Abstract
High energy demand due to boosted economic growth has led to heavy consumption of fossil fuels, thus causing massive emissions of carbon dioxide (CO2) in the air. A promising solution to reduce carbon emissions is to convert CO2 into methanol (CH3OH), which requires high-performance catalysts. Metal nanoparticles have been in the spotlight due to their under-coordinated active sites. Nonetheless, conventional catalytic substrates have emerged with a decline in catalytic performance due to agglomeration of MNPs. Metal–organic frameworks (MOFs) have been acknowledged as alternative platforms to preclude aggregation of MNPs by encapsulation. This review introduces conventional heterogeneous catalysts on CO2 hydrogenation to CH3OH as the first endeavor, then summarizes recent progress of MNPs@MOFs on the same reaction, and, finally, points out the problems that remain unsolved.
1. Introduction
The growing population and modern living standards have resulted in a rapid growth of energy demand. Since the industrial revolution in the late 1700s, energy consumption has heavily relied on the combustion of carbon-based fossil fuels and thus continually increased the atmospheric CO2 concentration [1], reaching 412 ppm in 2020 [2]. This has caused serious global warming and environmental problems [3]; consequently, there has been an urgent need to reduce carbon footprint. One solution is carbon capture and storage (CCS), and the other is carbon capture and utilization (CCU). CCS, as it is named, is composed of three main stages: (i) CO2 capture; (ii) transportation; and (iii) permanent CO2 storage by injecting into a ground or ocean [4]. CCU has gained more and more attention due to the mitigation of carbon emissions by making high-value-added chemical products [5]. Relying on well-understood technological components, CCS appears to be a mature and readily deployable solution towards carbon reduction [6]. Nevertheless, it is limited by cost-intensive operation [7] (e.g., requirement of high energy input for CO2 separation) and CO2 transportation [8]. With respect to CCS, among all potential products by CO2 hydrogenation, methanol (CH3OH) is highly attractive as it is a potential clean-burning fuel for automobiles [9,10]. Under this background, high-performance catalysts for such CO2 conversion have been targeted.
Because of the stable C=O bond [11], CO2 hydrogenation to CH3OH is particularly challenging [12], attracting global effort to develop advanced catalysts for CO2 activation and selective conversion towards the products. In contrast to the development of heterogeneous catalysts, there is a narrow portfolio towards homogeneous catalysis of CH3OH synthesis via CO2 hydrogenation [13]. The major challenges include formate species blocking the active site towards further hydrogenation [14], lengthy catalyst synthesis [15], catalyst incompatibility towards any additive, reaction pH, side products such as CO [16], energy-intensive operation, catalyst deactivation during recycling [17], etc., thus resulting in very limited choices of applicable molecular catalysts. Conversely, heterogeneous catalysts, which are commonly MNP (metal nanoparticle)-based catalysts, are expected to overcome these challenges and present a commercial-scale CH3OH production. Hereby, it still stands at a prominent position in the research trend. In thermal catalysis, which is more advantageous than electrocatalysis and photocatalysis in terms of kinetics and industry-scaled production [18,19,20], harsh operating conditions are often applied for efficient cleavage of a stable C=O bond [21,22,23]. Nevertheless, deactivation might emerge on conventional heterogeneous catalysts due to the rapid crystallization of nano-sized particles, with the intention of minimizing their surface energy [24]. This is particularly severe under high-temperature operation due to a drastic increment of chemical potential for MNPs, driving them to evolve toward an equilibrium shape mainly through coalescence and/or Ostwald ripening [25]. Coalescence is the migration of particles across a surface in a Brownian motion, while Ostwald ripening is the unleashing of individual atoms from particles followed by surface diffusion. These homeless particles or atoms will be prone to merge into a large nanoparticle for lowering chemical potential and finally approaching an equilibrium state [26,27]. Apparently, the formation of large nanoparticles is accompanied by a notable decrement in exposed surfaces and active sites, resulting in a remarkable decline in catalytic performance [28]. The presence of water accelerates sintering [29,30], due to which most catalysts may be stranded in the sintering event for CH3OH synthesis because of the concomitant water byproduct. In addition, the active sites of conventional catalysts can be occupied by undesired adsorbates as they are fully exposed to reactants in an open space, thus leading to lower product selectivity by side reactions [31].
To address these challenges, a core-shell strategy has been proposed [32]. The shell is of vital importance as it can provide high shape-selectivity catalysis by stopping the consecutive reaction due to limited space for reaction, allowing efficient transfer of size-selective molecules toward active sites [33], and acting as the capsule to stabilize MNPs in curbing their agglomeration—a hallmark of a core-shell catalyst [34]. In respect to this, metal–organic frameworks (MOFs), featured with highly tunable porosity and tailorable chemistry [35], have been recognized as an ideal platform for encapsulation of MNPs [36]. Herein, this review aims to provide a solid introduction to conventional support-based and MOFs-encapsulated MNPs for CO2 hydrogenation to methanol. Finally, suggestions for directions of future work on CO2-to-methanol conversion by MOF-based confined MNPs will be given.
2. Conventional Support-Based MNPs
The conventional heterogeneous catalysts are commonly metal nanoparticles (MNPs) supported by metal-oxide-based substrates, which can maximize the usage of under-coordinated active sites of MNPs by strong metal-support interactions (SMSI) and enhance their CO2 hydrogenation performance by synergistic effects [37]. Four main categories are introduced in this section: they are Cu-based catalysts, noble-metal-based catalysts, bimetallic catalysts, and metal oxide complexes.
2.1. Cu-Based Catalysts
Cu catalysts alone cannot provide high selectivity and catalytic activity to CH3OH synthesis [38,39], thus requiring an appropriate support to stabilize its active sites and tune its activity to advance catalytic performance [11]. The development of Cu-based catalysts for CH3OH synthesis dates back to 1905 when Paul Sabatier and Jean-Baptiste Senderens reported their discovery of Cu for effective CH3OH decomposition [40]. This discovery encouraged a number of pioneering research studies to look into how catalysts could promote effective CH3OH decomposition by assuming equally effective CH3OH formation over them under different operating conditions [40]. In 1921, George Patart filed the first patent for a Cu-based catalyst for CH3OH synthesis [41], but its vulnerability to sulfur poisoning made it unattractive for commercialization. In 1963, Phineas Davies and Frederick Snowdon from Imperial Chemical Industries (ICI) patented a metal oxide (copper, zinc, and chromium) composite for CH3OH production at 30–120 atm [42]. It enabled the production of CH3OH in high quantities without the need for high pressure. Based on the finding that substitution of alumina by chromium oxide could prolong the catalyst life, John Thomas Gallagher and John Michell Kidd [43] revised the patent in 1965 and developed Cu/ZnO/Al2O3, a highly selective catalyst that reigned supreme in the following CH3OH industry. Since then, the investigation of Cu-ZnO-based catalysts has dominated this topic, resulting in tremendous publications [44]. Until now, the conventional Cu/ZnO/Al2O3 catalyst remains prominent for the CH3OH industry owing to its low monetary price and high activity at mild operating conditions [44,45].
Although the incorporation of an Al promoter elevates CO2 hydrogenation performance for CH3OH synthesis [46], its strong hydrophilic character appears to be an issue during operation. For instance, the high production of water can induce a rapid particle sintering followed by a subsequent loss of active sites, finally leading to the deactivation of the catalyst [47]. In an attempt to address the issue, less hydrophilic promoters like ZrO2 have been proposed, which was confirmed by Arena et al. [48]. They performed a series of activity tests for Cu-ZnO/ZrO2 and commercial Cu-ZnO/Al2O3 at 160–260 °C and 1.0–3.0 MPa, revealing that enhanced CH3OH formation is essentially due to the less hydrophilic character of ZrO2. Nevertheless, this is not the only promotional effect gifted by ZrO2, under which stronger Cu-support interaction and improved Cu dispersion were also introduced with lower Cu coordination and increased Cu-O distance in contrast to the Cu/ZnO catalyst [49]. In addition, ZrO2 can improve Cu/Zn dispersion and Cu redox ability [46,50,51,52], plus bringing a strong modification on textural and chemical effects as well as adsorption properties [53,54]. As a result of multi-fold modifications by ZrO2, the ternary catalyst Cu/ZnO/ZrO2 can pose a much higher activity and selectivity towards CH3OH synthesis relative to conventional Cu/ZnO/Al2O3 catalysts [55].
Cu-ZnO/ZrO2 ternary catalyst shows competitive activity with the commercial catalyst. Nevertheless, introducing additional promoters, such as SiO2, Ga2O3, and TiO2, can revamp Cu-ZnO/ZrO2 into a more powerful and active catalyst [56,57,58]. For example, Sloczynski et al. [59] prepared a series of oxide additives of B, Ga, In, Gd, Y, Mn, and Mg on their promotional effects for Cu/ZnO/ZrO2. They found that the additives have a significant impact on textural and structural properties in terms of copper dispersion, ZrO2 loading, and hydrophobic character, and thus improved CH3OH yield, with Ga2O3 as the best among them. Phongamwong et al. [60] studied the activity of a series of CuO-ZnO-ZrO2-SiO2 in variation of 0–5% wt silica content, using the reverse co-precipitation method. The result showed that the addition of 1 wt% silica could outperform the silica-free system by an increase of 26% in CH3OH synthesis activity at 20 bar and 240 °C, based on which silica was believed to increase the exposed area of copper catalysts and surface basicity of parent catalysts.
2.2. Noble Metal and Bimetallic Catalysts
Other than Cu-based catalysts, Pd is often considered the second option to catalyze CO2 hydrogenation to CH3OH. Similarly, supports and promoters hold the key to CH3OH production as Pd sites promote CO formation, and it alone cannot stabilize the formate intermediate long enough to be converted into CH3OH, instead being decomposed into CO and H2O via RWGS reaction [61]. Despite 90% CO selectivity detected upon pure Pd, the addition of ZnO could form a bimetallic PdZn alloy, which acted as an active phase towards selective CH3OH production [61]. As a result, 11% CO2 conversion and 60% CH3OH selectivity were achieved at 250 °C and 20 bar. As revealed, PdZn alloy can passivate the Pd surface and strengthen the adsorption of formate species. Additionally, ZnO and Pd played their own roles with ZnO for CO2 adsorption and Pd in H2 dissociation, and such co-operation efficiently furnished this bifunctional catalyst [61].
In addition to ZnO, Ga2O3 is the other solid support pairing properly with Pd NPs [62]. The Pd/Ga2O3 catalyst involving active Pdn+ (0 < n < 2) sites could afford CO2 conversion of 19.6% with CH3OH yield in 10.1%, where the operating pressure and temperature are 5.0 MPa and 523 K, respectively [63]. The authors reckoned that GaxOy support generated an optimal amount of partially oxidized Pd species upon the catalyst surface; hence, Pd/Ga2O3 was endowed with the best activity in contrast to other Pd/metal oxides [63]. However, CH3OH selectivity is an issue in this case due to the in-parallel RWGS reaction leading to undesired CO. Interestingly, other study showed a suppressing effect on CO by Pd/SiO2. It enlightened the use of Ga2O3 as a promoter to Pd/SiO2, aiming for amelioration of CH3OH selectivity, overall showing a 500-fold increase in CH3OH production rate than the parent catalyst Pd/SiO2, where TOR (10−3 s−1) is 0.002 [64].
Au is scarcely studied since its bulk phase shows inertness in catalyzing hydrogenation, but it can become active when it is deposited as a nanoparticle on metal oxide support. For instance, the study by Yang et al. [65] demonstrated the activity of Au/CeOx/TiO2 on CO2 abstraction and hydrogenation at low pressure, in which CO and CH3OH production was promoted together with enhanced CH3OH selectivity by a small coverage of CeOx. Behm group [66] carried out a systematic study on different metal oxide supports (TiO2, Al2O3, ZnO, and ZrO2) for Au NPs at moderate operating conditions (5 bar pressure and 220–240 °C) and achieved the best CH3OH selectivity (i.e., 83.3% approximately) with ZnO support. Proceeding to the study of Au particle size effect over Au/ZnO, they disclosed that small-sized Au catalysts could result in a positive impact on CH3OH formation rate while imposing an adverse effect on CH3OH selectivity due to competitive CO production.
Given the above description for synergistic catalysis by PdZn and CuZn active sites, bimetallic systems have also been widely investigated for CH3OH production. For instance, Song et al. [67] reported a sequence of novel silica-supported PdxCu1-x (where x is within the range of 0.25–0.34) bimetallic catalysts, achieving a maximum rate of CH3OH production at x = 0.34. Such a bimetallic promotional effect was rationalized as the coexistence of PdCu and PdCu3 alloy phases, which delivered higher hydrogen absorption capacity. They subsequently extrapolated the optimal atomic ratio widely (2.4–18.7%) for the same catalyst and revealed that Pd-Cu(2.4)/SiO2 produced 1.6 times more CH3OH than commercial Cu-ZnO-Al2O3. Nevertheless, competitive CO production outpaced CH3OH, thus leading to regrettably low CH3OH selectivity [68]. In addition, Jaramilo’s group [69] synthesized silica-supported In-Pd bimetallic catalyst with In:Pd molar ratios varying by 1:0, 1:1, 2:1, and 0:1. Thanks to the In-Pd alloy phase and its synergy with indium oxide, all bimetallic samples outperformed individual metals in terms of CO2 conversion and CH3OH selectivity. Among them, In:Pd(2:1)/SiO2 won the championship by achieving the highest production (5.1 µmol/gInPds) and selectivity (61%) of CH3OH (Table 1). Similar enhancement was observed at the In-Ni/SiO2 system, indicating a translatable promotion mechanism on these alloy/oxide systems.

Table 1.
CH3OH selectivity and activity of studied samples under 573 K, 40 bar [69].
2.3. Metal Oxide Catalysts
Aside from metal-based catalysts, metal oxides have emerged as promising catalysts in CH3OH synthesis. For example, binary metal oxides ZnO-ZrO2 showed good stability and activity by achieving >10% CO2 conversion along with approximately 90% selectivity at 320 °C and 5 MPa after 500 h reaction [70]. Similarly, MZrOx (M = Cd, Ga) could deliver 4.3–12.4% and 80% for CO2 conversion and CH3OH selectivity, respectively, apparently performing better than individual oxides [71]. In both cases, the synergy between catalytic components is the main driving force for such amelioration of catalytic activity.
Indium oxide has been recognized and highlighted for its 100% CH3OH selectivity, but was limited by low CO2 conversion [72], which has motivated tremendous work to improve it. Javier’s group [73] demonstrated an enhancement with combined In2O3/ZrO2, highlighting the key role of ZrO2 to increase active sites. Witoon et al. [74] proposed an investigation of the promotional effect of GaxIn2-xO3 by varying the Ga/In ratio, with Ga0.4In1.6O3 giving the highest hydrogenation efficiency and CH3OH yield increasing from 1.1% (In2O3) to 2.4% at 340 °C and from 0.48% (In2O3) to 1.14% at 360 °C. Such enhancement was attributed to stronger adsorption of reaction intermediates by the catalyst. Pd-doping is also an available solution to enhance the catalytic property of In2O3 since the former is favorable for H2 split, a friendly H atom supplier as noted above [75].
3. MOF-Encapsulated MNPs
Since the work in 1995 by Yaghi [76], metal–organic frameworks (MOFs) as three-dimensional porous coordination polymers have rapidly grown their influence in the fields of chemistry and material science. The same group in 1999 developed [Zn4O(tpa)3]n (tpa = terephthalic acid), also called MOF-5, as one of the most well-known and studied MOFs [77]. In reticular chemistry, three core elements are required for the design of MOFs: building blocks, targeted nets, and iso-reticular chemistry [78]. Building blocks can be mainly denoted as secondary building units (SBUs) and organic linkers [79]. Topological nets are generally purposed to show the basic architectures of MOFs, by using dot-line expression to impart the spatial distribution of building blocks (e.g., Zr-MOFs shown in Figure 1) [80]. They usually serve as the basis and first phase for MOF design and construction. Under this scheme, iso-reticular chemistry can guide the regulation of chemical or physical properties through smart selection and combinations of building blocks while maintaining the underlying topology, making them desirable for broad applications under various environmental conditions [81]. In short, MOFs have been recognized as ultra-porous hybrid crystalline materials in which secondary building units are assembled by polydentate linkers (usually carboxylate, sulfonate, or phosphonate), integrating into well-defined networks.
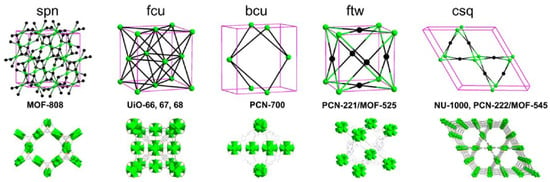
Figure 1.
Schematic presentation of selected Zr-MOF topological nets, where spn, fcu, bcu, ftw, and csq represent the topology of sodalite, face-centered cubic, body-centered cubic, Fluorite, and cubic square net, respectively [82]. (Copyright © 2016 American Chemical Society).
3.1. Structural Characteristics
The growing popularity of MOFs is mainly attributed to their high porosities and large pore volumes and surface areas, which provide the basis for various functions. Currently, most well-developed MOFs are microporous in nature as they have shown superior performance on gas adsorption; however, they are limited in emerging applications, such as drug delivery and catalysis, with strong demand to host large objects and to provide efficient molecular diffusion. Under this context, MOFs with large pores are requested, which can be easily decorated with different functional groups and provide large confined spaces, ideally dispersing and protecting active NPs. Stemming from the size of NPs and the nature of targeted reactions, proper MOF confinement can considerably improve the activity in various nanocatalysis [83]. This background highlights the importance of urgent development of mesoporous and macroporous MOFs [84], which is approachable through elongating the length of organic linkers [85].
3.2. MOF Stability
As the framework of active MNPs, MOF stability is of vital importance to all research objects as it can determine their lifespans for technical implementation, which is essentially determined by the strength of metal-ligand bonds [86,87,88]. On the ground of Pearson’s hard and soft acids and bases (HSAB) principle, a strong metal–ligand bond can only be derived by hard acid-hard base or soft acid-soft base combination, while alternative options (e.g., hard acids and soft bases, or soft acids and hard bases) will lead to a fragile and vulnerable interaction [89] Thence, MOFs synthesized by high-valency metals and carboxylate-based ligands exhibit outstanding stability in water and acidic solutions. The mechanism behind this is that the substitution of carboxylate-based ligands by water molecules [90,91] or the replacement of hard acid metal ions and hard-base ligands by protons and hydroxides through hydrolysis [92] becomes difficult in water solution or water vapor (Figure 2). Likewise, in an acidic solution, the proton is not strong enough to compete with the high-valent metal ion for chemically bonding with carboxylate linkers. Unfortunately, such MOFs are vulnerable to basic solutions. On the contrary, MOFs in soft-acid-base combination (lowly valent metal with azolate-based ligands) are effective on base and water resistance but weaker on combating acidic corrosion [89].
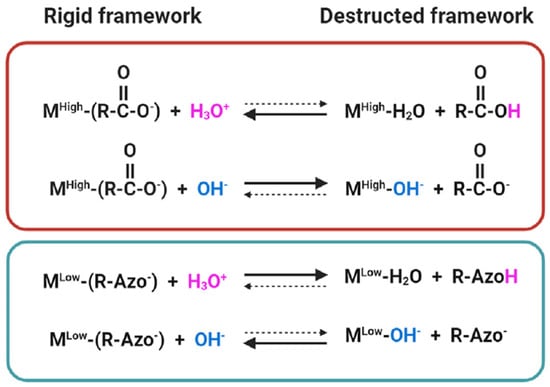
Figure 2.
Metal–ligand substitution reactions between MOFs and protons/hydroxides. Mhigh and Mlow denote high- and low-valence metal nodes, respectively. Solid arrows represent a more favored process, and dashed arrows represent a less favored process [93]. (Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany).
HSAB theory can guide researchers to obtain MOFs with high chemical and water stability by enhancing the node-linker bond strength. MIL-101-Cr, as a chromium terephthalate-based material, is a result of applying hard-acid-base theory. It showcases its chemical and water stability by staying intact for 5 days in acidic solution [94] and for months in a moist condition [95]. A strong metal-ligand bond is indeed conducive to MOF thermal stability. Nevertheless, it alone cannot firmly protect MOF intactness under harsh conditions. MIL-101-Cr was reported to decompose at 275 °C, attesting its relatively poor thermal stability even though its inorganic bricks are tightly bound by the linkers [96].
Connectivity [93], referring to the number of SBUs in connection with a single ligand and the number of ligands in connection with SBUs, is proposed as another key factor for thermal stability [84] Taking UiO-66 as an example, its SBU is an octahedral cluster with six vertices occupied by Zr4+ and eight triangular faces covered by four µ3-O and µ3-OH respectively [97]. Unlike MIL-101-Cr, which is constructed from 6-connected [Cr3(µ3-O)OH(H2O)2(COO)3] clusters and linear dicaboxylate linkers, UiO-66 has 12 organic linkers bridging to each of its SBUs, generating six more coordination bonds than MIL-101-Cr (Figure 3) [98]. Due to such higher connectivity, the thermal decomposition temperature for UiO-66 is significantly increased up to 540 °C [99].
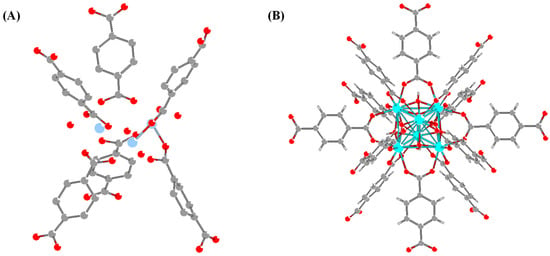
Figure 3.
Secondary building unit of (A) MIL-101-Cr (in linkage of 6 organic linkers) and (B) UiO-66 (in linkage of 12 organic linkers), where C, O, Cr, Zr, and H are grey, red, blue, light turquoise, and white atoms, respectively.
The high porosity and surface area are prominent features of MOFs, making them desirable for adsorption and other applications. However, it is indicative of many void spaces inside the structure and might lead to poor sustainability when MOFs are exposed to a pressured or vacuum environment [100]. Most MOFs can only withstand shear stress under 1 GPa, exhibiting their vulnerability in response to mechanical loading. However, UiO-type MOFs are a counterexample, as evidenced by the large shear modulus of UiO-66(Zr) and UiO-66(Hf) with an order of magnitude higher than that of other MOFs and similar to zeolites [101]. Such extraordinary mechanical stability is, ascribed to strong metal–ligand bonds and high connectivity, critical to host MNPs with long-term durability in harsh catalysis, as demonstrated below.
3.3. Case Studies
To the best of our knowledge, Yaghi et al. [102] were the first group to commence on CH3OH synthesis by CO2 reduction using MOF-encapsulated MNPs. They examined Cu@UiO-66 performance by comparing it with a series of catalysts, including Cu/UiO-66, Cu/ZnO/Al2O3, and Cu/ZrO2. Among these candidates, Cu@UiO-66 was found to steadily produce the highest output of CH3OH with 100% selectivity. The rationale behind this was more intimate interactions between the Cu surface and Zr oxide SBU, generating more Cu active sites for CO2 conversion. High CH3OH selectivity on Cu@UiO-66 was attributed to its intrinsic catalytic nature, meaning that the catalyst itself was disfavoured for CO production. The authors also found that Zr- and Zn-oxides were the main promoters for the Cu catalyst, thus improving CH3OH yield. Interestingly, this agrees with the findings discovered by Lercher et al. and Castaño et al. [103,104], where Cu-O-Zr and Zn-O-Cu interfaces are the smallest part of active sites responsible for improved activities of CO2 activation, thus increasing its conversion rate to CH3OH. In contrast to intact UiO-66 applied in Yaghi et al.’s work [102], Lercher et al. [103] entrapped Cu nanoparticles using defective UiO-66 instead. They assessed its catalytic performance at 523 K and 32 bar and found three times higher than their reference samples (i.e., Cu/ZrO2 and Cu/ZnO/Al2O3) due to its sufficiently small metallic Cu nanoparticles in the framework and large fractions of Cu-O-Zr interfacial sites. Castaño et al. [104] studied the reaction on ZIF-8-encapsulated 14-nm Cu NPs, which was 2-fold higher methanol productivity than the commercial Cu–Zn–Al catalyst and remarkably stable for over 150 h at reaction conditions (i.e., 500 MPa and 523 K). The supplemented DFT calculations further revealed inhibition of RWGS reaction on Cu@ZIF-8 and stronger adsorption of CO2 on the Cu-O-Zn interface than both Zn-O and bare Cu sites, thus making a very selective CO2-to-CH3OH conversion (>90%).
Pioneering work by Yaghi et al., [102] has also been supported by Wang and his co-workers [105], who successfully embedded Cu/ZnO nanocatalysts inside UiO-bpy for CO2 hydrogenation. The catalyst proved its splendid stability by diminishing less than 10% catalytic activity during 100 h on stream. At 250 °C and 4 MPa (CO2/H2 = 1:3), it exhibited 100% CH3OH selectivity and STY of 0.0809 mmolMeOH·gCu−1h−1, higher than 54.8% and 0.0259 mmolMeOH·gCu−1h−1 for Cu/ZnO/Al2O3, and 51.9% and 0.00406 mmolMeOH·gCu−1h−1 for Cu@UiO-bpy. Such performance was attributed to the synergism of Cu, ZnO, and Zr6 SBUs; moreover, they reported the importance of the chemical environment for particle confinement by evaluating the catalytic performance between Cu/ZnOx@UiO-bpy and CuZn@MIL-253-Al.
Different from narrow windows with a smaller cage in UiO-bpy, which could vastly suppress Cu agglomeration by strong NP/SBU interactions and steric hindrance, MIL-253-Al possessed a larger cavity and relatively expanded window size. Thereby, Cu nanoparticles in MIL-253-Al could grow into a larger size and even extend to the external surface of the MOF, resulting in only one-third CH3OH yield of Cu/ZnOx@UiO-bpy. The encapsulation of nanoparticles in MOFs is usually realized by the “ship-in-a-bottle” approach involving impregnation, coprecipitation, and deposition-precipitation [106]. However, it is rather challenging to precisely control the loading composition of active nanoparticles inside MOF cages since the precursors might precipitate on the external surface of MOFs, forming aggregated counterparts [107]. While using the traditional impregnation approach for preparing Cu/ZnOx@UiO-66, Li et al. [108] reported a similar issue and found an easy separation between Zn and Cu phases. Thereby, they proposed an otherwise double solvent method to overcome the synthesis hurdle. No agglomeration of metal particles and phase separation was observed while synthesizing Cu/ZnOx@UiO-66. The confinement effect endowed a catalyst with a stable hydrogenation process over a period of 100 h stream. The highly dispersed Cu/ZnO in UiO-66 cages, and appreciable interpenetration of Cu and ZnOx phases presented a rich Cu-ZnOx-Zr node interface, which enabled high CH3OH activity with STY of 39.7 mmolMeOH·gCu−1h−1 and 86.1% selectivity, higher than 7.19 mmolMeOH·gCu−1h−1 and 47.2% for Cu/ZnO/Al2O3 under exactly the same reaction conditions as performed by Wang et al. [105].
In addition to the Cu catalyst, Pd is the second most examined due to excellent hydrogen splitting capability; however, pure Pd favors CO production from CO2 hydrogenation, thus delivering poor selectivity of CH3OH. Alloying Pd with Zn can make the catalyst reversely favorable for CH3OH synthesis—an amelioration of CH3OH selectivity [109]. In 2019, Tsang et al. [110] developed an efficient PdZn alloy catalyst by depositing Pd nanoparticles on oxygen-defective ZnO nanorods coated by ZIF-8. The increase in ZIF-8 thickness could bring higher CO2 adsorption capacity owing to the concomitant increment in oxygen defects on ZnO; however, it discouraged the formation of PdZn alloy due to the spatial isolation effect. To obtain the optimum thickness of ZIF-8 jacket, a volcano-shaped correlation between CH3OH activity and etching time over Pd-ZnO@ZIF-8 was hence developed. Under the reaction condition of 563 K and 4.5 MPa (CO2/H2 = 1:3), CH3OH productivity reached a maximum (STY = 19.8 g gPd−1 h−1) when the etching time was 1 h. The authors extrapolated that there should be a trade-off between the PdZn phase and surface oxygen defects for CH3OH synthesis; thus, they proposed a synergistic catalysis mechanism for the fabrication of high-performance Pd-ZnO catalysts.
CH3OH is generally produced via two pathways from CO2 hydrogenation, including (i) CO formation from CO2 by reversed-water-gas-shift (RWSG) reaction, which can further be hydrogenated into CH3OH, and (ii) formate pathway [111,112], in which HCOO is recognized as the key intermediate and further hydrogenated into methoxy and CH3OH [113,114]. Similar to a Pd catalyst, Pt alone shows high activity for RWSG reaction, resulting in the inhibition of CH3OH synthesis due to intense competition with CO formation [111,114,115]. Though CH3OH can be synthesized from syngas, the presence of CO2 adversely affects the synthesis rate and might result in more unconverted CO gas retained upon the catalyst surface [116]. High CO production is not favored for CH3OH selectivity and is detrimental to active metal sites by poisoning [117]. Therefore, the key to selective CH3OH production is to avoid the RWSG pathway and stabilize HCOO* species.
To stabilize HCOO* species, UiO-series MOFs with abundant Zr oxides as Lewis acid sites have been explored, intending for further hydrogenation towards CH3OH [118]. Thermodynamically, low-temperature operation can help shift the equilibrium towards CH3OH production, hence probably preventing it from undergoing RWSG. Olsbye et al. [119] prepared a mixed-ligand-UiO-67-encapsulated Pt catalyst (UiO-67-Pt) and assessed its CO2 hydrogenation performance at 170 °C and 8 bar (CO2/H2 = 1:3). The CH3OH STY over UiO-67-Pt approached 0.072 mmolMeOH·gCu−1h−1 with a selectivity of 13% and outperformed UiO-67(LD)-Pt, a less defective UiO-67-encapsulated Pt catalyst with CH3OH STY 0.0216 mmolMeOH·gCu−1h−1 and selectivity of 7%. It is believed that the success originates from the unsaturated Zr sites serving as key participants for the catalytic promotion. They not only synergized with Pt nanoparticles to form rich Pt-Zr interfaces, which were accountable for CH3OH production, but also held HCOO species tightly for further hydrogenation into CH3OH (Figure 4). Thereby, UiO-67-Pt with 660 µmol·g−1 unsaturated Zr sites could deliver higher productivity of CH3OH than UiO-67(LD)-Pt with solely 70 µmol·g−1. It is also fascinating to note that the addition of water could further enhance CH3OH selectivity by promoting CH3OH desorption from Zr sites while inhibiting methane formation [120].
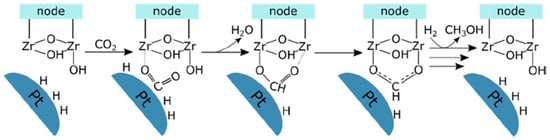
Figure 4.
Schematic presentation of the reaction mechanism of CO2 hydrogenation to HCOO intermediate in CH3OH formation at the Pt-Zr node interface [120] (Copyright © 2019 American Chemical Society).
Thus far, the major active sites of MNPs@MOFs, in accordance with published works above, are the interfacial sites between MNPs and metal nodes of MOFs, where synergistic catalysis is presented, as reported by other reviews [121,122,123]. Nevertheless, supplemented DFT calculations merely focus on CO2 activation. It remains unclear how synergism works along with each reaction step for thermal CO2-to-CH3OH conversion. By the rough comparison from Table 2, the catalytic activities might be impacted by the factors that need to be verified by further research, as follows:

Table 2.
MOF-confined NPs for hydrogenation of CO2 to CH3OH, where T, P, Smethanol, and STY are denoted to be temperature, pressure, methanol selectivity, and space time yield of CH3OH, respectively.
- Support effect, as implied by catalytic activities between Cu/ZnO@UiO-66 and Cu/Zn@UiO-bpy.
- Reaction conditions (i.e., pressure and temperature), as indicated by performance between Cu@UiO-66 and Cu@def-UiO-66, despite better CO2 activation on Cu@def-UiO-66, as the defected Zr node generates more interfacial sites.
- NP, Pd-ZnO@ZIF-8 shows significantly higher methanol productivity in contrast to Cu@ZIF-8.
4. Future Outlook
As summary, MOFs can be facilely tailored to meet the following requirements: (i) survive at hydrothermal conditions and withstand mechanical forces—outstanding robustness; (ii) have highly disperse active NPs in the pores of MOFs due to high porosity and surface area; (iii) offer selective-size molecules with access to active sites by shuffling off those irrelevant to desired reactions—molecular-sieving effect due to pre-selected pore matrix; (iv) inhibit the agglomeration of active NPs by encapsulation—pore confinement effect; (v) exhibit higher catalytic performance than individual counterparts by synergizing with active NPs—high catalytic activity, etc. Therefore, MOFs can act as eligible shells for cores (active metal NPs), and MNPs@MOF are promising for effective and stable CO2 hydrogenation to CH3OH. Despite tremendous efforts by the researchers on progressing developments of MNPs@MOF on CH3OH production, the interpretation of confinement effect by MOFs as well as the reaction mechanism at a molecular level is less than well understood for MNPs@MOF composites, the synthesis of catalysts is cost-ineffective, and the product selectivity, including the yield rate, is far from the industrial level. Therefore, more extensive efforts are needed to overcome these challenges.
Author Contributions
Z.Y.: writing—original draft, validation, formal analysis. W.X.: resources. H.C.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the conclusions of this review are summarized from published works. Please refer to the original works for further information.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Fang, C. Urbanization, Economic Growth, Energy Consumption, and CO2 Emissions: Empirical Evidence from Countries with Different Income Levels. Renew. Sustain. Energy Rev. 2018, 81, 2144–2159. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon Capture and Storage (CCS): The Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Sun, S.; Sun, H.; Williams, P.T.; Wu, C. Recent Advances in Integrated CO2 Capture and Utilization: A Review. Sustain. Energy Fuels 2021, 5, 4546–4559. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The Role of CO2 Capture and Utilization in Mitigating Climate Change. Nat. Clim. Change 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Zhao, M.; Minett, A.I.; Harris, A.T. A Review of Techno-Economic Models for the Retrofitting of Conventional Pulverised-Coal Power Plants for Post-Combustion Capture (PCC) of CO2. Energy Environ. Sci. 2013, 6, 25–40. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalón, S.; Primo, A.; García, H. Selective Gas-Phase Hydrogenation of CO2 to Methanol Catalysed by Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2024, 63, e202311241. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A Review of the Catalytic Hydrogenation of Carbon Dioxide into Value-Added Hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Shao, S.; Cui, C.; Tang, Z.; Li, G. Recent Advances in Metal-Organic Frameworks for Catalytic CO2 Hydrogenation to Diverse Products. Nano Res. 2022, 15, 10110–10133. [Google Scholar] [CrossRef]
- Wang, W.-H.; Bao, M.; Feng, X. Transformation of Carbon Dioxide to Formic Acid and Methanol, 1st ed.; SpringerBriefs in Green Chemistry for Sustainability Series; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.G.; Liu, P. Optimizing Binding Energies of Key Intermediates for CO2 Hydrogenation to Methanol over Oxide-Supported Copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.G.; Sen, R.; Goeppert, A. Homogeneous Hydrogenation of CO2 and CO to Methanol: The Renaissance of Low Temperature Catalysis in the Context of the Methanol Economy. Angew. Chem. Int. Ed. 2022, 61, anie.202207278. [Google Scholar] [CrossRef]
- Bai, S.-T.; De Smet, G.; Liao, Y.; Sun, R.; Zhou, C.; Beller, M.; Maes, B.U.W.; Sels, B.F. Homogeneous and Heterogeneous Catalysts for Hydrogenation of CO2 to Methanol under Mild Conditions. Chem. Soc. Rev. 2021, 50, 4259–4298. [Google Scholar] [CrossRef] [PubMed]
- Rayder, T.M.; Bensalah, A.T.; Li, B.; Byers, J.A.; Tsung, C.-K. Engineering Second Sphere Interactions in a Host–Guest Multicomponent Catalyst System for the Hydrogenation of Carbon Dioxide to Methanol. J. Am. Chem. Soc. 2021, 143, 1630–1640. [Google Scholar] [CrossRef]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core–Shell Structured Catalysts for Thermocatalytic, Photocatalytic, and Electrocatalytic Conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net Reduction of CO2 via Its Thermocatalytic and Electrocatalytic Transformation Reactions in Standard and Hybrid Processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Len, T.; Luque, R. Addressing the CO2 Challenge through Thermocatalytic Hydrogenation to Carbon Monoxide, Methanol and Methane. Green. Chem. 2023, 25, 490–521. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The Physical Chemistry and Materials Science behind Sinter-Resistant Catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Liang, B.; Ma, J.; Su, X.; Yang, C.; Duan, H.; Zhou, H.; Deng, S.; Li, L.; Huang, Y. Investigation on Deactivation of Cu/ZnO/Al2O3 Catalyst for CO2 Hydrogenation to Methanol. Ind. Eng. Chem. Res. 2019, 58, 9030–9037. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A Short Review of Recent Advances in CO2 Hydrogenation to Hydrocarbons over Heterogeneous Catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic Carbon Dioxide Hydrogenation to Methanol: A Review of Recent Studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Campbell, C.T. The Energetics of Supported Metal Nanoparticles: Relationships to Sintering Rates and Catalytic Activity. Acc. Chem. Res. 2013, 46, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Mao, Z. Chemical Potential of Metal Atoms in Supported Nanoparticles: Dependence upon Particle Size and Support. ACS Catal. 2017, 7, 8460–8466. [Google Scholar] [CrossRef]
- Rumptz, J.R.; Zhao, K.; Mayo, J.; Campbell, C.T. Size-Dependent Energy of Ni Nanoparticles on Graphene Films on Ni(111) and Adhesion Energetics by Adsorption Calorimetry. ACS Catal. 2022, 12, 12632–12642. [Google Scholar] [CrossRef]
- Goodman, E.D.; Schwalbe, J.A.; Cargnello, M. Mechanistic Understanding and the Rational Design of Sinter-Resistant Heterogeneous Catalysts. ACS Catal. 2017, 7, 7156–7173. [Google Scholar] [CrossRef]
- Sun, J.T.; Metcalfe, I.S.; Sahibzada, M. Deactivation of Cu/ZnO/Al2O3 Methanol Synthesis Catalyst by Sintering. Ind. Eng. Chem. Res. 1999, 38, 3868–3872. [Google Scholar] [CrossRef]
- Zhai, X.; Shamoto, J.; Xie, H.; Tan, Y.; Han, Y.; Tsubaki, N. Study on the Deactivation Phenomena of Cu-Based Catalyst for Methanol Synthesis in Slurry Phase. Fuel 2008, 87, 430–434. [Google Scholar] [CrossRef]
- Bao, J.; He, J.; Zhang, Y.; Yoneyama, Y.; Tsubaki, N. A Core/Shell Catalyst Produces a Spatially Confined Effect and Shape Selectivity in a Consecutive Reaction. Angew. Chem. 2008, 120, 359–362. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Towards a Molecular Understanding of Shape Selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting Functionality to a Metal–Organic Framework Material by Controlled Nanoparticle Encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef]
- Liu, J.; Goetjen, T.A.; Wang, Q.; Knapp, J.G.; Wasson, M.C.; Yang, Y.; Syed, Z.H.; Delferro, M.; Notestein, J.M.; Farha, O.K.; et al. MOF-Enabled Confinement and Related Effects for Chemical Catalyst Presentation and Utilization. Chem. Soc. Rev. 2022, 51, 1045–1097. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Dong, C.; Gao, R.; Xiao, D.; Liu, H.; Ma, D. Fully Exposed Cluster Catalyst (FECC): Toward Rich Surface Sites and Full Atom Utilization Efficiency. ACS Cent. Sci. 2021, 7, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A Review of Research Progress on Heterogeneous Catalysts for Methanol Synthesis from Carbon Dioxide Hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Le Valant, A.; Comminges, C.; Tisseraud, C.; Canaff, C.; Pinard, L.; Pouilloux, Y. The Cu–ZnO Synergy in Methanol Synthesis from CO2, Part 1: Origin of Active Site Explained by Experimental Studies and a Sphere Contact Quantification Model on Cu + ZnO Mechanical Mixtures. J. Catal. 2015, 324, 41–49. [Google Scholar] [CrossRef]
- Sheldon, D. Methanol Production—A Technical History. Johns. Matthey Technol. Rev. 2017, 61, 172–182. [Google Scholar] [CrossRef]
- Patart, G. Procédé de Production d’Alcools, d’Aldéhydes et d’Acides à Partir de Mélanges Gazeux Maintenus sous Pression et Soumis à l’Action d’Agents Catalytiques ou de l’Électricité. 540543. 1921. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=FR&NR=540543A&KC=A&FT=D&ND=3&date=19220712&DB=&locale=fr_EP# (accessed on 13 July 2022).
- Davies, P.; Snowdon, F. Water-Gas Conversion and Catalysts Therefor. GB1010871A, 3 June 1963. [Google Scholar]
- Gallagher, J.T.; Kidd, J.M. Methanol Synthesis. GB1159035A, 18 August 1965. [Google Scholar]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Bansode, A.; Tidona, B.; von Rohr, P.R.; Urakawa, A. Impact of K and Ba Promoters on CO2 Hydrogenation over Cu/Al2O3 Catalysts at High Pressure. Catal. Sci. Technol. 2013, 3, 767–778. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Likozar, B. The Role of Copper Oxidation State in Cu/ZnO/Al2O3 Catalysts in CO2 Hydrogenation and Methanol Productivity. Renew. Energy 2019, 140, 452–460. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef]
- Arena, F.; Barbera, K.; Italiano, G.; Bonura, G.; Spadaro, L.; Frusteri, F. Synthesis, Characterization and Activity Pattern of Cu–ZnO/ZrO2 Catalysts in the Hydrogenation of Carbon Dioxide to Methanol. J. Catal. 2007, 249, 185–194. [Google Scholar] [CrossRef]
- Yang, C.; Ma, Z.; Zhao, N.; Wei, W.; Hu, T.; Sun, Y. Methanol Synthesis from CO2-Rich Syngas over a ZrO2 Doped CuZnO Catalyst. Catal. Today 2006, 115, 222–227. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Xiao, F.; Zhao, N.; Sun, N.; Wei, W.; Zhong, L.; Sun, Y. Preparation and Activity of Cu/Zn/Al/Zr Catalysts via Hydrotalcite-Containing Precursors for Methanol Synthesis from CO2 Hydrogenation. Catal. Sci. Technol. 2012, 2, 1447. [Google Scholar] [CrossRef]
- Shaharun, S.; Shaharun, M.S.; Mohamad, D.; Taha, M.F. The Effect of Cu/Zn Molar Ratio on CO2 Hydrogenation over Cu/ZnO/ZrO2/Al2O3 Catalyst. In Proceedings of the 3rd International Conference on Fundamental and Applied Sciences, Kuala Lumpur, Malaysia, 3–5 June 2014; pp. 3–9. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, Y.-Z.; Han, M.-H.; Wang, J.-F.; Jin, Y.; Wei, F. Long Carbon Nanotubes Intercrossed Cu/Zn/Al/Zr Catalyst for CO/CO2 Hydrogenation to Methanol/Dimethyl Ether. Catal. Today 2010, 150, 55–60. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. How Oxide Carriers Control the Catalytic Functionality of the Cu–ZnO System in the Hydrogenation of CO2 to Methanol. Catal. Today 2013, 210, 39–46. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. Effects of Oxide Carriers on Surface Functionality and Process Performance of the Cu–ZnO System in the Synthesis of Methanol via CO2 Hydrogenation. J. Catal. 2013, 300, 141–151. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.G. CO2 Hydrogenation to Methanol over ZrO2-Containing Catalysts: Insights into ZrO2 Induced Synergy. ACS Catal. 2019, 9, 7840–7861. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Chen, S. Effect of Promoter SiO2, TiO2 or SiO2-TiO2 on the Performance of CuO-ZnO-Al2O3 Catalyst for Methanol Synthesis from CO2 Hydrogenation. Appl. Catal. A Gen. 2012, 415–416, 118–123. [Google Scholar] [CrossRef]
- Hu, X.; Qin, W.; Guan, Q.; Li, W. The Synergistic Effect of CuZnCeOx in Controlling the Formation of Methanol and CO from CO2 Hydrogenation. ChemCatChem 2018, 10, 4438–4449. [Google Scholar] [CrossRef]
- Yamamura, T.; Tada, S.; Kikuchi, R.; Fujiwara, K.; Honma, T. Effect of Sm Doping on CO2-to-Methanol Hydrogenation of Cu/Amorphous-ZrO2 Catalysts. J. Phys. Chem. C 2021, 125, 15899–15909. [Google Scholar] [CrossRef]
- Sloczynski, J.; Grabowski, R.; Olszewski, P.; Kozlowska, A.; Stoch, J.; Lachowska, M.; Skrzypek, J. Effect of Metal Oxide Additives on the Activity and Stability of Cu/ZnO/ZrO2 Catalysts in the Synthesis of Methanol from CO2 and H2. Appl. Catal. A Gen. 2006, 310, 127–137. [Google Scholar] [CrossRef]
- Phongamwong, T.; Chantaprasertporn, U.; Witoon, T.; Numpilai, T.; Poo-arporn, Y.; Limphirat, W.; Donphai, W.; Dittanet, P.; Chareonpanich, M.; Limtrakul, J. CO2 Hydrogenation to Methanol over CuO–ZnO–ZrO2–SiO2 Catalysts: Effects of SiO2 Contents. Chem. Eng. J. 2017, 316, 692–703. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Hutchings, G.; Dimitratos, N.; Wells, P.; Gibson, E.; Jones, W.; Brookes, C.; Morgan, D.; Lalev, G. Pd/ZnO Catalysts for Direct CO2 Hydrogenation to Methanol. J. Catal. 2016, 343, 133–146. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.-J. A Review on the Catalytic Conversion of CO2 Using H2 for Synthesis of CO, Methanol, and Hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Fujitani, T.; Saito, M.; Kanai, Y.; Watanabe, T.; Nakamura, J.; Uchijima, T. Development of an Active Ga2O3 Supported Palladium Catalyst for the Synthesis of Methanol from Carbon Dioxide and Hydrogen. Appl. Catal. A Gen. 1995, 125, L199–L202. [Google Scholar] [CrossRef]
- Bonivardi, A.L.; Chiavassa, D.L.; Querini, C.A.; Baltanás, M.A. Enhancement of the Catalytic Performance to Methanol Synthesis from CO2/H2 by Gallium Addition to Palladium/Silica Catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 3747–3752. [Google Scholar] [CrossRef]
- Yang, X.; Kattel, S.; Senanayake, S.D.; Boscoboinik, J.A.; Nie, X.; Graciani, J.; Rodriguez, J.A.; Liu, P.; Stacchiola, D.J.; Chen, J.G. Low Pressure CO2 Hydrogenation to Methanol over Gold Nanoparticles Activated on a CeOx/TiO2 Interface. J. Am. Chem. Soc. 2015, 137, 10104–10107. [Google Scholar] [CrossRef]
- Hartadi, Y.; Widmann, D.; Behm, R.J. CO2 Hydrogenation to Methanol on Supported Au Catalysts under Moderate Reaction Conditions: Support and Particle Size Effects. ChemSusChem 2015, 8, 456–465. [Google Scholar] [CrossRef]
- Jiang, X.; Koizumi, N.; Guo, X.; Song, C. Bimetallic Pd–Cu Catalysts for Selective CO2 Hydrogenation to Methanol. Appl. Catal. B Environ. 2015, 170–171, 173–185. [Google Scholar] [CrossRef]
- Jiang, X.; Jiao, Y.; Moran, C.; Nie, X.; Gong, Y.; Guo, X.; Walton, K.S.; Song, C. CO2 Hydrogenation to Methanol on Pd Cu Bimetallic Catalysts with Lower Metal Loadings. Catal. Commun. 2019, 118, 10–14. [Google Scholar] [CrossRef]
- Snider, J.L.; Streibel, V.; Hubert, M.A.; Choksi, T.S.; Valle, E.; Upham, D.C.; Schumann, J.; Duyar, M.S.; Gallo, A.; Abild-Pedersen, F.; et al. Revealing the Synergy between Oxide and Alloy Phases on the Performance of Bimetallic In–Pd Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 3399–3412. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A Highly Selective and Stable ZnO-ZrO2 Solid Solution Catalyst for CO2 Hydrogenation to Methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef]
- Wang, J.; Tang, C.; Li, G.; Han, Z.; Li, Z.; Liu, H.; Cheng, F.; Li, C. High-Performance MaZrOx (Ma = Cd, Ga) Solid-Solution Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 10253–10259. [Google Scholar] [CrossRef]
- Dang, S.; Qin, B.; Yang, Y.; Wang, H.; Cai, J.; Han, Y.; Li, S.; Gao, P.; Sun, Y. Rationally Designed Indium Oxide Catalysts for CO2 Hydrogenation to Methanol with High Activity and Selectivity. Sci. Adv. 2020, 6, eaaz2060. [Google Scholar] [CrossRef]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef]
- Akkharaphatthawon, N.; Chanlek, N.; Cheng, C.K.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Tuning Adsorption Properties of GaxIn2−xO3 Catalysts for Enhancement of Methanol Synthesis Activity from CO2 Hydrogenation at High Reaction Temperature. Appl. Surf. Sci. 2019, 489, 278–286. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Atomic-Scale Engineering of Indium Oxide Promotion by Palladium for Methanol Production via CO2 Hydrogenation. Nat. Commun. 2019, 10, 3377. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Jiang, H.; Alezi, D.; Eddaoudi, M. A Reticular Chemistry Guide for the Design of Periodic Solids. Nat. Rev. Mater. 2021, 6, 466–487. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kirlikovali, K.O.; Li, P.; Farha, O.K. Reticular Chemistry for Highly Porous Metal–Organic Frameworks: The Chemistry and Applications. Acc. Chem. Res. 2022, 55, 579–591. [Google Scholar] [CrossRef]
- Schukraft, G.E.M.; Ayala, S.; Dick, B.L.; Cohen, S.M. Isoreticular Expansion of polyMOFs Achieves High Surface Area Materials. Chem. Commun. 2017, 53, 10684–10687. [Google Scholar] [CrossRef]
- Rimoldi, M.; Howarth, A.J.; DeStefano, M.R.; Lin, L.; Goswami, S.; Li, P.; Hupp, J.T.; Farha, O.K. Catalytic Zirconium/Hafnium-Based Metal–Organic Frameworks. ACS Catal. 2017, 7, 997–1014. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally Stable Pt/Mesoporous Silica Core–Shell Nanocatalysts for High-Temperature Reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF Stability: Approaches and Applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.-S.; Lollar, C.T.; Zhou, H.-C. Stable Metal–Organic Frameworks with Group 4 Metals: Current Status and Trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal-Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, P.; Sharma, V.K.; Ma, X.; Zhou, H.-C. Metal-Organic Frameworks for Environmental Applications. Cell Rep. Phys. Sci. 2021, 2, 100348. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Bai, P.; Guo, X. Structure Stability of Metal-Organic Framework MIL-101(Cr) in Acetic Acid Solutions. Mod. Chem. Eng. 2018, 38, 129–134. [Google Scholar] [CrossRef]
- Liu, B.; Vikrant, K.; Kim, K.-H.; Kumar, V.; Kailasa, S.K. Critical Role of Water Stability in Metal–Organic Frameworks and Advanced Modification Strategies for the Extension of Their Applicability. Environ. Sci. Nano 2020, 7, 1319–1347. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hanna, S.L.; Redfern, L.R.; Alezi, D.; Islamoglu, T.; Farha, O.K. Reticular Chemistry in the Rational Synthesis of Functional Zirconium Cluster-Based MOFs. Coord. Chem. Rev. 2019, 386, 32–49. [Google Scholar] [CrossRef]
- Healy, C.; Patil, K.M.; Wilson, B.H.; Hermanspahn, L.; Harvey-Reid, N.C.; Howard, B.I.; Kleinjan, C.; Kolien, J.; Payet, F.; Telfer, S.G.; et al. The Thermal Stability of Metal-Organic Frameworks. Coord. Chem. Rev. 2020, 419, 213388. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional Mechanical Stability of Highly Porous Zirconium Metal–Organic Framework UiO-66 and Its Important Implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-Based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, J.; Ye, J.; Cui, Y.; Koh, K.; Kovarik, L.; Camaioni, D.M.; Fulton, J.L.; Truhlar, D.G.; Neurock, M.; et al. Copper-Zirconia Interfaces in UiO-66 Enable Selective Catalytic Hydrogenation of CO2 to Methanol. Nat. Commun. 2020, 11, 5849. [Google Scholar] [CrossRef] [PubMed]
- Velisoju, V.K.; Cerrillo, J.L.; Ahmad, R.; Mohamed, H.O.; Attada, Y.; Cheng, Q.; Yao, X.; Zheng, L.; Shekhah, O.; Telalovic, S.; et al. Copper Nanoparticles Encapsulated in Zeolitic Imidazolate Framework-8 as a Stable and Selective CO2 Hydrogenation Catalyst. Nat. Commun. 2024, 15, 2045. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal–Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Qi, Y.; Gao, H.; Zheng, N.; Wang, G. Synthesis of an Amino-Functionalized Metal–Organic Framework at a Nanoscale Level for Gold Nanoparticle Deposition and Catalysis. J. Mater. Chem. A 2014, 2, 20588–20596. [Google Scholar] [CrossRef]
- Volosskiy, B.; Niwa, K.; Chen, Y.; Zhao, Z.; Weiss, N.O.; Zhong, X.; Ding, M.; Lee, C.; Huang, Y.; Duan, X. Metal-Organic Framework Templated Synthesis of Ultrathin, Well-Aligned Metallic Nanowires. ACS Nano 2015, 9, 3044–3049. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ding, H.; Yang, D.; Cheng, E.; Hao, Y.; Wang, H.; Hong, Y.; Su, Y.; Wang, Y.; et al. Cu/ZnOx@UiO-66 Synthesized from a Double Solvent Method as an Efficient Catalyst for CO2 Hydrogenation to Methanol. Catal. Sci. Technol. 2021, 11, 4367–4375. [Google Scholar] [CrossRef]
- Brix, F.; Desbuis, V.; Piccolo, L.; Gaudry, É. Tuning Adsorption Energies and Reaction Pathways by Alloying: PdZn versus Pd for CO 2 Hydrogenation to Methanol. J. Phys. Chem. Lett. 2020, 11, 7672–7678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, G.; Xu, D.; Hong, X.; Edman Tsang, S.C. Confinement of Subnanometric PdZn at a Defect Enriched ZnO/ZIF-8 Interface for Efficient and Selective CO2 Hydrogenation to Methanol. J. Mater. Chem. A 2019, 7, 23878–23885. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO 2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and Challenges in the Selective Reduction of Carbon Dioxide to Methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the Art and Perspectives in Heterogeneous Catalysis of CO2 Hydrogenation to Methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Winter, L.R.; Chen, J.G.; Yan, B. CO2 Hydrogenation over Heterogeneous Catalysts at Atmospheric Pressure: From Electronic Properties to Product Selectivity. Green Chem. 2021, 23, 249–267. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Chen, J.G.; Liu, P. CO2 Hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of Synergy between Pt and Oxide Support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef]
- Tisseraud, C.; Comminges, C.; Belin, T.; Ahouari, H.; Soualah, A.; Pouilloux, Y.; Le Valant, A. The Cu–ZnO Synergy in Methanol Synthesis from CO2, Part 2: Origin of the Methanol and CO Selectivities Explained by Experimental Studies and a Sphere Contact Quantification Model in Randomly Packed Binary Mixtures on Cu–ZnO Coprecipitate Catalysts. J. Catal. 2015, 330, 533–544. [Google Scholar] [CrossRef]
- Chen, W.; Cao, J.; Fu, W.; Zhang, J.; Qian, G.; Yang, J.; Chen, D.; Zhou, X.; Yuan, W.; Duan, X. Molecular-Level Insights into the Notorious CO Poisoning of Platinum Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202200190. [Google Scholar] [CrossRef]
- Noh, G.; Lam, E.; Bregante, D.T.; Meyet, J.; Šot, P.; Flaherty, D.W.; Copéret, C. Lewis Acid Strength of Interfacial Metal Sites Drives CH3OH Selectivity and Formation Rates on Cu-Based CO2 Hydrogenation Catalysts. Angew. Chem. Int. Ed. 2021, 60, 9650–9659. [Google Scholar] [CrossRef]
- Gutterød, E.S.; Pulumati, S.H.; Kaur, G.; Lazzarini, A.; Solemsli, B.G.; Gunnæs, A.E.; Ahoba-Sam, C.; Kalyva, M.E.; Sannes, J.A.; Svelle, S.; et al. Influence of Defects and H2O on the Hydrogenation of CO2 to Methanol over Pt Nanoparticles in UiO-67 Metal–Organic Framework. J. Am. Chem. Soc. 2020, 142, 17105–17118. [Google Scholar] [CrossRef] [PubMed]
- Gutterød, E.S.; Lazzarini, A.; Fjermestad, T.; Kaur, G.; Manzoli, M.; Bordiga, S.; Svelle, S.; Lillerud, K.P.; Skúlason, E.; Øien-Ødegaard, S.; et al. Hydrogenation of CO2 to Methanol by Pt Nanoparticles Encapsulated in UiO-67: Deciphering the Role of the Metal–Organic Framework. J. Am. Chem. Soc. 2020, 142, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Tedeeva, M.A.; Kustov, A.L.; Batkin, A.M.; Garifullina, C.; Zalyatdinov, A.A.; Yang, D.; Dai, Y.; Yang, Y.; Kustov, L.M. Catalytic Systems for Hydrogenation of CO2 to Methanol. Mol. Catal. 2024, 566, 114403. [Google Scholar] [CrossRef]
- Lu, X.; Song, C.; Qi, X.; Li, D.; Lin, L. Confinement Effects in Well-Defined Metal–Organic Frameworks (MOFs) for Selective CO2 Hydrogenation: A Review. Int. J. Mol. Sci. 2023, 24, 4228. [Google Scholar] [CrossRef]
- Li, Y.-M.; Hu, J.; Zhu, M. Confining Atomically Precise Nanoclusters in Metal–Organic Frameworks for Advanced Catalysis. Coord. Chem. Rev. 2023, 495, 215364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).