Study on Photocatalytic Peroxone Process for Treating Organic Pollutants in Leachate Based on Modified Carbon Quantum Dots
Abstract
1. Introduction
2. Results and Discussion
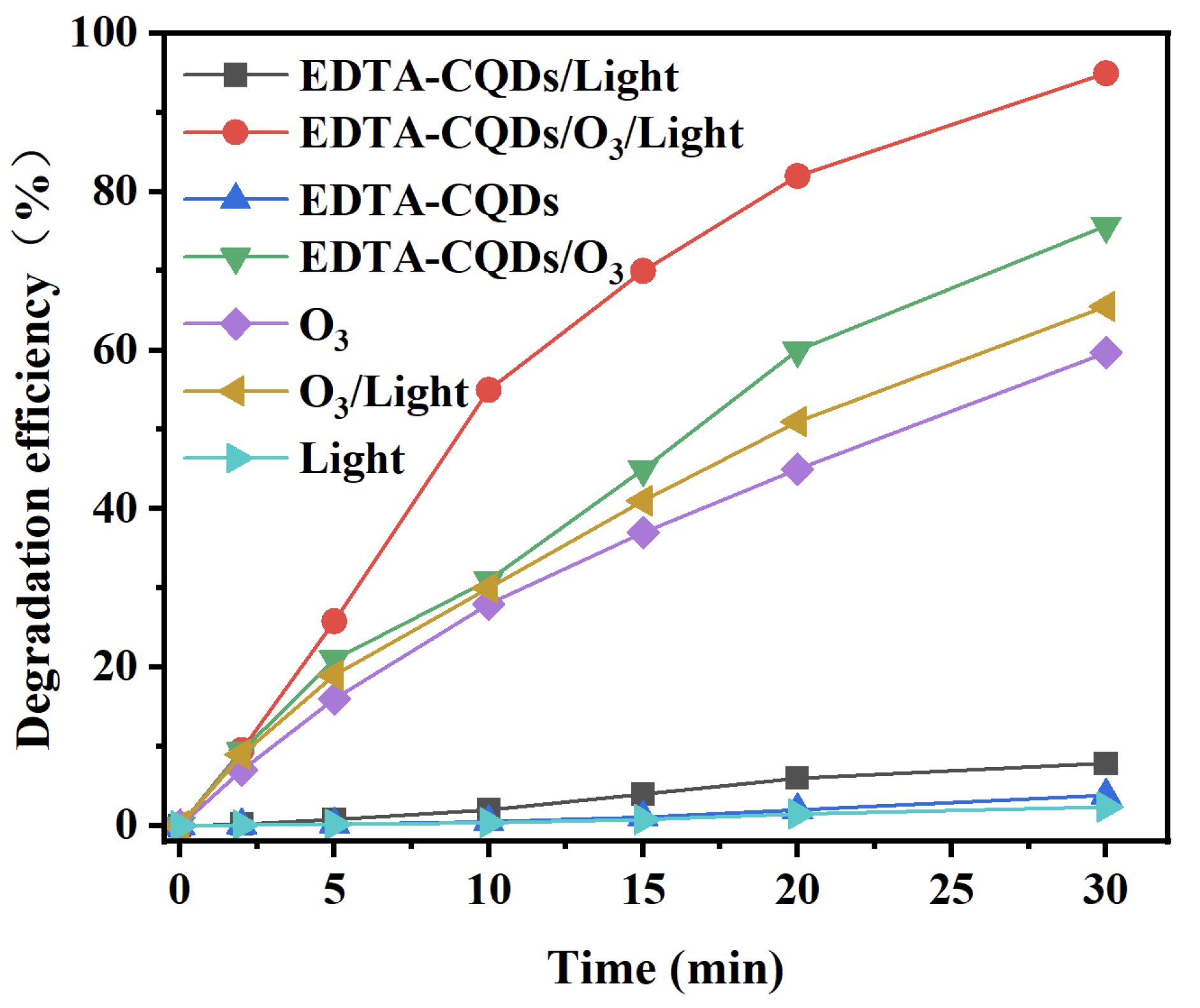
2.1. Degradation of Hydroquinone in Different Systems
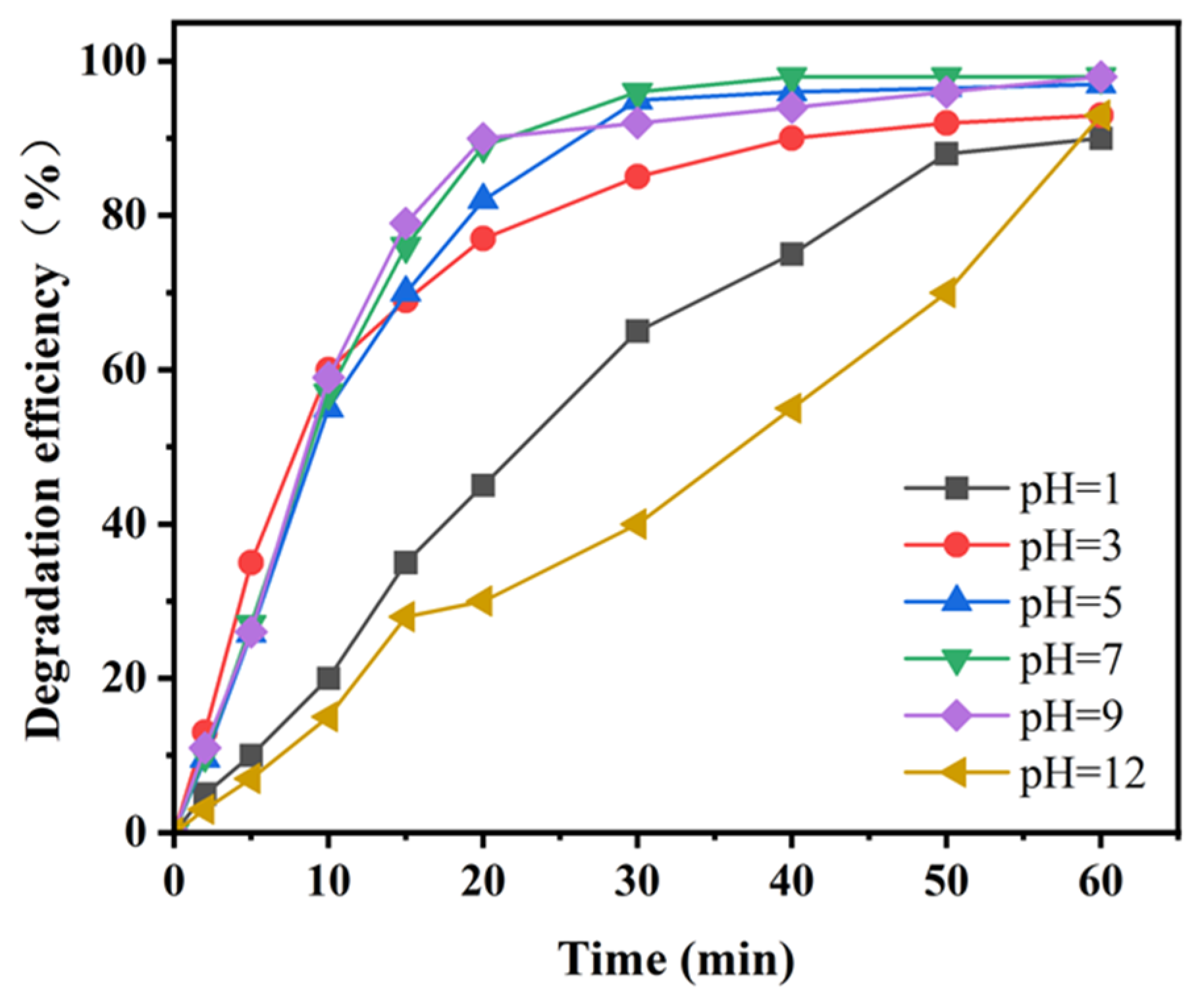
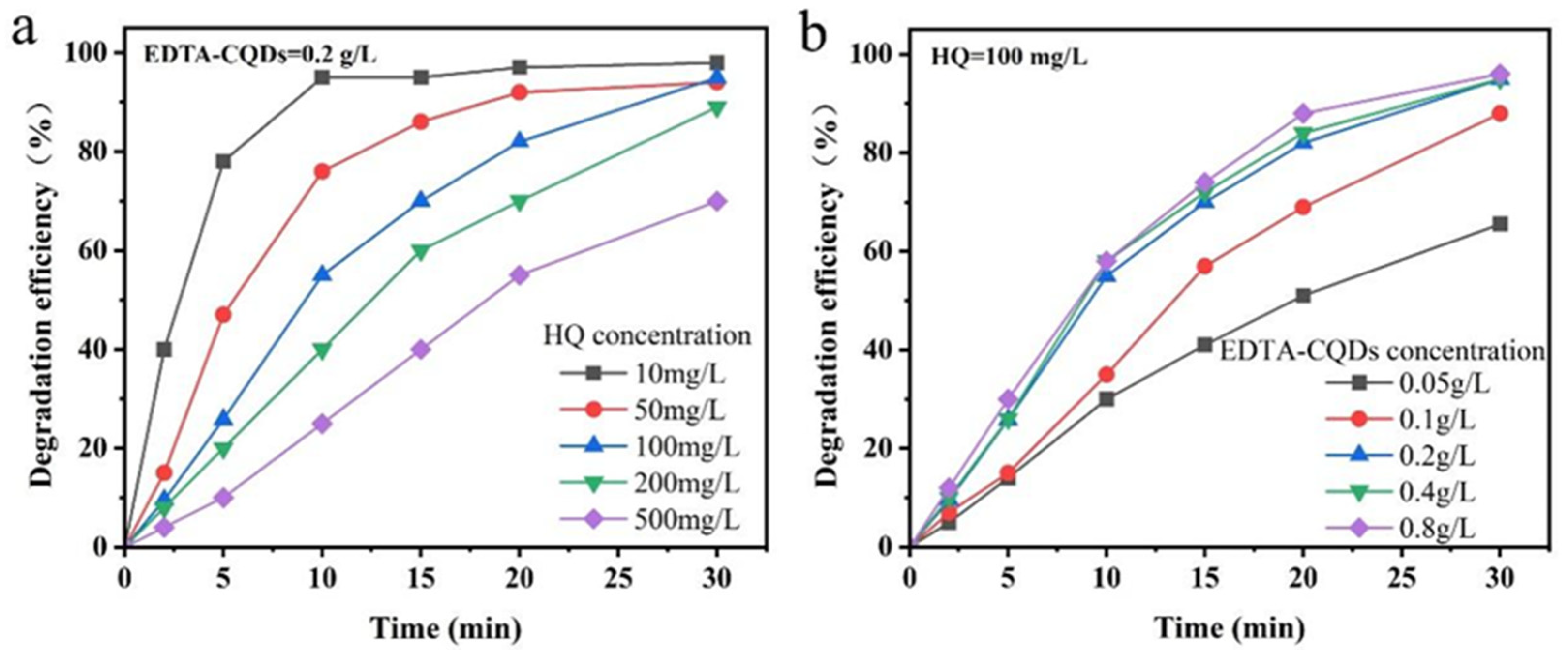
2.2. Degradation of Hydroquinone Under Different Reaction Conditions
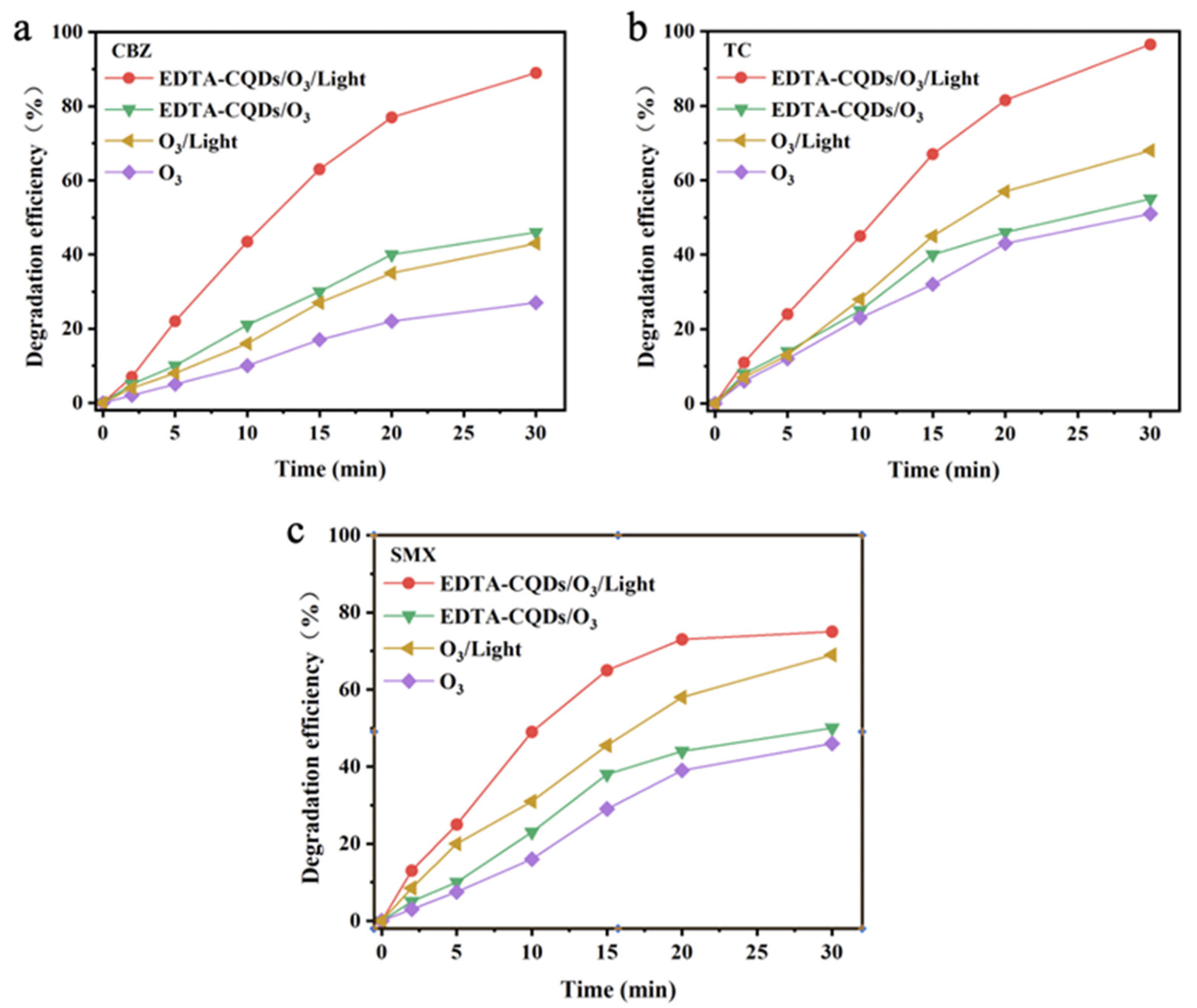
2.3. Degradation Effect of EDTA-CQD-Based H2O2/O3 System on Emerging Pollutants
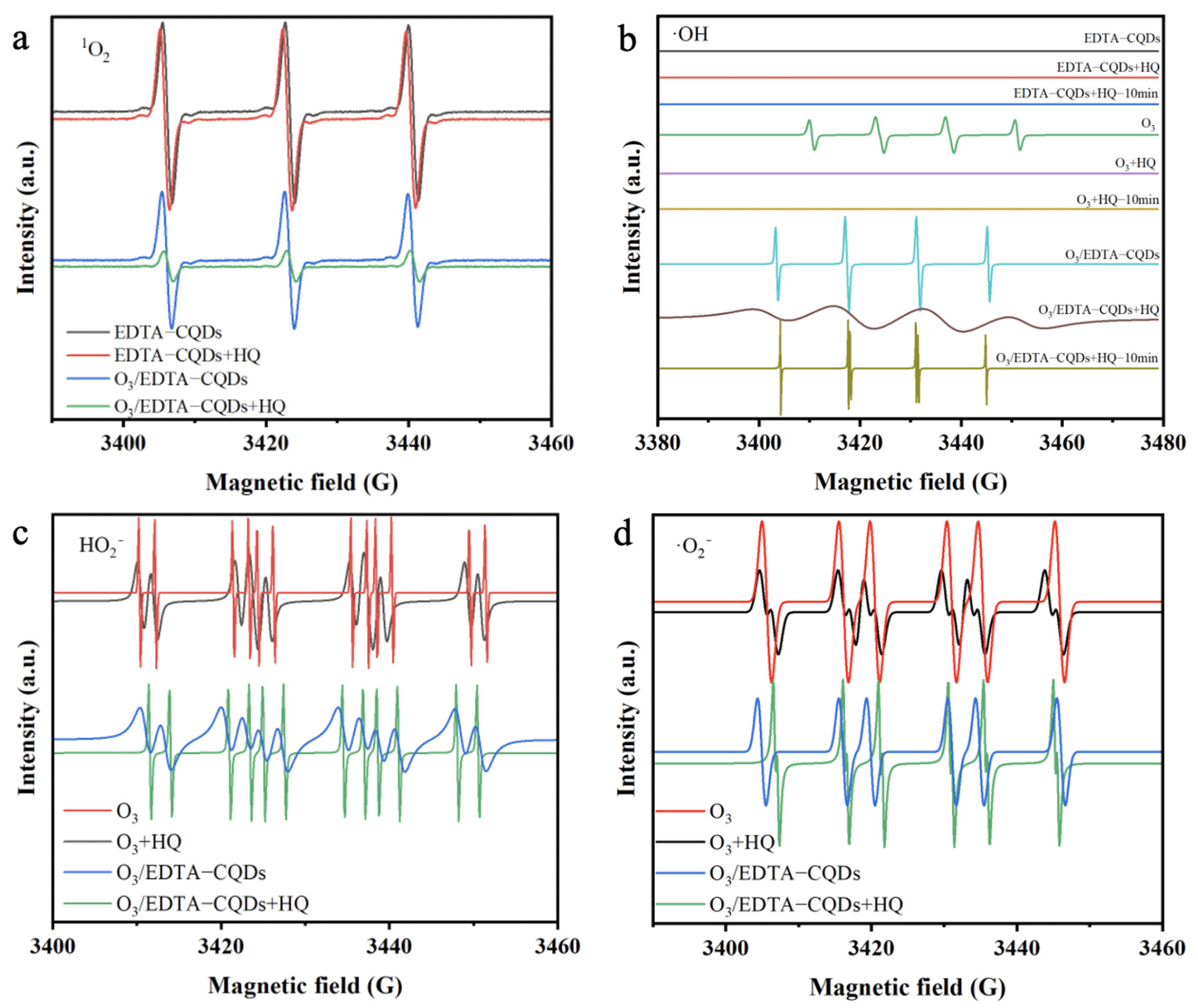
2.4. Mechanism Analysis of HQ Degradation by EDTA-CQD-Based H2O2/O3 System
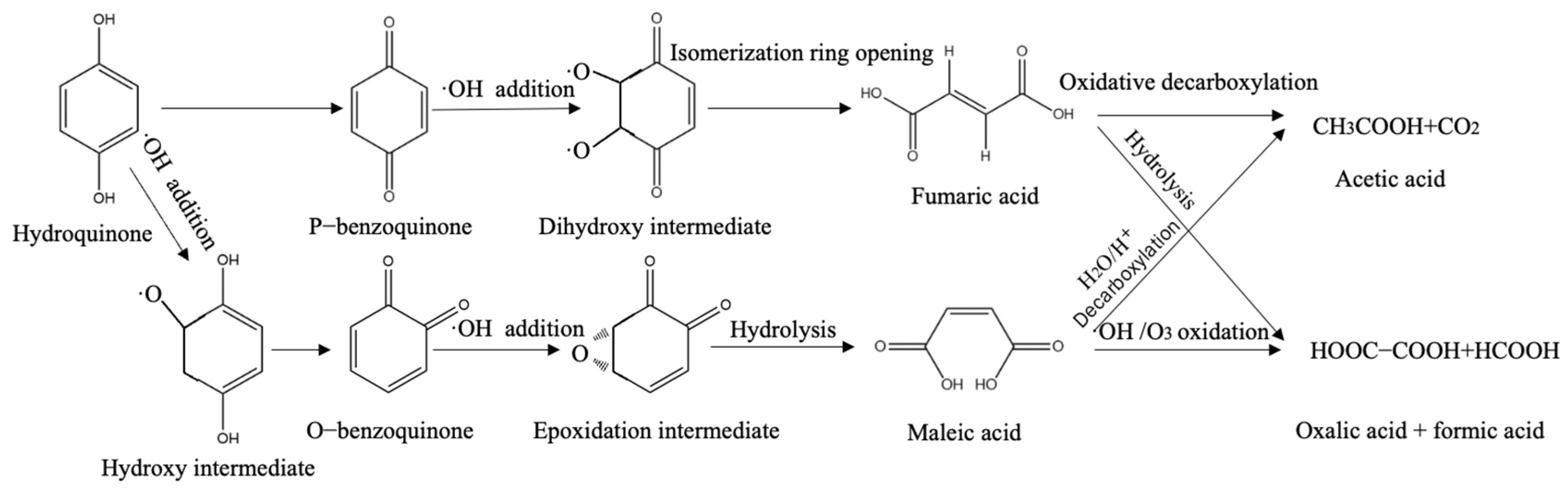
2.5. Analysis of the Degradation Path of HQ in the EDTA-CQD-Based H2O2/O3 System
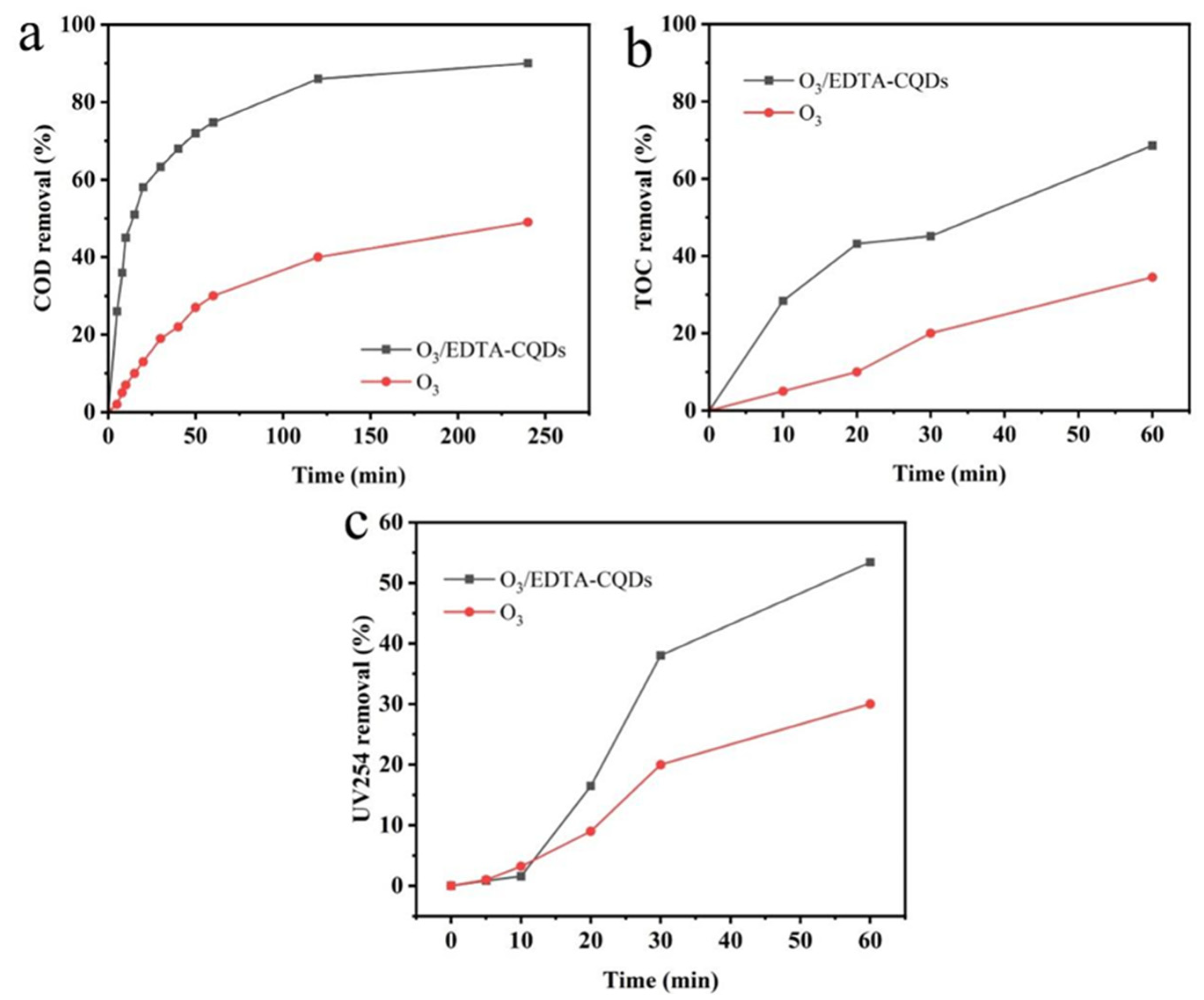
2.6. Treatment Effect of EDTA-CQD-Based H2O2/O3 System on Landfill Leachate
3. Experiment
3.1. Reagents and Materials
3.2. Preparation of EDTA-CQDs
3.3. Evaluation of Degradation Effect
3.4. Determination of Reactive Oxygen Species (ROS)
3.5. Degradation Pathway Analysis
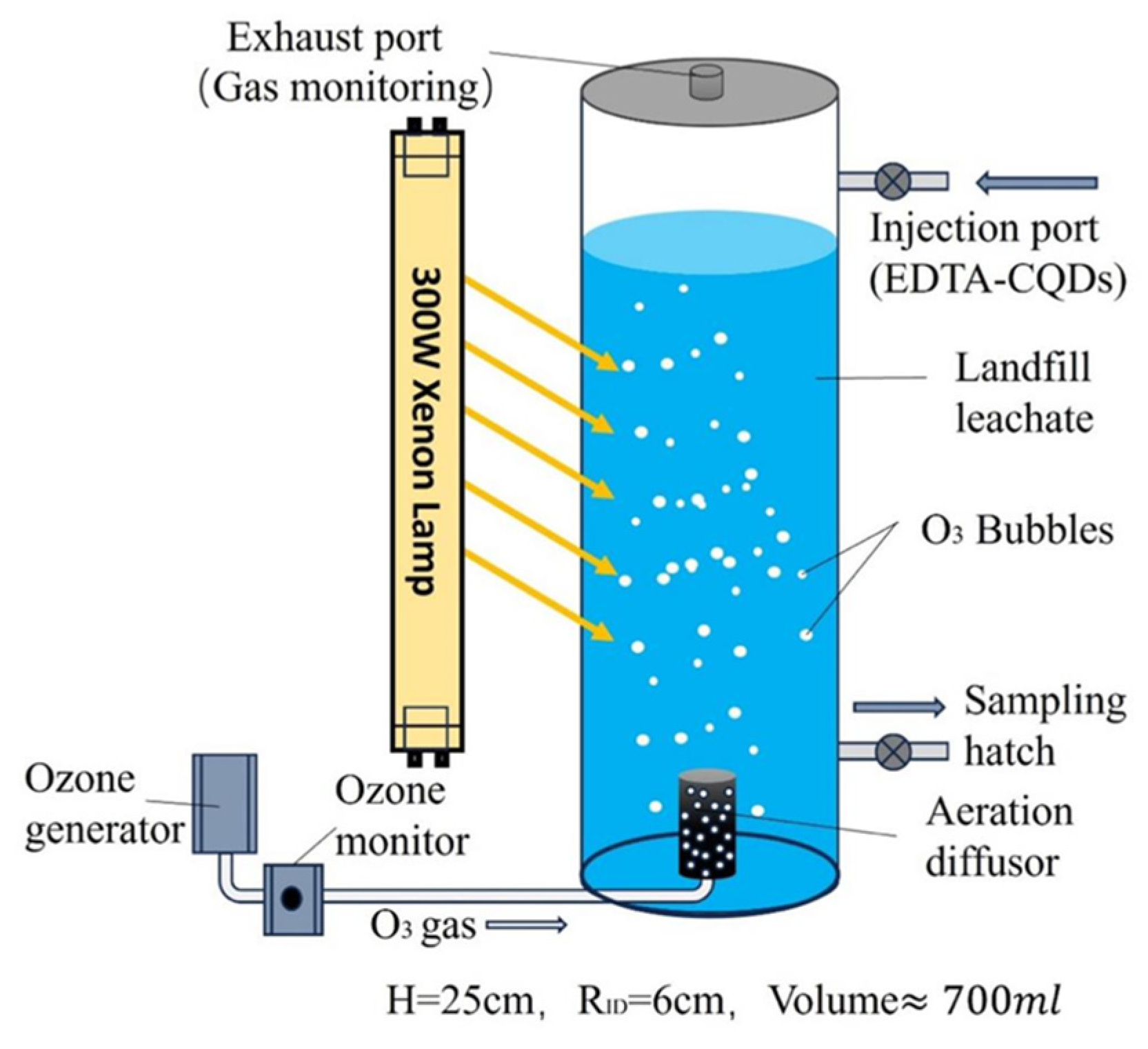
3.6. Construction of Actual Wastewater Treatment System for Landfill lLeachate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, M.; Wang, X.; Wang, F.; Wu, D.; Hussain, A.; Xie, B. Coupling ARB-Based Biological and Photochemical (UV/TiO2 and UV/S2O82−) Techniques to Deal with Sanitary Landfill Leachate. Waste Manag. 2017, 63, 292–298. [Google Scholar] [CrossRef]
- Hassan, M.; Zhao, Y.; Xie, B. Employing TiO2 Photocatalysis to Deal with Landfill Leachate: Current Status and Development. Chem. Eng. J. 2016, 285, 264–275. [Google Scholar] [CrossRef]
- Jia, C.Z.; Qin, Q.Y.; Wang, C.; Lv, F.L. Variation Characteristic of DOM from Landfill Leachate during Photocatalytic Degradation. In Proceedings of the World Automation Congress, Puerto Vallarta, Mexico, 24 June 2012; pp. 1–4. [Google Scholar]
- Deng, Y.; Zhu, X.; Chen, N.; Feng, C.; Wang, H.; Kuang, P.; Hu, W. Review on Electrochemical System for Landfill Leachate Treatment: Performance, Mechanism, Application, Shortcoming, and Improvement Scheme. Sci. Total Environ. 2020, 745, 140768. [Google Scholar] [CrossRef]
- Han, M.; Duan, X.; Cao, G.; Zhu, S.; Ho, S.-H. Graphitic Nitride-Catalyzed Advanced Oxidation Processes (AOPs) for Landfill Leachate Treatment: A Mini Review. Process Saf. Environ. Prot. 2020, 139, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, Y.; Zhang, C.; Qin, Q. UV-TiO2 Photocatalytic Degradation of Landfill Leachate. Water. Air. Soil Pollut. 2011, 217, 375–385. [Google Scholar] [CrossRef]
- Meeroff, D.E.; Bloetscher, F.; Reddy, D.V.; Gasnier, F.; Jain, S.; McBarnette, A.; Hamaguchi, H. Application of Photochemical Technologies for Treatment of Landfill Leachate. J. Hazard. Mater. 2012, 209–210, 299–307. [Google Scholar] [CrossRef]
- Qi, H.; Gao, B.; Liu, J.; Wang, Z.; Sillanpää, M. Enhanced Ozone Catalytic Treatment of Mature Landfill Leachate with Low C/N Ratio Using Transition Metal-Modified Biochar. J. Environ. Chem. Eng. 2025, 13, 118000. [Google Scholar] [CrossRef]
- de Sousa, T.A.T.; Dantas, E.R.B.; Lopes, W.D.S.; Leite, V.D.; Sousa, J.T.; Lopes, W.S. Toxicity Assessment of Sanitary Landfill Leachate before and after Fenton Treatment Process. Sci. Total Environ. 2023, 893, 164870. [Google Scholar] [CrossRef]
- Zaki, H.A.; Zaher, K. A Comparative Study of Landfill Leachate Treatment Using Advanced Oxidation Photochemical Processes, Ozonation Process and Hydrogen Peroxide Systems. Desalination Water Treat. 2022, 261, 107–119. [Google Scholar] [CrossRef]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on Landfill Leachate Treatment by Electrochemical Oxidation: Drawbacks, Challenges and Future Scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Gao, B.; Sillanpää, M. Landfill Leachate Treatment In-Depth by Bio-Chemical Strategy: Microbial Activation and Catalytic Ozonation Mechanism. Chem. Eng. J. 2022, 444, 136464. [Google Scholar] [CrossRef]
- Yang, Y.; Demeestere, K.; Van Hulle, S. Ozone-Based Advanced Oxidation of Biologically Treated Landfill Leachate: Oxidation Efficiency, Mechanisms, and Surrogate-Based Monitoring for Bulk Organics. J. Environ. Chem. Eng. 2021, 9, 106459. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, Y.; Bo, L.; Shen, Z.; Wang, D.; Shen, Z.; Tang, Y. Removal Performance and Mechanism of Aniline from Landfill Leachate by Ozone Oxidation Process Using Iron-Based Packed Catalyst. J. Environ. Manag. 2025, 375, 124397. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Hulle, S.V. Comparison and Performance Assessment of Ozone-Based AOPs in View of Trace Organic Contaminants Abatement in Water and Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, E.; Wang, J.; Zhang, X.; Huang, H.; Yu, G.; Wang, Y. Comparison of Emerging Contaminant Abatement by Conventional Ozonation, Catalytic Ozonation, O3/H2O2 and Electro-Peroxone Processes. J. Hazard. Mater. 2020, 389, 121829. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Lee, W.; Choi, S.; Kim, H.; Lee, W.; Lee, M.; Son, H.; Lee, C.; Cho, M.; Lee, Y. Efficiency of Ozonation and O3/H2O2 as Enhanced Wastewater Treatment Processes for Micropollutant Abatement and Disinfection with Minimized Byproduct Formation. J. Hazard. Mater. 2023, 454, 131436. [Google Scholar] [CrossRef]
- Abdul, J.M.; Vigneswaran, S.; Shon, H.K.; Nathaporn, A.; Kandasamy, J. Comparison of Granular Activated Carbon Bio-Sorption and Advanced Oxidation Processes in the Treatment of Leachate Effluent. Korean J. Chem. Eng. 2009, 26, 724–730. [Google Scholar] [CrossRef]
- Aarthi, A.; Umadevi, M.; Parimaladevi, R.; Sathe, G.V. Polyvinyl Thiol Assisted Ag NPs as an Efficient SERS Analyzer and Visible Light Photocatalyst for Tannery Waste Landfill Leachate. Vacuum 2019, 161, 125–129. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Abualnaja, K.M.; Ghareeb, R.Y.; Ibrahim, A.A.; Abdelsalam, N.R.; Emam, H.E. Technical Textiles Modified with Immobilized Carbon Dots Synthesized with Infrared Assistance. J. Colloid Interface Sci. 2021, 604, 15–29. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Mahmoud, N.E.; Mahdi, A.A.; Emam, H.E.; Abdelhameed, R.M. Affinity of Carbon Quantum Dots Anchored within Metal Organic Framework Matrix as Enhancer of Plant Nourishment. Heliyon 2022, 8, e12396. [Google Scholar] [CrossRef]
- Ahmed, H.B.; Mikhail, M.M.; Abdallah, A.E.M.; El-Shahat, M.; Emam, H.E. Pyrimidine-5-Carbonitrile Derivatives as Sprout for CQDs Proveniences: Antitumor and Anti-Inflammatory Potentiality. Bioorganic Chem. 2023, 141, 106902. [Google Scholar] [CrossRef]
- Kidak, R.; Qadir, M.N.; Aziz, F.H. Optimization of H2O2 and Ozone-Based Advanced Oxidation Processes as Pretreatment of Sanitary Landfill Leachate. Desalination Water Treat. 2020, 203, 119–128. [Google Scholar] [CrossRef]
- Slomczynska, B.; Wasowski, J.; Słomczyński, T. Effect of Advanced Oxidation Processes on the Toxicity of Municipal Landfill Leachates. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2018, 49, 273–277. [Google Scholar] [CrossRef]
- Tan, S.; Long, K.; Chen, W.; Liu, H.; Liang, S.; Zhang, Q. Synergistic Oxidation of Humic Acid Treated by H2O2/O3 Activated by CuCo/C with High Efficiency and Wide pH Range. J. Environ. Manag. 2024, 358, 120896. [Google Scholar] [CrossRef]
- You, Q.; Zhang, C.; Cao, M.; Wang, B.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Defects Controlling, Elements Doping, and Crystallinity Improving Triple-Strategy Modified Carbon Nitride for Efficient Photocatalytic Diclofenac Degradation and H2O2 Production. Appl. Catal. B Environ. 2023, 321, 121941. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Y.; Liu, Y.; Vequizo, J.J.M.; Sun, H.; Yang, C.; Yamakata, A.; Fan, F.; Lin, W.; Wang, X.; et al. Heteroatom Dopants Promote Two-Electron O2 Reduction for Photocatalytic Production of H2O2 on Polymeric Carbon Nitride. Angew. Chem. Int. Ed. 2020, 59, 16209–16217. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Tang, L.; Deng, Y.; Wang, J.; Luo, J.; Liu, Y.; Ouyang, X.; Yang, H.; Yu, J.; Wang, J. Synthesis of Leaf-Vein-Like g-C3 N4 with Tunable Band Structures and Charge Transfer Properties for Selective Photocatalytic H2O2 Evolution. Adv. Funct. Mater. 2020, 30, 2001922. [Google Scholar] [CrossRef]
- Meng, N.; Zhou, M.; Zhang, X.; Ma, L.; Ding, S.; Wang, W. Construction of Proton Relay on Carbon Quantum Dots for Highly Efficient Hydrogen Peroxide Photo-Production. Chem. Eng. J. 2025, 503, 158432. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, H.; Duan, X.; Ma, J.; Sun, H.; Tian, W.; Wang, S. Carbonaceous Materials in Structural Dimensions for Advanced Oxidation Processes. Chem. Soc. Rev. 2025, 54, 2436–2482. [Google Scholar] [CrossRef]
- Fan, X.; Restivo, J.; Órfão, J.J.M.; Pereira, M.F.R.; Lapkin, A.A. The Role of Multiwalled Carbon Nanotubes (MWCNTs) in the Catalytic Ozonation of Atrazine. Chem. Eng. J. 2014, 241, 66–76. [Google Scholar] [CrossRef]
- Song, Z.; Wang, M.; Wang, Z.; Wang, Y.; Li, R.; Zhang, Y.; Liu, C.; Liu, Y.; Xu, B.; Qi, F. Insights into Heteroatom-Doped Graphene for Catalytic Ozonation: Active Centers, Reactive Oxygen Species Evolution, and Catalytic Mechanism. Environ. Sci. Technol. 2019, 53, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Ma, J.; Cui, Y.H.; Zhao, L.; Zhang, B.P. Influence of Different Heat Treatments on the Surface Properties and Catalytic Performance of Carbon Nanotube in Ozonation. Appl. Catal. B Environ. 2010, 101, 74–80. [Google Scholar] [CrossRef]
- Yoon, Y.; Oh, H.; Ahn, Y.T.; Kwon, M.; Jung, Y.; Park, W.K.; Hwang, T.M.; Yang, W.S.; Kang, J.W. Evaluation of the O3/Graphene-Based Materials Catalytic Process: pH Effect and Iopromide Removal. Catal. Today 2017, 282, 77–85. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, R.; Chen, H.; Zhou, B.; Luo, S. Heterogeneous Catalytic Ozonation for the Removal of Antibiotics in Water: A Review. Environ. Res. 2024, 262, 119889. [Google Scholar] [CrossRef]
- Li, C.; Tan, X.; Ma, J. Concerted High Innergenerated-H2O2 Photocatalysis and Photo-Fenton Degradation of Organic Pollutants over SCNO@CdS. J. Photochem. Photobiol. Chem. 2021, 420, 113477. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Tan, P.; Meng, N.; Cao, X.; Zhang, Y. Study on the Kinetics and Mechanisms of Cr(VI) Removal by nZVI Modified with Four Modifiers. Sep. Purif. Technol. 2024, 342, 127022. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of Antibiotics by Advanced Oxidation Processes: An Overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Wei, B.; Yang, S.; Li, Q.; Zhao, H. Simultaneous Removal of Phosphate and Hydroquinone Using Fe3Ce1Ox(CA)/H2O2 Fenton-like System. Process Saf. Environ. Prot. 2025, 199, 107322. [Google Scholar] [CrossRef]
- Mohseni, M.; Demeestere, K.; Du Laing, G.; Yüce, S.; Keller, R.G.; Wessling, M. CNT Microtubes with Entrapped Fe3O4 Nanoparticles Remove Micropollutants through a Heterogeneous Electro-Fenton Process at Neutral pH. Adv. Sustain. Syst. 2021, 5, 2100001. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Xia, G.; Tang, Y.; Liu, Q.; Li, Y.; Fang, B.; Yao, J. Metal-Organic Framework Templated Z-Scheme ZnIn2S4/Bi2S3 Hierarchical Heterojunction for Photocatalytic H2O2 Production from Wastewater. Small 2024, 20, 2403268. [Google Scholar] [CrossRef]
- Xie, W.; Li, D.; Huang, R. Innovative Applications of Graphene-Based Materials for Sustainable Advanced Wastewater Treatment: A Comprehensive Review. Sep. Purif. Technol. 2025, 377, 134219. [Google Scholar] [CrossRef]
- Sossou, K.; Bala Prasad, S.; Agbotsou, K.E.; Saidou Souley, H.; Mudigandla, R. Characteristics of Landfill Leachate and Leachate Treatment by Biological and Advanced Coagulation Process: Feasibility and Effectiveness–An Overview. Waste Manag. Bull. 2024, 2, 181–198. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zhong, C.; Liu, M.; Yang, L.; He, J.; Sun, Y.; Zhao, C.; Wang, D. Treatment of Landfill Leachate by Coagulation: A Review. Sci. Total Environ. 2024, 912, 169294. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and Treatment of Landfill Leachate: A Review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, X.; Shan, H.; Zhang, H.; Xi, B. Degradation of Landfill Leachate Using UV-TiO2 Photocatalysis Combination with Aged Waste Reactors. Processes 2021, 9, 946. [Google Scholar] [CrossRef]
- Ghahrchi, M.; Rezaee, A. Electrocatalytic Ozonation Process Supplemented by EDTA-Fe Complex for Improving the Mature Landfill Leachate Treatment. Chemosphere 2021, 263, 127858. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Karimi-Jashni, A.; Javadpour, S.; Mahmoudian-Boroujerd, L. Photocatalytic Landfill Leachate Treatment Using P-Type TiO2 Nanoparticles under Visible Light Irradiation. Environ. Dev. Sustain. 2021, 23, 6047–6065. [Google Scholar] [CrossRef]
- Yasmin, C.; Lobna, E.; Mouna, M.; Kais, D.; Mariam, K.; Rached, S.; Abdelwaheb, C.; Ismail, T. New Trend of Jebel Chakir Landfill Leachate Pre-Treatment by Photocatalytic TiO2/Ag Nanocomposite Prior to Fermentation Using Candida Tropicalis Strain. Int. Biodeterior. Biodegrad. 2020, 146, 104829. [Google Scholar] [CrossRef]
- Becerra, D.; Soto, J.; Villamizar, S.; Machuca-Martínez, F.; Ramírez, L. Alternative for the Treatment of Leachates Generated in a Landfill of Norte de Santander–Colombia, by Means of the Coupling of a Photocatalytic and Biological Aerobic Process. Top. Catal. 2020, 63, 1336–1349. [Google Scholar] [CrossRef]
- Amigh, P.; Mokhtarani, N. Leachate Post Treatment, Using Ag-TiO2 Nanoparticles Immobilized on Rotating Vanes. J. Water Process Eng. 2022, 47, 102842. [Google Scholar] [CrossRef]
- Lalavi, R.; Salehiravesh, F.; Nasrollah Gavgani, J.; Adelnia, H.; Nikazar, M. Highly Efficient Photocatalytic Degradation of Landfill Leachate by Boron-Doped TiO2 Photocatalyst. Int. J. Environ. Stud. 2023, 80, 1673–1688. [Google Scholar] [CrossRef]
- Hindi, S.S.; Sabir, J.S.M.; Dawoud, U.M.; Ismail, I.M.; Asiry, K.A.; Mirdad, Z.M.; Abo-Elyousr, K.A.; Shiboob, M.H.; Gabal, M.A.; Albureikan, M.O.I.; et al. Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review. Polymers 2023, 15, 2660. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Du, Y.M.; Liu, R.; Yang, S.; Tang, H.; Yin, X.Z.; Xiao, Q.; Wang, X.; Wang, H. Alkali Metal Cation Adsorption–Induced Surface Polarization in Polymeric Carbon Nitride for Enhanced Photocatalytic Hydrogen Peroxide Production. J. Colloid Interface Sci. 2025, 679, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, H.; Li, D.; Qin, W.; Zhang, X.; Wang, S.; Duan, X. Surface Engineered Carbon Quantum Dots for Efficient Photocatalytic Hydrogen Peroxide Production. Appl. Catal. B Environ. Energy 2024, 350, 123918. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Qian, J.; Xu, K.; Lu, B.; Tang, S.; Liu, Y.; Shen, J. Efficient Photocatalytic H2O2 Production and Green Oxidation of Glycerol over a SrCoO3-Incorporated Catalyst. Appl. Catal. B Environ. Energy 2025, 361, 124565. [Google Scholar] [CrossRef]
- Liu, C.; Tong, H.; Wang, P.; Huang, P.; Yang, Z.; Huang, R.; Zhou, G. Coordination Engineering Regulating Metal Single-Atom Anchored on n-Doped Carbon as a Bifunctional Catalyst for H2O2 Production via Dual Channels. Chem. Eng. J. 2023, 476, 146573. [Google Scholar] [CrossRef]
- Liu, B.; Du, J.; Ke, G.; Jia, B.; Huang, Y.; He, H.; Zhou, Y.; Zou, Z. Boosting O2 Reduction and H2O Dehydrogenation Kinetics: Surface N -Hydroxymethylation of g -C3 N4 Photocatalysts for the Efficient Production of H2O2. Adv. Funct. Mater. 2022, 32, 2111125. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhou, Z.W.; Cai, C.X.; Wei, Y.J.; Yu, Z.P.; Wang, X.Y.; Wang, N. Photoenzyme Coupling System: Covalent Organic Frameworks In Situ Production of Hydrogen Peroxide Cascaded with Unspecific Peroxygenase to Achieve C–H Bonds Selective Activation. ACS Appl. Mater. Interfaces 2025, 17, 6347–6356. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Zhang, H.; Meng, X.; Hao, D.; Chang, K.; Li, P.; Kako, T.; Ye, J. Nature-Inspired Environmental “Phosphorylation” Boosts Photocatalytic H2 Production over Carbon Nitride Nanosheets under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2015, 54, 13561–13565. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fang, J.; Yang, H.; Zhao, Q.; Deng, J.; Gou, Y. Nanostructured G-C3N4 Synergistically with Hydrogen Peroxide as a Metal-Free Photocatalyst for Efficient Degradation of Organic Dye Pollutants. J. Porous Mater. 2025, 32, 1083–1096. [Google Scholar] [CrossRef]
- Liu, L.; Gao, M.Y.; Yang, H.; Wang, X.; Li, X.; Cooper, A.I. Linear Conjugated Polymers for Solar-Driven Hydrogen Peroxide Production: The Importance of Catalyst Stability. J. Am. Chem. Soc. 2021, 143, 19287–19293. [Google Scholar] [CrossRef] [PubMed]
| Landfill Leachate | Process | Reaction Conditions | Degradation Efficiency | References |
|---|---|---|---|---|
| COD: 680 mg L−1 TOC: 177 mg L−1 | EDTA-CQD-based H2O2/O3 system | EDTA-CQDs: 0.2 g/L Xe: 27 mW cm−2 O3: 0.1 L/h pH = 7.54 | 90% 70% | This study |
| COD: 255 mg L−1 | UV-TiO2 photocatalysis combination with aged waste reactors | TiO2: 4 mg L−1 pH = 8.88 | 32.5% | [48] |
| COD: 11378 mg L−1 | Integration of electrocoagulation and ozonation process | Current: 100 mA O3: 400 mg /h | 80% | [49] |
| COD: 600 mg L−1 | P-type TiO2 nanoparticle photocatalysis | Fluorescent lamp: 36 W Si-doped TiO2: 3.5 mg/mL pH = 6 | 85% | [50] |
| COD: 24,000 g O2 dm−3 TOC: 21 g L−1 | Combination TiO2/Ag nanocomposite photocatalysis and biological treatment using Candida tropicalis strain | Visible light: 33W cm−2 TiO2/Ag: 0.937 g dm−3 pH = 4.31 | 90% 85% | [51] |
| COD: 7920 g O2 L−3 | Coupling of photocatalysis and biological aerobic process | UV light: 20–60 kJ/L TiO2: 100–600 mg L−1 H2O2: 300 mg L−1 | 68% | [52] |
| COD: 1950 mg L−1 TOC: 590 mg L−1 | Photocatalytic degradation by Ag-TiO2 nanoparticles | UVC lamp: 25 W Aeration: 6 L/min pH = 4 | 62% 55% | [53] |
| COD: 2029 ppm | Photocatalytic degradation by Boron-doped TiO2 | TiO2: 2.5 g L−1 UV lamp: 33 W pH = 4.4 | 59% | [54] |
| Project | Unit | Value |
|---|---|---|
| pH | / | 7.54 |
| Conductivity | mS cm−1 | 36.9 |
| TDS | mg L−1 | 1690 |
| SS | mg L−1 | 8 |
| NH4+-N | mg L−1 | 67.92 |
| TN | mg L−1 | 258.39 |
| COD | mg L−1 | 6800 |
| TOC | mg L−1 | 1772.6 |
| Cl− | mg L−1 | 2722.16 |
| SO42− | mg L−1 | 22,271.68 |
| Ca2+ | g L−1 | 28.483 |
| Mg2+ | g L−1 | 62.986 |
| Total iron | mg L−1 | 24.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Meng, N.; Ma, L.; Zhang, X.; Ding, S.; Wang, W. Study on Photocatalytic Peroxone Process for Treating Organic Pollutants in Leachate Based on Modified Carbon Quantum Dots. Catalysts 2025, 15, 903. https://doi.org/10.3390/catal15090903
Wu S, Meng N, Ma L, Zhang X, Ding S, Wang W. Study on Photocatalytic Peroxone Process for Treating Organic Pollutants in Leachate Based on Modified Carbon Quantum Dots. Catalysts. 2025; 15(9):903. https://doi.org/10.3390/catal15090903
Chicago/Turabian StyleWu, Shuo, Nuo Meng, Lin Ma, Xiguo Zhang, Shihu Ding, and Wei Wang. 2025. "Study on Photocatalytic Peroxone Process for Treating Organic Pollutants in Leachate Based on Modified Carbon Quantum Dots" Catalysts 15, no. 9: 903. https://doi.org/10.3390/catal15090903
APA StyleWu, S., Meng, N., Ma, L., Zhang, X., Ding, S., & Wang, W. (2025). Study on Photocatalytic Peroxone Process for Treating Organic Pollutants in Leachate Based on Modified Carbon Quantum Dots. Catalysts, 15(9), 903. https://doi.org/10.3390/catal15090903








