Abstract
Ammonium perchlorate (AP), as the most commonly used oxidizer in composite solid propellants, achieving its rapid decomposition at lower temperatures, is one of the key items used to improve propellant performance. Copper-based catalysts, due to their good performance in promoting AP decomposition and improving propellant combustion characteristics, are currently one of the most widely used catalyst types. However, the catalytic performance of copper-based catalysts for the decomposition of ammonium perchlorate, including the decomposition products, changes in the kinetic process during the decomposition, and the combustion process needs further research and clarification in terms of the influencing factors and mechanisms. Based on this question, to further analyze the essence of copper-based catalysts and the decomposition mechanism of CuO-catalyzed ammonium perchlorate, as well as its relationship with particle size, this paper compared and studied the effects of two different particle size CuO catalysts (small-diameter CuO-S and large-diameter CuO-L) on the thermal decomposition and combustion performance of AP. The results indicate that the decomposition of AP catalyzed by CuO mainly includes two stages: the initial low-temperature decomposition stage accelerated by the electron transfer mechanism and the subsequent second stage accelerated by the adsorption and conversion of intermediates by the catalyst. The two stages are controlled by different properties and are related to the particle size of the catalyst. This work provides in-depth research on CuO catalysts for the thermal decomposition of AP.
1. Introduction
Ammonium perchlorate (AP) is one of the primary oxidizers in composite solid propellants (CSP), and its thermal decomposition characteristics, particularly the decomposition temperature and rate, critically influence key propellant performance parameters such as ignition delay, burning rate, and combustion stability [1,2,3]. The decomposition behavior of AP in the presence of catalysts is of the utmost importance for enhancing the efficiency and controllability of propellants [4,5,6]. For example, Zheng et al. [7] synthesized hierarchical Co-based hollow spheres (Co-HSs), and the Co-HSs@AP showed excellent thermal catalytic performances of AP, owing to their stably hierarchical structures. Thus, to investigate the relationship between the structure of catalysts and their catalytic performances towards AP decomposition is highly significant for the advancement of CSP [8,9,10].
To date, various catalysts, including transition metal oxides (such as Fe2O3, CuO, vanadium oxides, Co3O4), MOFs (metal–organic frameworks), HOFs (hydrogen-bonded organic frameworks), ferrites, and carbon-based materials, have been explored to enhance AP decomposition [11,12,13,14,15,16,17,18]. Among these, copper oxide (CuO) has attracted substantial research interest due to its demonstrated high catalytic activity [17,19,20]. Significant efforts have been dedicated to synthesizing CuO with various structures (e.g., nanoparticles, nanowires) and composite structures (e.g., supported on other metals). For instance, Guo et al. prepared CuO/SiO2 composites using a wet impregnation method and CuO nanorods uniformly dispersed throughout SiO2 aerogels without agglomeration, which could effectively improve the thermal behavior of AP, which is reflected in the reduction of the peak decomposition temperature of AP from 418.7 °C to 341.8 °C [21]. George Tzvetkov eta al. synthesized CuO/Cu(OH)2 structures with good AP decomposition properties [22]. The decomposition peak temperature of AP can be reduced by 96 °C, and the heat release can be increased by 38.6%. However, a critical challenge remains: the specific and isolated impact of the primary particle size (distinct from morphology or composite effects) on the catalytic process is often obscured or not systematically investigated [23,24,25].
For heterogeneous catalysts, the particle size of the catalyst affects the number of exposed catalytic active sites on the catalyst surface, thereby significantly influencing the catalytic effect, which is crucial for heterogeneous catalysis [26]. As particle size decreases, the surface-to-volume ratio increases dramatically, leading to a significantly higher density of accessible active sites where the AP decomposition reaction occurs [27]. This enhanced exposure of active sites is widely recognized as a crucial factor profoundly influencing catalytic effectiveness in heterogeneous catalysis [28,29,30]. Moreover, due to the complex decomposition process and intermediate reactions of AP, catalysts may accelerate the decomposition of AP through various means. Therefore, to clarify the potential impact of catalyst particle size on the catalytic process and mechanism, as well as to provide insights and benchmarks for subsequent catalyst design and mechanism research, it is necessary to investigate the relationship between catalyst particle size (exposure of catalytic active sites) and the catalytic process [31,32,33].
Based on the above assumptions, this study aims to investigate the catalytic mechanism of CuO on AP and the influence of particle size on its catalytic process by analyzing the CuO-catalyzed AP decomposition process. In order to eliminate the influence of morphology, we chose spherical CuO with particle sizes of 40 nm (referred to as CuO-S) and 100–200 nm (referred to as CuO-L) as catalysts for the catalytic testing of AP. The catalytic performance was rigorously evaluated through TG-DSC, isothermal TG, and TG-IR. This work mainly focuses on the thermal decomposition behavior, gas composition, activation energy, and combustion performance of CuO-catalyzed AP decomposition with different particle sizes, which have been rarely reported in the literature [19,34,35]. This work provides a foundation for the rational design of high-performance catalysts based on particle size control and deepens the understanding of potential catalytic mechanisms.
2. Results and Discussion
2.1. Characterization of CuO with Different Particle Sizes and Their Thermal Decomposition of AP
Figure 1a,b shows SEM images of the samples CuO-S (Figure 1a) and CuO-L (Figure 1b). From the images, it can be observed that the CuO was primarily composed of spherical particles, with the particle size of CuO-S being approximately 40 nm (Figure 1a) and that of CuO-L being approximately 100~200 nm (Figure 1b). The SEM results indicate that the morphologies of the two were basically similar, with only differences in particle size, which can largely exclude the influence of other factors and meet the experimental requirements. Figure 1c shows the particle size distribution of CuO with two different particle sizes, which is consistent with the SEM results. However, due to the presence of certain agglomeration phenomena, the particle size distribution test values were relatively large, but it did not affect the relative size of the two. Figure 1d depicts the XRD pattern of the purchased CuO-S and CuO-L. All diffraction peaks were consistent with the simulation results of CuO (COD ID 1011148) [36], and there were no impurity phases detected, indicating that the purchased materials were pure CuO. The XRD pattern exhibited relatively strong diffraction peaks, indicating that CuO has good crystallinity. The above-mentioned relevant characterizations demonstrate that the purchased CuO-S and CuO-L were pure CuO and had particle sizes in two ranges. The XPS results (Figure 1e,f) show that the test data of the two were basically consistent, with obvious Cu2+ characteristic peaks. The BET test results (Figure 1g) show a significant difference in the specific surface area between the two, with CuO-S having a significantly larger surface area than CuO-L, which is consistent with the results of SEM and particle size analysis. In addition, UV–VIS testing and calculation results show that due to size effects, CuO-S had a larger bandgap than CuO-L (Figure 1h,i), which is consistent with the testing and analysis results in related papers [37,38,39,40,41].
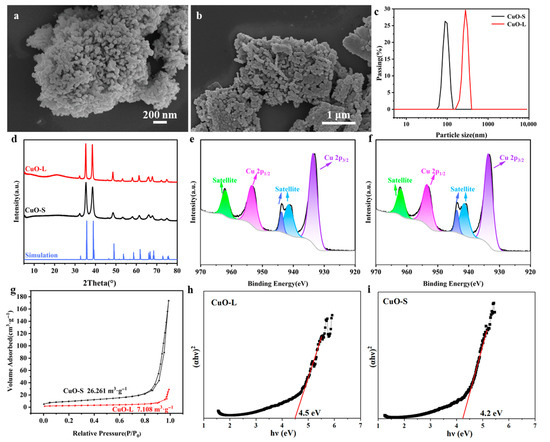
Figure 1.
(a,b) SEM images of CuO with different particle sizes: (a) CuO-L; (b) CuO-S. (c) Particle size distribution diagram of CuO-L and CuO-S; (d) XRD patterns of CuO-L and CuO-S. (e,f) XPS images of CuO with different particle sizes: (e) CuO-L; (f) CuO-S. (g) BET results of CuO-L and CuO-S. (h,i) Direct band gap estimations of CuO with different particle sizes: (h) CuO-L; (i) CuO-S.
The TG-DSC results of AP catalyzed by CuO with different particle sizes (CuO-S and CuO-L) are shown in Figure 2. The results indicate that the difference in CuO particle size did have a certain impact on the catalytic decomposition of AP, and this impact varied between the low-temperature decomposition stage and the high-temperature decomposition stage. Specifically, in the low-temperature decomposition stage, CuO-L decomposition was more dominant, while CuO-S decomposition had a better catalytic effect in the high-temperature decomposition stage. In the low-temperature decomposition stage, CuO-L, which possessed a smaller specific surface area, exhibited a superior catalytic effect. This phenomenon appeared to be at odds with the prevailing circumstances of heterogeneous catalysis. It is evident from a comprehensive review of the extant research literature that the decomposition mechanism of AP in the low-temperature decomposition stage can be divided into two distinct mechanisms: the electron transfer mechanism and the proton transfer mechanism [42,43,44]. It has been demonstrated that the proton transfer mechanism can play a role in the decomposition of AP itself, while the electron transfer mechanism is mostly achieved through catalysts [45,46,47]. It can be concluded from the experimental phenomena and the test results of the bandgap mentioned above that, due to the narrower bandgap of CuO-L, its electron transfer ability was stronger than that of CuO-S. This accelerated the electron transfer process during the initial decomposition of AP, ultimately leading to CuO-L being superior to CuO-S in the low-temperature decomposition stage.
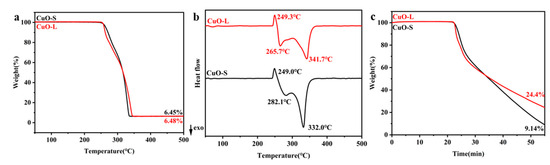
Figure 2.
TG-DSC curves of AP catalyzed by CuO-S and CuO-L: (a) TG curves; (b) DSC curves; (c) 280 °C isothermal experimental TG curve.
As the reaction progressed, the Cu active sites on CuO began to play a role in gas adsorption and conversion [48]. This process is somewhat closely related to the number of catalytically active sites on the surface; hence, CuO-S showed a significantly faster rate in the later stages of decomposition, completing the catalytic reaction at a lower temperature compared to CuO-L. Figure 2b shows the DSC curve of AP catalyzed by CuO-S and CuO-L. The particle size had little effect on the crystal transformation of at about 249 °C. The low-temperature decomposition (LTD) stage increased from 265.7 °C (CuO-S) to 282.1 °C (CuO-L) [9]. However, the high-temperature decomposition (HTD) stage decreased from 341.7 °C (CuO-S) to 332.0 °C (CuO-L). The results of the DSC curve (Figure 2b) were basically consistent with the above TG results, that is, CuO-L had a lower low-temperature decomposition peak temperature, while CuO-S had a higher high-temperature decomposition peak.
To further validate this viewpoint, this article conducted a 30-minute constant temperature experiment at 280 °C on the AP/catalyst mixed sample to further compare their catalytic effects. Figure 2c shows the isothermal TG curves of CuO-S/AP and CuO-L/AP, which further indicates that the main differences lay in their electron transfer capabilities and the number of exposed catalytic active sites of CuO with different particle sizes. The advantage of electron transfer capability lay in the faster initial decomposition rate of CuO-L. Conversely, the increase in catalytic active sites provided a significant advantage in the subsequent isothermal stage, facilitating the conversion of AP decomposition products and helping the reaction equilibrium shift to the right more quickly (CuO-S) [34]. The results correspond with the TG-DSC curves of AP catalyzed by CuO-S and CuO-L in Figure 2.
2.2. Analysis of Gas Products from AP Decomposition Catalyzed by CuO with Different Particle Sizes
The TG-IR were carried out to analyze the composition of the gases of the CuO-S/AP and CuO-L/AP, and their results are shown in Figure 3 and Figure 4, respectively. The initial decomposition product of CuO-S was N2O, demonstrating that CuO-S accelerated the decomposition process of AP through its electron transfer capability at the initial stage of the reaction (Figure 3a–c). As the decomposition reaction progressed, the system temperature rapidly rose, and subsequently, signals of NO, NOCl, and NO2 emerged. This may be attributed to the accelerated oxidation of active oxygen species due to the temperature increase, which aligned with the previous decomposition mechanism of AP catalyzed by other catalysts [7,35]. Furthermore, due to the high peak temperature of AP decomposition catalyzed by CuO-S, it can be observed that the proportion of NO2 increased rapidly in the later stages of the reaction, and it even reached an instantaneous proportion of over 50% in the later stages (Figure 3c). This confirms that under the influence of copper-based catalysts, there is a significant correlation between the degree of oxidation of NH3 oxidation gas and temperature. Within an appropriate temperature range, the higher the temperature, the higher the proportion of NO and its derivatives (Figure 3d). The proportion of NO and its derivatives in the total gas output significantly increased. The above results indicate that, as the reaction progressed and the catalytic active sites came into play, the increased number of catalytic active sites of CuO-S enabled better gas adsorption and conversion. Consequently, the oxidation degree of gas products, particularly those from NH3 oxidation, significantly increased (Figure 3d). An increase in the number of catalytic active sites benefitted the oxidation of N-containing gases to higher valence states. The exposure of more active sites facilitated heat accumulation and the formation of local hotspots. It can be inferred that the generation of NO2 at this time was also related to the formation of local hotspots. Ultimately, an analysis of the proportion of total NH3 oxidation gases in AP decomposition revealed a further decrease in the proportion of N2 and an increase in the production of NO2 gas.
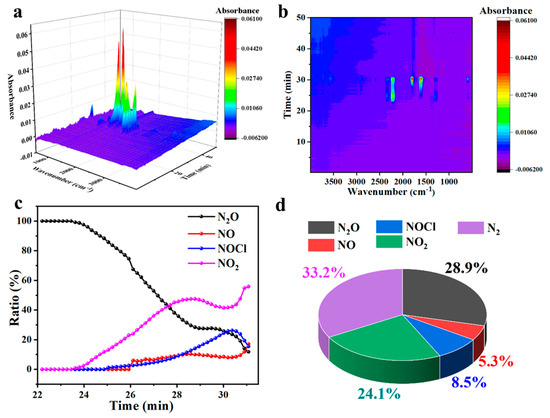
Figure 3.
Analysis results of gas products from AP decomposition catalyzed by CuO-S: (a) TG-IR 3D diagram; (b) TG-IR mapping diagram; (c) diagram of relative content changes of each gas over time in TG-IR; (d) diagram of total gas release proportion.
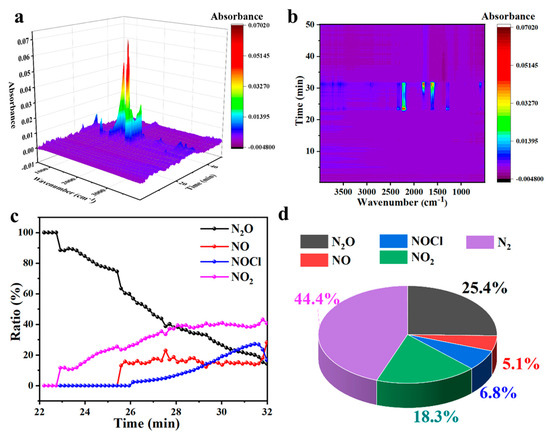
Figure 4.
Analysis results of gas products from AP decomposition catalyzed by CuO-L: (a) TG-IR 3D diagram; (b) TG-IR mapping diagram; (c) diagram of relative content changes of each gas over time in TG-IR; (d) diagram of total gas release proportion.
The test results of CuO-L further verified the above analysis (Figure 4). Compared to the results of CuO-L (Figure 4) and compared to CuO-S (Figure 3), the degree of NH3 oxidation in the system of CuO-L was lower than that of CuO-S. Due to the fewer catalytically active sites exposed in CuO-L compared to CuO-S, both the production and proportion of NO2 decreases significantly. In the graph depicting the proportion of total NH3 oxidation gas decomposed by AP, it can be observed that even though CuO-L had a higher peak temperature for decomposition, theoretically contributing to a higher degree of NH3 oxidation in the system, the oxidation degree was still lower than that catalyzed by CuO-S due to the insufficient number of active sites [26]. These results prove that the small size particle is good for the deep oxidation of NH3.
The isothermal experiments of AP catalyzed by CuO-S and CuO-L were studied by TG-IR, as shown in Figure 5. The results further demonstrate the adsorption and conversion processes of gas products on catalytic active sites. For CuO-S, the gas products during the isothermal process were mainly NO2 and NOCl, proving that there was a significant correlation between the composition of gas products and the number of active sites during the isothermal process. The gas production during the isothermal experiment of CuO-L was basically similar to that of CuO-S, except for a decrease in gas production during the isothermal process. This is consistent with the weaker catalytic efficiency of CuO-L compared to CuO-S in catalyzing AP during the isothermal process. Based on this, it can be speculated that the catalytic mechanism of CuO on AP in the later stage was mainly to enhance the adsorption and conversion processes of the initial decomposition products of AP, HClO4, and NH3, thereby breaking the phenomenon of slow decomposition rate caused by the similarity of reaction products during the decomposition of pure AP at this temperature and ultimately achieving a decrease in the peak decomposition temperature.
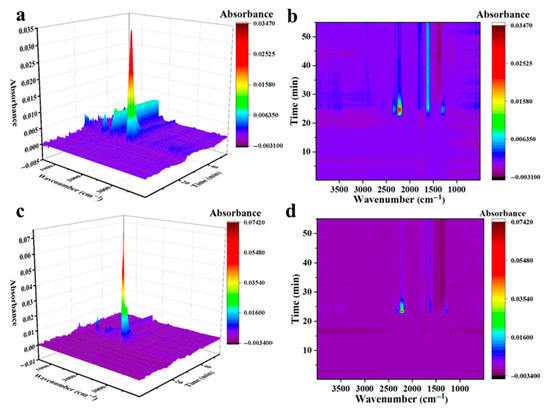
Figure 5.
TG-IR results of the AP isothermal experiment catalyzed by CuO-S (a,b) and CuO-L (c,d); (a,c) TG-IR 3D plots; (b,d) TG-IR mapping plots.
2.3. Kinetic Study of AP Catalyzed by CuO with Different Particle Sizes
To study the activation energy of AP catalyzed by CuO-S and CuO-L, the TG curves at different heating rates were tested, and the results are shown in Figure 6a and Figure 6c, respectively. These TG curves indicate that the heating rate did not affect the catalytic mechanism of CuO-S and CuO-L for AP. This is in line with the Arrhenius formula (the temperature rate increases, the reaction slows). According to the F-W-O formula [36], the activation energies were calculated at different reaction rates. Figure 6b and Figure 6d, respectively, show the activation energy curves of AP catalyzed by CuO-S and CuO-L. For CuO-S (Figure 6b) and CuO-L (Figure 6d), the value of α was about 0.2 for CuO-S to the extreme point, whereas this value was about 0.28 for CuO-L. This agrees well with how CuO-S showed a significantly faster rate in the later stages of decomposition. After the extreme point, the decomposition behavior of AP catalyzed by CuO-S and CuO-L continued until the end of the reaction, and the activation energy slowly increased. Generally, the variation patterns of the activation energies of the two were basically consistent, further verifying that the particle size did not significantly affect the decomposition mechanism of CuO catalyzing AP. Based on this, it was further verified that the catalytic decomposition mechanism of AP was mainly related to the composition or surface structure of the catalyst, while the morphological characteristics of the catalyst mainly affected the expression of the mechanism [24]. The main difference lay in the position of the extreme points (Figure 6b,d), which was consistent with the position of rate changes in the TG curves (Figure 6a,c).
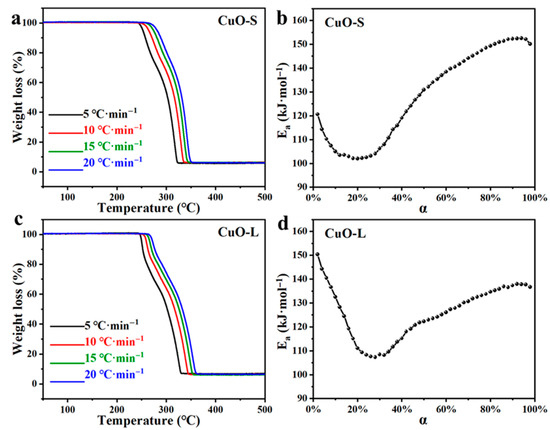
Figure 6.
Kinetic calculation results of AP decomposition catalyzed by CuO-S and CuO-L: (a) TG curves of AP catalyzed by CuO-S at different heating rates; (b) the change in activation energy of AP catalyzed by CuO-S with reaction conversion rate; (c) TG curves of AP catalyzed by CuO-L at different heating rates; (d) the change in activation energy of AP catalyzed by CuO-L with a reaction conversion rate.
2.4. Combustion Performances of CuO-S/AP and CuO-L/AP
Finally, the combustion experiments were conducted on mixed samples of CuO-S and CuO-L with AP. The combustion experiment apparatus used is based on the previous report [49]. The test results are shown in Figure 7 and Figure 8, respectively. Among them, the flame duration of CuO-S was 416 ms, while that of CuO-L was 725 ms. From the perspective of the flame, the flames catalyzed by the two catalysts for AP combustion were similar, but the flame area of CuO-S was larger and the flame color was brighter, which may be related to its faster decomposition rate and gas release rate. In addition, in the flame of CuO-L, the phenomenon of some particles being blown up due to the large amount of gas generated in the crucible could be observed, further indicating that during the catalytic combustion of AP, a large amount of gas could be generated in a short period of time. Overall, there was no significant difference in the flame structure and combustion process among these two CuO catalysts, which to some extent indicates that they shared similar catalytic mechanisms, in line with the above discussion.
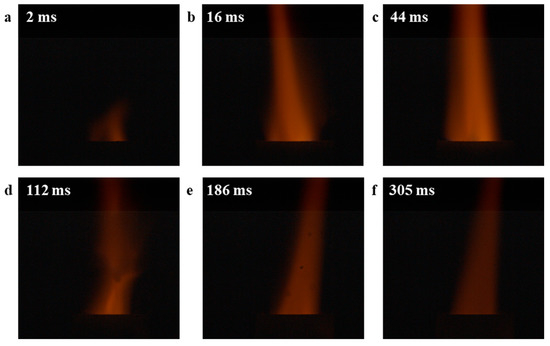
Figure 7.
The combustion experiment of AP catalyzed by CuO-S: (a) 2 ms; (b) 16 ms; (c) 44 ms; (d) 112 ms; (e) 186 ms; (f) 305 ms.
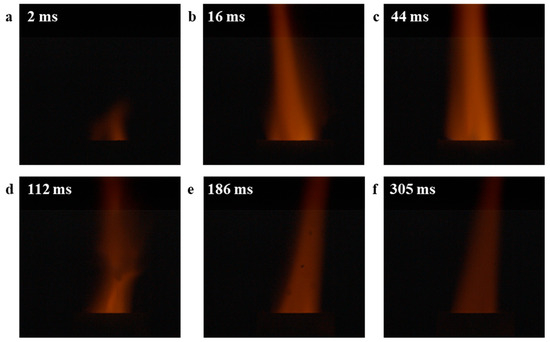
Figure 8.
The combustion experiment of AP catalyzed by CuO-L: (a) 2 ms; (b) 16 ms; (c) 44 ms; (d) 112 ms; (e) 186 ms; (f) 305 ms.
3. Materials and Methods
3.1. Catalysts
Nanometer-sized copper oxide 1 (CuO, with a particle size of approximately 40 nm, 99.5%), named as CuO-S, and nanometer-sized copper oxide 2 (CuO, with a particle size ranging from 100 to 200 nm, 99.5%), named as CuO-L, were purchased from Luoen Company (Wuhan, China). Ammonium perchlorate (NH4ClO4, Type II, Class 3), denoted as AP, was purchased from Tianyuan Chemical Co., Ltd. (Taiyuan, China) All reagents were used without any further purification. The preparation of the AP/CuO sample: AP and CuO samples were weighed and mixed at a mass ratio of 5% catalyst [7].
3.2. Materials Characterizations
X-ray diffraction (XRD) measurement was recorded on a Rigaku (Singapore) Smartlab 9 kW diffractometer. The diffractometer was tested under ambient temperature and pressure conditions of 45 kV, 200 mA, and Cu Kα1 (λ = 1.5406 Å), with a scanning speed of 10 °/min. Scanning electron microscopy (SEM) images were taken using Zeiss Merlin Compact (ZEISS, Jena, Germany), and before shooting, the sample was treated with platinum plating to enhance its conductivity, with an acceleration voltage of 5 kV.
3.3. Catalytic Performance Characterizations
Thermogravimetry–differential scanning calorimetry (TG-DSC) (NETZSCH STA-2500, Selb, Germany) was used to analyze the quality changes and heat absorption and release of samples under a programmed heating system. TG-DSC analyses from 30 to 500 °C were tested on a German NETZSCH STA-2500 synchronous thermal analyzer using a 10 °C/min heating rate. For AP/catalyst mixed samples, the testing atmosphere was nitrogen.
The constant temperature experiment was used to observe the physical and chemical changes that occur in the system at a certain temperature. The specific procedure uses a heating rate of 10 °C/min to raise the temperature to the specified temperature 280 °C and then maintains a constant temperature for 30 min. Thermogravimetry–infrared spectroscopy (TG-IR) testing was used to analyze the decomposition products of AP/catalyst samples and for relative quantitative comparison. The carrier gas flow rate decreased to 20 mL·min−1. The IR instruments were Thermo Fisher and IS-10 Fourier transform infrared spectrometers.
The kinetics were calculated based on TG data: if the total mass lost by the sample during the TG process is α = 1, then α is calculated based on the mass loss at different temperatures to obtain the α-T curve. Among them, α represents the degree of reaction. In order to further obtain the T value under a specific α, quadratic interpolation is used to process α-T [50].
After obtaining the α-T curve, the Ea values of the reaction at different α values were obtained using the OZAWA method [51].
3.4. Combustion Experiments
The A water-cooled CO2 laser manufactured by Shanghai Yuhong Laser Equipment Co., Ltd. (Shanghai, China), in China was used to generate enough flux to ignite the sample. The laser operates at a power of 80 W and a pulse duration of 2 s. Approximately 50 mg of the sample was used in each test, which was placed in an Al2O3 crucible. During ignition and combustion, the AvaSpec-2048 fiber-optic spectrometer from Avantes BV in Apeldoorn, The Netherlands was used to record the emission spectrum of the sample with a resolution of 0.8 nm. The American Phantom M340 high-speed digital camera was used to record the chemiluminescence emission at a speed of 1000 frames per second. During the test, N2 was injected into the combustion chamber to create an N2 environment, thereby displacing air and allowing the sample to burn in a 0.1 MPa N2 atmosphere.
4. Conclusions
In summary, this study comprehensively tested the decomposition process and products of CuO catalyzed AP, analyzed the catalytic mechanism of CuO on AP at different decomposition stages, and established a direct relationship between catalyst properties and catalytic processes by comparing the different catalytic processes of CuO with different particle sizes. The initial decomposition of AP was accelerated and controlled by the electron transfer mechanism of CuO, which was related to the conductivity of the catalyst. The subsequent decomposition was achieved through the adsorption and conversion of intermediates by the catalyst, which was related to the number of exposed active sites on the catalyst. This difference will affect the composition of NH3 oxidation gas products and the flame changes during AP decomposition but has little effect on the overall catalytic mechanism. This work provides guidance for the structural design of AP decomposition catalysts.
Author Contributions
Conceptualization, H.J. and X.T.; methodology, H.J. and X.T.; software, L.F.; validation, L.F., J.L. and Z.Z.; formal analysis, J.L. and C.D.; investigation, H.J. and X.T.; resources, Z.Z., H.J. and X.T.; data curation, H.J. and X.T.; writing—original draft preparation, H.J., X.T. and Y.Z.; writing—review and editing, Y.Z. and C.H.; visualization, C.D.; supervision, Y.Z. and C.H.; project administration, C.H.; funding acquisition, C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank the Core Research Facilities of College of Chemistry and Molecular Sciences at Wuhan University for the SEM and TEM characterization. Moreover, we thank the Core Facility of Wuhan University for EDS characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, J.; Liu, Y.; Fu, X.C.; Deng, N.M. Cu/Carbon Aerogels Derived from HKUST-1 for the Thermal Decomposition of Ammonium Perchlorate. ACS Appl. Nano Mater. 2024, 7, 17373–17378. [Google Scholar] [CrossRef]
- Zhou, P.; Ren, Z.; Tang, X.; Zheng, Z.; Zhang, K.; Liao, J.; Zhong, Y.; Zhang, Y.; Huang, C. Interaction Between Prussian Blue Ultrathin Nanosheet and Ammonium Perchlorate for Highly Efficient Thermal Decomposition. Adv. Funct. Mater. 2023, 33, 2300661. [Google Scholar] [CrossRef]
- Yu, J.; Kou, Y.; Luo, P.; Hu, Y.; Gao, H.; Zhao, F.; Jiang, W.; Xiao, L.; Hao, G. Facile Grinding Method for Preparing Nano-Cu-Cr-Fe Composite Metal Oxides with Enhanced Catalytic Activity for Thermal Decomposition of Ammonium Perchlorate. Combust. Sci. Technol. 2025, 197, 1920–1936. [Google Scholar] [CrossRef]
- Li, R.; Lin, Y.; Zhu, J.; Wang, Z.; Zeng, K.; Wang, J.; Pei, C.; Xie, R.; Ma, Y. Synthesis of Cu-MOF-derived complex copper–chromium oxides and their catalytic study on the thermal decomposition of ammonium perchlorate. J. Solid State Chem. 2024, 336, 124764. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Wu, M.; Li, J.; Feng, Y.; Ning, X.; Li, H.; Wang, N.; Shi, B. Micro-aluminum powder with bi- or tri-component alloy coating as a promising catalyst: Boosting pyrolysis and combustion of ammonium perchlorate. Def. Technol. 2024, 33, 100–113. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Tan, H.; Xia, Z.; Zhai, L.; Hu, J.; Yang, Q.; Xie, G.; Chen, Z.; Chen, S. In Situ MOF-74-Pyrolysis-Generated Porous Carbon Supporting Spinel Cu0.15Co2.85O4/C Boosts Ammonium Perchlorate Accelerating Decomposition: Precise Cu Doping Modulating Oxygen Vacancy Concentration. Small 2024, 20, 2400712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhou, P.; Tang, X.; Zeng, Q.; Yi, S.; Liao, J.; Hu, M.; Wu, D.; Zhang, B.; Liang, J.; et al. Hierarchical MOFs with Good Catalytic Properties and Structural Stability in Oxygen-Rich and High-Temperature Environments. Small 2024, 20, e2309302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, S.; Ren, Z.; Tang, X.; Zhang, K.; Zhou, R.; Wu, D.; Liao, J.; Zhang, Y.; Huang, C. In Situ Cutting of Ammonium Perchlorate Particles by Co-Bipy “scalpel” for High Efficiency Thermal Decomposition. Adv. Sci. 2022, 9, 2204109. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, S.; Ren, Z.; Wang, Y.; Zhang, Y.; Huang, C. Study on the thermal decomposition behavior of ammonium perchlorate catalyzed by Zn–Co cooperation in MOF. Inorg. Chem. Front. 2022, 9, 5195–5209. [Google Scholar] [CrossRef]
- Zhou, P.; Tang, X.; Yuan, B.; Zhou, Y.; Zheng, Z.; Ren, Z.; Liao, J.; Liang, J.; Huang, C. Selective conversion of thermal decomposition products of ammonium perchlorate by amorphous CoSnOx. J. Hazard. Mater. 2024, 480, 136111. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Song, R.; Chen, C.; Alomar, T.S.; Xiao, F.; AlMasoud, N.; El-Bahy, Z.M.; Yang, Y.; Algadi, H.; Sun, L. Graphene oxide–supported Cu/Co nano-catalysts for thermal decomposition of ammonium perchlorate composites. Adv. Compos. Hybrid Mater. 2023, 6, 188. [Google Scholar] [CrossRef]
- Yan, Y.; Jin, B.; Zhou, Q.; Peng, R. Copper loaded carbon aerogel from chitosan-precursor promotes thermal decomposition of ammonium perchlorate for solid propellants. Adv. Powder Technol. 2023, 34, 104188. [Google Scholar] [CrossRef]
- Zhang, G.-P.; Cheng, Y.-H.; Li, M.-M.; Xiao, L.; Guo, H.; Jiang, W.; Hao, G.-Z. Catalytic properties of CuO–Cr2O3 ternary nanocomposites favorable for the pyrolysis of ammonium perchlorate. Energetic Mater. Front. 2022, 3, 226–232. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Han, J.; Xia, Z.; Chen, S.; Xie, G.; Gao, S.; Lu, J.Y.; Yang, Q. In situ growth of copper-based energetic complexes on GO and an MXene to synergistically promote the thermal decomposition of ammonium perchlorate. Dalton Trans. 2023, 52, 17458–17469. [Google Scholar] [CrossRef]
- Wei, S.; Tan, H.; Zhang, Y.; Xia, Z.; Yang, Q.; Xie, G.; Chen, S. MOF-74 derivatives spinel CuCo2O4/C with d-band center modulation for accelerating ammonium perchlorate thermolysis. Fuel 2024, 370, 131814. [Google Scholar] [CrossRef]
- Gou, X.; Sun, X.; Yang, J.; Shi, J.; Yan, S.; Guo, X.; Yu, S.; Nie, J. Improvement of the Thermal Decomposition of Ammonium Perchlorate and Combustion of Aluminum Powder by Dual Core–Shell Ammonium Perchlorate-Based Composites Based on Self-Assembly Coating. Langmuir 2025, 41, 11674–11689. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, Y.; Hou, H.; Fan, N.; Yuan, F.; Shi, Y.; Meng, Q. Preparation and characterization of Cu(OH)2 and CuO nanowires by the coupling route of microemulsion with homogenous precipitation. Solid State Commun. 2010, 150, 585–589. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Huang, Y.; Wu, W.; Huang, C.; Meng, C. Fabrication and catalytic activity of ultra-long V2O5 nanowires on the thermal decomposition of ammonium perchlorate. Ceram. Int. 2014, 40, 11393–11398. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Li, H.; Guo, C.; Yang, L. Facile fabrication of well-dispersed CuxO nanoneedle on porous carbonized nano sponge and its promising application in the thermal decomposition of ammonium perchlorate. Powder Technol. 2021, 391, 206–213. [Google Scholar] [CrossRef]
- Zhang, Z.-K.; Guo, D.-Z.; Zhang, G.-M. Preparation, characterization and catalytic property of CuO nano/microspheres via thermal decomposition of cathode-plasma generating Cu2(OH)3NO3 nano/microspheres. J. Colloid Interface Sci. 2011, 357, 95–100. [Google Scholar] [CrossRef]
- Guo, C.; Lu, Y.; Tian, Y.; Guo, H.; Zhang, X. Porous SiO2 supported CuO as a promising catalyst on the thermal decomposition of ammonium perchlorate. Appl. Organomet. Chem. 2021, 35, e6215. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Spassov, T.; Tsvetkov, M.; Rangelova, V. Mesoporous cauliflower-like CuO/Cu(OH)2 hierarchical structures as an excellent catalyst for ammonium perchlorate thermal decomposition. Mater. Lett. 2021, 291, 129534. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z.; Lv, T.; Zhang, Z.; Zhou, Z.; Hu, T.; Meng, C.; Zhang, Y. Conjugated polyaniline as “conveyor” in tungstate boosting cation storage for high-performance aqueous batteries. Green Energy Environ. 2025, 10, 766–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Han, Z.; Wang, Y.; Jiang, H.; Zhang, F.; Zhu, X.; Meng, C.; Huang, C. Dual modification of cobalt silicate nanobelts by Co3O4 nanoparticles and phosphorization boosting oxygen evolution reaction properties. J. Colloid Interface Sci. 2025, 679, 1036–1045. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Zhao, Y.; Hu, T.; Gao, Z.; Meng, C. Fabrication of V2O5 with various morphologies for high-performance electrochemical capacitor. Appl. Surf. Sci. 2016, 377, 385–393. [Google Scholar] [CrossRef]
- Lv, T.-T.; Wang, H.-X.; Ren, X.-B.; Wang, L.-C.; Ding, R.-M.; Cao, J.-P.; Lv, B.-L. Protection of highly active sites on Cu2O nanocages: An efficient crystalline catalyst for ammonium perchlorate decomposition. CrystEngComm 2020, 22, 8214–8220. [Google Scholar] [CrossRef]
- Heng, B.; Xiao, T.; Hu, X.; Yuan, M.; Tao, W.; Huang, W.; Tang, Y. Catalytic activity of Cu2O micro-particles with different morphologies in the thermal decomposition of ammonium perchlorate. Thermochim. Acta 2011, 524, 135–139. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, S.; Dong, X.; Ding, C.; Mu, Y.; Cui, M.; Hu, T.; Meng, C.; Zhang, Y. Anion Structure Regulation of Cobalt Silicate Hydroxide Endowing Boosted Oxygen Evolution Reaction. Small 2024, 20, e2401394. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Lv, T.; Tan, X.; Wang, Q.; Wang, Y.; Meng, C. Core-shell cobalt-iron silicide electrocatalysts with enhanced bifunctional performance in hydrogen and oxygen evolution reactions. J. Colloid Interface Sci. 2025, 682, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Liu, Z.; Tan, H.; Xie, Z.; Li, J.; Zhang, G. Embedding of ferrocenes in the nanochannels of manganese energetic coordination polymers to retard their migration trends and enhance their catalytic efficiency in the thermal degradation of ammonium perchlorate. Appl. Surf. Sci. 2025, 711, 164042. [Google Scholar] [CrossRef]
- Hao, G.; Yang, R.; Kou, Y.; Wei, J.; Lu, Q.; Zhang, W.; Gao, H.; Zhao, F.; Jiang, W. Highly dispersed core–shell AP@AO energetic composites with good inhibitory effect on the low-temperature decomposition of AP and the burning rate of AP-based composite propellants. Fuel 2025, 402, 135967. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Xue, S.; Lv, W.; Qin, R.; Pan, W.; Lv, B. The facet-dependent catalytic performance of CeO2 nanocatalysts in the decomposition of ammonium perchlorate. Inorg. Chem. Front. 2025, 12, 3275–3284. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, B.; Hua, C.; Qiu, M.; Qiu, Y.; An, C.; Wang, J. Design of Polydopamine@iron Oxide-Coated Ammonium Perchlorate Core–Shell Composites for Enhancing the Combustion Performance of HTPB-Based Propellants. Langmuir 2025, 41, 1386–1399. [Google Scholar] [CrossRef]
- Ramdani, Y.; Liu, Q.; Huiquan, G.; Liu, P.; Zegaoui, A.; Wang, J. Synthesis and thermal behavior of Cu2O flower-like, Cu2O-C 60 and Al/Cu2O-C60 as catalysts on the thermal decomposition of ammonium perchlorate. Vacuum 2018, 153, 277–290. [Google Scholar] [CrossRef]
- Li, S.; Niu, Z.; Jiao, Y.; Jin, P.; Yang, D.; Bai, C.; Liu, J.; Li, G.; Luo, Y. Preparation of different morphology Cu/GO nanocomposites and their catalytic performance for thermal decomposition of ammonium perchlorate. RSC Adv. 2022, 12, 22806–22814. [Google Scholar] [CrossRef]
- Tunell, G.; Posnjak, Ε.; Ksanda, C.J. Geometrical and optical properties, and crystal structure of tenorite. Z. Für Krist.-Cryst. Mater. 1935, 90, 120–142. [Google Scholar] [CrossRef]
- Dagher, S.; Haik, Y.; Ayesh, A.I.; Tit, N. Synthesis and Optical Properties of Colloidal CuO Nanoparticles. J. Lumin. 2014, 151, 149–154. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829. [Google Scholar] [CrossRef]
- Luo, X.-L.; Wang, M.-J.; Yun, L.; Yang, J.; Chen, Y.-S. Structure-dependent activities of Cu2O cubes in thermal decomposition of ammonium perchlorate. J. Phys. Chem. Solids 2016, 90, 1–6. [Google Scholar] [CrossRef]
- Jing, X.; Mu, Y.; Gao, Z.; Dong, X.; Meng, C.; Huang, C.; Zhang, Y. Intermetallic ferric nickel silicide alloy derived from magadiite by magnesiothermic reaction as bifunctional electrocatalyst for overall water splitting. Nano Res. Energy 2024, 3, e9120104. [Google Scholar] [CrossRef]
- Rong, M.; Zhang, Y.; Tan, X.; Wang, Y.; Gao, N.; Huang, C.; Meng, C. Breath inspired multifunctional low-cost inorganic colloidal electrolyte for stable zinc metal anode. J. Energy Chem. 2024, 102, 218–229. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Khairetdinov, E.; Boldyrev, V.; Burshtein, A. On the mechanism of conductivity of ammonium salts. J. Solid State Chem. 1974, 10, 288–293. [Google Scholar] [CrossRef]
- Bircumshaw, L.L.; Newman, B.H. The thermal decomposition of ammonium perchlorate, II. The kinetics of the decomposition, the effect of particle size, and discussion of results. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1955, 227, 228–241. [Google Scholar] [CrossRef]
- Yang, J.; Ling, L.; Li, Y.; Lu, L. Density Functional Theory Study on Thermal Decomposition Mechanisms of Ammonium Perchlorate. Acta Chim. Sin. 2023, 81, 328–337. [Google Scholar] [CrossRef]
- Garrison, M.D.; Wallace, S.G.; Baldwin, L.C.; Guo, Z.; Kuo, L.; Estevez, J.E.; Briseno, A.L.; Hersam, M.C.; Baca, A.J. Accelerated decomposition kinetics of ammonium perchlorate via conformal graphene coating. Chem. Mater. 2021, 33, 9608–9617. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, C.; Liu, X.; Chen, W.; Tang, Q.; Li, Y. Tuning thermal decomposition of ammonium perchlorate by nanoporous Gd2O3 for improved safety and enhanced propellant efficiency. J. Rare Earths 2020, 38, 108–112. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, P.; Zhou, Y.; Yuan, B.; Zhan, F.; Gao, J.; Liang, T.; Ren, Z.; Hu, M.; Zhang, Y.; et al. Structural design and evolution of one-dimensional Cu hydrogen-bonded organic framework for catalyzing the rapid decomposition of ammonium perchlorate. J. Hazard. Mater. 2025, 486, 136961. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhao, J.; Yue, S.; Zhou, Y.; Yuan, B.; Zhou, P.; Ao, W.; Huang, C. Porous Cu2O hierarchical structure for promoting the decomposition of ammonium perchlorate and its combustion properties. Fuel 2026, 405, 136781. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J. Computation. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. Calorim. 1970, 2, 301–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).