, and attrition/crushing). The key features and considerations for each of these deactivation types is reviewed in detail with reference to the latest literature reports in these areas. Two case studies on the deactivation mechanisms of catalysts used for cobalt Fischer-Tropsch and selective catalytic reduction are considered to provide additional depth in the topics of sintering, coking, poisoning, and fouling. Regeneration considerations and options are also briefly discussed for each deactivation mechanism.
1. Introduction
Catalyst deactivation, the loss over time of catalytic activity and/or selectivity, is a problem of great and continuing concern in the practice of industrial catalytic processes. Costs to industry for catalyst replacement and process shutdown total billions of dollars per year. Time scales for catalyst deactivation vary considerably; for example, in the case of cracking catalysts, catalyst mortality may be on the order of seconds, while in ammonia synthesis the iron catalyst may last for 5–10 years. However, it is inevitable that all catalysts will decay.
Typically, the loss of activity in a well-controlled process occurs slowly. However, process upsets or poorly designed hardware can bring about catastrophic failure. For example, in steam reforming of methane or naphtha, great care must be taken to avoid reactor operation at excessively high temperatures or at steam-to-hydrocarbon ratios below a critical value. Indeed, these conditions can cause formation of large quantities of carbon filaments that plug catalyst pores and voids, pulverize catalyst pellets, and bring about process shutdown, all within a few hours.
While catalyst deactivation is inevitable for most processes, some of its immediate, drastic consequences may be avoided, postponed, or even reversed. Thus, deactivation issues (i.e., extent, rate, and reactivation) greatly impact research, development, design, and operation of commercial processes. Accordingly, there is considerable motivation to understand and treat catalyst decay. Over the past three decades, the science of catalyst deactivation has been steadily developing, while literature addressing this topic has expanded considerably to include books [1,2,3,4], comprehensive reviews [5,6,7,8], proceedings of international symposia [9,10,11,12,13,14], topical journal issues (e.g., [15]), and more than 20,000 U.S. patents for the period of 1976–2013. (In a U.S. patent search conducted in November 2013 for the keywords catalyst and deactivation, catalyst and life, and catalyst and regeneration, 14,712, 62,945, and 22,520 patents were found respectively.) This area of research provides a critical understanding that is the foundation for modeling deactivation processes, designing stable catalysts, and optimizing processes to prevent or slow catalyst deactivation.
The purpose of this article is to provide the reader with a comprehensive overview of the scientific and practical aspects of catalyst deactivation with a focus on mechanisms of catalyst decay, prevention of deactivation, and regeneration of catalysts. Case studies of deactivation and regeneration of Co Fischer-Tropsch catalysts and of commercial catalysts for selective catalytic reduction of nitrogen oxides in stationary sources have been included.
2. Mechanisms of Deactivation
There are many paths for heterogeneous catalyst decay. For example, a catalyst solid may be poisoned by any one of a dozen contaminants present in the feed. Its surface, pores, and voids may be fouled by carbon or coke produced by cracking/condensation reactions of hydrocarbon reactants, intermediates, and/or products. In the treatment of a power plant flue gas, the catalyst can be dusted or eroded by and/or plugged with fly ash. Catalytic converters used to reduce emissions from gasoline or diesel engines may be poisoned or fouled by fuel or lubricant additives and/or engine corrosion products. If the catalytic reaction is conducted at high temperatures, thermal degradation may occur in the form of active phase crystallite growth, collapse of the carrier (support) pore structure, and/or solid-state reactions of the active phase with the carrier or promoters. In addition, the presence of oxygen or chlorine in the feed gas can lead to formation of volatile oxides or chlorides of the active phase, followed by gas-phase transport from the reactor. Similarly, changes in the oxidation state of the active catalytic phase can be induced by the presence of reactive gases in the feed.
Thus, the mechanisms of solid catalyst deactivation are many; nevertheless, they can be grouped into six intrinsic mechanisms of catalyst decay: (1) poisoning, (2) fouling, (3) thermal degradation, (4) vapor compound formation and/or leaching accompanied by transport from the catalyst surface or particle, (5) vapor–solid and/or solid–solid reactions, and (6) attrition/crushing. As mechanisms 1, 4, and 5 are chemical in nature while 2 and 6 are mechanical, the causes of deactivation are basically threefold: chemical, mechanical, and thermal. Each of the six basic mechanisms is defined briefly in Table 1 and treated in some detail in the subsections that follow, with an emphasis on the first three. Mechanisms 4 and 5 are treated together, since 4 is a subset of 5.

Table 1.
Mechanisms of catalyst deactivation.
| Mechanism | Type | Brief definition/description |
|---|---|---|
| Poisoning | Chemical | Strong chemisorption of species on catalytic sites which block sites for catalytic reaction |
| Fouling | Mechanical | Physical deposition of species from fluid phase onto the catalytic surface and in catalyst pores |
| Thermal degradation and sintering | Thermal Thermal/chemical | Thermally induced loss of catalytic surface area, support area, and active phase-support reactions |
| Vapor formation | Chemical | Reaction of gas with catalyst phase to produce volatile compound |
| Vapor–solid and solid–solid reactions | Chemical | Reaction of vapor, support, or promoter with catalytic phase to produce inactive phase |
| Attrition/crushing | Mechanical | Loss of catalytic material due to abrasion; loss of internal surface area due to mechanical-induced crushing of the catalyst particle |
2.1. Poisoning
Poisoning [3,16,17,18,19,20,21,22] is the strong chemisorption of reactants, products, or impurities on sites otherwise available for catalysis. Thus, poisoning has operational meaning; that is, whether a species acts as a poison depends upon its adsorption strength relative to the other species competing for catalytic sites. For example, oxygen can be a reactant in partial oxidation of ethylene to ethylene oxide on a silver catalyst and a poison in hydrogenation of ethylene on nickel. In addition to physically blocking of adsorption sites, adsorbed poisons may induce changes in the electronic or geometric structure of the surface [17,21]. Finally, poisoning may be reversible or irreversible. An example of reversible poisoning is the deactivation of acid sites in fluid catalytic cracking catalysts by nitrogen compounds in the feed. Although the effects can be severe, they are temporary and are generally eliminated within a few hours to days after the nitrogen source is removed from the feed. Similar effects have been observed for nitrogen compound (e.g., ammonia and cyanide) addition to the syngas of cobalt Fischer-Tropsch catalysts, although these surface species require weeks to months before the lost activity is regained [23]. However, most poisons are irreversibly chemisorbed to the catalytic surface sites, as is the case for sulfur on most metals, as discussed in detail below. Regardless of whether the poisoning is reversible or irreversible, the deactivation effects while the poison is adsorbed on the surface are the same.
Many poisons occur naturally in feed streams that are treated in catalytic processes. For example, crude oil contains sulfur and metals, such as vanadium and nickel, that act as catalyst poisons for many petroleum refinery processes, especially those that use precious metal catalysts, like catalytic reforming, and those that treat heavier hydrocarbon fractions in which the sulfur concentrates and metals are almost exclusively found, such as fluid catalytic cracking and residuum hydroprocessing. Coal contains numerous potential poisons, again including sulfur and others like arsenic, phosphorous, and selenium, often concentrated in the ash, that can poison selective catalytic reduction catalysts as discussed later in Section 4.3.3.1. As a final example, some poisons may be added purposefully, either to moderate the activity and/or to alter the selectivity of fresh catalysts, as discussed as the end of this section, or to improve the performance of a product that is later reprocessed catalytically. An example of this latter case is lubricating oils that contain additives like zinc and phosphorous to improve their lubricating properties and stability, which become poisons when the lubricants are reprocessed in a hydrotreater or a fluid catalytic cracking unit.
Mechanisms by which a poison may affect catalytic activity are multifold, as illustrated by a conceptual two-dimensional model of sulfur poisoning of ethylene hydrogenation on a metal surface shown in Figure 1. To begin with, a strongly adsorbed atom of sulfur physically blocks at least one three- or fourfold adsorption/reaction site (projecting into three dimensions) and three or four topside sites on the metal surface. Second, by virtue of its strong chemical bond, it electronically modifies its nearest neighbor metal atoms and possibly its next-nearest neighbor atoms, thereby modifying their abilities to adsorb and/or dissociate reactant molecules (in this case H2 and ethylene molecules), although these effects do not extend beyond about 5 atomic units [21]. A third effect may be the restructuring of the surface by the strongly adsorbed poison, possibly causing dramatic changes in catalytic properties, especially for reactions sensitive to surface structure. In addition, the adsorbed poison blocks access of adsorbed reactants to each other (a fourth effect) and finally prevents or slows the surface diffusion of adsorbed reactants (effect number five).
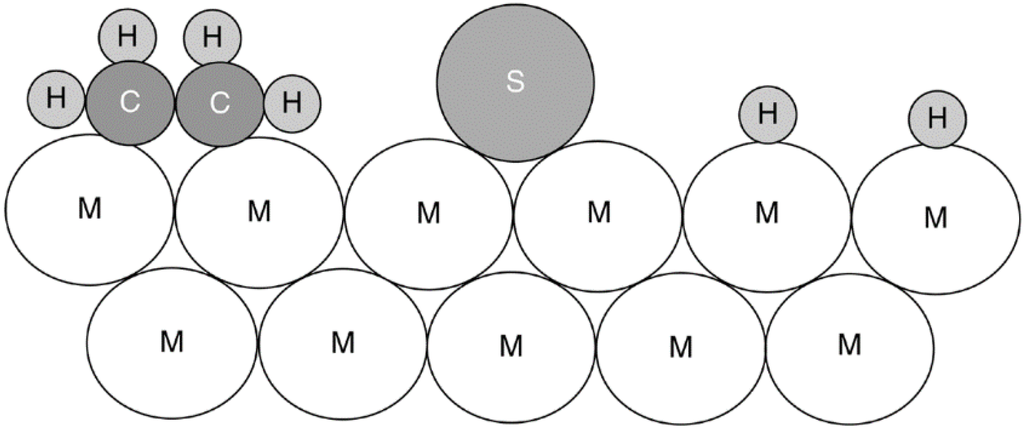
Figure 1.
Conceptual model of poisoning by sulfur atoms of a metal surface during ethylene hydrogenation. Reproduced from [8]. Copyright 2006, Wiley-Interscience.
Catalyst poisons can be classified according to their chemical makeup, selectivity for active sites, and the types of reactions poisoned. Table 2 lists four groups of catalyst poisons classified according to chemical origin and their type of interaction with metals. It should be emphasized that interactions of Group VA–VIIA elements with catalytic metal phases depend on the oxidation state of the former, e.g., how many electron pairs are available for bonding and the degree of shielding of the sulfur ion by ligands [16]. Thus, the order of decreasing toxicity for poisoning of a given metal by different sulfur species is H2S, SO2, SO42−, i.e., in the order of increased shielding by oxygen. Toxicity also increases with increasing atomic or molecular size and electronegativity, but decreases if the poison can be gasified by O2, H2O, or H2 present in the reactant stream [21]; for example, adsorbed carbon can be gasified by O2 to CO or CO2 or by H2 to CH4.

Table 2.
Common poisons classified according to chemical structure.
| Chemical type | Examples | Type of interaction with metals |
|---|---|---|
| Groups VA and VIA | N, P, As, Sb, O, S, Se, Te | Through s and p orbitals; shielded structures are less toxic |
| Group VIIA | F, Cl, Br, I | Through s and p orbitals; formation of volatile halides |
| Toxic heavy metals and ions | As, Pb, Hg, Bi, Sn, Cd, Cu, Fe | Occupy d orbitals; may form alloys |
| Molecules that adsorb with multiple bonds | CO, NO, HCN, benzene, acetylene, other unsaturated hydrocarbons | Chemisorption through multiple bonds and back bonding |
Table 3 lists a number of common poisons for selected catalysts in important representative reactions. It is apparent that organic bases (e.g., amines) and ammonia are common poisons for acidic solids, such as silica–aluminas and zeolites in cracking and hydrocracking reactions, while sulfur- and arsenic-containing compounds are typical poisons for metals in hydrogenation, dehydrogenation, and steam reforming reactions. Metal compounds (e.g., of Ni, Pb, V, and Zn) are poisons in automotive emissions control, catalytic cracking, and hydrotreating. Acetylene is a poison for ethylene oxidation, while asphaltenes are poisons in hydrotreating of petroleum residuum.

Table 3.
Poisons for selected catalysts in important representative reactions.
| Catalyst | Reaction | Poisons |
|---|---|---|
| Silica–alumina, zeolites | Cracking | Organic bases, hydrocarbons, heavy metals |
| Nickel, platinum, palladium | Hydrogenation/dehydrogenation | Compounds of S, P, As, Zn, Hg, halides, Pb, NH3, C2H2 |
| Nickel | Steam reforming of methane, naphtha | H2S, As |
| Iron, ruthenium | Ammonia synthesis | O2, H2O, CO, S, C2H2, H2O |
| Cobalt, iron | Fischer–Tropsch synthesis | H2S, COS, As, NH3, metal carbonyls |
| Noble metals on zeolites | Hydrocracking | NH3, S, Se, Te, P |
| Silver | Ethylene oxidation to ethylene oxide | C2H2 |
| Vanadium oxide | Oxidation/selective catalytic reduction | As/Fe, K, Na from fly ash |
| Platinum, palladium | Oxidation of CO and hydrocarbons | Pb, P, Zn, SO2, Fe |
Poisoning selectivity is illustrated in Figure 2, a plot of activity (the reaction rate normalized to initial rate) versus normalized poison concentration. “Selective” poisoning involves preferential adsorption of the poison on the most active sites at low concentrations. If sites of lesser activity are blocked initially, the poisoning is “antiselective”. If the activity loss is proportional to the concentration of adsorbed poison, the poisoning is “nonselective.” An example of selective poisoning is the deactivation of platinum by CO for the para-H2 conversion (Figure 3a) [24] while Pb poisoning of CO oxidation on platinum is apparently antiselective (Figure 3b) [25], and arsenic poisoning of cyclopropane hydrogenation on Pt is nonselective (Figure 3c) [26]. For nonselective poisoning, the linear decrease in activity with poison concentration or susceptibility (σ) is defined by the slope of the activity versus poison concentration curve. Several other important terms associated with poisoning are defined in Table 4. Poison tolerance, the activity at saturation coverage of the poison, and resistance (the inverse of deactivation rate) are important concepts that are often encountered in discussions of poisoning including those below.
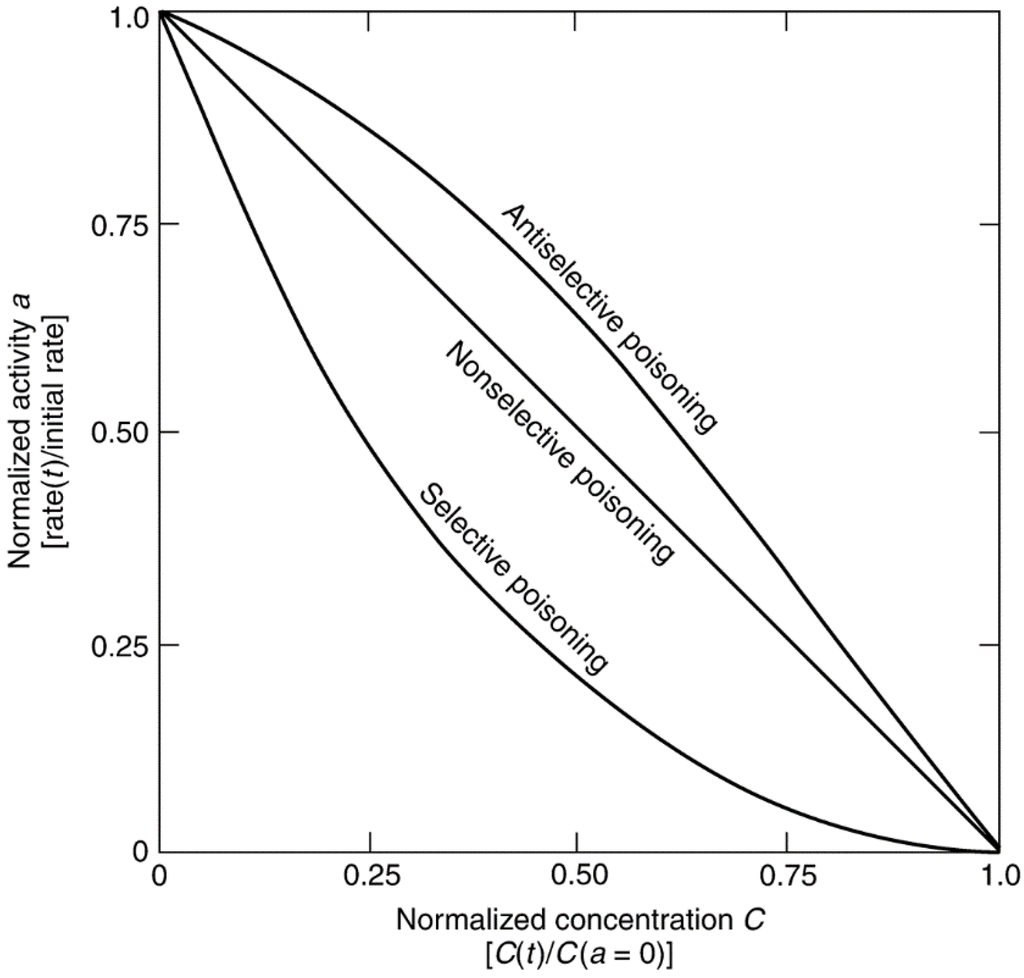
Figure 2.
Three kinds of poisoning behavior in terms of normalized activity versus normalized poison concentration. Reproduced from [8]. Copyright 2006, Wiley-Interscience.

Table 4.
Important Poisoning Parameters.
| Parameter | Definition |
|---|---|
| Activity (a) | Reaction rate at time t relative to that at t = 0 |
| Susceptibility (σ) | Negative slope of the activity versus poison concentration curve [σ = (a − 1)/C (t)]. Measure of a catalyst’s sensitivity to a given poison |
| Toxicity | Susceptibility of a given catalyst for a poison relative to that for another poison |
| Resistance | Inverse of the deactivation rate. Property that determines how rapidly a catalyst deactivates |
| Tolerance (a(Csat)) | Activity of the catalyst at saturation coverage (some catalysts may have negligible activity at saturation coverage) |
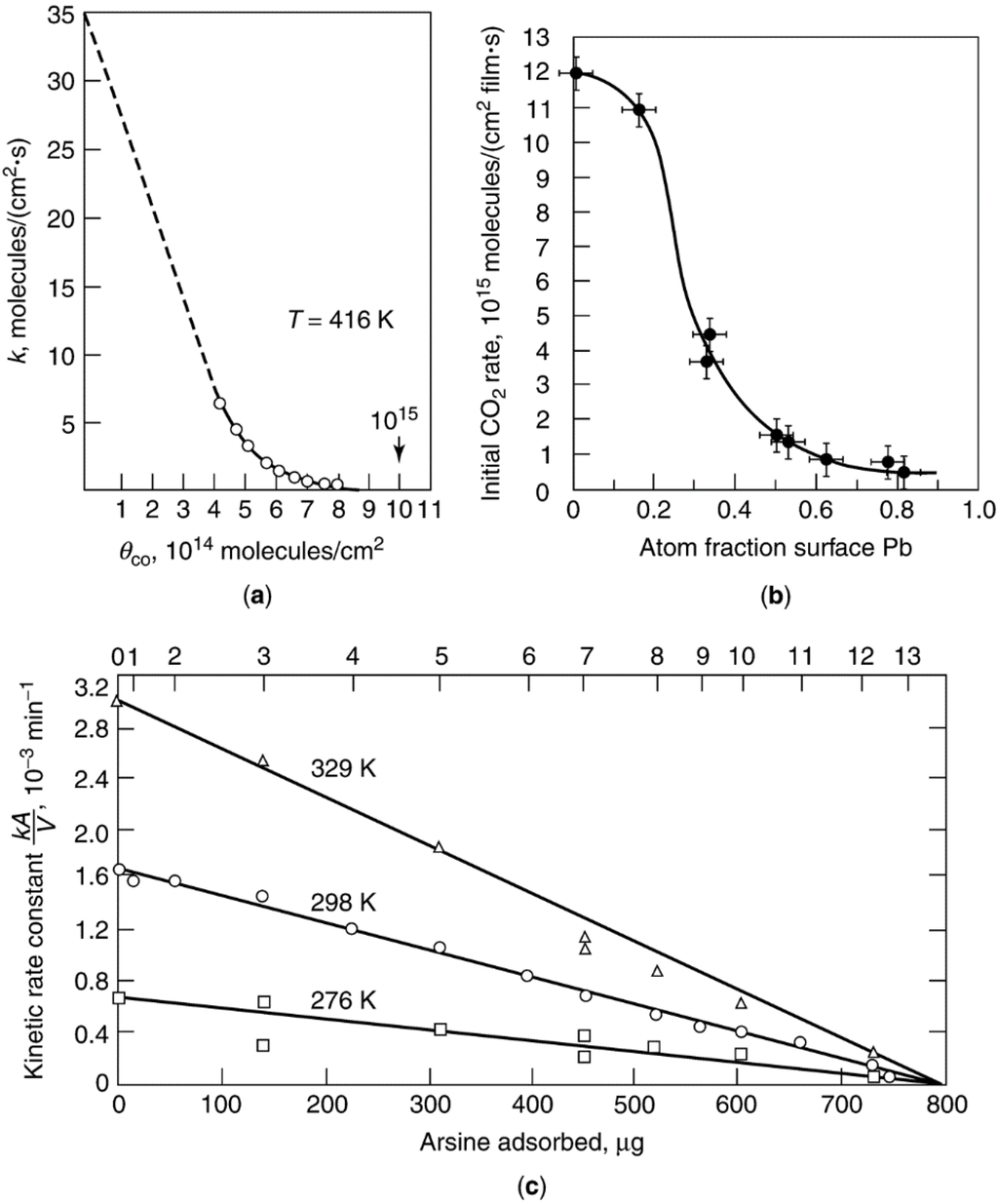
Figure 3.
(a) CO poisoning of para-H2 conversion over a Pt foil, reproduced from [24], copyright 1974, Wiley-VHC; (b) effect of lead coverage on the rate of CO oxidation of Pt film, reproduced from [25], copyright 1978, Elsevier; (c) rate constants of cyclopropane hydrogenolysis over a Pt film as a function of the amount of AsH3 adsorbed, reproduced from [26], copyright 1970, Elsevier.
The activity versus poison concentration patterns illustrated in Figure 2 are based on the assumption of uniform poisoning of the catalyst surface and surface reaction rate controlling, i.e., negligible pore-diffusional resistance. These assumptions, however, are rarely met in typical industrial processes because the severe reaction conditions of high temperature and high pressure bring about a high pore-diffusional resistance for either the main or poisoning reaction or both. In physical terms, this means that the reaction may occur preferentially in the outer shell of the catalysts particle, or that poison is preferentially adsorbed in the outer shell of the catalyst particle, or both. The nonuniformly distributed reaction and/or poison leads to nonlinear activity versus poison concentration curves that mimic the patterns in Figure 2 but do not represent truly selective or antiselective poisoning. For example, if the main reaction is limited to an outer shell in a pellet where poison is concentrated, the drop in activity with concentration will be precipitous. Pore diffusional effects in poisoning (nonuniform poison) are treated later in this review.
As sulfur poisoning is a difficult problem in many important catalytic processes (e.g., hydrogenation, methanation, Fischer–Tropsch synthesis, steam reforming, and fuel cell power production), it merits separate discussion as an example of catalyst poisoning phenomena. Studies of sulfur poisoning in hydrogenation and CO hydrogenation reactions have been thoroughly reviewed [8,21,27,28,29,30,31]. Much of the previous work focused on poisoning of nickel metal catalysts by H2S, the primary sulfur poison in many important catalytic processes, and thus provides some useful case studies of poisoning.
Previous adsorption studies [28,29,30] indicate that H2S adsorbs strongly and dissociatively on nickel metal surfaces. The high stability and low reversibility of adsorbed sulfur is illustrated by the data in Figure 4 [28], in which most of the previous equilibrium data for nickel are represented on a single plot of log (PH2S/PH2) versus reciprocal temperature. The solid line corresponds to the equilibrium data for formation of bulk Ni3S2. Based on the equation ΔG = RT ln(PH2S/PH2) = ΔH − TΔS, the slope of this line is ΔH/R, where ΔH = −75 kJ/mol and the intercept is −ΔS/R. Most of the adsorption data lie between the dashed lines corresponding to ΔH = −125 and −165 kJ/mol for coverages ranging from 0.5 to 0.9, indicating that adsorbed sulfur is more stable than the bulk sulfide. Indeed, extrapolation of high temperature data to zero coverage using a Tempkin isotherm [29] yields an enthalpy of adsorption of −250 kJ/mol; in other words, at low sulfur coverages, surface nickel–sulfur bonds are a factor of 3 more stable than bulk nickel–sulfur bonds. It is apparent from Figure 4 that the absolute heat of adsorption increases with decreasing coverage and that the equilibrium partial pressure of H2S increases with increasing temperature and increasing coverage. For instance, at 725 K (450 °C) and θ = 0.5, the values of PH2S/PH2 range from about 10−8 to 10−9. In other words, half coverage occurs at 1–10 ppb H2S, a concentration range at the lower limit of our present analytical capability. At the same temperature (450 °C), almost complete coverage (θ > 0.9) occurs at values of PH2S/PH2 of 10−7–10−6 (0.1–1 ppm) or at H2S concentrations encountered in many catalytic processes after the gas has been processed to remove sulfur compounds. These data are typical of sulfur adsorption on most catalytic metals. Thus, we can expect that H2S (and other sulfur impurities) will adsorb essentially irreversibly to high coverage in most catalytic processes involving metal catalysts.
Two important keys to reaching a deeper understanding of poisoning phenomena include (1) determining surface structures of poisons adsorbed on metal surfaces and (2) understanding how surface structure and hence adsorption stoichiometry change with increasing coverage of the poison. Studies of structures of adsorbed sulfur on single crystal metals (especially Ni) [3,28,32,33,34,35,36,37,38] provide such information. They reveal, for example, that sulfur adsorbs on Ni(100) in an ordered p(2 × 2) overlayer, bonded to four Ni atoms at S/Nis <0.25 and in a c(2 × 2) overlayer to two Ni atoms for S/Nis = 0.25–0.50 (see Figure 5; Nis denotes a surface atom of Ni); saturation coverage of sulfur on Ni(100) occurs at S/Nis = 0.5. Adsorption of sulfur on Ni(110), Ni(111), and higher index planes of Ni is more complicated; while the same p(2 × 2) structure is observed at low coverage, complex overlayers appear at higher coverages—for example, at S/Nis > 0.3 on Ni(111) a
overlayer is formed [32,33,34]. In more open surface structures, such as Ni(110) and Ni(210), saturation coverage occurs at S/Nis = 0.74 and 1.09 respectively; indeed, there is a trend of increasing S/Nis with decreasing planar density and increasing surface roughness for Ni, while the saturation sulfur concentration remains constant at 44 ng/cm2 Ni (see Table 5).
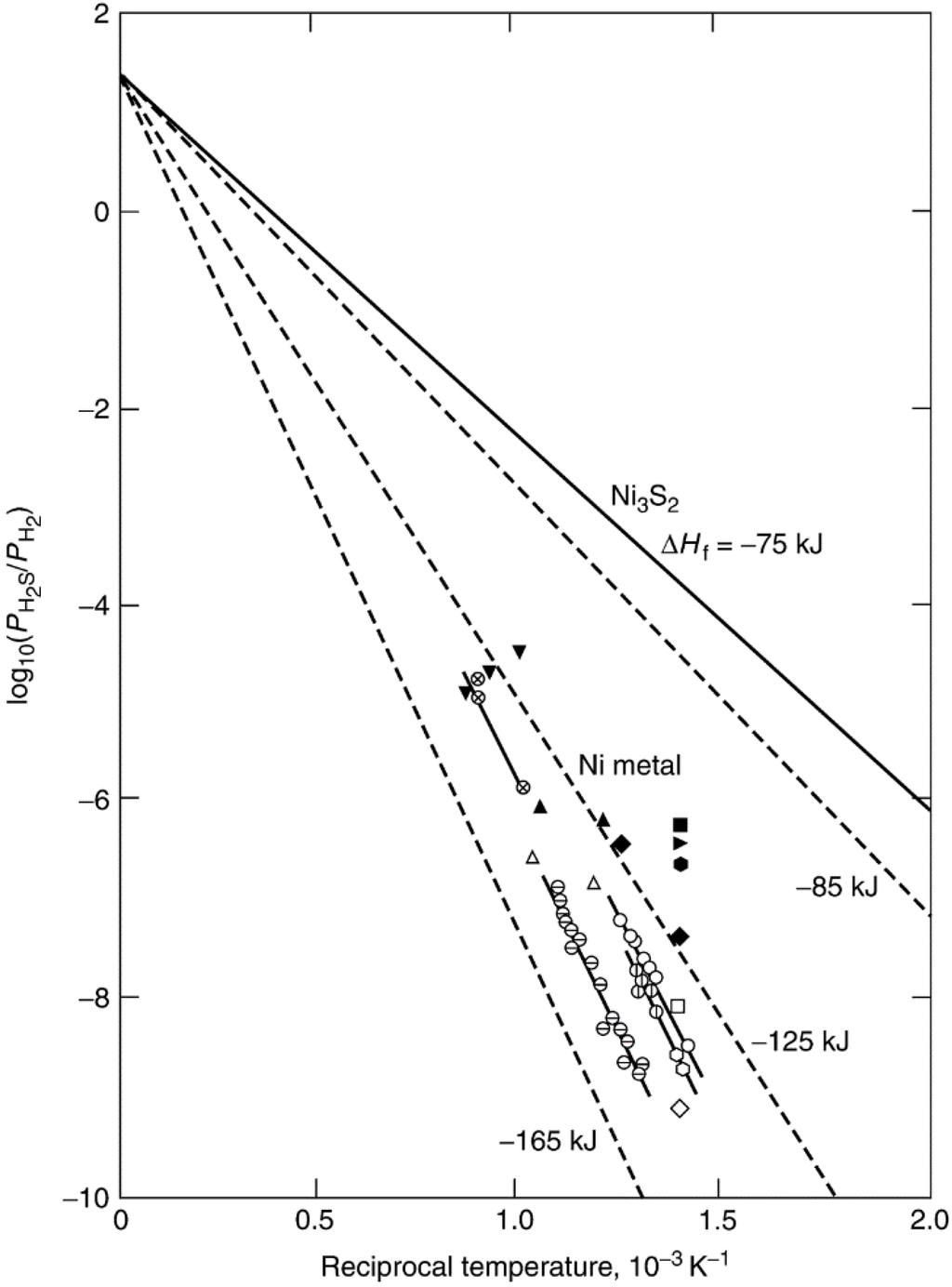
Figure 4.
Equilibrium partial pressure of H2S versus reciprocal temperature (values of ΔHf based on 1 mole of H2S); open symbols: θ = 0.5–0.6; closed symbols: θ = 0.8–0.9. Reproduced from [28]. Copyright 1982, Academic Press.
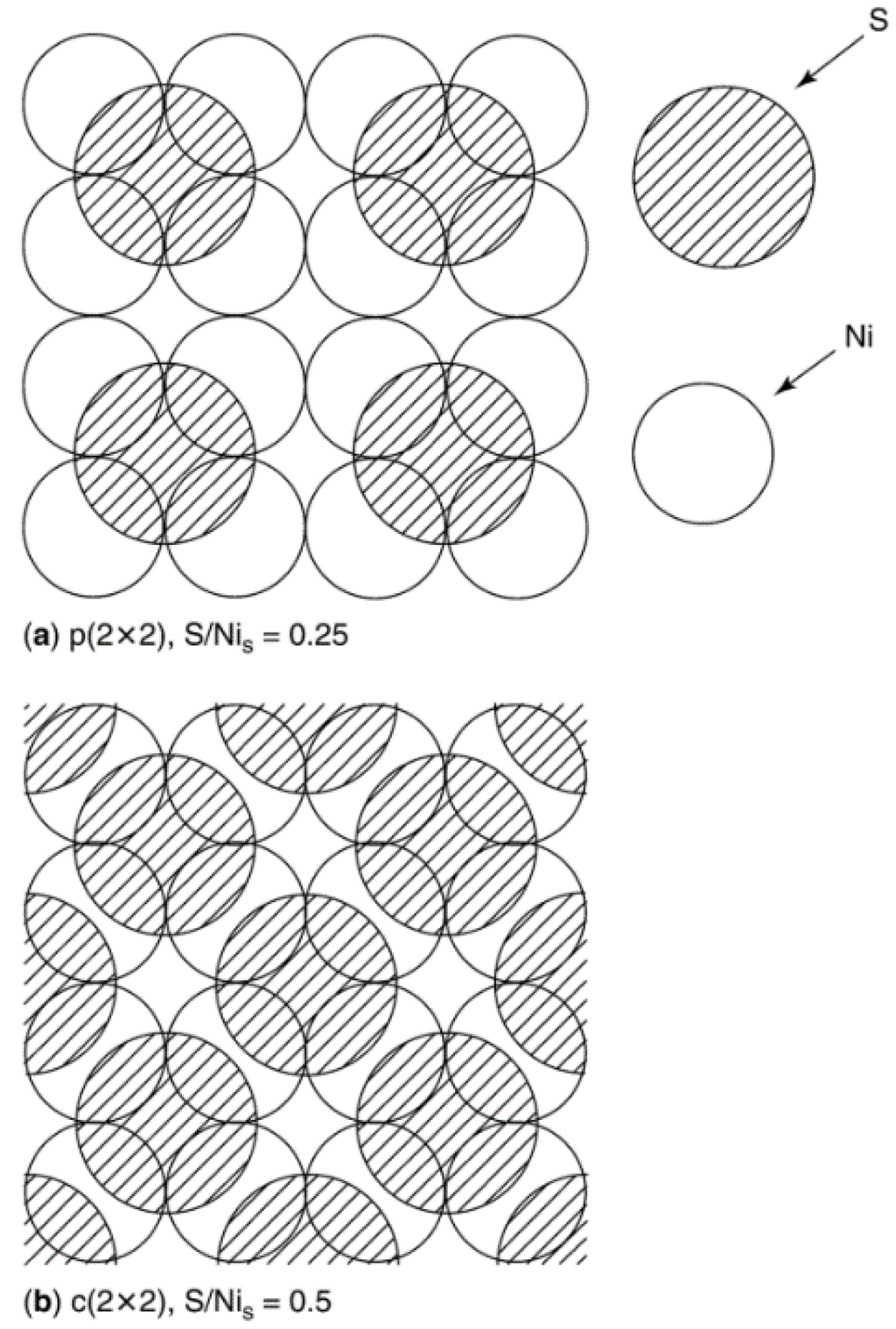
Figure 5.
Schematic view of sulfur adsorbed on a Ni(100) surface at a (a) S/Nis = 0.25 in a p(2 × 2) structure and (b) S/Nis = 0.50 in a c(2 × 2) structure. Reproduced from [39]. Copyright 2001, Elsevier.

Table 5.
Sulfur Adsorption Densities on Various Crystal Faces of Nickel a.
| Crystal face | Sulfur conc. at saturation, ng·S/cm2 | Number of S atoms/cm2 (×1015) | Number of Ni atoms/cm2 (×1015) | S atoms per surface Ni atom |
|---|---|---|---|---|
| (111) | 47 ± 1 | 0.86 | 1.8 | 0.48 |
| (100) | 43 ± 1 | 0.80 | 1.6 | 0.50 |
| (110) | 44.5 ± 1 | 0.82 | 1.1 | 0.74 |
| (210) | 42 ± 1 | 0.78 | 0.72 | 1.09 |
| Polycrystalline | 44.5 ± 1 | 0.82 | — | — |
a Data from [35].
Reported saturation stoichiometries for sulfur adsorption on polycrystalline and supported Ni catalysts (S/Nis) vary from 0.25 to 1.3 [28]. The values of saturation coverage greater than S/Nis = 0.5 may be explained by (1) a higher fractional coverage of sites of lower coordination number, i.e., atoms located on edges or corners of rough, high-index planes (Table 5); (2) enhanced adsorption capacity at higher gas phase concentrations of H2S in line with the observed trend of increasing saturation coverage with increasing H2S concentration in Figure 4; and/or (3) reconstruction of planar surfaces to rougher planes by adsorbed sulfur at moderately high coverages and adsorption temperatures.
The first effect would be favored, and in fact is observed, for supported metals of higher dispersion [28]. The second effect may explain the typically lower observed values of S/Nis for single crystal Ni, which are measured at extremely low pressures (high vacuum) relative to the higher values of S/Nis for polycrystalline and supported Ni, typically measured at orders of magnitude higher pressure; thus, in the case of the single crystal studies, the surface is not in equilibrium with gas phase H2S/H2.
The third effect, reconstruction of nickel surfaces by adsorbed sulfur, has been reported by a number of workers [28,32,33,36,37,38]; for example, McCarroll and co-workers [37,38] found that sulfur adsorbed at near saturation coverage on a Ni(111) face was initially in a hexagonal pattern, but upon heating above 700 K reoriented to a distorted c(2 × 2) (100) overlayer. Oudar [36] reported that sulfur adsorbed on a Ni(810) surface caused decomposition to (100) and (410) facets. During adsorption of H2S at RT, Ruan et al. [33] observed surface restructuring of Ni(111) from a p(2 × 2) at low coverage to a missing-row
terrace structure (0.4 monolayer) sparsely covered with small, irregular islands composed of sulfur adsorbed on disordered nickel; upon annealing to 460 K for 5 min, the islands ordered to the
phase and their size increased, suggesting further diffusion of Ni atoms from the terraces. The reconstruction of Ni (111) involving ejection and migration of Ni atoms was attributed to compressive surface stresses induced by sulfur adsorption; the role of compressive surface stress due to sulfur coverages exceeding 0.3 was confirmed by Grossmann et al. [32]. From these and similar studies, it is concluded that at moderately high temperatures (300–600 K) and coverages greater than 0.3, restructuring by sulfur of different facets of Ni to rougher, more open, stable structures is probably a general phenomenon. Thus, reconstruction probably accounts at least in part for observed increases in saturation S coverage with decreasing Ni site density.
The nature of reconstruction of a surface by a poison may depend on its pretreatment. For example, in a scanning tunneling microscopy (STM) study of room temperature H2S adsorption on Ni(110), Ruan and co-workers [40] found that the S/Ni structure at saturation varied with the initial state of the surface, i.e., whether clean or oxygen covered. Beginning with a clean Ni(110) surface, oxygen adsorbs dissociatively to form a (2 × 1)O overlayer at 1/2 monolayer coverage (Figure 6a); this is accompanied by a homogeneous nucleation of low-coordinated -Ni-O- rows along the [001] direction. As the oxygen-covered surface is exposed stepwise to 3 and then 8 Langmuirs (L) of H2S, oxygen atoms are removed by reaction with hydrogen to water; the surface is first roughened, after which white islands and black troughs having a c(2 × 2) structure are formed as sulfur atoms replace oxygen atoms (Figure 6b). Upon exposure to 25 L of H2S, the c(2 × 2) islands dissolve, while low-coordinated rows (periodicity of 1) form in the [001] direction, developing into ordered regions with a periodicity of 4 in the [1
0] direction (Figure 6c). After exposure to 50 L of H2S (Figure 6d), a stable, well-ordered (4 × 1)S structure appears, a surface clearly reconstructed relative to the original Ni(110). Moreover, the reconstructed surface in Figure 6d is very different from that observed upon direct exposure of the Ni(110) to H2S at room temperature, i.e., a c(2 × 2)S overlying the original Ni(110) (similar to Figure 5b); in other words, it appears that no reconstruction occurs by direct exposure to H2S at room temperature, rather only in the presence of O2 (or air). This emphasizes the complexities inherent in predicting the structure and stability of a given poison adsorbed on a given catalyst during a specified reaction as a function of different pretreatments or process disruptions, e.g., exposure to air.
In the previous discussion of Figure 4, −ΔHads was observed to decrease with increasing sulfur coverage; data in Figure 7 [41] show that –ΔHads decreases with increasing gas-phase H2S concentration and coverage. However, in contrast to the data in Figure 4, those in Figure 7 [41] show that at very high H2S concentrations and high adsorption temperatures, −ΔHads falls well below the −ΔHformation of bulk Ni3S2; at the same time, the S/Nis ratio approaches that of Ni2S3. This is a unique result, since all of the data obtained at lower temperatures and H2S concentrations [28] show −ΔHads to be greater than −ΔHformation of Ni3S2.
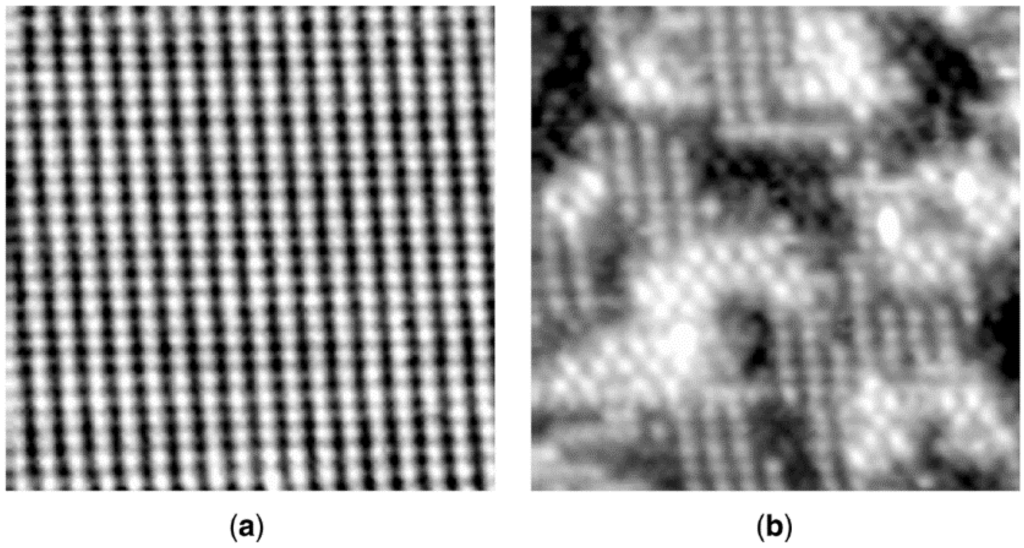
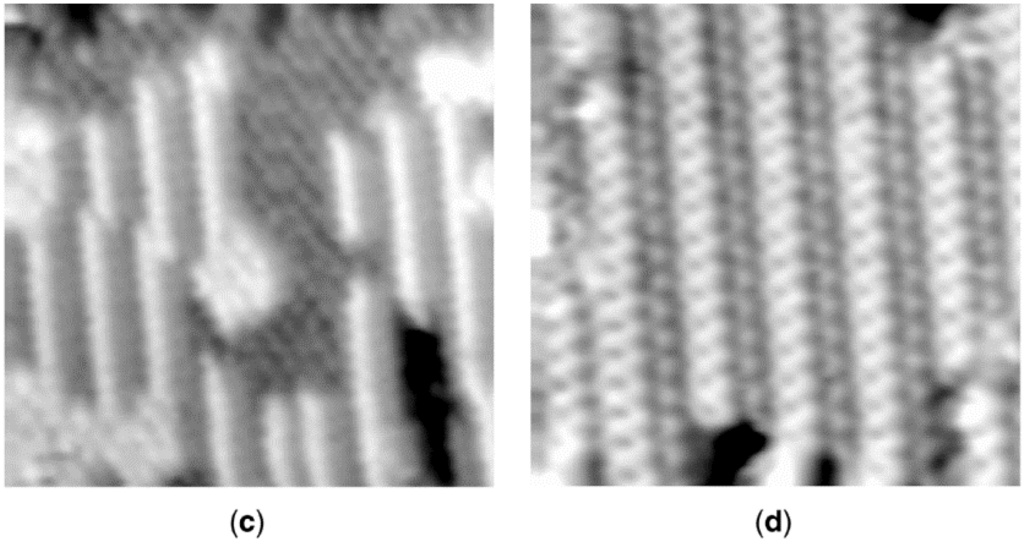
Figure 6.
A series of in situ scanning tunneling microscope (STM) images recorded after exposure of Ni(110) to oxygen and then progressively higher exposures of H2S: (a) (2 × 1)O overlayer; (b) white islands and black troughs with a c(2 × 2)S structure after exposure to 3 and 8 L of H2S; (c) 25 L, islands transform to low-coordinated rows in the [001] direction; and (d) 50 L, stable, well-ordered (4 × 1)S. Reproduced from [40]. Copyright 1992, American Physical Society.
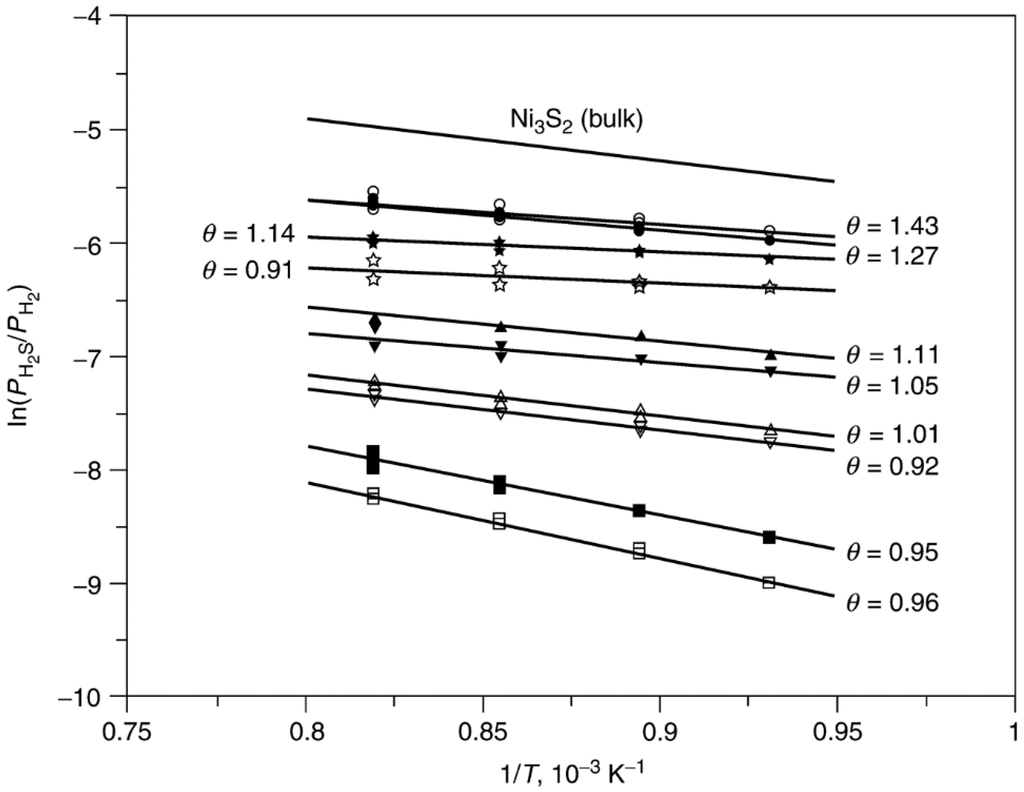
Figure 7.
Sulfur chemisorption isosteres on a Ni/α-Al2O3 catalyst at high temperatures and high H2S concentrations. Reproduced from [41]. Copyright 1999,Elsevier.
From the above discussion, the structure and stoichiometry of sulfur adsorbed on nickel evidently are complex functions of temperature, H2S concentration, sulfur coverage, and pretreatment, phenomena that account at least in part for the complex nature of nickel poisoning by sulfur. Could one expect similar complexities in the poisoning of other metals? Probably, since poisoning of nickel is prototypical, i.e., similar principles operate and similar poisoning behaviors are observed in other poison/metal systems, although none have been studied to the same depth as sulfur/nickel.
Since one of the necessary steps in a catalytic reaction is the adsorption of one or more reactants, investigation of the effects of adsorbed sulfur on the adsorption of other molecules, can provide useful insights into the poisoning process [21,28]. Previous investigations [28,42,43,44,45,46,47,48] indicate that both H2 and CO adsorptions on nickel are poisoned by adsorbed sulfur. For example, thermal desorption studies of CO from presulfided Ni(100) [44] reveal a weakening of the CO adsorption bond and a rapid, nonlinear decline in the most strongly bound β2 state (bridged CO) with increasing sulfur coverage, corresponding to a poisoning of about 8–10 Ni atoms for bridged CO adsorption per adsorbed sulfur atom at low sulfur coverage (see Figure 8); moreover, the β2 CO species is completely poisoned at about 0.2–0.4 mL of sulfur relative to a saturation coverage of 0.5 mL. Hydrogen adsorption is poisoned in a similar nonlinear fashion. On the other hand, the coverage of the β1 state (linear CO) is constant with increasing sulfur coverage. The sharp nonlinear drop in CO and hydrogen adsorptions at low sulfur coverages has been interpreted in terms of a combination of short-range electronic and steric effects operating over a range of less than 5 atomic units [13]. The different effects of sulfur on β1 and β2 states of CO have important implications for sulfur poisoning in reactions involving CO; that is, sulfur poisoning can affect reaction selectivity as well as activity [28].
Because sulfur adsorbs so strongly on metals and prevents or modifies the further adsorption of reactant molecules, its presence on a catalyst surface usually effects substantial or complete loss of activity in many important reactions. This is illustrated by the data in Figure 9 showing the steady-state methanation activities of Ni, Co, Fe, and Ru relative to the fresh, unpoisoned surface activity as a function of gas phase H2S concentration. These data indicate that Ni, Co, Fe, and Ru all suffer 3–4 orders of magnitude loss in activity at 15–100 ppb of H2S, i.e., their sulfur tolerances are extremely low. Moreover, the sharp drop in activity with increasing H2S concentration suggests highly selective poisoning. Nevertheless, the rate of sulfur poisoning and hence sulfur resistance varies from catalyst to catalyst and is apparently a function of catalyst composition [28] and reaction conditions [49]. Indeed, it is possible to significantly improve sulfur resistance of Ni, Co, and Fe with catalyst additives such as Mo and B that selectively adsorb sulfur. Because the adsorption of sulfur compounds is generally rapid and irreversible, surface sulfur concentrations in catalyst particles and beds are nonuniform, e.g., H2S adsorbs selectively at the entrance to a packed bed and on the outer surface of catalyst particles, making the experimental study and modeling of sulfur poisoning extremely difficult.
There are other complications in the study of sulfur poisoning. For example, the adsorption stoichiometry of sulfur in CO hydrogenation on Ni is apparently a function of the temperature, H2/CO ratio, and water partial pressure [49]. Moreover, at high CO partial pressures sulfur may be removed from the surface as COS, which is not as strongly adsorbed as H2S. At low temperature conditions, e.g., those representative of Fischer–Tropsch synthesis or liquid phase hydrogenations, the gas phase concentration of H2S in poisoning studies must be kept very low, i.e., below 0.1–5 ppm, to avoid formation of bulk metal sulfides—a phenomenon that seriously compromises the validity of the results. Thus, the importance of studying poisoning phenomena in situ under realistic reaction conditions, at low process-relevant poison concentrations, and over a process-representative range of temperature and concentration conditions is emphasized.
As mentioned earlier, there are a number of industrial processes in which one intentionally poisons the catalyst in order to improve its selectivity. For example, Pt-containing naphtha reforming catalysts are often pre-sulfided to minimize unwanted cracking reactions. On basic Pt/KL zeolite catalysts, these short term, low concentration exposures are beneficial to produce Pt ensemble sizes that promote aromatization, while longer term or higher concentration exposures poison the catalyst both by forming Pt-S bonds and producing large crystallites that block pores, as shown by transmission electron microscopy (TEM) and X-ray absorption fine structure spectroscopy (EXAFS), and favor only dehydrogenation [50,51,52,53]. Other examples are sulfur added to Fischer-Tropsch catalysts that have been reported to have either beneficial or negligibly harmful effects, which are important considerations in setting the minimum gas clean-up requirements [27,30,54,55,56]. S and P are added to Ni catalysts to improve isomerization selectivity in the fats and oils hydrogenation industry, while S and Cu are added to Ni catalysts in steam reforming to minimize coking. In catalytic reforming, sulfided Re or Sn is added to Pt to enhance the dehydrogenation of paraffins to olefins while poisoning hydrogenolysis/coking reactions. V2O5 is added to Pt to suppress SO2 oxidation to SO3 in diesel emissions control catalysts.
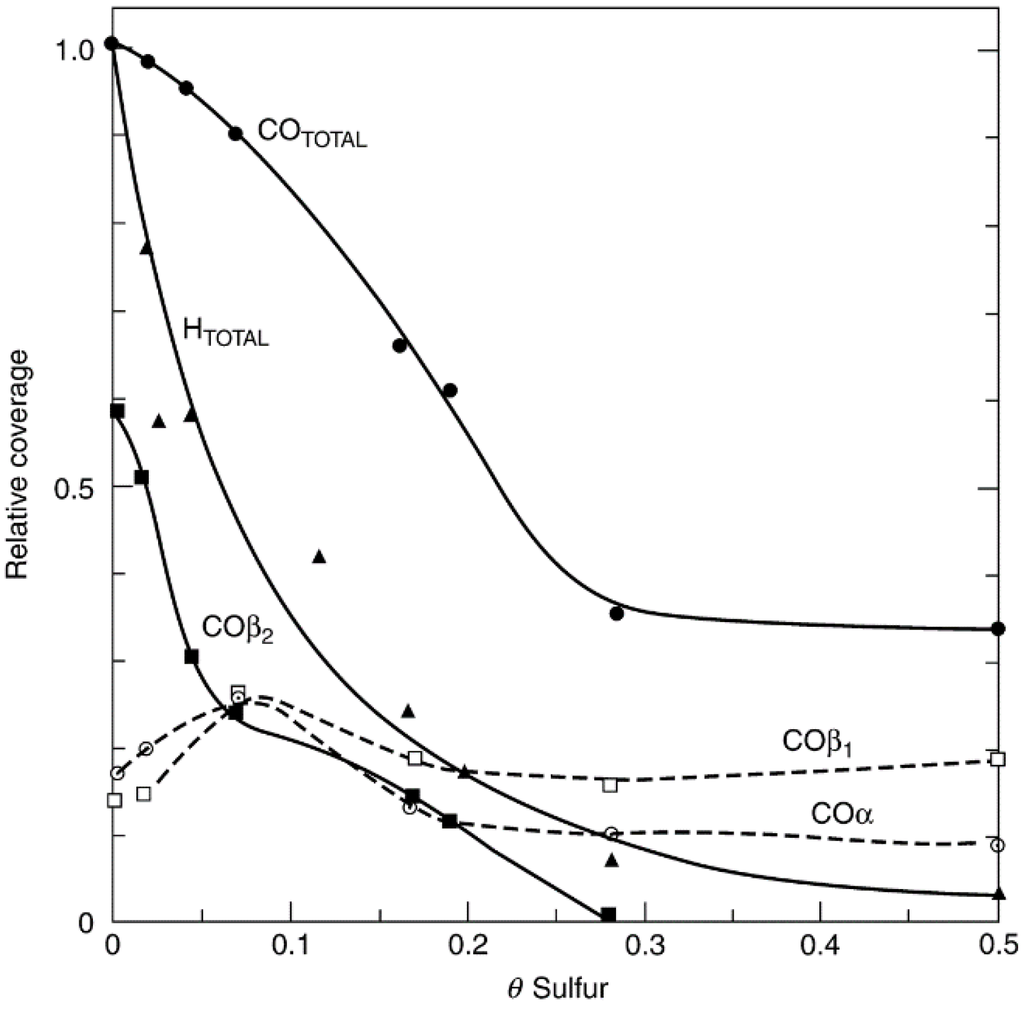
Figure 8.
Area under thermal programmed desorption spectra for H2 and the α, β1, β2, and total CO adsorption curves, as a function of sulfur precoverage. Reproduced from [44]. Copyright 1981, Elsevier.
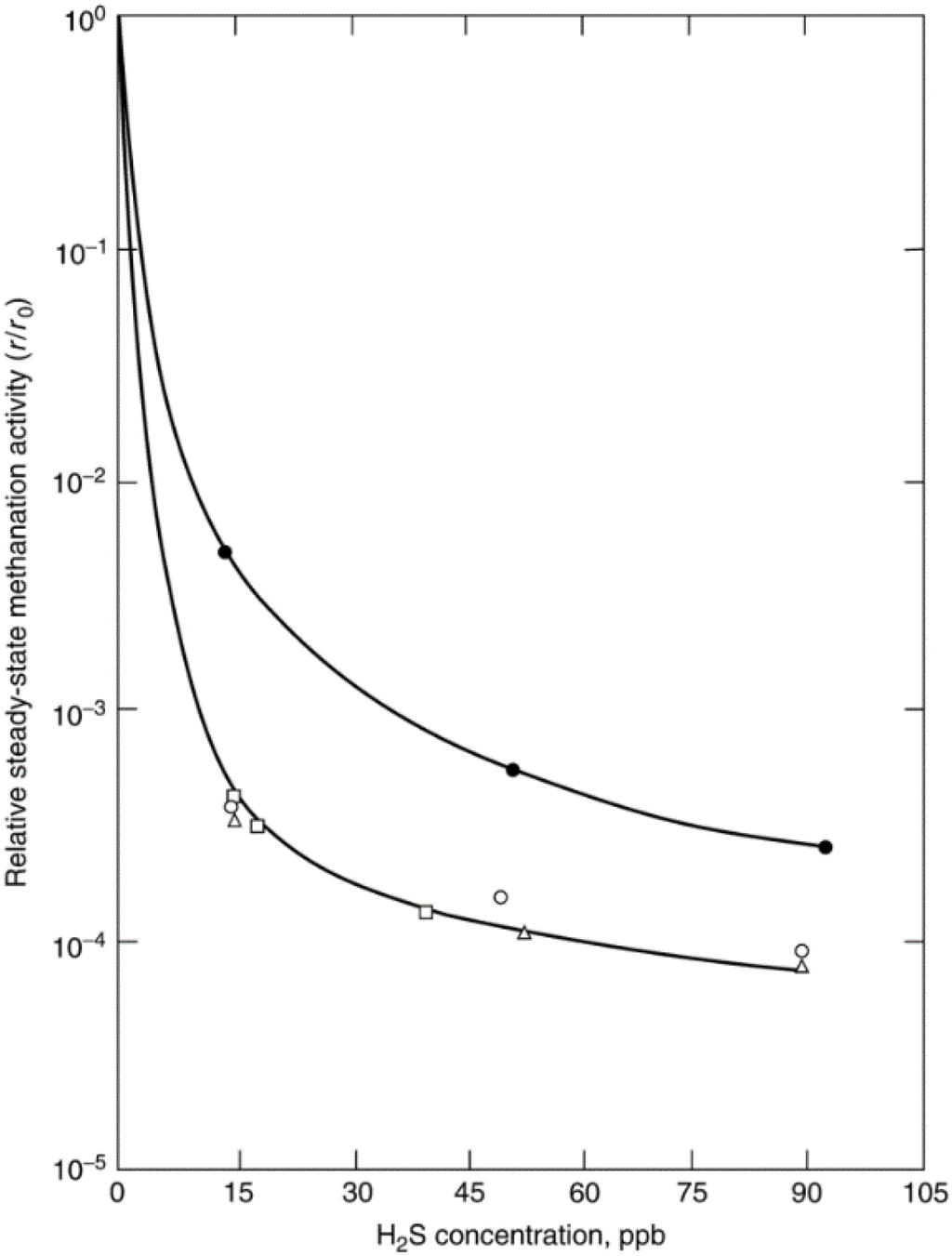
Figure 9.
Relative steady-state methanation activity profiles for Ni (●), Co (Δ), Fe (○), and Ru (□) as a function of gas-phase H2S concentration. Reaction conditions: 100 kPa, 400 °C, 1% CO/99% H2 for Co, Fe, and Ru, 4% CO/96% H2 for Ni. Reproduced from [28]. Copyright 1982, Academic Press.
2.2. Fouling, Coking, and Carbon Deposition
2.2.1. Fouling
Fouling is the physical (mechanical) deposition of species from the fluid phase onto the catalyst surface, which results in activity loss due to blockage of sites and/or pores. In its advanced stages, it may result in disintegration of catalyst particles and plugging of the reactor voids. Important examples include mechanical deposits of carbon and coke in porous catalysts, although carbon- and coke-forming processes also involve chemisorption of different kinds of carbons or condensed hydrocarbons that may act as catalyst poisons. The definitions of carbon and coke are somewhat arbitrary and by convention related to their origin. Carbon is typically a product of CO disproportionation while coke is produced by decomposition or condensation of hydrocarbons on catalyst surfaces and typically consists of polymerized heavy hydrocarbons. Nevertheless, coke forms may vary from high molecular weight hydrocarbons to primarily carbons such as graphite, depending upon the conditions under which the coke was formed and aged. A number of books and reviews treat the formation of carbons and coke on catalysts and the attendant deactivation of the catalysts [1,4,57,58,59,60,61,62].
The chemical structures of cokes or carbons formed in catalytic processes vary with reaction type, catalyst type, and reaction conditions. Menon [62] suggested that catalytic reactions accompanied by carbon or coke formation can be broadly classified as either coke-sensitive or coke-insensitive, analogous to Boudart’s more general classification of structure-sensitive and structure-insensitive catalytic reactions. In coke-sensitive reactions, unreactive coke is deposited on active sites, leading to activity decline, while in coke-insensitive reactions, relatively reactive coke precursors formed on active sites are readily removed by hydrogen (or other gasifying agents). Examples of coke-sensitive reactions include catalytic cracking and hydrogenolysis; on the other hand, Fischer–Tropsch synthesis, catalytic reforming, and methanol synthesis are examples of coke-insensitive reactions. On the basis of this classification, Menon [62] reasoned that the structure and location of a coke are more important than its quantity in affecting catalytic activity.
Consistent with Menon’s classification, it is also generally observed that not only structure and location of coke vary but also its mechanism of formation varies with catalyst type, e.g., whether it is a metal or metal oxide (or sulfide, sulfides being similar to oxides). Because of these significant differences in mechanism, formation of carbon and coke is discussed below separately for supported metals and for metal oxides and sulfides.
2.2.2. Carbon and Coke Formation on Supported Metal Catalysts
Possible effects of fouling by carbon (or coke) on the functioning of a supported metal catalyst are illustrated in Figure 10. Carbon may (1) chemisorb strongly as a monolayer or physically adsorb in multilayers and in either case block access of reactants to metal surface sites, (2) totally encapsulate a metal particle and thereby completely deactivate that particle, and (3) plug micro- and mesopores such that access of reactants is denied to many crystallites inside these pores. Finally, in extreme cases, strong carbon filaments may build up in pores to the extent that they stress and fracture the support material, ultimately causing the disintegration of catalyst pellets and plugging of reactor voids. For example, in steam methane reforming (SMR) catalysts, which are typically nickel supported on alumina with alkaline earth oxides, the carbon can diffuse through and begin to grow filaments from the back side of the nickel particles (structural type 3 in Table 6) especially at high reaction temperatures and low steam to methane ratios, which push the nickel particles off the support surface. Thermal or mechanical shock can then cause the carbon filaments to fall off the support, thus permanently deactivating the catalyst [8,60]. However, the behavior is complex because for other reaction conditions and other metals, the filaments may grow from the top surface of the metal particles or the carbon may diffuse into the metal and form bulk carbides [8].
An example of recent interest for biomass reactions that points to the complex interaction between the active metal and the support during carbon deposition is the steam reforming of light alcohols and other oxygenates, in which deactivation occurs primarily through coking. For traditional SMR catalysts (e.g., Ni/MgAl2O4) the coke is believed to originate primarily from alkene formation [63,64]. However, for the case of Ni/La2O3 catalysts, carbon appears to form at the interface between the active metal and the support to block the active phase [65].
Mechanisms of carbon deposition and coke formation on metal catalysts from carbon monoxide and hydrocarbons, including methane during SMR for hydrogen production [4,57,58,59,60,61], are illustrated in Figure 11 and Figure 12. Different kinds of carbon and coke that vary in morphology and reactivity are formed in these reactions (see Table 6 and Table 7). For example, CO dissociates on metals to form Cα, an adsorbed atomic carbon; Cα can react to Cβ, a polymeric carbon film. The more reactive, amorphous forms of carbon formed at low temperatures (e.g., Cα and Cβ) are converted at high temperatures over a period of time to less reactive, graphitic forms [60]
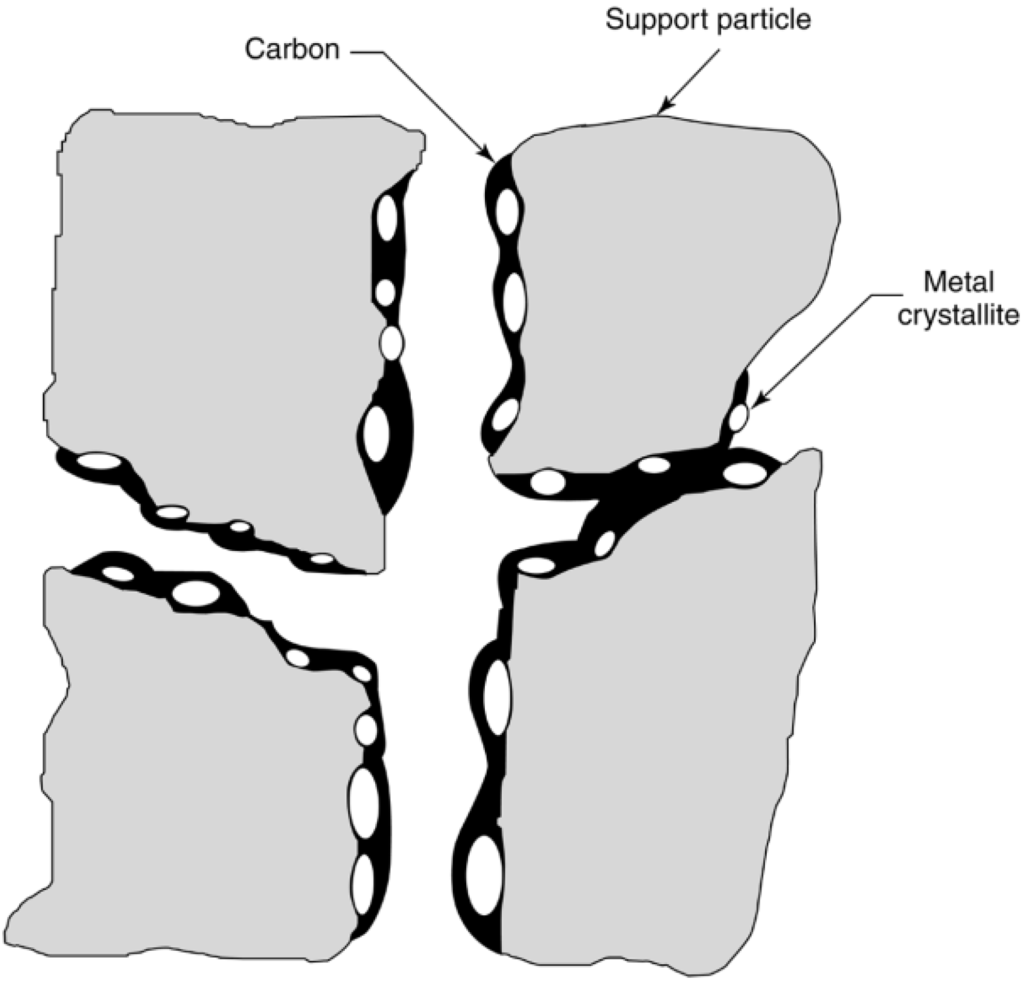
Figure 10.
Conceptual model of fouling, crystallite encapsulation, and pore plugging of a supported metal catalyst owing to carbon deposition. Reproduced from [8]. Copyright 2006, Wiley-Interscience.
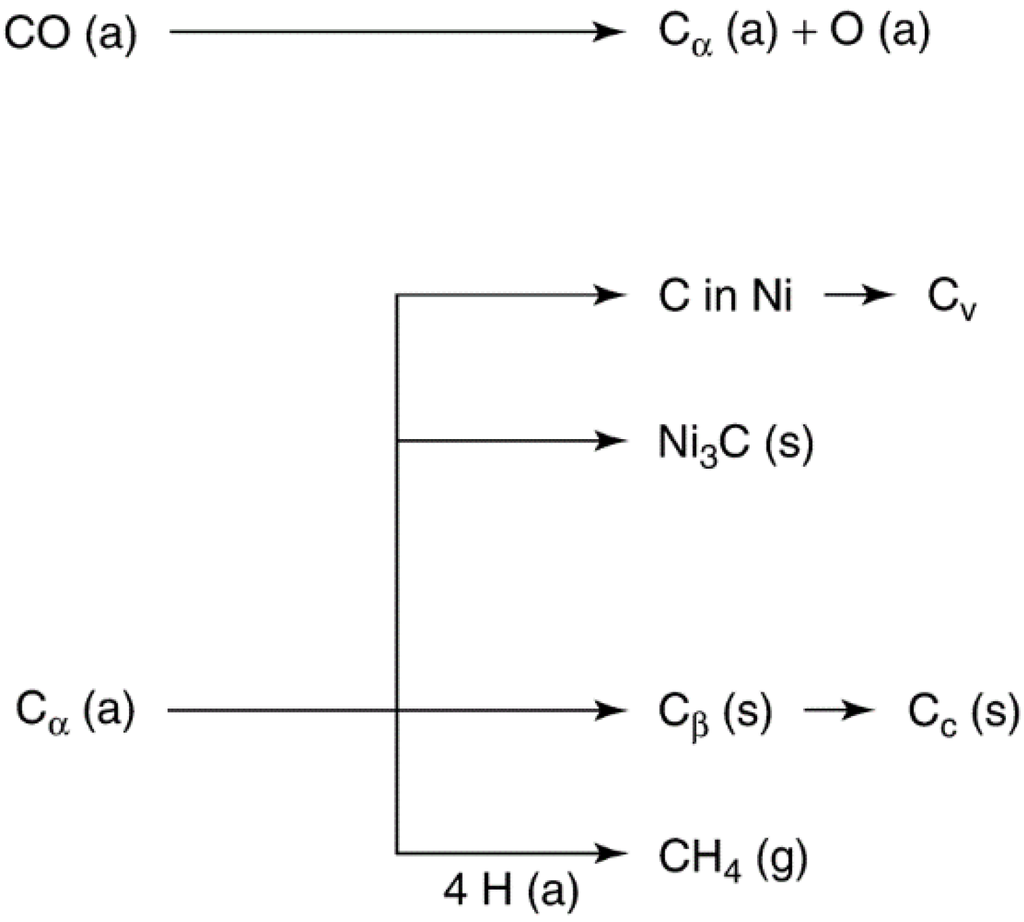
Figure 11.
Formation, transformation, and gasification of carbon on nickel (a, g, s refer to adsorbed, gaseous, and solid states respectively). Reproduced from [60]. Copyright 1983, Elsevier.
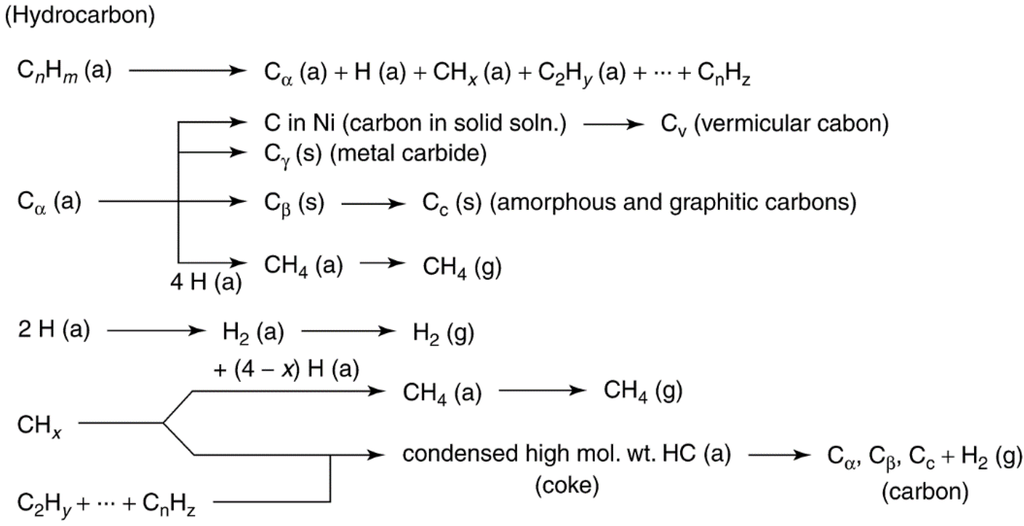
Figure 12.
Formation and transformation of coke on metal surfaces (a, g, s refer to adsorbed, gaseous, and solid states respectively); gas phase reactions are not considered. Reproduced from [60]. Copyright 1983, Elsevier.

Table 6.
Forms and Reactivities of Carbon Species Formed by Decomposition of CO on Nickel a.
| Structural type | Designation | Temp. formed, °C | Peak temp. for reaction with H2, °C |
|---|---|---|---|
| 1. Adsorbed, atomic (surface carbide) | Cα | 200–400 | 200 |
| 2. Polymeric, amorphous films or filaments | Cβ | 250–500 | 400 |
| 3. Vermicular filaments, fibers, and/or whiskers | Cv | 300–1000 | 400–600 |
| 4. Nickel carbide (bulk) | Cγ | 150–250 | 275 |
| 5. Graphitic (crystalline) platelets or films | Cc | 500–550 | 550–850 |
a Ref. [60].

Table 7.
Carbon Species Formed in Steam Reforming of Hydrocarbons on Nickel Catalysts a.
| Attribute | Encapsulating film | Whisker-like | Pyrolytic carbon |
|---|---|---|---|
| Formation | Slow polymerization of CnHm radicals on Ni surface, into encapsulating film | Diffusion of C through Ni crystal, nucleation and whisker growth with Ni crystal at top | Thermal cracking of hydrocarbon; deposition of C precursors on catalyst |
| Effects | Progressive deactivation | No deactivation of Ni surface. Breakdown of catalyst and increasing ΔP | Encapsulation of catalyst particle; deactivation and increasing ΔP |
| Temp. range, °C | <500 | >450 | >600 |
| Critical parameters | Low temperature, low H2O/CnHm, low H2/CnHm, aromatic feed | High temperature, low H2O/CnHm, no enhanced H2O adsorption, low activity, aromatic feed | High temperature, high void fraction, low H2O/CnHm, high pressure, acidic catalyst |
a Ref. [60].
It should also be emphasized that some forms of carbon result in loss of catalytic activity and some do not. For example, at low reaction temperatures (<300–375 °C) condensed polymer or β-carbon films and at high temperatures (>650 °C) graphitic carbon films encapsulate the metal surfaces of methanation and steam reforming catalysts [60]. Deactivation of steam reforming catalysts at high reaction temperatures (500–900 °C) may be caused by precipitation of atomic (carbidic) carbon dissolved in the Ni surface layers to a depth of more than 50–70 nm [62,66]. If it accumulates on the metal surface (at high or low temperatures), adsorbed atomic carbon can deactivate metal sites for adsorption and/or reaction. For example, Durer and co-workers [67] demonstrated that carbon atoms residing in the fourfold hollow sites of Rh(100) block the adsorption of hydrogen (and hence could block sites for hydrogenation). In the intermediate temperature range of 375–650 °C, carbon filaments (Figure 13) are formed by precipitation of dissolved carbon at the rear side of metal crystallites, causing the metal particles to grow away from the support [57]. Filament growth ceases when sufficient carbon accumulates on the free surface to cause encapsulation by a carbon layer; however, encapsulation of the metal particles does not occur if H2/CO or H2O/hydrocarbon ratios are sufficiently high. Thus, carbon filaments sometimes formed in CO hydrogenation or steam reforming of hydrocarbons would not necessarily cause a loss of intrinsic catalyst activity unless they are formed in sufficient quantities to cause plugging of the pores [60] or loss of metal occurs as the carbon fibers are removed during regeneration [68,69]. However, in practice, regions of carbon forming potential in steam reforming must be carefully avoided, since once initiated, the rates of filamentous carbon formation are sufficiently high to cause catastrophic pore plugging and catalyst failure within a few hours to days.

Figure 13.
Electron micrograph of 14% Ni/Al2O3 having undergone extensive carbon deposition during CO disproportionation at 673 K, PCO = 4.55 kPa (magnification of 200,000). Courtesy of the BYU Catalysis Laboratory.
The rate at which deactivation occurs for a given catalyst and reaction depends greatly on reaction conditions—especially temperature and reactant composition. A fundamental principle for coke-insensitive reactions on metals (e.g., methanation, Fischer–Tropsch synthesis, steam reforming, catalytic reforming, and methanol synthesis) is that deactivation rate depends greatly on the difference in rates of formation and gasification of carbon/coke precursors, i.e., rd = rf – rg. If the rate of gasification, rg, is equal to or greater than that of formation, rf, carbon/coke is not deposited. Rates of carbon/coke precursor formation and gasification both increase exponentially with temperature, although the difference between them varies a great deal with temperature because of differences in preexponential factors and activation energies. Thus, carbon/coke formation is avoided in regions of temperature in which precursor gasification rate exceeds deposition rate. This is illustrated in Figure 14, an Arrhenius plot for rates of formation and hydrogenation of alpha and beta carbons on nickel during CO methanation. Since at temperatures below 600 K (1/T > 1.66 × 10−3 K−1) the rate of Cα gasification exceeds that of Cα formation, no carbon is deposited. However above 600 K, Cα accumulates on the surface since the rate of Cα formation exceeds that of Cα gasification. As Cα accumulates (at 600–700 K), it is converted to a Cβ polymeric chain or film that deactivates the nickel catalyst; however, above 700 K (1/T < 1.43 × 10−3 K−1), the rate of Cβ hydrogenation exceeds that of formation and no deactivation occurs. Thus, the “safe” regions of methanation for avoiding deactivation by carbon are below 600 K and above 700 K; of course, these regions will vary somewhat with reactant concentrations and catalyst activity. A similar principle operates in steam reforming, i.e., at a sufficiently low reaction temperature, the rate of hydrocarbon adsorption exceeds the rate of hydrocracking and a deactivating polymer film is formed [70]; accordingly, it is necessary to operate above this temperature to avoid deactivation.
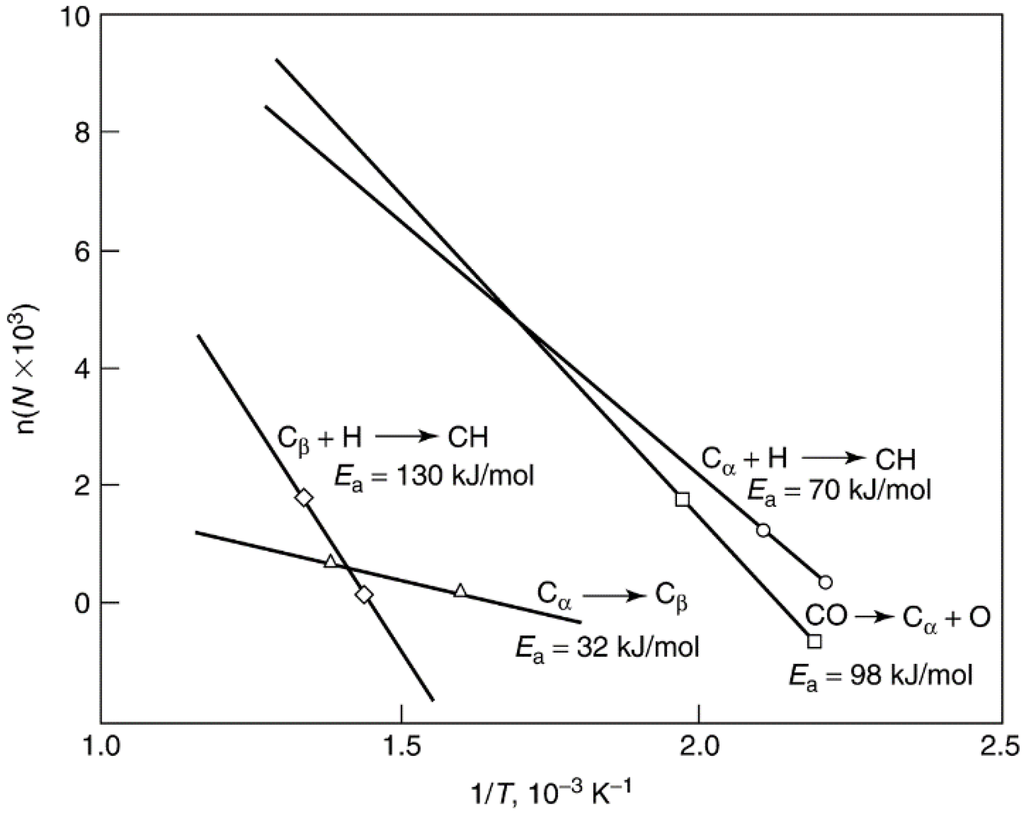
Figure 14.
Rates of formation (log scale) and hydrogenation of Cα and Cβ versus reciprocal temperature. Reproduced from [60]. Copyright 1983, Elsevier.
In steam reforming, filamentous carbon formation rate is a strong function of reactant hydrocarbon structure; for example, it decreases in the order acetylenes, olefins, paraffins, i.e., in order of decreasing reactivity, although activation energies for nickel are in the same range (125–139 kJ) independent of hydrocarbon structure and about the same as those observed for formation of filamentous carbon from decomposition of CO [60]. This latter observation suggests that the reactions of CO and different hydrocarbons to filamentous carbon proceed by a common mechanism and rate-determining step—probably the diffusion of carbon through the metal crystallites [60].
The rate at which a carbon or coke is accumulated in a given reaction under given conditions can vary significantly with catalyst structure, including metal type, metal crystallite size, promoter, and catalyst support. For example, supported Co, Fe, and Ni are active above 350–400 °C for filamentous carbon formation from CO and hydrocarbons; the order of decreasing activity is reportedly Fe > Co > Ni [60]. Pt, Ru, and Rh catalysts, on the other hand, while equally or more active than Ni, Co, or Fe in steam reforming, produce little or no coke or carbon. This is attributed to reduced mobility and/or solubility of carbon in the noble metals, thus retarding the nucleation process. Thus, it is not surprising that addition of noble metals to base metals retards carbon formation; for example, addition of Pt in Ni lowers carbon deposition rate during methanation, while addition of Cu or Au to Ni substantially lowers carbon formation in steam reforming [60,71]. In contrast to the moderating effects of noble metal additives, addition of 0.5% Sn to cobalt substantially increases the rate of carbon filament formation from ethylene [72], an effect desirable in the commercial production of carbon filament fibers.
Since carbon formation and gasification rates are influenced differently by modifications in metal crystallite surface chemistry, which are in turn a function of catalyst structure, oxide additives or oxide supports may be used to moderate the rate of undesirable carbon or coke accumulation. For example, Bartholomew and Strasburg [73] found the specific rate (turnover frequency) of filamentous carbon deposition on nickel during methanation at 350 °C to decrease in the order Ni/TiO2 > NiAl2O3 > Ni/SiO2, while Vance and Bartholomew [74] observed Cα hydrogenation rates at 170 °C to decrease in this same order (the same as for methanation at 225 °C). This behavior was explained in terms of promotional or inhibiting effects due to decoration of metal crystallites by the support, for example silica, inhibiting both CO dissociation and carbon hydrogenation. This hypothesis is consistent with observations [75,76] that silica evaporated on metal surfaces and supported metals inhibits formation of filamentous carbon. Similarly Bitter and co-workers [77] observed rates of carbon formation in CO2/CH4 reforming to decrease in the order Pt/γ-Al2O3→Pt/TiO2 > Pt/ZrO2; while 90% of the carbon deposited on the support, the authors linked deactivation to carbon accumulated on the metal owing to an imbalance between carbon formed by methane dissociation and oxidation by chemisorbed CO2. The rate of formation of coke in steam reforming is delayed and occurs at lower rates in nickel catalysts promoted with alkali or supported on basic MgO [78].
Since formation of coke, graphite, or filamentous carbon involves the formation of C-C bonds on multiple atoms sites, one might expect that coke or carbon formation on metals is structure-sensitive, i.e., sensitive to surface structure and metal crystallite size. Indeed, Bitter and co-workers [77] found that catalysts containing larger Pt crystallites deactivate more rapidly than those containing small crystallites. Moreover, a crystallite size effect, observed in steam reforming of methane on nickel [60,78], appears to operate in the same direction, i.e., formation of filamentous carbon occurs at lower rates in catalysts containing smaller metal crystallites.
In summary, deactivation of supported metals by carbon or coke may occur chemically, owing to chemisorption or carbide formation, or physically and mechanically, owing to blocking of surface sites, metal crystallite encapsulation, plugging of pores, and destruction of catalyst pellets by carbon filaments. Blocking of catalytic sites by chemisorbed hydrocarbons, surface carbides, or relatively reactive films is generally reversible in hydrogen, steam, CO2, or oxygen. Further details of the thermodynamics, kinetics, and mechanisms of carbon and coke formation in methanation and steam reforming reactions are available in reviews by Bartholomew [60] and Rostrup-Nielsen [70,78]. In recent reviews addressing deactivation of Co catalysts by carbon during Fischer-Tropsch synthesis [79,80], the same or similar carbon species, e.g., α, β, polymeric, and graphitic carbons, are observed on Co surfaces as on Ni; moreover, poisoning or fouling of the Co surfaces with β, polymeric, and graphitic carbon layers are found to be major causes of deactivation.
2.2.3. Coke Formation on Metal Oxide and Sulfide Catalysts
In reactions involving hydrocarbons, coke may be formed in the gas phase and on both noncatalytic and catalytic surfaces. Nevertheless, formation of coke on oxides and sulfides is principally a result of cracking reactions involving coke precursors (typically olefins or aromatics) catalyzed by acid sites [81,82]. Dehydrogenation and cyclization reactions of carbocation intermediates formed on acid sites lead to aromatics, which react further to higher molecular weight polynuclear aromatics that condense as coke (see Figure 15). Reactions 1–3 in Figure 15 illustrate the polymerization of olefins, reactions 4–8 illustrate cyclization from olefins, and reactions 9–14 illustrate chain reaction formation of polynuclear aromatics that condense as coke on the catalyst surface. Because of the high stability of the polynuclear carbocations (formed in reactions 10–13), they can continue to grow on the surface for a relatively long time before a termination reaction occurs through the back donation of a proton.
From this mechanistic scheme (Figure 15), it is clear that olefins, benzene and benzene derivatives, and polynuclear aromatics are precursors to coke formation. However, the order of reactivity for coke formation is clearly structure dependent, i.e., decreases in the order polynuclear aromatics > aromatics > olefins > branched alkanes > normal alkanes. For example, the weight percent coke formed on silica–alumina at 500 °C is 0.06, 3.8, 12.5, and 23% for benzene, naphthalene, fluoranthene, and anthracene respectively [83].
Coking reactions in processes involving heavy hydrocarbons are very complex; different kinds of coke may be formed and they may range in composition from CH to C and have a wide range of reactivities with oxygen and hydrogen, depending upon the time on stream and temperature to which they are exposed. For example, coke deposits occurring in hydrodesulfurization of residuum have been classified into three types [84]:
- (1)
- Type I deposits are reversibly adsorbed normal aromatics deposited during the first part of the cycle at low temperature.
- (2)
- Type II deposits are reversibly adsorbed asphaltenes deposited early in the coking process.
- (3)
- Type III deposits result from condensation of aromatic concentrates into clusters and then crystals that constitute a “mesophase.” This crystalline phase is formed after long reaction times at high temperature. This hardened coke causes severe deactivation of the catalyst [84].
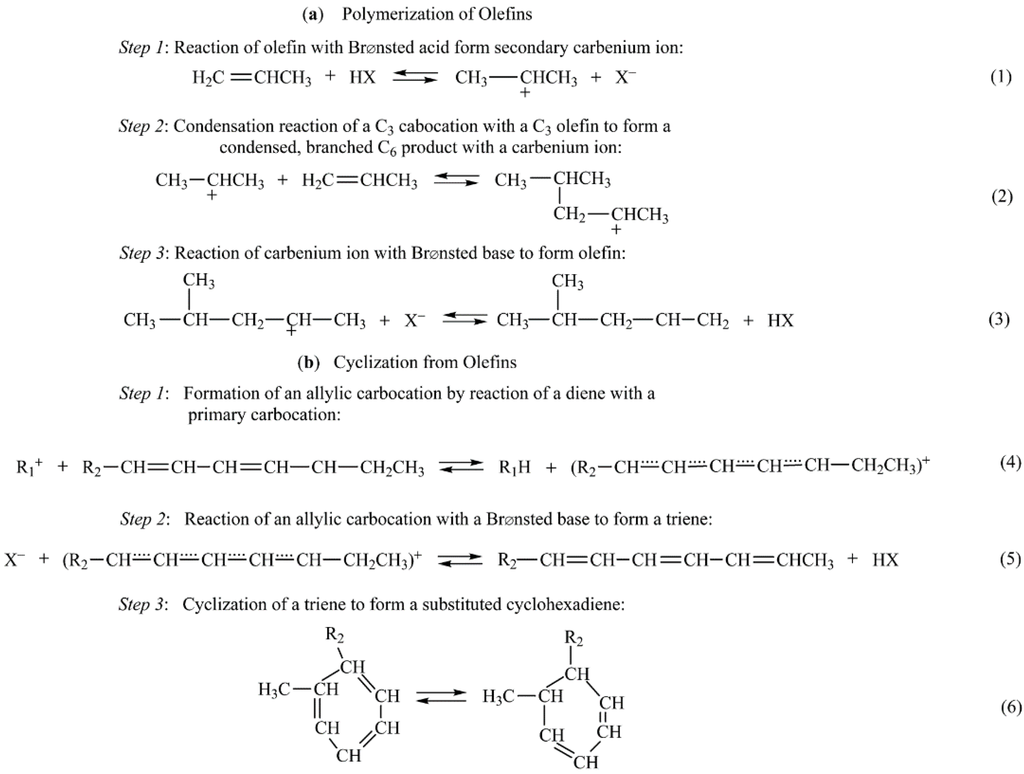
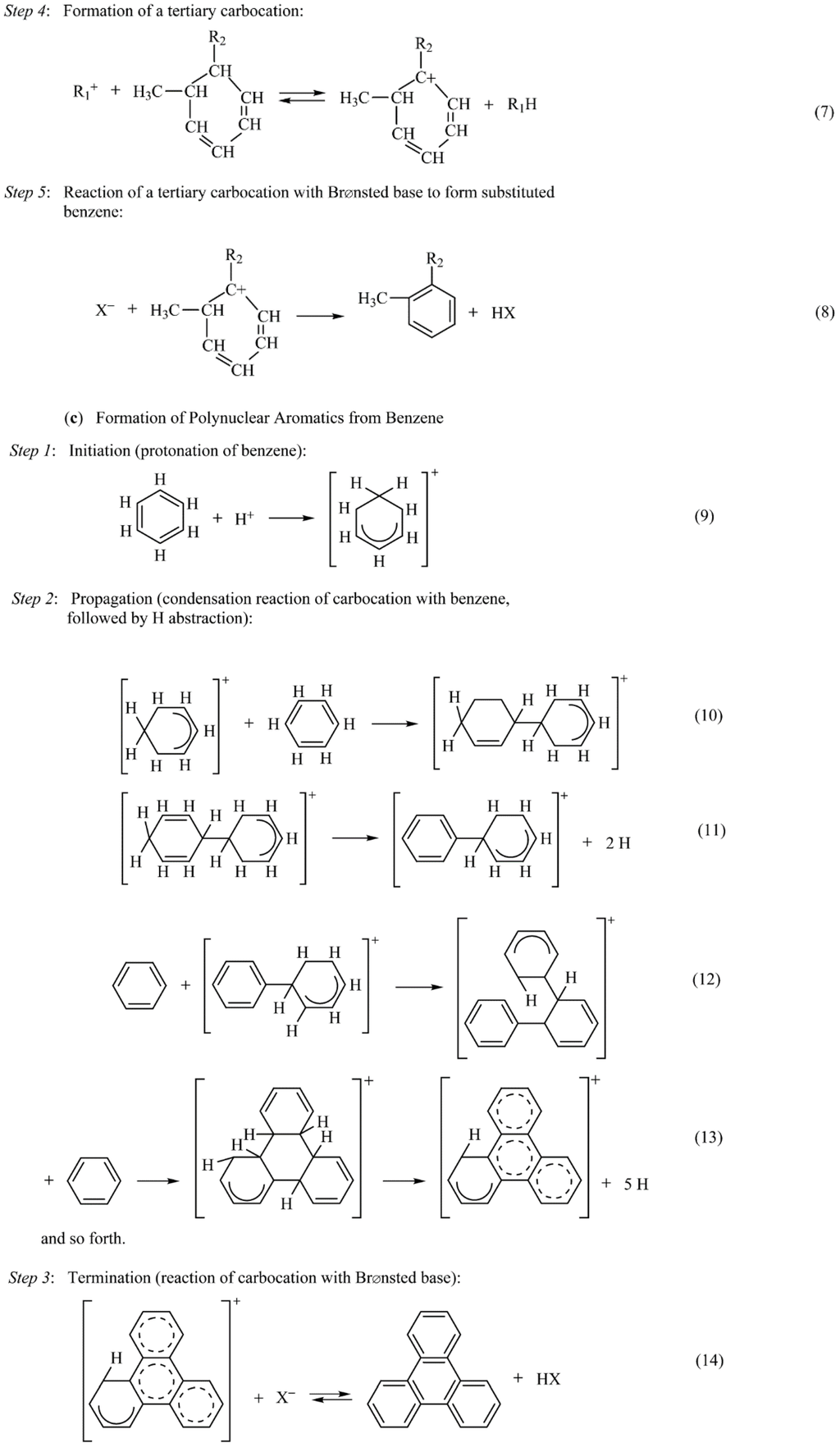
Figure 15.
Coke-forming reactions of alkenes and aromatics on oxide and sulfide catalysts: (a) polymerization of alkenes, (b) cyclization from alkenes, and (c) formation of polynuclear aromatics from benzene. Reproduced from [8], Copyright 2006, Wiley-Interscience.
In addition to hydrocarbon structure and reaction conditions, extent and rate of coke formation are also a function of the acidity and pore structure of the catalyst. Generally, the rate and extent of coke formation increase with increasing acid strength and concentration. Coke yield decreases with decreasing pore size (for a fixed acid strength and concentration); this is especially true in zeolites where shape selectivity plays an important role in coke formation. For example, coke yield in fluid catalytic cracking is only 0.4% for ZSM-5 (pore diameters of 0.54 × 0.56 nm) compared to 2.2% for Y-faujasite (aperture diameter of 0.72 nm) [82]. However, in pores of molecular diameter, a relatively small quantity of coke can cause substantial loss of activity. It should be emphasized that coke yield can vary considerably into the interior pores of a catalyst particle or along a catalyst bed, depending upon the extent to which the main and deactivation reactions are affected by film mass transport and pore diffusional resistance.
The mechanisms by which coke deactivates oxide and sulfide catalysts are, as in the case of supported metals, both chemical and physical. However, some aspects of the chemistry are quite different. The principal chemical loss of activity in oxides and sulfides is due to the strong adsorption of coke molecules on acidic sites. However, as discussed earlier, strong acid sites also play an important role in the formation of coke precursors, which subsequently undergo condensation reactions to produce large polynuclear aromatic molecules that physically coat catalytic surfaces. Physical loss of activity also occurs as coke accumulates, ultimately partially or completely blocking catalyst pores as in supported metal catalysts. For example, in isomerization of cis-butene on SiO2/Al2O3 [85] catalyst deactivation occurs by rapid, selective poisoning of strong acid sites; coke evolved early in the reaction is soluble in dichloromethane and pyridine and is slightly aromatic. Apparently, the blocking of active sites does not significantly affect porosity or catalyst surface area, as SiO2/Al2O3 contains relatively large mesopores.
In the case of supported bifunctional metal/metal oxide catalysts, different kinds of coke are formed on the metal and the acidic oxide support, e.g., soft coke (high H/C ratio) on Pt or Pt–Re metals and hard coke (low H/C ratio) on the alumina support in catalytic reforming [86]. In this case, coke precursors may be formed on the metal via hydrogenolysis, following which they migrate to the support and undergo polymerization and cyclization reactions, after which the larger molecules are dehydrogenated on the metal and finally accumulate on the support, causing loss of isomerization activity. Mild sulfiding of these catalysts (especially Pt–Re/alumina) substantially reduces the rate of hydrogenolysis and the overall formation of coke on both metal and support; it especially reduces the hard coke, which is mainly responsible for deactivation.
Several recent studies [82,87,88,89,90,91,92,93,94,95,96,97] have focused on coke formation during hydrocarbon reactions in zeolites including (1) the detailed chemistry of coke precursors and coke molecules formed in zeolite pores and pore intersections (or supercages) and (2) the relative importance of adsorption on acid sites versus pore blockage. The principal conclusions from these studies can be summarized as follows: (1) the formation of coke and the manner in which it deactivates a zeolite catalyst are shape-selective processes, (2) deactivation is mainly due to the formation and retention of heavy aromatic clusters in pores and pore intersections, and (3) while both acid-site poisoning and pore blockage participate in the deactivation, the former dominates at low coking rates, low coke coverages (e.g., in Y-zeolite below 2 wt%), and high temperatures, while the latter process dominates at high reaction rates, high coke coverages, and low temperatures. Thus, pore size and pore structure are probably more important than acid strength and density under typical commercial process conditions. Indeed, deactivation is typically more rapid in zeolites having small pores or apertures and/or a monodimensional structure [95]. Figure 16 illustrates four possible modes of deactivation of HZSM-5 by carbonaceous deposits with increasing severity of coking [95].
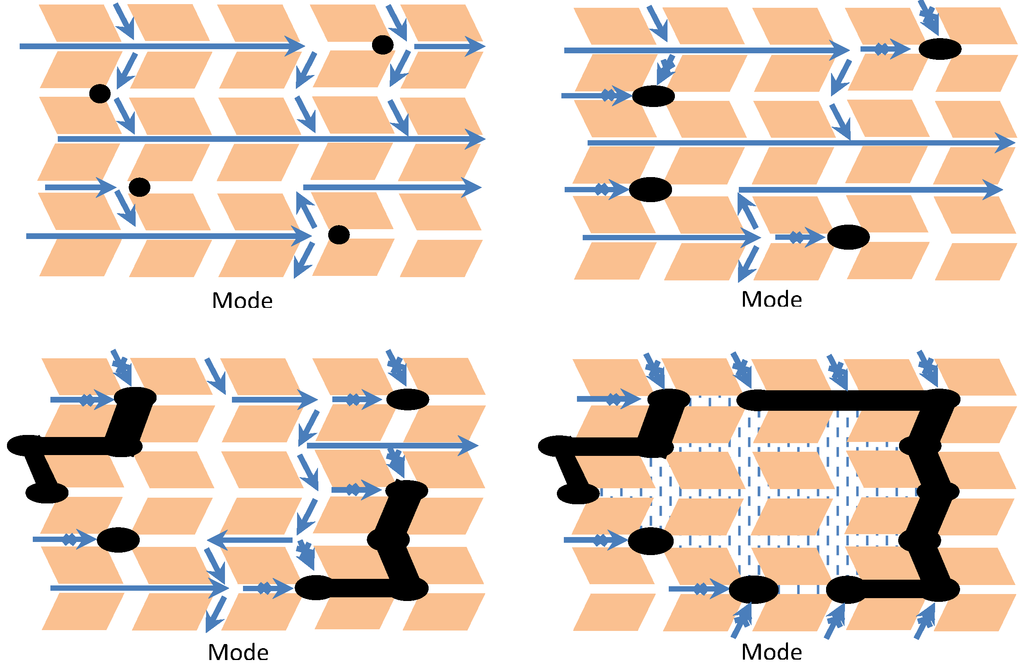
Figure 16.
Schematic of the four possible modes of deactivation by carbonaceous deposits in HZSM-5: (1) reversible adsorption on acid sites, (2) irreversible adsorption on sites with partial blocking of pore intersections, (3) partial steric blocking of pores, and (4) extensive steric blocking of pores by exterior deposits. Adapted from [95].
These conclusions (in the previous paragraph) are borne out, for example, in the study by Cerqueira and co-workers [97] of USHY zeolite deactivation during methylcyclohexane transformation at 450 °C, showing the following:
- (1)
- Coke is probably mainly formed by rapid transformation of toluenic C7 carbenium ions with lesser contributions from reactions of cyclopentadiene, C3–C6 olefins, and aromatics.
- (2)
- Soluble coke consists of polynuclear aromatic clusters containing three to seven five- and six-membered rings having a typical compositions of C30H40 to C40H44 and having dimensions of 0.9 × 1.1 nm to 1.1 × 1.5 nm, i.e., sizes that would cause them to be trapped in the supercages of Y-zeolite.
- (3)
- At short contact times, coking is relatively slow and deactivation is mainly due to acid-site poisoning, while at long contact times, coking is much faster because of the high concentrations of coke precursors; under these latter conditions coke is preferentially deposited at the outer pore openings of zeolite crystallites and deactivation is dominated by pore-mouth blockage.
That coke formed at large contact times not only blocks pores and/or pore intersections inside the zeolite, but also migrates to the outside of zeolite crystallites, where it blocks pore entrances, has been observed in several studies [91,93,94,97]. However, the amount, structure, and location of coke in ZSM-5 depends strongly on the coke precursor, e.g., coke formed from mesitylene is deposited on the external zeolite surface, whereas coking with isobutene leads to largely paraffinic deposits inside pores; coke from toluene, on the other hand, is polyaromatic and is deposited both on external and internal zeolite surfaces [91].
2.3. Thermal Degradation and Sintering
2.3.1. Background
Thermally induced deactivation of catalysts results from (1) loss of catalytic surface area due to crystallite growth of the catalytic phase, (2) loss of support area due to support collapse and of catalytic surface area due to pore collapse on crystallites of the active phase, and/or (3) chemical transformations of catalytic phases to noncatalytic phases. The first two processes are typically referred to as “sintering”. The third is discussed in the next section under solid–solid reactions. Sintering processes generally take place at high reaction temperatures (e.g., > 500 °C) and are generally accelerated by the presence of water vapor.
Most of the previous sintering and redispersion work has focused on supported metals. Experimental and theoretical studies of sintering and redispersion of supported metals published before 1997 have been reviewed fairly extensively [8,98,99,100,101,102,103,104,105,106,107]. Three principal mechanisms of metal crystallite growth have been advanced: (1) crystallite migration, (2) atomic migration, and (3) (at very high temperatures) vapor transport. The processes of crystallite and atomic migration are illustrated in Figure 17. Crystallite migration involves the migration of entire crystallites over the support surface, followed by collision and coalescence. Atomic migration involves detachment of metal atoms or molecular metal clusters from crystallites, migration of these atoms over the support surface, and ultimately, capture by larger crystallites. Redispersion, the reverse of crystallite growth in the presence of O2 and/or Cl2, may involve (1) formation of volatile metal oxide or metal chloride complexes that attach to the support and are subsequently decomposed to small crystallites upon reduction and/or (2) formation of oxide particles or films that break into small crystallites during subsequent reduction.
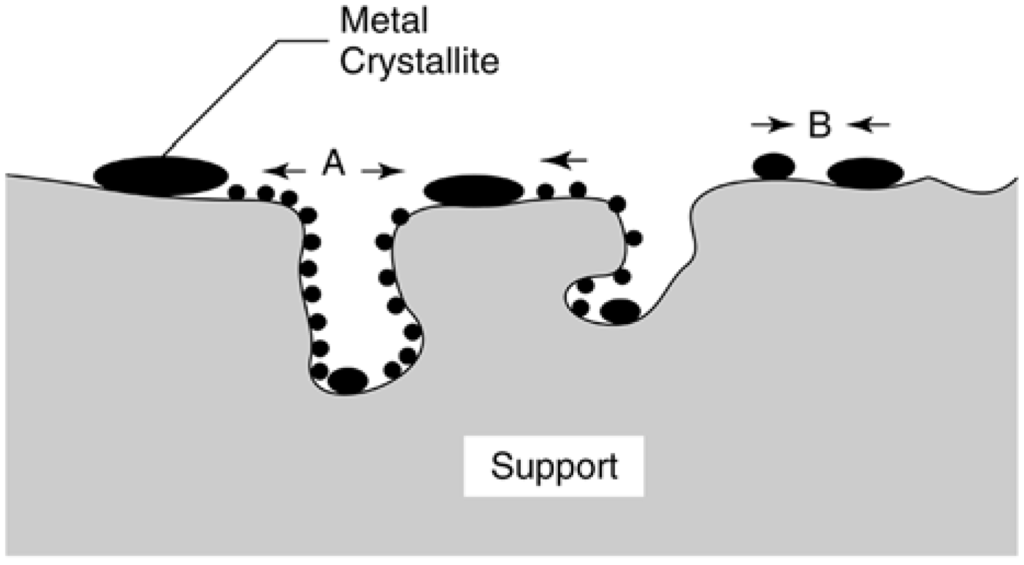
Figure 17.
Two conceptual models for crystallite growth due to sintering by (A) atomic migration or (B) crystallite migration. Reproduced from [8], Copyright 2006, Wiley-Interscience.
There is controversy in the literature regarding which mechanism of sintering (or redispersion) operates at a given set of conditions. Logically, atomic migration would be favored at lower temperatures than crystallite migration, since the higher diffusivities of atoms or small cluster would facilitate their migration, whereas the thermal energy necessary to induce motion of larger crystallites would only be available at higher temperatures. Moreover, migration of small crystallites might be favorable early in the sintering process but unfavorable as crystallites become larger. However, focusing on only one of the three sintering mechanisms (and two dispersion mechanisms) is a simplification that ignores the possibility that all mechanisms may occur simultaneously and may be coupled with each other through complex physicochemical processes, including the following: (1) dissociation and emission of metal atoms or metal-containing molecules from metal crystallites, (2) adsorption and trapping of metal atoms or metal-containing molecules on the support surface, (3) diffusion of metal atoms, metal-containing molecules and/or metal crystallites across support surfaces, (4) metal or metal oxide particle spreading, (5) support surface wetting by metal particles, (6) metal particle nucleation, (7) coalescence of, or bridging between, two metal particles, (8) capture of atoms or molecules by metal particles, (9) liquid formation, (10) metal volatilization through volatile compound formation, (11) splitting of crystallites in O2 atmosphere owing to formation of oxides of a different specific volume, and (12) metal atom vaporization. Depending upon reaction or redispersion conditions, a few or all of these processes may be important; thus, the complexity of sintering/redispersion processes is emphasized.
In general, thermal sintering processes are kinetically slow (at moderate reaction temperatures) and irreversible or difficult to reverse. Thus, sintering is more easily prevented than cured.
2.3.2. Factors Affecting Metal Particle Growth and Redispersion in Supported Metals
Temperature, atmosphere, metal type, metal dispersion, promoters/impurities, and support surface area, texture, and porosity are the principal parameters affecting rates of sintering and redispersion (see Table 8) [8,103,104,105,106,107]. Sintering rates increase exponentially with temperature. Metals sinter relatively rapidly in oxygen and relatively slowly in hydrogen, although depending upon the support, metal redispersion can be facilitated by exposure at high temperature (e.g., 500–550 °C for Pt/Al2O3) to oxygen and chlorine, followed by reduction. Water vapor also increases the sintering rate of supported metals, likely through chemical-assisted sintering effects similar to those described in Section 2.4.3.

Table 8.
Effects of Important Reaction and Catalyst Variables on Sintering Rates of Supported Metals Based on General Power-Law Expression (GPLE) Data a.
| Variable | Effect |
|---|---|
| Temperature | Sintering rates are exponentially dependent on T; Eact varies from 30 to 150 kJ/mol. Eact decreases with increasing metal loading; it increases in the following order with atmosphere: NO < O2 < H2 < N2 |
| Atmosphere | Sintering rates are much higher for noble metals in O2 than in H2 and higher for noble and base metals in H2 relative to N2. Sintering rate decreases for supported Pt in atmospheres in the following order: NO > O2 > H2 > N2 |
| Metal | Observed order of decreasing thermal stability in H2 is Ru > Ir Rh > Pt; thermal stability in O2 is a function of (1) volatility of metal oxide and (2) strength of metal oxide–support interaction |
| Support | Metal–support interactions are weak (bond strengths of 5–15 kJ/mol); with a few exceptions, thermal stability for a given metal decreases with support in the following order: Al2O3 > SiO2 > carbon |
| Promoters | Some additives decrease atom mobility, e.g., C, O, CaO, BaO, CeO2, GeO2; others increase atom mobility, e.g., Pb, Bi, Cl, F, or S. Oxides of Ba, Ca, or Sr are “trapping agents” that decrease sintering rate |
| Pore size | Sintering rates are lower for porous versus nonporous supports; they decrease as crystallite diameters approach those of the pores |
a Refs. [8,103,104,105,106,107]. For the definition of a GPLE, see Equation 2 later in this section.
Normalized dispersion (percentage of metal exposed at any time divided by the initial percentage exposed) versus time data in Figure 18 show that at temperatures of 650 °C or higher, rates of metal surface area loss (measured by hydrogen chemisorption) due to sintering of Ni/silica in hydrogen atmosphere are significant, causing 70% loss of the original metal surface area within 50 h at 750 °C. In reducing atmosphere, metal crystallite stability generally decreases with decreasing metal melting temperature, i.e., in the order Ru > Ir > Rh > Pt > Pd > Ni > Cu > Ag, although this order may be affected by relatively stronger metal–support interactions, e.g., the observed order of decreasing stability of supported platinum in vacuum is Pt/Al2O3 > Pt/SiO2 > Pt/C. In oxidizing atmospheres, metal crystallite stability depends on the volatility of metal oxides and the strength of the metal–oxide–support interaction. For noble metals, metal stability in air decreases in the order Rh > Pt > Ir > Ru; formation of volatile RuO4 accounts for the relative instability of ruthenium.
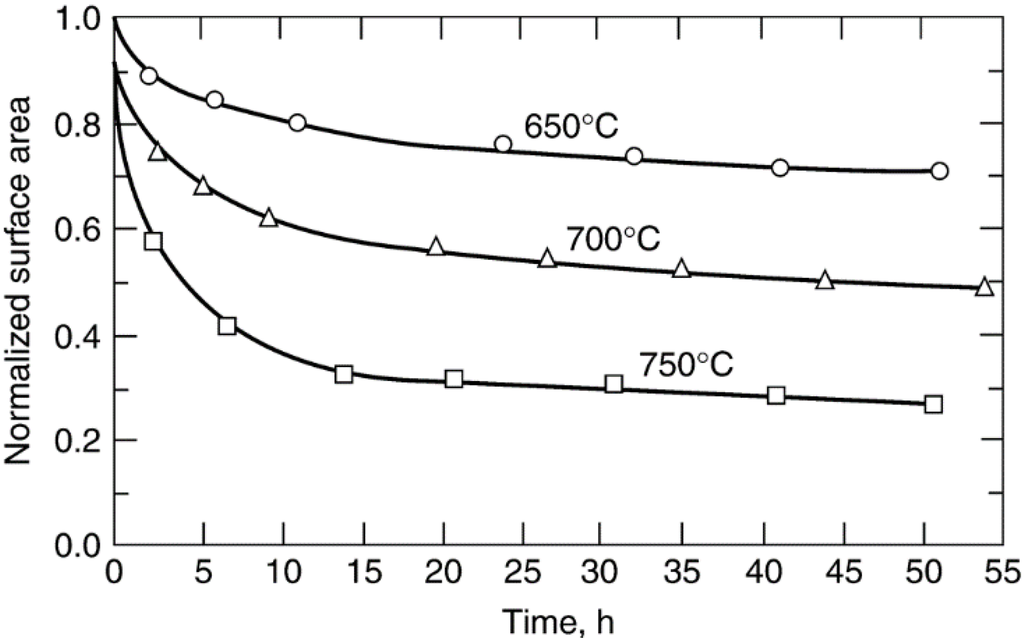
Figure 18.
Normalized nickel surface area (based on H2 adsorption) versus time data during sintering of 13.5% Ni/SiO2 in H2 at 650, 700, and 750 °C. Reproduced from [108]. Copyright 1983, Elsevier.
The effect of temperature on sintering of metals and oxides can be understood physically in terms of the driving forces for dissociation and diffusion of surface atoms, which are both proportional to the fractional approach to the absolute melting point temperature (Tmp). Thus, as temperature increases, the mean lattice vibration of surface atoms increases; when the Hüttig temperature (0.3Tmp) is reached, less strongly bound surface atoms at defect sites (e.g., edges and corner sites) dissociate and diffuse readily over the surface, while at the Tamman temperature (0.5Tmp), atoms in the bulk become mobile. Accordingly, sintering rates of a metal or metal oxide are significant above the Hüttig temperature and very high near the Tamman temperature; thus, the relative thermal stability of metals or metal oxides can be correlated in terms of the Hüttig or Tamman temperatures [109]. This can be illustrated from values of the melting and Tamman temperatures for noble and base metals and their compounds listed in Table 9. For example, sintering of copper catalysts for methanol synthesis is promoted by traces of chlorine in the feed, which react at about 225 °C (500 K) with the active metal/metal oxide surface to produce a highly mobile copper chloride phase having a Tamman temperature of only 79–174 °C (352–447 K) relative to 405–527 °C (678–800 K) for copper metal or metal oxides [110].

Table 9.
Values of Melting and Tamman Temperatures ( °C) for Common Catalytic Metals and Their Compounds a.
| Compound | Tmp, K | TTamman, K | THüttig, K |
|---|---|---|---|
| Ag | 1233 | 617 | 370 |
| Au | 1336 | 668 | 401 |
| Co | 1753 | 877 | 526 |
| Cu | 1356 | 678 | 407 |
| CuO | 1599 | 800 | 480 |
| Cu2O | 1508 | 754 | 452 |
| CuCl2 | 893 | 447 | 268 |
| Cu2Cl2 | 703 | 352 | 211 |
| Fe | 1808 | 904 | 542 |
| Mo | 2883 | 1442 | 865 |
| MoO3 | 1068 | 534 | 320 |
| MoS2 | 1458 | 729 | 437 |
| Ni | 1725 | 863 | 518 |
| NiO | 2228 | 1114 | 668 |
| NiCl2 | 1281 | 641 | 384 |
| Ni(CO)4 | 254 | 127 | 76 |
| Rh | 2258 | 1129 | 677 |
| Rh2O3 | 1373 | 687 | 412 |
| Ru | 2723 | 1362 | 817 |
| Pd | 1828 | 914 | 548 |
| PdO | 1023 | 512 | 307 |
| Pt | 2028 | 1014 | 608 |
| PtO | 823 | 412 | 247 |
| PtO2 | 723 | 362 | 217 |
| PtCl2 | 854 | 427 | 256 |
| PtCl4 | 643 | 322 | 193 |
| Zn | 693 | 347 | 208 |
| ZnO | 2248 | 1124 | 674 |
a Adapted from Ref. [109].
Promoters or impurities affect sintering and redispersion by either increasing (e.g., chlorine and sulfur) or decreasing (e.g., oxygen, calcium, cesium) metal atom mobility on the support; in the latter case, this is due to their high resistance to dissociation and migration due to high melting points, as well as their hindering dissociation and surface diffusion of other atoms. Similarly, support surface defects or pores impede surface migration of metal particles—especially micropores and mesopores with pore diameters about the same size as the metal crystallites.
Historically, sintering rate data were fitted to a simple power-law expression (SPLE) of the form
where ks is the sintering rate constant, D0 the initial dispersion, and n is the sintering order, which for typical catalyst systems may vary from 3 to 15; unfortunately, the SPLE is, in general, not valid for sintering processes because it assumes that surface area or dispersion ultimately reaches zero, given sufficient time, when in fact, for a given temperature and atmosphere, a nonzero or limiting dispersion is observed after long sintering times. Moreover, the use of the SPLE is further questionable because variations in sintering order are observed as a function of time and temperature for a given catalyst in a fixed atmosphere [105,106,107]; thus, data obtained for different samples and different reaction conditions cannot be quantitatively compared. Nevertheless, it has been shown by Fuentes [111,112] and Bartholomew [104,105,106] that the effects of temperature, atmosphere, metal, promoter, and support can be quantitatively determined by fitting sintering kinetic data to the general power-law expression (GPLE)
which adds a term −Deq/D0 to account for the observed asymptotic approach of the typical dispersion versus time curve to a limiting dispersion Deq at infinite time; m, the order of sintering, is found to be either 1 or 2. A recently compiled, comprehensive quantitative treatment of previous sintering rate data based on the GPLE with an order m of 2 [104,105,106] quantitatively addresses the effects of catalyst properties and reaction conditions on sintering rate. Some of these data are summarized in Table 10 [108,113,114,115]. These data show, for example, that the rate constant, and hence the rate of sintering, is less for Ni/Al2O3 than for Pt/Al2O3, an unexpected result in view of the lower heat of vaporization for Ni. This result is possibly explained by a greater metal support interaction for Ni with alumina.

Table 10.
Comparison of Second-Order Sintering Rate Constants and Activation Energies for Pt, Ni, and Ag Catalysts a.
| Catalyst | Atm. | D0 b | ks c (400 °C) | ks (650 °C) | ks (700 °C) | ks (750 °C) | Eact, d kJ/mol | Ref. |
|---|---|---|---|---|---|---|---|---|
| 0.6% Pt/γ-Al2O3 | H2 | ~0.85 | 0.007 | 0.310 | 0.530 | 1.32 | 79 | [113] |
| 5% Pt/γ-Al2O3 | H2 | 0.10 | 0.420 | 0.76 | 0.84 | 0.97 | 13 | [114] |
| 15% Ni/γ-Al2O3 | H2 | 0.16 | 0.004 | 0.083 | 0.13 | 0.27 | 66 | [108] |
| 0.6% Pt/γ-Al2O3 | Air | ~0.85 | 0.024 | 0.29 | 0.41 | 0.75 | 52 | [113] |
| 5% Pt/γ-Al2O3 | Air | 0.10 | 0.014 | 1.46 | 2.79 | 8.51 | 97 | [114] |
| 1.8% Ag/η-Al2O3 | Air | 0.36 | 0.69 | - | - | - | - | [115] |
a Refs. [105,106]; b Initial metal dispersion or percentage exposed; c Second-order sintering rate constant from general power-law expression (GPLE) with units of h−1; d Sintering activation energy for GPLE, −d(D/D0)/dt = ks[D/D0 − Deq/D0]m, where m = 2.
Sintering studies of supported metals are generally of two types: (1) studies of commercially relevant supported metal catalysts and (2) studies of model metal–support systems. The former type provides useful rate data that can be used to predict sintering rates, while the latter type provides insights into the mechanisms of metal particle migration and sintering, although the results cannot be quantitatively extrapolated to predict behavior of commercial catalysts. There is direct evidence from the previous studies of model-supported catalysts [104,107] for the occurrence of crystallite migration (mainly in well-dispersed systems early in the sintering process), atomic migration (mainly at longer sintering times), and spreading of metal crystallites (mainly in oxygen atmosphere). There is also evidence that under reaction conditions, the surface is dynamic, i.e., adsorbates and other adatoms rapidly restructure the surface and slowly bring about faceting; moreover, thermal treatments cause gradual changes in the distribution of coordination sites to minimize surface energy. There is a trend in increasing sophistication of spectroscopic tools used to study sintering and redispersion. In the next decade, we might expect additional insights into atomic and molecular processes during reaction at the atomic scale using STM, analytical high resolution transmission electron microscopy (HRTEM), and other such powerful surface science tools.
2.3.3. Sintering of Catalyst Carriers
Sintering of carriers (supports) has been reviewed by Baker and co-workers [103] and Trimm [116]. Single-phase oxide carriers sinter by one or more of the following processes: (1) surface diffusion, (2) solid-state diffusion, (3) evaporation/condensation of volatile atoms or molecules, (4) grain boundary diffusion, and (5) phase transformations. In oxidizing atmospheres, γ-alumina and silica are the most thermally stable carriers; in reducing atmospheres, carbons are the most thermally stable carriers. Additives and impurities affect the thermal properties of carriers by occupying defect sites or forming new phases. Alkali metals, for example, accelerate sintering; while calcium, barium, nickel, and lanthanum oxides form thermally stable spinel phases with alumina. Steam accelerates support sintering by forming mobile surface hydroxyl groups that are subsequently volatilized at higher temperatures. Chlorine also promotes sintering and grain growth in magnesia and titania during high temperature calcination. This is illustrated in Figure 19 [117]. By contrast, sulfuric acid treatment of hydrated alumina (gibbsite) followed by two-step calcination, results in a very stable transitional alumina with needle-like particle morphology [116]. Dispersed metals in supported metal catalysts can also accelerate support sintering; for example, dispersed nickel accelerates the loss of Al2O3 surface area in Ni/Al2O3 catalysts.
As an important example of support sintering through phase transformations, Al2O3 has a rich phase behavior as a function of temperature and preparation. A few among the many important phases that are stable or metastable, include boehmite, γ-alumina, and α-alumina [8,118,119]. Other phases are possible and the temperatures at which the phase transitions occur depend on crystal size and moisture content of the starting material, but as an example, as temperature is raised, boehmite, which is a hydrated or hydroxyl form of alumina, transforms to γ-alumina between 300 and 450 °C, then to δ-alumina at ~850°C, θ-alumina at ~1000°C, and finally α-alumina at ~1125 °C. The corresponding crystal structures for these five phases are orthorhombic, cubic defective spinel, orthorhombic, deformed monoclinic spinel, and hexagonal close pack (hcp with ABAB stacking) [8,118,119]. The approximate surface areas of these respective phases, as measured by nitrogen physisorption using Brunauer, Emmett, Teller (BET) analysis, are approximately 400, 200, 120, 50, and 1 m2/g [8]. The dramatic drop in surface area during the transition from θ to α is associated with collapse of the microporous structure and formation of the dense hcp phase.
Figure 19.
BET surface area of titania as a function of thermal treatment and chlorine content of fresh samples (before pretreatment). Samples were treated at the temperature indicated for 2 h. Reproduced from [117]. Copyright 1985, Elsevier. ● = Blank TiO2; ▲ =TiO2 soaked in H2O; Δ = TiO2 soaked in HCl/H2O (2.06 wt% Cl); ■ = TiO2 soaked in HCl/H2O (2.40 wt% Cl);○ = TiO2 soaked in HCl/H2O (2.55 wt% Cl); □ = TiO2 soaked in HCl/H2O (2.30 wt% Cl).
2.3.4. Effects of Sintering on Catalyst Activity
Baker and co-workers [103] have reviewed the effects of sintering on catalytic activity. Specific activity (based on catalytic surface area) can either increase or decrease with increasing metal crystallite size during sintering if the reaction is structure-sensitive, or it can be independent of changes in metal crystallite size if the reaction is structure-insensitive. Thus, for a structure-sensitive reaction, the impact of sintering may be either magnified or moderated; while for a structure insensitive-reaction, sintering has in principle no effect on specific activity (per unit surface area). In the latter case, the decrease in mass-based activity is proportional to the decrease in metal surface area. Ethane hydrogenolysis and ethane steam reforming are examples of structure-sensitive reactions, while CO hydrogenation on supported cobalt, nickel, iron, and ruthenium is largely structure-insensitive in catalysts of moderate loading and dispersion.
2.4. Gas/Vapor–Solid and Solid-State Reactions
In addition to poisoning, there are a number of chemical routes leading to catalyst deactivation: (1) reactions of the vapor phase with the catalyst surface to produce (a) inactive bulk and surface phases (rather than strongly adsorbed species), (b) volatile compounds that exit the catalyst and reactor in the vapor phase, or (c) sintering due to adsorbate interactions, that we call chemical-assisted sintering to distinguish it from thermal sintering previously discussed; (2) catalytic solid-support or catalytic solid-promoter reactions, and (3) solid-state transformations of the catalytic phases during reaction. Each of these routes is discussed in some detail below.
2.4.1. Gas/Vapor–Solid Reactions
2.4.1.1. Reactions of Gas/Vapor with Solid to Produce Inactive Phases
Dispersed metals, metal oxides, metal sulfides, and metal carbides are typical catalytic phases, the surfaces of which are similar in composition to the bulk phases. For a given reaction, one of these catalyst types is generally substantially more active than the others, e.g., only Fe and Ru metals are active for ammonia synthesis, while the oxides, sulfides, and carbides are inactive. If, therefore, one of these metal catalysts is oxidized, sulfided, or carbided, it will lose essentially all of its activity. While these chemical modifications are closely related to poisoning, the distinction here is that rather than losing activity owing to the presence of an adsorbed species, the loss of activity is due to the formation of a new phase altogether.
Examples of vapor-induced chemical transformations of catalysts to inactive phases are listed in Table 11 [8,120,121,122,123,124,125,126,127]. These include the formation of RhAl2O4 in the three-way Pt–Rh/Al2O3 catalyst during high temperature operation in an auto exhaust; oxidation of Fe by low levels of O2 during ammonia synthesis or by H2O during regeneration; dealumination (migration of Al from the zeolite framework) of Y-zeolite during high temperature catalytic cracking and regeneration in steam; reaction of SO3 with the alumina support to form aluminum sulfate leading to support breakdown and catalyst pore plugging in several processes, including CO oxidation in a gas turbine exhaust, conversion of CO and hydrocarbons in a diesel exhaust converter, and selective catalytic reduction (SCR) of NOx in utility boiler flue gases [8,122,123,124,127]; oxidation of Fe5C2 to Fe3O4 and of Co metal supported on alumina or silica to Co surface aluminates or silicates during Fischer–Tropsch synthesis at high conversions and hence high PH2O; and formation of NiAl2O4 during reaction and steam regeneration of Ni/Al2O3 in a slightly oxidizing atmosphere above about 500 °C, especially if more reactive aluminas, e.g., γ, δ, or θ forms, are used as supports. Because reaction of SO3 with γ-Al2O3 to produce Al2(SO4)3 is a serious cause of deactivation of alumina-supported catalysts in several catalytic processes (e.g., diesel exhaust abatement and SCR), TiO2 or SiO2 carriers are used rather than Al2O3 or in the diesel or automotive exhaust the alumina catalyst is stabilized by addition of BaO, SrO, or ZrO2 [8,122,123,124,125,126,127].

Table 11.
Examples of Reactions of Gases/Vapors with Catalytic Solids to Produce Inactive Phases.
| Catalytic process | Gas/vapor composition | Catalytic solid | Deactivating chemical reaction | Ref. |
|---|---|---|---|---|
| Auto emissions control | N2, O2, HCs, CO, NO, H2O, SO2 | Pt–Rh/Al2O3 | 2 Rh2O3 + γ-Al2O3→RhAl2O4 + 0.5 O2 | [120,121] |
| Ammonia synthesis and regeneration | H2, N2 | Fe/K/Al2O3 | Fe→FeO at >50 ppm O2 | [8] |
| Traces O2, H2O | Fe→FeO at >0.16 ppm H2O/H2 | |||
| Catalytic cracking | HCs, H2, H2O | La-Y-zeolite | H2O induced Al migration from zeolite framework causing zeolite destruction | [8] |
| CO oxidation, gas turbine exhaust | N2, O2, 400 ppm CO, 100–400 ppm SO2 | Pt/Al2O3 | 2 SO3 + γ-Al2O3→Al2(SO4)3 which blocks catalyst pores | [8] |
| Diesel HC/soot emissions control | N2, O2, HCs (gas and liquid), CO, NO, H2O, soot, SO2 | Pt/Al2O3 and β-zeolite; oxides of CaCuFeVK on TiO2 | Formation of Al2(SO4)3 or sulfates of Ca, Cu, Fe, or V, which block catalysts pores and lower activity for oxidation; Al2O3 stabilized by BaO | [122,123,124] |
| Fischer–Tropsch | CO, H2, H2O, CO2, HCs | Fe/K/Cu/SiO2 | Fe5C2→Fe3O4 due to oxidation at high XCO by product H2O, CO2 | [125] |
| Fischer–Tropsch | CO, H2, H2O, HCs | Co/SiO2 | Co + SiO2→CoO·SiO2 and collapse of SiO2 by product H2O | [126] |
| Selective catalytic reduction (SCR), stationary | N2, O2, NO, PM a, H2O, SO2 | V2O5/WO3/TiO2 | Formation of Al2(SO4)3 if Al2O3 is used | [127] |
| Steam reforming and regeneration in H2O | CH4, H2O, CO, H2, CO2 | Ni/Al2O3 | Ni + Al2O3→NiAl2O4 | [8] |
a Particulate matter.
2.4.1.2. Reactions of Gas/Vapor with Solid to Produce Volatile Compounds
Metal loss through direct vaporization is generally an insignificant route to catalyst deactivation. By contrast, metal loss through formation of volatile compounds, e.g., metal carbonyls, oxides, sulfides, and halides in CO, O2, H2S, and halogen-containing environments, can be significant over a wide range of conditions, including relatively mild conditions. Classes and examples of volatile compounds are listed in Table 12. Carbonyls are formed at relatively low temperatures but high pressures of CO; halides can be formed at relatively low temperatures and low concentration of the halogens. However, the conditions under which volatile oxides are formed vary considerably with the metal; for example, RuO3 can be formed at room temperature, while PtO2 is formed at measurable rates only at temperatures exceeding about 500 °C.

Table 12.
Types and Examples of Volatile Compounds Formed in Catalytic Reactions.
| Gaseous environment | Compound type | Example of compound |
|---|---|---|
| CO, NO | Carbonyls and nitrosyl carbonyls | Ni(CO)4, Fe(CO)5 (0–300 °C) a |
| O2 | Oxides | RuO3 (25 °C), PbO (>850 °C), PtO2 (>700 °C) |
| H2S | Sulfides | MoS2 (>550 °C) |
| Halogens | Halides | PdBr2, PtCl4, PtF6, CuCl2, Cu2Cl2 |
a Temperatures of vapor formation are listed in parentheses.
While the chemical properties of volatile metal carbonyls, oxides, and halides are well known, there is surprisingly little information available on their rates of formation during catalytic reactions. There have been no reviews on this subject and relatively few reported studies to define the effects of metal loss on catalytic activity [28,128,129,130,131,132,133,134,135,136,137,138,139,140,141]. Most of the previous work has focused on volatilization of Ru in automotive converters [128,129,130,131]; nickel carbonyl formation in nickel catalysts during methanation of CO [133,139] or during CO chemisorption at 25 °C [28,135], and formation of Ru carbonyls during Fischer–Tropsch synthesis [136,137]; volatilization of Pt during ammonia oxidation on Pt–Rh gauze catalysts [140,141]; and volatilization of Cu from methanol synthesis and diesel soot oxidation catalysts, leading to sintering in the former and better catalyst–soot contact but also metal loss in the latter case [109].
Results of selected studies are summarized in Table 13. Bartholomew [131] found evidence of significant (50%) Ru loss after testing of a Pd–Ru catalyst in an actual reducing automobile exhaust for 100 h, which he attributed to formation of a volatile ruthenium oxide and which was considered responsible at least in part for a significant loss (20%) of NO reduction activity.

Table 13.
Documented Examples of Reactions of Vapor with Solid to Produce Volatile Compounds.
| Catalytic process | Catalytic solid | Vapor formed | Comments on deactivation process | Ref. |
|---|---|---|---|---|
| Automotive converter | Pd–Ru/Al2O3 | RuO4 | 50% loss of Ru during 100-h test in reducing automotive exhaust | [131] |
| Methanation of CO | Ni/Al2O3 | Ni(CO)4 | PCO > 20 kPa and T < 425 °C due to Ni(CO)4 formation, diffusion and decomposition on the support as large crystallites | [133] |
| CO chemi-sorption | Ni catalysts | Ni(CO)4 | PCO > 0.4 kPa and T > 0 °C due to Ni(CO)4 formation; catalyzed by sulfur compounds | [134] |
| Fischer–Tropsch synthesis (FTS) | Ru/NaY zeolite, Ru/Al2O3, Ru/TiO2 | Ru(CO)5, Ru3(CO)12 | Loss of Ru during FTS (H2/CO = 1, 200–250 °C, 1 atm) on Ru/NaY zeolite and Ru/Al2O3; up to 40% loss while flowing CO at 175–275 °C over Ru/Al2O3 for 24 h. Rate of Ru loss less on titania-supported Ru and for catalysts containing large metal crystallites (3 nm) relative to small metal crystallites (1.3 nm). Surface carbon lowers loss | [136,137] |
| Ammonia oxidation | Pt–Rh gauze | PtO2 | Loss: 0.05–0.3 g Pt/ton HNO3; recovered with Pd gauze; loss of Pt leads to surface enrichment with inactive Rh | [8,142] |
| HCN synthesis | Pt–Rh gauze | PtO2 | Extensive restructuring and loss of mechanical strength | [8,143] |
| Methanol synthesis | CuZnO | CuCl2, Cu2Cl2 | Mobile copper chloride phase leads to sintering at reaction temperature (225 °C) | [109] |
| Diesel soot oxidation | Oxides of K, Cu, Mo, and trace Cl | CuCl2, Cu2Cl2 | Mobile copper chloride improves catalyst–soot contact; catalyst evaporation observed | [109] |
Shen and co-workers [133] found that Ni/Al2O3 methanation catalysts deactivate rapidly during methanation at high partial pressures of CO (>20 kPa) and temperatures below 425 °C because of Ni(CO)4 formation, diffusion, and decomposition on the support as large crystallites; under severe conditions (very high PCO and relatively low reaction temperatures) loss of nickel metal occurs. Thus, loss of nickel and crystallite growth could be serious problems at the entrance to methanation reactors where the temperature is low enough and PCO high enough for metal carbonyl formation. Agnelli and co-workers [139] investigated kinetics and modeling of sintering due to formation and migration of nickel carbonyl species. They found that the initially sharp crystallite size distribution evolved during several hours of sintering under low temperature (230 °C) reaction conditions to a bimodal system consisting of small spherical crystallites and large faceted crystals favoring (111) planes. The sintering process was modeled in terms of an Ostwald-ripening mechanism coupled with mass transport of mobile subcarbonyl intermediates. Long-term simulations were found to predict reasonably well the ultimate state of the catalyst. On the basis of their work, they proposed two solutions for reducing loss of nickel: (1) increasing reaction temperature and decreasing CO partial pressure in order to lower the rate of carbonyl formation, and (2) changing catalyst composition, e.g., alloying nickel with copper or adding alkali to inhibit carbonyl species migration.
Of note, Kuo and Hwang have shown that the particle morphology itself affects the rate of Ostwald ripening due to different relative chemical potential energies of the surfaces [144]. Using silver nanoparticles, they found that atoms at sharp edges and corners were removed first, resulting in more rounded particles for all starting geometries. Thus, initial particle geometry appears to have an effect in addition to the chemical atmosphere experienced by the particles.
Loss of nickel metal during CO chemisorption on nickel catalysts at temperatures above 0 °C is also a serious problem; moreover, this loss is catalyzed by sulfur poisoning [28]. In view of the toxicity of nickel tetracarbonyl, the rapid loss of nickel metal, and the ill-defined adsorption stoichiometries, researchers are advised to avoid using CO chemisorption for measuring nickel surface areas; instead, hydrogen chemisorption, an accepted ASTM method with a well-defined adsorption stoichiometry, is recommended [145]. Figure 20 illustrates a mechanism for the formation of Ni(CO)4 on a crystallite of nickel in CO atmosphere.
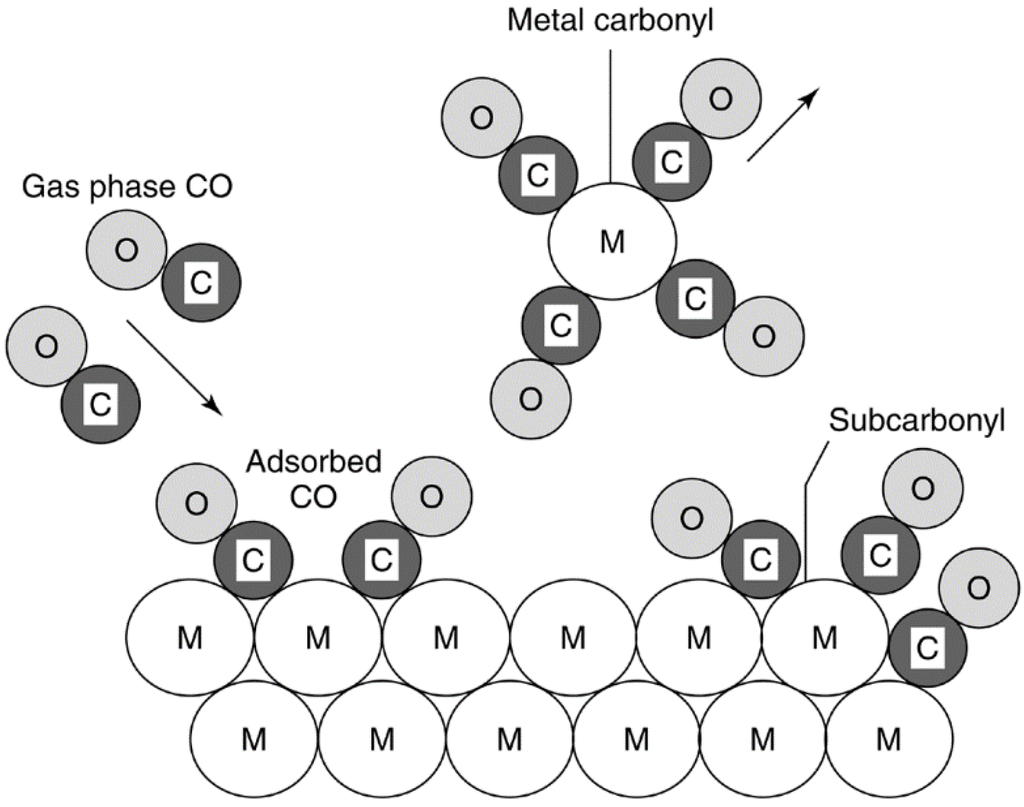
Figure 20.
Formation of volatile tetra-nickel carbonyl at the surface of nickel crystallite in CO atmosphere. Reproduced from [8]. Copyright 2006, Wiley-Interscience.
Goodwin and co-workers [136,137] studied the influence of reaction atmosphere, support, and metal particle size on the loss of Ru due to carbonyl formation. They found that the loss of Ru during CO hydrogenation (H2/CO = 1, 200–250 °C, 1 atm) on Ru/NaY zeolite and Ru/Al2O3 for extended periods of time was significant (e.g., up to 40% while flowing CO at 175–275 °C over Ru/Al2O3 for 24 h). The loss of Ru was significantly less on titania-supported Ru; moreover, the rate of loss was lower for catalysts containing large metal crystallites (3 nm) relative to those containing small metal crystallites (1.3 nm). Metal loss was inhibited in part at higher reaction temperatures as a result of carbon deposition. Thus, while it is clear that loss of ruthenium could be a serious problem in Fischer–Tropsch synthesis, there are measures in terms of catalyst design and choice of reaction conditions that can be taken to minimize loss.
One of the most dramatic examples of vapor phase loss of the catalyst occurs during NH3 oxidation on Pt–Rh gauze, an important reaction in the manufacture of nitric acid [8,140,141]. At the high reaction temperature (~900 °C), formation of a volatile platinum oxide (PtO2) occurs at a very significant rate; in fact, the rate of loss of 0.05–0.3 g Pt/ton of HNO3 is high enough to provide a substantial economic incentive for Pt recovery [8]. The most effective recovery process involves placing a woven Pd-rich alloy gauze immediately below the Pt–Rh gauze to capture the Pt through formation of a Pd–Pt alloy. Pt loss is also the most significant cause of catalyst deactivation as the gauze surface becomes enriched in nonvolatile but inactive rhodium oxide [142], requiring shutdown and catalyst replacement every 3–12 months [8].
Decomposition of volatile platinum oxide species formed during high temperature reaction may (similar to the previously discussed formation of large crystallites of Ni from Ni(CO)4) lead to formation of large Pt crystallites and/or substantial restructuring of the metal surface. For example, Wu and Phillips [146,147,148] observed surface etching, enhanced sintering, and dramatic surface restructuring of Pt thin films to faceted particles during ethylene oxidation over a relatively narrow temperature range (500–700 °C). The substantially higher rate of sintering and restructuring in O2/C2H4 relative to that in nonreactive atmospheres was attributed to the interaction of free radicals such as HO2, formed homogeneously in the gas phase, with the metal surface to form metastable mobile intermediates. Etching of Pt–Rh gauze in a H2/O2 mixture under the same conditions as Pt surfaces (600 °C, N2/O2/H2 = 90/7.5/2.5) has also been reported [143]. A significant weight loss was observed in a laminar flow reactor with little change in surface roughness, while in an impinging jet reactor, there was little weight loss, but substantial restructuring of the surface to particle-like structures, 1–10 μm in diameter; these particles were found to have the same Pt–Rh composition as the original gauze. The nodular structures of about 10-μm diameter formed in these experiments are strikingly similar to those observed on Pt–Rh gauze after use in production of HCN at 1100 °C in 15% NH3, 13% CH4, and 72% air (see Figure 21). Moreover, because of the high space velocities during HCN production, turbulent rather than laminar flow would be expected, as in the impinging jet reactor. While little Pt is volatilized from the Pt–Rh gauze catalyst during HCN synthesis, the extensive restructuring leads to mechanical weakening of the gauze [8].
Figure 21.
(a) SEM of Pt–Rh gauze after etching in N2/O2/H2 = 90/7.5/2.5 at 875 K for 45 h. Reproduced from [143]. Copyright 1992, Elsevier. (b) SEM of Pt–Rh gauze after use in production of HCN (magnification 1000×). Photographs courtesy of Ted Koch at DuPont, personal correspondence to the author.
Other examples of catalyst deactivation due to volatile compound formation include (1) loss of the phosphorus promoter from the VPO catalyst used in the fluidized-bed production of maleic anhydride, with an attendant loss of catalyst selectivity [8], (2) vapor-phase loss of the potassium promoter from steam-reforming catalysts in the high temperature, steam-containing environment [8], and (3) loss of Mo from a 12-Mo-V-heteropolyacid due to formation of a volatile Mo species during oxydehydrogenation of isobutyric acid to methacrylic acid [138].
While relatively few definitive studies of deactivation by volatile compound formation have been reported, the previous work does provide the basis for enumerating some general principles. A generalized mechanism of deactivation by formation of volatile metal compounds can be postulated (see Figure 22). In addition, the roles of kinetics and thermodynamics can be stated in general terms:
- (1)
- At low temperatures and partial pressures of the volatilization agent (VA), the overall rate of the process is limited by the rate of volatile compound formation.
- (2)
- At intermediate temperatures and partial pressures of the VA, the rate of formation of the volatile compound exceeds the rate of decomposition. Thus, the rate of vaporization is high, the vapor is stable, and metal loss is high.
- (3)
- At high temperatures and partial pressures of the VA, the rate of formation equals the rate of decomposition, i.e., equilibrium is achieved. However, the volatile compound may be too unstable to form or may decompose before there is an opportunity to be transported from the system. From the previous work, it is also evident that besides temperature and gas phase composition, catalyst properties (crystallite size and support) can play an important role in determining the rate of metal loss.
Figure 22.
Generalized mechanisms and kinetics for deactivation by metal loss. Reproduced from [8]. Copyright 2006, Wiley-Interscience.
2.4.2. Solid-State Reactions
Catalyst deactivation by solid-state diffusion and reaction appears to be an important mechanism for degradation of complex multicomponent catalysts in dehydrogenation, synthesis, partial oxidation, and total oxidation reactions [8,149,150,151,152,153,154,155,156,157,158,159,160]. However, it is difficult in most of these reactions to know the extent to which the solid-state processes, such as diffusion and solid-state reaction, are affected by surface reactions. For example, the rate of diffusion of Al2O3 to the surface to form an aluminate may be enhanced by the presence of gas-phase oxygen or water or the nucleation of a different phase may be induced by either reducing or oxidizing conditions. Recognizing this inherent limitation, the focus here is nevertheless on processes in which formation of a new bulk phase (and presumably the attendant surface phase) leads to substantially lower activity. There is probably some overlap with some of the examples given under Gas/Vapor–Solid Reactions involving reactions of gas/vapor with solid to produce inactive phases.
Examples from the literature of solid-state transformations leading to catalyst deactivation are summarized in Table 14. They include (1) the formation of KAlO2 during ammonia synthesis at the Fe/K/Al2O3 catalyst surface, (2) decomposition of the active phase PdO to inactive Pd metal during catalytic combustion on PdO/Al2O3 and PdO/ZrO2 catalysts, (3) transformation of active carbides to inactive carbides in Fischer–Tropsch synthesis on Fe/K/Cu catalysts, (4) formation of inactive V(IV) compounds in SO2 oxidation, and (5) reductive transformation of iron molybdate catalysts during partial oxidation of benzene, methanol, propene, and isobutene.

Table 14.
Examples of Solid-State Transformations Leading to Catalyst Deactivation.
| Catalytic process | Catalytic solid | Deactivating chemical reaction | Ref. | |
|---|---|---|---|---|
| Ammonia synthesis | Fe/K/Al2O3 | Formation of KAlO2 at catalyst surface | [159] | |
| Catalytic combustion | PdO/Al2O3, PdO/ZrO2 | PdO→Pd at T > 800 °C | [152] | |
| Catalytic combustion | Co/K on MgO, CeO2, or La2O3 | Formation of CoO–MgO solid soln., LaCoO3, or K2O film on CeO2 | [160] | |
| Dehydrogenation of ethyl benzene to styrene | Fe2O3/Cr2O3/K2O | K migration to center of pellet caused by thermal gradient | [8] | |
| Fischer–Tropsch | Fe/K, Fe/K/CuO | Transformation of active carbides to inactive carbides | [157,158] | |
| Oxidation of SO2 to SO3 | V2O5/K2O/Na2O/ | Formation of inactive V(IV) compounds at T < 420–430 °C | [155] | |
| Partial oxidation of benzene to maleic anhydride | V2O5–MoO3 | Decreased selectivity due to loss of MoO3 and formation of inactive vanadium compounds | [149] | |
| Partial oxidation of methanol to formaldehyde | Fe2(MoO4)3 plus MoO3 | Structural reorganization to β-FeMoO4; reduction of MoO3 | [150,156] | |
| Partial oxidation of propene to acrolein | Fe2(MoO4)3 | Reductive transformation of Mo18O52 to Mo4O11 | [153,156] | |
| Partial oxidation of isobutene to methacrolein | Fe2(MoO4)3 | Reduction to FeMoO4 and MoO3–x | [151,154] | |
There are basic principles underlying most solid-state reactions in working catalysts that have been enumerated by Delmon [156]: (1) the active catalytic phase is generally a high-surface-area defect structure of high surface energy and as such a precursor to more stable, but less active phases and (2) the basic reaction processes may itself trigger the solid-state conversion of the active phase to an inactive phase; for example, it may involve a redox process, part of which nucleates the inactive phase.
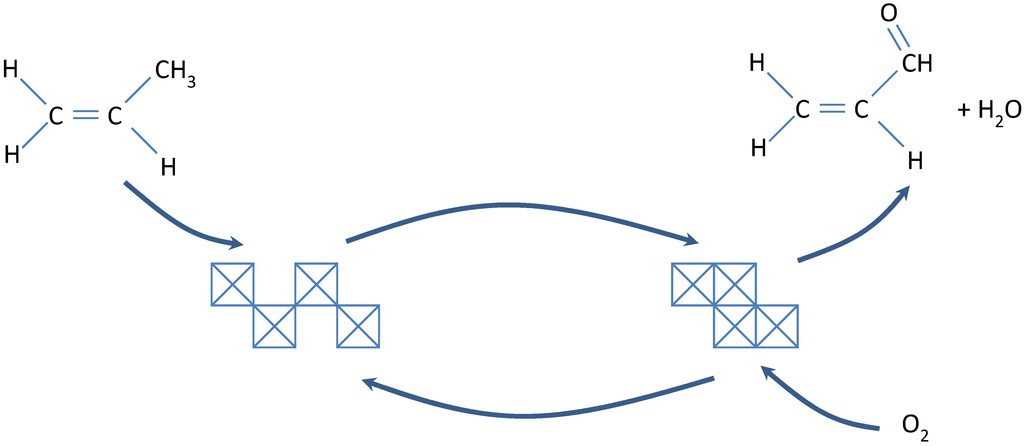
Figure 23.
Schematic representation of the cyclic reduction/oxidation of twin pairs of MoO6 octahedra between the corner and the edge-sharing arrangements (boxes represent MoO6 octahedra with sharing of oxygen atoms at corners for MoO3 or edges for MoO2). The figure is not completely accurate, because it cannot take into account the fact that the arrangements are not perpendicular to the main axes of the lattice. Adapted from [156].
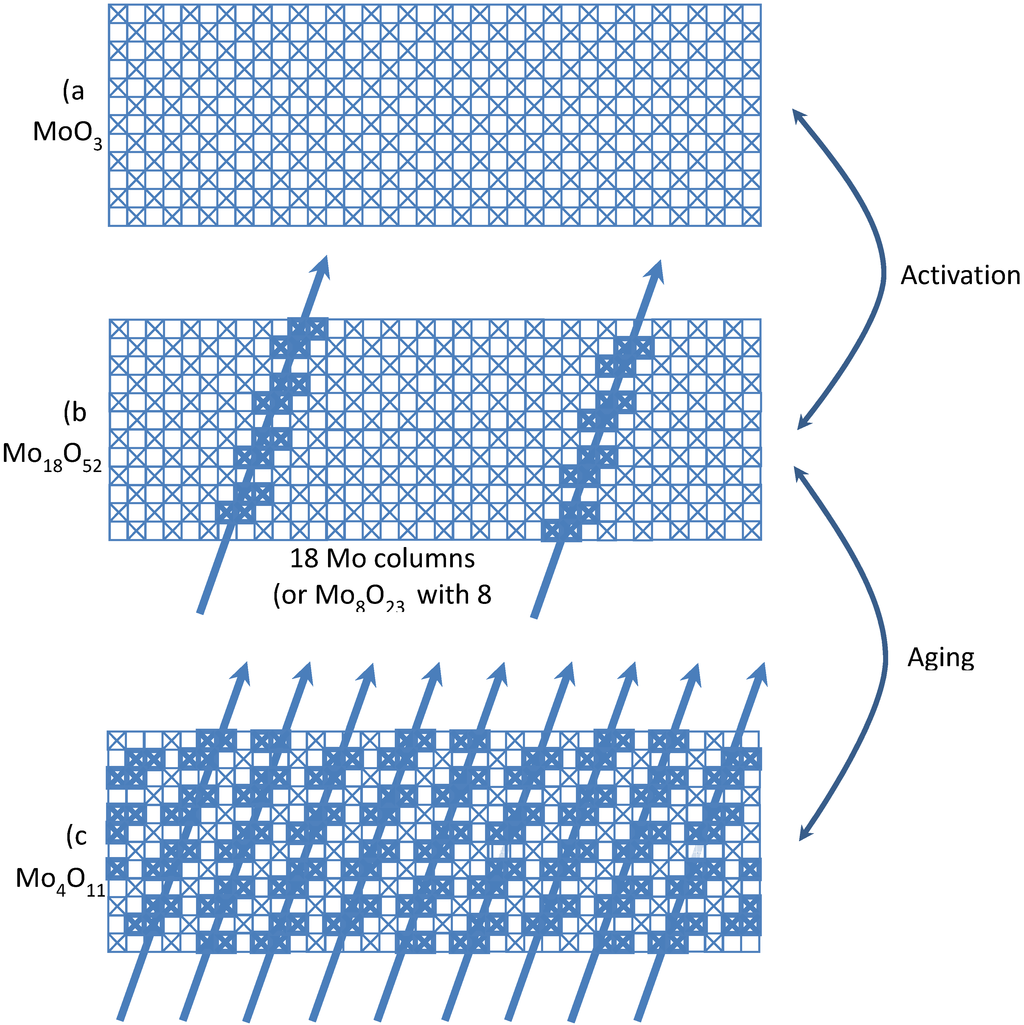
Figure 24.
Schematic representation of the structure of MoO3, Mo18O52, and Mo4O11. The shear planes in Mo18O52 and Mo4O11 are represented by the oblique arrows (boxes with an “X” represent MoO5 octahedra). Adapted from [156].
A well-documented example of these principles occurs in the partial oxidation of propene to acrolein on a Fe2(MoO4)3 catalyst [153,156]. This oxidation occurs by the “Mars van Krevelen” mechanism, i.e., a redox mechanism in which lattice oxygen reacts with the adsorbed hydrocarbon to produce the partially oxygenated product; the reduced catalyst is restored to its oxidized state through reaction with gaseous oxygen. In propene oxidation, two atoms of oxygen from the catalyst are used, one for removing two hydrogen atoms from the olefin and the other one in forming the unsaturated aldehyde. The fresh, calcined catalyst MoO3 consists of corner-sharing MoO6 octahedra (with Mo at the center and six oxygen atoms at the corners); but upon reduction to MoO2, octahedra share edges as shown in Figure 23. However, it has been reported [153,156] that only slightly reduced (relative to MoO3), open structures such as Mo18O52 and Mo8O23 are the most active, selective phases; more complete reduction of either of these structures leads to formation of Mo4O11 (see Figure 24) having substantially lower selectivity. Delmon and co-workers [154,156] have shown that addition of an oxygen donor such as Sb2O4 facilitates spillover of oxygen and thereby prevents overreduction and deactivation of the catalyst.
2.4.3. Reactions of Gas/Vapor with Solid to Restructure the Surface by Chemical Assisted Sintering
The surfaces of metallic catalysts can be greatly roughened by interactions with the reactants and/or products. However, as opposed to forming volatile species that are transported out of the reactor as discussed in the previous section, these interactions lead to a restructuring of the surface that is similar to that which occurs during thermal sintering, but at temperatures which are below the Tamman or Huttig temperatures, respectively defined as 0.5 and 0.3 of the melting point (Tm) of the material, at which thermal sintering might be expected. Therefore, this surface restructuring must be attributed to the interaction of the gas phase with the solid. The following three examples from the literature highlight the chemical-assisted sintering process caused by adsorbate-surface interactions on Ni, Co, and Pd surfaces.
Chemical sintering of Ni/alumina catalysts in methanation due to formation of volatile Ni(CO)4 followed by its decomposition downstream to large Ni crystallites has been well documented [8,105]. Moreover, deactivation of Ni/alumina by Ni aluminate formation is also observed at the exit of methanators where temperature is moderately high (T = 450 °C) and steam partial pressure is maximum [105].
Wilson and de Groot [161] reported that under high pressure (4 bar, H2/CO = 2) and moderate temperature (523 K) conditions, single crystal Co (0001) surfaces restructured significantly due to interaction with the CO, which they attributed to an etch-regrowth mechanism. The left hand panel of Figure 25a shows the scanning tunneling microscopy (STM) image of the single crystal surface, while Figure 25b shows the same location after exposure to the H2/CO atmosphere for 1 h The surface restructuring and roughening is profound, with the peaks approximately four atoms high relative to the previously smooth surface that had only well-defined steps interrupting the (0001) planar surface.
More recently, Parkinson et al. [162] have shown that chemical-assisted sintering occurs at room temperature for palladium supported on magnetite under ultra high vacuum conditions with CO partial pressures of only 5 × 10−10 mbar. Figure 26 shows four STM images from a movie that demonstrates the surface mobility of the Pd at these low CO partial pressures. Figure 26a is the surface prior to CO exposure, while Figure 26b–d show the surface as a function of time up to about an hour of exposure. The authors note that hydroxyl-Pd groups (OH-Pd), identified by the ×’s in the images, serve as anchoring points for the coalescence of larger Pd clusters. The full movie, available with the supplementary material for this article [162], is recommended to fully appreciate the unexpectedly high atomic mobility under these conditions.
Figure 25.
STM images of the Co (0001) surface (a) before and (b) after 1 h exposure to 4 MPa 2:1 H2:CO atmosphere at 523 K. Reproduced from [161]. Copyright 1995, American Chemical Society.
Figure 26.
“The CO-induced formation of a large Pd cluster. a – d, Four STM images (14 × 14 nm2, +1 V, 0.2 nA) selected from a 36-frame STM movie (duration 1 h 50 min) following the deposition of 0.2 ML Pd [on Fe3O4] at RT. Initially (a), isolated Pd atoms are present, together with hydroxyl groups and one OH–Pd (red cross). After three frames the background pressure of CO is raised to 5 × 10-10 mbar. Thirty minutes later (frame b), several mobile ‘fuzzy’ Pd carbonyl species, trapped at other Pd atoms, have formed. Shortly afterwards (c), three Pd carbonyls and four adatoms have formed a large cluster. Twenty-five minutes later (d), the cluster has captured another Pd carbonyl, and diffused to merge with an OH–Pd species.”. Reproduced from [162]. Copyright 2013, MacMillan Publishers.
2.5. Mechanical Failure of Catalysts
2.5.1. Forms and Mechanisms of Failure
Mechanical failure of catalysts is observed in several different forms that depend on the type of reactor, including (1) crushing of granular, pellet, or monolithic catalyst forms due to a load in fixed beds; (2) attrition, the size reduction, and/or breakup of catalyst granules or pellets to produce fines, especially in fluid or slurry beds; and (3) erosion of catalyst particles or monolith coatings at high fluid velocities in any reactor design. Attrition is evident by a reduction in the particle size or a rounding or smoothing of the catalyst particle easily observed under an optical or electron microscope. Washcoat loss is observed by scanning a cross section of the honeycomb channel with either an optical or an electron microscope. Large increases in pressure drop in a catalytic process are often indicative of fouling, masking, or the fracturing and accumulation of attritted catalyst in the reactor bed.
Commercial catalysts are vulnerable to mechanical failure in large part because of the manner in which they are formed; that is, catalyst granules, spheres, extrudates, and pellets ranging in diameter from 50 μm to several millimeters are in general prepared by agglomeration of 0.02–2 μm aggregates of much smaller primary particles having diameters of 10–100 nm by means of precipitation or gel formation, followed by spray drying, extrusion, or compaction. These agglomerates have, in general, considerably lower strengths than the primary particles and aggregates of particles from which they are formed.
Two principal mechanisms are involved in mechanical failure of catalyst agglomerates: (1) fracture of agglomerates into smaller agglomerates of approximately 0.2d0–0.8d0 and (2) erosion (or abrasion) from the surface of the agglomerate of aggregates of primary particles having diameters ranging from 0.1 to 10 μm [163]. While erosion is caused by mechanical stresses, fracture may be due to mechanical, thermal, and/or chemical stresses. Mechanical stresses leading to fracture or erosion in fluidized or slurry beds may result from (1) collisions of particles with each other or with reactor walls or (2) shear forces created by turbulent eddies or collapsing bubbles (cavitation) at high fluid velocities. Thermal stresses occur as catalyst particles are heated and/or cooled rapidly; they are magnified by temperature gradients across particles and by differences in thermal expansion coefficients at the interface of two different materials, e.g., catalyst coating/monolith interfaces; in the latter case the heating or cooling process can lead to fracture and separation of the catalyst coating. Chemical stresses occur as phases of different density are formed within a catalyst particle via chemical reaction; for example, carbiding of primary iron oxide particles increases their specific volume and micromorphology leading to stresses that break up these particles [164]. A further example occurs in supported metal catalysts when large quantities of filamentous carbon (according to reaction mechanisms discussed earlier) overfill catalysts pores, generating enormous stresses that can fracture primary particles and agglomerates.
2.5.2. Role of Physical and Chemical Properties of Ceramic Agglomerates in Determining Strength and Attrition Resistance
2.5.2.1. Factors Affecting the Magnitude of Stress Required for Agglomerate Breakage and the Mechanisms by Which It Occurs
The extent to which a mechanism, i.e., fracture or erosion, participates in agglomerate size reduction depends upon several factors: (1) the magnitude of a stress, (2) the strength and fracture toughness of the agglomerate, (3) agglomerate size and surface area, and (4) crack size and radius. Erosion (abrasion) occurs when the stress (e.g., force per area due to collision or cavitation pressure) exceeds the agglomerate strength, i.e., the strength of bonding between primary particles. Erosion rate is reportedly [163] proportional to the external surface area of the catalyst; thus, erosion rate increases with decreasing agglomerate size.
2.5.2.2. Fracture Toughness of Ceramic Agglomerates
Most heterogeneous catalysts are complex, multiphase materials that consist, in large part, of porous ceramic materials, i.e., are typically oxides, sulfides, or metals on an oxide carrier or support. When a tensile stress of a magnitude close to the yield point is applied, ceramics almost always undergo brittle fracture before plastic deformation can occur. Brittle fracture occurs through formation and propagation of cracks through the cross section of a material in a direction perpendicular to the applied stress. Agglomerate fracture due to a tensile stress occurs by propagation of internal and surface flaws; these flaws, created by external stresses or inherent defects, are stress multipliers, i.e., the stress is multiplied by 2(a/r)0.5, where a is the crack length and r is the radius of curvature of the crack tip; since a/r can vary from 2 to 1000, the effective stress at the tip of a crack can be 4–60 times the applied stress. Tensile stress multipliers may be microcracks, internal pores, and grain corners.
The ability of a material to resist fracture is termed fracture toughness. The plane strain fracture toughness, KIc, is defined as
where Y is a dimensionless parameter (often close to 1.0–2.0), the magnitude of which depends upon both specimen and crack geometries, σ is the applied stress, and a is the length of a surface crack or half the length of an internal crack. Crack propagation and fracture are likely if the right hand side of Equation 3 exceeds the experimental value of plane strain fracture toughness (left-hand side of Equation 3). Plane strain fracture toughness values for ceramic materials are significantly smaller than for metals and typically below 10 MPa(m)0.5; reported values for nonporous, crystalline alumina (99.9%), fused silica, and zirconia (3 mol% Y2O3) are 4–6, 0.8, and 7–12 MPa(m)0.5, respectively; flexural strengths (analogous to yield strengths for metals) for the same materials are 280–550, 100, and 800–1500 MPa [165]. Thus, on the basis of both fracture toughness and flexural strength, nonporous, crystalline zirconia is much stronger toward fracture than alumina, which in turn is much stronger than fused silica.
2.5.2.3. Effects of Porosity on Ceramic Agglomerate Strength
The introduction of porosity to crystalline or polycrystalline ceramic materials will, on the basis of stress amplification, significantly decrease elastic modulus and flexural strength for materials in tension. This is illustrated by data in Figure 27, showing that elastic modulus and flexural strength of a ceramic alumina (probably alpha form) are reduced by 75 and 85% respectively as porosity is increased from 0 to 50% [166]. Thus, according to Figure 27b, the flexural strength of typical porous aluminas used as catalyst supports might lie in the range of 30–40 MPa. However, yield strengths for γ-Al2O3 (shown in the next section) are factors of 3–50 lower. Nevertheless, the data in Figure 27b suggest that higher strengths may be possible.

Figure 27.
The influence of porosity on (a) the modulus of elasticity for aluminum oxide at room temperature and (b) the flexural strength for aluminum oxide at room temperature. Reproduced from [166]. Copyright 1956, Wiley.
2.5.2.4. Compressive Strengths of Ceramic Materials
Thus far, the discussion has focused mainly on tensile strength, the extent of which is greatly reduced by the presence of cracks or pores. However, for ceramic materials in compression, there is no stress amplification due to flaws or pores; thus ceramic materials (including catalytic materials) in compression are much stronger (approximately a factor of 10) than in tension. In addition, the strength of ceramic materials can be dramatically enhanced by imposing a residual compressive stress at the surface through thermal or chemical tempering. Moreover, introduction of binders, such as graphite, enables agglomerates of ceramic powders to undergo significant plastic deformation before fracture.
2.5.3. Tensile Strengths and Attrition Resistance of Catalyst Supports and Catalysts
2.5.3.1. Tensile Strength Data for Catalyst Support Agglomerates
The strengths cited above for nonporous, annealed crystalline or polycrystalline materials do not necessarily apply to porous catalyst agglomerates, even under compression; rather, agglomerate strength is dependent upon the strengths of chemical and physical bonds, including the cohesive energy, between primary particles. Agglomerate strength would depend greatly on the preparation of the compact. Representative data for catalyst agglomerates (see Table 15) suggest they are generally substantially weaker than polycrystalline ceramic materials prepared by high temperature sintering, such as the alumina cited in Figure 27 [163,165,167,168,169,170,171]. For example, Pham and co-workers [163] found that the breaking strength of a VISTA B alumina agglomerate during uniaxial compaction is in the range of 5–10 MPa—substantially lower than the reported values for heat-treated polycrystalline alumina of 280–550 MPa [165]. A large part of this difference (about 85–95%) can be attributed to porosity; however, the remaining 5–15% must be due to differences in bonding between primary particles. In other words, the bonds between primary particles in catalyst agglomerates (and some ceramic agglomerates prepared by similar methods) are typically physical in nature (e.g., involve van der Waals forces) while those in sintered polycrystalline ceramic agglomerates are principally chemical because of solid bridging of primary particles. Thus, there appears to be considerable potential for strengthening catalyst agglomerates, since their strengths are typically factors of 3–50 lower than for conventional, heat-treated ceramics of similar porosity.

Table 15.
Mechanical Strengths and Attrition Rates of Catalyst Supports Compared to Those of Sintered Ceramic Agglomerates.
| Catalyst support or ceramic | Preparation/pretreatment/properties | Strength, MPa | Attrition index, wt%/h | Ref. | ||
|---|---|---|---|---|---|---|
| High surface area catalyst supports | ||||||
| γ-Al2O3, 1.2–4.25-mm spheres | Sol–gel granulation/dried 10 h at 40 °C, calcined 3 h at 450 °C/389 m2/g, dpore = 3.5 nm | 11.6 ± 1.9 | 0.033 | [167] | ||
| γ-Al2O3, 4.25-mm spheres | Alcoa LD-350 | 0.7 | 0.177 | [167] | ||
| γ-Al2O3, 100 μm | VISTA-B-965-500C | 6.2 ± 1.3 | - | [163] | ||
| TiO2 (anatase), 30 μm | Thermal hydrolysis/dried 110 °C, calcined 2 h at 500 °C/ 92 m2/g, <10-nm primary crystallites | 28a | - | [168] | ||
| TiO2 (anatase), 90 μm | Basic precipitation/dried 110 °C, calcined 2 h at 500 °C/81 m2/g, 10–14-nm primary crystallites | 15a | - | [168] | ||
| TiO2 (75% anatase, 25% rutile) | Degussa P25, fumed/4-mm extrudates/48 m2/g, Vpore = 0.34 cm3/g, dpore = 21 nm | 0.9 | - | [169] | ||
| TiO2 (anatase) | Rhone-Poulenc DT51, ppt./4 mm extrudates/92 m2/g, Vpore = 0.40 cm3/g, dpore = 8, 65 nm | 0.9 | - | [169] | ||
| Low surface area ceramics | ||||||
| Al2O3 | Spray dried with organic binder; plastic deformation observed | 2.3 | - | [170] | ||
aRough estimates from break points on relative density versus log[applied pressure] curves; data are consistent with mass distribution versus pressure curves from ultrasonic tests.
2.5.3.2. Effects of Preparation and Pretreatment on Catalyst Agglomerate Strength
From the data in Table 15 it is evident that even subtle differences in preparation and pretreatment also affect agglomerate strength. For example, spheres of γ-Al2O3 prepared by sol–gel granulation are substantially (17 times) stronger than commercial γ-Al2O3 spheres [166]. Moreover, 30- and 90- μm diameter particles of TiO2 prepared by thermal hydrolysis or basic precipitation are 30 and 15 times stronger than commercially available 4-mm extrudates [169].
2.5.4. Attrition of Catalyst Agglomerates: Mechanisms, Studies, and Test Methods
Catalyst attrition is a difficult problem in the operation of moving-bed, slurry-bed, or fluidized-bed reactors. Generally, stronger materials have greater attrition resistance; this conclusion is supported by representative data in Table 15 for γ-Al2O3, showing that the strength of the alumina prepared by sol–gel granulation is 17 times higher, while its attrition rates is 5 times lower.
The mechanism by which attrition occurs (erosion or fracture) can vary with catalyst or support preparation, crush strength, and with reactor environment; it can also vary with the mechanical test method. There is some evidence in the attrition literature, supporting the hypothesis that in the presence of a large stress, weaker oxide materials are prone to failure by fracture, while stronger materials tend to erode. For example, in the fluid catalytic cracking process, as new silica–alumina/zeolite catalyst in the form of 50–150-μm spherical agglomerates is added to replace catalyst lost by attrition, the weaker agglomerates break up fairly rapidly by fracture into smaller subagglomerates, following which the stronger agglomerates are slowly abraded to produce fine particles of 1–10 μm [172]. However, there is also contrary evidence from Thoma and co-workers [168], showing that fracture may be the preferred mechanism for strong TiO2 agglomerates, while abrasion is favored for weaker agglomerates. That is, when subjected to ultrasonic stress, 30-μm-diameter agglomerates of amorphous anatase (TiO2) prepared by thermal hydrolysis were observed to undergo fracture to 5–15-μm fragments, while 90-μm agglomerates of polycrystalline anatase prepared by basic precipitation were found to break down by erosion to 0.1–5-μm fragments [168]; in this case, the amorphous anatase was apparently stronger by a factor of 2 (see Table 15). Supporting a third trend, data from Pham and co-workers [163] show that attrition mechanism and rate are independent of agglomerate strength, but depend instead on the type of material. That is, 100-μm-diameter agglomerates of precipitated Fe/Cu/K Fischer–Tropsch catalyst (prepared by United Catalyst Incorporated) and having nearly the same strength shown in Table 15 for Vista-B Al2O3 (6.3 vs. 6.2 MPa), were found to undergo substantial fracture to 5–30-μm fragments (an increase from 45 to 85%; see Figure 28) as well as substantial erosion to 1 μm or less fragments (increase from 2 to 50%). By comparison, under the same treatment conditions, 90-μm-diameter agglomerates of Vista-B Al2O3 underwent much less attrition, mainly by erosion (20% increase in 0.1–5-μm fragments). The very low attrition resistance of the Fe/Cu/K Universal Catalysts, Inc. (UCI) catalyst is further emphasized by the unsatisfactory outcome of a test of this catalyst by the U.S. Department of Energy (DOE) in a pilot-scale slurry-phase bubble-column reactor in LaPorte, TX.; following one day of operation, the filter system was plugged with catalyst fines, preventing catalyst–wax separation and forcing shutdown of the plant [173].
Thus, based on these three representative examples, it follows that which of the two attrition mechanisms predominates depends much more on material composition and type than on agglomerate strength. However, irrespective of mechanism, the rate of attrition is usually greater for the weaker material.
Figure 28.
Sedigraph particle size distribution for a United Catalysts, Inc. (UCI) Fischer-Tropsch catalyst (designated as UCI-LAPI-COMP-DRUMC), used previously in Department of Energy (DOE) pilot-plant tests. There is considerable particle breakdown and generation of fine particles after 15 min of ultrasonic irradiation. Reproduced from [163]. Copyright 1999, Elsevier. —□— 0 min; – –◊– –5 min; - -○- - 10 min; – – Δ – –15 min.
Figure 29 illustrates the large effect that catalyst preparation method can have on the attrition resistance of an Fe/Cu Fischer–Tropsch catalyst [174]. This catalyst, prepared by precipitation, undergoes severe attrition during a 25-min treatment with ultrasonic radiation; indeed, the mass fraction finer than 0.1–5 μm increases from 0 to 65%. However, after a spray drying treatment of the same catalyst, an increase of only 0 to 10% in the same fractions is evident.
Figure 29.
Sedigraph particle size distributions of a precipitated Fe–Cu catalyst, as-prepared and after spray-drying. The as-prepared catalyst (a) is weak and breaks down easily after 25 min of ultrasonic irradiation, while spray-drying (b) improves its attrition resistance. Reproduced from [174]. Copyright 2000, Elsevier. —□— 0 min; ♦ 5 min; —○— 10 min; —Δ— 15 min; —⊠— 20 min; — ⊕— 25 min.
In their review of attrition and attrition test methods, Bemrose and Bridgewater [175] discuss how attrition varies with reactor type, e.g., involves mainly particle–wall impacts in moving pellet bed reactors and particle–particle impacts in fluidized-bed reactors of high fluid velocity. In fact, jet attrition of catalyst particles in a gas fluidized-bed involving principally abrasion due to collision of high-velocity particles has been modeled in some detail [172,176]. Thus, given such important differences in attrition mechanism, realistic attrition test methods should attempt to model reactor operation as closely as possible. In addition, the ideal test would require only a small catalyst sample, a simple, inexpensive apparatus, and a few minutes to complete the test. Relatively quick, inexpensive single-particle crushing tests have been devised [175]; however, properties of a single particle are rarely representative of those for the bed; moreover, it is difficult to relate the results of this crushing test to the actual abrasion process. Realistic tests have been devised for two reactor types involving a moving catalyst, i.e., an air-jet test for fluidized-bed catalysts [177,178], and a rotating drum apparatus for moving-bed catalysts [179]; however, the air-jet test requires a large quantity (e.g., 50 g) of catalyst, an expensive apparatus, and about 20 h to run. In the past decade, a new jet-cup test has been developed for testing of fluidized-bed catalysts [177,178], which requires only a 5-g sample and about 1 h to complete; comparisons of results for the jet-cup and air-jet tests indicate that the two tests give comparable results [177,178]. Nevertheless, the mechanisms for the two tests are different, i.e., the air-jet (fluid-bed) test is abrasion- (erosion-) dominant, while the jet-cup test includes both abrasion and fracture mechanisms [178]. A 30-min, 10-g ultrasonic attrition test based on cavitation has also been developed in the past decade [168,174,180]; while it likewise involves both abrasion and fracture mechanisms, the results appear to correlate with other methods. For example, particle size distributions for the same Co/silica catalyst after ultrasonic, jet-cup, and laboratory-scale, slurry-bed column reactor (SBCR) tests are very similar (see Figure 30), indicating that both fracture and abrasion mechanisms operate in the small-scale SBCR. Moreover, the good agreement among the three methods suggests that both the jet-cup and ultrasonic tests may provide data representative of the attrition process in laboratory-scale SBCR reactors. It is evident that these two small-scale methods are especially useful for screening of a series of catalysts to determine relative strength.
Nevertheless, the more realistic large-scale tests are probably needed for accurately determining design attrition rates of a commercial catalyst to be used in a full-scale process. The observation that attrition of a fluid catalytic cracking (FCC) catalyst initially involves fracture of weak agglomerates followed by abrasion of strong agglomerates emphasizes the need to collect and analyze the particle size distribution of attrited fines as a function of time in order to define which mechanism (or mechanisms) operates at startup as well as in the steady-state process. Because the mechanism may be time dependent, rapid, small-scale tests may produce misleading results.
While realistic laboratory-scale tests have been developed for simulating attrition in large moving-bed and fluidized-bed reactors, no such laboratory test has been developed and demonstrated yet for simulation of large-scale SBCR reactors, although recent research has focused on the development of such tests. For example, in laboratory-scale, SBCR tests of supported cobalt catalysts over several days [180], the attrition resistance decreases in the order Co/Al2O3 > Co/SiO2 > Co/TiO2 (especially the anatase form underwent attrition at a high rate); attrition resistance was observed to increase with increasing cobalt loading from 10 to 40 wt%.
Figure 30.
Particle size distributions of Co/SiO2 catalyst. Adapted from [178]. ––––– Ultrasound 250 W (>10 μm);- - - jet cup L/min (>10 μm); —●— Co/SiO2 after SBCR;  Co/SiO2 fresh.
Co/SiO2 fresh.
 Co/SiO2 fresh.
Co/SiO2 fresh.
2.5.5. Implications of Mechanistic Knowledge of Attrition for Catalyst Design
The understanding of mechanisms important in attrition of catalyst supports and catalysts, the relationship between strength and attrition rate for a given material, and test data can be used to great advantage in the design of attrition resistant catalysts. Several alternatives follow from the previous discussion for increasing attrition resistance: (1) increasing aggregate/agglomerate strength by means of advanced preparation methods, e.g., sol–gel granulation, spray drying, and carefully controlled precipitation methods (see Table 15 and Figure 29 for examples), (2) adding binders to improve strength and toughness, e.g., the addition of a polyvinylpyrrolidone binder to agglomerates of quartz sand increases agglomerate strength from 0.1 to 3 MPa [181], (3) coating aggregates with a porous but very strong material such as ZrO2, e.g., embedding a fluidized-bed catalyst for partial oxidation of n-butane to maleic anhydride in a strong, amorphous matrix of zirconium hydrogen phosphate significantly improves its attrition resistance [182], and (4) chemical or thermal tempering of agglomerates to introduce compressive stresses that increase strength and attrition resistance, e.g., heating and cooling particles rapidly by passing them through a low-residence-time, high-temperature furnace to harden the agglomerate exterior, while preventing significant sintering of or phase changes in the porous interior. The subject of preventing mechanical degradation and other forms of catalyst deactivation is addressed in greater detail under Prevention of Catalyst Decay.
2.6. Summary of Deactivation Mechanisms for Solid Catalysts
Causes of solid (heterogeneous) catalyst deactivation are basically threefold: (1) chemical, (2) mechanical, and (3) thermal. Mechanisms of heterogeneous catalyst deactivation can be classified into five general areas: (1) chemical degradation including volatilization and leaching, (2) fouling, (3) mechanical degradation, (4) poisoning, and (5) thermal degradation. Poisoning and thermal degradation are generally slow processes, while fouling and some forms of chemical and mechanical degradation can lead to rapid, catastrophic catalyst failure. Some forms of poisoning and many forms of fouling are reversible; hence, reversibly poisoned or fouled catalysts are relatively easily regenerated. On the other hand, chemical, mechanical, and thermal forms of catalyst degradation are rarely reversible.
3. Prevention of Catalyst Decay
It is often easier to prevent rather than cure catalyst deactivation. Many poisons and foulants can be removed from feeds using guard beds, scrubbers, and/or filters. Fouling, thermal degradation, and chemical degradation can be minimized through careful control of process conditions, e.g., lowering temperature to lower sintering rate or adding steam, oxygen, or hydrogen to the feed to gasify carbon or coke-forming precursors. Mechanical degradation can be minimized by careful choice of carrier materials, coatings, and/or catalyst particle forming methods.
While treating or preventing catalyst deactivation is facilitated by an understanding of the mechanisms, additional perspectives are provided by examining the route by which each of the mechanisms causes loss of catalytic activity, i.e., how it influences reaction rate [109]. Thus, catalytic activity can be defined in terms of the observed site-based rate constant kobs, which is equal to the product of the active site density σ (number of sites per area of surface), the site-based intrinsic rate constant kintr, and the effectiveness factor η, i.e.,
Loss of catalytic activity may be due to a decrease in any of the three factors in Equation 4, whose product leads to kobs. Thus, catalyst deactivation can be caused by (1) a decrease in the site density σ, (2) a decrease in intrinsic activity (i.e., decrease in kintr), and/or (3) lowered access of reactants to active sites (decrease in η). Poisoning, for example, leads to a loss of active sites, i.e., σ = σ0(1 − α), where α is the fraction of sites poisoned; sintering causes loss of active sites through crystallite growth and reduction of active surface area. Fouling can cause both loss of active sites due to blocking of surface sites as well as plugging of pores, causing a decrease in the effectiveness η. Moreover, poisoning, as discussed earlier, can also lead to a decrease in intrinsic activity by influencing the electronic structure of neighboring atoms. Thus, each of the deactivation mechanisms affects one or more of the factors comprising observed activity (see Table 16); all of the mechanisms, however, can effect a decrease in the number of catalytic sites.

Table 16.
How Deactivation Mechanisms Affect the Rate of a Catalyzed Reaction and the Rapidity and Reversibility of Deactivation Process.
| Effects on reaction rate | |||||
|---|---|---|---|---|---|
| Deactivation mechanism | Decrease in number of active sites | Decrease in intrinisic activity (kintr) | Decrease in effectiveness factor (η) | Deactivation process | |
| Fast or slow a | Reversible | ||||
| Chemical degradation | × | × | × b,c | Varies | No |
| Fouling | × | × | - | Fast | Yes |
| Mechanical degradation | × | - | - | Varies | No |
| Poisoning | × | × | - | Slow | Usually |
| Thermal degradation/Sintering | × | × b,d | × b,e | Slow | Sometimes |
| Vaporization/leaching | × | × b,f | - | Fast | Sometimes |
a Generally; b In some cases; c Chemical degradation can cause breakdown of support, pore plugging, and loss of porosity; d If the reaction is structure-sensitive, sintering could either increase or decrease intrinsic activity; e Sintering of the support may cause support collapse and loss of porosity; it may also increase average pore diameter. f Leaching of aluminum or other cations from zeolites can cause buildup of aluminum or other oxides in zeolite pores.
3.1. General Principles of Prevention
The age-old adage that says “an ounce of prevention is worth a pound of cure” applies well to the deactivation of catalysts in many industrial processes. The catalyst inventory for a large plant may entail a capital investment of tens of millions of dollars. In such large-scale processes, the economic return on this investment may depend on the catalyst remaining effective over a period of up to 3–5 years. This is particularly true of those processes involving irreversible or only partially reversible deactivation (e.g., sulfur poisoning or sintering). Some typical industrial catalysts, approximate catalyst lifetimes, and factors that determine their life are listed as examples in Table 17. It is evident that in many processes more than one mechanism limits catalyst life. Moreover, there is a wide variation in catalyst lifetimes among different processes, i.e., from 10−6 to 15 years. While there is clearly greater interest in extending catalyst lifetimes in processes where life is short, it should be emphasized that great care must be exercised in protecting the catalyst in any process from process upsets (e.g., temperature runaway, short-term exposure to impure feeds, or changes in reactant composition) that might reduce typical catalyst life by orders of magnitude, e.g., from years to hours.

Table 17.
Typical Lifetimes and Factors Determining the Life of Some Important Industrial Catalysts a.
| Reaction | Operating conditions | Catalyst | Typical life (years) | Process affecting life of catalyst charge | Catalyst property affected |
|---|---|---|---|---|---|
| Ammonia synthesis N2 + 3 H2→2 NH3 | 450–470 °C 200–300 atm | Fe with promoters (K2O) and stabilizer (Al2O3) | 10–15 | Slow sintering | Activity |
| Methanation (ammonia and hydrogen plants) CO/CO2 + H2→CH4 + H2O | 250–350 °C 30 atm | Supported nickel | 5–10 | Slow poisoning by S, As, K2CO3 from plant upsets | Activity and pore blockage |
| Acetylene hydrogenation (“front end”) C2H2 + H2→C2H4 | 30–150 °C 20–30 atm | Supported palladium | 5–10 | Slow sintering | Activity/selectivity and temperature |
| Sulfuric acid manufacturing 2 SO2 + O2→2 SO3 | 420–600 °C 1 atm | Vanadium and potassium sulfates on silica | 5–10 | Inactive compound formation; pellet fracture; plugging by dust | Activity, pressure drop, and mass transfer |
| Methanol synthesis CO + 2 H2→CH3OH | 200–300 °C 50–100 atm | Copper on zinc and aluminum oxides | 2–5 | Slow sintering; poisoning by S, Cl, and carbonyls | Activity |
| Low temperature water gas shift CO + H2O→CO2 + H2 | 200–250 °C 10–30 atm | Copper on zinc and aluminum oxides | 2–4 | Slow poisoning and accelerated sintering by poisons | Activity |
| Hydrocarbon hydrodesulfurization R2S + 2 H2→H2S + R2 | 300–400 °C 30 atm | Cobalt and molybdenum sulfides on aluminum oxide | 1–10 | Slow coking, poisoning by metal deposits in residuum | Activity, mass transfer, and pressure drop |
| High temperature water gas shift CO + H2O→H2 + CO2 | 350–500 °C 20–30 atm | Fe3O4 and chromia | 1–4 | Slow sintering, pellet breakage due to steam | Activity and pressure drop |
| Steam reforming, natural gas CH4 + H2O→CO + 3 H2 | 500–850 °C 30 atm | Nickel on calcium aluminate or α-alumina | 1–3 | Sintering, sulfur-poisoning, carbon formation, and pellet breakage due to plant upsets | Activity and pressure drop |
| Ethylene partial oxidation 2 C2H4 + O2→2 C2H4O | 200–270 °C 10–20 atm | Silver on α-alumina with alkali metal promoters | 1–3 | Slow sintering, poisoning by Cl, S | Activity and selectivity |
| Butane oxidation to maleic anhydride C4H10 + 3.5 O2→C4H2O3 + 4 H2O | 400–520 °C 1–3 atm | Vanadium phosphorus oxide with transition metal additives | 1–2 | Loss of P; attrition or pellet breakage; S, Cl poisoning | Activity and selectivity |
| Reduction of aldehydes to alcohols RCHO + H2→RCH2OH | 220–270 °C 100–300 atm | Copper on zinc oxide | 0.5–1 | Slow sintering, pellet breakage (depends on feedstock) | Activity or pressure drop |
| Ammonia oxidation 2 NH3 + 5/2 O2→2 NO + 3 H2O | 800–900 °C 1–10 atm | Pt–Rh alloy gauze | 0.1–0.5 | Surface roughness, loss of platinum | Selectivity, fouling by Fe |
| Oxychlorination of ethylene to ethylene dichloride 2 C2H4 + 4 HCl + O2 →2 C2H4Cl2 + 2 H2O | 230–270 °C 1–10 atm | Copper chlorides on alumina (fluidized bed) | 0.2–0.5 | Loss by attrition and other causes resulting from plant upsets | Fluidized state and activity |
| Catalytic hydrocarbon reforming | 460–525 °C 8–50 atm | Platinum alloys on treated alumina | 0.01–0.5 | Coking, frequent regeneration | Activity and mass transfer |
| Catalytic cracking of oils | 500–560 °C 2–3 atm | Synthetic zeolites (fluidized bed) | 0.000002 | Very rapid coking, continuous regeneration | Activity and mass transfer |
Adapted from Ref. [9].
While complete elimination of catalyst deactivation is not possible, the rate of damage can be minimized in many cases through understanding of the mechanisms, thereby enabling control of the deactivation process, i.e., prevention is possible through control of catalyst properties, process conditions (i.e., temperatures, pressures), feedstock impurities, methods of contacting, and process design. Figure 31 illustrates general approaches to eliminating or moderating deactivation through modifications in catalyst and/or process. Examples of how deactivation can be prevented are discussed below in connection with the most important causes of deactivation: chemical degradation, fouling by coke and carbon, poisoning, sintering, and mechanical degradation. Principles for preventing deactivation by these mechanisms are summarized in Table 18, while representative results from studies focusing on prevention or minimization of catalyst deactivation are summarized in Table 19.
Figure 31.
Approaches to eliminating catalyst deactivation.

Table 18.
Methods for Preventing Catalyst Decay [8,41].
| Basic mechanism | Problem | Cause | Methods of minimization |
|---|---|---|---|
| Chemical degradation | Oxidation of metal catalysts to inactive oxides | Oxidation of metal by contaminant O2 or reactant/product water |
|
| Transformation of active phase to stable, inactive phase | Solid-state reaction of active phase with support or promoters |
| |
| Overreduction of active oxide phases |
| ||
| Fouling by coke or carbon | Loss of catalytic surface sites due to formation of carbon or coke films | Free radical reactions in gas phase |
|
| Free radical reactions at reactor walls |
| ||
| Formation and growth on metal surfaces |
| ||
| Fouling by coke or carbon (cont.) | Loss of catalytic surface sites due to formation of carbon or coke films | Formation and growth on metal oxides, sulfides |
|
| Loss of catalyst effectiveness; plugging of pores; destruction of catalyst | Formation of gas phase coke, vermicular carbons, and liquid or solid cokes in massive quantities |
| |
| Hot spots in pellet or bed |
| ||
| Mechanical failure | Crushing of granules, pellets, or monoliths in a fixed bed | Brittle fracture due to a mechanical load |
|
| Attrition and/or erosion in fixed or moving beds | Abrasion of catalyst coatings or particles due to mechanical, thermal, or chemical stresses |
| |
| Poisoning | Loss of catalytic surface sites | Blockage of sites by strong adsorption of impurity |
|
| Thermal degradation, sintering | Loss of metal area | Metal particle or subparticle migration at high temperatures |
|
| Loss of support area | Crystallization and/or structural modification or collapse | Same as for avoiding loss of metal area |

Table 19.
Representative Results from Studies Focusing on Prevention/Minimization of Catalyst Deactivation.
| Deactivation mechanism Process/Reaction Catalyst | Problem/cause | Method(s) of minimization | Ref. | ||
|---|---|---|---|---|---|
| Chemical degradation | |||||
| Auto emissions control Pt– or Pd–Rh/Al2O3 | In three-way catalyst, Rh is very active for NO reduction, but it forms a solid solution with Al2O3 that has no activity and alloys with Pt or Pd that reduce its activity | Place Rh in a separate catalyst layer from Pt or Pd to prevent alloying; support Rh on ZrO2, which is a noninteracting support for Rh. In general, multilayer strategies (up to 6 layers) are used to prevent undesirable interactions between different components of the catalyst | [183,184,185] | ||
| Fischer–Tropsch synthesis Co supported on Al2O3, SiO2, TiO2, and Fe/Cu/K/SiO2 | Oxidation of active Co metal crystallites to inactive Co oxides, aluminates, and silicates and of active iron carbides to inactive Fe3O4 or Fe3C in the presence of high pressure steam at high conversion |
| [8,126,186,187] | ||
| Partial oxidation of isobutene to methacrolein Fe2(MoO4)3, Mo12BixCeyOz | Overreduction of the catalyst during reaction leads to activity decrease |
| [154,156,188] | ||
| Steam reforming and steam-oxygen conversion of propane Pd/Al2O3 | In the absence of steam, PdO is reduced to less active, less thermally stable Pd metal | Adding steam to the reactants inhibits oxidation of propane at lower reaction temperatures while preventing reduction of PdO at higher temperatures (up to 700–900 °C) | [189] | ||
| Fouling by coke, carbon | |||||
| Alkene oligomerization Zeolites, esp. ZSM-5, –22, –23, beta-zeolite, ferrierite | Catalyst fouling by condensation of heavy oligomers to coke |
| [190,191,192] | ||
| Alkylation of isoparaffins on solid catalystsSulfated zirconia, USY a, Nafion | Rapid catalyst deactivation due to coke formation; unacceptable product quality, and thermal degradation of catalyst during regeneration |
| [193,194] | ||
| Catalytic reforming of naphtha Pt/Al2O3 promoted with Re, Sn, Ge, or Ir | Poisoning and fouling by coke produced by condensation of aromatics and olefins |
| [8,195,196,197,198] | ||
| Dehydrogenation of propane and butane Cr2O3/Al2O3, Cr2O3/ZrO2, FeO/K/MgO, Pt/Al2O3, Pt–Sn/Al2O3, Pt–Sn/KL-zeolite | Catalyst activity is low owing to equilibrium limitations and buildup of product H2; rapid loss of activity occurs owing to coke formation |
| [8,199,200,201,202,203] | ||
| Hydrocracking of heavy naphthaCoMo, NiW, MoW on Al2O3 or SiO2–Al2O3; Pt or Pd on Y-zeolite, mordenite or ZSM-5 | Loss of activity due to poisoning of sites and blocking of small zeolite pores by coke |
| [8,198,204] | ||
| Methane reforming CO2/Co/SiO2, Pt/SiO2, Pt/ZrO2, MgO-supported noble metals, NiO·MgO solid solution | High rates of carbon formation, which rapidly deactivate catalyst |
| [205,206,207,208] | ||
| Methanol to olefins or gasoline Silica–alumina, Y-zeolite, ZSM-5, other zeolites, and aluminophosphate molecular sieves | Severe coking and deactivation of silica–alumina and Y-zeolite catalysts observed during high conversions of MeOH; also substantial coking of ZSM-5, other zeolites, and alumino-phosphate molecular sieves |
| [209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236] | ||
| Steam reforming of light hydrocarbons or naphtha Ni on MgO, MgAl2O4 or CaAl2O4 promoted with S, Cu, or Au | High rates of carbon and coke formation, which rapidly deactivate catalyst |
| [8,60,70,71] | ||
| Poisoning | |||||
| Auto emissions control Pt–Rh/Al2O3 or Pd/Al2O3 | Poisoning of noble metal catalyst by P and S compounds and large hydrocarbons from lube oil | Optimize pore structure of alumina, deposit noble metals in layers below the support surface, or provide a diffusion barrier coating of zeolite or alumina; these measures prevent access of large poison molecules to catalyst layer | [18,212] | ||
| Fischer–Tropsch synthesis Co/Al2O3 | 100 ppb of HCN and NH3 poisons cobalt slurry catalyst within 4 days | Remove HCN and NH3 to less than 50 ppb total by (1) catalytic hydrolysis of HCN to NH3, followed by scrubbing with water or (2) guard bed containing acidic solid absorbent | [213] | ||
| Fluidized catalytic cracking (FCC) USY or REO-Y b in silica matrix |
|
| [8,198] | ||
| Poisoning | |||||
| Hydrotreating of gas oil; deep HDS Al2O3- supported CoMo, noble metals | Noble metal hydrogenation and high-activity HDS catalysts are poisoned by H2S |
| [198] | ||
| Hydrotreating of residuum Al2O3- supported Mo and CoMo | Pore-mouth poisoning and blockage by Ni, V, and Fe sulfides present in feed as organometallics |
| [8,214] | ||
| Thermal degradation | |||||
| Auto emissions control PdO/δ- or θ-Al2O3 doped with BaO, La2O3, Pr2O3, CeO2, and ZrO2 | In close-couple, fast-warm-up converters, exhaust temperatures reach 1000–1100 °C; conventional Pt–Rh/γ-Al2O3 catalysts sinter rapidly under these conditions; CeO2 used as oxygen storage material also sinters rapidly |
| [8,215,216,217,218,219,220,221,222] | ||
| Catalytic combustion of methane and LNG PdO/La2O3, Pr2O3, CeO2, and ZrO2 | Reaction temperatures ranging up to 1400 °C cause rapid sintering of most catalytic materials. Conversion above 800 °C of PdO to Pd metal is followed by rapid sintering of Pd and loss of activity |
| [8,224,225,226,227] | ||
| Dehydrogenation of butene to butadiene Cr2O3/Al2O3, Pt–Sn/Al2O3 | Permanent loss of catalytic activity by sintering at high reaction temperatures (550–650 °C) | Optimize the operation of staged catalytic reactors (cycle time between regenerations, temperature, and composition of the feed as variables) while placing a limit on the upper temperature | [8,228] | ||
| Fischer–Tropsch synthesis Co/Al2O3, Co/SiO2, Co/TiO2 | Sintering in hot spots and loss of hydrocarbon selectivity at higher reaction temperatures due to highly exothermic reaction |
| [186,229] | ||
| Fluid catalytic cracking (FCC) USY, REO-Y | Dealumination and destruction of zeolite crystallinity and loss of surface area/pore volume during high-temperature (650–760 °C, 3 atm) regeneration in steam/air |
| [8,198] | ||
| Methane steam reforming Ni on MgAl2O4 or CaAl2O4 | Sintering of Ni and support during high-temperature reaction (800–1000 °C) in high-pressure steam (20 atm) |
| [8,70] | ||
| Mechanical degradation | |||||
| Partial oxidation of n-butane to maleic anhydride VPO | Attrition in fluidized-bed process | Imbed catalyst particles in a strong, amorphous matrix of zirconium hydrogen phosphate | [182] | ||
| Fischer–Tropsch synthesis in a bubble-column slurry reactor Co/Al2O3, Co/SiO2, Co/TiO2 | Attrition in bubble column slurry reactor |
| [230,231,232,233,234,235] | ||
a USY: ultrastable Y-zeolite. b REO-Y: rare-earth exchanged Y-zeolite.
3.2. Prevention of Chemical Degradation (by Vapor–Solid and Solid–Solid Reactions)
The most serious problems of oxidation of metal catalysts, overreduction of oxide catalysts, and reaction of the active catalytic phase with carrier or promoter, can be minimized or prevented by careful catalyst and process design (as enumerated in Table 18 and illustrated in Table 19). For example, the loss of Rh due to solid-state reaction with alumina in the automotive three-way catalyst can be prevented by supporting Rh on ZrO2 in a separate layer from Pt and/or Pd on alumina [215,216,217,218,219,220,221,222] In Fischer–Tropsch synthesis, the oxidation of the active cobalt phase in supported cobalt catalysts to inactive oxides, aluminates, and silicates can be minimized by employing a two- or three-stage process in which product steam is moderated in the first stage by limiting conversion and in subsequent states by interstage removal of water [223] It can also be moderated by addition of noble metal promoters that facilitate and maintain high reducibility of the cobalt and by coating the alumina or silica support with materials such as ZrO2 that are less likely to react with cobalt to form inactive phases.
3.3. Prevention of Fouling by Coke and Carbon
Rostrup-Nielsen and Trimm [57], Trimm [59], and Bartholomew [60] have discussed principles and methods for avoiding coke and carbon formation. General methods of preventing coke or carbon formation are summarized in Table 18. Most of these are based on one important fundamental principle: carbon or coke results from a balance between the reactions that produce atomic carbon or coke precursors and the reactions of these species with H2, H2O, or O2 that remove them from the surface. If the conditions favor formation over gasification, these species accumulate on the surface and react further to form less active forms of carbon or coke, which either coat the surface with an inactive film or plug the pores, causing loss of catalyst effectiveness, pore plugging, or even destruction of the carrier matrix.
Methods to lower rates of formation of carbon or coke precursors relative to their rates of gasification vary with the mechanism of formation (i.e., gas, surface, or bulk phase) and the nature of the active catalytic phase (e.g., metal or oxide). For example, gas phase formation can be minimized by choosing reaction conditions that minimize the formation of free radicals, by using free-radical traps, by introducing gasifying agents (e.g., H2, H2O) or gas diluents, and by minimizing the void space available for homogeneous reaction. Similarly, the formation and growth of carbon or coke species on metal surfaces is minimized by choosing reaction conditions that minimize the formation of atomic carbon or coke precursors and by introducing gasifying agents. Selective membranes or supercritical conditions can also be used to lower the gas-phase and surface concentrations of coke precursors. Since carbon or coke formation on metals apparently requires a critical ensemble of surface metal atoms and/or dissolution of carbon into the bulk metal, introduction of modifiers that change ensemble sizes (e.g., Cu or S in Ni or Ru) or that lower the solubility of carbon (e.g., Pt in Ni) can be effective in minimizing these forms of deactivation.
For example, in a detailed STM study of submonolayers of Au on Ni(111), Besenbacher and co-workers [71] found that the electron density of Ni atoms in the vicinity of Au atoms was increased; from density functional theory (DFT) calculations they concluded that the strength of carbon adsorption (and hence the tendency to form graphite) was decreased on next-nearest neighbor Ni atoms; from studies of the effects of S adsorption on methane activation and graphite formation on pure Ni, they were able to infer that the ensemble size needed for methane dissociation is smaller than that for graphite formation. These fundamental insights were used in the design of a 0.3% Au-promoted 16% Ni/MgAl2O4 catalyst that loses no activity over 4000 h during steam reforming of n-butane, while the corresponding unpromoted Ni catalyst loses about 5% of its initial activity (see Figure 32). In contrast to the moderating effects of noble metal additives, addition of 0.5% Sn to cobalt substantially increases the rate of carbon filament formation from ethylene [72], an effect desirable in the commercial production of carbon filament fibers.
Figure 32.
Conversion of n-butane as a function of time during steam reforming in a 3% n–butane–7% hydrogen–3% water in helium mixture at a space velocity of 1.2 h−1. The dashed curve shows the n-butane conversion for the Ni catalyst (16.8% Ni) and the solid curve for the Au/Ni catalyst (16.4% Ni/0.3% Au). Reproduced from [71]. Copyright 1998, American Association for the Advancement of Science.
Coke deposition on oxide or sulfide catalysts occurs mainly on strongly acidic sites; accordingly the rate of coking can be lowered by decreasing the acidity of the support. For example, silanation of HY and HZSM-5 zeolites decreases their activities but improves catalyst life [236]. In steam reforming, certain catalyst additives, e.g., MgO, K2O, or U3O8, facilitate H2O or CO2 adsorption and dissociation to oxygen atoms, which in turn gasify coke precursors [8,60,70].
Similarly, for steam reforming catalysts used for light alcohol and oxygenate conversion, the addition of partially reducible oxides, like ceria, in nickel perovskite (La1−xCexNiO3) catalysts [237] or as a support for a cobalt catalyst [238], reduce the rate of carbon deposition. Alternatively, the reaction atmosphere may be modified to increase the gasification rate by adding oxidizing reactants (e.g., O2 and/or CO2) to reduce the rate of coke deposition [63]. This process is often described as autothermal reforming because it tends to balance the endothermic steam reforming reactions with exothermic reactions that make the process thermally neutral.
As in the case of poisoning (see below), there are certain reactor bed or catalyst geometries that minimize the effects of coking on the reaction. For example, specific film-mass transport or pore diffusion regimes favor coke or carbon deposition on either the outside or inside of the catalyst pellet [239,240]. Choosing supports with relatively large pores minimizes pore plugging; choice of large-diameter, mechanically-strong pellets avoids or delays reactor plugging. However, in view of the rapidity at which coke and carbon can deposit on, plug, and even destroy catalyst particles, the importance of preventing the onset of such formation cannot be overemphasized.
Reforming of naphtha provides an interesting case study of catalyst and process designs to avoid deactivation by coking [8,196,197,198,241]. The classical Pt/Al2O3 catalyst is bifunctional; that is, the metal catalyzes dehydrogenation, while the acid sites of the Al2O3 catalyze isomerization and hydrocracking. Together, the two functions catalyze dehydrocylization and aromatization. Addition of Re, Sn, or Ge, to Pt and sulfiding of the Pt–Re catalyst substantially reduce coke formation by diluting large Pt ensembles that would otherwise produce large amounts of coke, while addition of Sn and Ir improves selectivity for dehydrogenation relative to hydrogenolysis, the latter of which leads to coke formation. Naphtha reforming processes are designed for (1) high enough H2 pressure to favor gasification of coke precursors while minimizing hydrocracking, (2) maintenance of Cl and S contents throughout the bed to ensure optimum acidity and coke levels, and (3) low enough overall pressure to thermodynamically and kinetically favor dehydrogenation and dehydrocylization. Accordingly, optimal process conditions are a compromise between case 1 and case 3. The above-mentioned improvements in catalyst technologies, especially resistance to coking, have enabled important process improvements, such as optimal operation at lower pressure; thus, processes have evolved over the past two to three decades from conventional fixed-bed reactors at high pressure (35 bar) using nonregenerative Pt catalysts to low pressure (3.5 bar), slowly moving-bed, continuously regenerated units with highly selective Pt/Sn catalysts, resulting in substantial economic benefits [198,241].
3.4. Prevention of Poisoning
Since poisoning is generally due to strong adsorption of feed impurities and since poisoned catalysts are generally difficult or impossible to regenerate, it is best prevented by removal of impurities from the feed to levels that will enable the catalyst to operate at its optimal lifetime. For example, it is necessary to lower the feed concentration of sulfur compounds in conventional methanation and Fischer–Tropsch processes involving base metal catalysts to less than 0.1 ppm in order to ensure a catalyst lifetime of 1–2 years. This is typically accomplished using a guard bed of porous ZnO at about 200 °C. In cracking or hydrocracking reactions on oxide catalysts, it is important to remove strongly basic compounds, such as ammonia, amines, and pyridines, from the feed; ammonia in some feedstocks, for example, can be removed by aqueous scrubbing. The poisoning of catalysts by metal impurities can be moderated by selective poisoning of the unwanted metal. For example, in catalytic cracking of nickel-containing petroleum feedstocks, nickel sites, which would otherwise produce copious amounts of coke, are selectively poisoned by antimony [242]. The poisoning of hydrotreating catalysts by nickel and vanadium metals can be minimized by (1) using a guard bed of inexpensive Mo catalyst or graded catalyst bed with inexpensive, low-activity Mo at the top (bed entrance) and expensive, high-activity catalyst at the bottom (see Figure 33) and (2) by depositing coke prior to the metals, since these metal deposits can be physically removed from the catalyst during regeneration [243].
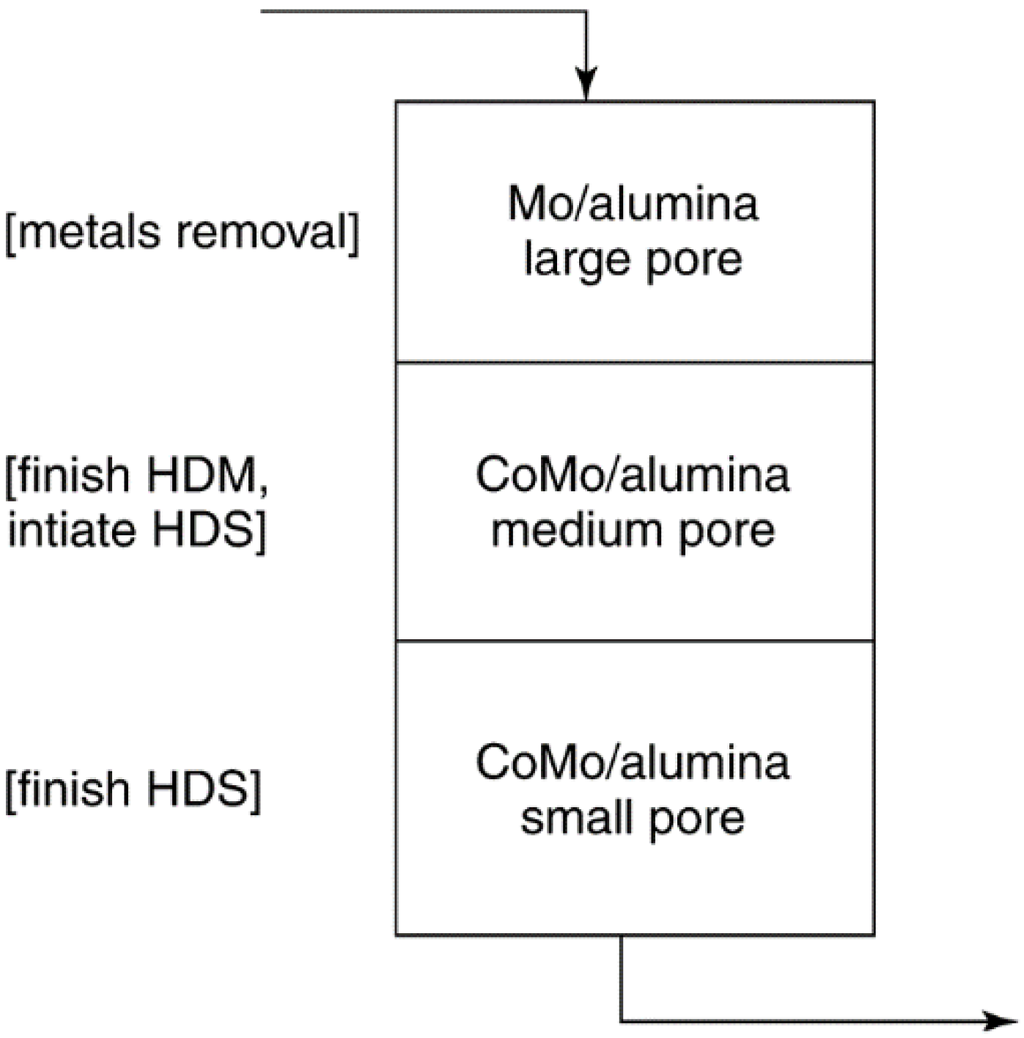
Figure 33.
Staged reactor system with decreasing pore size strategy for hydrodemetalization (HDM)/hydrodesulfurization (HDS) of residuum. Reproduced from [214]. Copyright 1993, Marcel Dekker.
It may be possible to lower the rate of poisoning through careful choice of reaction conditions that lower the strength of poison adsorption [60] or by choosing mass-transfer-limiting regimes that limit deposits to the outer shell of the catalyst pellet, while the main reaction occurs uninterrupted on the interior of the pellet [239]. The manner in which the active catalytic material is deposited on a pellet (e.g., uniformly or in an eggshell or egg yolk pattern) can significantly influence the life of the catalyst [17,244].
An example of reducing catalyst poisoning (and oxidation) through process design has been reported in a process patent for staged hydrocarbon synthesis via the Fischer–Tropsch reaction [245]. While cobalt catalysts are favored because of their high activities and while it is desirable to achieve high conversions of CO in the process, the one-pass conversion for cobalt is limited by (1) its tendency to be oxidized at high partial pressures of product water observed at high CO conversions and (2) its tendency under these conditions to form the oxygenated products (e.g., alcohols and aldehydes) that poison or suppress its synthesis activity. One alternative is to separate products and recycle the unused CO and H2, but this requires costly recompression and separation of the oxygenates. Costly separation and/or poisoning can be prevented by operating a first-stage reactor containing a cobalt catalyst to a moderately high conversion followed by reacting the remaining CO and H2 in a second stage to above 95% conversion on an iron catalyst, which is not sensitive to the oxygenates and which shifts some of the product water to H2 and CO2, thus minimizing its hydrothermal degradation.
An example of reducing catalyst poisoning through catalyst design occurs in abatement of emissions for automotive and motorcycle engines [18,212]. Application of an alumina or zeolite coating, or alternatively preparing the active phase in a sublayer, provides a diffusion barrier that prevents or slows the access of poisons from the fuel or oil (e.g., phosphorus and/or zinc from lubricating oil or corrosion products) to the catalyst surface. The principle is to optimize the pore size distribution of the diffusion barrier to provide access to the catalytic phase of relatively small hydrocarbon, CO, NO, and O2 molecules, while preventing access of larger molecules, such as from lubricating oil and/or particulates.
Finally, another strategy that has been employed to reduce the impact of poisoning, particularly for sulfur, is the inclusion of traps or “getters” as part of the catalyst. These species, including rare earth oxides of thulium (Tm) [50] or Ce [51] and simple zinc oxide, essentially act as sacrificial stoichiometric reactants to protect the active metal by preferentially adsorbing the poison. These traps can extend the catalyst life, but because they are not catalytic as they perform, they are necessarily temporary agents if the poison remains in the feed to the process.
3.5. Prevention of Sintering
Since most sintering processes are irreversible or are reversed only with great difficulty, it is important to choose reaction conditions and catalyst properties that avoid such problems. Metal growth is a highly activated process; thus, by choosing reaction temperatures lower than 0.3–0.5 times the melting point of the metal, rates of metal sintering can be greatly minimized. The same principle holds true in avoiding recrystallization of metal oxides, sulfides, and supports. Of course, one approach to lowering reaction temperature is to maximize activity and surface area of the active catalytic phase.
Although temperature is the most important variable in the sintering process, differences in reaction atmosphere can also influence the rate of sintering. Water vapor, in particular, accelerates the crystallization and structural modification of oxide supports. Accordingly, it is vital to minimize the concentration of water vapor in high temperature reactions on catalysts containing high surface area supports.
Besides lowering temperature and minimizing water vapor, it is possible to lower sintering rates through addition of thermal stabilizers to the catalyst. For example, the addition of higher melting noble metals (such as rhodium or ruthenium) to a base metal (such as nickel) increases the thermal stability of the base metal [106]. Addition of Ba, Zn, La, Si, and Mn oxide promoters improves the thermal stability of alumina [246]. These additives can affect product selectivity, but generally positively toward desired products, and always through extending the productive life of the catalysts [8].
Designing thermally stable catalysts is a particular challenge in high temperature reactions, such as automotive emissions control, ammonia oxidation, steam reforming, and catalytic combustion. The development of thermally stable automotive catalysts has received considerable attention, thus providing a wealth of scientific and technological information on catalyst design (e.g., Refs. [8,215,216,217,218,219,220,221,222]). The basic design principles are relatively simple: (1) utilize thermally and hydrothermally stable supports, e.g., high-temperature δ- or θ-aluminas or alkaline-earth or rare-earth oxides that form ultrastable spinels with γ-alumina; (2) use PdO rather than Pt or Pt–Rh for high temperature converters, since PdO is considerably more thermally stable in an oxidizing atmosphere because of its strong interaction with oxide supports; and (3) use multilayer strategies and/or diffusion barriers to prevent thermally induced solid-state reactions (e.g., formation of Rh aluminate) and to moderate the rate of highly exothermic CO and hydrocarbon oxidations. For example, a typical three-way automotive catalyst may contain alkaline-earth metal oxides (e.g., BaO) and rare-earth oxides (e.g., La2O3 and CeO2), for stabilizing Pt and/or PdO on alumina, and ZrO2 as a thermal stabilizer for the CeO2 (an oxygen storage material) and as a noninteracting support for Rh in a separate layer or in a separate phase in a composite layer (see Figure 34).
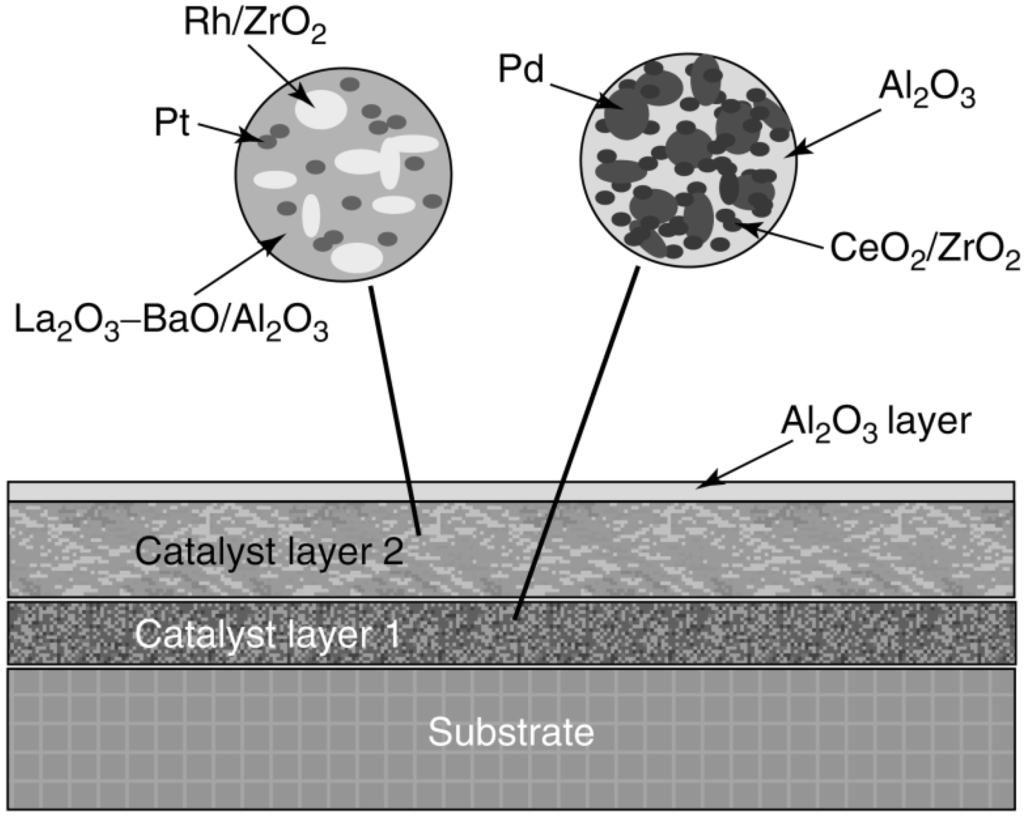
Figure 34.
Conceptual design (by C. H. Bartholomew) of an advanced three-way catalyst for auto emissions control. Catalyst layer 1 is wash-coated first onto the monolithic substrate and consists of (a) well-dispersed Pd, which serves to oxidize CO/hydrocarbons and to reduce NO and (b) CeO2/ZrO2 crystallites (in intimate contact with Pd), which store/release oxygen respectively, thereby improving the performance of the Pd. Catalyst layer 2 (added as a second wash coat) is a particle composite of Rh/ZrO2 (for NO reduction) and Pt/La2O3–BaO/Al2O3 (with high to moderately-high activity for oxidation of CO and hydrocarbons). A thin (50–80 μm) coat of Al2O3, deposited over catalyst layer 2, acts as a diffusion barrier to foulants and/or poisons. Both the Al2O3 layer and catalyst layer 2 protect the sulfur-sensitive components of catalyst layer 1 from poisoning by SO2.
Often, ideal metal dispersions require metal nanoparticles to be distributed closely together, but these particles are thermodynamically unstable on the surface and undergo rapid sintering, as described in Section 2.3 above. Recently, in an attempt to reduce sintering rates, researchers have attempted to stabilize the metal nanoparticles by first dispersing them on a support, encapsulating them in the same or another metal oxide, and then opening porosity to the particles (e.g., [247,248]). These approaches have met with varying degrees of success, but point to promising new areas of synthesis techniques that have the potential to reduce or to eliminate deactivation by sintering.
3.6. Prevention of Mechanical Degradation
While relatively few studies have focused on this topic, there are nevertheless principles that guide the design of processes and catalysts in preventing or minimizing mechanical degradation (see Table 19). In terms of catalyst design, it is important to (1) choose supports, support additives, and coatings that have high fracture toughness, (2) use preparation methods that favor strong bonding of primary particles and agglomerates in pellets and monolith coatings, (3) minimize (or rather optimize) porosity (thus maximizing density), and (4) use binders, such as carbon, to facilitate plastic deformation and thus protect against brittle fracture. Processes (and to some extent preparation procedures) should be designed to minimize (1) highly turbulent shear flows or cavitation that lead to fracture of particles or separation of coatings, (2) large thermal gradients or thermal cycling leading to thermal stresses, and (3) formation of chemical phases of substantially different densities or formation of carbon filaments leading to fracture of primary particles and agglomerates. Nevertheless, thermal or chemical tempering can be used in a controlled fashion to strengthen catalyst particles or agglomerates.
Examples of catalyst design to minimize attrition can be found in the recent scientific [230,231] and patent [232,233,234,235] literature focusing on the Fischer–Tropsch synthesis in slurry reactors. These studies indicate that (1) spray drying of particles improves their density and attrition resistance; (2) addition of silica and/or alumina into titania improves its attrition resistance, while addition of only 2000–3000 ppm of titania to γ-alumina improves alumina’s attrition resistance; and (3) preformed alumina spheres promoted with La2O3 provide greater attrition resistance relative to silica. Increasing attrition resistance is apparently correlated with increasing density [230,231,235]. According to Singleton and co-workers [235], attrition resistance of Co/Al2O3 is improved when the γ-alumina support is (1) formed from synthetic boehmite having a crystallite diameter of 4–5 nm and (2) is pretreated in acidic solution having a pH of 1–3 (see Figure 35); moreover, attrition resistance decreases in the order Co/Al2O3 > Co/SiO2 > Co/TiO2 and is greater for catalysts prepared by aqueous versus nonaqueous impregnation.
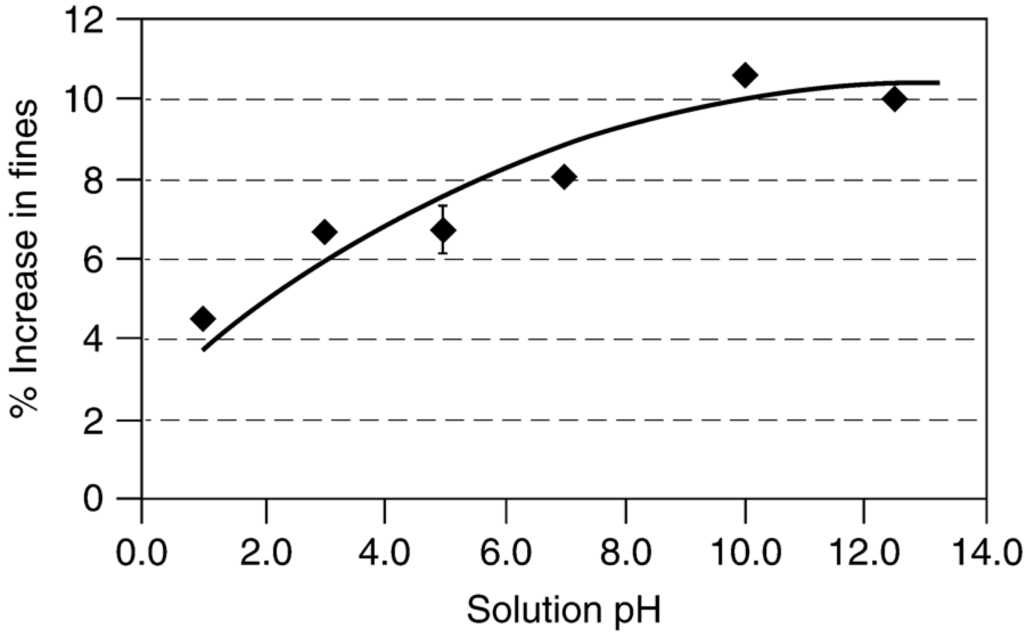
Figure 35.
Effect of solution pH on the attrition resistance of 70-μm γ-Al2O3 particles measured in jet-cup tests [235]. The % increase in fines is defined at the % increase of particles of less than 11 μm.
4. Regeneration of Deactivated Catalysts
Despite our best efforts to prevent it, the loss of catalytic activity in most processes is inevitable. When the activity has declined to a critical level, a choice must be made among four alternatives: (1) restore the activity of the catalyst, (2) use it for another application, (3) reclaim and recycle the important and/or expensive catalytic components, or (4) discard the catalyst. The first alternative (regeneration and reuse) is almost always preferred; catalyst disposal is usually the last resort, especially in view of environmental considerations.
The ability to reactivate a catalyst depends upon the reversibility of the deactivation process. For example, carbon and coke formation is relatively easily reversed through gasification with hydrogen, water, or oxygen. Sintering on the other hand is generally irreversible, although metal redispersion is possible under certain conditions in selected noble metal systems. Some poisons or foulants can be selectively removed by chemical washing, mechanical treatments, heat treatments, or oxidation [249,250]; others cannot be removed without further deactivating or destroying the catalyst.
The decision to regenerate/recycle or discard the entire catalyst depends largely on the rate of deactivation. If deactivation is very rapid, as in the coking of cracking catalysts, repeated or continuous regeneration becomes an economic necessity. Precious metals are almost always reclaimed where regeneration is not possible. Disposal of catalysts containing nonnoble heavy metals (e.g., Cr, Pb, or Sn) is environmentally problematic and should be a last resort; if disposal is necessary, it must be done with great care, probably at great cost. Accordingly, a choice to discard depends upon a combination of economic and legal factors [250]. Indeed, because of the scarcity of landfill space and an explosion of environmental legislation, both of which combine to make waste-disposal prohibitively expensive, there is a growing trend to regenerate or recycle spent catalysts [251,252]. A sizeable catalyst regeneration industry benefits petroleum refiners by helping to control catalyst costs and to limit liabilities [253,254]; it provides for ex situ regeneration of catalyst and recovery/recycling of metals, e.g., of cobalt, molybdenum, nickel, and vanadium from hydroprocessing catalysts [251].
Consistent with its importance, the scientific literature treating catalyst regeneration is significant and growing (includes nearly 1000 journal articles since 1990). Regeneration of sulfur-poisoned catalysts has been reviewed by Bartholomew and co-workers [28]. Removal of coke and carbon from catalysts has received attention in reviews by Trimm [59,250], Bartholomew [60], and Figueiredo [1]. Redispersion of sintered catalysts has been discussed by Ruckenstein and Dadyburjor [101], Wanke [102], and Baker and co-workers [103]. Useful case studies of regeneration of hydrotreating [255] and hydrocarbon-reforming catalysts [256] have also been reported. The proceedings of the 9th International Symposium on Catalyst Deactivation (2001) contains 12 papers treating catalyst regeneration [257]. Regeneration, recycling, and disposal of deactivated heterogeneous catalysts have been reviewed briefly by Trimm [250].
The patent literature treating catalyst regeneration/reactivation is enormous (more than 17,000 patents); the largest fraction of this literature describes processes for regeneration of catalysts in three important petroleum refining processes, i.e., FCC, catalytic hydrocarbon reforming, and alkylation. However, a significant number of patents also claim methods for regenerating absorbents and catalysts used in aromatization, oligomerization, catalytic combustion, SCR of NO, hydrocracking, hydrotreating, halogenation, hydrogenation, isomerization, partial oxidation of hydrocarbons, carbonylations, hydroformylation, dehydrogenation, dewaxing, Fisher–Tropsch synthesis, steam reforming, and polymerization.
Conventional methods for regenerating (largely in situ) coked, fouled, poisoned, and/or sintered catalysts in some of these processes and representative examples thereof [258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297] are summarized in Table 20, while the basic principles and limitations involved in regeneration of coked, poisoned, and sintered catalysts are briefly treated in the subsections that follow.

Table 20.
Conventional Methods for and Representative Examples of Catalyst Regeneration from Scientific and Patent Literatures.
| Deactivation mechanism Process/Reaction Catalyst | Problem/cause | Method(s) of regeneration/phenomena studied/conclusions | Ref. |
|---|---|---|---|
| Deactivation by coke, carbon | |||
| Alkene aromatization oligomerization Zeolites, esp. ZSM-5, -22, -23, beta-zeolite, ferrierite | Catalyst fouling by condensation of heavy oligomers to coke |
| [258,259] |
| Alkylation of isoparaffins on solid catalysts Sulfated zirconia, USY a, Nafion, silicalite, ZSM-5 | Rapid catalyst deactivation due to coke formation; unacceptable product quality, and thermal degradation of catalyst during regeneration |
| [260,261,262,263] |
| Catalytic reforming of naphtha Pt/Al2O3 promoted with Re, Sn, Ge, or Ir | Poisoning and fouling by coke produced by condensation of aromatics and olefins |
| [264,265,266,267,268,269] |
| Dehydrogenation of propane and butane Cr2O3/Al2O3, Cr2O3/ZrO2, FeO/K/MgO, Pt/Al2O3, Pt–Sn/Al2O3, Pt–Sn/KL-zeolite | Catalyst activity is low due to equilibrium limitations and build-up of product H2; rapid loss of activity occurs due to coke formation |
| [270,271] |
| Fischer–Tropsch synthesis Co/Al2O3 | Loss of activity due to blocking of sites by carbon overlayers and heavy hydrocarbons |
| [272–274] |
| Fluid catalytic cracking (FCC) of heavy hydrocarbons USY or REO-Y b in silica matrix | Rapid loss of activity due to poisoning of acid sites and blocking of small zeolite pores by coke |
| [275,276] |
| Hydrocracking of heavy naphtha CoMo, NiW, MoW on Al2O3 or SiO2–Al2O3; Pt or Pd on Y-zeolite, mordenite, or ZSM-5 | Loss of activity due to poisoning of acid sites and blocking of small zeolite pores by coke |
| [277,278] |
| Hydrotreating of gas oil Al2O3-supported Mo and CoMo, NiMo, NiCoMo, MoW, NiW | Loss of activity due to formation of types I, II, and III coke on metal sulfide and alumina surfaces and in pores |
| [279,280,294,295,296,297] |
| Methanol to olefins or gasoline Silica– alumina, Y-zeolite, ZSM-5, other zeolites, and aluminophosphate molecular sieves | Severe coking and deactivation of silica–alumina and Y-zeolite catalysts observed during high conversions of MeOH; also substantial coking of ZSM-5, other zeolites, and aluminophosphate molecular sieves |
| [281,282] |
| Poisoning | |||
| FCC of residuum USY or REO-Y in silica matrix |
|
| [281,282,298,299,300,301] |
| Hydrogenation or dechlorination Ni/SiO2, Pd/Al2O3 | Poisoning of metal sites by arsenic, sulfur, and other poisons |
| [285,286] |
| Hydrotreating of residuum Al2O3-supported Mo and CoMo | Pore-mouth poisoning and blockage by Ni, V, and Fe sulfides present in feed as organometallics |
| [287,288] |
| Thermal degradation | |||
| Catalytic reforming of naphtha Pt/Al2O3 promoted with Re, Sn, Ge, or Ir; Pt/KL-zeolite | Sintering of Pt causing formation of large metal crystallites and loss of active surface area |
| [266,269,289,290] |
| Hydrocracking of heavy naphtha CoMo, NiW, MoW on Al2O3 or SiO2–Al2O3; Pt or Pd on Y-zeolite, mordenite, or ZSM-5 | Sintering of noble metal causing formation of large metal crystallites and loss of active surface area | Redispersion of noble metals on molecular sieves including silica-aluminates, ALPOS, SAPOS | [291] |
| Hydrotreating of gas oil and residuum Al2O3-supported Mo and CoMo | Sintering of Mo and Co sulfides causing formation of large sulfide crystals and loss of active surface area |
| [292,293] |
a USY: ultrastable Y-zeolite. b REO-Y: rare-earth exchanged Y-zeolite.4.1. Regeneration of Catalyst Deactivated by Coke or Carbon
4.1. Regeneration of Catalyst Deactivated by Coke or Carbon
Carbonaceous deposits can be removed by gasification with O2, H2O, CO2, and H2. The temperature required to gasify these deposits at a reasonable rate varies with the type of gas, the structure and reactivity of the carbon or coke, and the activity of the catalyst. Walker and co-workers [302] reported the following order (and relative magnitudes) for rates of uncatalyzed gasification at 10 kN/m3 and 800 °C: O2 (105) > H2O (3) > CO2 (1) > H2 (3 × 10−3). However, this activity pattern does not apply in general for other conditions and for catalyzed reactions [1]. Nevertheless, the order of decreasing reaction rate of O2 > H2O > H2 can be generalized.
Rates of gasification of coke or carbon are greatly accelerated by the same metal or metal oxide catalysts upon which carbon or coke deposits. For example, metal-catalyzed coke removal with H2 or H2O can occur at a temperature as low as 400 °C [1]; β-carbon deposited in methanation can be removed with H2 over a period of a few hours at 400–450 °C and with oxygen over a period of 15–30 min at 300 °C [60]. However, gasification of more graphitic or less reactive carbons or coke species in H2 or H2O may require temperatures as high as 700–900 °C [1], conditions, of course, that result in catalyst sintering.
Because catalyzed removal of carbon with oxygen is generally very rapid at moderate temperatures (e.g., 400–600 °C), industrial processes typically regenerate catalysts deactivated by carbon or coke in air. Indeed, air regeneration is used to remove coke from catalysts in catalytic cracking [81], hydrotreating processes [255], and catalytic reforming [256].
One of the key problems in air regeneration is avoiding hot spots or overtemperatures which could further deactivate the catalyst. The combustion process is typically controlled by initially feeding low concentrations of air and by increasing oxygen concentration with increasing carbon conversion [255,303]; nitrogen gas can be used as a diluent in laboratory-scale tests, while steam is used as a diluent in full-scale plant operations [303]. For example, in the regeneration of hydrotreating catalysts, McCulloch [255] recommends keeping the temperature at less than 450 °C to avoid the γ- to α-alumina conversion, MoO3 sublimation, and cobalt or nickel aluminate formation, which occur at 815, 700, and 500–600 °C respectively.
Because coke burn-off is a rapid, exothermic process, the reaction rate is controlled to a large extent by film heat and mass transfer. Accordingly, burn-off occurs initially at the exterior surface and then progresses inward, with the reaction occurring mainly in a shrinking shell consistent with a “shell-progressive” or “shrinking-core” model, as illustrated in Figure 36 [304]. As part of this same work, Richardson [304] showed how experimental burn-off rate data can be fitted to various coking transport models, e.g., parallel or series fouling. Burn-off rates for coke deposited on SiO2/Al2O3 catalysts were reported by Weisz and Goodwin [305]; the burning rate was found to be independent of initial coke level, coke type, and source of catalyst.
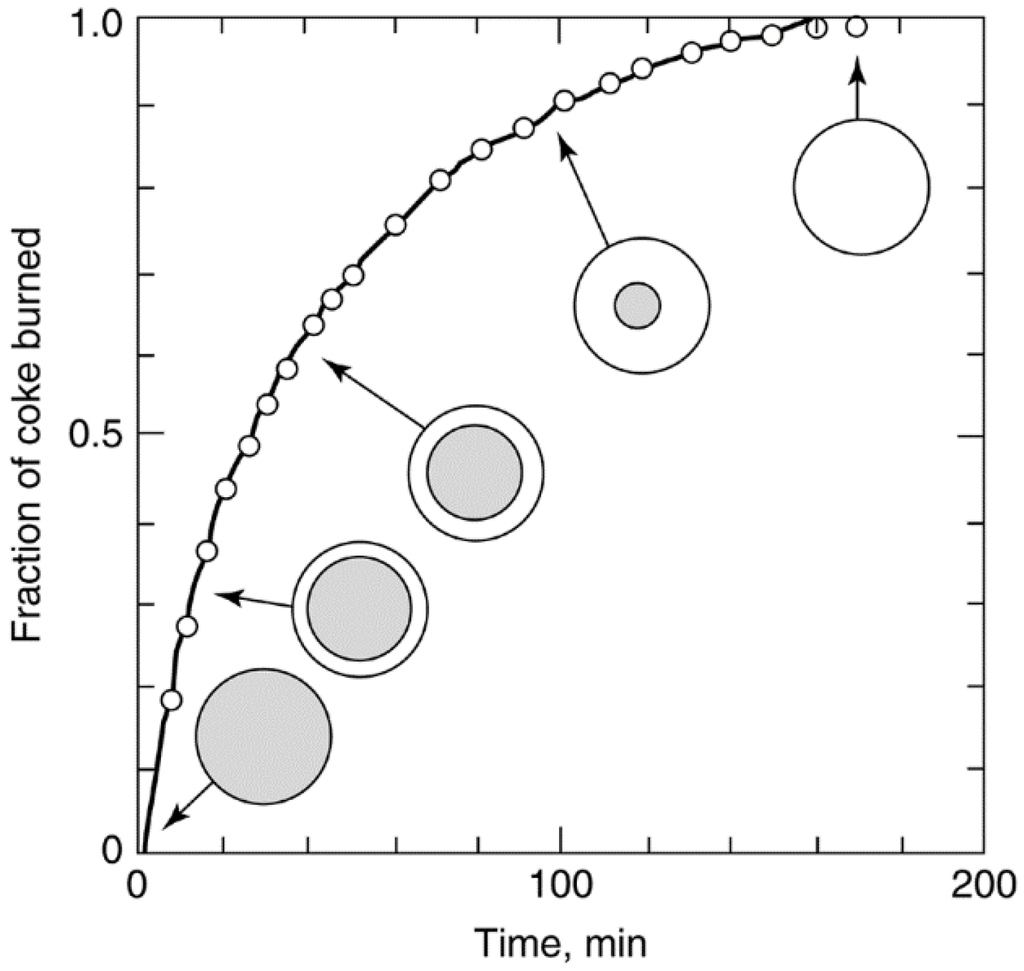
Figure 36.
Shell-progressive regeneration of fouled pellet Reproduced from [304]. Copyright 1972, American Chemical Society.
4.2. Regeneration of Poisoned Catalysts
Much of the previous literature has focused on regeneration of sulfur-poisoned catalysts used in hydrogenations and steam reforming. Studies of regeneration of sulfur-poisoned Ni, Cu, Pt, and Mo with oxygen/air, steam, hydrogen, and inorganic oxidizing agents have been reported [28]. Rostrup-Nielsen [306] indicates that up to 80% removal of surface sulfur from Mg- and Ca-promoted Ni, steam reforming catalysts occurs at 700 °C in steam. The presence of both SO2 and H2S in the gaseous effluent suggests that the following reactions occur:
Ni-S + H2O→NiO + H2S
H2S + 2H2O→SO2 + 3H2
Although this treatment is partially successful in the case of low-surface-area steam reforming catalysts, the high temperatures required for these reactions would cause sintering of most high-surface-area nickel catalysts.
Regeneration of sulfur-poisoned catalysts, particularly base metal catalysts, in air or oxygen has been largely unsuccessful. For example, the treatment of nickel steam-reforming catalysts in steam and air results in the formation of sulfates, which are subsequently reduced back to nickel sulfide upon contact with hydrogen. Nevertheless, sulfur can be removed as SO2 at very low oxygen partial pressures, suggesting that regeneration is possible under carefully controlled oxygen atmospheres, including those provided by species such as CO2 or NO that dissociate to oxygen. Apparently, at low oxygen pressures, the oxidation of sulfur to SO2 occurs more rapidly than the formation of nickel oxide, while at atmospheric pressure the converse is true, i.e., the sulfur or sulfate layer is rapidly buried in a nickel oxide layer. In the latter circumstance, the sulfur atoms diffuse to the nickel surface during reduction, thereby restoring the poisoned surface. Regeneration of sulfur-poisoned noble metals in air is more easily accomplished than with steam, although it is frequently attended by sintering. Regeneration of sulfur-poisoned nickel catalysts using hydrogen is impractical because (1) adsorption of sulfur is reversible only at high temperatures at which sintering rates are also high and (2) rates of removal of sulfur in H2 as H2S are slow even at high temperature.
Inorganic oxidizing agents such as KMnO4 can be used to oxidize liquid phase or adsorbed sulfur to sulfites or sulfates [16]. These electronically shielded structures are less toxic than the unshielded sulfides. This approach has somewhat limited application, i.e., in partial regeneration of metal catalysts used in low temperature liquid-phase hydrogenation reactions or in liquid-phase destruction of chlorinated organic compounds. For example, Lowrey and Reinhard [286] reported successful regeneration in dilute hypochlorite solution of a Pd/Al2O3 catalyst deactivated during the aqueous-phase dechlorination of trichloroethylene (TCE) in the presence of sulfite or HS− ions. These poisons are formed by sulfate-reducing bacteria present in natural groundwater and are apparently adsorbed on the alumina or Pd surfaces more strongly than sulfate ions. Figure 37 illustrates how readily the poisoned catalyst is regenerated by dilute hypochlorite solutions; indeed, it is evident in Figure 37b that regeneration every 5–10 days successfully maintains the catalytic conversion of TCE around 25% (a value only slightly less than that observed for reaction in distilled water).
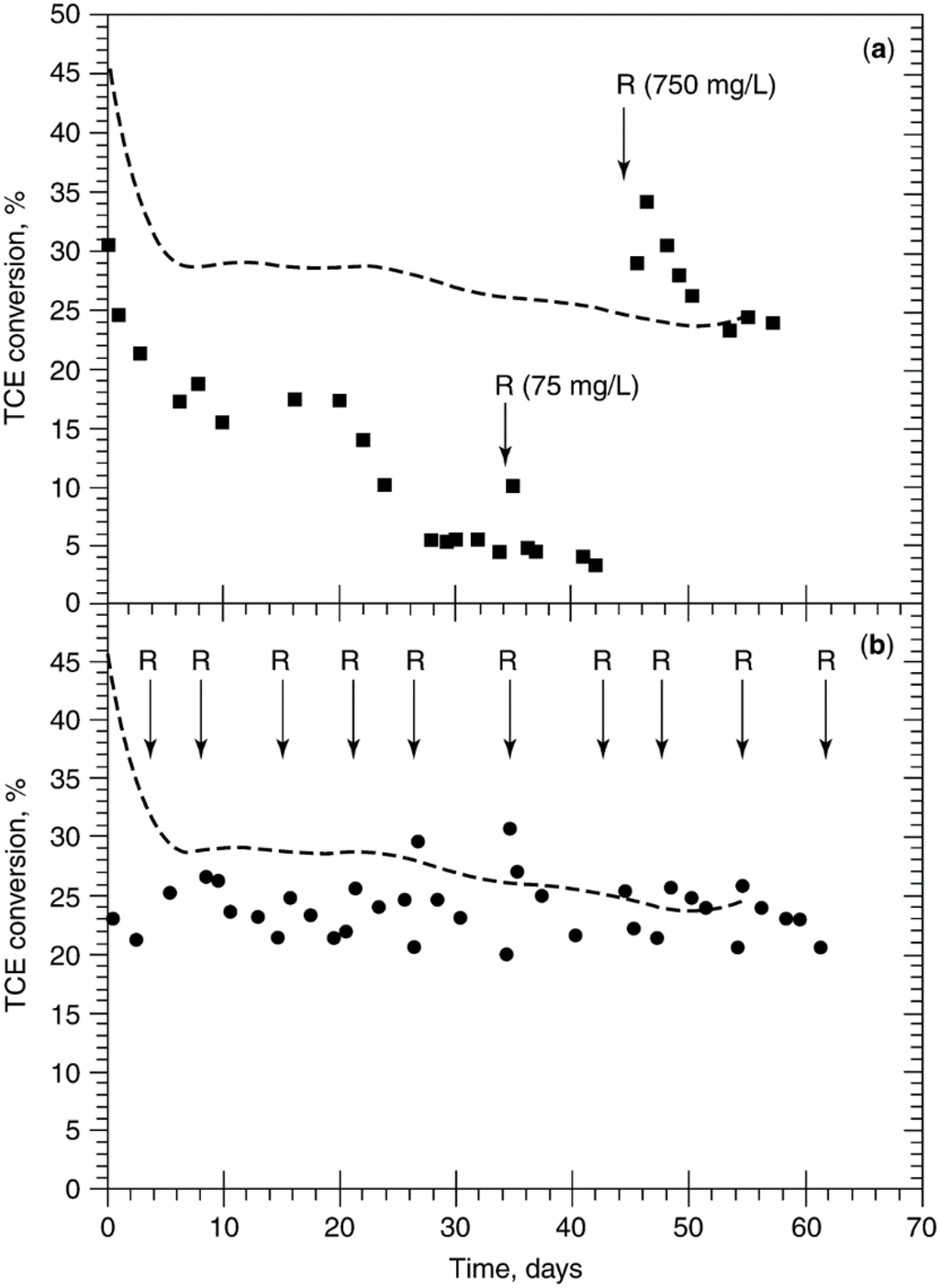
Figure 37.
Effect of regeneration (R) with hypochlorite of Pd/Al2O3 catalysts used for aqueous phase dechlorination of trichloroethylene in the presence of HS−/SO32. Reproduced from [286]. Copyright 1992, American Chemical Society.
4.3. Detailed Case Study on Regeneration of Selective Catalytic Reduction (SCR) Catalysts
4.3.1. Introduction to SCR: Key to Abatement of NOx from Coal Utility Boilers
NOx, generally defined as NO and NO2, emissions from coal utility boilers (approximately 30% of total NOx emissions in the U.S.) contribute substantially to the formation of acid rain and photochemical smog, which in turn damage human health, property, agriculture, lakes, and forests. Selective catalytic reduction (SCR) technology has been used in utility boilers since the 1980s in Japan and Europe in response to stringent NOx removal regulations. By 2000, SCR systems had been installed in coal-fired boilers totaling roughly 25 and 55 GW in Japan and Europe respectively [307,308]. Equivalent stringent NOx abatement regulations were enacted later in the U.S. by the EPA, including
- (1)
- the 1990 ARP and OTC mandates, requiring states to reduce NOx emissions by 80%;
- (2)
- the 1995 OTC-Phase 1 requiring Reasonably Available Control Technology (RACT);
- (3)
- the 1998 NOx SIP Call setting up a regional cap-and-trade program for 20 eastern states based on an equivalent NOx emission rate of 0.15 lb/106-Btu; and
- (4)
- the 2005 Clean Air Interstate Rule (CAIR) requiring all states to meet Best Available Retrofit Technology (BART) for existing plants, equivalent to emission rates of less than 0.05–0.10 lb/106-Btu [309,310].
By 2006, about 100 GW of coal-fired steam boilers in the U.S. used SCR. Presently, the U.S. has about 140 GW [309] of coal-boiler SCR capacity; world-wide, an estimated 300 GW of coal-boiler SCR is in operation.
Prior to the more recent stringent U.S. emissions regulations, boiler and engine manufacturers successfully reduced NOx emissions by 30–60% using modifications to combustion processes, including reducing excess air, adding two-stage combustion features, altering burner design, etc. However, meeting the new reduction targets of 80–90% is, in general, only possible through catalytic after-treatment (SCR). Given ever more restrictive NOx emission standards and the fact that worldwide power production from coal could double or triple in the next decade to an estimated 1500 GW [311], total installed SCR unit capacity is expected to grow commensurately, providing continued investment and design challenges in this area.
4.3.2. Selective Catalytic Reduction of NOx
4.3.2.1. Reaction Chemistry and Preferred Catalysts
Selective catalytic reduction (SCR) is a process in which a reducing agent, typically NH3, reacts selectively with the NOx to produce N2 without consumption of the excess O2 present in the flue gas. Desirable stoichiometric reactions for SCR of NO and NO2 (Equations 7 and 8) occur with high activity and selectivity to N2 within a narrow temperature window of 300–400 °C on preferred commercial catalysts.
4NH3 + 4NO + O2→4N2 + 6H2O
4NH3 + 2NO2 + O2→3N2 + 6H2O
Undesirable side reactions include oxidations of SO2 (present in the flue gas) and the reducing agent NH3. While only a small fraction of the SO2 present in the flue gas is catalytically oxidized to SO3, this acid precursor either corrodes downstream heat-exchange surfaces or reacts with NH3 to form ammonium sulfates, which in turn can foul catalyst and/or heat exchange surfaces. Oxidation of NH3 to either NO or N2 may also occur at temperatures above 400 °C.
A typical commercial vanadia catalyst consists of 1 wt% V2O5 and 10 wt% WO3 (alternatively 6 wt% MoO3) supported on high-surface-area TiO2 (mostly anatase, 60–80 m2/g). TiO2 has the decided advantage over Al2O3 as a support, since the former stabilizes the active vanadia species and does not form a bulk sulfate in the presence of SO2-containing flue gases; thus TiO2 promotes activity and extends catalyst life. WO3 and MoO3 prevent the transformation of anatase to rutile; they reside on basic sites of TiO2, blocking adsorption of SO3, thereby preventing sulfation of the support. Additionally, WO3 and MoO3 increase Brønsted acidity, promoting NOx reduction while lowering SO2 oxidation rate. Commercial vanadia-titania catalysts are typically supplied in the form of extruded monoliths or plates (see Figure 38), forms which minimize pressure drop [8].
Figure 38.
SCR catalyst support geometries: (a) extruded ceramic monolith; and (b) plate. Reproduced from [8]. Copyright 2006, Wiley-Interscience.
4.3.2.2. SCR Process Options
Two process options in terms of SCR reactant placement have found broad use for SCR units installed in coal-fired plants:
- (1)
- the high dust unit (HDU) involving placement of the SCR unit after the economizer and prior to the air heater, particulate collector, and SO2 scrubber; and
- (2)
- the tail end unit (TEU) involving placement of the SCR unit following the SO2 scrubber.
The HDU is used more widely in the U.S. and the TEU more frequently in Europe and Japan.
The HDU has the advantage of providing flue gas to the SCR unit at its ideal temperature range of 300–400 °C and disadvantages of
- (1)
- deactivation of the catalyst due to erosion, fouling, and poisoning by fly ash thereby limiting its useful life to about 3–4 years;
- (2)
- large monolith channel design to limit plugging by fly ash, but which also limits the amount of active catalyst per reactor volume; and
- (3)
- requirement for a low activity catalyst to limit oxidation of SO2 to SO3 and the attendant formation of ammonium sulfates which foul and corrode downstream heat exchangers.
The TEU enables use of a smaller volume of high activity catalyst with small diameter channels, since particulates and SO2 have already been removed upstream; moreover, since deactivation rate is much lower due to the absence of fly ash and other poisons, catalyst life is substantially extended (i.e., to 15–20 years). A significant disadvantage is that the outlet scrubber gas, which is only about 120 °C, must be reheated to at least 200–250 °C for the SCR to occur at reasonable rates. The energy cost of reheating only 100 °C can be as much as 4–6% of the boiler capacity, unless a regenerative heat exchanger is used. In addition, the SCR catalyst must be designed to operate at significantly lower temperatures (200–290 °C relative to a typical 300–400 °C for an HDU).
Given the long life of the TEU catalyst, no regeneration is necessary. However, regeneration of the HDU catalyst is highly desirable, since the regeneration cost is significantly lower than the cost of a new catalyst. With this background, further discussion focuses on the deactivation and regeneration of the HDU catalyst.
4.3.3. Catalyst Deactivation, Rejuvenation, and Regeneration
4.3.3.1. Catalyst Deactivation
SCR catalysts have typical process lifetimes around 2–7 years, depending upon their application and placement in a power plant or other such facility. The principal causes of SCR catalyst deactivation [8,312] are fourfold:
- (1)
- fouling/masking of (deposition of solids on) catalyst surfaces, pores, and channels by fly ash components (e.g., sulfates and phosphates of Ca, K, and Na) or ammonium bisulfate;
- (2)
- chemical poisoning of active sites by elements present in upstream lubricants or originating in the fuel such as As, Se, and P and alkali and alkaline earth metals;
- (3)
- hydrothermal sintering of the titania, especially as a result of high-temperature excursions; and
- (4)
- abrasion or erosion by fly ash.
Erosion, fouling, and masking from fly ash and poisoning by As and alkali metals are specific to SCR catalysts installed near the hot, high-particulate side of a coal-fired boiler, accounting for the significantly lower catalyst life of 2–4 years for this configuration.
Formation of ammonium bisulfate depends on flue gas temperature, SO3 concentration and NH3 concentration [313]. Deposition of ammonium bisulfate is more likely to occur in catalyst pores at lower reactor temperatures in low-dust or tail-end (TEU) SCR units and on cooler surfaces of heat exchangers. Figure 39a shows typical activity loss versus time performance for a set of commercial V/Ti catalysts tested in a DOE pilot SCR unit installed in a slip-stream near the exit of a coal-fired boiler (HDU location) using high sulfur, Eastern U.S. coals; 20% of the initial catalyst activity is lost in about 14,000 h (1.6 years); however, the plant will not shut down until 50–60% of the initial activity has been lost (around 3–4 years). Activity and NH3 slip are plotted against NH3/NO ratio for the same catalysts in Figure 39b. To maintain NH3 slip (exit NH3 concentration) below a target maximum of 2–5 ppm (2 is highly preferred), the NH3/NO ratio must be maintained near 0.8; under these conditions NO conversion is about 88%.
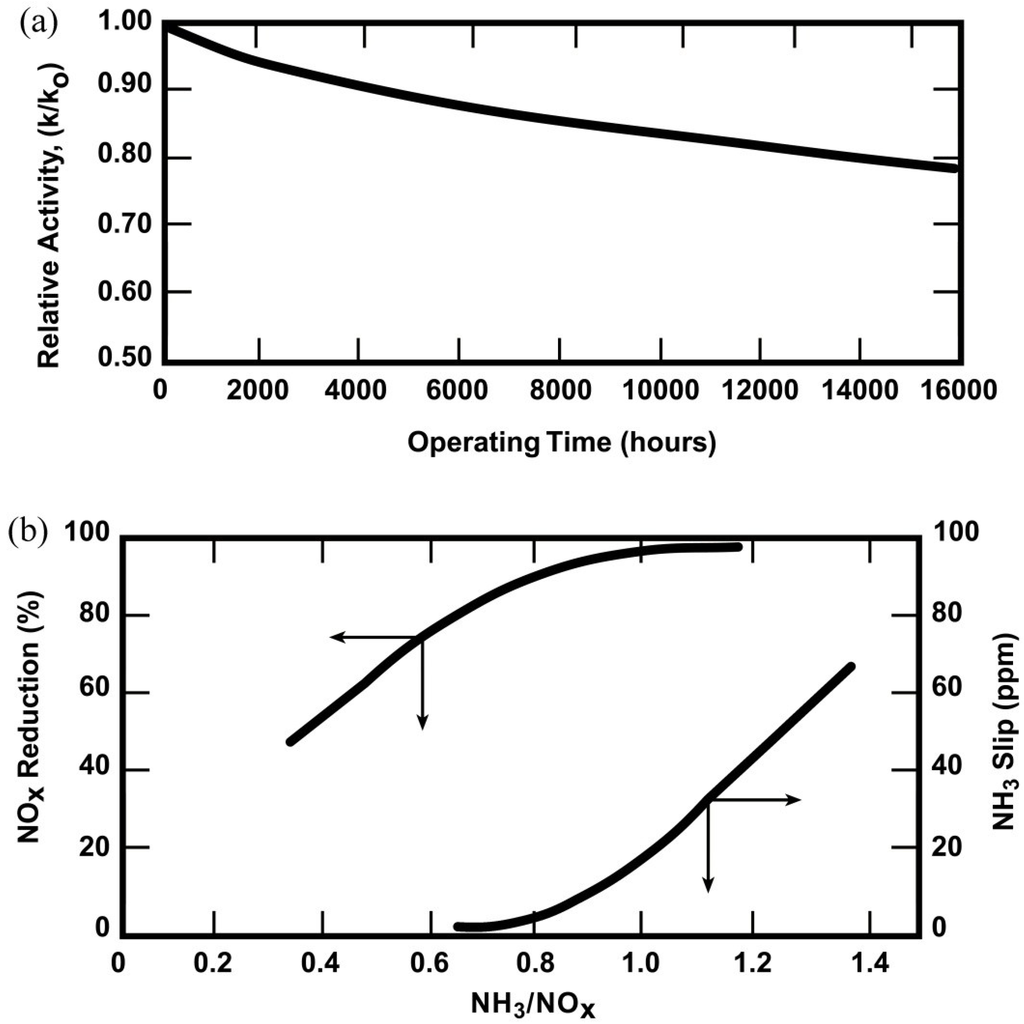
Figure 39.
(a) Catalyst activity (k/ko) vs. time; (b) Typical SCR performance. Reproduced from [313].
Prevention of deactivation requires optimal choices of catalyst design and process conditions. Abrasion, fouling, and/or poisoning by fly ash can be prevented by installation of a hot-side electrostatic precipitator or installing an active, low-temperature catalyst at the tail end of the process. Sintering is minimized by using catalyst promoters that enhance thermal stability and by maintaining reaction temperatures below critical values. The MoO3 promoter extends catalyst life (in coal boilers) by preferentially adsorbing vapor-phase As which would otherwise adsorb on active V4+ sites. Free CaO in the fly ash (up to 3%) also scavenges As to low levels, forming calcium arsenide particles which are collected with the fly ash. Many U.S. coals contain adequate CaO; however, if the CaO content of the coal is too low, it can be added to the boiler or fuel. However, CaO levels above 3% of the fly ash are undesirable, since CaO reacts with SO2 to form CaSO4 which masks the exterior surface of the catalyst. Fouling by ammonium bisulfate is minimized by keeping exit SO3 and NH3 concentrations low and maintaining reaction temperatures above about 230 °C; SO3 formation is minimized by keeping reaction temperatures below 350 °C or by using lower activity V2O5/TiO2 or zeolite catalysts that have low selectivities for SO3. Ultimately, however, extra catalyst volume is typically added to SCR reactors to extend periods between catalyst replacements.
For plants fueled by coal, substantial carry-over of inorganic ash occurs to HDU SCR units, a small, but significant fraction of which deposits on monolith walls, masks or blocks catalyst macropores, and plugs flow channels [314]. Extensive fouling necessitates the use of air lancing to purge the ash out of the catalyst channels. Figure 40 reveals the extent of serious channel plugging and erosion of an SCR catalyst in a pilot plant following several thousand hours of operation in flue gas containing coal fly ash. Plugging and excessive pressure drop are avoided by keeping monolith cell width at or above 7 mm.
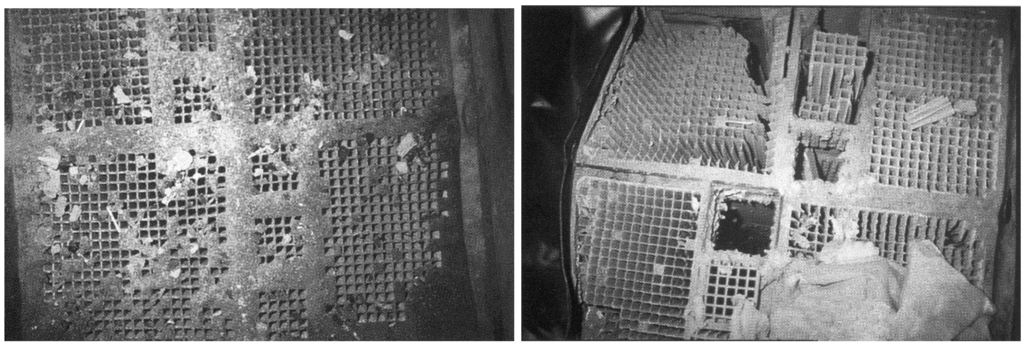
Figure 40.
Catalyst channel plugging (left) and damage due to erosion (right) during operation in an SCR facility. Reproduced from [313].
The type and extent of chemical deactivation depends on operating conditions, fuel type, catalyst geometry, shut-downs for boiler maintenance, etc. Mini-pilot tests and subsequent full-scale SCR operating experience have provided little evidence of poisoning by basic minerals from Western United States coals; rather they indicate that deactivation occurs principally by masking of catalyst layers and plugging of catalyst pores by CaSO4 and other fly ash minerals. Moreover, laboratory analysis of catalysts exposed to power plant slip streams indicates that mineral poisons do not penetrate deep into catalyst pores [315,316] nor do they adsorb on Brønsted acid sites unless plant conditions cause moisture to condense on the catalyst.
4.3.3.2. Plant Operating Strategy to Maximize Catalyst Life
A typical SCR unit consists of a series of two to four catalyst layers (three is most common for coal boiler cleanup) through which the flue gas usually flows downward (see Figure 41). A layer of fresh catalyst can be added as catalyst performance declines over time [317]. Two general schemes are followed for replacing the spent catalyst, both of which take into consideration the relative activity or design activity level, a parameter that is usually defined as the ratio of NOx conversion at any time divided by that produced by the fresh catalyst. Once the NOx reduction performance declines to the minimum design activity level (typically 65–75% of fresh activity), the catalyst can either be replaced entirely (simultaneous replacement scheme) or one layer can be replaced at a time (sequential replacement scheme), usually beginning at the top and working down [313,318]. The sequential method results in increased overall catalyst life (on a per-volume-replaced basis), while annual replacement cost would be 60% lower for the simultaneous scheme (see Figure 42 [319]). Thus, optimal, cost-effective design of an SCR unit requires considering both the initial capital and annual costs.
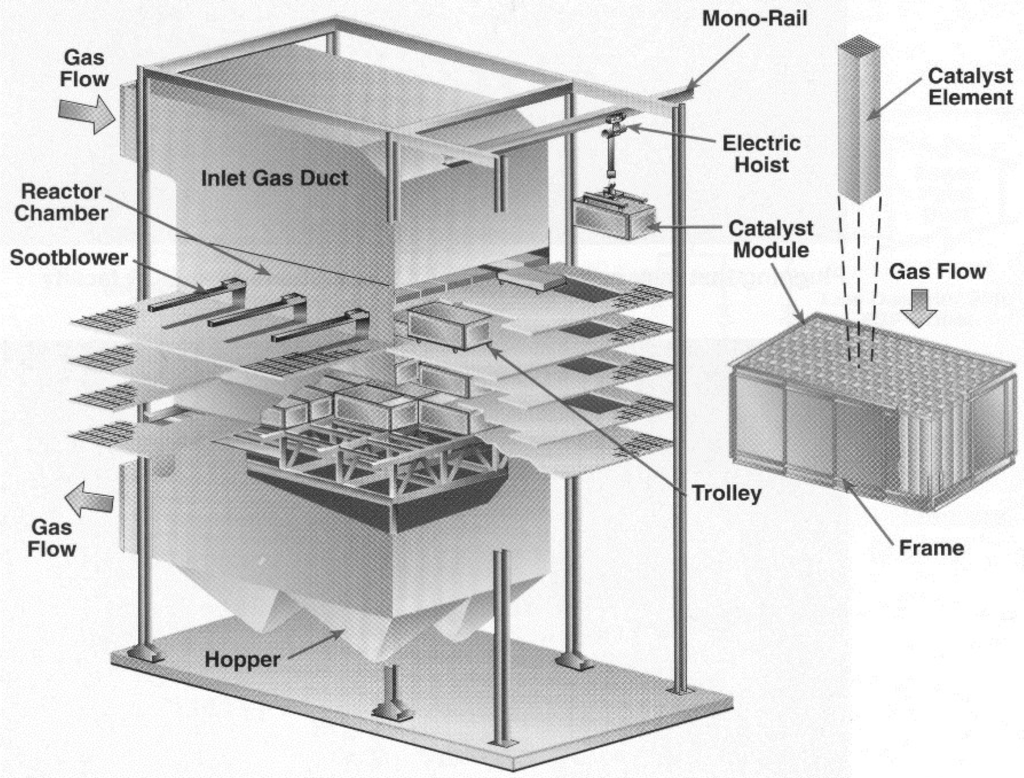
Figure 41.
Vertical-flow fixed-bed SCR reactor. DOE SCR demonstration facility at Gulf Power Company’s Plant Crist. Reproduced from [313].
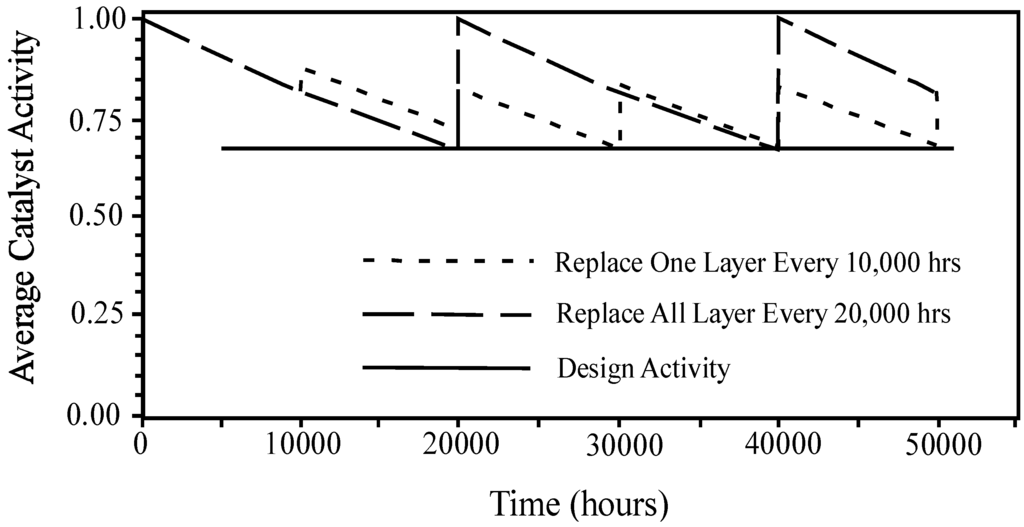
Figure 42.
SCR replacement strategies: comparison of total replacement on a 20,000 h cycle relative to sequential replacement on a 10,000 h cycle while maintaining constant catalyst volume. Reproduced from [319].
Operating experience for commercial SCR installations has been better than anticipated. Catalyst lifetimes of 3–4 years at overall efficiencies of 75–90% for HDU’s have been observed for electric boiler installations [312]. The principal contributors to operating cost include catalyst replacement cost, shutdown cost for catalyst replacement, and plant derating cost associated with catalyst pressure drop. Catalyst replacement or regeneration was typically required within 2–3 years and catalyst replacement times varied from 2–7 days. Pressure drop ranged from 0.8–15 cm of water for the various catalyst configurations and volumes. Pressure drops for plate type catalysts were significantly lower than for monolithic catalysts.
4.3.3.3. Catalyst Rejuvenation and Regeneration
While high-dust-catalyst life of 2–3 years is acceptable, advances in SCR catalyst regeneration technologies make it possible to extend life by several additional years. Recent experience indicates that even after long-term exposure to fly ash, foulants, and poisons, SCR catalysts may be successfully regenerated to the original performance or better [307,308,320,321,322].
4.3.3.4. Methods of Renewing Catalysts
Deactivated catalysts may be cleaned, rejuvenated, and/or regenerated. Cleaning commonly refers to removal of physical restrictions such as monolith channels plugged with fly ash or channel surfaces covered with a loose dust layer; these restrictions are easily removed in situ using compressed air, although cleaning will also be done as a first step in the other methods. Rejuvenation refers to relatively mild treatments that remove catalyst poisons or foulants inside the catalyst pores and restore part of the catalytic activity; these treatments are often done in situ or on-site. Rejuvenation involves removal of blinding layers and partial removal of some poisons; thus, activity is partly recovered, but none is added. Regeneration involves the off-site, complete restoration of catalytic activity through a series of relatively sophisticated treatments, some of which remove not only poisons and foulants, but also a part or much of the active catalytic materials from the support; hence, regeneration also involves restoration of the catalytically active materials bringing the catalyst to its original state or one of even higher activity. SCR catalysts are routinely and regularly cleaned or “blown out” during operation, while rejuvenation or regeneration is typically done after approximate 50–60% of the initial activity of the catalyst has been lost. In situ rejuvenation (ISR) treatments were practiced early (e.g., 1990s and early 2000s), while off-site regeneration (OSR) is now the predominant practice because of its greater effectiveness.
4.3.3.5. Rejuvenation or Regeneration?
According to McMahon [322], rejuvenating SCR catalyst may be more cost-effective than regenerating, if the catalyst is fairly new or the SCR system does not operate year around (as in the case of plants operating only during high pollutant levels, known as the “ozone season”). Otherwise, the choice between rejuvenation and regeneration depends largely on economics, i.e.,
- (1)
- the plant’s dispatch economics, including transportation costs;
- (2)
- length of catalyst service;
- (3)
- costs of removing and replacing the catalyst;
- (4)
- the impact of the fuels combusted, i.e., coal, oil, or gas; and
- (5)
- the location of the catalyst in the plant, i.e., HDU or TGU.
Examples of rejuvenation treatments are found in the scientific and patent literature. For example, work by Zheng and Johnsson [323] and others (e.g., [324,325]) indicates that activity of poisoned catalysts might be partially regenerated by washing with water, sulfuric acid, NH4Cl, and/or catalyst precursor solutions (e.g., ammonium paratungstate and vanadyl sulfate), as well as a combination of washing and treatment with gaseous SO2. The extent to which these rejuvenation methods are effective in restoring a significant fraction of the original catalyst activity varies significantly.
4.3.3.5.1. Rejuvenation
On-site rejuvenation methods generally include the following procedural types: (i) removal of dust in the monolith channels with compressed air followed by (ii) washing catalyst in a tank containing agitated, deionized water to remove the CaSO4 coating and alkali metal salts deposited by fly ash (the solution is generally mildly acidic due to impurities on the catalyst) or acidic aqueous solution (pH = 1–2 in either case) in a tank; (iii) rinsing vigorously with deionized water (usually in the same tank) to remove the dissolved and suspended deposits; and (iv) drying slowly in clean air at room temperature followed by drying gently in hot air. Examples of on-site regeneration methods include those developed and practiced in the time frame of 1995–2002 by SCR-Tech, SBW, Saar Energie, Steag, EnBW, HEW, BHK, and Integral [326,327,328]. The method described by Schneider and Bastuck [327] provided for adding catalytic materials, i.e., vanadium and tungsten oxides (via impregnation of the V and W salts) to the cleaned catalyst.
The patent of Budin et al. [328] provides for more sophisticated treatments, including use of (i) nonionic surfactants and complex-forming or ion-exchange additives, (ii) washing with an acid or base, (iii) using acoustic radiation to remove fly-ash components, and (iv) addition of catalytic materials (oxides of V, W, Mo free of alkali and alkaline-earth metals, halogen, and sulfur) to restore activity, although few details or conditions of use are provided. In fact, no examples are provided in any of the patents cited directly above; accordingly, it is unclear to what extent and under what conditions the more sophisticated methods were used for on-site regeneration. The methods claimed by Budin et al. [328] are clearly more readily applied in off-site regeneration, as will be clear from the discussion below.
4.3.3.5.2. Regeneration
Bullock & Hartenstein [320], Cooper et al. [329], and McMahon [322] build a strong case for off-site regeneration and a comprehensive catalyst management program.
4.3.3.6. A Comprehensive Approach to Catalyst Management
The approach [320,322] includes
- (1)
- strategies for extending catalyst life and reusability and planning for catalyst removal/rotation to coincide with power plant outages;
- (2)
- catalyst inspection and testing before and following regeneration with replacement of badly damaged catalyst which is unregenerable;
- (3)
- off-site regeneration using a series of robust washing and chemical treatments to remove channel blockages, deactivated catalyst metals, and poisons, followed by chemical treatments to restore active catalytic materials; and
- (4)
- gentle drying/calcination in air to high temperatures to produce catalytically active oxides.
4.3.3.7. Common Regeneration Practices
Normal regeneration procedures [307,308,320,322,330,331,332,333] are designed to enhance removal of blockages, deactivated catalyst, and poisons and restore active catalytic material. These typically include the following steps:
- (1)
- pressurized wet and dry treatments to remove channel blockages and outer dust layers;
- (2)
- washing of catalyst units in tanks containing agitated water augmented with surfactants, dispersants, ion-exchange materials, emulsifiers, acid, base, and/or acoustic radiation to remove the outer CaSO4 coating, alkali metal salts deposited in the catalyst pores, and deactivated (e.g., As-poisoned) catalyst;
- (3)
- rinsing repeatedly in deionized water and repeating ultrasonic treatments between or in concert with chemical treatments, with a final rinse to finish removal of any catalyst or fouling residue;
- (4)
- reimpregnation of the clean support with salts of the active catalytic materials (V, Mo, and W); and
- (5)
- drying (calcining) at low heating rates to decompose the salts of the active catalytic materials to active metal oxides of V, Mo, and W.
4.3.3.8. Regeneration Process Profile: SCR-Tech Regeneration Process
SCR-Tech is the most prominent and experienced off-site regeneration company with 13 years of experience in the regeneration business and a documented record of research and development, going back to their German parent company ENVICA, who in 1997 began developing an offsite regeneration process. SCR-Tech was the first and until 2008 the only company in the U.S. to perform off-site regeneration. In September 2007, Evonik Energy Services (formerly Steag) opened an SCR catalyst regeneration facility in the U.S.
The SCR-Tech regeneration process involves a number of different process steps illustrated in Figure 43. Upon receipt of a shipment of catalyst, catalyst elements from several modules are inspected and analyzed; results of the analysis provide a basis for determining the precise protocol for treatment, i.e., the number and order of processing steps [334,335]. A large catalyst module is then led through a protocol of soaking, washing, ultrasonic treatment, arsenic and/or phosphorus removal (as needed), replenishment of V and Mo, neutralization, and rinsing in various soaking pits, as shown in Figure 43; all of these wet chemical steps are performed at controlled pH and temperature. Finally, the catalyst is dried, inspected, and packaged for shipment. Performance guarantees are provided for complete removal of blinding layers, catalyst activity (typically higher after regeneration), SO2 to SO3 conversion rate (typically lower), mechanical stability (the same), and deactivation rate (the same) such that all properties of the regenerated catalyst are as good or better than the new catalyst.
A comparison of the physical appearances of SCR monoliths and plates before and after regeneration in Figure 44 reveals the rigor of the SCR-Tech cleaning process. The nearly complete removal of poisons originally in high concentrations by the regeneration process is demonstrated in Figure 45. Surface concentrations of CaO, P2O5, SiO2, and SO4 were also substantially reduced.
Table 21 compares the costs of regenerating versus buying a new catalyst [322]. This case is for a typical 500 MW unit with 650 m3 of catalyst contained in 450 modules (150 modules in each of 3 layers). The purchase cost of new catalyst in 2006 was $3500 to 4500 per m3. The cost to regenerate the catalyst is approximately 60% of this price. Thus, the purchase cost of one layer is $758,000 to $975,000 as compared to a regeneration cost of $455,000 to $585,000 resulting in savings per layer of $303,000 to $390,000 or $910,000 to $1.2 million for three layers. Assuming the SCR unit runs year around (as most do now) and catalyst life is three years, the annual savings due to regeneration is in the range of $300,000 to $600,000. The disposal cost for an SCR catalyst can range from $50 to $2,000/ton, the upper figure based on the cost of treating the vanadium as hazardous waste. Hence the disposal cost could be as high as $500,000 for a layer of catalyst. According to McMahon, SCR catalysts can be regenerated from 3 to 7 times.
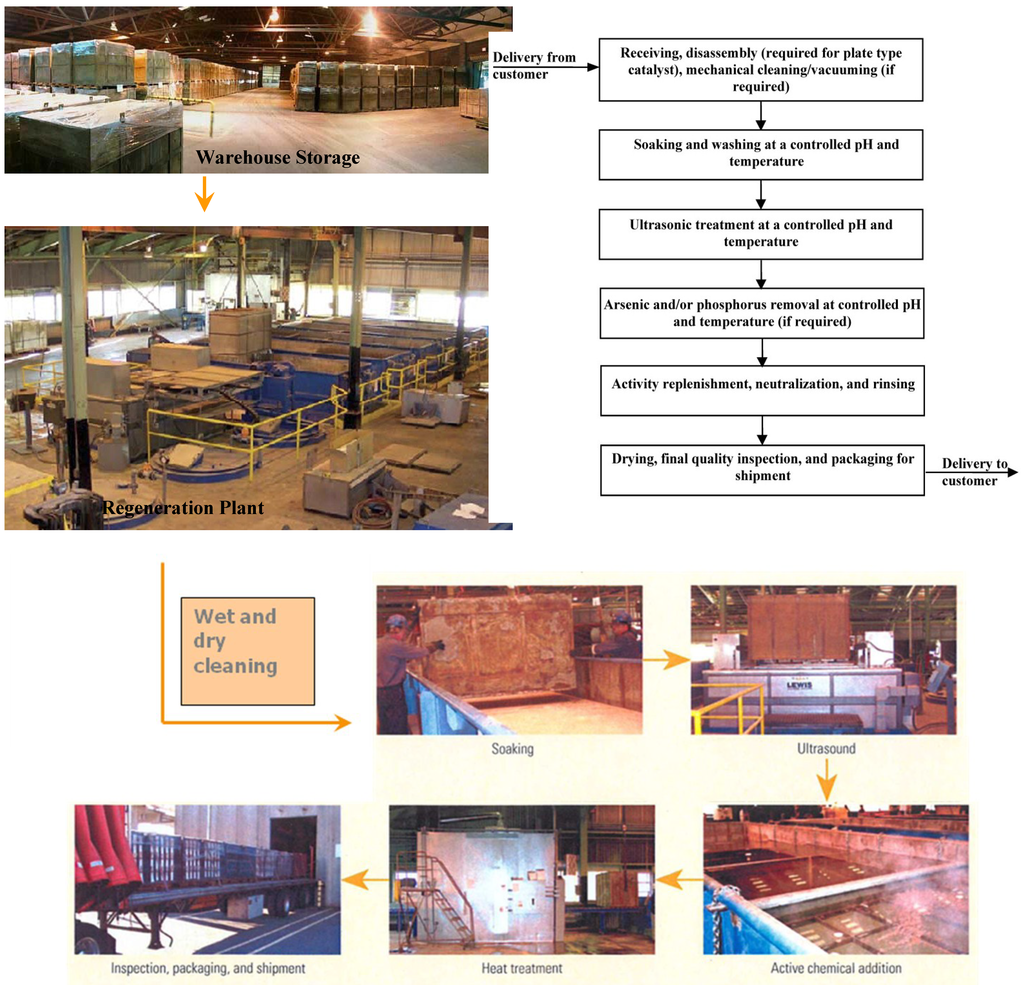
Figure 43.
SCR-Tech catalyst regeneration process. Reproduced from [322,335,336,337]. Reproduced with permission of Electric Power and CoaLogix, Inc.
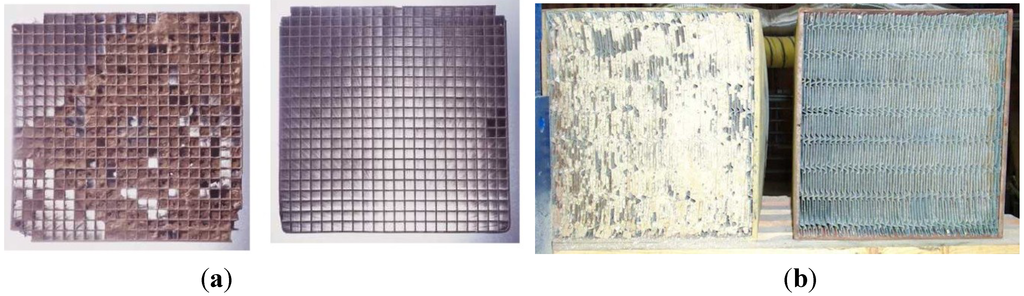
Figure 44.
(a) Monolith and (b) plate SCR catalysts before and after SCR-Tech regenerative treatment. Reproduced from [334]. Courtesy CoaLogix, Inc.
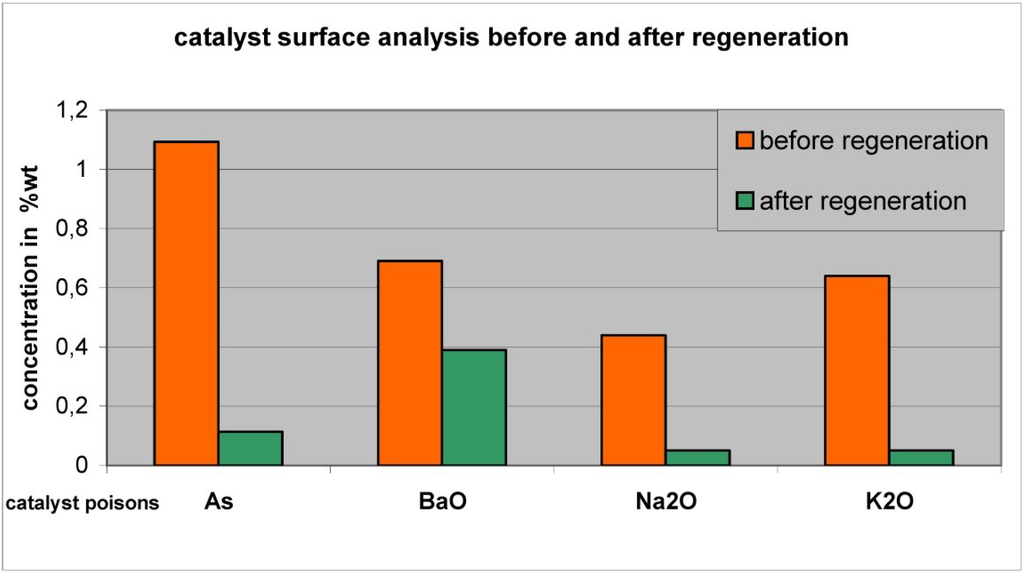
Figure 45.
Concentration of principle poisons before and after regeneration. Reproduced from [320]. Courtesy CoaLogix, Inc.

Table 21.
Cost per layer (217 m3 or 150 modules) of new versus regenerated SCR catalyst. Adapted from [322]. Copyright 2006, Electric Power.
| Catalyst Handling Step | New | Regenerated |
|---|---|---|
| Removal from SCR system | Comparable | Comparable |
| Transport out | Comparable | Comparable |
| Purchase price | $758,000–$975,000 | $455,000–$585,000 |
| Shipping | Comparable | Comparable |
| Installation | Comparable | Comparable |
| Net savings from regeneration | $303,000–$390,000 pls disposal cost | |
| Disposal cost | $20,000–$500,000 | 0 |
4.3.4. SCR Catalyst Case Study Summary Observations and Conclusions
- Off-site regeneration processes are more sophisticated and demanding than on-site rejuvenation processes; the off-site regeneration processes provide significantly more efficient cleaning and reconstitution of the catalyst with full recovery of activity—sometimes greater than the fresh catalyst activity. Rejuvenation provides only partial (up to 85%) recovery of the original activity.
- The development of offsite processes for regeneration of SCR catalysts is relatively new, having occurred largely over the past 10–15 years. SCR-Tech was the first and until 2008 the only company to operate an off-site regeneration facility in the U.S.
- Because surface deposits are a primary deactivation mechanism, especially in HDU catalysts, extensive multi-step treatments are required, but rejuventation or regeneration appear to be a cost-effective method of catalyst management for SCR catalysts.
4.4. Redispersion of Sintered Catalysts
During catalytic reforming of hydrocarbons on platinum-containing catalysts, growth of 1-nm platinum metal clusters to 5–20-nm crystallites occurs. An important part of the catalyst regeneration procedure is the redispersion of the platinum phase by a high temperature treatment in oxygen and chlorine, generally referred to as “oxychlorination.” A typical oxychlorination treatment involves exposure of the catalyst to HCl or CCl4 at 450–550 °C in 2–10% oxygen for a period of 1–4 h (see details in Table 22). During coke burning, some redispersion occurs, e.g., dispersion (D) increases from 0.25 to 0.51, while during oxychlorination the dispersion is further increased, e.g., from 0.51 to 0.81 [256]. A mechanism for platinum redispersion by oxygen and chlorine is shown in Figure 46 [256]. It involves the adsorption of oxygen and chlorine on the surface of a platinum crystallite and formation of AlCl3, followed by the formation of PtCl2(AlCl3)2 complexes that dissociatively adsorb on alumina to oxychloro-platinum complexes. These latter complexes form monodisperse platinum clusters upon subsequent reduction.

Table 22.
Typical Regeneration Procedure for Reforming Catalysts a.
|
a Ref. [255,256].
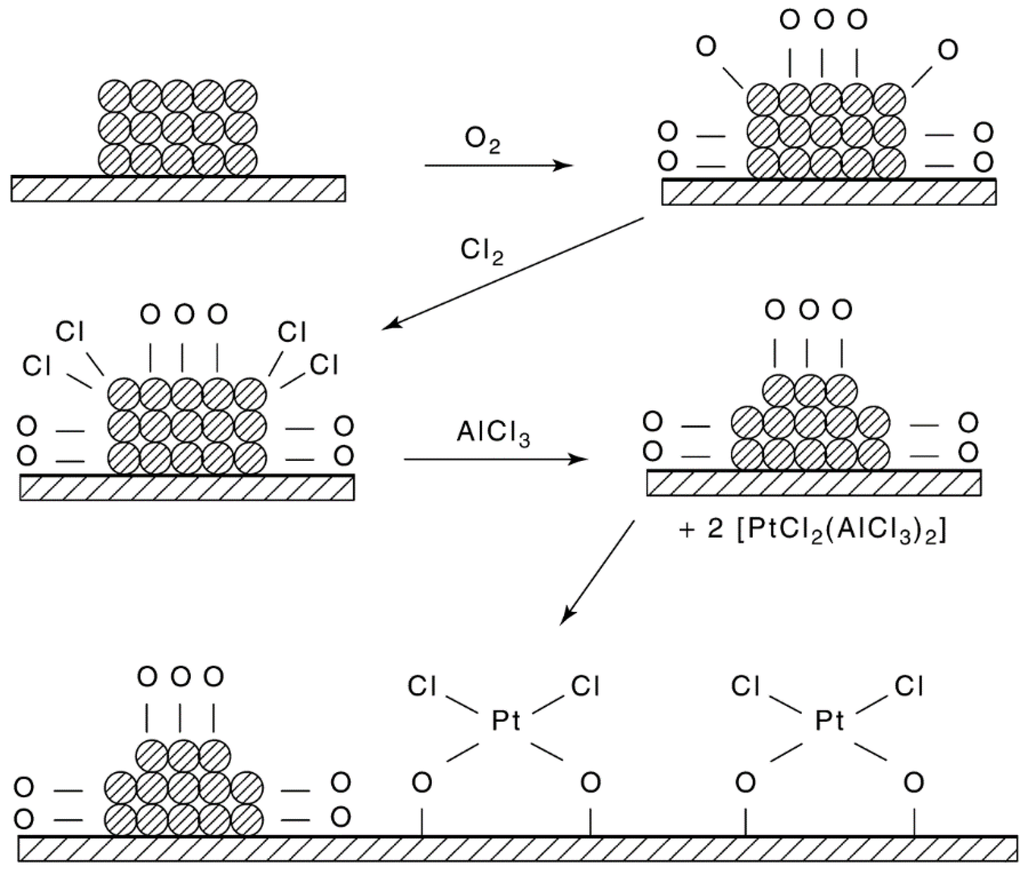
Figure 46.
Proposed mechanism for redispersion by oxychlorination of alumina-supported platinum. Reproduced from [256]. Copyright 1982, Brill Nijhoff Publishers.
Some guidelines and principles regarding the redispersion process are worth enumerating:
- (1)
- In cases involving a high degree of Pt sintering or poisoning, special regeneration procedures may be required. If large crystallites have been formed, several successive oxychlorinations are performed [256].
- (2)
- Introducing oxygen into reactors in parallel rather than in series results in a significant decrease in regeneration time [101].
- (3)
- Introduction of hydrocarbons present in the reactor recycle after regeneration is said to stabilize the catalyst; solvents such as ammonium acetate, dilute nitric acid containing lead nitrate, and EDTA and its diammonium salt are reported to dissolve out metal aggregates without leaching out the dispersed metal [101].
- (4)
- The procedures for redispersion of Pt/alumina are not necessarily applicable to Pt on other supports or to other metals. For example, Pt/silica is redispersed at lower temperature and higher Cl2 concentration (150–200 °C and 25% Cl2). Pd/alumina can be redispersed in pure O2 at 500 °C. While Pt–Re/alumina is readily redispersed by oxychlorination at 500 °C, Pt–Ir/alumina is not redispersed in the presence of O2, unless the catalyst is pretreated with HCl [266].
An extensive scientific and patent literature of redisperson describes the use of chlorine, oxygen, nitric oxide, and hydrogen as agents for redispersion of sintered catalysts (summarized in Table 23). Most of the early literature shows positive effects for chlorine compounds in the presence of oxygen in redispersing alumina-supported platinum and other noble metals. Recent literature demonstrates the need for understanding the detailed surface chemistry in order to successfully develop and improve redispersion processes, especially in more complex catalyst systems such as alumina-supported bimetallics. For example, on the basis of a fundamental study of the redispersion surface chemistry, Fung [266] developed a redispersion procedure for Pt–Ir bimetallic catalysts using a wet HCl/air treatment, since the conventional oxychlorination is not effective for this catalyst.

Table 23.
Representative Patents Prior to 1990 Treating Catalyst Redispersion.
| Dispersing agent class | Dispersing agent | Metals/support | Patent No. | Ref. |
|---|---|---|---|---|
| Chlorine-Containing | ||||
| Cl2, Cl + halogen | Pt/zeolite | U.S. 4,645,751 | [338] | |
| Cl, H2O, O2 | Pt/zeolite | U.S. 4,657,874 | [339] | |
| HCl, Cl–O | Ir | U.S. 4,491,636 | [340] | |
| Cl, O2 | Pt–Ir, Ir | U.S. 4,467,045 | [341] | |
| HCl, Cl | Pt–Ir–Re, Pt–Ir/zeolites | U.S. 4,359,400 | [342] | |
| Cl, halogen | Ir, Pt–Ir/Al2O3 | U.S. 4,480,046 | [343] | |
| Cl–H2O | Pt–Ir–Se/Al2O3 | U.S. 4,492,767 | [344] | |
| HCl–O–He | Pt–Ir–Se/Al2O3 | U.S. 4,491,635 | [345] | |
| Cl, O2 | Pt/zeolite | U.S. 4,855,269 | [346] | |
| HCl, Cl, H2O, O | Pt/zeolite | U.S. 4,925,819 | [347] | |
| HCl, O | Ir, Pt–Ir/Al2O3 | U.S. 4,444,896 | [348] | |
| Cl, halogen | Ir, Pt–Ir/Al2O3 | U.S. 4,444,895 | [349] | |
| HCl | Ir, Pt–Ir/Al2O3 | U.S. 4,517,076 | [350] | |
| Oxygen | ||||
| O2 | Pt, Re/Al2O3 | U.S. 4,482,637 | [351] | |
| Oxygen/N2 | ||||
| O2, N2 | Cu/Cr, Mn, Ru, Pd, Zn, Si, Mg, Ca, Sr, Ba | U.S. 4,855,267 | [352] | |
| Other | ||||
| NO, NO + halogen | Pt, Pd/zeolite | Eu 0,306,170 | [353] | |
| Halogen | Ru, Os, Rh, Pd/Al2O3 | U.S. 4,891,346 | [354] | |
| Halide | Ir, Pt–Ir/Al2O3 | U.S. 4,447,551 | [355] | |
| Halide, halogen/H2O | Ir, Pt–Ir/Al2O3 | U.S. 4,472,514 | [356] | |
| Halogen | Ir, Pt–Ir/Al2O3 | U.S. 4,473,656 | [357] | |
| NO, NO + halogen, Cl | Group VIII metals/Al2O3, SiO2, zeolites | U.S. 4,952,543 | [358] | |
| H2-halides, O2 | Ir, Pt–Ir/Al2O3 | U.S. 4,444,897 | [359] | |
| Halogen, H2O | Ir, Pt–Ir/Al2O3 | U.S. 4,472,515 | [360] | |
Redispersion of alumina-supported platinum and iridium crystallites is also possible in a chlorine-free oxygen atmosphere, if chlorine is present on the catalyst. The extent of redispersion depends on the properties of the Pt/Al2O3 catalyst and temperature; for example, the data in Figure 47 [102] for two different catalysts [catalyst 1 is a commercial Pt/Al2O3 (Engelhard); catalyst 2 is Pt/Al2O3 (Kaiser KA-201) impregnated with chloroplatinic acid] show that the maximum increases in dispersion occur at about 550 °C. The data also show that redispersion does not occur in a hydrogen environment. The question whether redispersion of platinum occurs only in oxygen without chlorine present on the catalyst remains controversial.
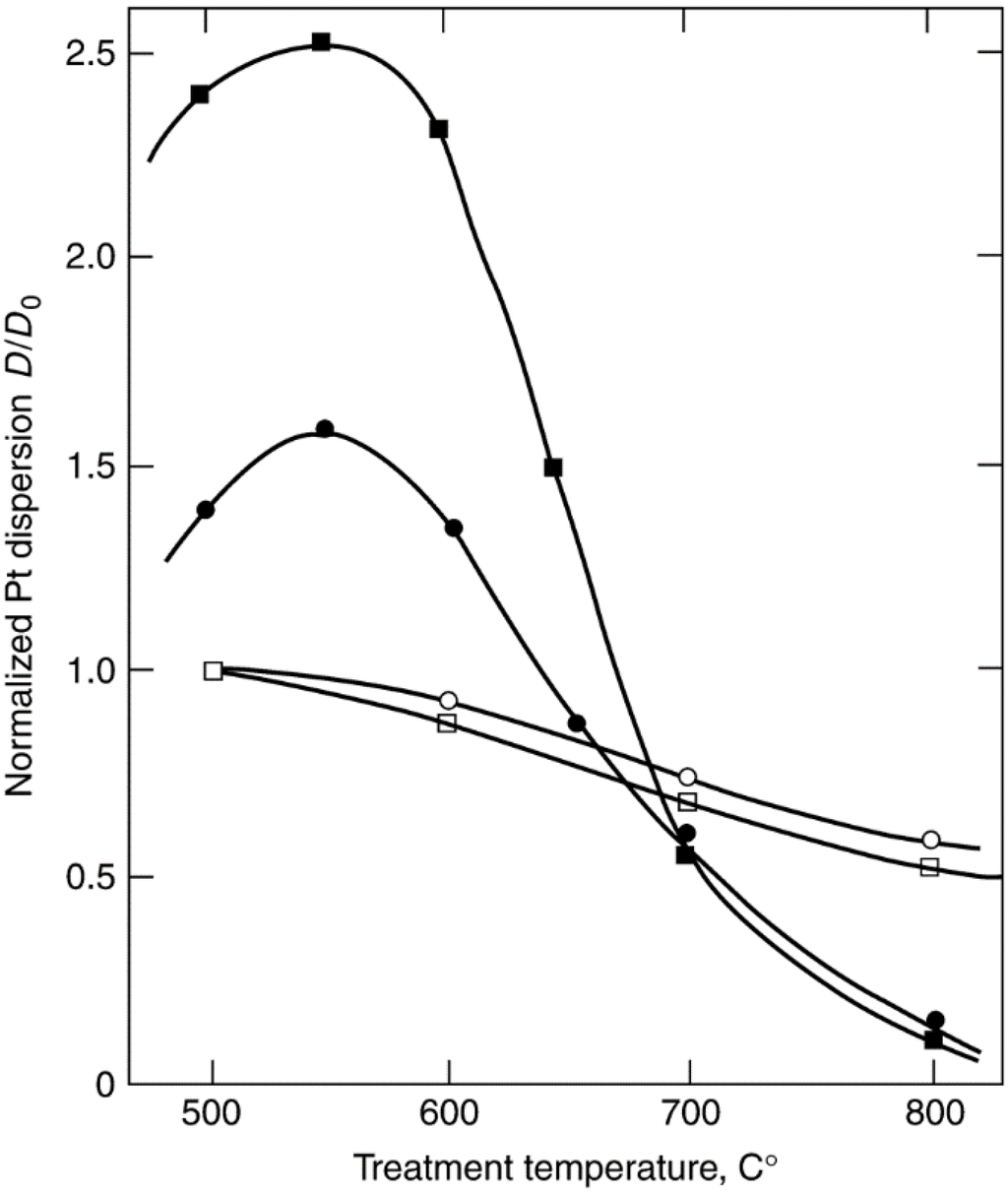
Figure 47.
Effects of 1-h treatments in O2 (closed symbols) and H2 (open symbols) on the dispersion of Pt/Al2O3 catalysts: ○,● Pt/Al2O3 (Engelhard), □,■ Pt/KA-201 alumina (Kaiser). Reproduced from [102]. Copyright 1982, Brill Nijhoff Publishers.
Two models, “the thermodynamic redispersion model” and “the crystallite splitting model,” have been advanced to explain the redispersion in oxygen [101,102,361]. The “thermodynamic” redispersion model hypothesizes the formation of metal oxide molecules that detach from the crystallite, migrate to active sites on the support, and form surface complexes with the support. Upon subsequent reduction, the metal oxide complexes form monodisperse metal clusters. In the “crystallite splitting” model, exposure of a platinum crystallite to oxygen at 500 °C leads to formation of a platinum oxide scale on the outer surface of the crystallite, which stresses and ultimately leads to splitting of the particle [361]. Dadyburjor hypothesizes that the crystallite splitting model is most applicable to the behavior of large crystallites and to all particles at relatively small regeneration times, while the thermodynamic migration model is useful for small particles and most particles after longer regeneration times.
4.4.1. Case Study: Cobalt based Fischer-Tropsch (FT) Catalyst Regeneration
Fischer-Tropsch (FT) synthesis is a catalytic process used to produce long chain hydrocarbons from synthesis gas consisting of carbon monoxide and hydrogen. Cobalt catalysts were initially developed by Franz Fischer and Hans Tropsch in the 1920s and similar cobalt-based catalysts are still in use today [8]. Although more expensive than iron based catalysts that are also used for FTS, supported cobalt FT catalysts are more active and selective for the desired liquid and wax products.
A recent review by the Davis group at the Center for Applied Energy Research at the University of Kentucky with Bukur at the University of Texas A&M in Qatar [362] focused on the results of studies using synchrotron radiation to characterize Co FT catalysts. The review includes a detailed consideration and analysis of the mechanisms and processes of sintering, oxidation, aluminate formation, and coking and carbide formation and under what operating conditions each is important. They summarize their and others’ previous findings that oxidation primarily occurs on small (<2 nm) cobalt crystallites and at high partial pressures of water [362,363,364,365,366]. Further, they highlight the potentially complicated transformations between CoO and aluminates [362,364,367]. These complications highlight a complex mechanism that may be related to chemical-assisted sintering of Co FTS catalysts through a combination of the effect of CoO reduction during the initial activation of the catalysts and water exposure during operation. First, CoO, present either due to incomplete reduction of the catalysts [368] or oxidation of the small (<2 nm) crystallites as suggested by Davis’ group [369,370] can apparently increase the sintering rate due to mobility that allows them to aggregate into larger CoO clusters that are subsequently reduced to metallic Co, as inferred from evidence presented in a number of studies [79,362,368,369,370,371]. Primarily, X-ray absorption near edge (XANES) analysis shows simultaneous increasing extent of reduction and increasing Co-Co coordination, due both to removal of oxygen and increases in particle size. Second, water is believed to cause chemical-assisted sintering [80,367,372,373,374], especially at high partial pressures that occur at CO conversions above about 65% [223], although the exact mechanisms are debated. Minor surface oxidation [373,374] and surface wetting [375] have been proposed, although Saib et al. have shown that cobalt oxidation is not an important deactivation route [79] in catalysts with Co particles >~8 nm, which are typical in commercial FTS catalysts.
A number of articles by researchers at Sasol, Eindhoven University of Technology, and the University of South Africa detailed the causes of deactivation and demonstrated the regenerability of alumina-supported cobalt FT catalysts [79,368,371,376,377,378,379,380,381,382]. Through a combination of studies on single crystal [377] and actual catalysts from pilot plants operated under industrial FT conditions [368,371], they concluded that contrary to prior hypotheses, neither formation of cobalt aluminates nor oxidation of the cobalt were significant deactivation mechanisms. In fact, extent of Co oxidation actually decreased with time on stream [371]. However, Co sintering and carbon deposition were identified as the primary means of deactivation. In unpublished presentations by these authors, the relative contributions of carbon deposition and sintering to the deactivation were reported as roughly equal. More interestingly, both of these deactivation mechanisms could be largely reversed through high pressure oxidation treatment [376,378], which removes both inactive carbon and redisperses the cobalt. Through high resolution transmission electron micrographs (HRTEM), the mechanism of redispersion of the cobalt was identified as the Kirkendall effect, which results in the formation of spherical shells of cobalt oxide that during subsequent reduction disperse into smaller crystallites of cobalt (see Figure 48). Bezemer et al. have previously shown that unpromoted Co FT catalysts require Co crystallites of at least 6 nm in diameter to achieve maximum turnover frequency, but this is the optimum size because larger crystallites display the same surface activity as the 6 nm particles [383]. The oxidative regeneration and reduction process described by Hauman et al. [376] and Weststrate et al. [377] recovers ~95% of the fresh catalyst activity by removing the carbon deposits and returning the sintered cobalt particles to near the optimum 6 nm size. While the rate per mass of catalyst is nearly constant following regeneration, some smaller particles are produced on model catalysts because the rate on a turnover frequency basis decreases by roughly 1/3 compared to the fresh catalysts [376].
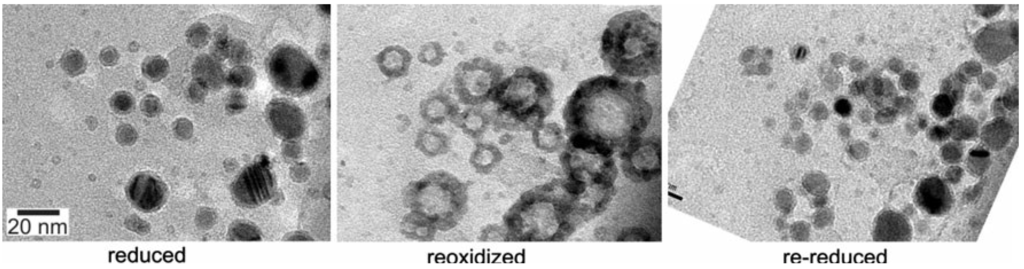
Figure 48.
Bright field TEM images showing redispersion of cobalt particles supported on a flat model silica by oxidative treatment. The center image shows hollow spheres created by the Kirkendall effect, which form dispersed smaller particles upon re-reduction in the right hand image. Reproduced from [378]. Copyright 2011, Springer.
These results are significant because they show the power of careful evaluation of the root causes of deactivation in an important catalytic system and then show how proper choice of regeneration conditions can extend the life of the catalysts by redispersion of the active metal. However, promoters may not be redispersed as completely as the cobalt during repeated regeneration. Although traditional promoters, like Pt and Ru, appear to remain with the Co and maintain their effect, some promoters like Au tend to segregate and lose their promotion effect, as indicated by TPR peaks shifting to higher temperatures [384].
5. Summary
This article focuses on the causes, mechanisms, prevention, modeling, and treatment (experimental and theoretical) of deactivation. Several general, fundamental principles are evident:
- (1)
- The causes of deactivation are basically of three kinds: chemical, mechanical, and thermal. The five intrinsic mechanisms of catalyst decay, (a) poisoning, (b) fouling, (c) thermal degradation, (d) chemical degradation, and (e) mechanical failure, vary in their reversibility and rates of occurrence. Poisoning and thermal degradation are generally slow, irreversible processes, while fouling with coke and carbon is generally rapid and reversible by regeneration with O2 or H2.
- (2)
- Catalyst deactivation is more easily prevented than cured. Poisoning by impurities can be prevented through careful purification of reactants or mitigated to some extent by adding traps or “getters” as components of the catalyst. Carbon deposition and coking can be prevented by minimizing the formation of carbon or coke precursors through gasification, careful design of catalysts and process conditions, and by controlling reaction rate regimes, e.g., mass transfer regimes, to minimize effects of carbon and coke formation on activity. Sintering is best avoided by minimizing and controlling the temperature of reaction, although recent developments have focused on encapsulating metal crystallites to eliminate mobility, while still allowing access for reactants and products.
- (3)
- Catalyst regeneration is feasible in some circumstances, especially to recover activity loss due to rapid coking or longer term deactivation associated with loss of active metal dispersion. Typically, regeneration or rejuvenation strategies are dictated by process or economic necessity to obtain desired process run lengths. Life cycle operating strategies are important considerations when evaluating catalyst regeneration/rejuvenation versus replacement decisions. Rejuvenation treatments can extend the useful life of catalysts. Selective catalytic reduction catalysts provide an example of rejuvenation practiced in a commercial process.
Acknowledgments
The authors wish to acknowledge Brigham Young University for its support and the employees of MDPI, for their untiring assistance in publishing this review.
Author Contributions
Calvin H. Bartholomew was the primary author of this review. Morris D. Argyle provided assistance in writing and revising the case studies and updating the article in response to the reviewers’ comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Figueiredo, J.L. Carbon formation and gasification on nickel. In Progress in Catalyst Deactivation; (NATO Advanced Study Institute Series E, No. 54); Martinus Nijhoff Publishers: Boston, MA, USA, 1982; pp. 45–63. [Google Scholar]
- Hughes, R. Deactivation of Catalysts; Academic Press: London, UK, 1984. [Google Scholar]
- Oudar, J.; Wise, H. Deactivation and Poisoning of Catalysts; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Butt, J.B.; Petersen, E.E. Activation, Deactivation, and Poisoning of Catalysts; Academic Press: San Diego, CA, USA, 1988. [Google Scholar]
- Denny, P.J.; Twigg, M.V. Factors determining the life of industrial heterogeneous catalysts. In Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nerthelands, 1980; Volume 6, pp. 577–599. [Google Scholar]
- Bartholomew, C.H. Catalyst Deactivation. Chem. Eng. 1984, 91, 96–112. [Google Scholar]
- Butt, J.B. Catalyst deactivation and regeneration. In Catalysis—Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer-Verlag: New York, NY, USA, 1984; Volume 6, pp. 1–63. [Google Scholar]
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Delmon, B.; Froment, G.F. Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Elsevier: Amsterdam, The Nerthelands, 1980; Volume 6. [Google Scholar]
- Delmon, B.; Froment, G.F. Catalyst Deactivation 1987 (Studies in Surface Science and Catalysis); Elsevier: Amsterdam, The Nerthelands, 1987; Volume 34. [Google Scholar]
- Bartholomew, C.H.; Butt, J.B. Catalyst Deactivation 1991 (Studies in Surface Science and Catalysis); Elsevier: Amsterdam, The Nerthelands, 1991; Volume 68. [Google Scholar]
- Delmon, B.; Froment, G.F. Catalyst Deactivation 1994 (Studies in Surface Science and Catalysis); Elsevier: Amsterdam, The Nerthelands, 1994; Volume 88. [Google Scholar]
- Bartholomew, C.H.; Fuentes, G.A. Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Elsevier: Amsterdam, The Nethelands, 1997; Volume 111. [Google Scholar]
- Delmon, B.; Froment, G.F. Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Elsevier: Amsterdam, The Nethelands, 1999; Volume 126. [Google Scholar]
- Moulijn, J.A. Catalyst Deactivation. Appl. Catal. A 2001, 212, 1–255. [Google Scholar] [CrossRef]
- Maxted, E.B. The poisoning of metallic catalysts. Adv. Catal. 1951, 3, 129–177. [Google Scholar]
- Hegedus, L.L.; McCabe, R.W. Catalyst poisoning. In Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1980; Volume 6, pp. 471–505. [Google Scholar]
- Hegedus, L.L.; McCabe, R.W. Catalyst Poisoning; Marcel Dekker: New York, NY, USA, 1984. [Google Scholar]
- Butt, J.B. Catalyst poisoning and chemical process dynamics. In Progress in Catalyst Deactivation (NATO Advanced Study Institute Series E, No. 54); Figueiredo, J.L., Ed.; Martinus Nijhoff Publishers: Boston, MA, USA, 1982; pp. 153–208. [Google Scholar]
- Barbier, J. Effect of poisons on the activity and selectivity of metallic catalysts. In Deactivation and Poisoning of Catalysts; Oudar, J., Wise, H., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 109–150. [Google Scholar]
- Bartholomew, C.H. Mechanisms of nickel catalyst poisoning. In Catalyst Deactivation 1987 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1987; Volume 34, pp. 81–104. [Google Scholar]
- Rostrup-Nielsen, J.R. Promotion by poisoning. In Catalyst Deactivation 1991 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Butt, J.B., Eds.; Elsevier: Amsterdam, The Nethelands, 1991; Volume 68, pp. 85–101. [Google Scholar]
- Inga, J.; Kennedy, P.; Leviness, S. A1 Fischer-tropsch process in the presence of nitrogen contaminants. WIPO Patent WO 2005/071044, 4 August 2005. [Google Scholar]
- Völiter, V.J.; Hermann, M. Katalytische Wirksamkeit van reinem und von CO-vergiftetem Platin bei der p-H2-Umwandlung. Z. Anorg. Allg. Chem. 1974, 405, 315. [Google Scholar] [CrossRef]
- Baron, K. Carbon monoxide oxidation on platinum-lead films. Thin Solid Films 1978, 55, 449–462. [Google Scholar] [CrossRef]
- Clay, R.D.; Petersen, E.E. Catalytic activity of an evaporated platinum film progressively poisoned with arsine. J. Catal. 1970, 16, 32–43. [Google Scholar]
- Madon, R.J.; Seaw, H. Effect of Sulfur on the Fischer-Tropsch Synthesis. Catal. Rev.—Sci. Eng. 1977, 15, 69–106. [Google Scholar]
- Bartholomew, C.H.; Agrawal, P.K.; Katzer, J.R. Sulfur poisoning of metals. Adv. Catal. 1982, 31, 135–242. [Google Scholar]
- Rostrup-Nielsen, J.R. Sulfur Poisoning. In Progress in Catalyst Deactivation (NATO Advanced Study Institute Series E, No. 54); Figueiredo, J.L., Ed.; Martinus Nijhoff Publishers: Boston, MA, USA, 1982; pp. 209–227. [Google Scholar]
- Wise, H.; McCarty, J.; Oudar, J. Sulfur and carbon interactions with metal surfaces. In Deactivation and Poisoning of Catalysts; Oudar, J., Wise, H., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 1–50. [Google Scholar]
- Rostrup-Nielsen, J.R.; Nielsen, P.E.H. Catalyst deactivation in synthesis gas production, and important syntheses. In Deactivation and Poisoning of Catalysts; Oudar, J., Wise, H., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 259–323. [Google Scholar]
- Grossmann, A.; Erley, W.; Ibach, H. Adsorbate-induced surface stress and surface reconstruction: oxygen, sulfur and carbon on Ni(111). Surf. Sci. 1995, 337, 183–189. [Google Scholar]
- Ruan, L.; Stensgaard, I.; Besenbacher, F.; Lægsgaard, E. Observation of a missing-row structure on an fcc (111) surface: The (5 √3 ×2)S phase on Ni(111) studied by scanning tunneling microscopy. Phys. Rev. Lett. 1993, 71, 2963–2966. [Google Scholar]
- Kitajima, Y.; Yokoyama, T.; Ohta, T.; Funabashi, M.; Kosugi, N.; Kuroda, H. Surface EXAFS and XANES studies of (5v3× 2)S/Ni(111). Surf. Sci. 1989, 214, L261–L269. [Google Scholar] [CrossRef]
- Perdereau, M.; Oudar, J. Structure, mécanisme de formation et stabilité de la couche d'adsorption du soufre sur le nickel. Surf. Sci. 1970, 20, 80–98. [Google Scholar]
- Oudar, J. Sulfur adsorption and poisoning of metallic catalysts. Catal. Rev.—Sci. Eng. 1980, 22, 171–195. [Google Scholar]
- McCarroll, J.J.; Edmonds, T.; Pitkethly, R.C. Interpretation of a complex low energy electron diffraction pattern: Carbonaceous and sulphur-containing structures on Ni(111). Nature 1969, 223, 1260–1262. [Google Scholar]
- Edmonds, T.; McCarroll, J.J.; Pitkethly, R.C. Surface structures formed during the interaction of sulphur compounds with the (111) face of nickel. J. Vac. Sci. Technol. 1971, 8, 68–74. [Google Scholar]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60. [Google Scholar]
- Ruan, L.; Besenbacher, F.; Stensgaard, I.; Lægsgaard, E. Atom-resolved studies of the reaction between H2S and O on Ni(110). Phys. Rev. Lett. 1992, 69, 3523–3526. [Google Scholar]
- Hepola, J.; McCarty, J.; Krishnan, G.; Wong, V. Elucidation of behavior of sulfur on nickel-based hot gas cleaning catalysts. Appl. Catal. B 1999, 20, 191–203. [Google Scholar]
- Erley, W.; Wagner, H. Sulfur poisoning of carbon monoxide adsorption on Ni(111). J. Catal. 1978, 53, 287–294. [Google Scholar]
- Rendulic, K.D.; Winkler, A. The initial sticking coefficient of hydrogen on sulfur- and oxygen-covered polycrystalline nickel surfaces. Surf. Sci. 1978, 74, 318–320. [Google Scholar]
- Goodman, D.W.; Kiskinova, M. Chemisorption and reactivity studies of H2 and CO on sulfided Ni(100). Surf. Sci. 1981, 105, L265–L270. [Google Scholar]
- Kiskinova, M.; Goodman, D.W. Modification of chemisorption properties by electronegative adatoms: H2 and CO on chlorided, sulfided, and phosphided Ni(100). Surf. Sci. 1981, 108, 64–76. [Google Scholar]
- Johnson, S.; Madix, R.S. Desorption of hydrogen and carbon monoxide from Ni(100), Ni(100)p(2 × 2)S, and Ni(100)c(2 × 2)S surfaces. Surf. Sci. 1981, 108, 77–98. [Google Scholar]
- Madix, R.J.; Thornberg, M.; Lee, S.B. CO-sulfur interaction on Ni(110); evidence for local interactions, not long range electronic effects. Surf. Sci. 1983, 133, L447–L451. [Google Scholar]
- Hardegree, E.L.; Ho, P.; White, J.M. Sulfur adsorption on Ni(100) and its effect on CO chemisorption: I. TDS, AES and work function results. Surf. Sci. 1986, 165, 488–506. [Google Scholar]
- Erekson, E.J.; Bartholomew, C.H. Sulfur poisoning of nickel methanation catalysts: II. Effects of H2S concentration, CO and H2O partial pressures and temperature on reactivation rates. Appl. Catal. 1983, 5, 323–336. [Google Scholar]
- Jacobs, G.; Ghadiali, F.; Pisanu, A.; Padro, C.L.; Borgna, A.; Alvarez, W.E.; Resasco, D.E. Increased Sulfur Tolerance of Pt/KL Catalysts Prepared by Vapor-Phase Impregnation and Containing a Tm Promoter. J. Catal. 2000, 191, 116–127. [Google Scholar]
- Jongpatiwut, S.; Sackamduang, P.; Rirksomboon, T.; Osuwan, S.; Alvarez, W.E.; Resasco, D.E. Sulfur- and water-tolerance of Pt/KL aromatization catalysts promoted with Ce and Yb. Appl. Catal. A 2002, 230, 177–193. [Google Scholar]
- Jacobs, G.; Ghadiali, F.; Pisanu, A.; Borgna, A.; Alvarez, W.E.; Resasco, D.E. Characterization of the morphology of Pt clusters incorporated in a KL zeolite by vapor phase and incipient wetness impregnation. Influence of Pt particle morphology on aromatization activity and deactivation. Appl. Catal. A 1999, 188, 79–98. [Google Scholar]
- McVicker, G.B.; Kao, J.L.; Ziemiak, T.J.J.; Gates, W.E.; Robbins, J.L.; Treacy, M.M.J.; Rice, S.B.; Vanderspurt, T.H.; Cross, V.R.; Ghosh, A.K. Effect of Sulfur on the Performance and on the Particle Size and Location of Platinum in Pt/KL Hexane Aromatization Catalysts. J. Catal. 1993, 139, 48–61. [Google Scholar]
- Farbenindustrie, I.G. Improvements in the Manufacture and Production of Unsaturated Hydrocarbons of Low Boiling Point. British Patent 322,284, 5 December 1929. [Google Scholar]
- Kritzinger, J.A. The role of sulfur in commercial iron-based Fischer–Tropsch catalysis with focus on C2-product selectivity and yield. Catal. Today 2002, 71, 307–318. [Google Scholar]
- Sparks, D.E.; Jacobs, G.; Gnanamani, M.K.; Pendyala, V.R.R.; Ma, W.; Kang, J.; Shafer, W.D.; Keogh, R.A.; Graham, U.M.; Gao, P.; et al. Poisoning of cobalt catalyst used for Fischer–Tropsch synthesis. Catal. Today 2013, 215, 67–72. [Google Scholar]
- Rostrup-Nielsen, J.R.; Trimm, D.L. Mechanisms of carbon formation on nickel-containing catalysts. J. Catal. 1977, 48, 155–165. [Google Scholar]
- Trimm, D.L. The Formation and Removal of Coke from Nickel Catalyst. Catal. Rev.—Sci. Eng. 1977, 16, 155–189. [Google Scholar]
- Trimm, D.L. Catalyst design for reduced coking (review). Appl. Catal. 1983, 5, 263–290. [Google Scholar]
- Bartholomew, C.H. Carbon deposition in steam reforming and methanation. Catal. Rev.—Sci. Eng. 1982, 24, 67–112. [Google Scholar]
- Albright, L.F.; Baker, R.T.K. Coke Formation on Metal Surfaces (ACS Symposium Series 202); American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Menon, P.G. Coke on catalysts—harmful, harmless, invisible and beneficial types. J. Mol. Catal. 1990, 59, 207–220. [Google Scholar]
- Rostrup-Nielsen, J.R. Conversion of hydrocarbons and alcohols for fuel cells. Phys. Chem. Chem. Phys. 2001, 3, 283–288. [Google Scholar]
- Trane-Restrup, R.; Resasco, D.E.; Jensen, A.D. Steam reforming of light oxygenates. Catal. Sci. Technol. 2013, 3, 3292–3302. [Google Scholar]
- De Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Assaf, J.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Evaluation of the performance of Ni/La2O3 catalyst prepared from LaNiO3 perovskite-type oxides for the production of hydrogen through steam reforming and oxidative steam reforming of ethanol. Appl. Catal. A 2010, 377, 181–190. [Google Scholar]
- Deken, J.D.; Menon, P.G.; Froment, G.F.; Haemers, G. On the nature of carbon in Niα-Al2O3 catalyst deactivated by the methane-steam reforming reaction. J. Catal. 1981, 70, 225–229. [Google Scholar]
- Durer, W.G.; Craig, J.H., Jr.; Lozano, J. Surface carbon and its effects on hydrogen adsorption on Rh(100). Appl. Surf. Sci. 1990, 45, 275–277. [Google Scholar]
- Moeller, A.D.; Bartholomew, C.H. Deactivation by carbon of nickel and nickel-molybdenum methanation catalysts. Prepr.—Am. Chem. Soc., Div. Fuel Chem. 1980, 25, 54–70. [Google Scholar]
- Marschall, K.-J.; Mleczko, L. Short-contact-time reactor for catalytic partial oxidation of methane. Ind. Eng. Chem. Res. 1999, 38, 1813–1821. [Google Scholar]
- Rostrup-Nielsen, J.R. Catalytic steam reforming. In Catalysis—Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer-Verlag: New York, NY, USA, 1984; Volume 5, pp. 1–117. [Google Scholar]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Nørskov, J.K.; Stensgaard, I. Design of a surface alloy catalyst for steam reforming. Science 1998, 279, 1913–1915. [Google Scholar]
- Nemes, T.; Chambers, A.; Baker, R.T.K. Characteristics of carbon filament formation from the interaction of Cobalt−Tin particles with ethylene. J. Phys. Chem. B 1998, 102, 6323–6330. [Google Scholar]
- Bartholomew, C.H.; Strasburg, M.V.; Hsieh, H. Effects of support on carbon formation and gasification on nickel during carbon monoxide hydrogenation. Appl. Catal. 1988, 36, 147–162. [Google Scholar]
- Vance, C.K.; Bartholomew, C.H. Hydrogenation of carbon dioxide on group viii metals: III, Effects of support on activity/selectivity and adsorption properties of nickel. Appl. Catal. 1983, 7, 169–177. [Google Scholar]
- Baker, R.T.K.; Chludzinski, J.J., Jr. Filamentous carbon growth on nickel-iron surfaces: The effect of various oxide additives. J. Catal. 1980, 64, 464–468. [Google Scholar]
- Brown, D.E.; Clark, J.T.K.; Foster, A.I.; McCarroll, J.J.; Sims, M.L. Inhibition of coke formation in ethylene steam cracking. In Coke Formation on Metal Surfaces (ACS Symposium Series 202); Albright, L.F., Baker, R.T.K., Eds.; American Chemical Society: Washington, DC, USA, 1982; pp. 23–43. [Google Scholar]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Deactivation and coke accumulation during CO2/CH4 reforming over Pt catalysts. J. Catal. 1999, 183, 336–343. [Google Scholar]
- Rostrup-Nielsen, J.R. Coking on nickel catalysts for steam reforming of hydrocarbons. J. Catal. 1974, 33, 184–201. [Google Scholar]
- Saib, A.M.; Moodley, D.J.; Ciobica, I.M.; Hauman, M.M.; Sigwebela, B.H.; Weststrate, C.J.; Niemanstsverdriet, J.W.; van de Loosdrecht, J. Fundamental understanding of deactivation and regeneration of cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2010, 154, 271–282. [Google Scholar]
- Tsakoumis, N.E.; Rønning, M.; Borg, O.; Rytter, E.; Holmen, A. Deactivation of cobalt based Fischer–Tropsch catalysts: A review. Catal. Today 2010, 154, 162–182. [Google Scholar]
- Gates, B.C.; Katzer, J.R.; Schuit, G.C.A. Chemistry of Catalytic Processes; McGraw-Hill: New York, NY, USA, 1979. [Google Scholar]
- Naccache, C. Deactivation of acid catalysts. In Deactivation and Poisoning of Catalysts; Oudar, J., Wise, H., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 185–203. [Google Scholar]
- Appleby, W.G.; Gibson, J.W.; Good, G.M. Coke Formation in Catalytic Cracking. Ind. Eng. Chem. Process Des. Dev. 1962, 1, 102–110. [Google Scholar] [CrossRef]
- Beuther, H.; Larson, O.H.; Perrotta, A.J. The mechanism of coke formation on catalysts. In Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nerthelands, 1980; Volume 6, pp. 271–282. [Google Scholar]
- Gayubo, A.G.; Arandes, J.M.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Deactivation and acidity deterioration of a silica/alumina catalyst in the isomerization of cis-butene. Ind. Eng. Chem. Res. 1993, 32, 588–593. [Google Scholar]
- Augustine, S.M.; Alameddin, G.N.; Sachtler, W.M.H. The effect of Re, S, and Cl on the deactivation of Pt γ-Al2O3 reforming catalysts. J. Catal. 1989, 115, 217–232. [Google Scholar]
- Guisnet, M.; Magnoux, P. Coking and deactivation of zeolites: Influence of the Pore Structure. Appl. Catal. 1989, 54, 1–27. [Google Scholar]
- Bauer, F.; Kanazirev, V.; Vlaev, C.; Hanisch, R.; Weiss, W. Koksbildung in ZSM-5-Katalysatoren. Chem. Tech. 1989, 41, 297–301. [Google Scholar]
- Grotten, W.A.; Wojciechowski, B.W.; Hunter, B.K. On the relationship between coke formation chemistry and catalyst deactivation. J. Catal. 1992, 138, 343–350. [Google Scholar]
- Bellare, A.; Dadyburjor, D.B. Evaluation of modes of catalyst deactivation by coking for cumene cracking over zeolites. J. Catal. 1993, 140, 510–525. [Google Scholar]
- Uguina, M.A.; Serrano, D.P.; Grieken, R.V.; Vènes, S. Adsorption, acid and catalytic changes induced in ZSM-5 by coking with different hydrocarbons. Appl. Catal. A 1993, 99, 97–113. [Google Scholar]
- Li, C.; Chen, Y.-W.; Yang, S.-J.; Yen, R.-B. In-situ FTIR investigation of coke formation on USY zeolite. Appl. Surf. Sci. 1994, 81, 465–468. [Google Scholar]
- Buglass, J.G.; de Jong, K.P.; Mooiweer, H.H. Analytical studies of the coking of the zeolite ferrierite. J. Chem. Soc. Abstr. 1995, 210. 105-PETR. [Google Scholar]
- Chen, D.; Rebo, H.P.; Moljord, K.; Holmen, A. Effect of coke deposition on transport and adsorption in zeolites studied by a new microbalance reactor. Chem. Eng. Sci. 1996, 51, 2687–2692. [Google Scholar]
- Guisnet, M.; Magnoux, P.; Martin, D. Roles of acidity and pore structure in the deactivation of zeolites by carbonaceous deposits. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 1–19. [Google Scholar]
- Masuda, T.; Tomita, P.; Fujikata, Y.; Hashimoto, K. Deactivation of HY-type zeolite catalyst due to coke deposition during gas-oil cracking. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 89–96. [Google Scholar]
- Cerqueira, H.S.; Magnoux, P.; Martin, D.; Guisnet, M. Effect of contact time on the nature and location of coke during methylcyclohexane transformation over a USHY zeolite. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 105–112. [Google Scholar]
- Wanke, S.E.; Flynn, P.C. The sintering of supported metal catalysts. Catal. Rev.—Sci. Eng. 1975, 12, 93–135. [Google Scholar]
- Wynblatt, P.; Gjostein, N.A. Supported metal crystallites. Prog. Solid State Chem. 1975, 9, 21–58. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Pulvermacher, B. Kinetics of crystallite sintering during heat treatment of supported metal catalysts. AIChE J. 1973, 19, 356–364. [Google Scholar]
- Ruckenstein, E.; Dadyburjor, D.B. Sintering and redispersion in supported metal catalysts. Rev. Chem. Eng. 1983, 1, 251–356. [Google Scholar]
- Wanke, S.E. Sintering of commercial supported platinum group metal catalysts. In Progress in Catalyst Deactivation (NATO Advanced Study Institute Series E, No. 54); Figueiredo, J.L., Ed.; Martin Nijhoff Publishers: Boston, MA, USA, 1982; pp. 315–328. [Google Scholar]
- Baker, R.T.; Bartholomew, C.H.; Dadyburjor, D.B. Sintering and Redispersion: Mechanisms and Kinetics. In Stability of Supported Catalysts: Sintering and Redispersion; Horsley, J.A., Ed.; Catalytica: Mountain View, CA, USA, 1991; pp. 169–225. [Google Scholar]
- Bartholomew, C.H. Model catalyst studies of supported metal sintering and redispersion kinetics. In Catalysis (Specialist Periodical Report); Spivey, J.J., Agarwal, S.K., Eds.; Royal Society of Chemistry: Cambridge, UK, 1993; Volume 10, pp. 41–82. [Google Scholar]
- Bartholomew, C.H. Sintering kinetics of supported metals: new perspectives from a unifying GPLE treatment. Appl. Catal. A 1993, 107, 1–57. [Google Scholar]
- Bartholomew, C.H. Sintering kinetics of supported metals: perspectives from a generalized power law approach. In Catalyst Deactivation 1994 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1994; Volume 88, pp. 1–18. [Google Scholar]
- Bartholomew, C.H. Sintering and redispersion of supported metals: Perspectives from the literature of the past decade. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 585–592. [Google Scholar]
- Bartholomew, C.H.; Sorenson, W. Sintering kinetics of silica- and alumina-supported nickel in hydrogen atmosphere. J. Catal. 1983, 81, 131–141. [Google Scholar]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: is it predictable?: What to do? Appl. Catal. A 2001, 212, 3–16. [Google Scholar]
- Bridger, G.W.; Spencer, M.S. Methanol synthesis. In Catalyst Handbook, 2nd ed.; Twigg, M.V., Ed.; Manson Publishing: London, UK, 1996; pp. 441–468. [Google Scholar]
- Fuentes, G.A. Catalyst deactivation and steady-state activity: A generalized power-law equation model. Appl. Catal. 1985, 15, 33–40. [Google Scholar]
- Fuentes, G.A.; Ruiz-Trevino, F.A. Towards a better understanding of sintering phenomena in catalysis. In Catalyst Deactivation 1991 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Butt, J.B., Eds.; Elsevier: Amsterdam, The Nethelands, 1991; Volume 68, pp. 637–644. [Google Scholar]
- Bournonville, J.P.; Martino, G. Sintering of Alumina Supported Platinum. In Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1980; Volume 6, pp. 159–166. [Google Scholar]
- Somorjai, G.A. Small-Angle X-Ray Scattering and Low Energy Electron Diffraction Studies on Catalyst Surfaces. In X-ray and Electron Methods of Analysis (Progress in Analytical Chemistry); Van Olphen, H., Parrish, W., Eds.; Plenum Press: New York, NY, USA, 1968; Volume 1, pp. 101–126. [Google Scholar]
- Seyedmonir, S.R.; Strohmayer, D.E.; Guskey, G.J.; Geoffroy, G.L.; Vannice, M.A. Characterization of supported silver catalysts: III. Effects of support, pretreatment, and gaseous environment on the dispersion of Ag. J. Catal. 1985, 93, 288–302. [Google Scholar] [CrossRef]
- Trimm, D.L. Thermal stability of catalyst supports. In Catalyst Deactivation 1991 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Butt, J.B., Eds.; Elsevier: Amsterdam, The Nethelands, 1991; Volume 68, pp. 29–51. [Google Scholar]
- Shastri, A.G.; Datye, A.K.; Schwank, J. Influence of chlorine on the surface area and morphology of TiO2. Appl. Catal. 1985, 14, 119–131. [Google Scholar] [CrossRef]
- Oberlander, R.K. Aluminas for catalysts: Their preparations and properties. In Applied Industrial Catalysis; Leach, B.E., Ed.; Academic Press: Orlando, FL, USA, 1984; Volume 3, pp. 64–112. [Google Scholar]
- Wefers, K.; Misra, C. Oxides and Hydroxides of Aluminum; Alcoa Technical Paper No. 19; Alcoa Laboratories: Pittsburg, PA, USA, 1987. [Google Scholar]
- Hegedus, L.L.; Baron, K. Phosphorus accumulation in automotive catalysts. J. Catal. 1978, 54, 115–119. [Google Scholar] [CrossRef]
- Summers, J.; Hegedus, L.L. Modes of catalyst deactivation in stoichiometric automobile exhaust. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 318–324. [Google Scholar] [CrossRef]
- Peter-Hoblyn, J.D.; Valentine, J.M.; Sprague, B.N.; Epperly, W.R. Methods for reducing harmful emissions from a diesel engine. U.S. Patent 6,003,303, 21 December 1999. [Google Scholar]
- Manson, I. Self-regenerating diesel exhaust particulate filter and material. U.S. Patent 6,013,599, 11 January 2000. [Google Scholar]
- Deeba, M.; Lui, Y.K.; Dettling, J.C. Four-way diesel exhaust catalyst and method of use. U.S. Patent 6,093,378, 25 July 2000. [Google Scholar]
- Dry, M.E. The fischer-tropsch synthesis. In Catalysis—Science and Technology; Anderson, J., Boudart, M., Eds.; Springer-Verlag: New York, NY, USA, 1981; pp. 159–218. [Google Scholar]
- Huber, G.W.; Guymon, C.G.; Stephenson, B.C.; Bartholomew, C.H. Hydrothermal stability of Co/SiO2 Fischer-Tropsch synthesis catalysts. In Catalyst Deactivation 2001 (Studies in Surface Science and Catalysis); Spivey, J.J., Roberts, G.W., Davis, B.H., Eds.; Elsevier: Amsterdam, The Netheland, 2001; Volume 139, pp. 423–430. [Google Scholar]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Kobylinski, T.P.; Taylor, B.W.; Yong, J.E. Stabilized ruthenium catalysts For NO x reduction. Proc. SAE, 1974; Paper 740250. [Google Scholar]
- Shelef, M.; Gandhi, H.S. The reduction of nitric oxide in automobile emissions: Stabilisation of catalysts containing ruthenium. Platinum Met. Rev. 1974, 18, 2–14. [Google Scholar]
- Gandhi, H.S.; Stepien, H.K.; Shelef, M. Optimization of ruthenium-containing, stabilized, nitric oxide reduction catalysts. Mat. Res. Bull. 1975, 10, 837–845. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Reduction of nitric oxide by monolithic-supported palladium-nickel and palladium-ruthenium alloys. Ind. Eng. Chem. Prod. Res. Dev. 1975, 14, 29–33. [Google Scholar] [CrossRef]
- Clark, R.W.; Tien, J.K.; Wynblatt, P. Loss of palladium from model platinum-palladium supported catalysts during annealing. J. Catal. 1980, 61, 15–18. [Google Scholar] [CrossRef]
- Shen, W.M.; Dumesic, J.A.; Hill, C.G. Criteria for stable Ni particle size under methanation reaction conditions: Nickel transport and particle size growth via nickel carbonyl. J. Catal. 1981, 68, 152–165. [Google Scholar] [CrossRef]
- Pannell, R.B.; Chung, K.S.; Bartholomew, C.H. The stoichiometry and poisoning by sulfur of hydrogen, oxygen and carbon monoxide chemisorption on unsupported nickel. J. Catal. 1977, 46, 340–347. [Google Scholar] [CrossRef]
- Lohrengel, G.; Baerns, M. Determination of the metallic surface area of nickel and its dispersion on a silica support by means of a microbalance. Appl. Catal. 1981, 1, 3–7. [Google Scholar] [CrossRef]
- Qamar, I.; Goodwin, J.G. Fischer-Tropsch Synthesis over Composite Ru Catalysts. In Proceedings of 8th North American Catalysis Society Meeting, Philadelphia, PA, USA; 1983. Paper C-22. [Google Scholar]
- Goodwin, J.G.; Goa, D.O.; Erdal, S.; Rogan, F.H. Reactive metal volatilization from Ru/Al2O3 as a result of ruthenium carbonyl formation. Appl. Catal. 1986, 24, 199–209. [Google Scholar] [CrossRef]
- Watzenberger, O.; Haeberle, T.; Lynch, D.T.; Emig, G. Deactivation of Heteropolyacid Catalysts. In Catalyst Deactivation 1991 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Butt, J.B., Eds.; Elsevier: Amsterdam, The Nethelands, 1991; Volume 68, pp. 441–448. [Google Scholar]
- Agnelli, M.; Kolb, M.; Mirodatos, C. Co hydrogenation on a nickel catalyst: 1. Kinetics and modeling of a low-temperature sintering process. J. Catal. 1994, 148, 9–21. [Google Scholar] [CrossRef]
- Lee, H.C.; Farrauto, R.J. Catalyst deactivation due to transient behavior in nitric acid production. Ind. Eng. Chem. Res. 1989, 28, 1–5. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Lee, H.C. Ammonia oxidation catalysts with enhanced activity. Ind. Eng. Chem. Res. 1990, 29, 1125–1129. [Google Scholar] [CrossRef]
- Sperner, F.; Hohmann, W. Rhodium-platinum gauzes for ammonia oxidation. Platinum Met. Rev. 1976, 20, 12–20. [Google Scholar]
- Hess, J.M.; Phillips, J. Catalytic etching of Pt/Rh gauzes. J. Catal. 1992, 136, 149–160. [Google Scholar] [CrossRef]
- Kuo, C.L.; Hwang, K.C. Does morphology of a metal nanoparticle play a role in ostwald ripening processes? Chem. Mater. 2013, 25, 365–371. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Hydrogen adsorption on supported cobalt, iron, and nickel. Catal. Lett. 1990, 7, 27–51. [Google Scholar] [CrossRef]
- Wu, N.L.; Phillips, J. Catalytic etching of platinum during ethylene oxidation. J. Phys. Chem. 1985, 89, 591–600. [Google Scholar] [CrossRef]
- Wu, N.L.; Phillips, J. Reaction‐enhanced sintering of platinum thin films during ethylene oxidation. J. Appl. Phys. 1986, 59, 769–779. [Google Scholar] [CrossRef]
- Wu, N.L.; Phillips, J. Sintering of silica-supported platinum catalysts during ethylene oxidation. J. Catal. 1988, 113, 129–143. [Google Scholar] [CrossRef]
- Bielanski, A.; Najbar, M.; Chrzaoszcz, J.; Wal, W. Deactivation of the V2O5-MoO3, catalysts in the selective oxidation of eenzene to maleic anhydride and the changes in its morphology and chemical composition. In Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1980; Volume 6, pp. 127–140. [Google Scholar]
- Burriesci, N.; Garbassi, F.; Petrera, M.; Petrini, G.; Pernicone, N. Solid state reactions in Fe-Mo oxide catalysts for methanol oxidation during aging in industrial plants. In Catalyst Deactivation 1980 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1980; Volume 6, pp. 115–126. [Google Scholar]
- Xiong, Y.L.; Castillo, R.; Papadopoulou, C.; Dada, L.; Ladriere, J.; Ruiz, P.; Delmon, B. The protecting role of antimony oxide against deactivation of iron molybdate in oxidation catalysts. In Catalyst Deactivation 1991 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Butt, J.B., Eds.; Elsevier: Amsterdam, The Nethelands, 1991; Volume 68, pp. 425–432. [Google Scholar]
- Farrauto, R.J.; Hobson, M.; Kennelly, T.; Waterman, E. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Gai-Boyes, P.L. Defects in Oxide Catalysts: Fundamental Studies of Catalysis in Action. Catal. Rev.—Sci. Eng. 1992, 34, 1–54. [Google Scholar] [CrossRef]
- Delmon, B. Solid-state reactions in catalysts during ageing: Beneficial role of spillover. In Catalyst Deactivation 1994 (Studies in Surface Science and Catalysis, Volume 88); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1994; pp. 113–128. [Google Scholar]
- Erickson, K.M.; Karydis, D.A.; Boghosian, S.; Fehrmann, R. Deactivation and compound formation in sulfuric-acid catalysts and model systems. J. Catal. 1995, 155, 32–42. [Google Scholar] [CrossRef]
- Delmon, B. Solid state reactions in catalysts: An approach to real active systems and their deactivation. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 39–51. [Google Scholar]
- Jackson, N.B.; Datye, A.K.; Mansker, L.; O’Brien, R.J.; Davis, B.H. Deactivation and attrition of iron catalysts in synthesis gas. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 501–516. [Google Scholar]
- Eliason, S.A.; Bartholomew, C.H. Temperature-programmed reaction study of carbon transformations on iron fischer-tropsch catalysts during steady-state synthesis. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 517–526. [Google Scholar]
- Baranski, A.; Dziembaj, R.; Kotarba, A.; Golebiowski, A.; Janecki, Z.; Pettersson, J.B.C. Deactivation of iron catalyst by water-potassium thermal desorption studies. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 229–236. [Google Scholar]
- Querini, C.A.; Ravelli, F.; Ulla, M.; Cornaglia, L.; Miró, E. Deactivation of Co, K catalysts during catalytic combustion of diesel soot: Influence of the support. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 257–264. [Google Scholar]
- Wilson, J.; de Groot, C. Atomic-scale restructuring in high-pressure catalysis. J. Phys. Chem. 1995, 99, 7860–7866. [Google Scholar] [CrossRef]
- Parkinson, G.S.; Novotny, Z.; Argentero, G.; Schmid, M.; Pavelec, J.; Kosak, R.; Blaha, P.; Diebold, U. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 2013, 12, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.; Reardon, J.; Datye, A.K. Measuring the strength of slurry phase heterogeneous catalysts. Powder Technol. 1999, 103, 95–102. [Google Scholar] [CrossRef]
- Kalakkad, D.S.; Shroff, M.D.; Kohler, S.; Jackson, N.; Datye, A.K. Attrition of precipitated iron Fischer-Tropsch catalysts. Appl. Catal. A 1995, 133, 335–350. [Google Scholar] [CrossRef]
- Callister, W.D. Materials Science and Engineering: An Introduction; John Wiley, & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Coble, R.L.; Kingery, W.D. Effect of porosity on physical properties of sintered alumina. J. Am. Ceram. Soc. 1956, 39, 377–385. [Google Scholar] [CrossRef]
- Deng, S.G.; Lin, Y.S. Granulation of sol-gel-derived nanostructured alumina. AIChE J. 1997, 43, 505–514. [Google Scholar] [CrossRef]
- Thoma, S.G.; Ciftcioglu, M.; Smith, D.M. Determination of agglomerate strength distributions: Part 1. Calibration via ultrasonic forces. Powder Technol. 1991, 68, 53–61. [Google Scholar] [CrossRef]
- Bankmann, M.; Brand, R.; Engler, B.H.; Ohmer, J. Forming of high surface area TiO2 to catalyst supports. Catal. Today 1992, 14, 225–242. [Google Scholar] [CrossRef]
- Kenkre, V.M.; Endicott, M.R. A theoretical model for compaction of granular materials. J. Am. Ceram. Soc. 1996, 79, 3045–3054. [Google Scholar] [CrossRef]
- Song, H.; Evans, J.R.G. A die pressing test for the estimation of agglomerate strength. J. Am. Ceram. Soc. 1994, 77, 806–814. [Google Scholar] [CrossRef]
- Werther, J.; Xi, W. Jet attrition of catalyst particles in gas fluidized beds. Powder Technol. 1993, 76, 39–46. [Google Scholar] [CrossRef]
- Bhatt, B.L.; Schaub, E.S.; Hedorn, E.C.; Herron, D.M.; Studer, D.W.; Brown, D.M. Liquid phase Fischer-Tropsch synthesis in a bubble column. In Proceedings of Liquefaction Contractors Review Conference; Stiegel, G.J., Srivastava, R.D., Eds.; U.S. Department of Energy: Pittsburgh, PA, USA, 1992; pp. 402–423. [Google Scholar]
- Pham, H.N.; Datye, A.K. The synthesis of attrition resistant slurry phase iron Fischer-Tropsch catalysts. Catal. Today 2000, 58, 233–240. [Google Scholar] [CrossRef]
- Bemrose, C.R.; Bridgewater, J. A review of attrition and attrition test methods. Powder Technol. 1987, 49, 97–126. [Google Scholar] [CrossRef]
- Ghadiri, M.; Cleaver, J.A.S.; Tuponogov, V.G.; Werther, J. Attrition of FCC powder in the jetting region of a fluidized bed. Powder Technol. 1994, 80, 175–178. [Google Scholar] [CrossRef]
- Weeks, S.A.; Dumbill, P. Method speeds FCC catalyst attrition resistance determinations. Oil Gas J. 1990, 88, 38–40. [Google Scholar]
- Zhao, R.; Goodwin, J.G.; Jothimurugesan, K.; Spivey, J.J.; Gangwal, S.K. Comparison of attrition test methods: ASTM standard fluidized bed vs jet cup. Ind. Eng. Chem. Res. 2000, 39, 1155–1158. [Google Scholar] [CrossRef]
- Doolin, P.K.; Gainer, D.M.; Hoffman, J.F. Laboratory testing procedure for evaluation of moving bed catalyst attrition. J. Test. Eval. 1993, 21, 481. [Google Scholar] [CrossRef]
- Oukaci, R.; Singleton, A.H.; Wei, D.; Goodwin, J.G. Attrition resistance of Al2O3-supported cobalt F-T catalysts. In Proceedings of the 217th National Meeting, ACS Division of Petroleum Chemistry, Anaheim, CA, USA, 21–25 March 1999; p. 91.
- Adams, M.J.; Mullier, M.A.; Seville, J.P.K. Agglomerate strength measurement using a uniaxial confined compression test. Powder Technol. 1994, 78, 5–13. [Google Scholar] [CrossRef]
- Emig, G.; Martin, F.G. Development of a fluidized bed catalyst for the oxidation of n-butane to maleic anhydride. Ind. Eng. Chem. Res. 1991, 30, 1110–1116. [Google Scholar] [CrossRef]
- Silver, R.G.; Summers, J.C.; Williamson, W.B. Catalysis and Automotive Pollution Control II; Elsevier: Amsterdam, The Nethelands, 1991; p. 167. [Google Scholar]
- Fisher, G.B.; Zammit, M.G.; LaBarge, J. Investigation of Catalytic Alternatives to Rhodium in Emissions Control; SAE Report 920846; SAE International: Warrendale, PA, USA, 1992. [Google Scholar]
- Farrauto, R.J.; Heck, R.M. Catalytic converters: state of the art and perspectives. Catal. Today 1999, 51, 351–360. [Google Scholar] [CrossRef]
- Beer, G.L. Extended catalyst life two stage hydrocarbon synthesis process. U.S. Patent 6,169,120, 2 January 2001. [Google Scholar]
- Bartholomew, C.H.; Stoker, M.W.; Mansker, L.; Datye, A. Effects of pretreatment, reaction, and promoter on microphase structure and Fischer-Tropsch activity of precipitated iron catalysts. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 265–272. [Google Scholar]
- Nagano, O.; Watanabe, T. Method for producing methacrolein. U.S. Patent 5,728,894, 17 March 1998. [Google Scholar]
- Maillet, T.; Barbier, J.; Duprez, D. Reactivity of steam in exhaust gas catalysis III. Steam and oxygen/steam conversions of propane on a Pd/Al2O3 catalyst. Appl. Catal. B 1996, 9, 251–266. [Google Scholar] [CrossRef]
- Mathys, G.M.K.; Martens, L.R.M.; Baes, M.A.; Verduijn, J.P.; Huybrechts, D.R.C. Alkene oligomerization. WIPO Patent WO 1993/016020 (A3), 16 September 1993. [Google Scholar]
- Mathys, G.M.K.; Martens, L.R.M.; Baes, M.A.; Verduijn, J.P.; Huybrechts, D.R.C. Alkene oligomerization. U.S. Patent 5,672,800, 30 September 1997. [Google Scholar]
- Stine, L.O.; Muldoon, B.S.; Gimre, S.C.; Frame, R.R. Process for oligomer production and saturation. U.S. Patent 6,080,903, 26 June 2000. [Google Scholar]
- Subramaniam, B.; Arunajatesan, V.; Lyon, C.J. Coking of solid acid catalysts and strategies for enhancing their activity. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 63–77. [Google Scholar]
- Ginosar, D.M.; Fox, R.V.; Kong, P.C. Solid catalyzed isoparaffin alkylation at supercritical fluid and near-supercritical fluid conditions. WIPO Patent WO 1999/033769 (A1), 8 July 1999. [Google Scholar]
- Ribeiro, F.H.; Bonivardi, A.L.; Kim, C. Transformation of platinum into a stable, high-temperature, dehydrogenation-hydrogenation catalyst by ensemble size reduction with rhenium and sulfur. J. Catal. 1994, 150, 186–198. [Google Scholar] [CrossRef]
- Ginosar, D.; Subramaniam, B. Coking and activity of a reforming catalyst in near-critical and dense supercritical reaction mixtures. In Catalyst Deactivation 1994 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1994; Volume 88, pp. 327–334. [Google Scholar]
- Petersen, E.E. Catalyst deactivation: Opportunity amidst woe. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 87–98. [Google Scholar]
- Gosselink, J.W.; Veen, J.A.R.V. Coping with catalyst deactivation in hydrocarbon processing. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 3–16. [Google Scholar]
- Lin, L.; Zao, T.; Zang, J.; Xu, Z. Dynamic process of carbon deposition on Pt and Pt–Sn catalysts for alkane dehydrogenation. Appl. Catal. 1990, 67, 11–23. [Google Scholar] [CrossRef]
- Resasco, D.E.; Haller, G.L. Catalytic dehydrogenation of lower alkanes. In Catalysis (Specialist Periodiodical Report; Spivey, J.J., Agarwal, S.K., Eds.; Royal Society of Chemistry: Cambridge, UK, 1994; Volume 11, pp. 379–411. [Google Scholar]
- Cortright, R.D.; Dumesic, J.A. Microcalorimetric, Spectroscopic, and Kinetic Studies of Silica Supported Pt and Pt/Sn Catalysts for Isobutane Dehydrogenation. J. Catal. 1994, 148, 771–778. [Google Scholar] [CrossRef]
- Weyten, H.; Keizer, K.; Kinoo, A.; Luyten, J.; Leysen, R. Dehydrogenation of propane using a packed-bed catalytic membrane reactor. AIChE J. 1997, 43, 1819–1827. [Google Scholar] [CrossRef]
- Praserthdam, P.; Mongkhonsi, T.; Kunatippapong, S.; Jaikaew, B.; Lim, N. Determination of coke deposition on metal active sites of propane dehydrogenation catalysts. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 153–158. [Google Scholar]
- Rose, B.H.; Kiliany, T.R. Dual catalyst system. WIPO Pat. App. WO 2000/069993 (A1), 12 May 2000. [Google Scholar]
- Guerrero-Ruiz, A.; Sepulveda-Escribano, A.; Rodriguez-Ramos, I. Cooperative action of cobalt and MgO for the catalysed reforming of CH4 with CO2. Catal. Today 1994, 21, 545–550. [Google Scholar] [CrossRef]
- Qin, D.; Lapszewicz, J. Study of mixed steam and CO2 reforming of CH4 to syngas on MgO-supported metals. Catal. Today 1994, 21, 551–560. [Google Scholar] [CrossRef]
- Stagg, S.; Resasco, D. Effects of promoters and supports on coke formation on Pt catalysts during CH4 reforming with CO2. In Catalyst Deactivation 1997 (Studies in Surface Science and Catalysis); Bartholomew, C.H., Fuentes, G.A., Eds.; Elsevier: Amsterdam, The Nethelands, 1997; Volume 111, pp. 543–550. [Google Scholar]
- Fujimoto, K.; Tomishige, K.; Yamazaki, O.; Chen, Y.; Li, X. Development of catalysts for natural gas reforming: Nickel-magnesia solid solution catalyst. Res. Chem. Intermed. 1998, 24, 259–271. [Google Scholar] [CrossRef]
- Marshall, C.L.; Miller, J.T. Process for converting methanol to olefins or gasoline. U.S. Patent 5,191,142, 2 March 1993. [Google Scholar]
- Gayubo, A.G.; Aguayo, A.T.; Campo, A.E.S.D.; Benito, P.L.; Bilbao, J. The role of water on the attenuation of coke deactivation of a SAPO-34 catalyst in the transformation of methanol into olefins. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 129–136. [Google Scholar]
- Barger, P.T. SAPO catalysts and use thereof in methanol conversion processes. U.S. Patent 5,248,647, 28 Sepetember 1993. [Google Scholar]
- Cox, J.P.; Evans, J.M. Exhaust gas catalyst for two-stroke engines. WIPO Patent WO 1999/042202 (A1), 20 February 1998. [Google Scholar]
- Leviness, S.C.; Mart, C.J.; Behrmann, W.C.; Hsia, S.J.; Neskora, D.R. Slurry hydrocarbon synthesis process with increased catalyst life. WIPO Patent WO 1998/050487 (A1), 2 May 1997. [Google Scholar]
- Bartholomew, C.H. Catalyst deactivation in hydrotreating of residua: A review. In Catalytic Hydroprocessing of Petroleum and Distillates; Oballa, M., Shih, S., Eds.; Marcel Dekker: New York, NY, USA, 1993; pp. 1–32. [Google Scholar]
- Summers, J.; Williamson, W.B. Palladium-only catalysts for closed-loop control. In Environmental Catalysis 1993; Armor, J.N., Ed.; American Chemical Society: Washington, DC, USA, 1993; Volume 552, pp. 94–113. [Google Scholar]
- Dettling, J.; Hu, Z.; Lui, Y.K.; Smaling, R.; Wan, C.Z.; Punke, A. Smart Pd TWC technology to meet stringent standards. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Nethelands, 1995; Volume 96, pp. 461–472. [Google Scholar]
- Berndt, M.; Ksinsik, D. Catalyst and process for its preparation. U.S. Patent 4,910,180, 20 March 1990. [Google Scholar]
- Prigent, M.; Blanchard, G.; Phillippe, P. Catalyst supports/catalysts for the treatment of vehicular exhaust gases. U.S. Patent 4,985,387, 15 January 1991. [Google Scholar]
- Williamson, W.B.; Linden, D.G.; Summers, J.C., II. High-temperature three-way catalyst for treating automotive exhaust gases. U.S. Patent 5,041,407, 20 August 1991. [Google Scholar]
- Williamson, W.B.; Linden, D.G.; Summers, J.C., II. High durability and exhuast catalyst with low hydrogen sulfide emissions. U.S. Patent 5,116,800, 26 May 1992. [Google Scholar]
- Narula, C.K.; Watkins, W.L.H.; Chattha, M.S. Binary La-Pd oxide catalyst and method of making the catalyst. U.S. Patent 5,234,881, 10 August 1993. [Google Scholar]
- Wan, C.-Z.; Tauster, S.J.; Rabinowitz, H.N. Catalyst composition containing platinum and rhodium components. U.S. Patent 5,254,519, 19 October 1993. [Google Scholar]
- Argyle, M.D.; Frost, T.S.; Bartholomew, C.H. Modeling cobalt fischer tropsch catalyst deactivation with generalized power law expressions. Top. Catal. 2014, 57, 415–429. [Google Scholar] [CrossRef]
- Furuya, T.; Yamanaka, S.; Hayata, T.; Koezuka, J.; Yoshine, T.; Ohkoshi, A. Hybrid catalytic combustion for stationary gas turbine—Concept and small scale test results. In Proceedings of Gas Turbine Conference and Exhibition, Anaheim, CA, USA, 31 May–4 June 1987. ASME Paper 87-GT-99.
- Kawakami, T.; Furuya, T.; Sasaki, Y.; Yoshine, T.; Furuse, Y.; Hoshino, M. Feasibility study on honeycomb ceramics for catalytic combustor. In Proceedings of Gas Turbine and Aeroengine Congress and Exposition, Toronto, ON, Canada, 4–8 June 1989. ASME Paper 89-GT-41.
- Betta, R.D.; Ribeiro, F.; Shoji, T.; Tsurumi, K.; Ezawa, N.; Nickolas, S. Catalyst structure having integral heat exchange. U.S. Patent 5,250,489, 5 October 1993. [Google Scholar]
- Fujii, T.; Ozawa, Y.; Kikumoto, S. High pressure test results of a catalytic combustor for gas turbine. J. Eng. Gas Turbines Power 1998, 120, 509–513. [Google Scholar] [CrossRef]
- Borio, D.O.; Schbib, N.S. Simulation and optimization of a set of catalytic reactors used for dehydrogenation of butene into butadiene. Comput. Chem. Eng. 1995, 19, S345–S350. [Google Scholar] [CrossRef]
- Fiato, R.A. Two stage process for hydrocarbon synthesis. U.S. Patent 5,028,634, 2 July 1991. [Google Scholar]
- Zhao, R.; Goodwin, J.G., Jr.; Jothimurugesan, K.; Gangwal, S.K.; Spivey, J.J. Spray-dried Iron Fischer-Tropsch catalysts. 1. Effect of structure on the attrition resistance of the catalysts in the calcined state. Ind. Eng. Chem. Res. 2001, 40, 1065–1075. [Google Scholar] [CrossRef]
- Zhao, R.; Goodwin, J.G., Jr.; Jothimurugesan, K.; Gangwal, S.K.; Spivey, J.J. Spray-dried iron Fischer-Tropsch catalysts. 2. Effect of carburization on catalyst attrition resistance. Ind. Eng. Chem. Res. 2001, 40, 1320–1328. [Google Scholar] [CrossRef]
- Singleton, A.H.; Oukaci, R.; Goodwin, J.G. Processes and catalysts for conducting fischer-tropsch synthesis in a slurry bubble column reactor. U.S. Patent 5,939,350, 17 August 1999. [Google Scholar]
- Plecha, S.; Mauldin, C.H.; Pedrick, L.E. Titania catalysts, their preparation and use in Fischer-Tropsch synthesis. U.S. Patent 6,087,405, 11 July 2000. [Google Scholar]
- Plecha, S.; Mauldin, C.H.; Pedrick, L.E. Titania catalysts, their preparation and use in fischer-tropsch synthesis. U.S. Patent 6,124,367, 26 September 2000. [Google Scholar]
- Singleton, A.H.; Oukaci, R.; Goodwin, J.G. Reducing fischer-tropsch catalyst attrition losses in high agitation reaction systems. WIPO Patent WO 2000/071253 (A3), 30 November 2000. [Google Scholar]
- Seitz, M.; Klemm, E.; Emig, G. Poisoning and regeneration of NOx adsorbing catalysts for automotive applications. In Catalyst Deactivation 1999 (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1999; Volume 126, pp. 211–218. [Google Scholar]
- de Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Assaf, J.M.; Mattos, L.V.; Sarkari, R.; Venugopal, A.; Noronha, F.B. Hydrogen production through oxidative steam reforming of ethanol over Ni-based catalysts derived from La1−xCexNiO3 perovskite-type oxides. Appl. Catal. B 2012, 121–122, 1–9. [Google Scholar]
- Song, H.; Ozkan, U.S. Ethanol steam reforming over Co-based catalysts: Role of oxygen mobility. J. Catal. 2009, 261, 66–74. [Google Scholar] [CrossRef]
- Masamune, S.; Smith, J.M. Performance of fouled catalyst pellets. AIChE J. 1966, 12, 384–394. [Google Scholar] [CrossRef]
- Murakami, Y.; Kobayashi, T.; Hattori, T.; Masuda, M. Effect of intraparticle diffusion on catalyst fouling. Ind. Eng. Chem. Fundam. 1968, 7, 599–605. [Google Scholar] [CrossRef]
- Stork, W.H.J. Molecules, catalysts and reactors in hydroprocessing of oil fractions. In Hydrotreatment and Hydrocracking of Oil Fractions (Studies in Surface Science and Catalysis); Froment, G.F., Delmon, B., Grange, P., Eds.; Elsevier: New York, NY, USA, 1997; Volume 106, pp. 41–67. [Google Scholar]
- Parks, G.D.; Schaffer, A.M.; Dreiling, M.J.; Shiblom, C.M. Surface Studies on the Interaction of Nickel and Antimony on Cracking Catalysts. Prepr.—Am. Chem. Soc., Div. Petr. Chem. 1980, 25, 335. [Google Scholar]
- Trimm, D.L. Introduction to Catalyst Deactivation. In Progress in Catalyst Deactivation (NATO Advanced Study Institute Series E, No. 54); Figueiredo, J.L., Ed.; Martinus Nijhoff Publishers: Boston, MA, USA, 1982; pp. 3–22. [Google Scholar]
- Becker, E.R.; Wei, J.J. Nonuniform distribution of catalysts on supports: I. Bimolecular Langmuir reactions. J. Catal. 1977, 46, 365–381. [Google Scholar] [CrossRef]
- Long, D.C. Staged hydrocarbon synthesis process. U.S. Patent 5,498,638, 12 March 1996. [Google Scholar]
- Powell, B.R., Jr. Stabilitzation of high surface area aluminas. In Proceedings of the Materials Research Society Annual Meeting, Boston, MA, USA, 16–21 November 1980. Paper H9.
- Dai, Y.; Lim, B.; Yang, Y.; Cobley, C.M.; Li, W.; Cho, E.C.; Grayson, B.; Fanson, P.T.; Campbell, C.T.; Sun, Y.; et al. A sinter-resistant catalytic system based on platinum nanoparticles supported on TiO2 nanofibers and covered by porous silica. Angew. Chem. Int. Ed. 2010, 49, 8165–8168. [Google Scholar] [CrossRef]
- Shang, L.; Bian, T.; Zhang, B.; Zhang, D.; Wu, L.-Z.; Tung, C.-H.; Yin, Y.; Zhang, T. Graphene-supported ultrafine metal nanoparticles encapsulated by mesoporous silica: Robust catalysts for oxidation and reduction reactions. Angew. Chem. Int. Ed. 2014, 53, 250–254. [Google Scholar] [CrossRef]
- Heck, R.; Farrauto, R. Catalytic Air Pollution Control: Commercial Technology; Van Nostrand Reinhold: New York, NY, USA, 1995. [Google Scholar]
- Trimm, D.L. The regeneration or disposal of deactivated heterogeneous catalysts. Appl. Catal. A 2001, 212, 153–160. [Google Scholar] [CrossRef]
- Berrebi, G.; Dufresne, P.; Jacquier, Y. Recycling of spent hydroprocessing catalysts: EURECAT technology. Environ. Prog. 1993, 12, 97–100. [Google Scholar] [CrossRef]
- D’Aquino, R.L. For catalyst regeneration, incentives anew. Chem. Eng. 2000, 107, 32–33, 35. [Google Scholar]
- Chang, T. Regeneration industry helps refiners control costs, limit liabilities. Oil Gas J. 1998, 96, 49. [Google Scholar]
- Blashka, S.R.; Duhon, W. Catalyst regeneration. Int. J. Hydrocarbon Eng. 1998, 4, 60–64. [Google Scholar]
- McCulloch, D.C. Catalytic hydrotreating in petroleum refining. In Applied Industrial Catalysis; Leach, B.E., Ed.; Academic Press: New York, NY, USA, 1983; pp. 69–121. [Google Scholar]
- Franck, J.P.; Martino, G. Deactivation and Regeneration of Catalytic-Reforming Catalysts. In Progress in Catalyst Deactivation (NATO Advanced Study Institute Series E., No. 54); Figueiredo, J.L., Ed.; Martinus Nijhoff Publishers: Boston, MA, USA, 1982; pp. 355–397. [Google Scholar]
- Spivey, J.J.; Roberts, G.W.; Davis, B.H. Catalyst Deactivation 2001 (Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Nethelands, 2001; Volume 139. [Google Scholar]
- Haddad, J.H.; Harandi, M.N.; Owen, H. Upgrading light olefin fuel gas in a fluidized bed catalyst reactor and regeneration of the catalyst. U.S. Patent 5,043,517, 27 August 1991. [Google Scholar]
- Ueda, M.; Murakami, T.; Shibata, S.; Hirabayashi, K.; Kondoh, T.; Adachi, K.; Hoshino, N.; Inoue, S. Process for the regeneration of coke-deposited, crystalline silicate catalyst. U.S. Patent 5,306,682, 26 April 1994. [Google Scholar]
- Zhang, S.Y.-F.; Gosling, C.D.; Sechrist, P.A.; Funk, G.A. Dual regeneration zone solid catalyst alkylation process. U.S. Patent 5,675,048, 7 October 1997. [Google Scholar]
- Panattoni, G.; Querini, C.A. Isobutane alkylation with C4 olefins: regeneration of metal-containing catalysts. In Catalyst Deactivation 2001 (Studies in Surface Science and Catalysis); Spivey, J.J., Roberts, G.W., Davis, B.H., Eds.; Elsevier: Amsterdam, The Nethelands, 2001; Volume 139, p. 181. [Google Scholar]
- Thompson, D.N.; Ginosar, D.M.; Burch, K.C. Regeneration of a deactivated USY alkylation catalyst using supercritical isobutene. Appl. Catal. A 2005, 279, 109–116. [Google Scholar] [CrossRef]
- Petkovic, L.M.; Ginosar, D.M.; Burch, K.C. Supercritical fluid removal of hydrocarbons adsorbed on wide-pore zeolite catalysts. J. Catal. 2005, 234, 328–339. [Google Scholar] [CrossRef]
- Dufresne, P.; Brahma, N. Offsite regeneration process for a catalyst containing at least one precious metal. U.S. Patent 5,854,162, 29 December 1998. [Google Scholar]
- Innes, R.A.; Holtermann, D.L.; Mulaskey, B.F. Low temperature regeneration of coke deactivated reforming catalysts. U.S. Patent 5,883,031, 16 March 1999. [Google Scholar]
- Fung, S.C. Regenerating a reforming catalyst—this bimetallic noble-metal catalyst is used in a refining process to convert saturated-hydrocarbons to aromatics - simply burning off the carbon was not enough—a procedure had to be found for redispersing the metals on the alumina support as well. Chemtech 1994, 24, 40–44. [Google Scholar]
- Alfonso, J.C.; Aranda, D.A.G.; Schmal, M.; Frety, R. Regeneration of a Pt-Sn/Al2O3 catalyst: Influence of heating rate, temperature and time of regeneration. Fuel Proc. Technol. 1997, 50, 35–48. [Google Scholar] [CrossRef]
- Arteaga, G.J.; Anderson, J.A.; Rochester, C.H. Effects of Catalyst Regeneration with and without Chlorine on Heptane Reforming Reactions over Pt/Al2O3 and Pt–Sn/Al2O3. J. Catal. 1999, 187, 219–229. [Google Scholar] [CrossRef]
- Pieck, C.L.; Vera, C.R.; Parera, J.M. Study of industrial and laboratory regeneration of Pt-Re/Al2O3 catalysts. In Catalyst Deactivation 2001 (Studies in Surface Science and Catalysis); Spivey, J.J., Roberts, G.W., Davis, B.H., Eds.; Elsevier: Amsterdam, The Nethelands, 2001; Volume 139, pp. 279–286. [Google Scholar]
- Acharya, D.R.; Hughes, R.; Kennard, M.A.; Liu, Y.P. Regeneration of fixed beds of coked chromia—alumina catalyst. Chem. Eng. Sci. 1992, 47, 1687–1693. [Google Scholar] [CrossRef]
- Sikkenga, D.L.; Zaenger, I.C.; Williams, G.S. Palladium catalyst reactivation. U.S. Patent 4,999,326, 12 March 1991. [Google Scholar]
- Ekstrom, A.; Lapszewicz, J. On the role of metal carbides in the mechanism of the Fischer-Tropsch reaction. J. Phys. Chem. 1984, 88, 4577–4580. [Google Scholar] [CrossRef]
- Ekstrom, A.; Lapszewicz, J. The reactions of cobalt surface carbides with water and their implications for the mechanism of the Fischer-Tropsch reaction. J. Phys. Chem. 1987, 91, 4514–4619. [Google Scholar] [CrossRef]
- Pedrick, L.E.; Mauldin, C.H.; Behrmann, W.C. Draft tube for catalyst rejuvenation and distribution. U.S. Patent 5,268,344, 7 December 1993. [Google Scholar]
- Owen, H.; Schipper, P.H. Process and apparatus for regeneration of FCC catalyst with reduced NOx and or dust emissions. U.S. Patent 5,338,439, 16 August 1994. [Google Scholar]
- Raterman, M.F. Two-stage fluid bed regeneration of catalyst with shared dilute phase. U.S. Patent 5,198,397, 30 March 1993. [Google Scholar]
- Apelian, M.R.; Fung, A.S.; Hatzikos, G.H.; Kennedy, C.R.; Lee, C.-H.; Kiliany, T.R.; Ng, P.K.; Pappal, D.A. Regeneration of noble metal containing zeolite catalysts via partial removal of carbonaceous deposits. U.S. Patent 5,393,717, 28 February 1995. [Google Scholar]
- Clark, D.E. Hydrocracking process using a reactivated catalyst. U.S. Patent 5,340,957, 23 August 1994. [Google Scholar]
- Yoshimura, Y.; Sato, T.; Shimada, H.; Matsubayashi, N.; Imamura, M.; Nishijima, A.; Yoshitomi, S.; Kameoka, T.; Yanase, H. Oxidative regeneration of spent molybdate and tungstate hydrotreating catalysts. Energy Fuels 1994, 8, 435–445. [Google Scholar] [CrossRef]
- Oh, E.-S.; Park, Y.-C.; Lee, I.-C.; Rhee, H.-K. Physicochemical changes in hydrodesulfurization catalysts during oxidative regeneration. J. Catal. 1997, 172, 314–321. [Google Scholar] [CrossRef]
- Aquavo, A.T.; Gayubo, A.G.; Atutxa, A.; Olazar, M.; Bilbao, J. Regeneration of a catalyst based on a SAPO-34 used in the transformation of methanol into olefins. J. Chem. Tech. Biotech. 1999, 74, 1082–1088. [Google Scholar] [CrossRef]
- Forbus, N.P.; Wu, M.M.-S. Regeneration of methanol/methyl ether conversion catalysts. U.S. Patent 4,777,156, 11 October 1988. [Google Scholar]
- Krishna, A.; Hsieh, C.; English, A.E.; Pecoraro, T.; Kuehler, C. Additives improve FCC process; increase catalyst life, lower regeneration temperatures and improve yield and quality of products cost effectively. Hydrocarbon Processing 1991, 59–66. [Google Scholar]
- Altomare, C.; Koermer, G.; Schubert, P.; Suib, S.; Willis, W. A designed fluid cracking catalyst with vanadium tolerance. Chem. Mater. 1989, 1, 459–463. [Google Scholar] [CrossRef]
- Aguinaga, A.; Montes, M. Regeneration of a nickel/silica catalyst poisoned by thiophene. Appl. Catal. A 1992, 90, 131–144. [Google Scholar] [CrossRef]
- Lowry, G.V.; Reinhard, M. Pd-Catalyzed TCE Dechlorination in Groundwater: Solute Effects, Biological Control, and Oxidative Catalyst Regeneration. Environ. Sci. Technol. 2000, 34, 3217–3223. [Google Scholar] [CrossRef]
- Trinh, D.C.; Desvard, A.; Martino, G. Process for regenerating used catalysts by means of hydrogen peroxide aqueous solution stabilized with an organic compound. U.S. Patent 4,830,997, 16 May 1989. [Google Scholar]
- Sherwood, D.E. Process for the reactivation of spent alumina-supported hydrotreating catalysts. U.S. Patent 5,230,791, 27 July 1993. [Google Scholar]
- Fung, S.C. Deactivation and regeneration/redispersion chemistry of Pt/KL-zeolite. In Catalyst Deactivation 2001 (Studies in Surface Science and Catalysis); Spivey, J.J., Roberts, G.W., Davis, B.H., Eds.; Elsevier: Amsterdam, The Nethelands, 2001; Volume 139, pp. 399–406. [Google Scholar]
- Didillon, B. Catalyst regeneration process and use of the catalyst in hydrocarbon conversion processes. U.S. Patent 5,672,801, 30 September 1997. [Google Scholar]
- Tsao, Y.-Y.P.; von Ballmoos, R. Reactivating catalysts containing noble metals on molecular sieves containing oxides of aluminum and phosphorus. U.S. Patent 4,929,576, 29 May 1990. [Google Scholar]
- Dufresne, P.; Brahma, N.; Girardier, F. Off-site regeneration of hydroprocessing catalysts. Rev. Inst. Fr. Pet. 1995, 50, 283–293. [Google Scholar]
- Clark, F.T.; Hensley, A.L., Jr. Process for regenerating a spent resid hydroprocessing catalyst using a group IIA metal. U.S. Patent 5,275,990, 4 January 1994. [Google Scholar]
- Brito, A.; Arvelo, R.; Gonzalez, A.R. Variation in structural characteristics of a hydrotreatment catalyst with deactivation/regeneration cycles. Ind. Eng. Chem. Res. 1998, 37, 374–379. [Google Scholar] [CrossRef]
- Teixeira-da-Silva, V.L.S.; Lima, F.P.; Dieguez, L.C. Regeneration of a deactivated hydrotreating catalyst. Ind. Eng. Chem. Res. 1998, 37, 882–886. [Google Scholar] [CrossRef]
- Snape, C.E.; Diaz, M.C.; Tyagi, Y.R.; Martin, S.C.; Hughes, R. Characterisation of coke on deactivated hydrodesulfurisation catalysts and a novel approach to catalyst regeneration. In Catalyst Deactivation 2001 (Studies in Surface Science and Catalysis); Spivey, J.J., Roberts, G.W., Davis, B.H., Eds.; Elsevier: Amsterdam, The Nethelands, 2001; Volume 139, pp. 359–365. [Google Scholar]
- Sherwood, D.E., Jr.; Hardee, J.R., Jr. Method for the reactivation of spent alumina-supported hydrotreating catalysts. U.S. Patent 5,445,728, 29 August 1995. [Google Scholar]
- Maholland, M.K.; Fu, C.-M.; Lowery, R.E.; Kubicek, D.H.; Bertus, B.J. Demetallization and passivation of spent cracking catalysts. U.S. Patent 5,021,377, 4 June 1991. [Google Scholar]
- Kubicek, D.H.; Fu, C.-M.; Lowery, R.E.; Maholland, K.M. Reactivation of spent cracking catalysts. U.S. Patent 5,141,904, 25 August 1992. [Google Scholar]
- Fu, C.-M.; Maholland, M.; Lowery, R.E. Reactivation of spent, metal-containing cracking catalysts. U.S. Patent 5,151,391, 29 September 1992. [Google Scholar]
- Hu, Y.; Luo, B.; Sun, K.; Yang, Q.; Gong, M.; Hu, J.; Fang, G.; Li, Y. Dry regeneration-demetalization technique for catalyst for residuum and/or heavy oil catalytic cracking. U.S. Patent 6,063,721, 16 May 2000. [Google Scholar]
- Walker, P.L., Jr.; Rusinko, F., Jr.; Austin, L.G. Gas Reactions of Carbon. Adv. Catal. 1959, 11, 133–221. [Google Scholar]
- Fulton, J.W. Regenerating Spent Catalyst. Chem. Eng. 1988, 96, 111–114. [Google Scholar]
- Richardson, J.T. Experimental determination of catalyst fouling parameters. Carbon profiles. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 8–11. [Google Scholar] [CrossRef]
- Weisz, P.B.; Goodwin, R.B. Combustion of carbonaceous deposits within porous catalyst particles: II. Intrinsic burning rate. J. Catal. 1966, 6, 227–236. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Some principles relating to the regeneration of sulfur-poisoned nickel catalyst. J. Catal. 1971, 21, 171–178. [Google Scholar] [CrossRef]
- Hartenstein, H. Feasibility of SCR Technology for NOx Control Technology for the Milton R. Young Station, Center, North Dakota; Expert Report on behalf of the United States Department of Justice: Washington, DC, USA, July 2008. [Google Scholar]
- Hartenstein, H.; Ehrnschwender, M.; Sibley, A.F. SCR Regeneration-10 Years of R&D and Commercial Application. In Proceedings of Power Plant Air Pollution Control Mega Symposium, Baltimore, MD, USA, 24–28 August 2008. Paper No. 104.
- EPA; Office of Air and Radiation. Update on Cap and Trade Programs for SO2 and NOx. In Presented at the Enviornmental Markets Association, 12 Annual Spring Conference, Miami, FL, USA, 29 April–2 May 2008.
- EPA. NOx Budget Trading Program—Basic Information. Available online: http://www.epa.gov/airmarkt/progsregs/nox/docs/NBPbasicinfo.pdf (accessed on 31 October 2014).
- McIlvaine Company. News Release: Coal to Play Bigger Role in Future Power Generation With World Capacity of 1450 GW by 2012. Available online: http://www.mcilvainecompany.com/brochures/Alerts_for_Internet/s9%20moni/1104coal%20to%20play%20bigger%20role%20in%20future%20power%20generation.htm (accessed on 31 October 2014).
- Hjalmarsson, A.-K. NOx Control Technologies for Coal Combustion; IEACR/24; IEA Coal Research: Lexington, KY, USA, 1990. [Google Scholar]
- U.S. Department of Energy; Southern Company Services. Control of Nitrogen Oxide Emissions: Selective Catalytic Reduction. In Clean Coal Technology; Topical Report 9; U.S. Department of Energy: Pittsburgh, PA, USA; Southern Company Services: Birmingham, AL, USA, 1997. [Google Scholar]
- Janssen, F.; Meijer, R. Quality control of DeNOx catalysts: Performance testing, surface analysis and characterization of DeNOx catalysts. Catal. Today 1993, 16, 157–185. [Google Scholar] [CrossRef]
- Ashton, J.; Nackos, A.; Bartholomew, C.H.; Hecker, W.C.; Baxter, L. Poisoning/Deactivation of Vanadia/Titanium Dioxide SCR Catalysts in Coal Systems. In Presented at the 19th Annual Western States Catalysis Club Symposium, Albuquerque, NM, USA, 25 February 2005.
- Guo, X.; Nackos, A.; Ashton, J.; Bartholomew, C.H.; Hecker, W.C.; Baxter, L. Poisoning Study of V2O5-WO3/TiO2 Catalysts by Na, K, and Ca. In Presented at 19th Annual Western States Catalysis Club Symposium; Albuquerque, NM, USA: 25 February 2005.
- Forzatti, P.; Lietti, L.; Tronconi, E. Nitrogen Oxides Removal–Industrial. In Encyclopedia of Catalysis; Horváth, T.I., Ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Rubin, E.S.; Kalagnanam, J.R.; Frey, C.H.; Berkenpas, M.B. Integrated Environmental Control Modeling of Coal-Fired Power Systems. Journal of the Air and Waste Management Association 1997, 47, 1180–1188. [Google Scholar] [CrossRef]
- Cochran, J.R.; Ferguson, A.W. Selective Catalytic Reduction for NOx Emission Control. In Proceedings of the First International Conference on Air Pollution, Monterrey, Mexico, 16-18 Feb. 1993; Zannetti, P., Brebbia, C.A., Garcia Gardea, J.E., Ayala Milian, G., Eds.; Volume 1, pp. 703–718.
- Bullock, P.E.; Hartenstein, H.U. O&M Cost Reduction of a Coal-Fired US Merchant Plant Through an Optimized SCR Management Strategy Involving Catalyst Regeneration. In Proceedings of the Conference on Selective Catalytic Reduction and Selective Non-Catalytic Reduction for NOx Control, Pittsburgh, PA, USA, 15–17 May 2002.
- Babcock Hitachi’s Rejuvenation of Mehrum SCR Catalyst. In FGD and DeNOx Newsletter; No. 315; The McIlvaine Company: Northfield, IL, USA, 2004.
- McMahon, B. Catalyst regeneration: The business case. Power 2006, 150, 36–39. [Google Scholar]
- Zheng, Y.; Jensen, A.; Johnsson, J. Laboratory investigation of selective catalytic reduction catalysts: Deactivation by potassium compounds and catalyst regeneration. Ind. Eng. Chem. Res. 2004, 43, 941–947. [Google Scholar] [CrossRef]
- Khodayari, R.; Odenbrand, C. Regeneration of commercial SCR catalysts by washing and sulphation: effect of sulphate groups on the activity. Appl. Catal. B 2001, 33, 277–291. [Google Scholar] [CrossRef]
- Khodayari, R.; Odenbrand, C. Regeneration of commercial TiO2-V2O5-WO3 SCR catalysts used in bio fuel plants. Appl. Catal. B 2001, 30, 87–89. [Google Scholar] [CrossRef]
- Dörr, H.-K.; Koch, G.; Bastuck, W. Method for renewed activation of honeycomb-shaped catalyst elements for denitrating flue gases. U.S. Patent 6,387,836 (B1), 14 May 2002. [Google Scholar]
- Schneider, P.; Bastuck, W. Method to re-enable honeycomb catalyst elements constructed for the denitrification of flue gases. DE Patent 10222915 (B4), 15 January 2004. [Google Scholar]
- Budin, R.; Krotla, K.; Rabitsch, H. Process for regenerating used deNOx or dedioxin catalytic converters. U.S. Patent 6,484,733 (B2), 26 November 2002. [Google Scholar]
- Cooper, M.; Harrison, K.; Lin, C. An Experimental Program to Optimize SCR Catalyst Regeneration for Lower Oxidation of SO2 to SO3. In Proceedings of the 2006 Environmental Controls Conference, Pittsburgh, PA, USA, 16–18 May 2006.
- Patel, N.; Hartenstein, H.-U.; Wenz, F. Process for decoating a washcoat catalyst substrate. U.S. Patent 7,559,993 (B1), 14 July 2009. [Google Scholar]
- Patel, N.; Hartenstein, H.-U.; Wenz, F. Process for decoating a washcoat catalyst substrate. U.S. Patent 6,929,701, 16 August 2005. [Google Scholar]
- Tate, A.; Skipper, J.; Wenz, F. Environmentally sound handling of deactivated SCR catalyst. Coal Power, 31 July 2008. [Google Scholar]
- Hartenstein, H.U.; Hoffman, T. Method of regeneration of SCR catalyst. U.S. Patent 7,723,251, 25 May 2010. [Google Scholar]
- Servatius, P.; Schluttig, A. Lifetime extension of SCR-DeNOx catalysts using SCR-Tech’s high efficiency ultrasonic regeneration process. In Proceedings of 2001 Conference of Selective Catalytic Reduction and Selective Non-Catalytic Reduction for NOx Control, Pittsburgh, PA, USA, 16–18 May 2001.
- Hartenstein, H.; Gutberlet, H. Catalyst Regeneration—An Integral Part of Proper Catalyst Management. In Proceedings of the 2001 Workshop on Selective Catalytic Reduction, Baltimore, MD, USA, 13–15 November 2001.
- Wenz, F.; Deneault, R.; Franklin, H.N. The Goals, Challenges and Successes of Regenerating Selective Catalytic Reduction Catalyst. In Proceedings of the Electric Power 2006 Conference–NOx Control III–SCR Technologies, Atlanta, GA, USA, 2–4 May 2006.
- CoaLogix. Available online: http://www.coalogix.com (accessed on 31 October 2014).
- McCullen, S.B.; Wong, S.S.; Huang, T.J. Regeneration of noble metal-highly siliceous zeolite with sequential hydrogen halide and halogen or organic-halogen compound treatment. U.S. Patent 4,645,751, 24 February 1987. [Google Scholar]
- Borghard, W.S.; Huang, T.J.; McCullen, S.B.; Schoennagel, H.J.; Tsao, Y.-.P.; Wong, S.S. Redispersion of agglomerated noble metals on zeolite catalysts. U.S. Patent 4,657,874, 14 April 1987. [Google Scholar]
- Fung, S.C.; Rice, R.W. Process using halogen/oxygen for reactivating iridium and selenium containing catalysts. U.S. Patent 4,491,636, 1 January 1985. [Google Scholar]
- Fung, S.C. Redispersion of Ir catalysts by low temperature reduction step. U.S. Patent 4,467,045, 21 August 1984. [Google Scholar]
- Landolt, G.R.; McHale, W.D.; Schoennagel, H.J. Catalyst regeneration procedure. U.S. Patent 4,359,400, 16 November 1982. [Google Scholar]
- Fung, S.C.; Weissman, W.; Carter, J.L. Reactivation process for iridium-containing catalysts using low halogen flow rates. U.S. Patent 4,480,046, 30 October 1984. [Google Scholar]
- Fung, S.C. Low temperature decoking process for reactivating iridium and selenium containing catalysts. U.S. Patent 4,492,767, 8 January 1985. [Google Scholar]
- Fung, S.C.; Weissman, W.; Carter, J.L.; Kmak, W.S. Reactivating iridium and selenium containing catalysts with hydrogen halide and oxygen. U.S. Patent 4,491,635, 1 January 1985. [Google Scholar]
- Mohr, D.H. Process for regenerating a monofunctional large-pore zeolite catalyst having high selectivity for paraffin dehydrocyclization. U.S. Patent 4,855,269, 8 August 1989. [Google Scholar]
- Fung, S.C.; Tauster, S.J.; Koo, J.Y. Method of regenerating a deactivated catalyst. U.S. Patent 4,925,819, 15 May 1990. [Google Scholar]
- Fung, S.C.; Kmak, W.S. Reactivation of iridium-containing catalysts by halide pretreat and oxygen redispersion. U.S. Patent 4,444,896, 24 April 1984. [Google Scholar]
- Fung, S.C.; Weissman, W.; Carter, J.L. Reactivation process for iridium-containing catalysts using low halogen flow rates. U.S. Patent 4,444,895, 24 April 1984. [Google Scholar]
- Boyle, J.P.; Gilbert, J.B. Reactivation of iridium-containing catalysts. U.S. Patent 4,517,076, 14 May 1985. [Google Scholar]
- Buss, W.D.; Hughes, T.R. In situ hydrocarbon conversion catalyst regeneration and sulfur decontamination of vessels communicating with catalyst reactor. U.S. Patent 4,482,637, 13 November 1984. [Google Scholar]
- Cheng, W.-H. Regeneration of methanol dissociation catalysts. U.S. Patent 4,855,267, 8 August 1989. [Google Scholar]
- Huang, Y.-Y.; LaPierre, R.B.; McHale, W.D. Process for dispersing or redispersing a group VIII noble metal species on a porous inorganic support. European Patent 0,306,170 (B1), 8 March 1989. [Google Scholar]
- Hucul, D.A. Redispersal of Ru, Os, Rh and Pd catalysts and processes therewith. U.S. Patent 4,891,346, 2 January 1990. [Google Scholar]
- Fung, S.C.; Rice, R.W. Process for reactivating iridium-containing catalysts. U.S. Patent 4,447,551, 8 May 1984. [Google Scholar]
- Fung, S.C. Process for reactivating iridium-containing catalysts in series. U.S. Patent 4,472,514, 18 September 1984. [Google Scholar]
- Fung, S.C.; Rice, R.W. Process for reactivating iridium-containing catalysts. U.S. Patent 4,473,656, 25 September 1984. [Google Scholar]
- Huang, Y.-Y.; LaPierre, R.B.; McHale, W.D. Process for dispersing or redispersing a Group VIII noble metal species on a porous inorganic support. U.S. Patent 4,952,543, 28 August 1990. [Google Scholar]
- Fung, S.C.; Weissman, W.; Carter, J.L.; Kmak, W.S. Reactivating iridium-containing catalysts with hydrogen halide and oxygen. U.S. Patent 4,444,897, 24 April 1984. [Google Scholar]
- Fung, S.C. Low temperature decoking process for reactivating iridium containing catalysts. U.S. Patent 4,472,515, 18 September 1984. [Google Scholar]
- Dadyburjor, D.B. Regions of validity for models of regeneration of sintered supported metal catalysts. In Catalyst Deactivation (Studies in Surface Science and Catalysis); Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Nethelands, 1980; Volume 6, pp. 341–351. [Google Scholar]
- Jacobs, G.; Ma, W.; Gao, P.; Todic, B.; Bhatelia, T.; Bukur, D.B.; Davis, B.H. The application of synchrotron methods in characterizing iron and cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2013, 214, 100–139. [Google Scholar] [CrossRef]
- Van Steen, E.; Claeys, M.; Dry, M.E.; van de Loosdrecht, J.; Viljoen, E.L.; Visagie, J.L. Stability of nanocrystals: Thermodynamic analysis of oxidation and re-reduction of cobalt in water/hydrogen mixtures. J. Phys. Chem. B 2005, 109, 3575–3577. [Google Scholar]
- Ma, W.; Jacobs, G.; Ji, Y.; Bhatelia, T.; Bukur, D.B.; Khalid, S.; Davis, B.H. Fischer–Tropsch synthesis: Influence of CO conversion on selectivities, H2/CO usage ratios, and catalyst stability for a Ru promoted Co/Al2O3 catalyst using a slurry phase reactor. Top. Catal. 2011, 54, 757–767. [Google Scholar] [CrossRef]
- Jacobs, G.; Sarkar, A.; Ji, Y.; Patterson, P.M.; Das, T.K.; Luo, M.; Davis, B.H. Fischer-Tropsch synthesis: Characterization of interactions between reduction promoters and Co for Co/Al2O3–based GTL catalysts. In Proceedings of AIChE Annual Meeting, San Francisco, CA, USA, 12–17 November 2006.
- Das, T.K.; Jacobs, G.; Patterson, P.M.; Conner, W.A.; Davis, B.H. Fischer–Tropsch synthesis: characterization and catalytic properties of rhenium promoted cobalt alumina catalysts. Fuel 2003, 82, 805–815. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Patterson, P.M.; Luo, M.; Conner, W.A.; Davis, B.H. Fischer–Tropsch synthesis: Effect of water on Co/Al2O3 catalysts and XAFS characterization of reoxidation phenomena. Appl. Catal. A 2004, 270, 65–76. [Google Scholar] [CrossRef]
- Moodley, D.J.; Saib, A.M.; van de Loosdrecht, J.; Welker-Nieuwoudt, C.A.; Sigwebela, B.H.; Niemanstsverdriet, J.W. The impact of cobalt aluminate formation on the deactivation of cobalt-based Fischer–Tropsch synthesis catalysts. Catal. Today 2011, 171, 192–200. [Google Scholar] [CrossRef]
- Jermwongratanachai, T.; Jacobs, G.; Shafer, W.D.; Ma, W.; Pendyala, V.R.R.; Davis, B.H.; Kitiyanan, B.; Khalid, S.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L. Fischer–Tropsch synthesis: Oxidation of a fraction of cobalt crystallites in research catalysts at the onset of FT at partial pressures mimicking 50% CO conversion. Top. Catal. 2014, 57, 479–490. [Google Scholar] [CrossRef]
- Azzam, K.; Jacobs, G.; Ma, W.; Davis, B.H. Effect of cobalt particle size on the catalyst intrinsic activity for Fischer–Tropsch synthesis. Catal. Lett. 2014, 144, 389–394. [Google Scholar] [CrossRef]
- Saib, A.M.; Borgna, A.; van de Loosdrecht, J.; van Berge, P.J.; Niemanstsverdriet, J.W. XANES study of the susceptibility of nano-sized cobalt crystallites to oxidation during realistic Fischer–Tropsch synthesis. Appl. Catal. A 2006, 312, 12–19. [Google Scholar] [CrossRef]
- Dalai, A.K.; Davis, B.H. Fischer–Tropsch synthesis: A review of water effects on the performances of unsupported and supported Co catalysts. Appl. Catal. A 2008, 348, 1–15. [Google Scholar] [CrossRef]
- Sadeqzadeh, M.; Chambrey, S.; Piché, S.; Fongarland, P.; Luck, F.; Curulla-Ferré, D.; Schweich, D.; Bousquet, J.; Khodakov, A.Y. Deactivation of a Co/Al2O3 Fischer–Tropsch catalyst by water-induced sintering in slurry reactor: Modeling and experimental investigations. Catal. Today 2013, 215, 52–59. [Google Scholar] [CrossRef]
- Sadeqzadeh, M.; Hong, J.; Fongarland, P.; Curulla-Ferre, D.; Luck, F.; Bousquet, J.; Schweich, D.; Khodakov, A.Y. Mechanistic modeling of cobalt based catalyst sintering in a fixed bed reactor under different conditions of Fischer–Tropsch synthesis. Ind. Eng. Chem. Res. 2012, 51, 11955–11964. [Google Scholar] [CrossRef]
- Soled, S.L.; Kiss, G.; Kliewer, C.; Baumgartner, J.; El-Malki, E.-M. Learnings from Co Fischer-Tropsch catalyst studies. In Present at 245th ACS National Meeting & Exposition, New Orleans, LA, USA, 7–11 April 2013.
- Hauman, M.M.; Saib, A.M.; Moodley, D.J.; Plessis, E.D.; Claeys, M.; van Steen, E. Re-dispersion of cobalt on a model fischer–tropsch catalyst during reduction–oxidation–reduction cycles. Chem. Catal. Chem. 2012, 4, 1411–1419. [Google Scholar]
- Weststrate, C.J.; Kizilkaya, A.C.; Rossen, E.T.R.; Verhoeven, M.W.G.M.; Ciobica, I.M.; Saib, A.M.; Niemanstsverdriet, J.W. Atomic and Polymeric Carbon on Co(0001): Surface Reconstruction, Graphene Formation, and Catalyst Poisoning. J. Phys. Chem. C 2012, 116, 11575–11583. [Google Scholar] [CrossRef]
- Weststrate, C.J.; Hauman, M.M.; Moodley, D.J.; Saib, A.M.; van Steen, E.; Niemanstsverdriet, J.W. Cobalt Fischer–Tropsch catalyst regeneration: The crucial role of the kirkendall effect for cobalt redispersion. Top. Catal. 2011, 54, 811–816. [Google Scholar] [CrossRef]
- Moodley, D.J.; van de Loosdrecht, J.; Saib, A.M.; Overett, M.J.; Datye, A.K.; Niemanstsverdriet, J.W. Carbon deposition as a deactivation mechanism of cobalt-based Fischer–Tropsch synthesis catalysts under realistic conditions. Appl. Catal. A 2009, 354, 102–110. [Google Scholar] [CrossRef]
- van de Loosdrecht, J.; Balzhinimaev, B.; Dalmon, J.-A.; Niemanstsverdriet, J.W.; Tsybulya, S.V.; Saib, A.M.; van Berge, P.J.; Visagie, J.L. Cobalt Fischer-Tropsch synthesis: Deactivation by oxidation? Catal. Today 2007, 123, 293–302. [Google Scholar]
- Saib, A.M.; Borgna, A.; van de Loosdrecht, J.; van Berge, P.J.; Niemanstsverdriet, J.W. In Situ surface oxidation study of a planar Co/SiO2/Si(100) model catalyst with nanosized cobalt crystallites under model Fischer−Tropsch synthesis conditions. J. Phys. Chem. B 2006, 110, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Moodley, D.J.; van de Loosdrecht, J.; Saib, A.M.; Niemanstsverdriet, J.W. The formation and influence of carbon on cobalt-based Fischer-Tropsch synthesis catalysts: An integrated review. In Advances in Fischer-Tropsch Synthesis, Catalysts, and Catalysis; Davis, B.H., Occelli, M.L., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 49–81. [Google Scholar]
- Bezemer, G.L.; Herman, J.H.; Kuipers, P.C.E.; Oosterbeek, H.; Holewign, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer−Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.; Ribeiro, M.C.; Ma, W.; Ji, Y.; Khalid, S.; Sumodjo, P.T.A.; Davis, B.H. Group 11 (Cu, Ag, Au) promotion of 15%Co/Al2O3 Fischer–Tropsch synthesis catalysts. Appl. Catal. A 2009, 361, 137–151. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).