Abstract
Photocatalytic technology offers significant potential for pollutant remediation through efficient, cost-effective mineralization but faces inherent limitations, including catalyst agglomeration and rapid charge recombination. To address these challenges, we developed activated carbon-modified porous graphitic carbon nitride (APCN) synthesized through the co-polycondensation of dicyandiamide with NH4Cl and fir-wood-derived activated carbon (AC). The incorporated AC effectively prevented the agglomeration of carbon nitride frameworks, thereby enhancing the specific surface area (SBET) of APCN. This matrix was subsequently composited with hydrothermally prepared (1T/2H) mixed-phase MoS2 through ultrasonication, forming a MoS2/APCN heterostructure. Characterizations including Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), and N2 adsorption–desorption isotherms (BET) confirmed that MoS2 was successfully loaded onto APCN via an ultrasonic synthesis method. The composite exhibited outstanding photocatalytic activity, degrading 95.5% RhB in 40 min (pH = 7) and 97.4% in 25 min (pH = 3.5), with 87.3% efficiency retention after four cycles (pH = 7). Crucially, AC enhanced visible-light absorption and functioned as an electron-mediating component. Photoelectrochemical analyses and radical-trapping experiments confirmed a direct Z-scheme charge transfer mechanism, wherein conductive AC accelerates electron transport and suppresses carrier recombination. This study establishes both an efficient RhB degradation photocatalyst and a sustainable strategy for valorizing agricultural waste in advanced material design.
1. Introduction
In recent years, rapid industrialization has exacerbated environmental pollution, particularly due to the discharge of dye-contaminated wastewater. Among the various synthetic dyes, Rhodamine B (RhB) has been widely employed in multiple industries, including textile dyeing, paper manufacturing, leather processing, and colored-glass production. However, the release of RhB into aquatic ecosystems poses significant environmental and health risks. It not only increases water chroma and disrupts aquatic biodiversity, but is also well documented to exhibit toxicity to human health, particularly affecting the reproductive and respiratory systems. Acute exposure to RhB can induce symptoms such as nausea, vomiting, abdominal pain, and diarrhea. Furthermore, RhB exhibits potential carcinogenic risks to human health. Given these hazards, the development of efficient and sustainable strategies for RhB removal from wastewater has become a critical priority in environmental remediation [1]. Conventional treatment methods, including physical adsorption, flocculation precipitation, and biodegradation, have been applied for RhB degradation. However, these techniques have inherent limitations, such as high operational costs, incomplete degradation, and the generation of secondary toxic byproducts. In contrast, photocatalysis—an advanced oxidation process—represents a promising alternative that enables the complete mineralization of RhB into harmless H2O and CO2 through redox reactions mediated by photogenerated charge carriers. Compared to traditional water treatment technologies, photocatalysis demonstrates superior degradation efficiency, lower energy consumption, and the absence of hazardous byproducts, making it a highly attractive solution for sustainable wastewater treatment [2].
Graphitic carbon nitride is a polymeric semiconductor characterized by the sp2 hybridization of all constituent atoms. Its structure comprises interconnected rings chemically bonded via terminal nitrogen atoms, forming extended π-conjugated planes. These planar units stack layer-wise, resulting in a two-dimensional layered architecture analogous to graphene [3,4]. As a metal-free organic semiconductor photocatalyst, carbon nitride possesses a moderate band gap (~2.70 eV) and demonstrates favorable light-responsiveness [5]. However, pristine carbon nitride has significant limitations, including particle agglomeration and rapid electron–hole recombination, which substantially impede its photocatalytic activity [6]. Consequently, extensive research has focused on enhancing carbon nitride performance through modification strategies such as morphological control, elemental doping (metal/nonmetal), and heterojunction construction with complementary functional materials [7,8,9]. Among the conventional modification approaches, the soft-template method utilizing NH4Cl as a blowing agent is one of the most well established. During polycondensation, NH4Cl decomposition generates gaseous species (NH3 and HCl), creating pores that significantly increase the specific surface area. This expansion enhances the density of active sites, thereby improving photocatalytic efficiency [10].
Activated carbon, a porous carbonaceous material produced via pyrolysis and the activation of biomass under oxygen-limited conditions at elevated temperatures, exhibits unique physicochemical properties that make it useful in many applications [11]. Activated carbon can be derived from diverse renewable precursors, including agricultural and forestry residues (e.g., wood, bamboo, rice husks), marine byproducts (e.g., lobster shells, crab shells), and industrial waste streams such as end-of-life tires and post-consumer plastics. These raw materials are converted into porous activated carbon through carbonization followed by physical or chemical activation processes [12]. Substantial evidence demonstrates that coupling photocatalysts with AC remarkably enhances the photocatalytic degradation efficiency of a variety of semiconductor systems, including carbon nitride-based materials [13,14,15]. Currently, modified semiconductor photocatalysts with AC are widely applied to enhance the degradation efficiency of organic wastewater (e.g., dyes and antibiotics) and improve the hydrogen production of semiconductors [13,16,17].
Molybdenum disulfide (MoS2), a prevalent transition metal dichalcogenide, features a graphite-like layered structure. Naturally occurring MoS2 predominantly exists in the semiconducting 2H phase; however, its limited number of active sites significantly constrains its photocatalytic performance. Consequently, the metallic 1T phase is frequently introduced to improve its catalytic properties [18]. However, MoS2 suffers from pronounced agglomeration that constrains its intrinsic photocatalytic activity, thereby driving heterostructure formation through integration with semiconductors to enhance photocatalytic performance [19]. The well-aligned band structures of carbon nitride and MoS2 accelerate charge carrier migration, enabling effective heterostructure formation [20]. The heterojunctions significantly promote charge separation and suppress electron-hole recombination, substantially boosting the composite’s photocatalytic activity.
Based on these principles, we developed an activated carbon-modified carbon nitride composite photocatalyst (APCN), which exhibits excellent photocatalytic degradation performance towards RhB. Characterization and photocatalytic performance results confirmed that the fir-wood-derived activated carbon (AC) significantly enhanced the properties of the APCN composite, in contrast to the porous carbon nitride (PCN) synthesized solely from NH4Cl and dicyandiamide, which showed inferior performance. Building on this, we prepared a composite catalyst, MoS2/APCN, by incorporating mixed-phase 1T/2H MoS2 with APCN via ultrasonic-assisted synthesis. The direct Z-scheme heterojunction formed between MoS2 and APCN greatly facilitates interfacial charge transfer, thereby further improving the photocatalytic performance. Notably, AC played a critical role in enhancing the photocatalytic efficiency of the composite. It not only effectively inhibited the disordered stacking of carbon nitride layers during the thermal polymerization process, resulting in a porous APCN structure with higher specific surface area and more exposed active sites, but also—due to its inherent physical properties—enhanced the charge transfer capability across the heterojunction and improved visible-light absorption of the MoS2/APCN composite. These synergistic effects collectively enhanced the overall photocatalytic performance through multiple mechanisms. This study provides valuable insights into the practical application of biomass-derived activated carbon as a “one material, multiple functions” platform in photocatalysis, while also proposing a referential strategy for extending the use of waste biomass in high-performance catalytic systems.
2. Result and Discussion
2.1. Characterization of the Photocatalysts
XRD analysis was used to investigate the phase structure of the photocatalysts (Figure 1a). PCN exhibited two obvious peaks at 13.30° and 27.60°, corresponding to the interlayer stacking surface of carbon nitride and the interplanar stacking surface of the aromatic system, respectively [21]. Compared with PCN, the (002) diffraction peak of AC-modified carbon nitride APCN shifts from 27.60° to 27.38° (Figure 1b), indicating enlarged interlayer spacing. This is because AC inhibits the normal stacking of carbon nitride, which increases the interlayer distance of carbon nitride to a certain extent. The resulting enlarged interlayer distance contributes to an increased specific surface area, providing more exposed active sites and consequently enhancing photocatalytic activity [22]. For the MoS2/APCN composite, diffraction peaks observed at 33.4° and 59.0° correspond to the (100) and (110) planes of hexagonal MoS2 (JCPDS No. 37-1492) [23]. Importantly, the (002) diffraction peak of MoS2 appears at approximately 9.7°, exhibiting a significant low-angle shift compared to the standard position (14.0°). This shift likely originates from the intercalation of ammonium ions (NH4+) derived from the (NH4)6Mo7O24·4H2O precursor during hydrothermal synthesis [24]. The comparative analysis confirmed that both APCN and MoS2/APCN retained the characteristic diffraction features of pristine carbon nitride, demonstrating structural integrity throughout the compounding processes.
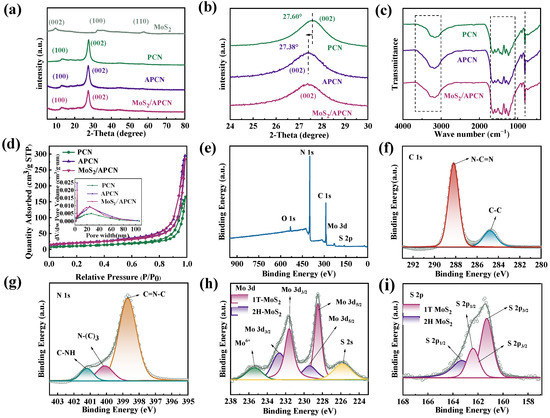
Figure 1.
(a) XRD patterns of PCN, APCN, and MoS2/APCN; (b) partial enlarged view of (a); (c) FTIR of PCN, APCN, and MoS2/APCN; (d) N2 adsorption–desorption isotherms and BJH pore size distributions of PCN, APCN, and MoS2/APCN. XPS spectra of (e) Survey; (f) C 1s; (g) N 1s; (h) Mo 3d; and (i) S 2p of MoS2/APCN.
FTIR spectroscopy was conducted to analyze the functional groups of PCN, APCN, and MoS2/APCN (Figure 1c). All spectra exhibit a broad peak spanning 3000–3700 cm−1, attributed to the stretching vibrations of N–H in free amino groups and O–H bonds [25]. Within the range of 1200–1700 cm−1, a series of absorption bands are observed, primarily corresponding to the breathing and stretching vibrational modes of the heptazine heterocyclic rings inherent to the carbon nitride structure [26]. A sharp characteristic peak at approximately 809 cm−1 appeared in all samples, corresponding to the out-of-plane bending vibration of tri-s-triazine rings in the carbon nitride framework [27]. Notably, both APCN and MoS2/APCN maintain highly similar FTIR spectra to that of PCN. This indicates that neither the incorporation of AC during the thermal polymerization of the carbon nitride precursor nor the ultrasonic compositing of APCN with MoS2 significantly altered the fundamental functional group structure of the carbon nitride matrix, confirming its remarkable structural stability [27].
The specific surface area and pore size information of the materials were obtained from a BET test. Figure 1d displays the N2 adsorption–desorption isotherms and corresponding pore size distribution curves of PCN, APCN, and MoS2/APCN. According to IUPAC classification, all samples exhibited type IV isotherms with H3-type hysteresis loops, characteristic of mesoporous materials [28]. The mesoporous structure plays a pivotal role in enhancing pollutant diffusion kinetics, which is critical for both efficient contaminant adsorption from aqueous solutions and improved accessibility to catalytic active sites [13]. The specific surface areas, pore volumes, and average pore diameters of all samples are summarized in Table 1. This enhancement could be attributed to two synergistic effects: (1) the incorporated AC effectively prevents the agglomeration and disordered stacking of carbon nitride, and (2) ammonium chloride acts as a gas bubble template during precursor preparation. These combined mechanisms have been found to promote the formation of a well-developed porous architecture in APCN, ultimately giving it a larger surface area [29]. Notably, MoS2/APCN exhibited a specific surface area (71.28 m2/g) comparable to that of APCN (70.34 m2/g), representing only a marginal increase of 0.94 m2/g, while its pore volume decreased slightly from 0.43 cm3/g to 0.40 cm3/g. This minimal textural alteration may be attributed to the formation of macroporous structures during ultrasonic compositing, wherein fragmented MoS2 becomes embedded within macropores. Since standard BET analysis primarily characterizes mesopores, these macroporous regions containing MoS2 fragments were not fully investigated, resulting in a negligible net change in the detectable surface area despite MoS2 incorporation.

Table 1.
BET specific surface area value (SBET), pore volume and average pore width of PCN, APCN and MoS2/APCN.
To further elucidate the surface chemical properties of MoS2/APCN, X-ray photoelectron spectroscopy (XPS) analysis was performed to investigate its elemental composition, surface functional groups, and chemical states. The XPS survey spectrum (Figure 1e) exhibits characteristic peaks for C 1s, N 1s, Mo 3d, and S 2p, confirming the coexistence of C, N, Mo, and S within the MoS2/APCN composite. Deconvolution of the C 1s spectrum (Figure 1f) reveals two distinct peaks at 288.1 eV and 284.8 eV, attributed to sp2-hybridized carbon in aromatic rings (N=C–N) and C–C single bonds, respectively [30]. Similarly, the N 1s spectrum (Figure 1g) was deconvoluted into three peaks centered at 398.7 eV, 400.1 eV, and 401.2 eV, corresponding to sp2-hybridized nitrogen in aromatic C=N–C groups, tertiary nitrogen (N–(C)3), and amino functional groups (C–N–H), respectively [31]. The analysis of the Mo 3d spectrum (Figure 1h) yielded six distinct peaks. The doublet at 229.4 eV (Mo 3d5/2) and 232.5 eV (Mo 3d3/2) is characteristic of 2H MoS2 [32,33]. Importantly, an additional doublet observed at lower binding energies of 228.5 eV (Mo 3d5/2) and 231.6 eV (Mo 3d3/2), exhibiting a negative shift relative to 2H MoS2, provides definitive evidence for the coexistence of the metallic 1T MoS2 phase [34]. The peak at 235.5 eV is attributed to Mo6+ species, indicating an oxidized molybdenum state [35]. Furthermore, the peak at 226.0 eV corresponds to the S 2s orbital, consistent with metal sulfide materials [34]. Deconvolution of the S 2p spectrum (Figure 1i) shows four components. The doublet at 162.1 eV (S 2p3/2) and 163.3 eV (S 2p1/2) is characteristic of 2H MoS2 [32]. Concurrently, a doublet observed at 161.3 eV (S 2p3/2) and 162.4 eV (S 2p1/2), exhibiting a negative binding-energy shift relative to 2H MoS2, provides compelling evidence for the presence of the metallic 1T MoS2 phase again [34]. These results provide unambiguous evidence of the successful formation of the MoS2/APCN composite, as demonstrated through the comprehensive characterization of its elemental composition and chemical states.
The morphology of the composite catalyst was analyzed using scanning electron microscopy (SEM). As depicted in Figure 2a, PCN exhibits a characteristic layered-stacking lamellar structure, attributed to van der Waals forces [36]. Furthermore, the SEM images reveal a porous structure within the carbon nitride matrix, resulting from the templating effect of ammonium chloride during its synthesis, which promote pore formation [10]. Figure 2b shows that MoS2 formed well-defined flower-like microspheres, composed of numerous intercrossed and curled nanosheets [37]. The morphological characteristics of AC are displayed in Figure 2c, revealing a loosely packed porous structure. This distinctive morphology provides direct evidence for the high specific surface area characteristic of AC. Following the modification of AC into the thermal polymerization precursor, the resulting APCN (Figure 2d) exhibited a thin lamellar structure. This structural change is related to the introduction of AC into the thermal polymerization system, which reduces the disordered stacking of carbon nitride [38]. For the MoS2/APCN composite (Figure 2e), the characteristic layered structure of carbon nitride remains clearly discernible. Compared to APCN, the MoS2/APCN composite exhibits significantly more macroporous structures. Additionally, the SEM images reveal that the MoS2 within the composite displays a fragmented flower-like morphology. This fragmentation likely stems from the disruption of intact flower-like MoS2 spheres during the ultrasonication process [39]. This fractured MoS2 is dispersed both on the surface of the layered APCN structure and within the macroporous structure. This structural configuration may account for the similar specific surface areas observed between MoS2/APCN and APCN in the BET measurements. TEM imaging (Figure 2f) further confirms the dispersion of MoS2 on the APCN surface, consistent with the SEM observations. Furthermore, characteristic lattice distances of 0.62 nm and 0.27 nm were clearly resolved in Figure 2g, corresponding to the (002) plane of 2H MoS2 and the (101) plane of 1T MoS2, respectively [40,41]. Moreover, distinct boundaries observed at the interfaces between MoS2, carbon nitride, and AC suggest the formation of close contact among these components [42]. EDS analysis results (Figure 2h–k) confirmed the uniform distribution of C, N, Mo, and S elements in the MoS2/APCN composite catalyst, providing a direct evidence of element distribution for the successful synthesis of this hybrid material [37].
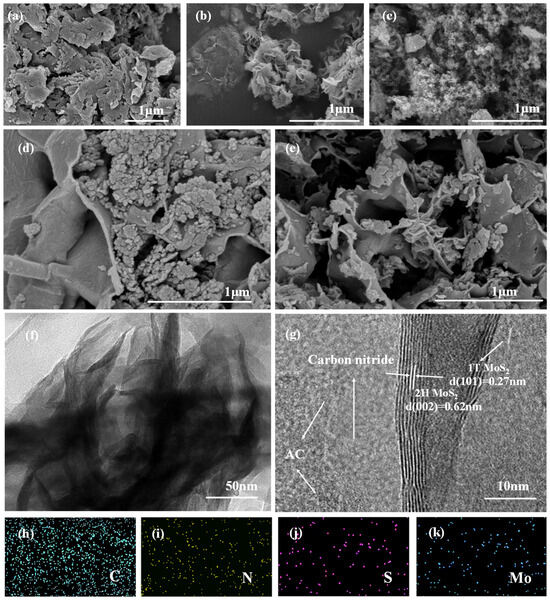
Figure 2.
SEM images of (a) PCN, (b) MoS2, (c) AC, (d) APCN, and (e) MoS2/APCN; (f) TEM image of MoS2/APCN; (g) HRTEM image of MoS2/APCN; (h–k) EDS spectra of MoS2/APCN.
2.2. Photodegradation Studies of Catalysts
To investigate the influence of AC incorporation on the photocatalytic performance of carbon nitride, we conducted degradation experiments using RhB as a model pollutant. The photocatalytic activity was evaluated by monitoring the changes in absorbance in the RhB solution under Xe lamp irradiation (Figure 3a). Prior to illumination, adsorption equilibria were established during a standardized 30 min dark period (Figure 3b), with the following capacities: MoS2/APCN > APCN > MoS2/PCN > PCN. The enhanced adsorption of AC-containing composites (APCN, MoS2/APCN) is attributed to their increased specific surface areas. All photocatalytic evaluations were performed at pH = 7.0 to simulate natural aquatic conditions. As shown in Figure 3c, negligible RhB degradation occurred without a photocatalyst. After 40 min of illumination, PCN and APCN achieved degradation efficiencies of 36.1% and 84.6%, respectively, confirming the significant enhancement resulting from AC incorporation. The enhanced photocatalytic performance of APCN originates from AC effectively suppressing carbon nitride layer stacking during thermal polycondensation, which generates a porous architecture with increased specific surface area, thereby augmenting the density of photocatalytically active sites [38].
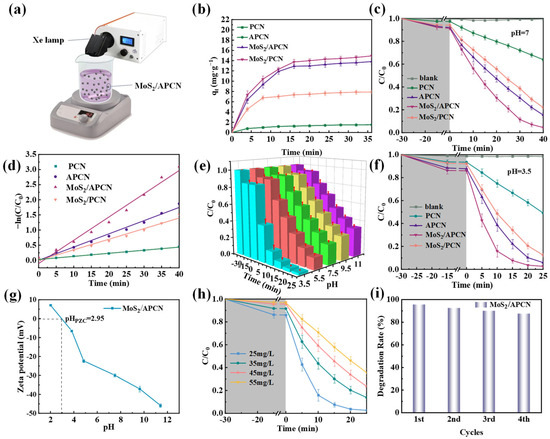
Figure 3.
(a) The photocatalytic degradation device; (b) The change in RhB adsorption capacity with increasing contact time; (c) Degradation curves of RhB for PCN, APCN, MoS2/APCN, and MoS2/PCN at pH = 7; (d) Pseudo-first-order kinetics of RhB at pH = 7; (e) Degradation curves of RhB for MoS2/APCN at different initial concentrations; (f) Degradation curves of RhB for MoS2/APCN at different pH values; (g) Zeta potential diagram of MoS2/APCN; (h) The photocatalytic degradation performance of MoS2/APCN on RhB under different initial concentrations; (i) Recycle experiment on MoS2/APCN at pH = 7.
The MoS2/APCN composite exhibited superior photocatalytic performance relative to APCN, achieving 95.5% RhB degradation within 40 min versus 84.6% for APCN. This enhancement confirms that the MoS2/APCN heterojunction accelerates photogenerated charge carrier separation, thereby improving degradation efficiency [43]. Furthermore, MoS2/APCN significantly outperformed MoS2/PCN (95.5% vs. 78.0%) degradation. This performance differential unequivocally establishes the critical function of AC in facilitating charge separation and transfer during photocatalysis.
In order to identify the optimum mass ratio of MoS2 to APCN, we performed a series of photocatalytic degradation experiments using different catalyst loadings, specifically with MoS2-to-APCN mass ratios ranging from 1:100 to 20:100. The corresponding results are displayed in Figure S1. When the mass ratios of MoS2 to APCN were 1:100, 2.5:100, 5:100, 10:100, and 20:100, the degradation rates of RhB within 40 min reached 90.8%, 95.5%, 92.7%, 88.8%, and 85.3%, respectively. Therefore, the composite catalyst with a mass ratio of 2.5:100 exhibited the highest photocatalytic performance. This can be attributed to the following reasons: When the MoS2 content is too low, its ability to suppress the recombination of photogenerated electron-hole pairs is limited, thereby reducing the photocatalytic degradation efficiency of RhB [27]. However, when MoS2 is present in excessive amounts, its inherent drawback of rapid recombination of photogenerated electron-hole pairs—which leads to poor photocatalytic efficiency—becomes predominant [44]. The surplus MoS2 forms a continuous overlayer on the APCN support, which significantly attenuates light absorption by the composite material. Moreover, excessive MoS2 may cover the surface of PCN, blocking active photocatalytic sites and diminishing light absorption by PCN, ultimately leading to a decrease in the overall photocatalytic degradation rate [22].
To quantitatively analyze the photocatalytic performances of the catalysts, the degradation kinetics were systematically evaluated using a pseudo-first-order reaction model. This approach provides fundamental insights into the reaction rates and mechanistic differences between catalyst systems. The kinetic behavior can be mathematically described as follows:
where C0 (mg/L) represents the initial concentration of RhB solution at t = 0, C (mg/L) denotes the residual concentration at reaction time t (min), and k (min−1) is the apparent pseudo-first-order rate constant. The magnitude of k directly reflects the reaction kinetics, with higher values indicating superior photocatalytic efficiency. The kinetic analysis revealed the following rate constants: MoS2/APCN (0.078 min−1) > APCN (0.044 min−1) > MoS2/PCN (0.035 min−1) > PCN (0.010 min−1) (Figure 3d) (Table 2). The MoS2/APCN composite exhibited 7.8-fold, 1.77-fold, and 2.23-fold enhancements relative to PCN, APCN, and MoS2/PCN, respectively. These kinetic results align precisely with the degradation profiles in Figure 3c, confirming that the MoS2/APCN heterostructure achieves optimal photocatalytic activity through superior charge separation and transfer efficiency.
–ln(C/C0) = kt

Table 2.
Pseudo-first-order kinetic parameters for RhB degradation by various catalysts at pH = 7.
To systematically investigate the pH-dependent photocatalytic performance of MoS2/APCN, RhB degradation experiments were conducted across pH levels of 3.5–11. As shown in Figure 3e, the composite exhibited broad pH tolerance with efficient degradation throughout this range. The photocatalytic degradation of RhB proceeded most rapidly at pH = 3.5, achieving degradation efficiency of 97.4% within 25 min. However, as the pH increased to 5.5, 7.5, 9.5, and 11, the degradation efficiencies within the same period significantly decreased to 86.2%, 75.7%, 71.4%, and 66.6%, respectively, indicating that acidic conditions are more conducive to the degradation of RhB. Under optimal acidic conditions (pH = 3.5) (Figure 3f), the RhB photocatalytic degradation performance of the catalysts was as follows: MoS2/APCN > APCN > MoS2/PCN > PCN. MoS2/APCN achieved 97.4% RhB removal within 25 min, substantially outperforming the other catalysts. Notably, this identical activity ranking observed under acidic conditions remained fully consistent with the degradation trend at neutral pH (Figure 3c), demonstrating that acidic conditions do not alter the relative photocatalytic performance hierarchy across the catalyst series.
A zeta potential test was performed on MoS2/APCN to determine the surface charge of the material. As shown in Figure 3g, the isoelectric point of MoS2/APCN is 2.95. Therefore, the surface of MoS2/APCN is negatively charged at pH > 2.95, and its surface is positively charged at pH < 2.95. Previous studies have demonstrated that RhB has a pKa value of 3.7 [45]. At pH = 3.5, the negatively charged MoS2/APCN surface and positively charged RhB molecules enable electrostatic attraction, optimizing adsorption. Conversely, at higher pH (pH = 5.5, 7.5, 9.5, and 11), both components exhibit negative surface charges. With increasing pH, the enhanced negative charge density strengthens electrostatic repulsion, progressively weakening RhB adsorption and consequently diminishing photocatalytic efficiency. Additionally, acidic conditions facilitate RhB degradation through two mechanisms, (1) preferential demethylation and hydroxylation pathways [46] and (2) synergistic interactions between the “–N=” groups in g–C3N4’s conjugated network and “–COOH” groups in RhB, promoting degradation by active species [47]. These collective mechanisms explain the optimal photocatalytic performance of MoS2/APCN at pH = 3.5.
Degradation experiments were conducted at pH = 3.5 across initial RhB concentrations of 25 mg/L, 35 mg/L, 45 mg/L, and 55 mg/L to systematically evaluate the concentration-dependent photocatalytic performance of MoS2/APCN (Figure 3h). The composite exhibited optimal efficiency at 25 mg/L, achieving 97.4% degradation within 25 min. Photocatalytic efficiency progressively declined with increasing RhB concentration, reaching 64.5% at 55 mg/L after equivalent irradiation. This inverse relationship primarily stems from the competitive occupation of active sites by excessive RhB molecules, which inhibits active species formation and consequently restricts electron-hole pair generation. In addition, an increase in the optical density of the solution attenuates incident light penetration, reducing photon utilization efficiency. These synergistic factors collectively diminish photocatalytic performance at elevated pollutant concentrations [48].
The recyclability of photocatalysts is critical for practical wastewater treatment applications. To evaluate the degradation stability of MoS2/APCN in actual water bodies, cycling experiments were conducted through four consecutive degradation cycles at a neutral pH (pH = 7.0) under identical operational parameters. As shown in Figure 3i, MoS2/APCN maintained high photocatalytic activity throughout the testing, achieving 95.5% RhB removal in the initial cycle and retaining 87.3% efficiency after four cycles, thus demonstrating excellent reusability. The slight performance decrease is likely attributable to the partial occupation of active sites by residual RhB degradation intermediates from previous cycles, which may moderately inhibit subsequent photocatalytic reactions [49]. These results confirm that MoS2/APCN maintains significant operational stability under neutral aqueous conditions, demonstrating strong potential for practical implementation in wastewater remediation. Furthermore, the photocatalytic performance of MoS2/APCN for the degradation of RhB was compared with that of other activated carbon-composite photocatalytic semiconductors reported in the literature, as presented in Table S1 [16,50,51,52,53,54]. The results indicate that the as-prepared catalyst exhibits excellent and comparable photocatalytic activity for RhB degradation.
Table S2 provides a comprehensive comparison of the sustainability of the MoS2/APCN composite photocatalyst with previously reported photocatalysts [55,56,57,58,59,60]. The evaluation was conducted based on four key aspects: the environmental friendliness of the precursor materials, the economic feasibility of the catalyst, its photocatalytic performance toward RhB, and its cycling stability. AC in MoS2/APCN is derived from fir wood, a renewable resource. Moreover, the synthesis process of this composite material uses or generates relatively fewer toxic and harmful substances, with moderate overall cost. Furthermore, MoS2/APCN exhibits exceptional photocatalytic efficiency and outstanding recyclability. These findings demonstrate that the MoS2/APCN composite is not only highly efficient and stable but also offers a sustainable pathway for the valorization of agricultural and forestry waste in catalytic applications.
2.3. Photocatalytic Mechanism Research
UV–vis DRS spectroscopy was employed to characterize the optical properties and electronic structures of 2H MoS2, PCN, MoS2/APCN, APCN, and MoS2/PCN (Figure 4a). PCN displayed an absorption edge at 478 nm, while AC incorporation induced a distinct red-shift to 501 nm in APCN. AC significantly extended the visible-light-harvesting capabilities of the carbon nitride matrix. MoS2/PCN exhibited a distinct absorption edge at 488 nm, while that of MoS2/APCN is red-shifted to 511 nm. The 23 nm difference between MoS2/APCN and MoS2/PCN further demonstrated the critical role of AC in enhancing the visible light absorption capacity of composite catalysts. Notably, the 10 nm red-shift in MoS2/APCN relative to APCN (511 nm vs. 501 nm) originated from MoS2’s strong light-harvesting properties [61].
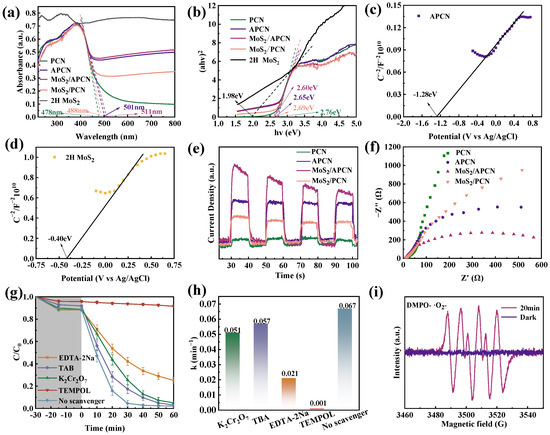
Figure 4.
(a) DRS spectra of PCN, APCN, MoS2/APCN, and MoS2/PCN; (b) Tauc spectra of PCN, APCN, MoS2/APCN, and MoS2/PCN; (c) Mott–Schottky curves of APCN; (d) Mott–Schottky curves of 2H MoS2; (e) transient photocurrent and (f) impedance of PCN, APCN, MoS2/APCN, and MoS2/PCN; (g) free-radical-trapping experiments and (h) pseudo-first-order kinetics of RhB; (i) ESR spectrum of DMPO–·O2− with MoS2/APCN.
The band gap energy of the prepared photocatalysts can be estimated via the Kubelka–Munk equation:
where α, h, ν, A, and Eg represent the absorption coefficient, Planck’s constant, the light frequency, a proportionality constant, and the band gap energy, respectively [48,62]. As shown in Figure 4b, Tauc plots of (αhν)2 versus photon energy (hν) revealed band gap energies of 2.76 eV (PCN), 2.65 eV (APCN), 2.69 eV (MoS2/PCN), and 2.60 eV (MoS2/APCN), demonstrating that both AC incorporation and MoS2 hybridization effectively modulated the electronic structures of the carbon nitride-based material. The narrower band gap suggests stronger visible-light responsiveness, which may bring about more e−-h+ pairs, thereby enhancing photocatalytic efficiency [63,64].
αhν = A (hν − Eg)n
Mott–Schottky (MS) measurements were performed to determine the conduction band (CB) positions of APCN and MoS2 (Figure 4c,d). Given the coexistence of metallic 1T and semiconducting 2H phases in MoS2, the analysis was specifically conducted on the 2H MoS2 component [65]. Both APCN and 2H MoS2 exhibited positive MS plot slopes, confirming their n-type semiconductor characteristics [66]. According to the established methodology, the flat-band potential (E fb) of n-type semiconductors approximates the CB position [48,67]. A tangent line was established relative to the MS curves of APCN and 2H MoS2, and the values of the tangent points relative to the horizontal coordinates were −1.28 eV (vs. Ag/AgCl) and −0.40 eV (vs. Ag/AgCl), respectively, which could be considered the Efb values of APCN and 2H MoS2. These potentials were converted to the Normal Hydrogen Electrode (NHE) scale using the following equation:
E(NHE) = E (Ag/AgCl) + 0.197
Thus, the CB positions were determined to be −1.08 eV for APCN and −0.20 eV for 2H MoS2 (vs. NHE). Based on the CB positions, the valence band (VB) positions were calculated using the following equation:
E(VB) = Eg + E(CB)
UV−vis DRS data yielded Eg = 1.98 eV for 2H MoS2 and 2.65 eV for APCN (Figure 4b). Consequently, the VB potentials were determined to be 1.78 eV (vs. NHE) for 2H MoS2 and 1.57 eV (vs. NHE) for APCN.
To assess the charge carrier migration capabilities of PCN, APCN, MoS2/APCN, and MoS2/PCN, transient photocurrent response measurements were performed under periodic illumination. As depicted in Figure 4e, APCN exhibited enhanced photocurrent density compared to PCN. This enhancement clearly demonstrates that AC modification significantly promotes charge separation and transport efficiency within the carbon nitride matrix. Furthermore, the photocurrent response of MoS2/APCN surpassed that of APCN, indicating that the formation of a composite heterostructure between MoS2 and APCN facilitates the separation of photogenerated electron-hole pairs. Notably, MoS2/APCN demonstrated a substantially stronger photocurrent response than MoS2/PCN. This pronounced improvement unambiguously confirms the pivotal role of AC in enhancing the overall electrical conductivity of the composite system and effectively suppressing charge recombination through the establishment of efficient electron transport pathways [68]. Collectively, these results indicate that the heterostructure formed between MoS2 and APCN enhances photocatalytic degradation performance, with the presence of AC playing a pivotal role in facilitating this enhancement. The interfacial charge transfer properties of the photocatalysts were further investigated using electrochemical impedance spectroscopy (EIS). The smaller semicircle radius in the Nyquist plot corresponds to lower charge transfer resistance. Figure 4f reveals the following order of charge transfer resistance: MoS2/APCN < APCN < MoS2/PCN < PCN. This trend is attributed to the enhanced conductivity facilitated by AC within both APCN and MoS2/APCN, coupled with improved interfacial charge transfer capability in the MoS2/APCN heterostructure. These results are highly consistent with the trends observed in both the aforementioned transient photocurrent response measurements and the RhB degradation experiments.
Radical-trapping experiments were conducted to identify the dominant active species responsible for RhB degradation. Specifically, TEMPOL, TBA, K2Cr2O7, and EDTA–2Na were individually introduced into the photocatalytic system. The irradiation conditions remained consistent with those of previous experiments. As illustrated in Figure 4g, the photocatalytic degradation of RhB was most significantly inhibited in the presence of TEMPOL, achieving only 9.3% degradation after 60 min. The addition of EDTA–2Na also substantially suppressed photocatalytic activity, indicating that h+ played a significant role in RhB degradation. In contrast, the presence of TBA and K2Cr2O7 exhibited minimal inhibitory effects, with their degradation efficiencies remaining as high as 96.9% and 95.0% after 60 min, respectively. These results clearly demonstrate that neither ·OH nor e− acts as a primary active species in the degradation process. Pseudo-first-order kinetic fitting of the radical-trapping degradation curves yielded rate constants (k) of 0.051 min−1 (K2Cr2O7), 0.057 min−1 (TBA), 0.021 min−1 (EDTA–2Na), and 0.001 min−1 (TEMPOL). Collectively, these findings confirm that·O2− and h+ are the primary active species responsible for RhB degradation (Figure 4h).
In order to further elucidate the photocatalytic mechanism of the MoS2/APCN system, electron spin resonance (ESR) spectroscopy was employed to identify reactive oxygen species generated during the degradation process. Using 5,5–dimethyl–1–pyrroline N–oxide (DMPO) as a spin-trapping agent for superoxide radicals (·O2−), characteristic DMPO–·O2− adduct signals were monitored under specific conditions. As shown in Figure 4i, no characteristic DMPO–·O2– signals were detected in the dark. However, upon irradiation with a Xe lamp for 20 min, intense characteristic signals emerged, unambiguously confirming the photogeneration of ·O2− species. This result demonstrates that ·O2− serves as a critical active species in the photocatalytic degradation process, which is consistent with the radical quenching experiments, where TEMPOL significantly inhibited RhB degradation.
Based on comprehensive evidence from the radical-trapping experiments, UV–vis DRS analysis, and Mott–Schottky measurements, we propose a direct Z–scheme charge transfer mechanism for the MoS2/APCN photocatalytic system (Figure 5). In the APCN composite, AC acts as an efficient electron transfer mediator due to its superior electrical conductivity, facilitating electron migration from the CB of carbon nitride to the AC matrix. This process significantly enhances interfacial charge separation kinetics.
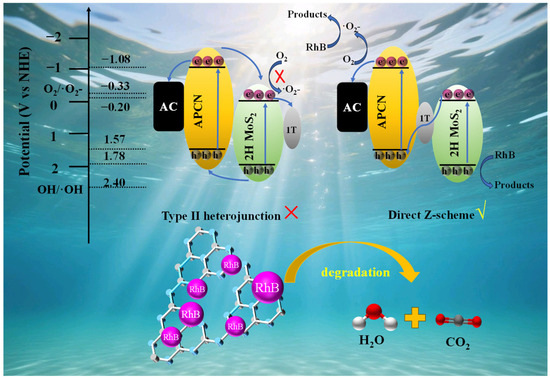
Figure 5.
The charge transfer pathways of MoS2/APCN under light irradiation and the possible mechanisms for photocatalytic degradation of RhB.
Upon illumination, electrons in both APCN and 2H MoS2 components are photoexcited from their VB to their CB. The band structures exhibit a staggered alignment, with the CB/VB potentials of APCN at −1.08 eV/+1.57 eV, and those of 2H MoS2 at −0.20 eV/+1.78 eV (vs. NHE). Although this alignment initially suggests a Type–II heterojunction pathway (where electrons transfer from APCN–CB to 2H MoS2–CB, while holes move from 2H MoS2–VB to APCN–VB), previous study reveals a critical inconsistency: the 2H MoS2 CB potential (−0.20 eV) is insufficiently negative to reduce O2 to ·O2− (E (O2/·O2−) = −0.33 eV) [66]. This contradicts the radical-trapping experiments, which conclusively identified ·O2− as the dominant reactive species.
Consequently, we propose a direct Z–scheme charge transfer mechanism wherein photoexcited electrons from the CB of 2H MoS2 migrate through the 1T MoS2 phase to recombine with holes in the VB of APCN. This recombination leads to the accumulation of highly reductive electrons in the APCN CB (−1.08 eV vs. NHE), which readily reduce adsorbed molecular oxygen (E (O2/·O2−) = −0.33 V) to generate superoxide radicals (·O2−). These radicals subsequently initiate RhB degradation through bond cleavage and ring-opening reactions, ultimately mineralizing organic pollutants to CO2 and H2O. Concurrently, oxidative holes accumulate in the VB of 2H MoS2 (+1.78 eV). Nevertheless, due to their insufficient oxidative potential relative to the OH−/·OH redox couple (E (·OH/OH−) = +2.40 eV), these holes cannot oxidize H2O/OH− to hydroxyl radicals. Instead, they directly oxidize RhB molecules via non-radical pathways, forming biodegradable intermediates that undergo complete mineralization [48]. Throughout the charge migration process, AC significantly facilitates charge transfer kinetics due to its superior electrical conductivity, thereby substantially enhancing the photocatalytic performance of the catalyst toward RhB degradation.
3. Materials and Methods
3.1. Materials
Fir sawdust was purchased from Alibaba Network Technology Co., Ltd. (Hangzhou, China); phosphoric acid (H3PO4), thiourea (CS(NH2)2), and ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China); dicyandiamide (C2H4N4), tert-butanol (TBA), potassium dichromate (K2Cr2O7), disodium ethylenediaminetetraacetate (EDTA-2Na), 4-hydroxypiperidine pure oxygen radical (TEMPOL), 5,5-Dimethyl-1-pyrroline N-oxide (DMPO), and Rhodamine B were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China); ammonium chloride and hydrochloric acid were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China); and ethanol was purchased from Wuxi Yasheng Chemical Co., Ltd. (Wuxi, China). All the reagents and solvents were of analytical purity.
3.2. Synthesis of AC
Fir-wood powder was thoroughly mixed with an 85 wt% H3PO4 solution at a mass ratio of 1:1.5 (wood/acid). The mixture was then soaked at 85 °C for 1 h to ensure complete impregnation. Subsequently, the impregnated product was transferred to a tube furnace and heated to 450 °C at a rate of 5 °C/min under an inert atmosphere, then held at that temperature for 1 h for activation. The resulting carbonized material was sequentially washed with dilute hydrochloric acid and deionized water until a neutral pH was achieved. After drying at 105 °C overnight, the sample was ground into a fine powder, yielding a black AC product.
3.3. Synthesis of APCN, MoS2, and MoS2/APCN
The synthetic routes for APCN, MoS2, and MoS2/APCN composites are graphically summarized in Figure 6. APCN was synthesized via calcination. Briefly, 3 g of dicyandiamide, 3 g of NH4Cl, and 0.18 g of AC were thoroughly ground in an agate mortar to ensure homogeneity. The mixture was then transferred to a covered 50 mL alumina crucible and calcined in a muffle furnace at 600 °C for 2 h (heating rate: 2.3 °C/min), followed by natural cooling to room temperature. The resulting product was ground into a fine gray powder.
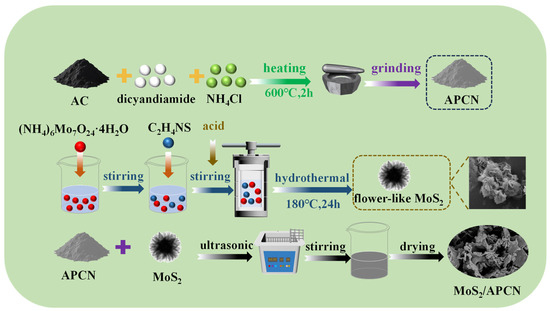
Figure 6.
A schematic diagram of the process of MoS2/APCN preparation.
(1T/2H) mixed-phase MoS2 was synthesized via a hydrothermal method. A homogeneous precursor solution was prepared by dissolving 0.58 g ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O) in 30 mL deionized water, followed by addition of 1.78 g thiourea (CH4N2S). After 50 min of vigorous stirring with concurrent pH adjustment to acidic conditions, the mixture was transferred into a Teflon-lined stainless steel autoclave and subjected to hydrothermal treatment at 180 °C for 24 h. The reactor was cooled naturally to room temperature post-reaction. The resulting precipitate was collected and washed repeatedly with deionized water and absolute ethanol to remove residual impurities. Finally, the purified product was dried overnight at 60 °C in a vacuum oven, yielding black MoS2 powder.
The MoS2/APCN composite was prepared via a solution-mixing method. Initially, 100 mg of APCN powder was uniformly mixed with a certain amount of MoS2 in 40 mL of ethanol. The mixture was ultrasonicated (40 kHz) at 40 °C for 4 h to achieve homogeneous dispersion. Subsequently, the suspension was subjected to extended magnetic stirring to ensure sufficient interfacial contact. The resulting suspension was washed three times with ethanol and deionized water (with supernatant decantation after each wash) to remove unreacted species or impurities. Finally, the collected powder was dried overnight in a muffle furnace to obtain the MoS2/APCN composite.
For comparison, porous graphitic carbon nitride (denoted as PCN) was synthesized using the same procedure as for APCN but without the addition of AC. Similarly, the MoS2/PCN composite was prepared following the same procedure as for MoS2/APCN, using PCN instead of APCN.
3.4. Material Characterization
The crystal structures of the samples were characterized by X-ray powder diffraction (XRD) using a Rigaku Ultima–IV diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation. Scans were performed over a 2θ range of 5° to 80° at a rate of 5° min−1. Fourier transform infrared (FTIR) spectra were acquired using a Thermo Fisher spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) in the range of 4000–400 cm−1 with a resolution of 4 cm−1. X-ray photoelectron spectroscopy (XPS) analysis was conducted using a JEOL AXIS UltraDLD spectrometer (JEOL Ltd., Tokyo, Japan) to determine the elemental composition and chemical states of the samples, and their binding energies were referenced to the C 1s peak at 284.8 eV for charge correction. Scanning electron microscopy (SEM) images were obtained using a JEOL JSM-7600F instrument (JEOL Ltd., Japan) to examine the surface morphology and microstructure. Transmission electron microscopy (TEM) was performed using a JEOL JEM-2100F UHR field emission microscope (JEOL Ltd., Japan) to investigate the lattice spacing of the samples and obtain a detailed description of their microscopic morphology. N2 adsorption–desorption isotherms were measured at 77 K using a Micromeritics ASAP 2460 analyzer (Micromeritics Instrument Corporation, Norcross, GA, USA) to determine the specific surface area and porosity (BET method). Prior to the analysis, the samples were degassed at 160 °C for 2 h under vacuum conditions. Electron paramagnetic resonance (ESR) spectra were recorded at room temperature using a Bruker E500-9.5/12 spectrometer (Bruker Corporation, Ettlingen, Germany) equipped with an X–band field scanning system. UV–vis diffuse reflectance spectra (UV–vis DRS) were obtained using a Lambda 950 UV–vis spectrophotometer (PerkinElmer Inc., Waltham, MA, USA).
3.5. Photocatalytic Measurements
Photocatalytic degradation experiments were conducted in a 100 mL beaker using a 300 W Xe lamp equipped with an AM 1.5 optical cut-off filter as the light source. For each test, 10 mg of the photocatalyst was dispersed in 50 mL of an aqueous Rhodamine B (RhB) solution (initial concentration: 25 mg/L). Prior to illumination, the suspension was magnetically stirred in the dark for 30 min to establish an adsorption–desorption equilibrium between RhB and the catalyst surface. During illumination, 1.5 mL aliquots of the reaction mixture were periodically extracted at 5 min intervals. These aliquots were centrifuged to separate the catalyst, and the supernatant absorbance was measured at 554 nm using a UV–6100 UV–vis spectrophotometer. The RhB concentration was determined based on the Beer–Lambert law. All photocatalytic degradation data were obtained from multiple independent replicates, and the variations between experiments are presented as error bars in the figures. To identify the predominant reactive species involved in the photocatalytic mechanism, scavenger experiments were performed. EDTA–2Na, TBA, K2Cr2O7, and TEMPOL were introduced as specific quenchers for holes (h+), hydroxyl radicals (·OH), electrons (e−), and superoxide radicals (·O2−), respectively.
3.6. Photoelectrochemical Characterization
Catalyst powder (10 mg) was ultrasonically dispersed in a 0.5% nafion solution for 1 h. The resulting slurry was then spin-coated onto a 1 × 1 cm2 indium tin oxide (ITO) film. Photocurrent response, electrochemical impedance spectroscopy (EIS), and Mott–Schottky (MS) plots were measured using a CHI660E electrochemical workstation (Shanghai Chenhua Co., Ltd., Shanghai, China). The coated film served as the working electrode, alongside an Ag/AgCl reference electrode and a Pt foil counter-electrode. The flat-band potentials (Efb) of APCN and 2H MoS2 were estimated from the Mott–Schottky analysis to determine their conduction band (CB) positions. All the measurements were performed in a 0.5 M Na2SO4 aqueous electrolyte under illumination using a 300 W Xe lamp.
4. Conclusions
In this study, a synergistic strategy was developed for synthesizing activated carbon-modified porous carbon nitride (APCN) by incorporating NH4Cl and fir-wood-derived AC into the calcination precursor dicyandiamide. The obtained material effectively suppressed the layer stacking of carbon nitride, increased the specific surface area, and exposed more photocatalytic active sites. Based on this optimized substrate, a MoS2/APCN composite catalyst was successfully constructed via an ultrasonic-assisted method. The introduced AC enhanced visible light absorption and facilitated charge migration. Furthermore, a direct Z-scheme heterojunction mechanism was proposed between MoS2 and APCN, which effectively suppressed charge recombination while maintaining high charge-transfer capability, thereby significantly enhancing the photocatalytic degradation efficiency of RhB (achieving 95.5% degradation within 40 min under simulated natural water conditions). The composite also exhibited broad pH tolerance, demonstrating outstanding degradation performance under acidic conditions (97.4% removal within 25 min at pH = 3.5). Moreover, MoS2/APCN displayed excellent cycling stability under conditions simulating real wastewater, highlighting its potential for practical applications. This work emphasizes the importance of precursor design and heterojunction engineering in photocatalysis, demonstrates the promising role of biomass-derived AC in this field, and provides valuable insights for future applications of activated carbon in photocatalytic systems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15090875/s1, Figure S1: The photocatalytic performance curves of RhB for MoS2/APCN with different mass ratios. Table S1: Performance comparison of the MoS2/APCN catalyst with other reported activated carbon-composite photocatalysts for RhB dye treatment. Table S2: Comparison of sustainability aspects between the present work and previous studies on photocatalytic degradation of RhB.
Author Contributions
Conceptualization, resources, and funding acquisition: Y.Y.; Writing (original draft) and methodology: K.L.; Writing (review and editing): D.W.; Data curation and Investigation: N.T. and Z.Z.; Validation: W.Z. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31901274) and the Agricultural Science and Technology Independent Innovation Foundation of Jiangsu Province, China (No. CX(24)3049).
Data Availability Statement
Data is available on request.
Acknowledgments
The Advanced Analysis and Testing Center of Nanjing Forestry University is acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.; Al-shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef]
- Guo, T.; Fan, X.; Jiang, X.; Qi, Y.; Du, J.; Zhang, A.; Wang, H. Engineering shape of BiOCl nanosheets with improved visible-light response for superior photocatalytic degradation of Rhodamine B. J. Alloys Compd. 2023, 948, 169586. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S. Engineered Graphitic Carbon Nitride-Based Photocatalysts for Visible-Light-Driven Water Splitting: A Review. Energy Fuels 2021, 35, 6504–6526. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Z.; Zou, Y.; Chen, J.; Shi, J.-W. The progress of g-C3N4 in photocatalytic H2 evolution: From fabrication to modification. Coord. Chem. Rev. 2024, 500, 215489. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Z.; Jing, B.; Ao, Z.; Shang, C.; Ling, L. Visible light-driven NH2Cl activation by g-C3N4 photocatalysis producing reactive nitrogen species to degrade bisphenol A. Water Res. 2023, 235, 119889. [Google Scholar] [CrossRef]
- Karthik, P.; Gowthaman, P.; Venkatachalam, M.; Saroja, M. Design and fabrication of g-C3N4 nanosheets decorated TiO2 hybrid sensor films for improved performance towards CO2 gas. Inorg. Chem. Commun. 2020, 119, 108060. [Google Scholar] [CrossRef]
- Arumugam, M.; Tahir, M.; Praserthdam, P. Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4. Chemosphere 2022, 286 Pt 2, 131765. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Guo, Z.; Kong, L.; Song, P. g-C3N4/MnFe2O4 p-n hollow stratified heterojunction to improve the photocatalytic CO2 reduction activity. Chem. Phys. Lett. 2023, 827, 140698. [Google Scholar] [CrossRef]
- Fei, B.; Tang, Y.; Wang, X.; Dong, X.; Liang, J.; Fei, X.; Xu, L.; Song, Y.; Zhang, F. One-pot synthesis of porous g-C3N4 nanomaterials with different morphologies and their superior photocatalytic performance. Mater. Res. Bull. 2018, 102, 209–217. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, J.; Zuo, S.; Lin, S.; Chen, G. Preparation, Modification, and Application of Biochar in the Printing Field: A Review. Materials 2023, 16, 5081. [Google Scholar] [CrossRef] [PubMed]
- Gloria, D.C.S.; Brito, C.H.V.; Mendonça, T.A.P.; Brazil, T.R.; Domingues, R.A.; Vieira, N.C.S.; Santos, E.B.; Gonçalves, M. Preparation of TiO2/activated carbon nanomaterials with enhanced photocatalytic activity in paracetamol degradation. Mater. Chem. Phys. 2023, 305, 127947. [Google Scholar] [CrossRef]
- Rungsawang, T.; Krobthong, S.; Paengpan, K.; Kaewtrakulchai, N.; Manatura, K.; Eiad-Ua, A.; Boonruang, C.; Wongrerkdee, S. Synergy of functionalized activated carbon and ZnO nanoparticles for enhancing photocatalytic degradation of methylene blue and carbaryl. Radiat. Phys. Chem. 2024, 223, 111924. [Google Scholar] [CrossRef]
- Lu, S.; Liu, F.; Qiu, P.; Qiao, M.; Li, Y.; Cheng, Z.; Xue, N.; Hou, X.; Xu, C.; Xiang, Y.; et al. Photothermal-assisted photocatalytic degradation with ultrahigh solar utilization: Towards practical application. Chem. Eng. J. 2020, 379, 122382. [Google Scholar] [CrossRef]
- Shi, J.; Chen, T.; Guo, C.; Liu, Z.; Feng, S.; Li, Y.; Hu, J. The bifunctional composites of AC restrain the stack of g-C3N4 with the excellent adsorption-photocatalytic performance for the removal of RhB. Colloids Surf. A 2019, 580, 123701. [Google Scholar] [CrossRef]
- Sekar, S.; Preethi, V.; Srivishnu, V.S.; Saravanan, S.; Lee, S. Highly-efficient photocatalytic activity of TiO2-AC nanocomposites for hydrogen production from sulphide wastewater. Int. J. Hydrogen Energy 2022, 47, 40275–40285. [Google Scholar] [CrossRef]
- Hua, X.Y.; Chen, H.J.; Rong, C.; Addison, F.; Dong, D.M.; Qu, J.; Liang, D.P.; Guo, Z.Y.; Zheng, N.; Liu, H.Y. Visible-light-driven photocatalytic degradation of tetracycline hydrochloride by Z-scheme Ag3PO4/1T@2H-MoS2 heterojunction: Degradation mechanism toxicity assessment, and potential applications. J. Hazard. Mater. 2023, 448, 130951. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Song, Y.; Guo, S.; Zhang, Y.; Wang, B.; Wang, Y.; Li, X. One-step synthesis of P-doped MoS2 for efficient photocatalytic hydrogen production. J. Alloys Compd. 2020, 829, 154635. [Google Scholar] [CrossRef]
- Senthilnathan, S.; Ganesh Kumar, K.; Sugunraj, S.; Dhanalakshmi, M.A.; M, R.; Suganthi, M.; Shree Kesavan, K.; Sasikumar, P.; Abbas, M.; Dator, W.L.T. MoS2 modified g-C3N4 composite: A potential candidate for photocatalytic applications. J. Saudi Chem. Soc. 2023, 27, 101717. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Cleaner Prod. 2020, 276, 124319. [Google Scholar] [CrossRef]
- Jiao, Y.; Huang, Q.; Wang, J.; He, Z.; Li, Z. A novel MoS2 quantum dots (QDs) decorated Z-scheme g-C3N4 nanosheet/N-doped carbon dots heterostructure photocatalyst for photocatalytic hydrogen evolution. Appl. Catal. B 2019, 247, 124–132. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhu, X.; Liu, X.; Li, H. 1T and 2H mixed phase MoS2 nanobelts coupled with Ti3+ self-doped TiO2 nanosheets for enhanced photocatalytic degradation of RhB under visible light. Appl. Surf. Sci. 2021, 556, 149768. [Google Scholar] [CrossRef]
- Huang, J.; Meng, T.; Yi, F.; Cui, Z.; Shu, D.; Gao, A. NH4+ intercalated 1T/2H hybrid MoS2@AlOOH for long-term aqueous ammonium-ion storage. J. Solid State Chem. 2024, 338, 124837. [Google Scholar] [CrossRef]
- Yang, H.; Cao, R.; Sun, P.; Yin, J.; Zhang, S.; Xu, X. Constructing electrostatic self-assembled 2D/2D ultra-thin ZnIn2S4/protonated g-C3N4 heterojunctions for excellent photocatalytic performance under visible light. Appl. Catal. B 2019, 256, 117862. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, C.; An, Q.; Yu, J.; Xu, S.; Li, L. The preparation of Al2O3/g-C3N4 composites in aluminum–water self-assembly system and its improved photocatalytic properties. New J. Chem. 2021, 45, 16750–16759. [Google Scholar] [CrossRef]
- Zhu, K.; Luan, X.; Matras-Postolek, K.; Yang, P. 2D/2D MoS2/g-C3N4 layered heterojunctions with enhanced interfacial electron coupling effect. J. Electroanal. Chem. 2021, 893, 115350. [Google Scholar] [CrossRef]
- Buttersack, C. Modeling of type IV and V sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. 2019, 21, 5614–5626. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wen, H.; Guo, K.; Brendon, H.; Liu, D. A green, efficient reductive N-formylation of nitro compounds catalyzed by metal-free graphitic carbon nitride supported on activated carbon. Appl. Catal. B 2023, 321, 122042. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, X.; Shu, S.; Zhang, R.; Chen, J.; Wang, Y. Constructing a Z-scheme β-Bi2O3/g-C3N4 heterojunction with enhancing photocatalytic activity for sulfamethoxypyridazine degradation. Mater. Sci. Semicond. Process. 2025, 189, 109273. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Shao, L.; Du, J.; Gao, Q.; Fu, S.; Li, L.; Hu, M.; Zhao, F.; Zhou, J. Promoting charge separation in dual defect mediated Z-scheme MoS2/g-C3N4 photocatalysts for enhanced photocatalytic degradation activity: Synergistic effect insight. Colloids Surf. A 2020, 594, 124668. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Guo, Y.; Zhang, G.; Cui, H.; Tian, J. Rationalizing and controlling the phase transformation of semi-metallic 1T′-phase and semi-conductive 2H-phase MoS2 as cocatalysts for photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 396, 125344. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, R.; Chang, F.; Liu, M.; Deng, J.; Hu, J.; He, J.; Feng, D.; Gao, J.; Chen, C.; et al. Ultrathin 1T/2H mixed phase MoS2 decorated TiO2 nanorod arrays for effective photocatalytic hydrogen evolution. CrystEngComm 2021, 23, 3710–3716. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Zhang, X.; Gong, X.; Chen, C.; Nie, G.; Liu, X.; Liu, P. MoS2-Decorated/Integrated Carbon Fiber: Phase Engineering Well-Regulated Microwave Absorber. Nanomicro Lett. 2021, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, B.; Liu, M.; Liu, S.; Chen, W.; Gao, L.; Li, X. In situ growth of vertically aligned ultrathin MoS2 on porous g-C3N4 for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2021, 554, 149617. [Google Scholar] [CrossRef]
- Wen, M.Q.; Xiong, T.; Zang, Z.G.; Wei, W.; Tang, X.S.; Dong, F. Synthesis of MoS2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO). Opt. Express 2016, 24, 10205–10212. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Ren, J.; Wu, J.; Wang, G.; Chen, L. Photocatalytic H2 production with simultaneous wastewater purification over flower-like 1T/2H-MoS2-decorated CNT/CNU isotype heterojunction photocatalyst. Appl. Surf. Sci. 2021, 569, 151072. [Google Scholar] [CrossRef]
- Li, S.; Niu, Z.; Pan, D.; Cui, Z.; Shang, H.; Lian, J.; Wu, W. Efficient photoreduction strategy for uranium immobilization based on graphite carbon nitride/activated carbon nanocomposites. Chin. Chem. Lett. 2022, 33, 3581–3584. [Google Scholar] [CrossRef]
- Qin, H.; Guo, R.T.; Liu, X.Y.; Pan, W.G.; Wang, Z.Y.; Shi, X.; Tang, J.Y.; Huang, C.Y. Z-Scheme MoS2/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Dalton Trans. 2018, 47, 15155–15163. [Google Scholar] [CrossRef]
- Mehtab, A.; Ali, S.A.; Ingole, P.P.; Mao, Y.; Alshehri, S.M.; Ahmad, T. MoS2 Nanoflower-Deposited g-C3N4 Nanosheet 2D/2D Heterojunction for Efficient Photo/Electrocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2023, 6, 12003–12012. [Google Scholar] [CrossRef]
- Zhu, C.; Xian, Q.; He, Q.; Chen, C.; Zou, W.; Sun, C.; Wang, S.; Duan, X. Edge-Rich Bicrystalline 1T/2H-MoS2 Cocatalyst-Decorated 110 Terminated CeO2 Nanorods for Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 35818–35827. [Google Scholar] [CrossRef]
- Han, X.; Liu, Q.; Qian, A.; Ye, L.; Pu, X.; Liu, J.; Jia, X.; Wang, R.; Ju, F.; Sun, H.; et al. Transition-Metal Single Atom Anchored on MoS2 for Enhancing Photocatalytic Hydrogen Production of g-C3N4 Photocatalysts. ACS Appl. Mater. Interfaces 2023, 15, 26670–26681. [Google Scholar] [CrossRef]
- Azhar, A.; Basit, M.A.; Mehmood, W.; Ali, M.A.; Zahid, S.; Ahmad, M.; Zaidi, S.J.A.; Park, T.J. Synchronized wet-chemical development of 2-dimensional MoS2 and g-C3N4/MoS2 QDs nanocomposite as efficient photocatalysts for detoxification of aqueous dye solutions. Colloids Surf. A 2023, 657, 130581. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Li, Z.S.; Lin, Z.D.; Pan, W.G. Recent advances and perspectives of MoS2 based materials for photocatalytic dyes degradation: A review. Colloids Surf. A 2021, 611, 125836. [Google Scholar] [CrossRef]
- Yue, Y.; Hou, K.; Chen, J.; Cheng, W.; Wu, Q.; Han, J.; Jiang, J. Ag/AgBr/AgVO3 Photocatalyst-Embedded Polyacrylonitrile/Polyamide/Chitosan Nanofiltration Membrane for Integrated Filtration and Degradation of RhB. ACS Appl. Mater. Interfaces 2022, 14, 24708–24719. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fang, W.X.; Wang, J.C.; Qiao, X.; Wang, B.; Guo, X. pH-controlled mechanism of photocatalytic RhB degradation over g-C3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ćirković, J.; Radojković, A.; Luković Golić, D.; Tasić, N.; Čizmić, M.; Branković, G.; Branković, Z. Visible-light photocatalytic degradation of Mordant Blue 9 by single-phase BiFeO3 nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104587. [Google Scholar] [CrossRef]
- Pan, H.; Gu, J.; Hou, K.; Li, J.; Wang, Y.; Yue, Y. High-efficiency, compressible, and recyclable reduced graphene oxide/chitosan composite aerogels supported g-C3N4/BiOBr photocatalyst for adsorption and degradation of rhodamine B. J. Environ. Chem. Eng. 2022, 10, 107157. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yue, Y. A Transparent, High-Strength, and Recyclable Core–Shell Structured Wood Hydrogel Integrated with Carbon Dots for Photodegradation of Rhodamine B. ACS Appl. Nano Mater. 2023, 6, 2894–2907. [Google Scholar] [CrossRef]
- Doumbia, M.; Guenfoud, F.; Boudriche, L.; Giannakis, S. UVA-assisted photocatalytic removal of rhodamine B using green-synthesized activated carbon derived from date pits and impregnated with Ag/Ag3PO4. J. Coord. Chem. 2024, 77, 2791–2817. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Doan, V.; Nguyen, T.L.H.; Nguyen, A.T.; Tran, Q.H.; Tran, V.A.; Le, V.T. Mechanistic pathway and optimization of rhodamine B degradation using Mn3O4/ZnO nanocomposite on microalgae-based carbon. RSC Adv. 2025, 15, 6241–6259. [Google Scholar] [CrossRef]
- Bhaysar, K.S.; Labhane, P.K.; Dhake, R.B.; Sonawane, G.H. Crystal structures, morphological, optical, adsorption, kinetic and photocatalytic degradation studies of activated carbon loaded BiOBr nanoplates prepared by solvothermal method. Inorg. Chem. Commun. 2019, 104, 134–144. [Google Scholar] [CrossRef]
- Sumi, K.; Supraja, K.S.; Aashika, R.C.; Arivanandhan, M. Biomass-derived activated carbon/cerium oxide nanocomposite as adsorptive photocatalyst for effective removal of carcinogenic dye. Mater. Res. Bull. 2025, 183, 113212. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.X.; Li, J.F.; Li, B.Y.; Wang, X.L.; Shi, Q.W. Synthesis of black titanium dioxide/activated carbon composites for enhanced visible-light photocatalytic properties. J. Mater. Sci.-Mater. Electron. 2024, 35, 1050. [Google Scholar] [CrossRef]
- Mittal, M.; Gupta, A.; Pandey, O.P. Role of oxygen vacancies in Ag/Au doped CeO2 nanoparticles for fast photocatalysis. Sol. Energy 2018, 165, 206–216. [Google Scholar] [CrossRef]
- Mei, X.Q.; Yuan, H.; Li, C.H. Study on the MOF Frame Pt-TiO2 Hybrid Photocatalyst and Its Photocatalytic Performance. Sustainability 2023, 15, 1403. [Google Scholar] [CrossRef]
- Alshammari, A.S.; Bagabas, A.; Alarifi, N.; Altamimi, R. Effect of the Nature of Metal Nanoparticles on the Photocatalytic Degradation of Rhodamine B. Top. Catal. 2019, 62, 786–794. [Google Scholar] [CrossRef]
- Ali, G.; Ahmad, W.; Mullani, N.; Basit, M.A.; Park, T.J. Contrasting photocatalytic performance of quantum-dot sensitized p-type and n-type TiO2 hierarchical nanocomposites under visible light. Mater. Chem. Phys. 2023, 308, 128233. [Google Scholar] [CrossRef]
- Chen, P.F.; Zhang, L.; Wu, Q.; Yao, W.F. Novel synthesis of Ag3PO4/CNFs/silica-fiber hybrid composite as an efficient photocatalyst. J. Taiwan Inst. Chem. Eng. 2016, 63, 506–511. [Google Scholar] [CrossRef]
- Kumar, Y.; Sharma, K.; Sudhaik, A.; Raizada, P.; Thakur, S.; Nguyen, V.; Van Le, Q.; Ahamad, T.; Alshehri, S.M.; Singh, P. Fabrication of magnetically retrievable ZnIn2S4/Bi2O2CO3/ ZnFe2O4 dual S-scheme heterojunction for superior photocatalytic activity. Appl. Nanosci. 2023, 13, 4129–4142. [Google Scholar] [CrossRef]
- Zhu, K.; Luan, X.; Wang, B.; Yang, P. MoS2 (1T/2H)/g-C3N4 heterojunctions created via Mo seed growth in situ towards high photo- and electro-chemical performance. J. Electronal. Chem. 2021, 899, 115683. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Ding, Z.; Zhang, J.; Wang, X. Optimal synthesis of platinum-free 1D/2D CdS/MoS2 (CM) heterojunctions with improved photocatalytic hydrogen production performance. J. Alloys Compd. 2020, 813, 152234. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. Two-dimensional hexaphosphate BiMP6 (M = Al, Ga, In) with desirable band gaps and ultrahigh carrier mobility for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 517, 146116. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Ding, D.; Zhang, S.; Deng, X. Research progress of 1T-MoS2 in electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 39771–39795. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, S.; Cheng, W.; Han, G.; Wu, Q.; Jiang, J. Construction of mechanically robust and recyclable photocatalytic hydrogel based on nanocellulose-supported CdS/MoS2/Montmorillonite hybrid for antibiotic degradation. Colloids Surf. A 2022, 636, 128035. [Google Scholar] [CrossRef]
- Liang, J.; Ning, X.-A.; Sun, J.; Song, J.; Hong, Y.; Cai, H. An integrated permanganate and ozone process for the treatment of textile dyeing wastewater: Efficiency and mechanism. J. Clean. Prod. 2018, 204, 12–19. [Google Scholar] [CrossRef]
- Vaishali, M.S.; Priyadarshini, N.; Nagapandiselvi, P.; Chandraleka, C. Calcium molybdate/activated carbon nanocomposite: A dual-functional material for energy storage and photocatalytic applications. Colloids Surf. A 2025, 721, 137207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
