Two-Dimensional Multilayered Ferroelectric with Polarization-Boosted Photocatalytic Hydrogen Evolution
Abstract
1. Introduction
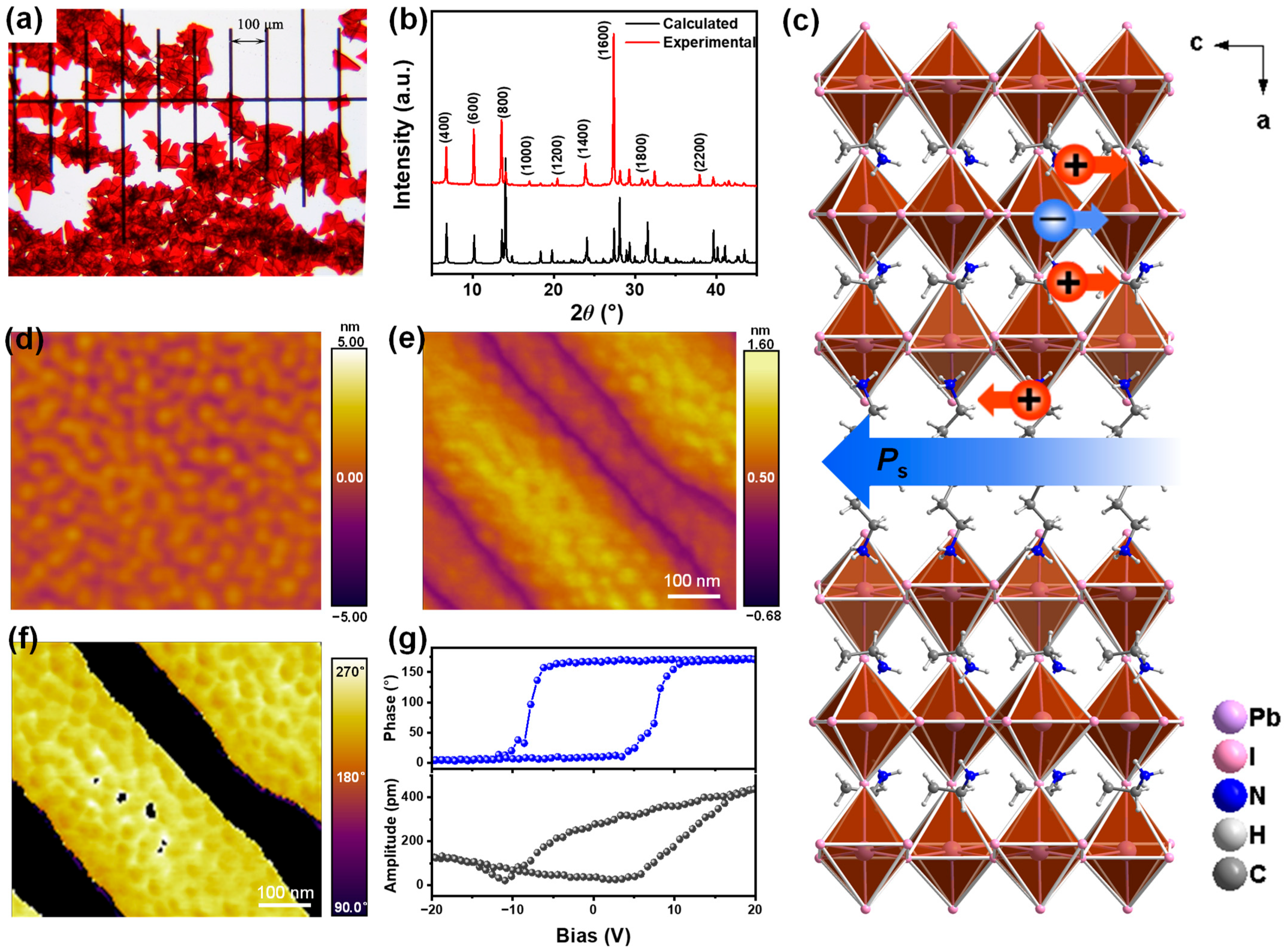
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of BAPI Microcrystals
3.3. Material Characterization
3.4. Photocatalytic H2 Evolution Measurements
3.5. Calculations of AQYs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, H.; Sun, X.; Jia, S.; Li, X.; Miao, T.J.; Xiong, L.; Wang, S.; Zhang, X.; Liu, X.; et al. High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu-TiO2 photocatalysts. Nat. Mater. 2023, 22, 619–626. [Google Scholar]
- Li, Z.; Li, R.; Jing, H.; Xiao, J.; Xie, H.; Hong, F.; Ta, N.; Zhang, X.; Zhu, J.; Li, C. Blocking the reverse reactions of overall water splitting on a Rh/GaN–ZnO photocatalyst modified with Al2O3. Nat. Catal. 2023, 6, 80–88. [Google Scholar] [CrossRef]
- Pornrungroj, C.; Mohamad Annuar, A.B.; Wang, Q.; Rahaman, M.; Bhattacharjee, S.; Andrei, V.; Reisner, E. Hybrid photothermal–photocatalyst sheets for solar-driven overall water splitting coupled to water purification. Nat. Water 2023, 1, 952–960. [Google Scholar]
- Hisatomi, T.; Yamada, T.; Nishiyama, H.; Takata, T.; Domen, K. Materials and systems for large-scale photocatalytic water splitting. Nat. Rev. Mater. 2025. [Google Scholar] [CrossRef]
- Zhen, C.; Zhu, H.; Chen, R.; Zheng, Z.; Fan, F.; Li, B.; Xu, X.; Du, Y.; Cheng, H.-M.; Domen, K.; et al. An artificial leaf with patterned photocatalysts for sunlight-driven water splitting. J. Am. Chem. Soc. 2024, 146, 28482–28490. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kang, Y.; Liu, J.; Yao, T.; Qiu, J.; Du, P.; Huang, B.; Hu, W.; Liang, Y.; Xie, T.; et al. Gradient tungsten-doped Bi3TiNbO9 ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting. Nat. Commun. 2023, 14, 7948. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Qu, J.; Xie, H.; Lu, R.; Fan, F.; Li, C. Boosting photocatalytic water oxidation on photocatalysts with ferroelectric single domains. Adv. Mater. 2023, 35, 2210374. [Google Scholar] [CrossRef]
- Kang, Y.; Qi, H.; Wan, G.; Zhen, C.; Xu, X.; Yin, L.-C.; Wang, L.; Liu, G.; Cheng, H.-M. Ferroelectric polarization enabled spatially selective adsorption of redox mediators to promote Z-scheme photocatalytic overall water splitting. Joule 2022, 6, 1876–1886. [Google Scholar] [CrossRef]
- Fridkin, V.M. Photoferroelectrics; Springer: New York, NY, USA, 1979. [Google Scholar]
- Choi, T.; Lee, S.; Kiryukhin, V.; Cheong, S.-W. Switchable ferroelectric diode and photovoltaic effect in BiFeO3. Science 2009, 324, 63–66. [Google Scholar] [CrossRef]
- Nakamura, M.; Horiuchi, S.; Kagawa, F.; Ogawa, N.; Kurumaji, T.; Tokura, Y.; Kawasaki, M. Shift current photovoltaic effect in a ferroelectric charge-transfer complex. Nat. Commun. 2017, 8, 281. [Google Scholar] [CrossRef]
- Paillard, C.; Bai, X.; Infante, I.C.; Guennou, M.; Geneste, G.; Alexe, M.; Kreisel, J.; Dkhil, B. Photovoltaics with ferroelectrics: Current status and beyond. Adv. Mater. 2016, 28, 5153–5168. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, S.; Xie, H.; Zhu, J.; Shi, Q.; Ta, N.; Chen, R.; Gao, Y.; An, H.; Nie, W.; et al. Internal-field-enhanced charge separation in a single-domain ferroelectric PbTiO3 photocatalyst. Adv. Mater. 2020, 32, e1906513. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, J.; Song, W.; Peng, S.; Sun, M.; Xing, G.; Yu, L. Atomic heterointerface engineering and nonequilibrium carrier dynamics for enhanced photocatalysis in halide perovskite/MoS2 systems. Angew. Chem. Int. Ed. 2025, 64, e202506436. [Google Scholar] [CrossRef]
- Fu, H.; Wu, Y.; Guo, Y.; Sakurai, T.; Zhang, Q.; Liu, Y.; Zheng, Z.; Cheng, H.; Wang, Z.; Huang, B.; et al. A scalable solar-driven photocatalytic system for separated H2 and O2 production from water. Nat. Commun. 2025, 16, 990. [Google Scholar] [CrossRef] [PubMed]
- Chemmangat, A.; Chen, H.-T.; Kamat, P.V. Bandgap engineering of halide perovskite nanocrystals for maximizing hole transfer: Accessing the Marcus inverted region. J. Am. Chem. Soc. 2025, 147, 25727–25737. [Google Scholar] [CrossRef]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 16185. [Google Scholar] [CrossRef]
- Vesborg, P.C.K. Photocatalysis: HI-time for perovskites. Nat. Energy 2017, 2, 16205. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Zhao, S.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B.; Wang, P. Integrating mixed halide perovskite photocatalytic HI splitting and electrocatalysis into a loop for efficient and robust pure water splitting. Adv. Mater. 2023, 35, 2208915. [Google Scholar] [CrossRef]
- Xu, T.; Xie, Y.; Qi, S.; Zhang, H.; Ma, W.; Wang, J.; Gao, Y.; Wang, L.; Zong, X. Simultaneous defect passivation and Co-catalyst engineering leads to superior photocatalytic hydrogen evolution on metal halide perovskites. Angew. Chem. Int. Ed. 2024, 63, e202409945. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X.; Fu, J.; Wu, Y.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. 2D/Quasi-2D Ruddlesden–Popper perovskite: A high-performance photocatalyst for hydrogen evolution. ACS Catal. 2023, 13, 14716–14724. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Wang, J.; Gao, Y.; Fan, F.; Wu, K.; Zong, X.; Li, C. Mechanistic understanding of efficient photocatalytic H2 evolution on two-dimensional layered lead iodide hybrid perovskites. Angew. Chem. Int. Ed. 2021, 60, 7376–7381. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Wang, X.; Sui, X.Y.; Lin, M.Y.; Zhu, Y.; Jing, C.; Yuan, H.Y.; Yang, S.; Liu, P.F.; et al. Polar aromatic two-dimensional Dion-Jacobson halide perovskites for efficient photocatalytic H2 evolution. Angew. Chem. Int. Ed. 2024, 63, e202319882. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Q.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B.; Wang, P. Photocatalytic overall water splitting with a solar-to-hydrogen conversion efficiency exceeding 2 % through halide perovskite. Angew. Chem. Int. Ed. 2024, 63, e202411016. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Wang, P.; Hu, W.; Wang, S.; Ji, C.; Hong, M.; Luo, J. Tailored engineering of an unusual (C4H9NH3)2(CH3NH3)2Pb3Br10 two-dimensional multilayered perovskite ferroelectric for a high-performance photodetector. Angew. Chem. Int. Ed. 2017, 56, 12150–12154. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhang, Z.X.; Song, X.J.; Chen, X.G.; Xiong, R.G. Two-dimensional hybrid perovskite ferroelectric induced by perfluorinated substitution. J. Am. Chem. Soc. 2020, 142, 20208–20215. [Google Scholar] [CrossRef]
- Chen, X.G.; Song, X.J.; Zhang, Z.X.; Zhang, H.Y.; Pan, Q.; Yao, J.; You, Y.M.; Xiong, R.G. Confinement-driven ferroelectricity in a two-dimensional hybrid lead iodide perovskite. J. Am. Chem. Soc. 2020, 142, 10212–10218. [Google Scholar] [CrossRef]
- Fan, C.C.; Han, X.B.; Liang, B.D.; Shi, C.; Miao, L.P.; Chai, C.Y.; Liu, C.D.; Ye, Q.; Zhang, W. Chiral Rashba ferroelectrics for circularly polarized light detection. Adv. Mater. 2022, 34, e2204119. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ye, L.; Gong, Z.X.; Ma, J.J.; Wang, Q.W.; Jiang, J.Y.; Hua, M.M.; Wang, C.F.; Yu, H.; Zhang, Y.; et al. Two-dimensional organic-inorganic hybrid rare-earth double perovskite ferroelectrics. J. Am. Chem. Soc. 2020, 142, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Weng, W.; Ji, C.; Liu, X.; Sun, Z.; Lin, W.; Hong, M.; Luo, J. Trilayered lead chloride perovskite ferroelectric affording self-powered visible-blind ultraviolet photodetection with large zero-bias photocurrent. J. Am. Chem. Soc. 2020, 142, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, X.; Sun, Z.; Ji, C.; Li, L.; Wu, Z.; Wang, S.; Yao, Y.; Hong, M.; Luo, J. Exploiting the bulk photovoltaic effect in a 2D trilayered hybrid ferroelectric for highly sensitive polarized light detection. Angew. Chem. Int. Ed. 2020, 59, 3933–3937. [Google Scholar] [CrossRef]
- Peng, Y.; Bie, J.; Liu, X.; Li, L.; Chen, S.; Fa, W.; Wang, S.; Sun, Z.; Luo, J. Acquiring high-TC layered metal halide ferroelectrics via cage-confined ethylamine rotators. Angew. Chem. Int. Ed. 2021, 60, 2839–2843. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, L.; Ji, C.; Liu, X.; Wang, G.-E.; Xu, G.; Sun, Z.; Luo, J. Visible-photoactive perovskite ferroelectric-driven self-powered gas detection. J. Am. Chem. Soc. 2023, 145, 12853–12860. [Google Scholar] [CrossRef]
- Ji, C.; Dey, D.; Peng, Y.; Liu, X.; Li, L.; Luo, J. Ferroelectricity-driven self-powered ultraviolet photodetection with strong polarization sensitivity in a two-dimensional halide hybrid perovskite. Angew. Chem. Int. Ed. 2020, 59, 18933–18937. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Xiao, L.B.; Zhao, J.; Pan, B.K.; Li, J.; Liao, W.Q.; Xiong, R.G.; Zou, G.F. Molecular ferroelectrics-driven high-performance perovskite solar cells. Angew. Chem. Int. Ed. 2020, 59, 19974–19982. [Google Scholar] [CrossRef]
- Han, S.; Liu, X.; Liu, Y.; Xu, Z.; Li, Y.; Hong, M.; Luo, J.; Sun, Z. High-temperature antiferroelectric of lead iodide hybrid perovskites. J. Am. Chem. Soc. 2019, 141, 12470–12474. [Google Scholar] [CrossRef]
- Xu, Z.; Dong, X.; Wang, L.; Wu, H.; Liu, Y.; Luo, J.; Hong, M.; Li, L. Precisely tailoring a FAPbI3-derived ferroelectric for sensitive self-driven broad-spectrum polarized photodetection. J. Am. Chem. Soc. 2023, 145, 1524–1529. [Google Scholar] [CrossRef]
- Ye, H.; Peng, Y.; Wei, M.; Zhang, X.; Zhu, T.; Guan, Q.; Li, L.; Chen, S.; Liu, X.; Luo, J. Bulk photovoltaic effect in chiral layered hybrid perovskite enables highly sensitive near-infrared circular polarization photodetection. Chem. Mater. 2023, 35, 6591–6597. [Google Scholar] [CrossRef]
- Li, X.; Ke, W.; Traore, B.; Guo, P.; Hadar, I.; Kepenekian, M.; Even, J.; Katan, C.; Stoumpos, C.C.; Schaller, R.D.; et al. Two-dimensional Dion-Jacobson hybrid lead iodide perovskites with aromatic diammonium cations. J. Am. Chem. Soc. 2019, 141, 12880–12890. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Li, H.; Xue, J.; Jiang, S.; Zhang, Q.; Bao, J. Promoting photocatalytic H2 evolution through retarded charge trapping and recombination by continuously distributed defects in methylammonium lead iodide perovskite. Angew. Chem. Int. Ed. 2023, 62, e202308140. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Q.; Zhang, Q.; Lou, Z.; Liu, K.; Ma, Y.; Wang, Z.; Zheng, Z.; Cheng, H.; Liu, Y.; et al. An organometal halide perovskite supported Pt single-atom photocatalyst for H2 evolution. Energy Environ. Sci. 2022, 15, 1271–1281. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Hisatomi, T.; Vequizo, J.J.M.; Suzuki, S.; Chen, S.; Nakabayashi, M.; Lin, L.; Pan, Z.; Kariya, N.; et al. Sequential cocatalyst decoration on BaTaO2N towards highly-active Z-scheme water splitting. Nat. Commun. 2021, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, R.; Matsunaga, H.; Nakashima, T.; Wada, K.; Yamakata, A.; Ishitani, O.; Maeda, K. Nature-inspired, highly durable CO2 reduction system consisting of a binuclear ruthenium(II) complex and an organic semiconductor using visible light. J. Am. Chem. Soc. 2016, 138, 5159–5170. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Fan, F.; Li, C. Unraveling charge-separation mechanisms in photocatalyst particles by spatially resolved surface photovoltage techniques. Angew. Chem. Int. Ed. 2022, 61, e202117567. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z.; Zhang, J.; Li, S.; Zhao, Z.; Zhu, L.; Liu, W.; Lin, Y.; Liu, H.; Zhang, Z. Enhanced catalytic performance by multi-field coupling in KNbO3 nanostructures: Piezo-photocatalytic and ferro-photoelectrochemical effects. Nano Energy 2019, 58, 695–705. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.; Li, C.; Yan, F.; Bai, H.; Shen, B.; Zeng, H.; Zhai, J. Piezophototronic effect in enhancing charge carrier separation and transfer in ZnO/BaTiO3 heterostructures for high-efficiency catalytic oxidation. Nano Energy 2019, 66, 104127. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Wan, G.; Yin, L.; Chen, X.; Xu, X.; Huang, J.; Zhen, C.; Zhu, H.; Huang, B.; Hu, W.; Ren, Z.; et al. Photocatalytic overall water splitting over PbTiO3 modulated by oxygen vacancy and ferroelectric polarization. J. Am. Chem. Soc. 2022, 144, 20342–20350. [Google Scholar] [CrossRef]
- Jiang, L.; Ni, S.; Liu, G.; Xu, X. Photocatalytic hydrogen production over Aurivillius compound Bi3TiNbO9 and its modifications by Cr/Nb co-doping. Appl. Catal. B Environ. 2017, 217, 342–352. [Google Scholar] [CrossRef]
- Huang, J.; Kang, Y.; Liu, J.A.; Chen, R.; Xie, T.; Liu, Z.; Xu, X.; Tian, H.; Yin, L.; Fan, F.; et al. Selective exposure of robust perovskite layer of Aurivillius-type compounds for stable photocatalytic overall water splitting. Adv. Sci. 2023, 10, 2302206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Dittrich, T.; Wang, Z.; Ji, P.; Li, M.; Ta, N.; Zhang, H.; Zhen, C.; Xu, Y.; et al. Unveiling charge utilization mechanisms in ferroelectric for water splitting. Nat. Commun. 2025, 16, 1515. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Li, L.; Xu, Y.; Wang, X.; Hou, Y. Two-Dimensional Multilayered Ferroelectric with Polarization-Boosted Photocatalytic Hydrogen Evolution. Catalysts 2025, 15, 910. https://doi.org/10.3390/catal15090910
Peng Y, Li L, Xu Y, Wang X, Hou Y. Two-Dimensional Multilayered Ferroelectric with Polarization-Boosted Photocatalytic Hydrogen Evolution. Catalysts. 2025; 15(9):910. https://doi.org/10.3390/catal15090910
Chicago/Turabian StylePeng, Yu, Liangyao Li, Yilin Xu, Xing Wang, and Yu Hou. 2025. "Two-Dimensional Multilayered Ferroelectric with Polarization-Boosted Photocatalytic Hydrogen Evolution" Catalysts 15, no. 9: 910. https://doi.org/10.3390/catal15090910
APA StylePeng, Y., Li, L., Xu, Y., Wang, X., & Hou, Y. (2025). Two-Dimensional Multilayered Ferroelectric with Polarization-Boosted Photocatalytic Hydrogen Evolution. Catalysts, 15(9), 910. https://doi.org/10.3390/catal15090910





