Maximally Exploiting the Fe2+ at the Interface of Micro and Nano Bubbles in the Fenton-Coupled Micro and Nano Bubble System for Organic Pollutant Degradation
Abstract
1. Introduction
2. Results and Discussion
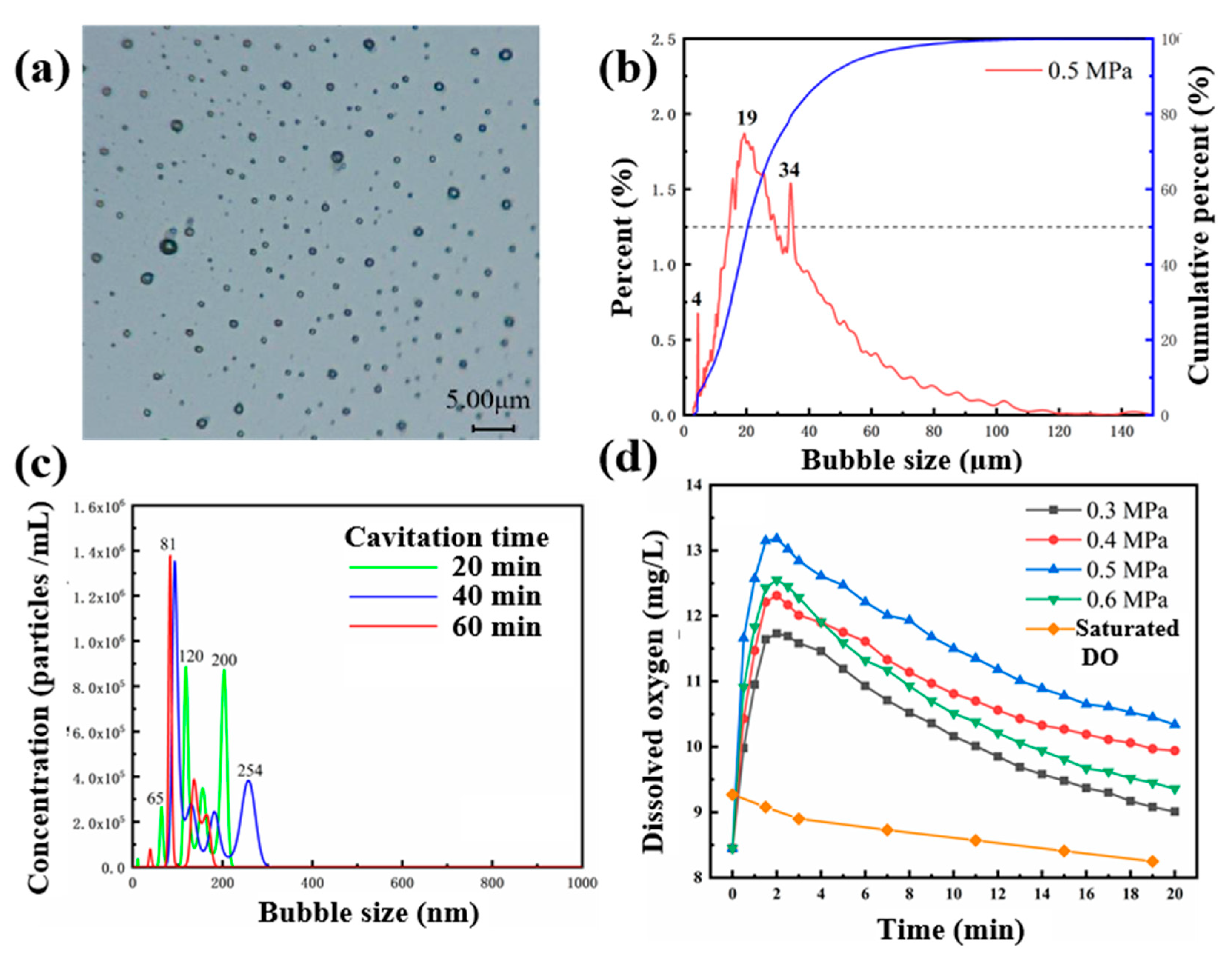
2.1. MNBs Properties
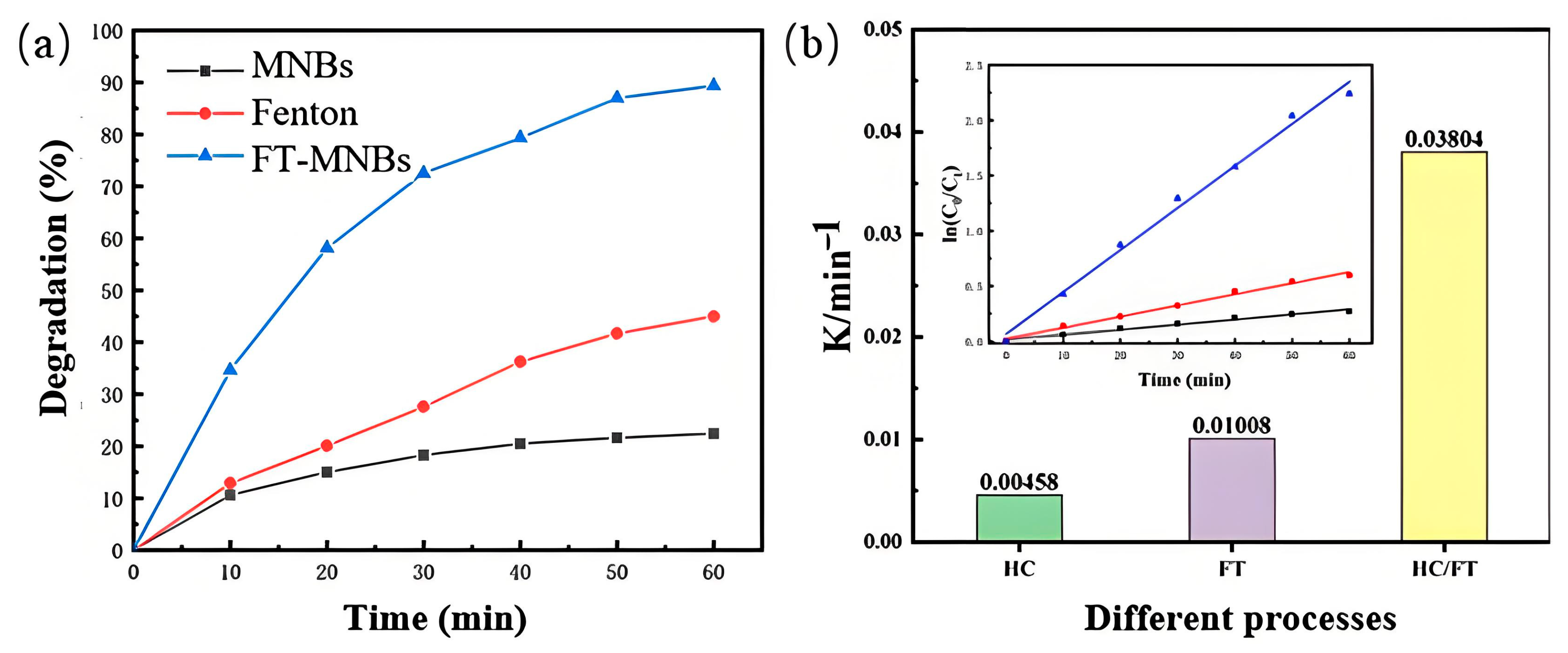
2.2. Degradation of Indole by Fenton Combined MNBs
2.2.1. The Degradation Kinetics of Indole by FT-MNBs Methods
2.2.2. Influence of Experimental Parameters on Indole Degradation
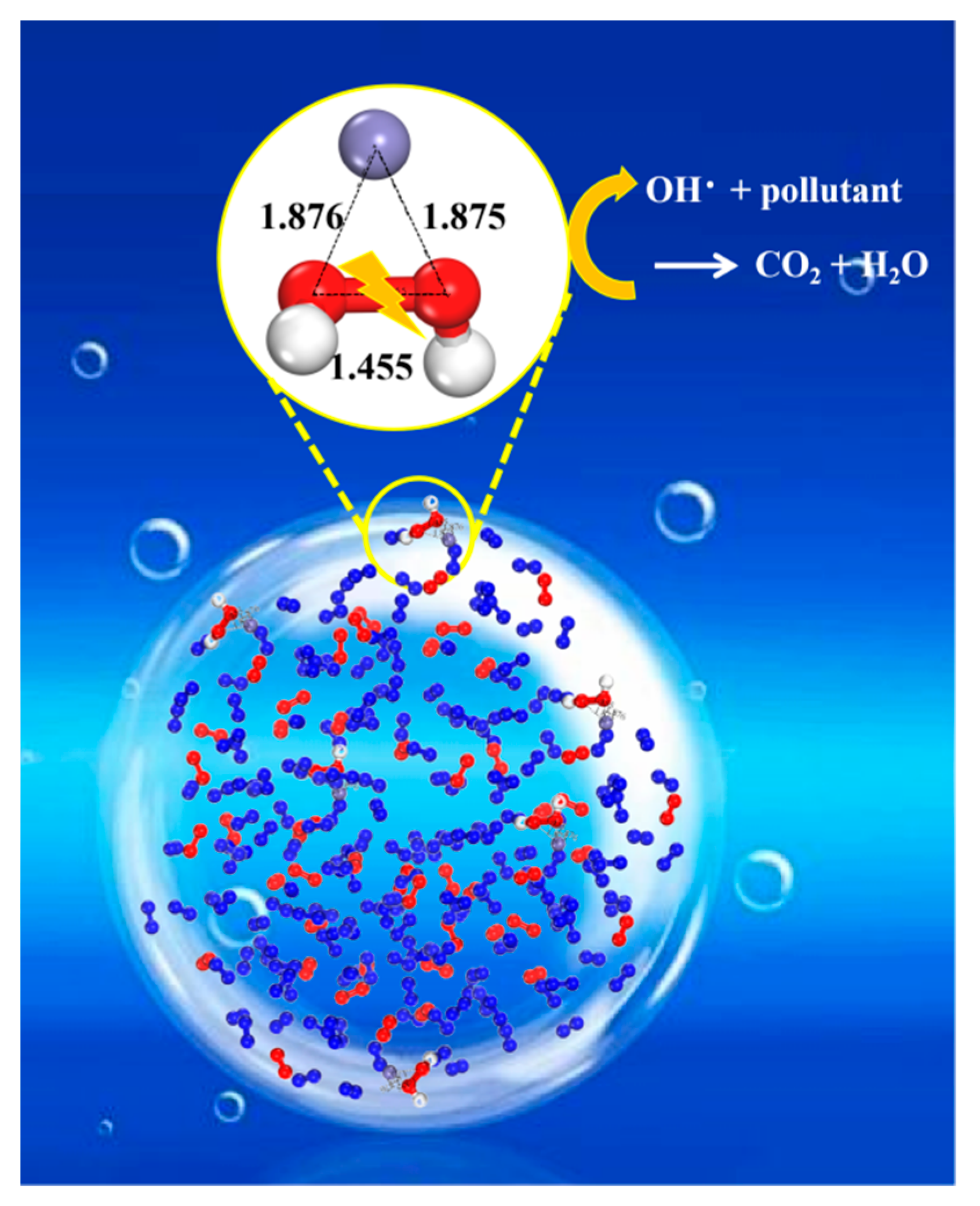
2.3. Degradation Mechanism
2.3.1. The Detection of Free Radicals
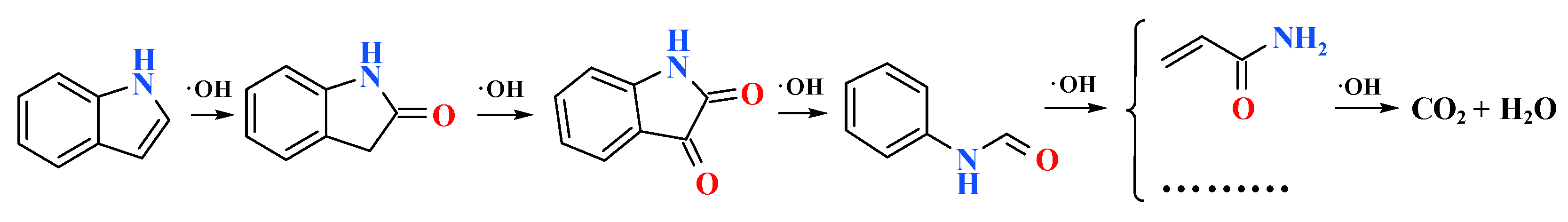
2.3.2. Indole Degradation Products and Pathways
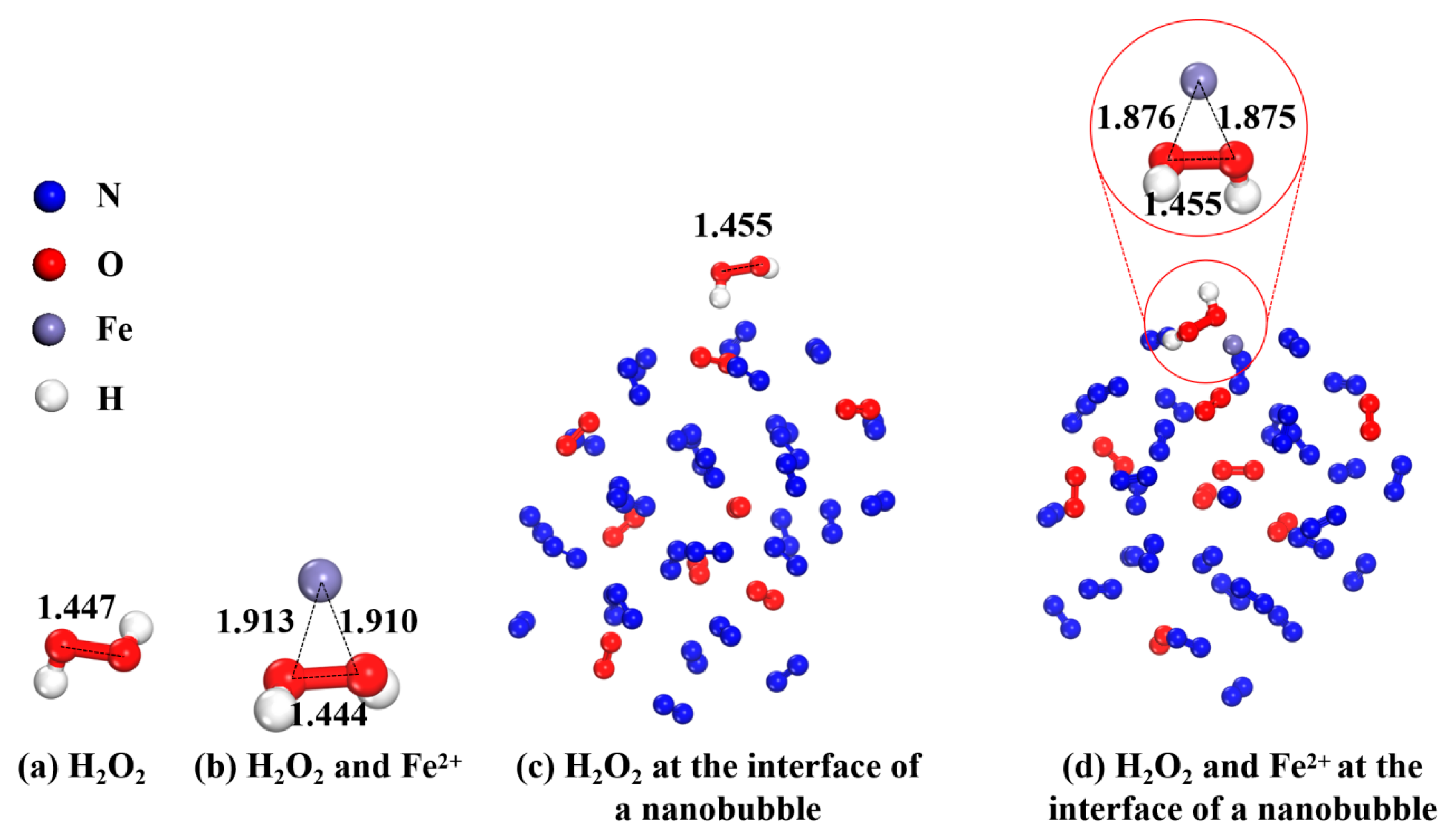
2.3.3. Indole Degradation Mechanism by Theoretical Calculation
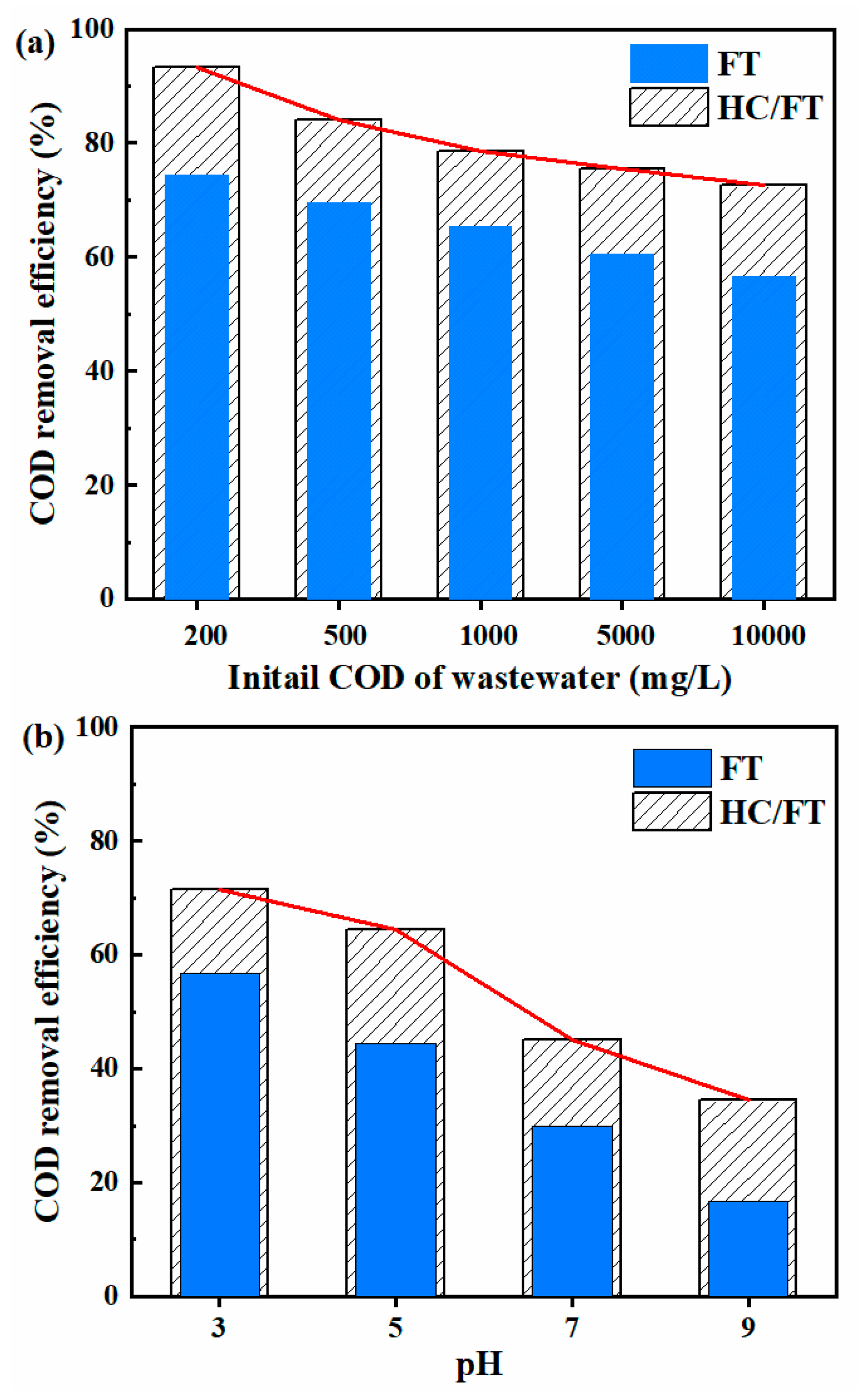
2.4. Treatment of Industrial Coking Wastewater
3. Experimental Sections
3.1. Materials and Reagents
3.2. Production of Bulk Micro–Nano Bubbles and Size Measurements
3.3. Experimental Procedures and Intermediate Product Analysis
3.4. Analysis of Reactive Oxygen Species and Intermediate Product Analysis
3.5. Simulation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble Technologies Offer Opportunities to Improve Water Treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-i.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Ma, P.; Han, C.; He, Q.; Miao, Z.; Gao, M.; Wan, K.; Xu, E. Oxidation of Congo red by Fenton coupled with micro and nanobubbles. Environ. Technol. 2023, 44, 2539–2548. [Google Scholar] [CrossRef]
- Wang, L.; Ali, J.; Wang, Z.; Oladoja, N.A.; Cheng, R.; Zhang, C.; Mailhot, G.; Pan, G. Oxygen nanobubbles enhanced photodegradation of oxytetracycline under visible light: Synergistic effect and mechanism. Chem. Eng. J. 2020, 388, 124227. [Google Scholar] [CrossRef]
- He, Q.; Cui, R.; Miao, Z.; Xing, Y.; Wan, K.; Gao, M.; Zhang, M. Improved removal of Congo Red from wastewater by low-rank coal using micro and nanobubbles. Fuel 2021, 291, 120090. [Google Scholar] [CrossRef]
- Lee, S.; Anwer, H.; Park, J.W. Oxidative power loss control in ozonation: Nanobubble and ultrasonic cavitation. J. Hazard. Mater. 2023, 455, 131530. [Google Scholar] [CrossRef]
- John, A.; Carra, I.; Jefferson, B.; Jodkowska, M.; Brookes, A.; Jarvis, P. Are microbubbles magic or just small? A direct comparison of hydroxyl radical generation between microbubble and conventional bubble ozonation under typical operational conditions. Chem. Eng. J. 2022, 435, 134854. [Google Scholar] [CrossRef]
- Xiang, P.; Ma, P.; He, Q.; Song, Z.; Miao, Z. Enhanced removal of phenol and chemical oxygen demand from coking wastewater using micro and nano bubbles: Microbial community and metabolic pathways. Bioresour. Technol. 2024, 394, 130207. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of OH radical production from ozone bubbles in water after stopping cavitation. Ultrason. Sonochem. 2019, 58, 104707. [Google Scholar] [CrossRef]
- Adhikari, U.; Goliaei, A.; Berkowitz, M.L. Mechanism of Membrane Poration by Shock Wave Induced Nanobubble Collapse: A Molecular Dynamics Study. J. Phys. Chem. B 2015, 119, 6225–6234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.B.; Cao, D.P.; Zhang, X.R. Degradation Mechanism of Micro-Nanobubble Technology for Organic Pollutants in Aqueous Solutions. Nanomaterials 2022, 12, 2654. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Tang, L.; Hu, N.; Zhang, D.; Pan, X. Fenton micro-reactor on a bubble: A novel microbubble-triggered simultaneous capture and catalytic oxidation strategy for recalcitrant organic pollutant removal. Sci. Total Environ. 2022, 835, 155556. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Ning, R.; Ratul, R.; Zhang, X.; Ma, J. Mechanisms on stability of bulk nanobubble and relevant applications: A review. J. Clean. Prod. 2023, 426, 139153. [Google Scholar] [CrossRef]
- Goyal, R.N.; Kumar, N. Oxidation chemistry and biochemistry of 5-hydroxyindole. Oxid. Commun. 2001, 24, 563–575. [Google Scholar]
- Zhang, B.; You, H.; Wang, F. Microwave-enhanced catalytic wet peroxide oxidation of quinoline: The influence of pH and H2O2 dosage and identification of reactive oxygen species. RSC Adv. 2017, 7, 14769–14775. [Google Scholar] [CrossRef]
- Ning, R.; Yu, S.; Li, L.; Snyder, S.A.; Li, P.; Liu, Y.; Togbah, C.F.; Gao, N. Micro and nanobubbles-assisted advanced oxidation processes for water decontamination: The importance of interface reactions. Water Res. 2024, 265, 122295. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, Y.; Pan, Y.; Yang, Y.; Ma, H. A cost-effective production of hydrogen peroxide via improved mass transfer of oxygen for electro-Fenton process using the vertical flow reactor. Sep. Purif. Technol. 2020, 241, 116695. [Google Scholar] [CrossRef]
- Qiu, S.; Tang, W.; Yang, S.; Xie, J.; Yu, D.; Garcia-Rodriguez, O.; Qu, J.; Bai, S.; Deng, F. A microbubble-assisted rotary tubular titanium cathode for boosting Fenton’s reagents in the electro-Fenton process. J. Hazard. Mater. 2022, 424, 127403. [Google Scholar] [CrossRef]
- He, S.; Zhao, S.; Chen, Z.; Li, X.; Ma, Y.; Ren, Y.; Duan, X. Enhanced in situ production of Fenton reagent’s by nanobubble aeration and sacrificial iron anodes in the electro-Fenton process. Electrochem. Commun. 2024, 158, 107640. [Google Scholar] [CrossRef]
- Jia, M.; Farid, M.U.; Kharraz, J.A.; Kumar, N.M.; Chopra, S.S.; Jang, A.; Chew, J.; Khanal, S.K.; Chen, G.; An, A.K. Nanobubbles in water and wastewater treatment systems: Small bubbles making big difference. Water Res. 2023, 245, 120613. [Google Scholar] [CrossRef]
- Lyu, T.; Wu, S.; Mortimer, R.J.G.; Pan, G. Nanobubble Technology in Environmental Engineering: Revolutionization Potential and Challenges. Environ. Sci. Technol. 2019, 53, 7175–7176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, J.; Wang, Y. Combination of photocatalysis with hydrodynamic cavitation for degradation of tetracycline. Chem. Eng. J. 2017, 315, 274–282. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Bond Order Analysis Based on the Laplacian of Electron Density in Fuzzy Overlap Space. J. Phys. Chem. A 2013, 117, 3100–3108. [Google Scholar] [CrossRef] [PubMed]



| COD (mg/L) | pH | Chroma | K+ (mg/L) | NH4+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | F− (mg/L) | Cl− (mg/L) | SO42− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| 10,020~10,760 | 9.10 | 50 | 26.77 | 53.73 | 1698.23 | 100.26 | 106.79 | 1043.04 | 397.34 |
| Reagents | Price (¥/kg) | Conventional Fenton | FT-MNBs | ||
|---|---|---|---|---|---|
| Dosage | Cost (¥) | Dosage | Cost (¥) | ||
| H2O2 | 1 | 0.18 | 0.18 | 0.18 | 0.18 |
| FeSO4 | 0.63 | 0.02 | 0.01 | 0.02 | 0.01 |
| H2SO4 | 0.95 | 0.6 | 0.57 | 0.3 | 0.29 |
| NaOH | 2.85 | 0.25 | 0.71 | 0.08 | 0.23 |
| Energy (kWh) | 0.85 | 0 | 0 | 0.4 | 0.34 |
| Total | 1.47 | 1.05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Song, Z.; Huang, S.; Gao, R.; Han, C.; Miao, Z. Maximally Exploiting the Fe2+ at the Interface of Micro and Nano Bubbles in the Fenton-Coupled Micro and Nano Bubble System for Organic Pollutant Degradation. Catalysts 2025, 15, 888. https://doi.org/10.3390/catal15090888
He Q, Song Z, Huang S, Gao R, Han C, Miao Z. Maximally Exploiting the Fe2+ at the Interface of Micro and Nano Bubbles in the Fenton-Coupled Micro and Nano Bubble System for Organic Pollutant Degradation. Catalysts. 2025; 15(9):888. https://doi.org/10.3390/catal15090888
Chicago/Turabian StyleHe, Qiongqiong, Zhaoyang Song, Shaomeng Huang, Ruize Gao, Chao Han, and Zhenyong Miao. 2025. "Maximally Exploiting the Fe2+ at the Interface of Micro and Nano Bubbles in the Fenton-Coupled Micro and Nano Bubble System for Organic Pollutant Degradation" Catalysts 15, no. 9: 888. https://doi.org/10.3390/catal15090888
APA StyleHe, Q., Song, Z., Huang, S., Gao, R., Han, C., & Miao, Z. (2025). Maximally Exploiting the Fe2+ at the Interface of Micro and Nano Bubbles in the Fenton-Coupled Micro and Nano Bubble System for Organic Pollutant Degradation. Catalysts, 15(9), 888. https://doi.org/10.3390/catal15090888






