Citrus aurantiifolia Peel-Facilitated Synthesis of Zinc Oxide, Interfaced with Biomass-Assisted Graphene Oxide for Enhanced Photocatalytic Degradation of Dye
Abstract
1. Introduction
2. Result and Discussion
2.1. Scanning Electron Microscopy/Energy-Dispersive X-Ray (EDX) Analysis of ZnO (Commercial, Synthesized) and ZnO/GO
2.2. Transmission Electron Microscope (TEM) Analysis of ZnO (Commercial, Synthesized), and ZnO/GO
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of GO, ZnO (Commercial, Synthesized), and ZnO/GO
2.4. UV–Visible Diffuse Reflectance Spectroscopy (UV–Vis DRS) Analysis of ZnO (Commercial, Synthesized) and ZnO/GO
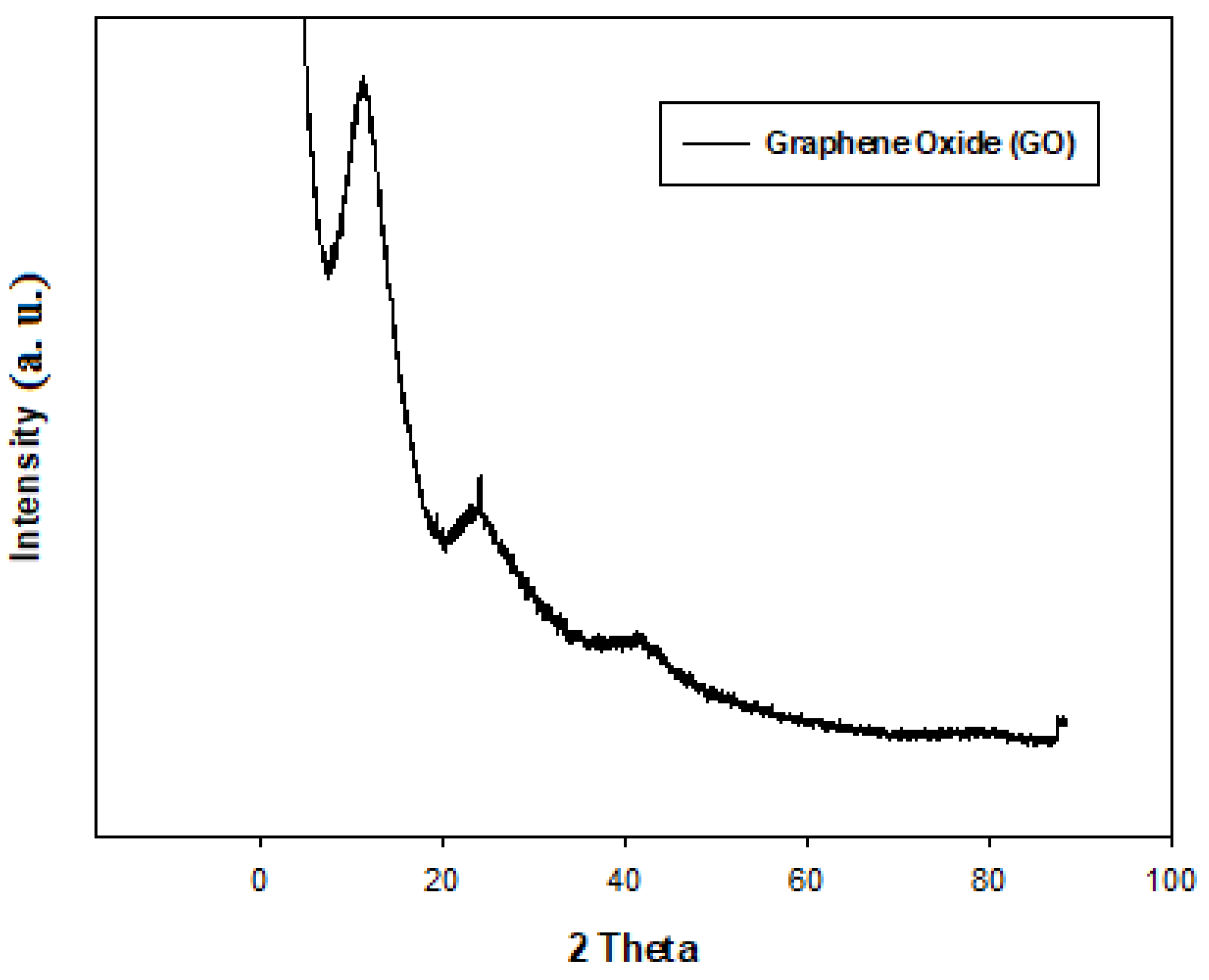
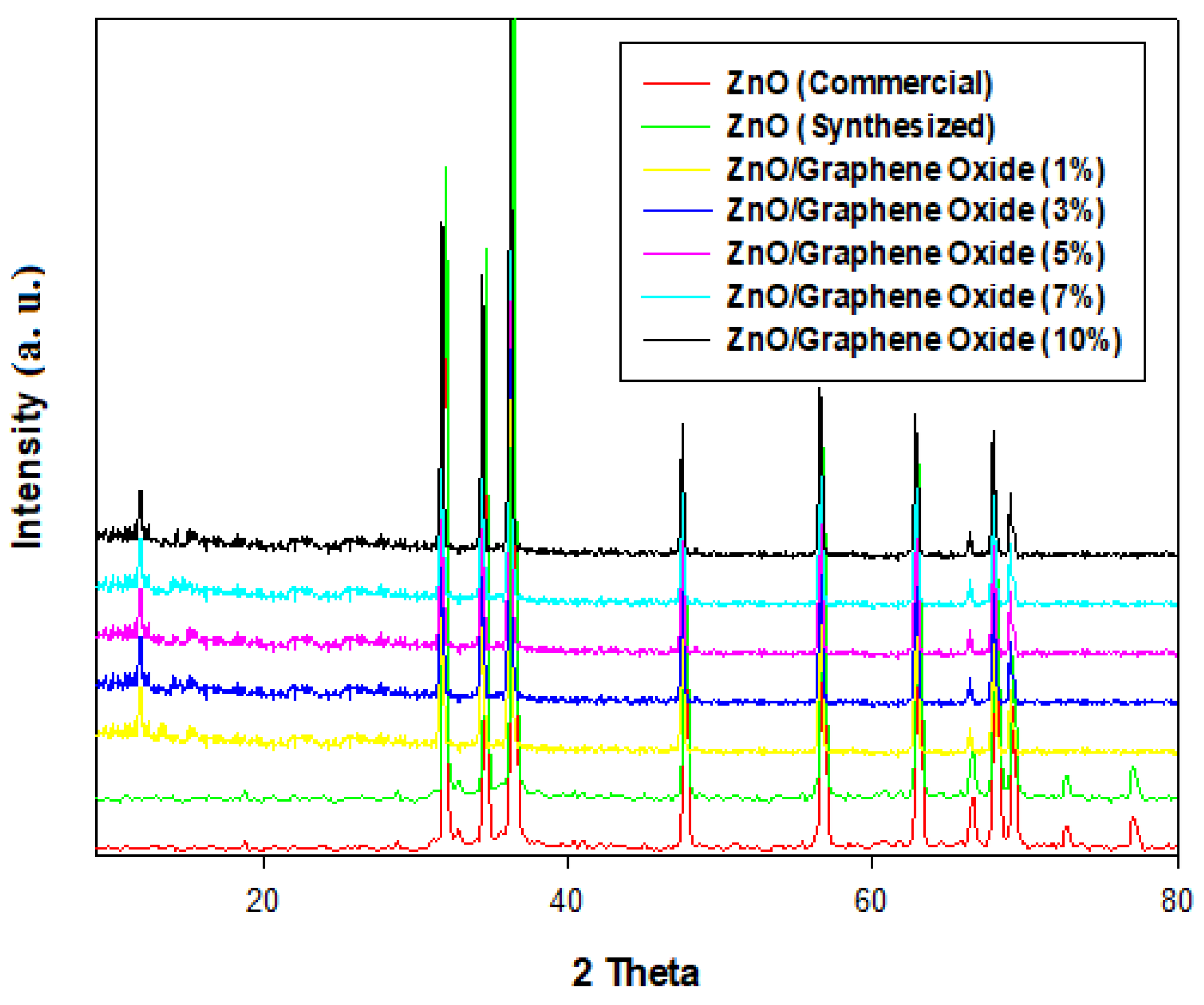
2.5. X-Ray-Diffraction (XRD) Analysis of GO, ZnO (Commercial, Synthesized) and ZnO/GO
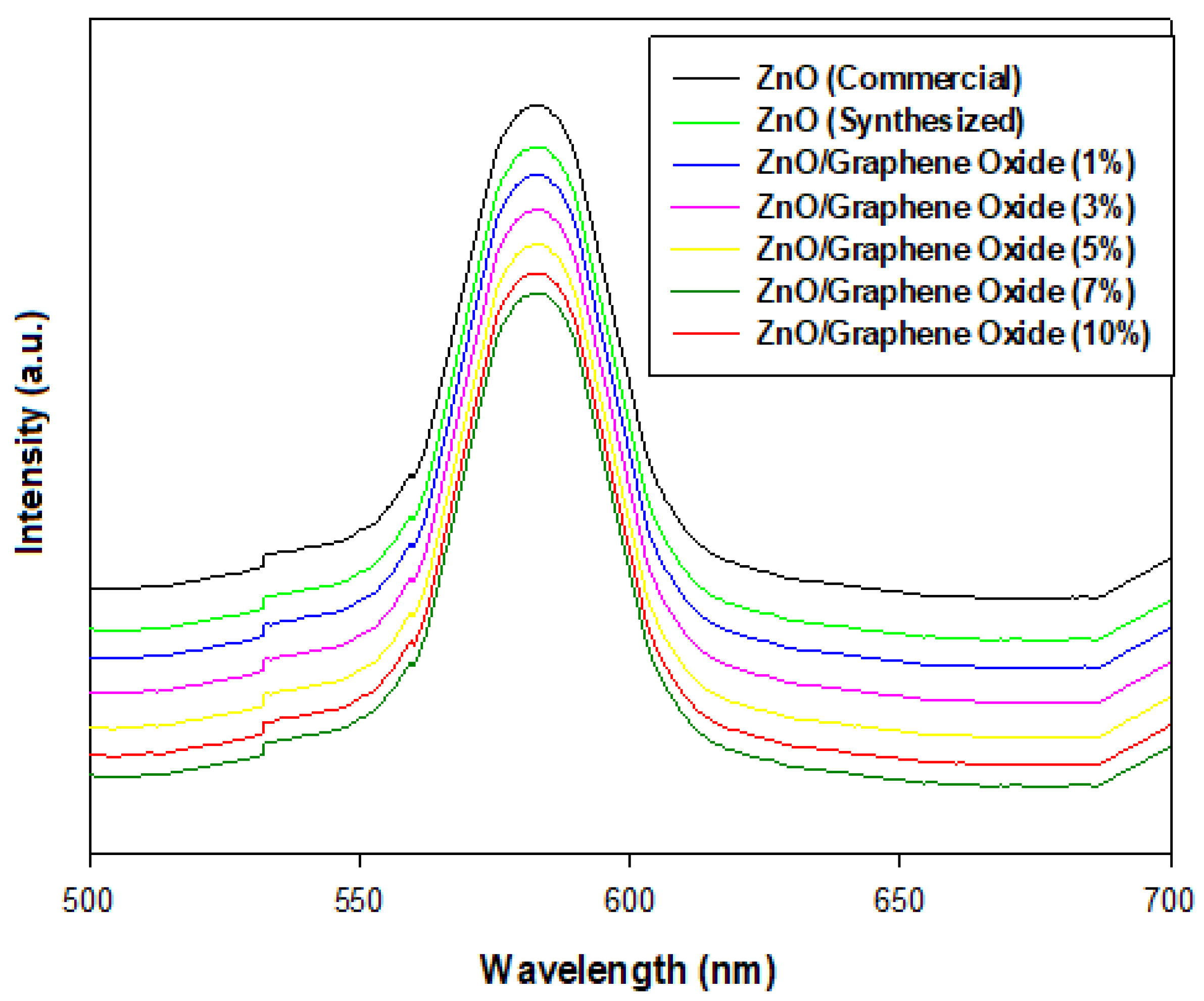
2.6. Photoluminescence (PL) Analysis of ZnO (Commercial, Synthesized) and ZnO/GO
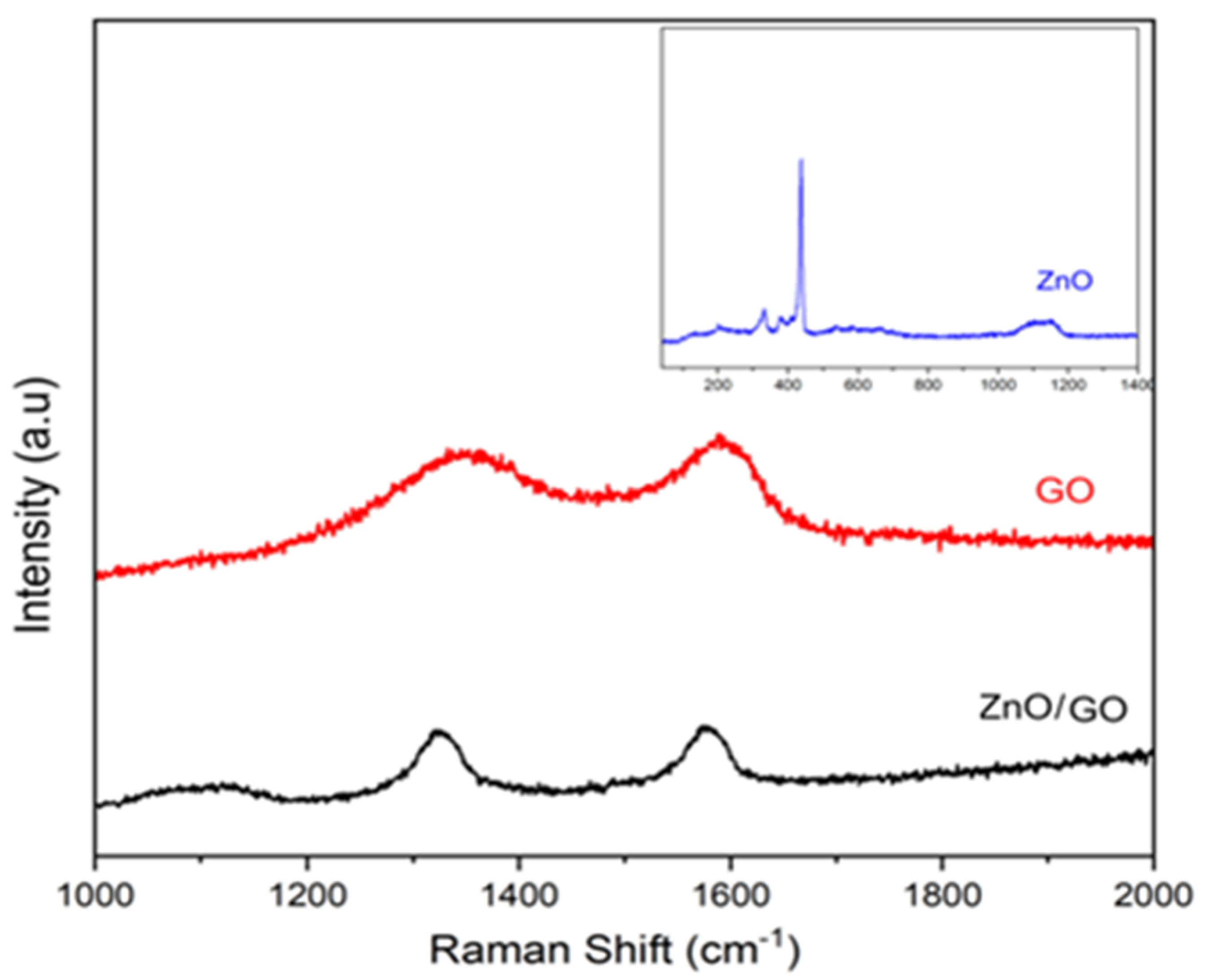
2.7. Raman Analysis of GO, ZnO (Synthesized) and ZnO/GO
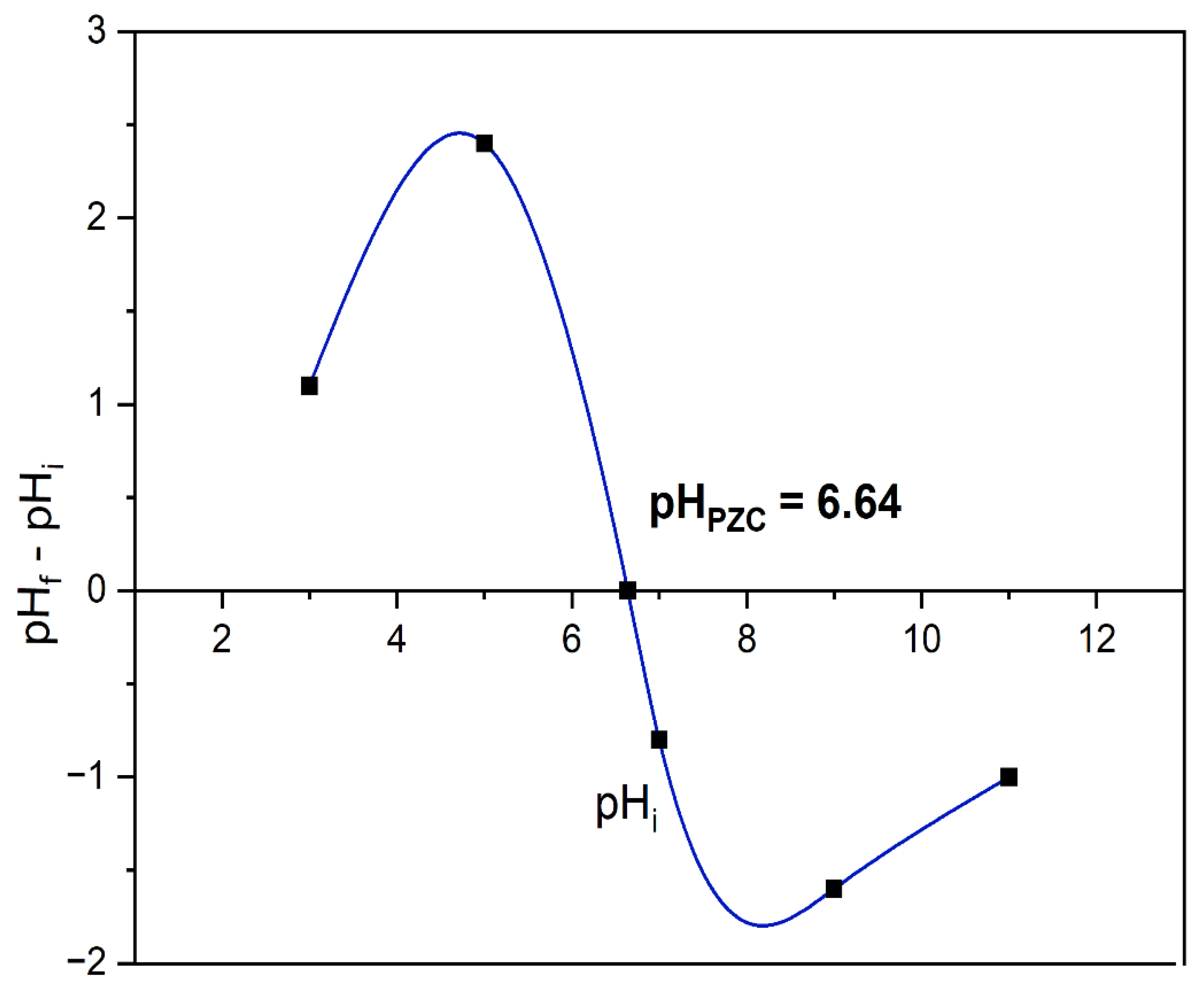
2.8. Point of Zero Charge (PZC) of ZnO/GO
2.9. Photocatalytic Activity
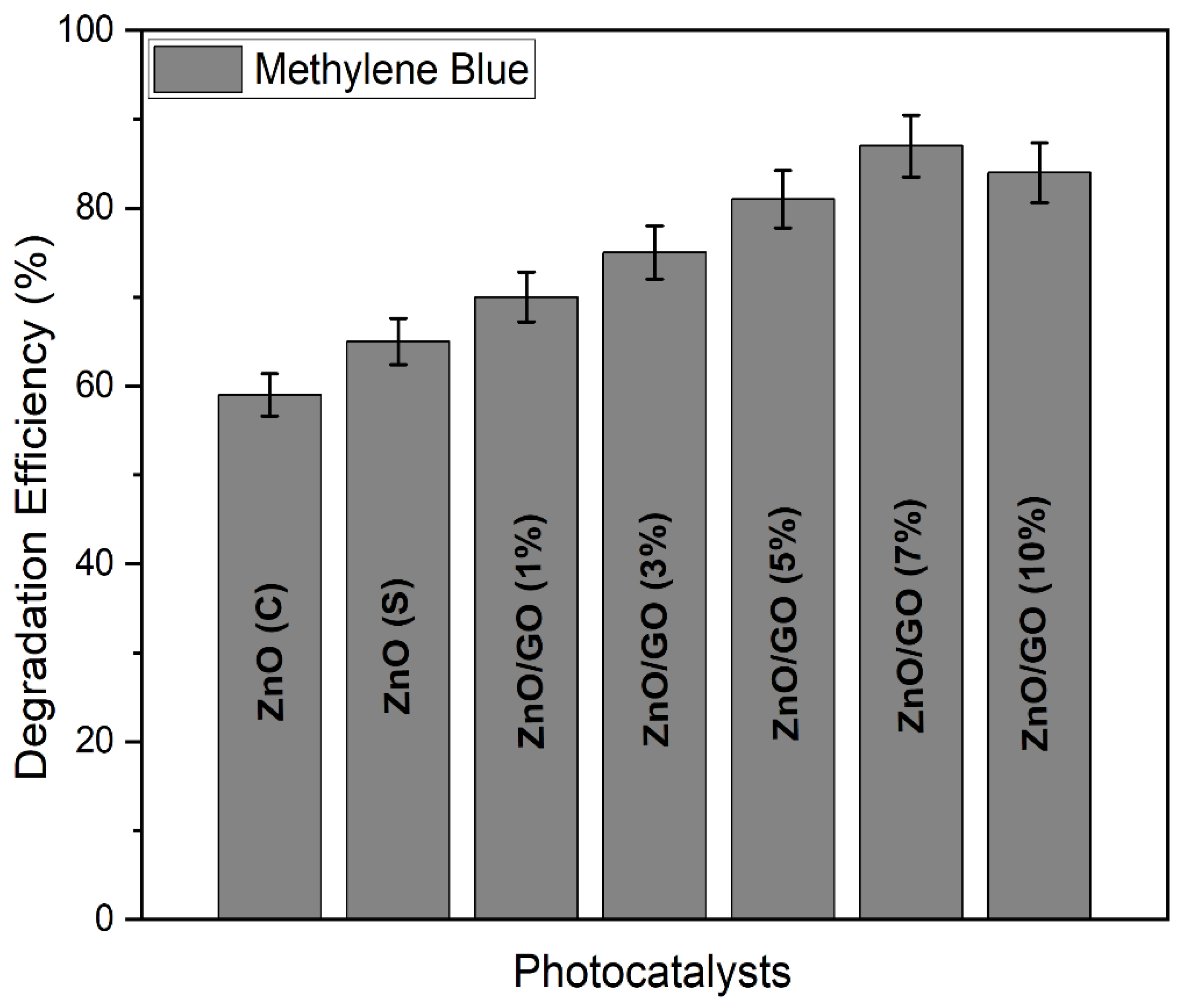
2.9.1. Photocatalytic Activity of ZnO (Commercial), ZnO (Synthesized), and ZnO with Varying GO Concentrations for the Photocatalytic Degradation of Methylene Blue
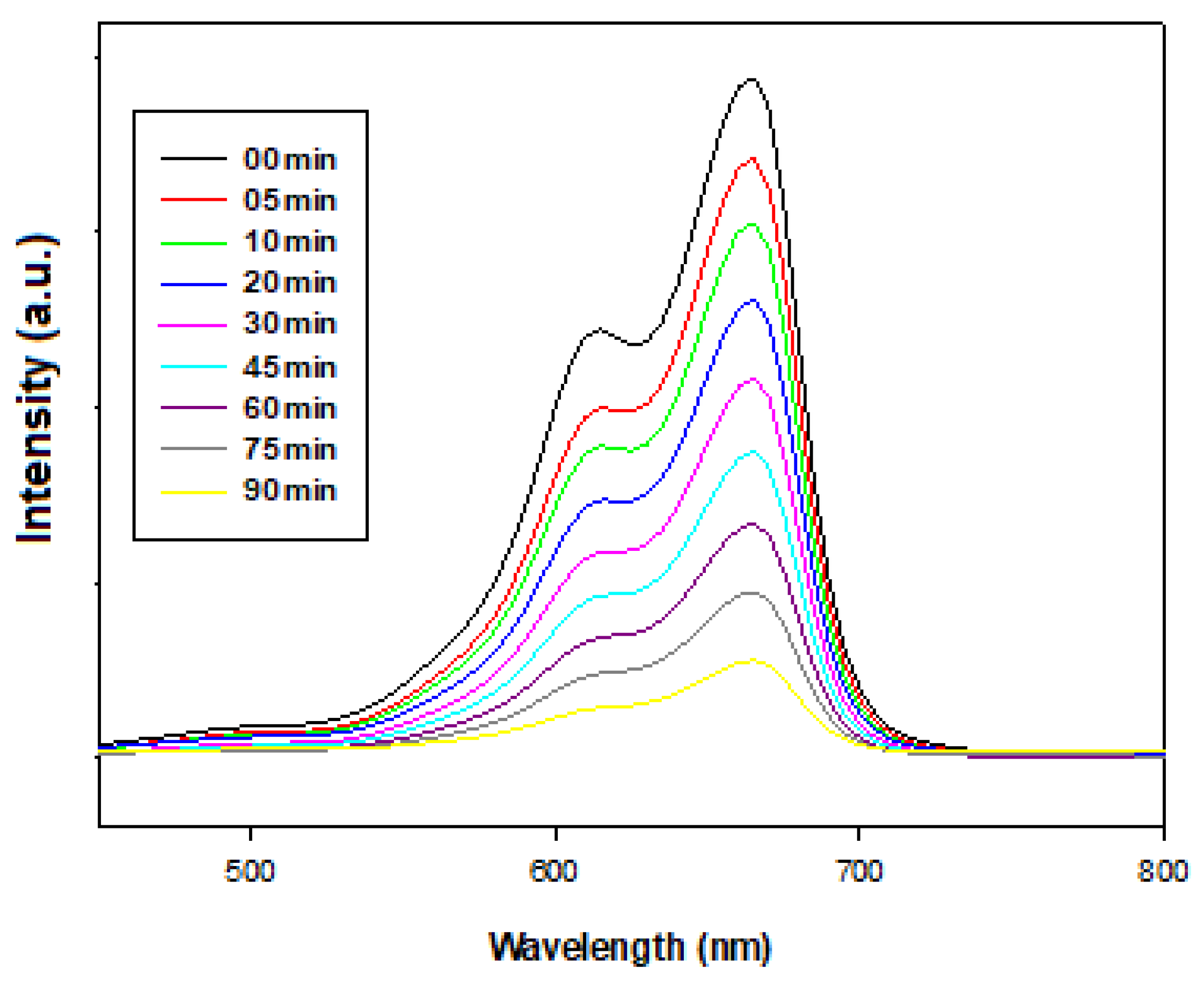
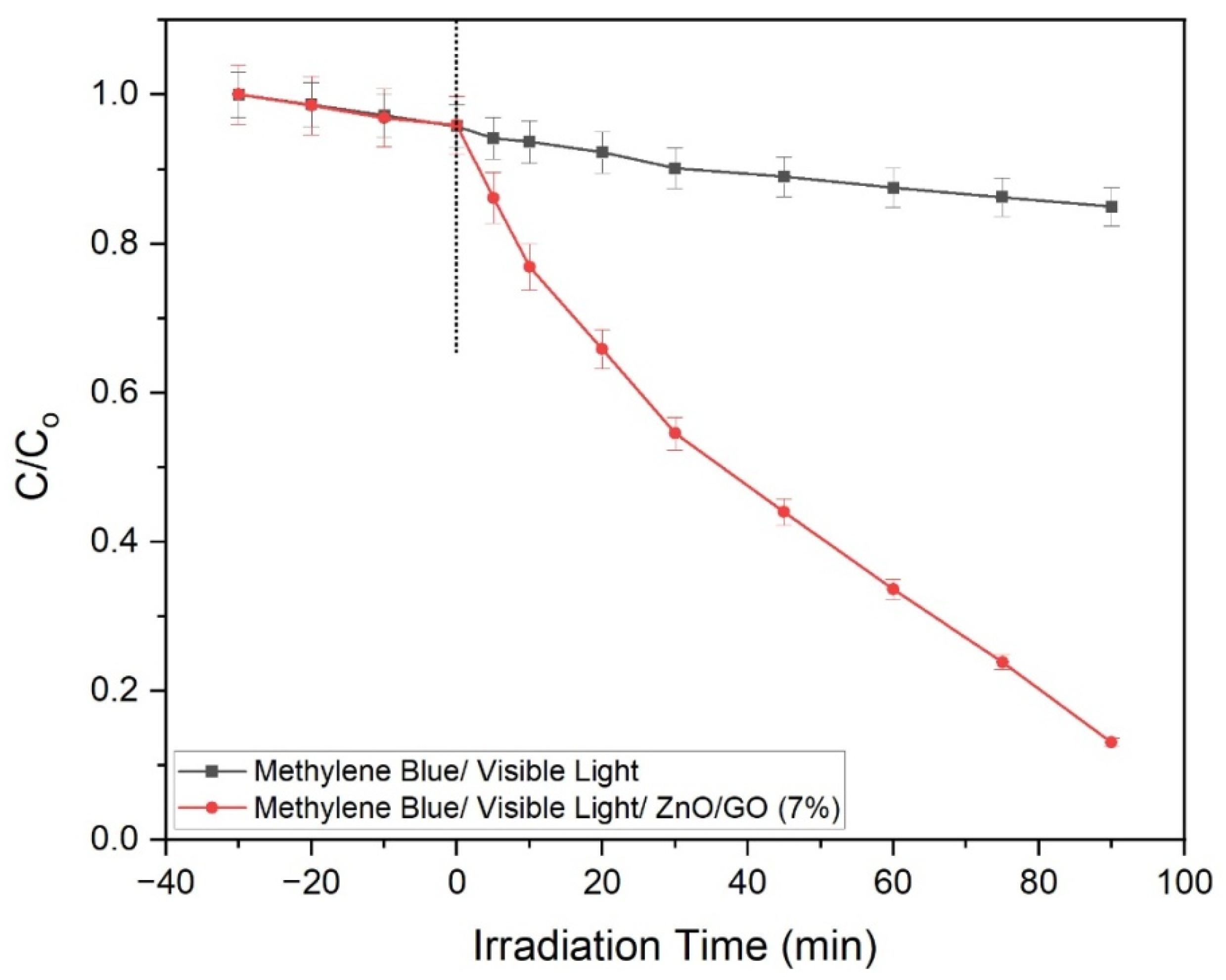
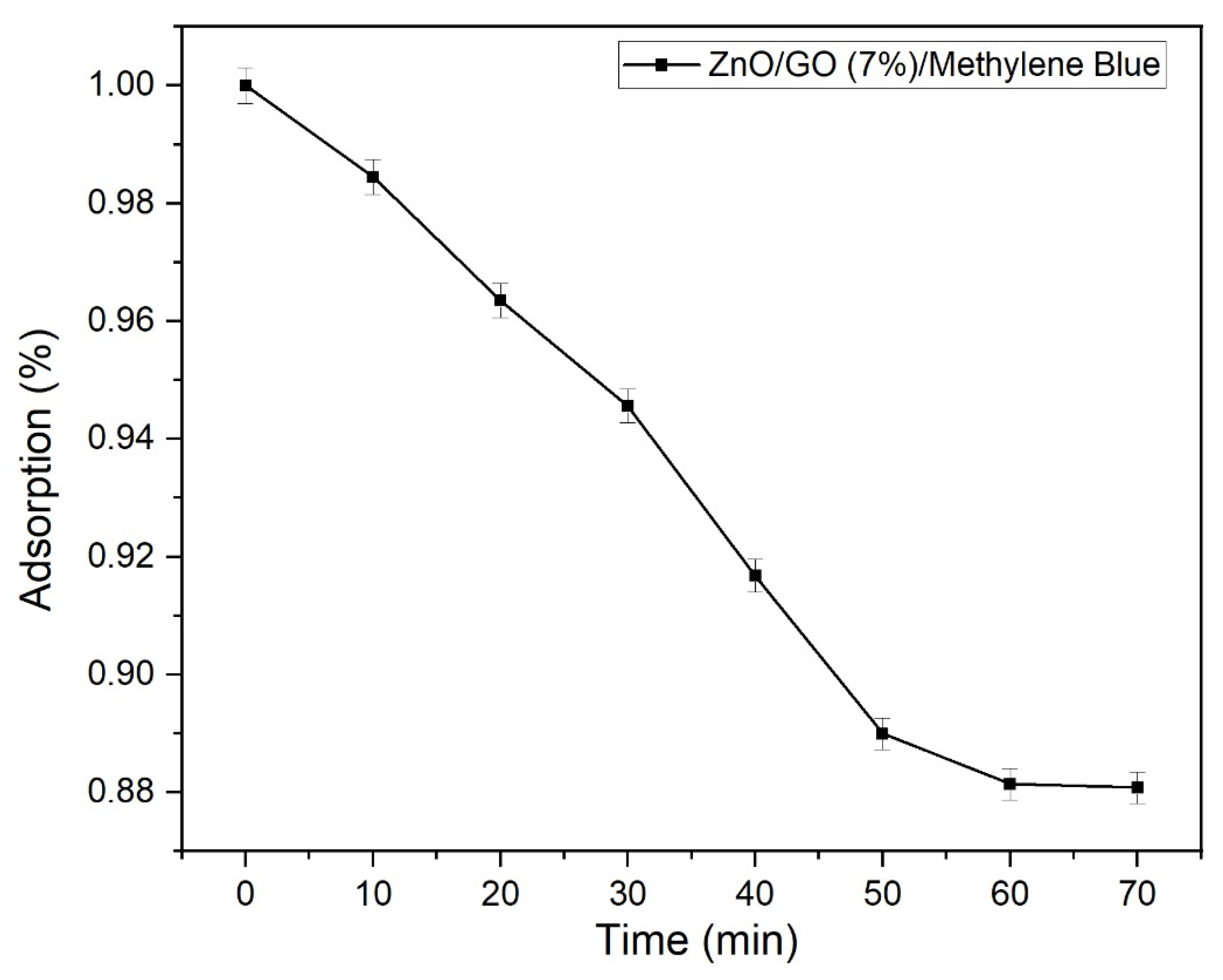
2.9.2. Impact of Time Duration on the Photocatalytic Degradation of Methylene Blue with ZnO/GO (7%) Photocatalyst
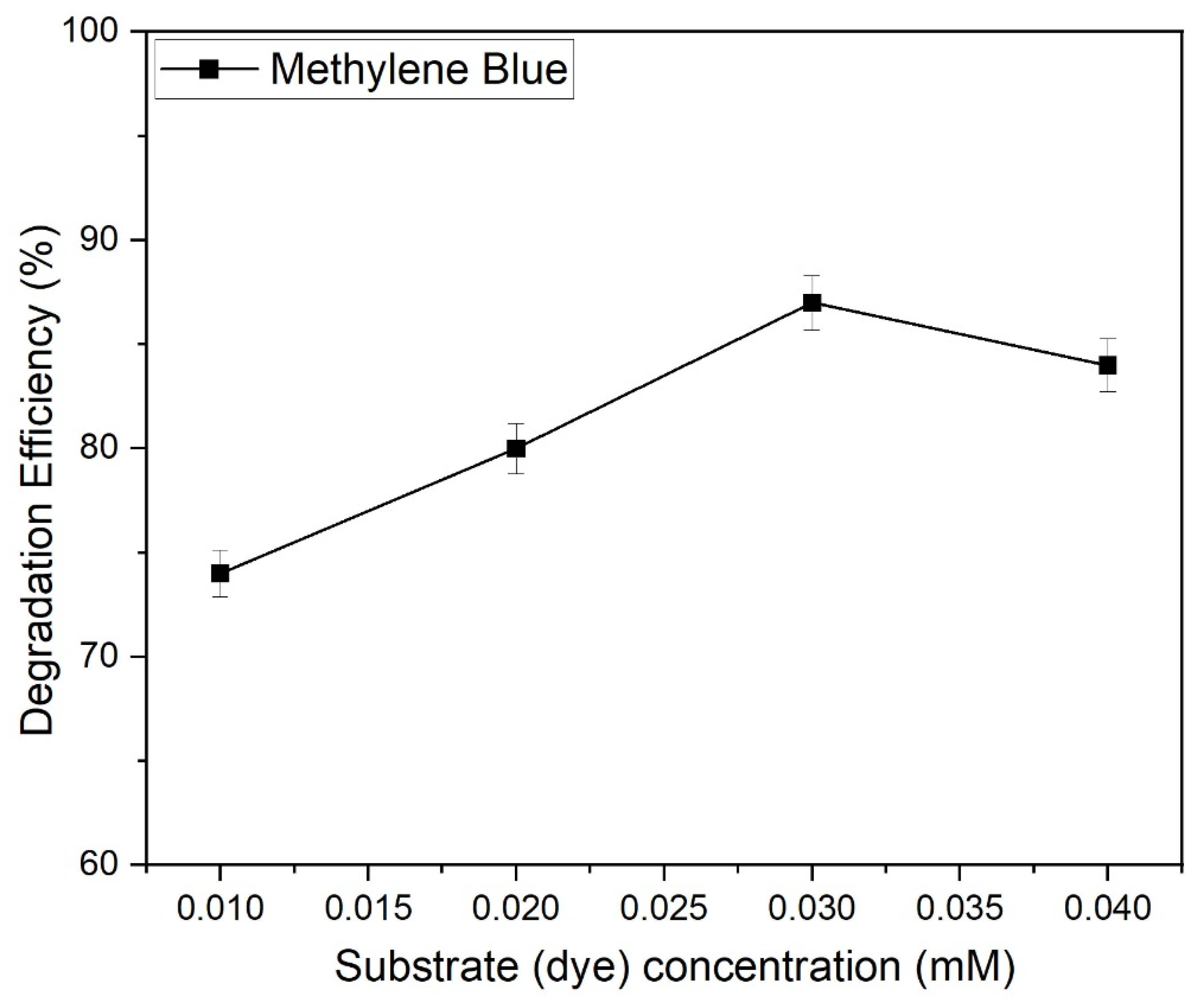
2.9.3. The Impact of Varying Concentrations of the Substrate (Dye) on the Photocatalytic Degradation of Methylene Blue
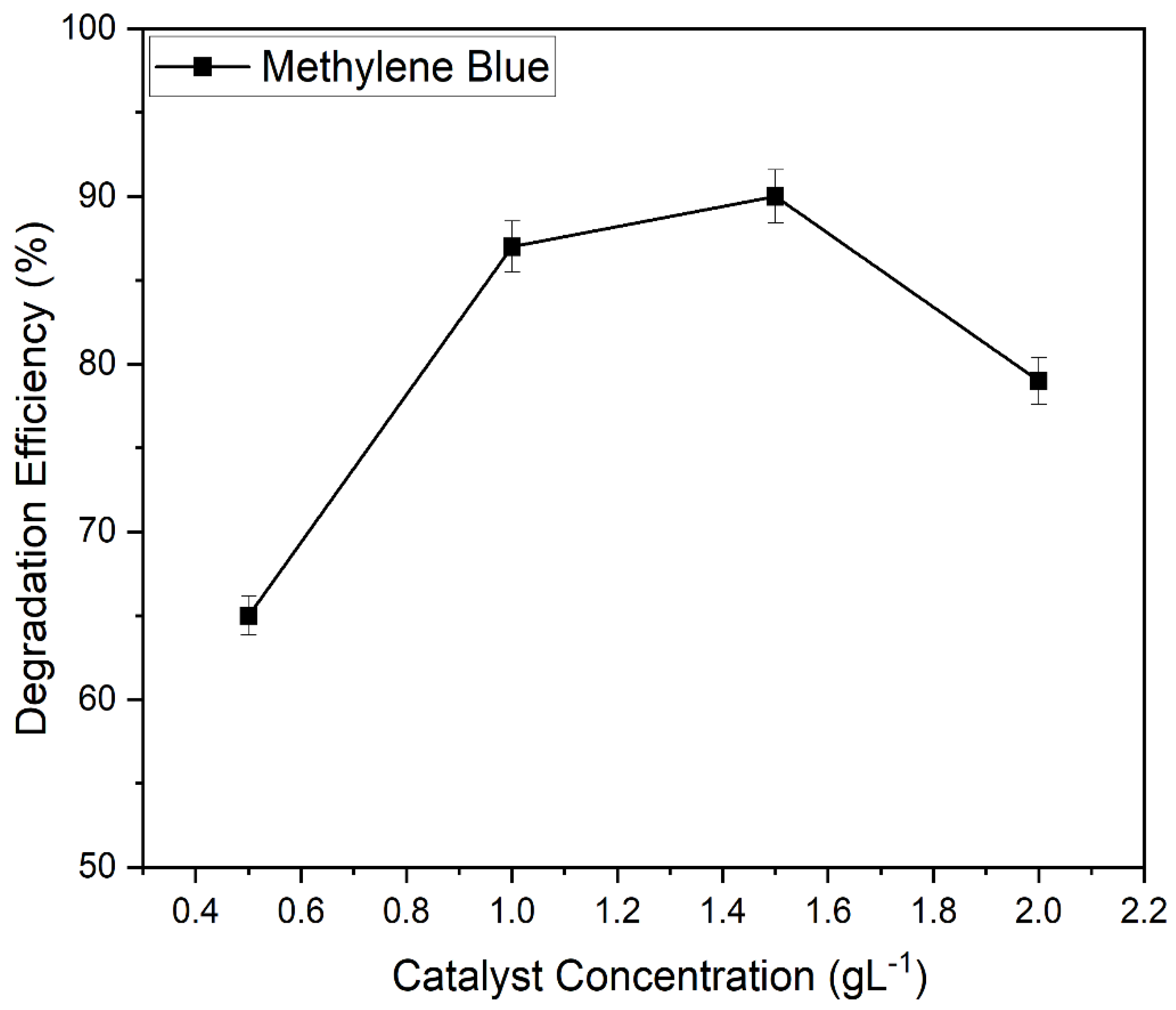
2.9.4. Impact of Catalyst Loading on the Photocatalytic Degradation of Methylene Blue
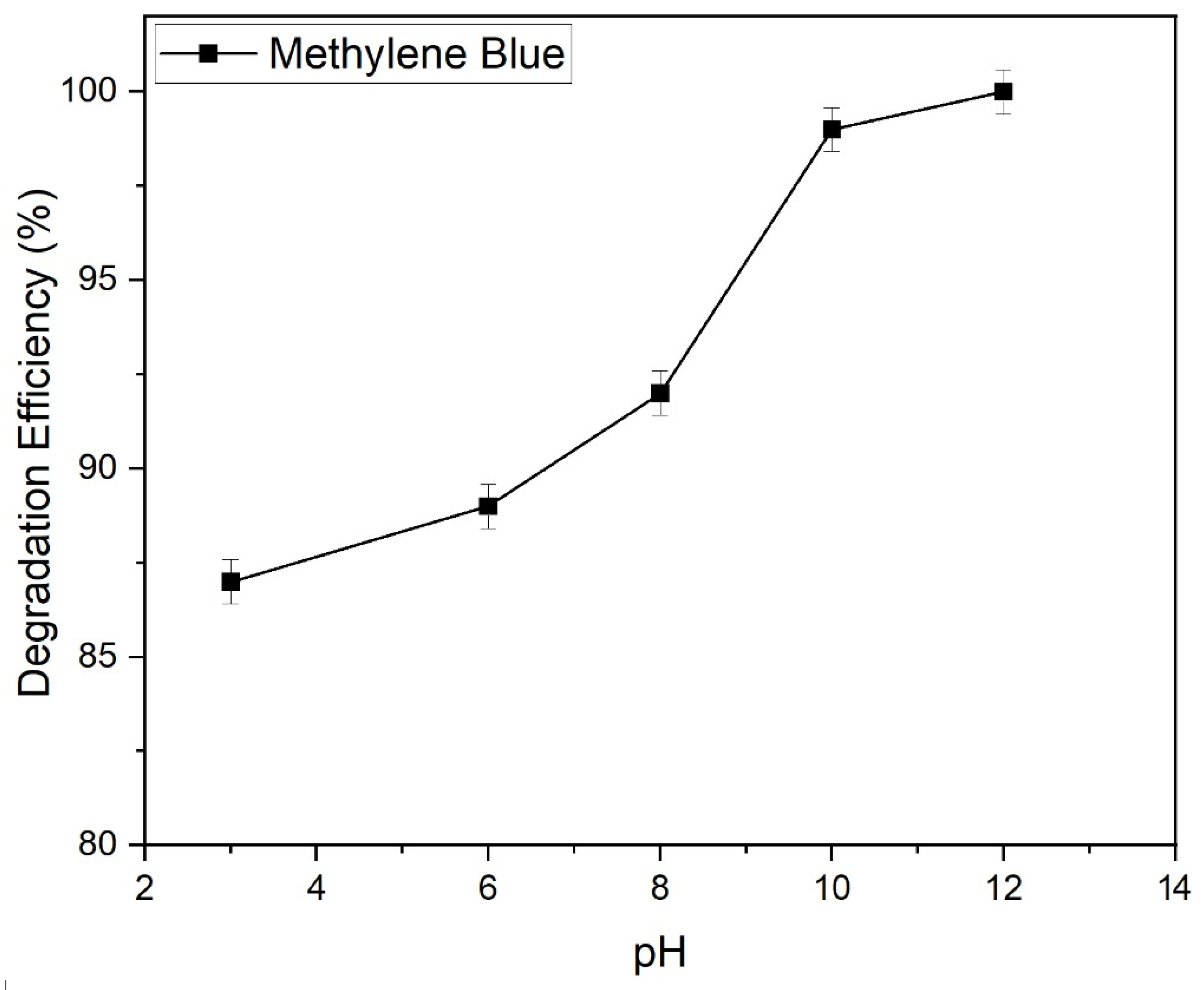
2.9.5. Effect of pH on the Photocatalytic Degradation of Methylene Blue
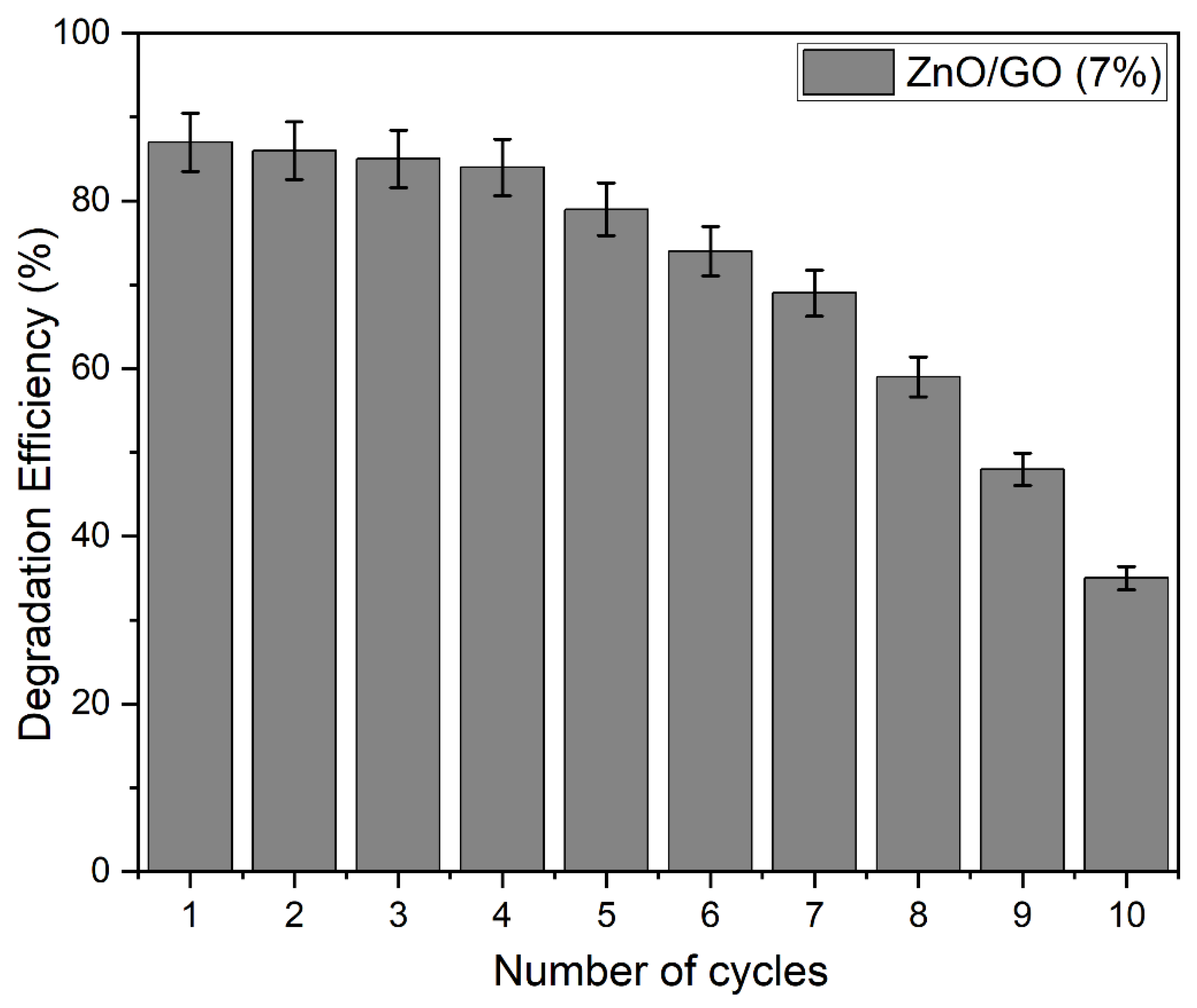
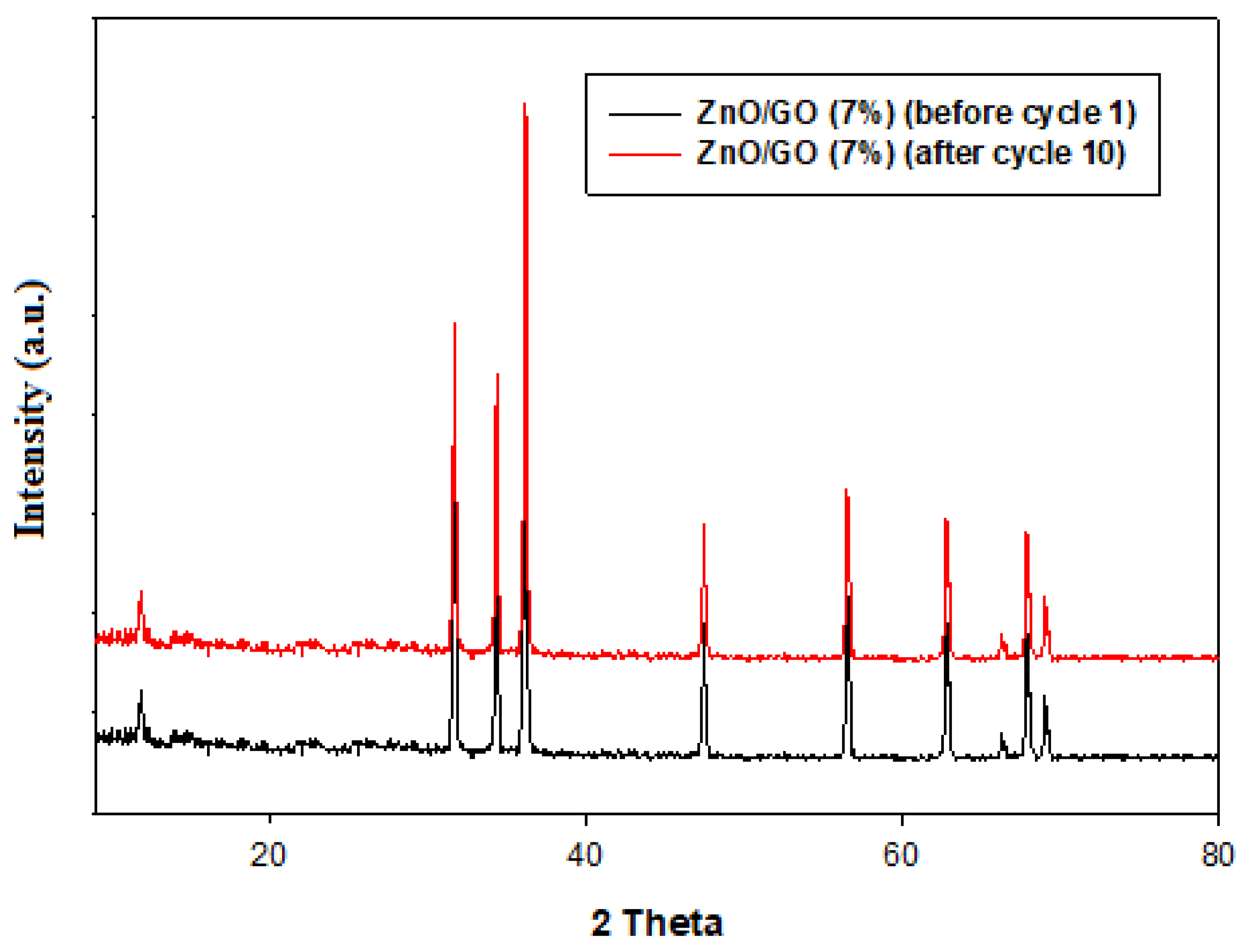
2.9.6. Reusability of the Photocatalyst for Photocatalytic Degradation of Methylene Blue
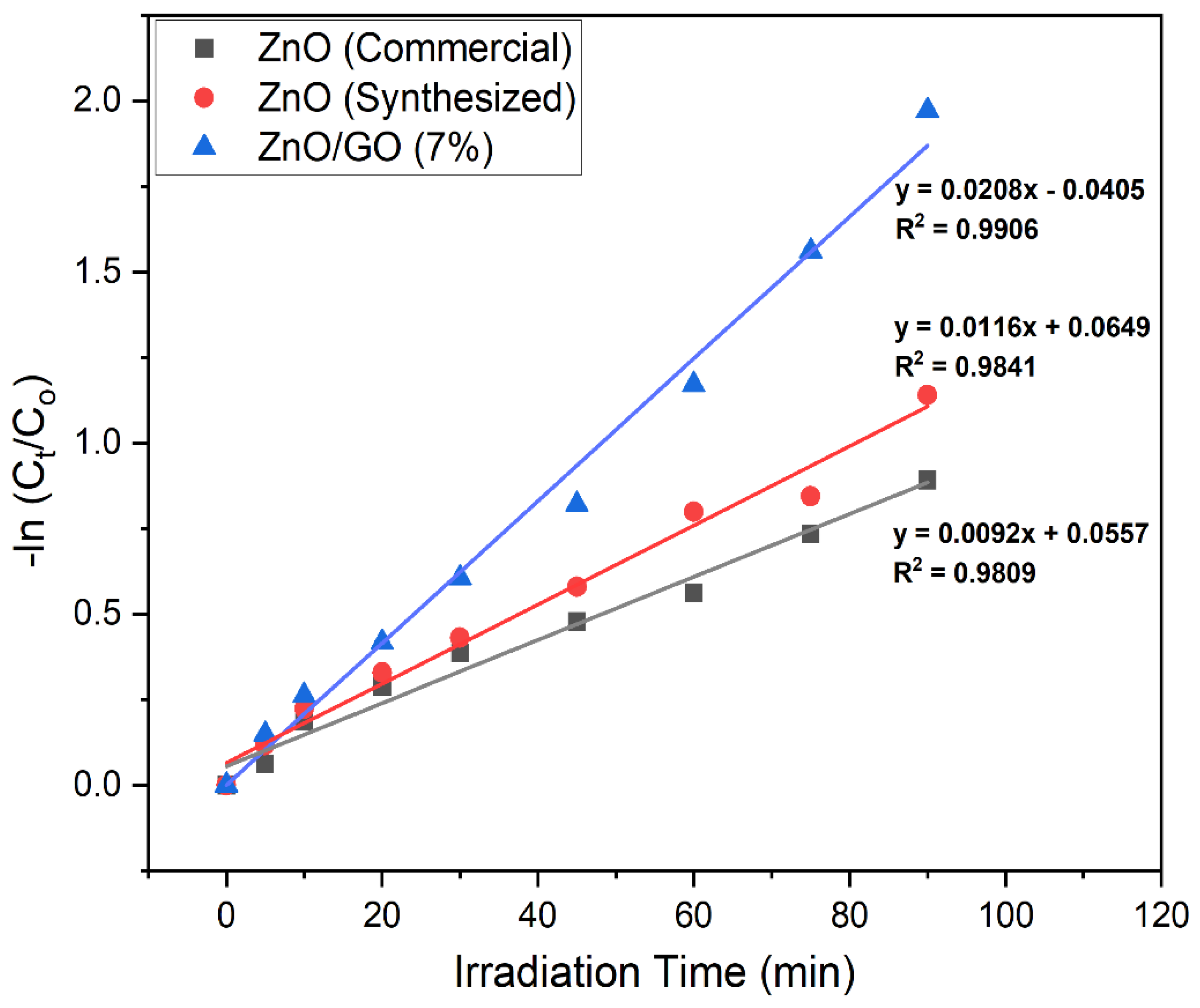
2.9.7. Reaction Kinetics for the Degradation of Methylene Blue Using ZnO (Commercial and Synthesized) and ZnO/GO (7%)
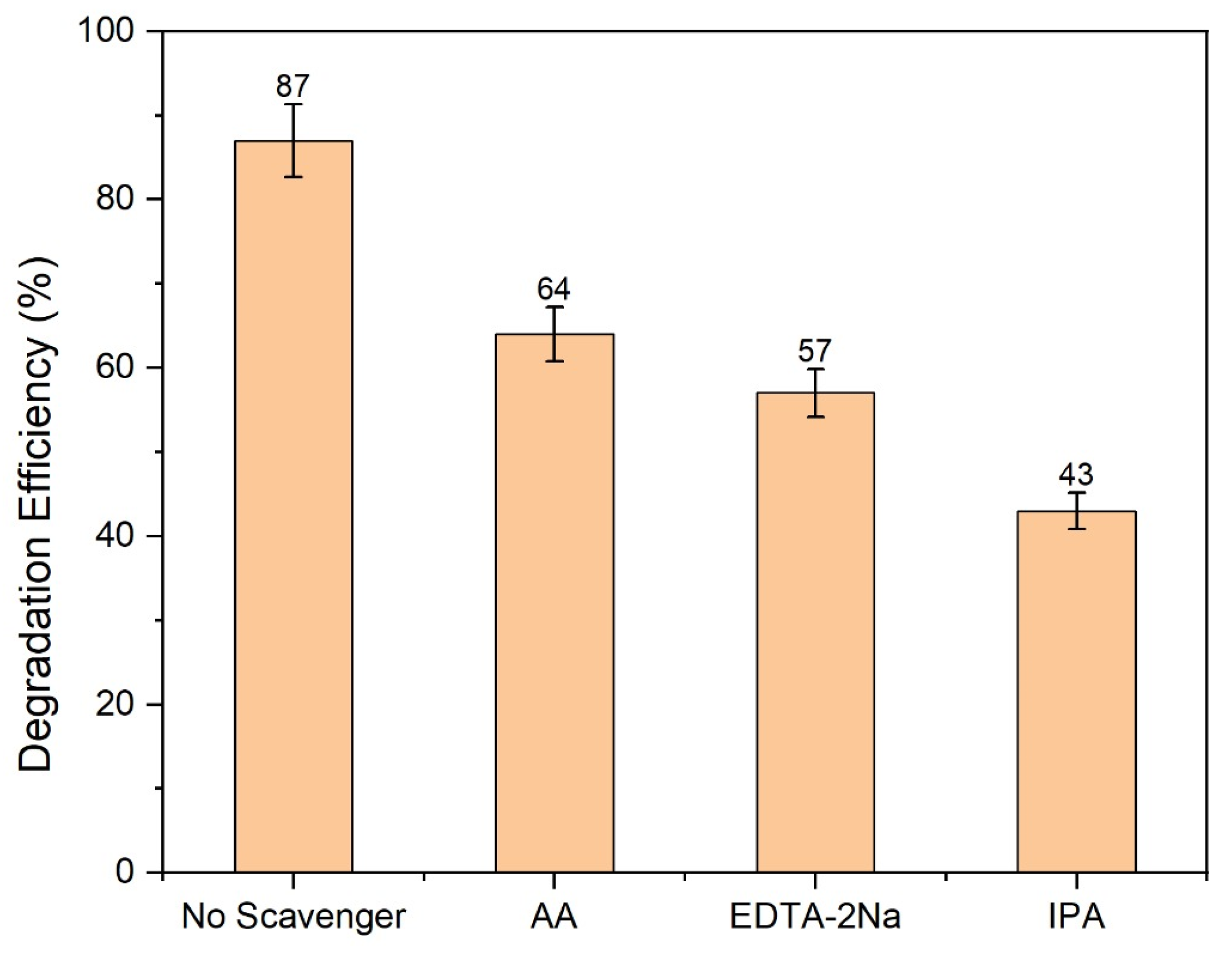
2.9.8. Scavenger Test for the Degradation Methylene Blue Using ZnO/GO (7%)
3. Material and Methods
3.1. Materials
3.2. Methods
3.2.1. Carbonization of Oil Palm Empty Fruit Bunch Fibre (OPEFB)
3.2.2. Preparation of Graphene Oxide (GO)
3.2.3. Synthesis of ZnO
3.2.4. Preparation of ZnO/GO Nanocomposites
3.2.5. Photocatalytic Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Afridi, S.K.; Umar, K. Comprehensive Exploration of Photocatalytic Degradation: Eradication of Various Dye Classes Using Elemental-Doped and Graphene-Interfaced Semiconductor Nanocomposites in a Sustainable Framework. Catal. Lett. 2025, 155, 167. [Google Scholar] [CrossRef]
- Ashtekar, V.S.; Bhandari, V.M.; Shirsath, S.R.; Sai, C.P.L.; Jolhe, P.D.; Ghodke, S.A. Dye wastewater treatment: Removal of reactive dyes using inorganic and organic coagulants. J. Ind. Pollut. Control 2014, 1, 30. [Google Scholar]
- Shakoor, M.H.; Shakoor, M.B.; Jilani, A.; Ahmed, T.; Rizwan, M.; Dustgeer, M.R.; Iqbal, J.; Zahid, M.; Yong, J.W.H. Enhancing the Photocatalytic Degradation of Methylene Blue with Graphene Oxide-Encapsulated g-C3N4/ZnO Ternary Composites. ACS Omega 2024, 9, 16187. [Google Scholar] [CrossRef] [PubMed]
- Foteinis, S.; Chatzisymeon, E. Heterogeneous Photocatalysis for Water Purification. In Nanostructured Photocatalysts; Boukherroub, R., Ogale, S.B., Robertson, N., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Al-Zahrani, S.A.; Umar, K.; Tweib, S.A.; Rashd, J.A.M.; Afridi, S.K.; Bhawani, S.A.; Otaibi, A.A.; Masood, N.; Mansour, D.; Khan, A.; et al. Biomass Mediated Synthesis of ZnO and ZnO/GO for the Decolorization of Methylene Blue under Visible Light Source. Catalysts 2023, 13, 409. [Google Scholar] [CrossRef]
- Afridi, S.K.; Umar, K. A Novel Bio-Facilitated Synthesis of GO/ZnO/Ag Nanocomposite via Citrulline Lanatus and OPEFB for Enhanced Photocatalytic Degradation of Imidacloprid: Kinetics, Mechanism and Byproduct Analysis. J. Water Process Eng. 2025, 72, 107603. [Google Scholar] [CrossRef]
- Baig, A.; Siddique, M.; Panchal, S. A Review of Visible-Light-Active Zinc Oxide Photocatalysts for Environmental Application. Catalysts 2025, 15, 100. [Google Scholar] [CrossRef]
- Umar, K.; Afridi, S.K.; Al-Ghurabi, Z.A.S.; Parveen, T.; Adnan, R.; Jameel, M. Biochar in Redox-Mediated Reactions for the Removal of Organic Pollutants from Water Resources. In Biochar: A Sustainable Approach; Bhawani, S.A., Bhat, A.H., Wahi, R., Ngaini, Z., Eds.; Springer Nature: Singapore, 2024. [Google Scholar]
- Afridi, S.K.; Umar, K.; Adnan, R.; Parveen, T. Biochar-Based Photocatalysts and Their Application. In Biochar-Based Catalysts: Preparation, Characterization and Applications; Bhawani, S.A., Umar, K., Mohamad Ibrahim, M.N., Alotaibi, K.M., Eds.; Springer Nature: Singapore, 2024. [Google Scholar]
- Molla, A.; Hoque, M.M.; Richi, F.T.; Kabir, M.H.; Alam, S.; Ahmed, N.U.; Emon, N.U.; Shao, C.; Zeng, C.; Wang, S. Biosynthesis and Characterization of ZnO Nanoparticles Using Citrus reticulata Peel Followed by Photocatalytic, Antibacterial, and Antioxidative Nanotherapeutic Attributes Assessment Supported by Computer Simulation. Int. J. Nanomed. 2025, 20, 4803. [Google Scholar] [CrossRef]
- Mao, T.; Liu, M.; Lin, L.; Cheng, Y.; Fang, C. A Study on Doping and Compound of Zinc Oxide Photocatalysts. Polymers 2022, 14, 4484. [Google Scholar] [CrossRef]
- Umar, K.; Aris, A.; Parveen, T.; Jaafar, J.; Majid, Z.A.; Reddy, A.V.; Talib, J. Synthesis, characterization of Mo and Mn doped ZnO and their photocatalytic activity for the decolorization of two different chromophoric dyes. Appl. Catal. A: Gen. 2015, 505, 507. [Google Scholar] [CrossRef]
- Afridi, S.K.; Umar, K.; Parveen, T.; Razali, M.R. Nanocomposites in the Degradation of Organic Pollutants. In Nanocomposites-Advanced Materials for Energy and Environmental Aspects; Khan, M.E., Aslam, J., Verma, C., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2023. [Google Scholar]
- Poorna, A.R.; Saravanathamizhan, R.; Balasubramanian, N. Graphene and graphene like structure from biomass for Electrochemical Energy Storage application: A Review. Electrochem. Sci. Adv. 2021, 1, 2000028. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Noor, N.H.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291. [Google Scholar] [CrossRef]
- Chiang, A.K.M.; Ng, L.Y.; Ng, C.Y.; Lim, Y.P.; Mahmoudi, E.; Tan, L.S.; Mah, S.K. Conversion of Palm Oil Empty Fruit Bunches to Highly Stable and Fluorescent Graphene Oxide Quantum Dots: An Eco-Friendly Approach. Mater. Chem. Phys. 2023, 309, 128433. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Facile Synthesis of Zinc Oxide Nanoparticles Decorated Graphene Oxide Composite via Simple Solvothermal Route and Their Photocatalytic Activity on Methylene Blue Degradation. J. Photochem. Photobiol. B Biol. 2016, 162, 500. [Google Scholar] [CrossRef]
- Posa, V.R.; Annavaram, V.; Koduru, J.R.; Ammireddy, V.R.; Somala, A.R. Graphene-ZnO Nanocomposite for Highly Efficient Photocatalytic Degradation of Methyl Orange Dye under Solar Light Irradiation. Korean J. Chem. Eng. 2016, 33, 456. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Yang, C.; Cao, M.; Ma, M.; Liu, R. ZnO Microspheres-Reduced Graphene Oxide Nanocomposite for Photocatalytic Degradation of Methylene Blue Dye. Appl. Surf. Sci. 2017, 392, 196. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Zhou, M.; Qian, H.; Hu, X. Microwave-Assisted Non-Aqueous Route to Deposit Well-Dispersed ZnO Nanocrystals on Reduced Graphene Oxide Sheets with Improved Photoactivity for the Decolorization of Dyes under Visible Light. Appl. Catal. B Environ. 2012, 125, 425. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohd Noor, N.H.; binti; Serrà, A.; Mohamad Ibrahim, M.N. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Thi, T.U.D.; Thoai Nguyen, T.; Dang Thi, Y.; Thi, K.H.T.; Thang Phan, B.; Ngoc Pham, K. Green Synthesis of ZnO Nanoparticles Using Orange Fruit Peel Extract for Antibacterial Activities. RSC Adv. 2020, 10, 23899. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wojnarowicz, J.; Morawski, A.W.; Narkiewicz, U.; Sobczak, K.; Gierlotka, S.; Lojkowski, W. Size-Dependent Effects of ZnO Nanoparticles on the Photocatalytic Degradation of Phenol in a Water Solution. Appl. Surf. Sci. 2021, 541, 148416. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Moussavi, G.; Mahjoub, A.; Yaghmaeian, K.; Srivastava, V.; Sillanpää, M.; Satari, M. Novel Magnetic Fe3O4@rGO@ZnO Onion-like Microspheres Decorated with Ag Nanoparticles for the Efficient Photocatalytic Oxidation of Metformin: Toxicity Evaluation and Insights into the Mechanisms. Catal. Sci. Technol. 2019, 9, 5819. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Kiran, G.R.; Acharyya, S.G. Remarkable Performance of GO/ZnO Nanocomposites under Optimized Parameters for Remediation of Cd (II) from Water. Appl. Surf. Sci. 2023, 626, 157238. [Google Scholar] [CrossRef]
- Verma, N.; Chundawat, T.S.; Chandra, H.; Verma, M.; Vaya, D. Role of GO Content in ZnO/@ x Wt% GO NCs for the Reduction of Cr(VI) Pollutant from Wastewater Stream. Int. J. Environ. Sci. Technol. 2025, 22, 1123. [Google Scholar] [CrossRef]
- Habibi Jetani, G.; Rahmani, M.B. TiO2/GO Nanocomposites: Synthesis, Characterization, and DSSC Application. Eur. Phys. J. Plus 2020, 135, 720. [Google Scholar] [CrossRef]
- Cheshme Khavar, A.H.; Moussavi, G.; Mahjoub, A.R. The Preparation of TiO2@rGO Nanocomposite Efficiently Activated with UVA/LED and H2O2 for High Rate Oxidation of Acetaminophen: Catalyst Characterization and Acetaminophen Degradation and Mineralization. Appl. Surf. Sci. 2018, 440, 963. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Abbaspour, S.; Masood, M.; Mirsattari, S.N.; Vahedi, A.; Mackenzie, K.J.D. Photocatalytic Properties of Mesoporous TiO2 Nanocomposites Modified with Carbon Nanotubes and Copper. Ceram. Int. 2016, 42, 11901. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and Characterization of Ag-Doped TiO2 Nanomaterials and Their Photocatalytic Reduction of Cr(VI) under Visible Light. Appl. Surf. Sci. 2014, 321, 396. [Google Scholar] [CrossRef]
- Raja, K.; Ramesh, P.S.; Geetha, D. Structural, FTIR and Photoluminescence Studies of Fe Doped ZnO Nanopowder by Co-Precipitation Method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 183. [Google Scholar] [CrossRef]
- Reddy, A.J.; Kokila, M.K.; Nagabhushana, H.; Chakradhar, R.P.S.; Shivakumara, C.; Rao, J.L.; Nagabhushana, B.M. Structural, Optical and EPR Studies on ZnO:Cu Nanopowders Prepared via Low Temperature Solution Combustion Synthesis. J. Alloys Compd. 2011, 509, 5349. [Google Scholar] [CrossRef]
- Achehboune, M.; Khenfouch, M.; Boukhoubza, I.; Leontie, L.; Doroftei, C.; Carlescu, A.; Bulai, G.; Mothudi, B.; Zorkani, I.; Jorio, A. Microstructural, FTIR and Raman Spectroscopic Study of Rare Earth Doped ZnO Nanostructures. Mater. Today Proc. 2022, 53, 319. [Google Scholar] [CrossRef]
- Bano, N.; Hussain, I.; EL-Naggar, A.M.; Albassam, A.A. Reduced Graphene Oxide Nanocomposites for Optoelectronics Applications. Appl. Phys. A 2019, 125, 215. [Google Scholar] [CrossRef]
- Ching, L.W.; Keesan, F.W.M.; Muhamad, I.I. Optimization of ZnO/GO Nanocomposite-Loaded Polylactic Acid Active Films Using Response Surface Methodology. J. King Saud Univ.-Sci. 2022, 34, 101835. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-Yield Preparation of Graphene Oxide from Small Graphite Flakes via an Improved Hummers Method with a Simple Purification Process. Carbon 2015, 81, 826. [Google Scholar] [CrossRef]
- Mututu, V.; Sunitha, A.K.; Thomas, R.; Pandey, M.; Manoj, B. An Investigation on Structural, Electrical and Optical Properties of GO/ZnO Nanocomposite. Int. J. Electrochem. Sci. 2019, 14, 375. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814. [Google Scholar] [CrossRef]
- Lv, N.; Li, Y.; Huang, Z.; Li, T.; Ye, S.; Dionysiou, D.D.; Song, X. Synthesis of GO/TiO2/Bi2WO6 Nanocomposites with Enhanced Visible Light Photocatalytic Degradation of Ethylene. Appl. Catal. B Environ. 2019, 246, 303. [Google Scholar] [CrossRef]
- Olatunde, O.C.; Onwudiwe, D.C. Graphene-Based Composites as Catalysts for the Degradation of Pharmaceuticals. Int. J. Environ. Res. Public Health 2021, 18, 1529. [Google Scholar] [CrossRef]
- Rahimi, K.; Yazdani, A.; Ahmadirad, M. Facile Preparation of Zinc Oxide Nanorods Surrounded by Graphene Quantum Dots Both Synthesized via Separate Pyrolysis Procedures for Photocatalyst Application. Mater. Res. Bull. 2018, 98, 148. [Google Scholar] [CrossRef]
- Paul, R.; Gayen, R.N.; Biswas, S.; Bhat, S.V.; Bhunia, R. Enhanced UV Detection by Transparent Graphene Oxide/ZnO Composite Thin Films. RSC Adv. 2016, 6, 61661. [Google Scholar] [CrossRef]
- Ahamad, T.; Ahmed, A.S. Influence of graphene oxide on the dielectric properties of biogenically synthesized ZnO nanoparticles. Hybrid Adv. 2023, 3, 100059. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Leontie, L.; Galca, A.C.; Enculescu, M.; Carlescu, A.; Guerboub, M.; Mothudi, B.M.; Jorio, A.; et al. Graphene Oxide Concentration Effect on the Optoelectronic Properties of ZnO/GO Nanocomposites. Nanomaterials 2020, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Yadav, M.; Gupta, S.; Popat, Y.; Gangan, A.; Chakraborty, B.; Ramaniah, L.M.; Fernandes, R.; Miotello, A.; Press, M.R.; et al. Effect of Graphene Oxide Loading on TiO2: Morphological, Optical, Interfacial Charge Dynamics-A Combined Experimental and Theoretical Study. Carbon 2019, 143, 51. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Sadighikia, S.; Khataee, A. UV-Assisted Synthesis of Reduced Graphene Oxide–ZnO Nanorod Composites Immobilized on Zn Foil with Enhanced Photocatalytic Performance. Res. Chem. Intermed. 2016, 42, 4479. [Google Scholar] [CrossRef]
- Silambarasan, M.; Shanmugam, S.; Soga, T. Raman and Photoluminescence Studies of Ag and Fe-Doped ZnO Nanoparticles. Int. J. ChemTech Res. 2015, 7, 1644. [Google Scholar]
- How, G.T.S.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly Exposed {001} Facets of Titanium Dioxide Modified with Reduced Graphene Oxide for Dopamine Sensing. Sci. Rep. 2014, 4, 5044. [Google Scholar] [CrossRef]
- Ghanbari Shohany, B.; Khorsand Zak, A. Doped ZnO Nanostructures with Selected Elements-Structural, Morphology and Optical Properties: A Review. Ceram. Int. 2020, 46, 5507. [Google Scholar] [CrossRef]
- Abbasi, Z.; Farrokhnia, A.; Garcia-Lopez, E.I.; Zargar Shoushtari, M.; Aghaie, E. Synthesis of ZnO–Ag2CO3–Fe3O4@rGO Core–Shell Structure: Magnetically Separable Photocatalyst for Degradation of MB Using the Box–Behnken Design. J. Mater. Sci. Mater. Electron. 2020, 31, 19554. [Google Scholar] [CrossRef]
- Pham, T.A.T.; Tran, V.A.; Le, V.D.; Nguyen, M.V.; Truong, D.D.; Do, X.T.; Vu, A.-T. Facile Preparation of ZnO Nanoparticles and Ag/ZnO Nanocomposite and Their Photocatalytic Activities under Visible Light. Int. J. Photoenergy 2020, 2020, 8897667. [Google Scholar] [CrossRef]
- Chauhan, H.A.; Rafatullah, M.; Ali, K.A.; Umar, M.F.; Khan, M.A.; Jeon, B.-H. Photocatalytic Activity of Graphene Oxide/Zinc Oxide Nanocomposite Derived from Rice Husk for the Degradation of Phenanthrene under Ultraviolet-Visible Light. J. Water Process Eng. 2022, 47, 102714. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. Preparation and Photocatalytic Activity of TiO2/PPy/GO for the Degradation of Rose Bengal and Victoria Blue Dye in Visible Light in Aqueous Solution. Desalination Water Treat. 2018, 114, 265. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.; Zhang, C.; Yu, Z. Decomposition of Acetaminophen in Water by a Gas Phase Dielectric Barrier Discharge Plasma Combined with TiO2-rGO Nanocomposite: Mechanism and Degradation Pathway. J. Hazard. Mater. 2017, 323, 719. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, H.; Mahjoub, A.R.; Aghayan, H. Effective Removal of Methylene Blue and Cerium by a Novel Pair Set of Heteropoly Acids Based Functionalized Graphene Oxide: Adsorption and Photocatalytic Study. Chem. Eng. Res. Des. 2017, 120, 303. [Google Scholar] [CrossRef]
- Minella, M.; Sordello, F.; Minero, C. Photocatalytic Process in TiO2/Graphene Hybrid Materials. Evidence of Charge Separation by Electron Transfer from Reduced Graphene Oxide to TiO2. Catal. Today 2017, 281, 29. [Google Scholar] [CrossRef]
- Lin, C.J.; Yang, W.-T.; Chou, C.-Y.; Liou, S.Y.H. Hollow Mesoporous TiO2 Microspheres for Enhanced Photocatalytic Degradation of Acetaminophen in Water. Chemosphere 2016, 152, 490. [Google Scholar] [CrossRef]
- Akpotu, S.O.; Oseghe, E.O.; Ayanda, O.S.; Skelton, A.A.; Msagati, T.A.M.; Ofomaja, A.E. Photocatalysis and Biodegradation of Pharmaceuticals in Wastewater: Effect of Abiotic and Biotic Factors. Clean Technol. Environ. Policy 2019, 21, 1701. [Google Scholar] [CrossRef]
- Umar, K.; Dar, A.A.; Haque, M.M.; Mir, N.A.; Muneer, M. Photocatalysed Decolourization of Two Textile Dye Derivatives, Martius Yellow and Acid Blue 129, in UV-Irradiated Aqueous Suspensions of Titania. Desalination Water Treat. 2012, 46, 205. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Insight in to the Degradation of Bisphenol A by Doped ZnO@ZnHCF Nanocubes: High Photocatalytic Performance. J. Colloid Interface Sci. 2018, 530, 16. [Google Scholar] [CrossRef]
- Göktaş, S. Synergic effects of pH, reaction temperature, and various light sources on the photodegradation of methylene blue without photocatalyst: A relatively high degradation efficiency. Chem. Afr. 2024, 7, 4425. [Google Scholar] [CrossRef]
- Cano, F.J.; Coste, S.; Reyes-Vallejo, O.; Makowska-Janusik, M.; Velumani, S.; Olvera, M.d.l.L.; Kassiba, A. Influence of GO Oxidation Degrees on the Organization and Physical Features of TiO2–GO-Based Nanocomposites for Water Dye Removal. Surf. Interfaces 2024, 46, 104004. [Google Scholar] [CrossRef]
- Ramos-Álvarez, D.; Hernández-Rodríguez, Y.M.; Vega-Gómez, J.; Cigarroa-Mayorga, O.E. Influence of Copper Support on the Charge Transfer Enhancement of Zinc Oxide Nanoflakes. Mater. Lett. 2023, 349, 134875. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, B.; Kaur, D. Modified Ag-ZnO Coated Graphene Oxide Ternary Composite for Superior Photocatalytic Degradation of Crystal Violet Dye under Visible Light Irradiation. Diam. Relat. Mater. 2024, 143, 110935. [Google Scholar] [CrossRef]
- Anjum, F.; Asiri, A.M.; Khan, M.A.; Khan, M.I.; Khan, S.B.; Akhtar, K.; Chakraborty, S. Photo-degradation, thermodynamic and kinetic study of carcinogenic dyes via zinc oxide/graphene oxide nanocomposites. J. Mater. Res. Technol. 2021, 15, 3171. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Ramaila, S.; Mavuru, L.; Onwudiwe, D.C. Enhanced photocatalytic degradation of methyl orange using Sn-ZnO/GO nanocomposite. J. Photochem. Photobiol. 2022, 11, 100131. [Google Scholar] [CrossRef]
- Chaudhary, K.; Aadil, M.; Zulfiqar, S.; Ullah, S.; Haider, S.; Agboola, P.O.; Shakir, I. Graphene oxide and reduced graphene oxide supported ZnO nanochips for removal of basic dyes from the industrial effluents. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 915. [Google Scholar] [CrossRef]
- Puneetha, J.; Kottam, N.; Rathna, A. Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorg. Chem. Commun. 2021, 125, 108460. [Google Scholar]
- Al-Rawashdeh, N.A.; Allabadi, O.; Aljarrah, M.T. Photocatalytic activity of graphene oxide/zinc oxide nanocomposites with embedded metal nanoparticles for the degradation of organic dyes. ACS Omega 2020, 5, 28046. [Google Scholar] [CrossRef]
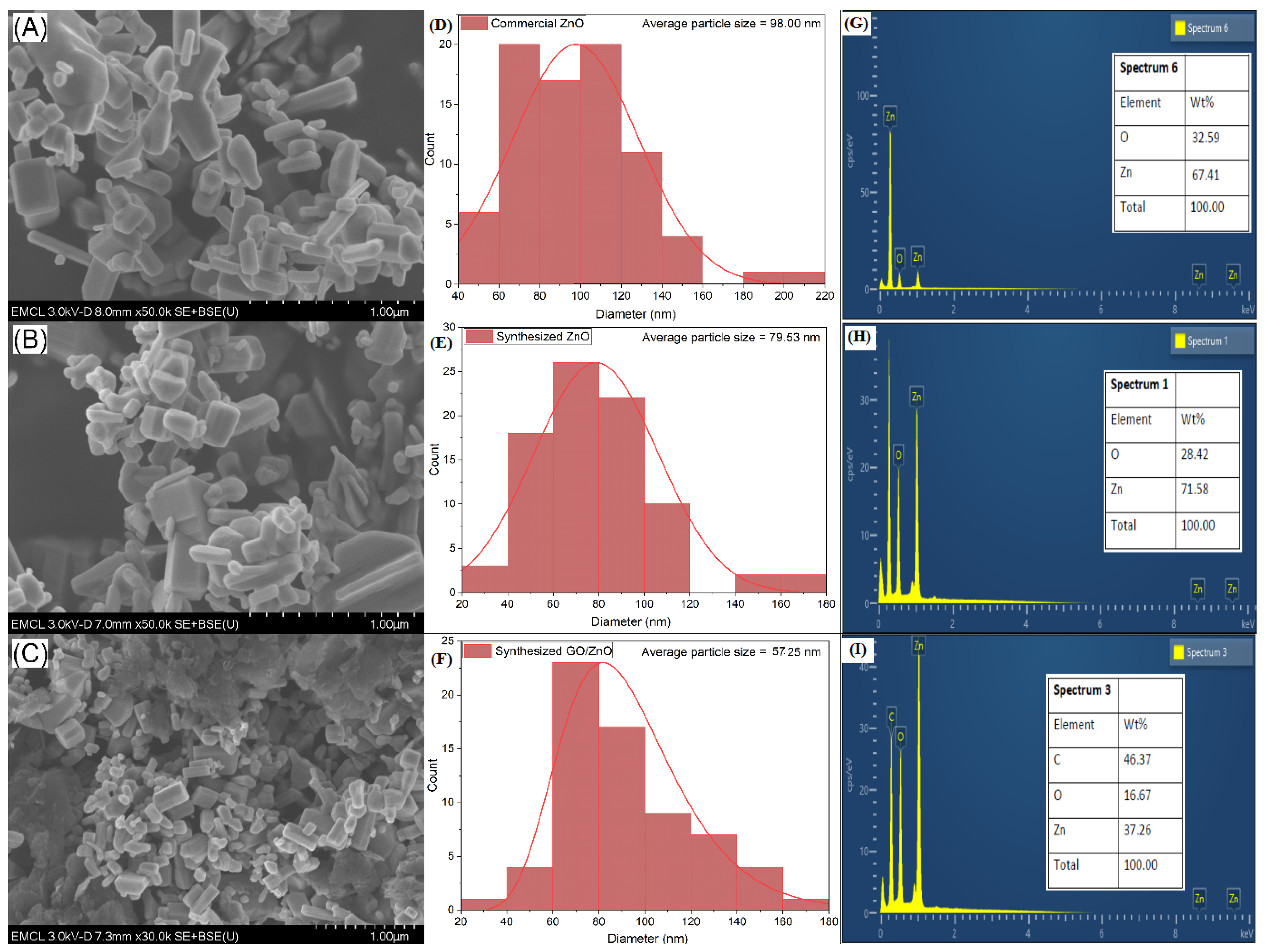
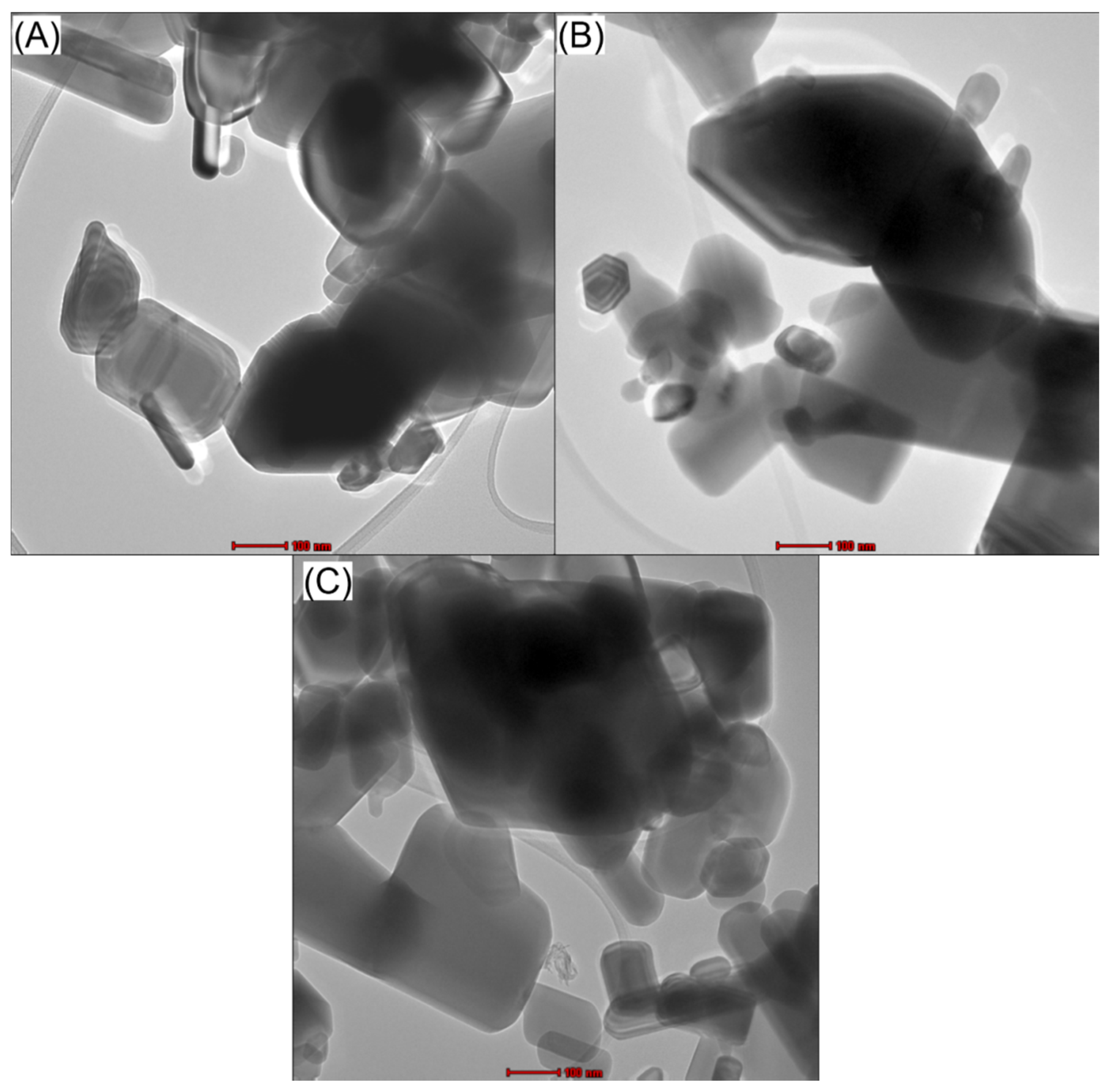
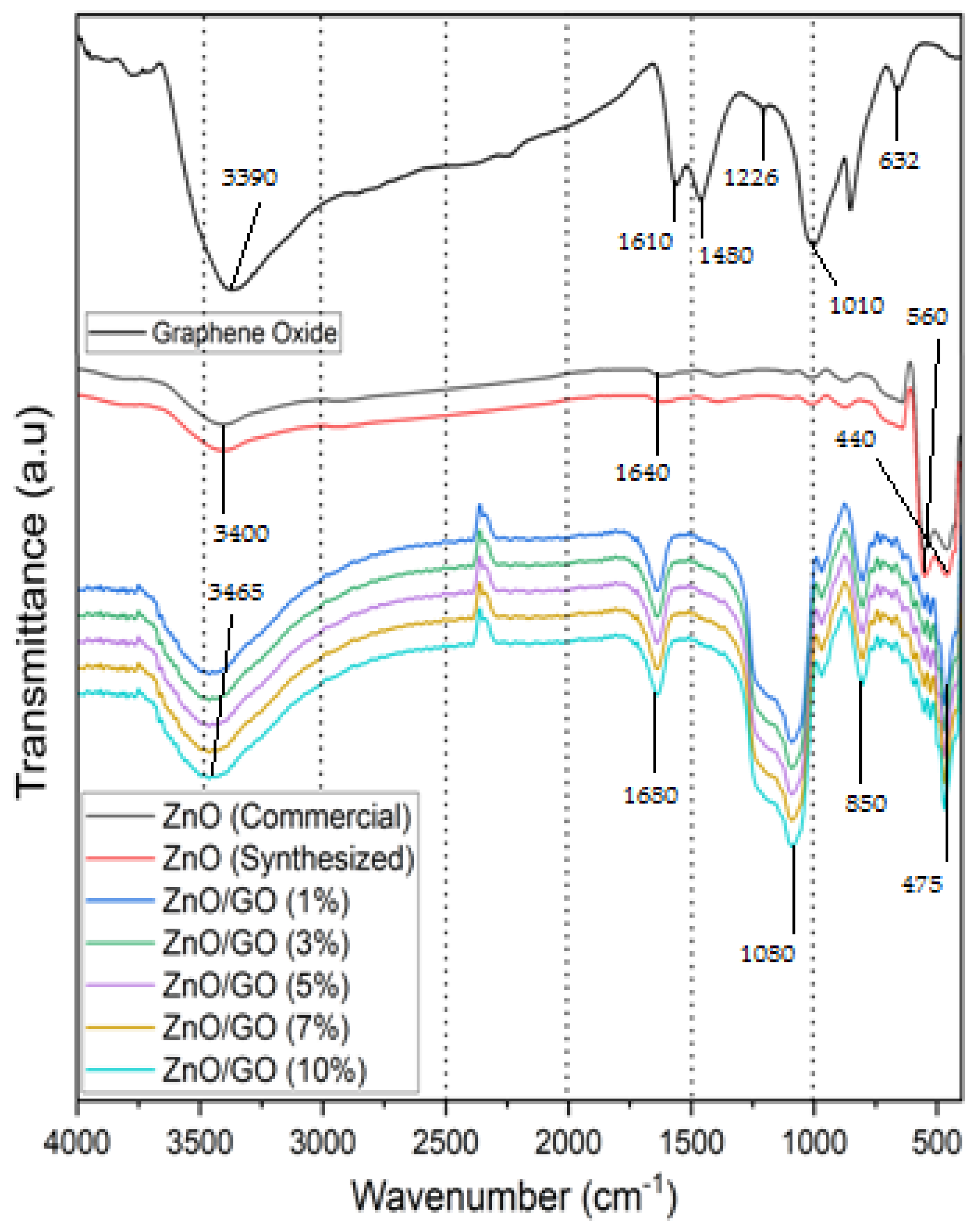
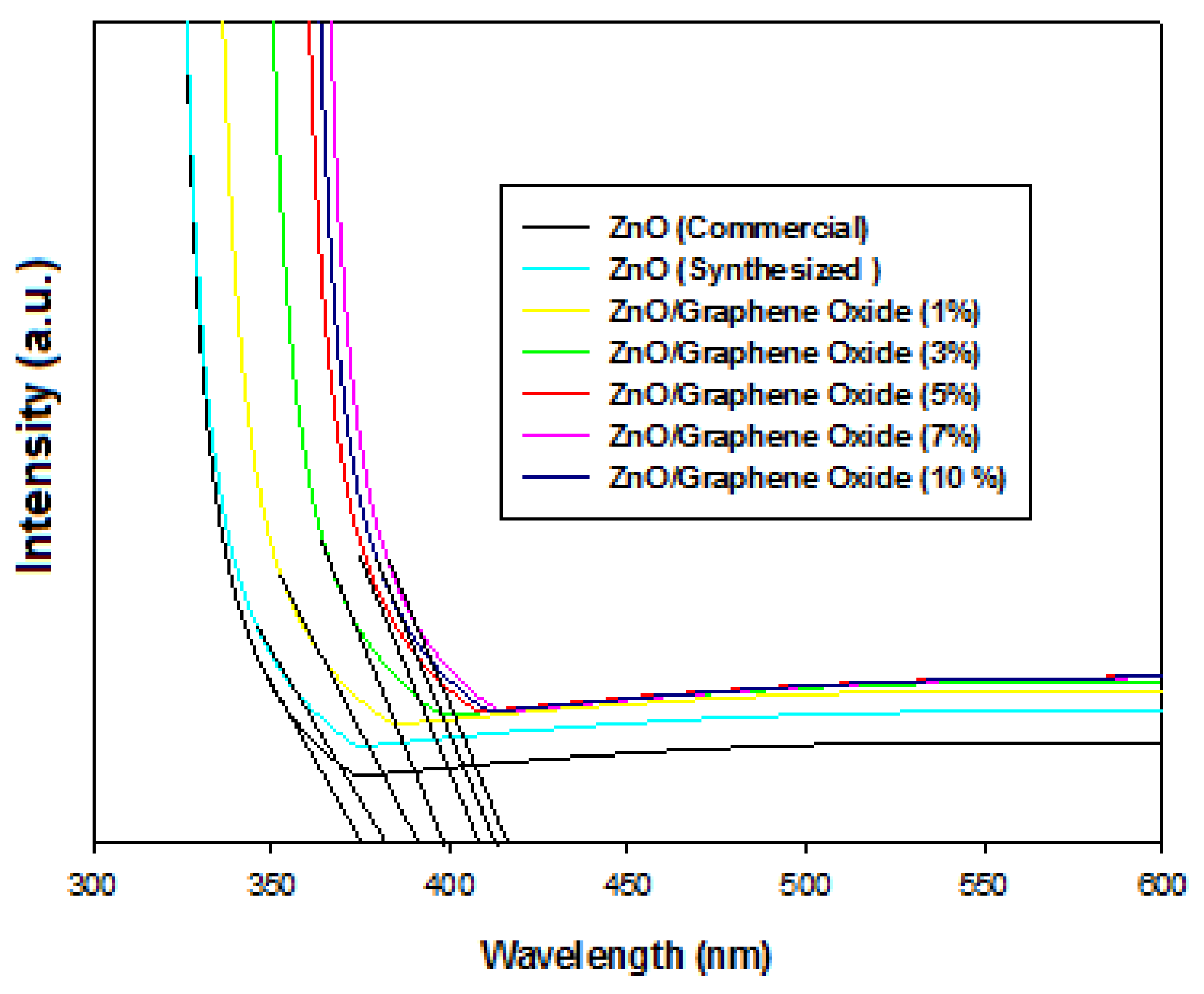
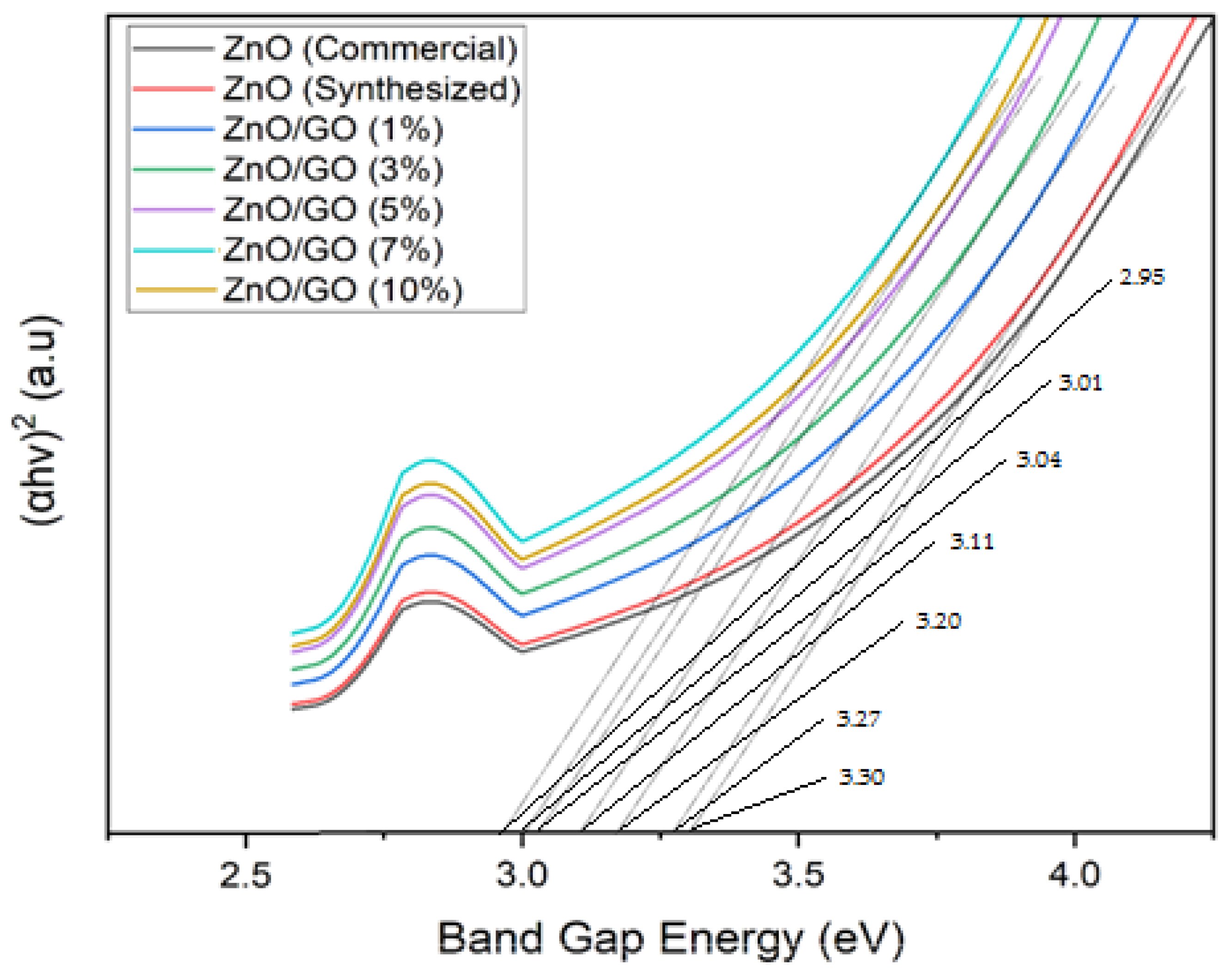
| No. | Photocatalyst Material | Bandgap Energy (eV) |
|---|---|---|
| 1 | ZnO (Commercial) | 3.30 |
| 2 | ZnO (Synthesized) | 3.27 |
| 3 | ZnO/Graphene Oxide (1%) | 3.20 |
| 4 | ZnO/Graphene Oxide (3%) | 3.11 |
| 5 | ZnO/Graphene Oxide (5%) | 3.04 |
| 6 | ZnO/Graphene Oxide (7%) | 2.95 |
| 7 | ZnO/Graphene Oxide (10%) | 3.01 |
| Photocatalysts | Rate Constant (k, min−1) | Regression Coefficient (R2) | Degradation Efficiency (%) | Bandgap Energy (eV) |
|---|---|---|---|---|
| ZnO (Commercial) | 0.0092 | 0.9809 | 59 | 3.30 |
| ZnO (Synthesized) | 0.0116 | 0.9841 | 65 | 3.27 |
| ZnO/GO (7%) | 0.0208 | 0.9906 | 87 | 2.95 |
| Photocatalyst | Pollutant | Pollutant Concentration | Removal (%) | Light Source | Dosage | Ref |
|---|---|---|---|---|---|---|
| ZnO/GO | Methyl red | 25 ppm | 98 | Xenon Lamp | 10 mg | [66] |
| ZnO/GO | Methyl orange | 50 ppm | 96 | Xenon lamp | 100 mg | [67] |
| ZnO/GO | Rhodamine-B | 25 ppm | 77 | Xenon Lamp | 50 mg | [68] |
| ZnO/GO | Crystal violet | 5 ppm | 99 | Mercury vapour lamp | 80 mg | [69] |
| ZnO/GO | Methylene blue | 3.13 × 10−5 M | 84 | Xenon Lamp | 50 mg | [70] |
| ZnO/GO | Methylene Blue | 0.03 mM | 87 | Halogen linear lamp | 100 mg | [Present study] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamjoum, H.A.A.; Umar, K.; Afridi, S.K.; Ahmad, H.; Parveen, T.; Haseen, U. Citrus aurantiifolia Peel-Facilitated Synthesis of Zinc Oxide, Interfaced with Biomass-Assisted Graphene Oxide for Enhanced Photocatalytic Degradation of Dye. Catalysts 2025, 15, 874. https://doi.org/10.3390/catal15090874
Jamjoum HAA, Umar K, Afridi SK, Ahmad H, Parveen T, Haseen U. Citrus aurantiifolia Peel-Facilitated Synthesis of Zinc Oxide, Interfaced with Biomass-Assisted Graphene Oxide for Enhanced Photocatalytic Degradation of Dye. Catalysts. 2025; 15(9):874. https://doi.org/10.3390/catal15090874
Chicago/Turabian StyleJamjoum, Hayfa Alajilani Abraheem, Khalid Umar, Saima Khan Afridi, Hilal Ahmad, Tabassum Parveen, and Uzma Haseen. 2025. "Citrus aurantiifolia Peel-Facilitated Synthesis of Zinc Oxide, Interfaced with Biomass-Assisted Graphene Oxide for Enhanced Photocatalytic Degradation of Dye" Catalysts 15, no. 9: 874. https://doi.org/10.3390/catal15090874
APA StyleJamjoum, H. A. A., Umar, K., Afridi, S. K., Ahmad, H., Parveen, T., & Haseen, U. (2025). Citrus aurantiifolia Peel-Facilitated Synthesis of Zinc Oxide, Interfaced with Biomass-Assisted Graphene Oxide for Enhanced Photocatalytic Degradation of Dye. Catalysts, 15(9), 874. https://doi.org/10.3390/catal15090874








